Endocrinology of the Menopause (original) (raw)
. Author manuscript; available in PMC: 2020 Jan 26.
Published in final edited form as: Endocrinol Metab Clin North Am. 2015 Sep;44(3):485–496. doi: 10.1016/j.ecl.2015.05.010
Abstract
In women, age-related changes in ovarian function begin in the mid-thirties with decreased fertility and compensatory hormonal changes in the hypothalamic-pituitary-gonadal axis that maintain follicle development and estrogen secretion in the face of a waning pool of ovarian follicles. The menopause transition is characterized by marked variability in follicle development, ovulation, bleeding patterns and symptoms of hyper- and hypoestrogenism. The menopause, which is clinically defined by the last menstrual period, is followed by the consistent absence of ovarian secretion of estradiol.
Keywords: ovary, hypothalamus, pituitary, menopause, estradiol, follicle stimulating hormone
Menopause is a signal event in a woman’s life that marks the end of reproductive competence. While the age-related loss of vaginal bleeding in women has been described throughout history, the studies of Block, published in 1952, documented the dramatic decrease in the number of follicles within the ovary as a function of age 1, demonstrating that the loss of both germ cells and the hormone-producing cells that support them is key to the the loss of menstrual function in women. Menopause is defined retrospectively by the final menstrual period and occurs at an average age of 51. However, the process of reproductive aging is gradual, begins much earlier than the final menstrual period, and can be conceptualized as encompassing: 1) an initial period in which compensatory changes in the hypothalamus, pituitary and ovary help to maintain both reproductive competence and gonadal hormone secretion; 2) a period characterized by marked variability in follicle development, ovarian secretion and consequent symptomatology leading up to the final menstrual period; and 3) stable and low ovarian hormone secretion. In the following chapter these three phases of reproductive aging will be discussed on the background of a discussion of normal reproductive cycle function and the changes that occur at all levels of the reproductive axis with aging.
Reproductive Function in Normal Women
Regular reproductive cycles are established within the first 1–3 years after the first menstrual period in normal women2. Although cycles between 25 and 35 days (from the first day of menses in one cycle to the first day of menses in the subsequent cycle) are considered normal3, cycle-to-cycle variability in an individual woman is considerably smaller (+/− 2 days).
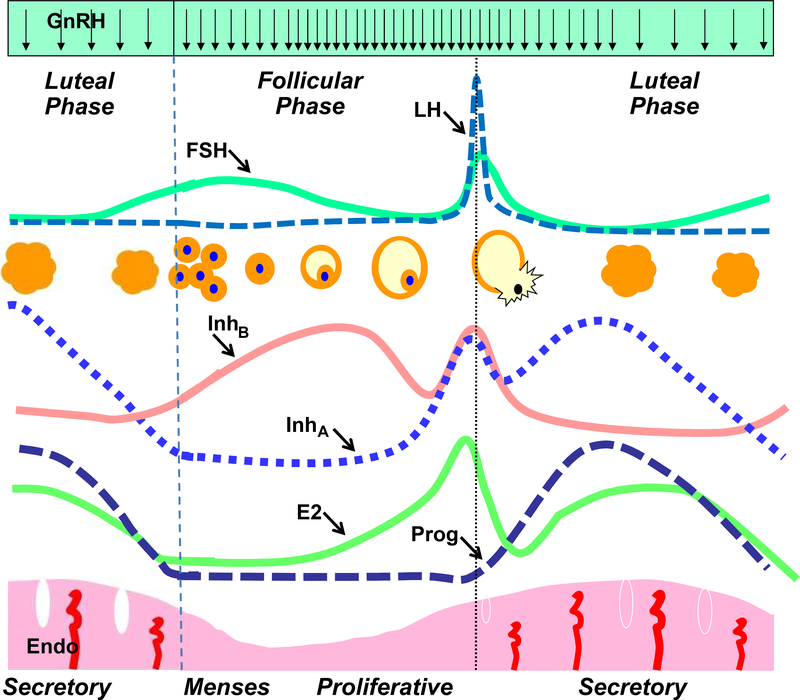
Normal menstrual cycle function requires tightly integrated interactions between the hypothalamus, pituitary and ovary while the endometrium serves as a gonadal steroid end organ and clinical marker of reproductive cycles (Figure 1). Estradiol secretion from developing follicles causes follicular phase proliferation of the endometrium. After ovulation, the combination of progesterone and estrodial produces the secretory changes that prepare the endometrium for implantation if conception occurs. In the absence of pregnancy, the function of the corpus luteum declines, hormonal support of the endometrium is lost and menses results (for review see Hall et al 4).
Figure 1 –
The hormonal, follicular, and endometrial (Endo) dynamics of the normal menstrual cycle from the late luteal phase through menses and the beginning of a new cycle of follicle development, ovulation, and corpus luteum function and decline. inhibin B (InhB) inhibin A (InhA) From Hall JE. Neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology, 7th Edition. Philadelphia, PA: Elsevier Publishing, pp 141–156; 2013, with permission.
At the beginning of each cycle, increasing levels of FSH are necessary for recruitment of a new cohort of follicles while restraint of FSH is required to ensure that only a single follicle reaches the preovulatory stage to ensure optimum pregnancy outcomes for women. Estradiol secretion from developing follicles contributes to the negative feedback control of FSH, acting at both the hypothalamus and, to a lesser degree, the pituitary. Ovarian secretion of inhibin B and inhibin A provide an additional level of control through selective inhibition of FSH at the pituitary. In the late follicular phase, rising levels of estradiol result in generation of the preovulatory LH surge that is necessary for ovulation. The LH surge requires ongoing pulsatile GnRH secretion, but is otherwise fully dependent on the marked augmentation of gonadotropin responses to GnRH that occur at the level of the pituitary in response to the exponential rise in estradiol secretion from the dominant follicle4.
Progesterone secretion from the corpus luteum is maintained by LH secretion and inhibits GnRH pulse frequency5, 6. In the early and mid- luteal phase, slow-frequency GnRH pulses are hypothesized to increase synthesis of FSH over that of LH, as has been shown in animal models,7 although secretion of FSH is inhibited by estradiol and probably inhibin A. In the late luteal phase, the loss of this negative feedback from the waning corpus luteum8 results in a preferential rise in FSH over LH. Increased hypothalamic GnRH secretion, made possible by the loss of progesterone and estradiol negative feedback, is also necessary during the luteal-follicular transition to achieve levels of FSH that are adequate for the next wave of follicular recruitment9.
Ovarian Aging in Women
In women, the finite pool of resting follicles in the ovary reaches its maximum in neonatal life. Thereafter, there is a steady decline due to atresia such that, at birth, only one million follicles remain with a further reduction to 250,000 by the time of puberty (for review see Gougeon et al10). During and after puberty, follicles will leave the pool of resting follicles by activation of further growth or by degeneration. Rising levels of FSH provide a critical stimulus for recruitment of resting follicles into the growing follicle pool while antimullerian hormone (AMH), produced in granulosa cells from small growing follicles, restrains this effect of FSH within the ovary11. Throughout early reproductive life the number of growing follicles is highly correlated with the size of the resting pool. However, between the ages of 30 and 35, the percentage of growing follicles increase12–14 and the trajectory of follicle loss is accelerated until the pool of resting follicles is reduced to between 100 and 1000 when there is cessation of reproductive cycles15. Age-related changes in oocyte quality parallel the decrease in follicle number with reported decrease in fertilization and conception rates and higher rates of pregnancy loss. Chemotherapy, radiation and smoking are all factors that accelerate follicle loss through damage to the oocyte and/or dividing granulosa cells16–19.
However, one of the most powerful predictors of age at menopause is family history, with twin studies estimating that a remarkable 44–85% of the variance in age at natural menopause is heritable20–22. At least 17 genes functioning in diverse pathways including hormonal regulation, DNA repair, and immune function have been associated with the age at natural menopause in genome wide association studies (GWAS)23–26. There is further evidence of gene-environment interactions27 raising the possibility that the wide range in age at menopause in normal women will be found to result from such interactions.
The Hypothalamus and Pituitary Changes with Repductive Aging
Response to the Loss of Ovarian Feedback
During normal reproductive life, the ovarian hormones and peptides restrain gonadotropin secretion through mechanisms that are operative at both the hypothalamus and pituitary. At the hypothalamus, progesterone has a profound inhibitory effect on LH, and therefore GnRH, pulse frequency. Estradiol does not appear to influence pulse frequency in women, but does decrease the overall quantity of GnRH secretion and thus the amount of GnRH secreted with each pulse. At the level of the pituitary, estradiol decreases the gonadotrope response to GnRH, with an effect that is greater for FSH than LH while inhibin B plays an additional pituitary role in the selective inhibition of FSH over LH.
The earliest hormonal evidence of ovarian aging is the selective increase in FSH that results from decreasing levels of inhibin B, a marker of granulosa cell number. With a further decrease in ovarian function and the loss of ovulatory cycles, GnRH pulses occur more frequently28 while declining levels of estradiol permit increases in both GnRH29 and in the gonadotropin responses to GnRH30. Autopsy studies confirm an increase in GnRH expression in the medial basal hypothalamus after menopause31 and suggest that this effect is mediated by an increase in the stimulatory neuropeptides, neurokinin B and kisspeptin, and a decrease in the inhibitory neuropeptide, dynorphin32. Finally, the half-life of LH and FSH disappearance is prolonged in postmenopausal women as a result of alterations in the isoform composition of these two glycoprotein hormones with the loss of estradiol33, 34. Taken together, declining levels of ovarian steroids and peptides with ovarian aging result in a 15-fold increase in FSH and a 10-fold increase in LH in postmenopausal women in the early years after their LMP35.
Potential Roles of the Hypothalamus and Pituitary in Menopause
In rodents, ovaries from old donors undergo folliculogenesis and ovulation when transplanted to young ovariectomized hosts36, providing evidence for a central contribution to reproductive failure with aging in rodents. Further studies have pointed to the importance of age-related alterations in estrogen positive feedback on GnRH secretion as at least one contributing mechanism37. An important question is whether similar central mechanisms contribute to reproductive failure in women.
Studies in younger and older postmenopausal women suggest that there are effects of aging on the hypothalamus and pituitary that are independent of the loss of steroid feedback. After menopause there is a 30–40% decrease in LH and FSH between the ages of 50 and 7535, 38. Underlying these gonadotropin changes are complex effects of aging on GnRH secretion with a 22% decrease in GnRH pulse frequency39 that is partially compensated by a 14% increase in the overall amount of GnRH secreted over that due to the loss of ovarian function alone29. There are also age-related effects at the pituitary with a 30% decrease in both LH and FSH responses to GnRH in older compared to younger postmenopausal women30.
Estrogen negative feedback at the hypothalamic level remains intact in older compared with younger postmenopausal women; low-dose estrogen administration is associated with a significant decline in circulating levels of LH, FSH and FAS and a parallel decrease in the overall amount of GnRH, with no effect on pulse frequency28. Addition of progesterone decreased pulse frequency in postmenopausal women with a concomitant decrease in overall amount of GnRH, neither of which effects differed with aging28, 29. The effect of estrogen negative feedback on the LH response to GnRH is not influenced by aging athough the FSH response to GnRH is attenuated with aging30.
Several studies have suggested that sensitivity to estrogen positive feedback may be lost with aging in women 40, 41 as it is in rodents42. An LH surge and increased progesterone were not evident in a small percentage of women who collected daily urine samples for variable periods of time during the menopause transition, despite similar peak late follicular phase estradiol levels43. In further studies, a controlled and graded steroid infusion paradigm that recreated early and late follicular phase levels of estradiol in younger and older postmenopausal women demonstrated attenuation of the LH surge with age in the face of identical preovulatory patterns of estradiol was attenuated with age44. There is now ample evidence for the overriding importance of increased pituitary responsiveness to GnRH in generation of the LH surge in women and thus, attenuation of the steroid induced-surge with aging is quite consistent with the decrease in pituitary responsiveness to GnRH with aging described earlier45.
Integration of Hormonal Changes with Aging in Women
Our clinical and mechanistic understanding of the process of ovarian aging has progressed dramatically since the classic studies of Block in the 1950’s demonstrated follicle loss across the reproductive lifespan in women1 and markers of ovarian aging have also changed. FSH levels are increased in older women even before their FMP46 and for many years the selective rise in FSH on day 3 of the menstrual cycle was the only clinically available marker of fertility potential. Although the discovery of inhibin B elucidated its critical roles in ovarian negative feedback on FSH and as a marker of ovarian aging47, 48, measurement of inhibin B was unable to serve as a better marker of fertility potential than FSH itself49. However, since that time anti-mullerian hormone (AMH) has been established as an unexpected marker of the number of ovarian follicles and AMH and antral follicle count have been used as prognostic markers in infertility programs (for review see Broekmans et al50). Our understanding of these new ovarian factors has not only contributed to fertility prognosis, but has also provided important insights into the integretated changes that occur with reproductive aging.
Maintenance of Follicle Development and Estrogen Secretion in Early Ovarian Aging
The existence of compensatory hormonal and intra-ovarian mechanisms that are operative in the early stages of ovarian aging is evidenced by continued follicle development and maintenance of estradiol levels well beyond the time at which other markers indicate declining ovarian function 51–54. The age-related decrease in the number of follicles in the ovary reviewed above is reflected in the number of antral follicles seen on ultrasound55, in declining levels of AMH12 which is expressed only in small growing follicles11, and in decreased levels of inhibin B which is constitutively secreted from granulosa cells in FSH-dependent growing follicles56.
Inhibin B plays an extremely important role as a gonadal feedback modulator of FSH secretion in early ovarian aging with declining levels of inhibin B associated with increasing FSH48, 57. Higher levels of FSH drive increased recruitment of follicles into the growing pool, the percentage of growing follicles increases13, 14 and, as indicated above, there is an increase in the rate of follicle loss15. It is likely that increased FSH is responsible both for the increased rate of spontaneous dizygotic twinning in older women58 and for the increased follicular phase estradiol levels in early reproductive aging even in the absence of development of more than one preovualtory follicle59. AMH restrains the stimulatory actions of FSH on recruitment of primordial follicles into the growing pool11 and on the FSH-dependent stimulation of aromatase60. Upregulation of aromatase in older cycling women is suggested by an increase in the ratio of serum levels of estrone to androstendione in the follicular phase in regularly cycling women ≥ 35 compared with their younger counterparts61 and increased aromatase expression in granulosa cells aspirated from the dominant follicle in older compared with younger normal women.
Taken together, these data support that hypothesis that lower levels of AMH in conjunction with elevated levels of FSH drive the accelerated depletion of the resting follicle pool after age 35, that these hormonal and autocrine/paracrine changes are important for maintaining estradiol levels in the face of ovarian function and serve to extend fertility and maintain reproductive cycles and estrogen levels early in reproductive aging.
Hormonal Variability of the Menopause Transition
With ongoing follicle loss, the compensatory mechanisms described above are no longer adequate and cycles become irregular, signifying the onset of the menopause transition. Large scale cohort studies describe the overall hormonal changes, characterized by the reciprocal relationship between decreasing inhibin B and increasing FSH levels early on and a later decline in estradiol that may not reach its nadir until one to two years after the FMP52, 62, 63.
However, these aggregate changes do not reflect the marked variability in hormone levels that occur between women and within an individual woman (Figure 2) from month to month over the two to five years of the menopause transition. Estrogen levels may fluctuate between undetectable and many times normal for variable periods of time with these anovulatory hormonal patterns interspersed with ovulatory cycles64. While the inconsistent estrogen response to FSH is not well understood, there is evidence of a mismatch between the progressively decreasing and scattered follicles in the ovary and their blood supply; Doppler studies indicate reduced ovarian blood supply in the aging ovary65, 66 and there are higher levels of vascular endothelial growth factor (VEGF), a marker of tissue hypoxia, in older reproductive aged women67.
Figure 2 –
Daily urine samples over 6 months in a perimenopausal woman indicate marked variability in the pattern of LH, FSH, estrone conjugates (E1C) and progesterone diglucuronide (PDG). The dashed line in the upper panel indicates the upper limit of normal for FSH in young women while the dotted line in the lower panel indicates the upper limit of normal E1G in young women. Shaded bars indicate cycles in which levels of PDG are consistent with ovulatory cycles. Data from Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996; 81(4): 1495–501.
FSH levels are dramatically increased when estrogen levels are low due to the loss of steroid and inhibin feedback at the pituitary, and also because of a marked decrease in gonadotropin clearance in the face of hypoestrogenism34. Age related slowing of GnRH pulse frequency would also favor synthesis and secretion of FSH over LH7, 39. Prior to the complete loss of ovarian follicles, consistently increased FSH levels will drive follicle development and estrogen synthesis and secretion to levels that may be many times higher than ever seen in normal cycles64.
In the year before the FMP, 60–70% of cycles are either anovulatory or have prolonged follicular phases68, 69 consistent with hormonal data indicating that a significant number of increases in estradiol are not followed by an LH surge and an increase in progesterone. Generation of a preovulatory surge requires a highly specific pattern of increasing estrogen levels over an adequate duration70, 71, both of which are likely to be altered in the face of asynchronous FSH and ovarian function43. Attenuation of the pituitary response to GnRH with aging45 may further contribute to the abnormal cycle dynamics or the perimenopause. Irregular bleeding patterns, varying breast tenderness, hot flashes, sleep disturbance and possible mood changes are a frequent accompaniment of the variability in hormonal patterns in the menopause transition.
Stability of Hormone Secretion after Menopause
In the first one to two years after the FMP, occasional follicle development is evident in individual women. Consistent with these observations, epidemiologic studies using sensitive estradiol assays54 demonstrate a further decrease from the FMP to the estradiol nadir two years later. Thereafter, estradiol levels remain low and stable. FSH levels also remain stable between two and eight years after the FMP54, but decline over time such that FSH decreases by approximately 30% by approximately age 75, as does LH72. While women are no longer bothered by irregular bleeding and breast tenderness, hot flashes may persist for up to 7 years after the FMP73 and with prolonged hypoestrogenism, genitourinary syndrome of menopause (GSM) may emerge as a new clinical symptom.
Clinical Assessment of Reproductive Aging and Menopause
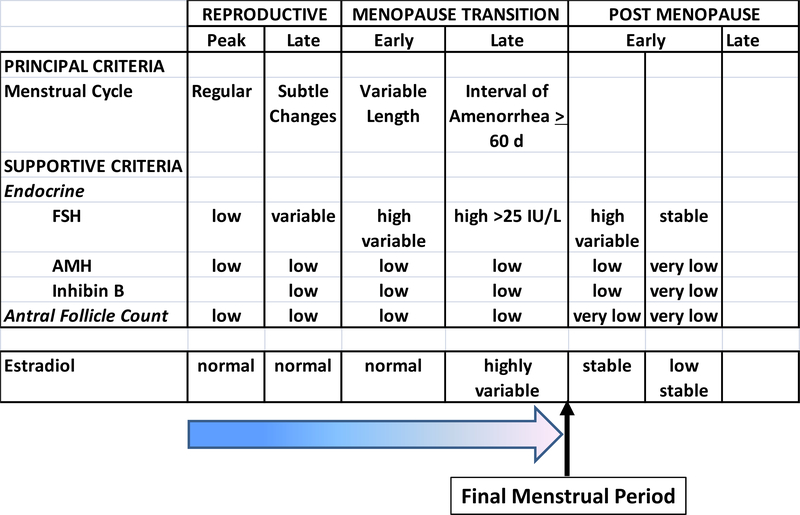
In 2001, the first Stages or Reproductive Aging Workshop (STRAW), utilized the findings from important cohort studies of women across the menopause transition, based primarily on changes in menstrual bleeding patterns and qualitative changes in FSH, and introduced standardized terminology referenced to the final menstrual period74. This staging system provided a critical scaffold for further studies and was updated a decade later (STRAW+10) to incorporate the results of longitudinal studies across the menopause transition and studies of fertility in older women75. FSH cut-off levels are now possible because of international standardization of this measure. AFC and AMH have been limited to qualitative descriptors as these measures have not been standardized across centers and are used primarily in infertility populations and particularly in women over 3576.
Addition of changes in estradiol to this schema permits us to understand the the clinical symptons of changes in menstrual cyclicity, vasomotor symptoms and GSM, which will be discussed in Chapter 2 in the context of evolving hormonal changes with progressive loss of ovarian function (Figure 3).
Figure 3 –
Qualitative changes in estradiol superimposed in relation to the STRAW+10 system for reproductive aging75 reflect the result of initial compensatory changes that preserve folliculogenesis and estrogen section and the progressive loss of compensation (graded arrow) with marked hormonal variability leading up to the final menstrual period, followed by low and stable estrogen secretion.
Conclusions
Reproductive aging in women is primarily due the progressive, and ultimately accelerating, loss of ovarian follicles. The associated decline in inhibin B secretion from the ovaries results in the loss of negative feedback on FSH. Within the ovary, low levels of AMH serve to facilitate FSH-stimulated follicle growth and estrogen synthesis and secretion. With further follicle loss, these compensatory hormonal mechanisms are no longer adequate, follicle development becomes unpredictable before further loss of ovarian function results in the stable but very low estradiol levels that characterize the postmenopause.
Key Points.
- Menopause is defined by the final menstrual period, but this diagnosis can only be made retrospectively after a year of amenorrhea.
- Reproductive aging in women is largely due to the loss of ovarian function, which is marked by decreased levels of inhibin B and anti-mullerian hormone (AMH).
- In the early stages of reproductive aging, there is an increase in FSH, as inhibin B levels decline, which serves to maintain folliculogenesis and estradiol secretion.
- With further loss of ovarian function, these compensatory changes are not always sufficient and result in marked variability in cycle dynamics and in estradiol and FSH levels.
- Estradiol levels stabilize at very low levels after the final menstrual period.
Footnotes
The author has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Block E Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat(Basel) 1952; 14(1–2): 108–23. [DOI] [PubMed] [Google Scholar]
- 2.Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab 2000; 85(3): 1021–5. [DOI] [PubMed] [Google Scholar]
- 3.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. IntJ Fertil 1967; 12(1 Pt 2): 77–126. [PubMed] [Google Scholar]
- 4.Hall JE. Neuroendocrine control of the menstrual cycle In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology, 7th Edition. Philadelphia, PA: Elsevier Publishing, pp 141–156; 2013. [Google Scholar]
- 5.Filicori M, Butler JP, Crowley WF. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest 1984; 73(6): 1638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 1984; 58(2): 378–83. [DOI] [PubMed] [Google Scholar]
- 7.Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology 1989; 125(2): 917–24. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Schoenfeld DA, Martin KA, Crowley WF Jr. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab 1992; 74(3): 600–7. [DOI] [PubMed] [Google Scholar]
- 9.Welt CK, Martin KA, Taylor AE, Lambert-Messerlian GM, Crowley WF Jr., Smith JA, et al. Frequency modulation of follicle-stimulating hormone (FSH) during the luteal-follicular transition: evidence for FSH control of inhibin B in normal women. J Clin Endocrinol Metab 1997; 82(8): 2645–52. [DOI] [PubMed] [Google Scholar]
- 10.Gougeon A Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996; 17(2): 121–55. [DOI] [PubMed] [Google Scholar]
- 11.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001; 142(11): 4891–9. [DOI] [PubMed] [Google Scholar]
- 12.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab 2013. April; 98(4): 1602–11. [DOI] [PubMed] [Google Scholar]
- 13.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. BiolReprod 1994; 50(3): 653–63. [DOI] [PubMed] [Google Scholar]
- 14.Gougeon A Ovarian follicular growth in humans: ovarian ageing and population of growing follicles Regulation of ovarian follicular development in primates: facts and hypotheses. Maturitas 1998; 30(2): 137–42. [DOI] [PubMed] [Google Scholar]
- 15.Gosden RG, Faddy MJ. Ovarian aging, follicular depletion, and steroidogenesis. ExpGerontol 1994; 29(3–4): 265–74. [DOI] [PubMed] [Google Scholar]
- 16.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011. August; 3(8): 782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattison DR, Shiromizu K, Nightingale MS. The mechanisms of action of reproductive toxins Oocyte destruction by polycyclic aromatic hydrocarbons Effects of toxic substances on female reproduction. Am J Ind Med 1983. February; 4(1–2): 65–79. [PubMed] [Google Scholar]
- 18.Cramer DW, Xu H. Predicting age at menopause. Maturitas 1996. April; 23(3): 319–26. [DOI] [PubMed] [Google Scholar]
- 19.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001; 153(9): 865–74. [DOI] [PubMed] [Google Scholar]
- 20.de Bruin JP, Nikkels PG, Bruinse HW, van Haaften M, Looman CW, te Velde ER. Morphometry of human ovaries in normal and growth-restricted fetuses. Early Hum Dev 2001. January; 60(3): 179–92. [DOI] [PubMed] [Google Scholar]
- 21.Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet 1998. October 3; 352(9134): 1084–5. [DOI] [PubMed] [Google Scholar]
- 22.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 1998. June; 83(6): 1875–80. [DOI] [PubMed] [Google Scholar]
- 23.He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 2009. June; 41(6): 724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic markers of ovarian follicle number and menopause in women of multiple ethnicities. Hum Genet Nov; 131(11): 1709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 2012. March; 44(3): 260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titus SA, Southall N, Marugan J, Austin CP, Zheng W. High-Throughput Multiplexed Quantitation of Protein Aggregation and Cytotoxicity in a Huntington’s Disease Model. Curr Chem Genomics 2012; 6: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butts SF, Sammel MD, Greer C, Rebbeck TR, Boorman DW, Freeman EW. Cigarettes, genetic background, and menopausal timing: the presence of single nucleotide polymorphisms in cytochrome P450 genes is associated with increased risk of natural menopause in European-American smokers. Menopause 2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab 2002; 87(5): 2297–302. [DOI] [PubMed] [Google Scholar]
- 29.Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 2002; 87(5): 2290–6. [DOI] [PubMed] [Google Scholar]
- 30.Shaw ND, Histed SN, Srouji SS, Yang J, Lee H, Hall JE. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J Clin Endocrinol Metab 2010; 95(4): 1955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab 1996; 81(10): 3540–6. [DOI] [PubMed] [Google Scholar]
- 32.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 2010; 1364: 116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpless JL, Supko JG, Martin KA, Hall JE. Disappearance of endogenous luteinizing hormone is prolonged in postmenopausal women. J Clin Endocrinol Metab 1999; 84(2): 688–94. [DOI] [PubMed] [Google Scholar]
- 34.Wide L, Eriksson K, Sluss PM, Hall JE. Serum half-life of pituitary gonadotropins is decreased by sulfonation and increased by sialylation in women. J Clin Endocrinol Metab 2009; 94(3): 958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall JE. Neuroendocrine physiology of the early and late menopause. EndocrinolMetab ClinNorth Am 2004; 33(4): 637–59. [DOI] [PubMed] [Google Scholar]
- 36.Krohn PL. Review lectures on senescence. II. Heterochronic transplantation in the study of ageing. Proc R Soc Lond B Biol Sci 1962. December 18; 157: 128–47. [DOI] [PubMed] [Google Scholar]
- 37.Wise PM, Smith MJ, Dubal DB, Wilson ME, Krajnak KM, Rosewell KL. Neuroendocrine influences and repercussions of the menopause. EndocrRev 1999; 20(3): 243–8. [DOI] [PubMed] [Google Scholar]
- 38.Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol 1998; 178(4): 732–41. [DOI] [PubMed] [Google Scholar]
- 39.Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab 2000; 85(5): 1794–800. [DOI] [PubMed] [Google Scholar]
- 40.van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol (Oxf) 1977; 7(1): 13–31. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto VY, Klein NA, Battaglia DE, Bremner WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive-age women. Fertil Steril 1996; 65(3): 539–44. [PubMed] [Google Scholar]
- 42.Wise PM, Kashon ML, Krajnak KM, Rosewell KL, Cai A, Scarbrough K, et al. Aging of the female reproductive system: a window into brain aging. Recent ProgHormRes 1997; 52: 279–303. [PubMed] [Google Scholar]
- 43.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 2004; 292(24): 2991–6. [DOI] [PubMed] [Google Scholar]
- 44.Shaw ND, Srouji SS, Histed SN, Hall JE. Differential effects of aging on estrogen negative and positive feedback. AmJ Physiol Endocrinol Metab 2011; 301(2): E351–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin releasing hormone. J Clin Endocrinol Metab 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab 1976; 42(4): 629–36. [DOI] [PubMed] [Google Scholar]
- 47.Muttukrishna S, Sharma S, Barlow DH, Ledger W, Groome N, Sathanandan M. Serum inhibins, estradiol, progesterone and FSH in surgical menopause: a demonstration of ovarian pituitary feedback loop in women. Hum Reprod 2002; 17(10): 2535–9. [DOI] [PubMed] [Google Scholar]
- 48.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 1999; 84(1): 105–11. [DOI] [PubMed] [Google Scholar]
- 49.Hall JE, Welt CK, Cramer DW. Inhibin A and inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Hum Reprod 1999; 14(2): 409–15. [DOI] [PubMed] [Google Scholar]
- 50.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009. August; 30(5): 465–93. [DOI] [PubMed] [Google Scholar]
- 51.Burger HG. The endocrinology of the menopause. Maturitas 1996; 23(2): 129–36. [DOI] [PubMed] [Google Scholar]
- 52.Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF Jr. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab 2008; 93(10): 3847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 2008; 93(9): 3478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Randolph JF Jr., Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 2011. March; 96(3): 746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La Marca A, Spada E, Sighinolfi G, Argento C, Tirelli A, Giulini S, et al. Age-specific nomogram for the decline in antral follicle count throughout the reproductive period. Fertil Steril 2011. February; 95(2): 684–8. [DOI] [PubMed] [Google Scholar]
- 56.Welt CK, Schneyer AL. Differential regulation of inhibin B and inhibin a by follicle-stimulating hormone and local growth factors in human granulosa cells from small antral follicles. J Clin Endocrinol Metab 2001; 86(1): 330–6. [DOI] [PubMed] [Google Scholar]
- 57.Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril 1999; 71(4): 658–62. [DOI] [PubMed] [Google Scholar]
- 58.Beemsterboer SN, Homburg R, Gorter NA, Schats R, Hompes PG, Lambalk CB. The paradox of declining fertility but increasing twinning rates with advancing maternal age. Hum Reprod 2006. June; 21(6): 1531–2. [DOI] [PubMed] [Google Scholar]
- 59.Hansen KR, Thyer AC, Sluss PM, Bremner WJ, Soules MR, Klein NA. Reproductive ageing and ovarian function: is the early follicular phase FSH rise necessary to maintain adequate secretory function in older ovulatory women? HumReprod 2005; 20(1): 89–95. [DOI] [PubMed] [Google Scholar]
- 60.di Clemente N, Goxe B, Remy JJ, Cate RL, Josso N, Vigier B, et al. Inhibitory effect of AMH upon aromatase activity and LH receptors of granulosa cells of rat and porcine immature ovaries. Endocirne 1994; 2: 553–8. [Google Scholar]
- 61.Welt CK, Jimenez Y, Sluss PM, Smith PC, Hall JE. Control of estradiol secretion in reproductive ageing. HumReprod 2006; 21(8): 2189–93. [DOI] [PubMed] [Google Scholar]
- 62.Randolph JF Jr., Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 2003; 88(4): 1516–22. [DOI] [PubMed] [Google Scholar]
- 63.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update 2007. Nov-Dec; 13(6): 559–65. [DOI] [PubMed] [Google Scholar]
- 64.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996; 81(4): 1495–501. [DOI] [PubMed] [Google Scholar]
- 65.Pan HA, Cheng YC, Li CH, Wu MH, Chang FM. Ovarian stroma flow intensity decreases by age: a three-dimensional power doppler ultrasonographic study. Ultrasound Med Biol 2002. April; 28(4): 425–30. [DOI] [PubMed] [Google Scholar]
- 66.Ng EH, Chan CC, Yeung WS, Ho PC. Effect of age on ovarian stromal flow measured by three-dimensional ultrasound with power Doppler in Chinese women with proven fertility. Hum Reprod 2004; 19(9): 2132–7. Epub 004 Jul 8. [DOI] [PubMed] [Google Scholar]
- 67.Klein NA, Battaglia DE, Woodruff TK, Padmanabhan V, Giudice LC, Bremner WJ, et al. Ovarian follicular concentrations of activin, follistatin, inhibin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-2 (IGFBP-2), IGFBP-3, and vascular endothelial growth factor in spontaneous menstrual cycles of normal women of advanced reproductive age. J Clin Endocrinol Metab 2000; 85(12): 4520–5. [DOI] [PubMed] [Google Scholar]
- 68.Van Voorhis BJ, Santoro N, Harlow S, Crawford SL, Randolph J. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol 2008. July; 112(1): 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, et al. Progesterone and ovulation across stages of the transition to menopause. Menopause 2009. Nov-Dec; 16(6): 1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keye WR Jr., Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. I. Effects of varying duration of estradiol administration. J Clin Endocrinol Metab 1975; 41(06): 1003–8. [DOI] [PubMed] [Google Scholar]
- 71.Young JR, Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J Clin Endocrinol Metab 1976; 42(3): 432–42. [DOI] [PubMed] [Google Scholar]
- 72.Hall JE. Neuroendocrine changes with reproductive aging in women. SeminReprodMed 2007; 25(5): 344–51. [DOI] [PubMed] [Google Scholar]
- 73.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015. April 1; 175(4): 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001; 76(5): 874–8. [DOI] [PubMed] [Google Scholar]
- 75.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril 2012. April; 97(4): 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 2012. May; 97(5): 1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]