Current treatment paradigms and emerging therapies for NAFLD/NASH (original) (raw)
. Author manuscript; available in PMC: 2021 Jan 1.
Published in final edited form as: Front Biosci (Landmark Ed). 2021 Jan 1;26:206–237. doi: 10.2741/4892
Abstract
Non-alcoholic fatty liver disease (NAFLD) is one the fastest emerging manifestations of the metabolic syndrome worldwide. Non-alcoholic steatohepatitis (NASH), the progressive form of NAFLD, may culminate into cirrhosis and hepatocellular cancer (HCC) and is presently a leading cause of liver transplant. Although a steady progress is seen in understanding of the disease epidemiology, pathogenesis and identifying therapeutic targets, the slowest advancement is seen in the therapeutic field. Currently, there is no FDA approved therapy for this disease and appropriate therapeutic targets are urgently warranted. In this review we discuss the role of lifestyle intervention, pharmacological agents, surgical approaches, and gut microbiome, with regard to therapy for NASH. In particular, we focus the role of insulin sensitizers, thyroid hormone mimetics, antioxidants, cholesterol lowering drugs, incretins and cytokines as therapeutic targets for NASH. We highlight these targets aiming to optimize the future for NASH therapy.
Keywords: NAFLD, NASH, Therapy, Insulin Sensitizers, Antioxidants, Statins, Incretins, Cytokines, Bariatric Surgery, Gut Microbiome, Review
2. Introduction
Parallel to the global increase in metabolic syndrome, diabetes and obesity, a sharp rise is seen in the prevalence of NAFLD. Non-alcoholic steatohepatitis (NASH) is the clinically serious form of non-alcoholic fatty liver disease (NAFLD), characterized by excessive accumulation of triglycerides (steatosis), inflammation, injury and apoptosis in the liver cells which in extreme cases may lead to cirrhosis and liver cancer (1). NAFLD is currently recognized as the most chronic liver disease, and globally affects around 25% of the adult population (2). It is one the most prominent cause of liver related morbidity and mortality (3).
Owing to its high prevalence and potential risk of progression to cirrhosis and hepatocellular carcinoma (HCC) (4), NASH has become a major health concern worldwide. While it’s adverse hepatic consequences include cirrhosis culminating into liver failure and HCC, it has non-liver associated outcomes such as cardiovascular disease (5), (6). The number of NAFLD cases in the United States is expected to increase from 83.1 million in 2015 to 100.9 million in 2030, a large proportion of which will be NASH (7). This rise will lead to an increase in the number of patients with cirrhosis and end stage liver disease, necessitating liver transplantation (8), (9) and leading to a steep rise in HCC (10).
Despite this high prevalence and growing impact on world health, there are currently no approved treatments for NASH (11). Among all aspects of NASH, the slowest advancement has been seen in the therapeutic field, although a steady progress is seen in understanding disease epidemiology, pathophysiology and identifying therapeutic targets. Even after years of intense research worldwide, presently there is no approved drug for its treatment. Additionally, liver biopsy, a costly and invasive procedure, is still the gold standard for histological evaluation, warranting effective non-invasive imaging and biochemical tests to prevent the need for liver biopsy. This review highlights the treatment strategies for NAFLD/NASH in view of the ongoing rapid progress and unmet challenges, focusing on the critical assessment and understanding of the key elements with regard to therapy for NASH.
3. Lifestyle Intervention
Given the fact that presently no drug or surgery is approved for the treatment of NASH, lifestyle modifications (diet, physical activity, and exercise) remain the cornerstone approach for its management. These approaches mainly aim at controlling bodyweight and metabolic disorders (12), (13), (14).
3.1. Physical activity and exercise
Physical activity is recommended for people with NAFLD, as it is a key determinant of metabolic control. People inclined to excessive adiposity, metabolic syndrome and type 2 diabetes (T2D) show higher sedentary behaviour (15). An increase in sedentary time may lead to a predisposition towards NAFLD. Interestingly, the increased breaks to sedentary time are reported to be beneficial for glucose and fatty acid metabolism, and obesity control (16). Several cross-sectional studies have reported lower levels of physical activity in people with NAFLD (17) (18) (19), and that they are more prone to fatigue (20). Individuals with a healthy lifestyle are less predisposed to develop insulin resistance, diabetes and impaired glucose tolerance (21), (22).
Exercise reduces the possibility of developing NASH (23). It lowers the risk of diabetes, hypertension and metabolic syndrome (24), (25). A meta-analysis reports that exercise reduces hepatic fat content, with little or no weight loss (26). It reduces visceral adipose tissue and plasma free fatty acids. The effect of exercise on histological lesions in NASH is not clearly known, however, preliminary data suggest that exercise has an effect on hepatic markers of apoptosis (27). The effect of physical activity shows a dose-effect relationship (24). Vigorous activity is much more beneficial for NASH and fibrosis, as compared to moderate activity (23). The Compendium of Physical Activities defines specific physical activity (PA) based on the rate of energy expenditure. It defines the intensity of physical activity as the ratio of work metabolic rate to a standard resting metabolic rate (MET). One MET (4.184 kJ)·kg−1·h−1, is defined as the rate of energy expenditure while a person is at rest or the resting metabolic rate during quiet sitting. Activities having MET values between 3 and 5.9 are classified as moderate activity while those with MET values ≥6 are defined as vigorous activities (23).
3.2. Caloric restriction
Excess caloric consumption causes obesity, which is a leading risk factor for NAFLD (2). Caloric restriction (CR) exerts metabolic reprogramming and effective utilization of body fuel, lowering the oxidative damage to cells (28). Carbohydrates (CHO) are the main source of energy in the body. CHO intake is linked to NAFLD (29), and CHO restriction in the diet reduces the glycemic load, and improves insulin resistance and pancreatic β- cell insulin secretion (30). Low CHO diets increase high density lipoprotein (HDL) and reduce serum triglycerides, and glucose (31). In a post hoc analysis of 52 obese and insulin resistant patients, a hypocaloric low-CHO/high-fat diet (40% and 45% total calories per day, respectively) significantly reduced alanine transaminase (ALT) and serum insulin as compared to a hypercaloric high-CHO/low fat diet (60% and 25%, respectively) (32).
The intake of fructose derived from sugars (table sugar) and corn syrup, commonly used in sweetened beverages, has increased (33). Fructose metabolism enhances free radical production, gut permeability, bacterial growth, lipogenesis, and the level of serum lipopolysaccharides (29), (34). A study reported a significant rise in cholesterol (11.4%), serum triglycerides (32.7%), liver fat (150%), visceral adipose fat (25%), and muscle fat (200%) in 47 overweight patients subjected to a daily intake of 1 litre of sugar-sweetened soft drinks up to 6 months (35). High fructose consumption increases the risk of fibrosis in NASH patients (36). A diet rich in omega-3 polyunsaturated fatty acids (PUFA) reduces intrahepatic triglycerides, increases insulin sensitivity (37), and improves steatohepatitis (38), (39). Therefore, CR is recommended for improvement of NAFLD and the overall calorie intake is recommended to be 1,200-1,500 kcal/day for women and 1,500-1,800 kcal/day for men (40).
3.3. Time restricted feeding
Time-restricted feeding (TRF) constitutes a circadian synchronised dietary approach wherein food access is restricted within a particular time window (8-16 hours) (41). A recent study on NAFLD patients demonstrated that TRF helps to significantly achieve weight loss and reduce triglyceride levels after 12 weeks as compared to the control group (42). Furthermore, TRF regimen mice show reduction in severity of hyperinsulinemia, hepatic steatosis and inflammation, despite the consumption of similar quantities of high-fat diet (HFD) as compared to the ad libitum group (43). Interestingly, TRF also mitigates the effect of maternal HFD feeding on fetal hepatic steatosis (44). However, results from clinical trials to test the efficacy of TRF in human NAFLD/NASH are still awaited.
Pharmacological Therapy
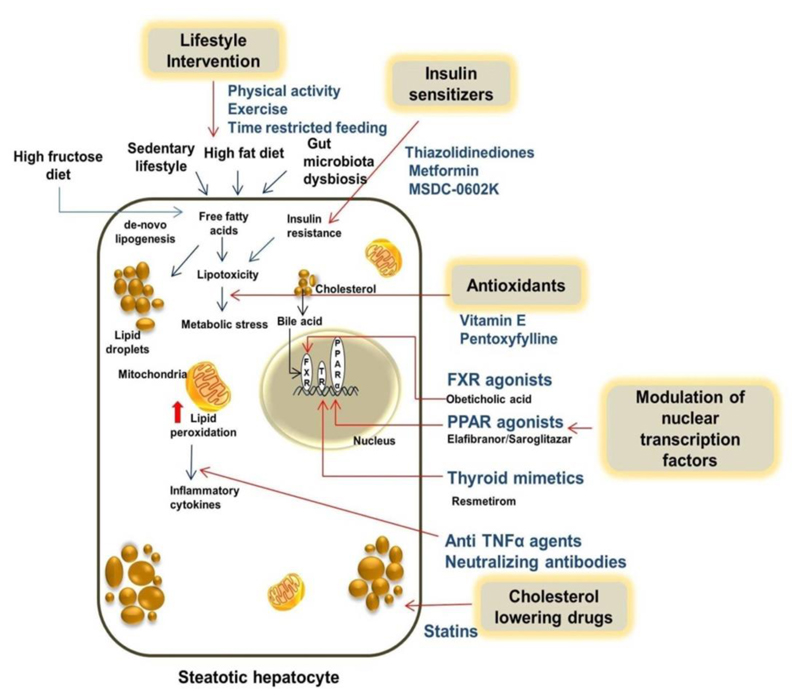
Diet and lifestyle intervention measures cannot be successfully or sustainably implemented in most patients. The patients who fail to benefit from lifestyle intervention or those with already advanced disease (significant fibrosis), need pharmacological treatments which are specifically aimed at improving hepatic inflammation, fibrosis and steatohepatitis (45), as illustrated in Figure 1. Steatohepatitis increases liver-related mortality and reduces survival by progression into cirrhosis, liver failure and HCC, therefore, pharmacological therapy is restricted to patients with NASH (46), (45), (47). Several randomized controlled trials (RCTs) have tested pharmacological agents, which show histological improvements in NASH. An effective therapy for NASH is still in infancy; well conducted RCTs aimed at relevant therapeutic targets are needed.
Figure 1.
Mechanism of action of lifestyle modification and pharmacological therapy in NAFLD and NASH. NAFLD: nonalcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; TNF: tumor necrosis factor; FXR: farnesoid X receptor; PPAR: peroxisome proliferator-activated receptor.
4.1. Insulin sensitizers
Insulin resistance is a major driving force for excessive fat accumulation in the liver and plays a key role in the initiation and progression of steatohepatitis and fibrosis. Several pharmacological agents listed below are focused to improve insulin resistance.
4.1.1. Thiazolidinediones
Thiazolidinediones (TZD), also known as glitazones, are the strongest evidence-based drugs tested for the treatment of NASH (47). Current guidelines recommend the use of glitazones in NASH (45), (46). Glitazones promote the differentiation of insulin-resistant large pre-adipocytes into small, proliferative, insulin-sensitive adipocytes (49) (50) (51). Upon the induction of lipogenic genes (52) and lipoprotein lipase (LPL), they elevate fatty acid synthesis and uptake in adipose tissue (53), thereby diverting the free fatty acid (FFA) burden towards adipocytes, instead of the liver. Despite the expansion in fat mass, insulin sensitivity is subsequently improved. Alternatively, glitazones upregulate the production of the insulin-sensitizing and anti-steatogenic adipokine, adiponectin (54), which enhances fatty acid beta-oxidation in liver and muscles (55). Thus, they act by redistributing fat from ectopic tissues to the adipose tissue, and by increasing levels of adiponectin. They also contribute to the reduction of insulin resistance by increasing the expression of AMP-activated protein kinase (56), (57) and upregulating glucose transporter type -4 (GLUT-4) in muscle and adipose tissue.
Some studies based on rodents also report anti-inflammatory actions of glitazones, showing decreased hepatic fibrogenesis in response to recurrent liver injury (58), (59). Interestingly, these studies show a reduction in the nuclear expression of peroxisome proliferator-activated receptor (PPAR)-γ, upon activation and trans-differentiation of stellate cells into fibrogenic myofibroblasts (58). PPAR-γ expression was restored upon treatment with PPAR-γ agonists and hepatic scar-forming response was elevated (60). This suggests a hepatoprotective effect of glitazones in addition to their insulin-sensitizing property. Rosiglitazone and pioglitazone are the two available glitazones, which exhibit similar cellular action, except lipoprotein metabolism which favours pioglitazone (61). The widespread use of glitazones is restricted by their safety concerns and adverse effects including congestive heart failure (62), bone fractures in women (63), and increased risk of bladder cancer for pioglitazone (64), owing to which, it was withdrawn from the market in France and Germany. As of now, the European Medicines Agency and the FDA continue the licence pioglitazone, except in patients with, or at the risk of developing bladder cancer. Pioglitazone is recommended for treatment of steatohepatitis in biopsy-proven NASH patients. However, the long term benefits and safety issues remain unknown.
4.1.2. Metformin
Another insulin-sensitizing agent is metformin, an oral biguanide, which reduces hepatic glucose production and increases peripheral glucose utilization (65). Metformin is approved for use in patients with type T2D (66). It causes a reduction in endogenous glucose production, activates AMP-activated protein kinase (67) and inhibits the mitochondrial glycerophosphate dehydrogenase shuttle with change in redox state (46). Metformin is considered as a safe drug, however, it is not recommended for the treatment of NASH (69) as there is no proof of its efficacy on hepatic histology.
4.1.3. MSDC-0602K
Pioglitazone, the first-generation insulin sensitizer improved NASH but proved to be of little use owing to its side effects (68). Another second-generation insulin sensitizer, MSDC-0602K, an inhibitor of mitochondrial pyruvate carrier (MPC) with PPAR-γ binding, is reported to enhance lipid oxidation and downregulate de novo lipid synthesis and gluconeogenesis in liver, in vivo and in vitro. MSDC-0602K does not exhibit the side effects associated with the first generation insulin sensitizers (68). MSDC-0602K leads to hepatic histological improvement and reduction in ballooning or lobular inflammation with no increase in fibrosis at 12 months, as reported in the phase 2b, 52-week double-blind study (68).
4.2. Antioxidants
Increased oxidative stress and defected antioxidant defence mechanisms have been studied extensively across the progressive stages of NAFLD, and are believed to contribute to liver injury and development of NASH. Some of the agents with antioxidant activity being used as a therapy for NASH are listed below.
4.2.1. Vitamin E
Vitamin E is a fat soluble compound present in a variety of compounds which belong to two main families, tocopherol and tocotrienol. Vitamin E is present in the phospholipid bilayer of the cell membranes, where it helps to protect against oxidative damage caused by free radicals (55). It protects against mitochondrial toxicity and blocks intrinsic apoptotic pathways, thereby protecting against liver injury (69), (70). It is also reported to have non-antioxidant properties and may alter cell signaling, gene expression (71) or down-regulate nuclear factor kappa B (NF-kB) dependent inflammatory pathways (72).
The largest RCT until now, the PIVENS trial (73) where vitamin E (800 IU/day) was tested along with pioglitazone and placebo in non-diabetic and non-cirrhotic patients with NASH for 2 years, showed significant histological improvement in patients. Vitamin E improved steatosis, inflammation and ballooning, and resolution of NASH was induced in 36% of the patients receiving vitamin E. The safety concerns related to vitamin E include overall mortality (74), haemorrhagic stroke (75), and prostate cancer in males older than 50 years (76). However, the long term safety of vitamin E remains a concern.
4.2.2. Pentoxifylline
Pentoxifylline is a non-selective phosphodiesterase inhibitor used for the treatment of peripheral vascular disease. Animal studies report that pentoxifylline exhibits antioxidant effect (77) and inhibits the production of pro-inflammatory cytokines (78). These effects were confirmed in vivo, where pentoxifylline significantly decreased steatohepatitis in methionine-choline-deficient dietary NASH model (79). In a 1 year long RCT in 55 patients with NASH receiving pentoxifylline or placebo, pentoxifylline improved liver enzyme levels and histological features of NASH, and displayed a marginal effect on fibrosis (80). Good quality human data regarding the use of pentoxifylline in NASH are lacking.
4.3. Lipotoxicity based targets
The progression from steatosis to NASH may be determined by the accumulation of lipotoxic intermediates of triglyceride synthesis and/or lipolysis (81), (82). De novo lipogenesis (DNL) refers to the biochemical process of synthesis of fatty acids from acetyl-CoA subunits commonly produced by carbohydrate catabolism, which occurs primarily in the liver. Fructose is a profound lipogenic substrate which drives DNL, in addition to glucose. Increased hepatic DNL contributes significantly to NASH. The rate-limiting step in DNL is catalysed by acetyl-CoA carboxylase (ACC), which occurs in two major isoforms in mammals, ACC1 and ACC2. The pharmacological inhibition of ACC is another attractive approach to control fatty acid synthesis in lipogenic tissues. GS-0976 is an inhibitor of acetyl-CoA carboxylase. In an open-label study, NASH patients administered with GS-0976 for 12 weeks showed reduction in hepatic DNL, hepatic steatosis and improvement in markers of liver injury (ClinicalTrials.gov no: NCT02856555) (83). NDI-010976, an allosteric inhibitor of ACC1 and ACC2, reduces hepatic de DNL and is reported to improve steatosis, inflammation, and fibrosis in animal models with fatty liver disease (84).
4.3.1. Cholesterol lowering drugs
Cholesterol is a key toxic lipid mediator of liver injury, which may be targeted by specific pharmacological interventions. The synthesis of mono-unsaturated fatty acids such as oleic acid is catalysed by the enzyme stearoyl-CoA desaturase 1 (SCD-1) (85). The deficiency or inhibition of SCD-1 leads to a reduction in liver steatosis and improvement in insulin sensitivity (86), (87), (88). Enhanced SCD-1 activity has been reported in obese patients with NASH (89). A double blind placebo-controlled clinical trial in 60 NAFLD patients reported a reduction in hepatic fat content with the administration of Aramchol, the SCD-1 inhibitor (90).
Statins, also known as HMG-CoA reductase inhibitors, are a class of cholesterol lowering drugs. Statins are reported to show marginal improvement of steatosis (91). They are the first choice agents used to reduce plasma cholesterol and reduce cardiovascular disease (CVD) risk (92). CVD is a leading cause of death in patients suffering from NAFLD and NASH (93). Statins do not show any beneficial effect on liver histology in patients with NASH (91). Therefore, statins are not currently recommended for NASH treatment (46). Concurrent metabolic comorbidities recommend the use of statins for cardiovascular end points in NAFLD patients. A number of studies have focused on the safety of statins in hepatotoxicity (94) as these drugs are reported to lower aminotransferase levels in patients with the metabolic syndrome (95).
4.4. Modulation of nuclear transcription factors
Nuclear transcription factors are also believed to have therapeutic potential for the treatment of NASH. These are molecules, which upon binding to their specific ligand regulate transcription of specific genes.
4.4.1. Farnesoid X receptor (FXR) agonists
The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily, and has emerged as a key regulator of metabolic pathways including glucose homeostasis and the inflammatory and fibrogenic processes. The hepatic expression level of FXR is inversely correlated with the severity of disease in patients with NASH (96). The FXR is expressed in the liver, kidneys, intestines, and adrenal glands. It is involved in the synthesis and enterohepatic circulation of bile acids and regulates lipoprotein metabolism [97]. FXR activation affects bile acid production, hepatic lipogenesis, cholesterol synthesis and glucose homoeostasis, and protects hepatocytes against bile acid-induced cytotoxicity [98, 99]. This finding is corroborated by FXR-deficient mice fed on a high-fat diet, which exhibit severe hepatic steatosis, necrotic inflammation and fibrosis (100). Furthermore, FXR agonists have been shown to prevent the development of NASH in rodent models of diet-induced NASH, where they also promoted the resolution of steatohepatitis and fibrosis (101). Therefore, a number of synthetic FXR agonists are being tested for the treatment of NASH and other hepatic disorders (102), (103).
4.4.1.1. Obeticholic acid
Obeticholic acid (OCA) is a modified bile acid which is derived from the primary human bile acid, chenodeoxycholic acid. It stimulates the FXR in humans (104). The FXR plays a key role in the synthesis and entero-hepatic circulation of bile acids (105) and is expressed in the liver, adrenal glands, kidneys and intestines. OCA is a synthetic 6α-ethyl derivative of chenodeoxycholic acid, the primary human bile acid. It is a potent agonist of the FXR, a nuclear hormone receptor which regulates glucose and lipid metabolism. This chemical modification stimulates the activity of FXR approximately 100-fold more than the natural FXR agonist in humans (106), chenodeoxycholic acid. It shows high selectivity and minimal activity with other bile acid receptors (107).
The FLINT trial, a phase 2b study (108) and double-blind, placebo-controlled, randomized clinical trial in the USA, assessed NASH patients without cirrhosis and a NAFLD activity score (NAS) ≥4, for 72 weeks. Improvement in centrally scored liver histology by 2 points in NASH was observed, without any worsening of fibrosis. In FLINT trial, the administration of OCA was associated with significantly great increase in the concentration of total cholesterol and low-density lipoprotein cholesterol (LDLc) and decrease in the concentration of high-density lipoprotein cholesterol (HDLc) as compared to placebo. However, the increase in LDLc concentrations observed upon treatment with OCA were reversed with concomitant statin therapy (e.g. atorvastatin).
Another ongoing phase 3 REGENERATE trial (109) aims to compare the effects of OCA and placebo in the histological improvements and clinical outcomes in NASH and liver fibrosis stage 2 and 3. The patients are randomized and treated with OCA 10 mg, OCA 25 mg and placebo. The primary analysis included 931 patients suffering from F2-F3 fibrosis (311 placed in the placebo group, 312 in OCA 10 mg treated group and 308 in OCA 25 mg treated group). An improvement in fibrosis was seen in 37 (12%) patients of the placebo group, 55 (18%) patients of the OCA 10 mg group (P = 0.045), and 71 (23%) patients of the OCA 25 mg group (P = 0.0002). The resolution of NASH was not attained in any of the patients.
4.4.1.2. Tropifexor (LJN-452)
Tropifexor (LJN-452) is a very potent non-bile acid agonist of FXR, which is reported to induce target genes at very low concentrations. Tropifexor has been shown to be very effective in preclinical NASH models (110). An ongoing phase 2 RCT, FLIGHTFXR, is evaluating the efficacy and safety of tropifexor and cenicriviroc in patients with fibrosis and biopsy-proven NASH (111).
4.4.2. Peroxisome proliferator-activated receptors (PPARs)
PPARs are a group of nuclear receptors, which are expressed in the liver, adipose tissue, heart, skeletal muscle and kidney, and transcriptionally regulate multiple metabolic processes including β-oxidation, lipid transport and gluconeogenesis (112). They are classified into three isotypes, designated as PPARα, PPARβ (also known as PPARδ) and PPARγ, which differ in tissue distribution; however, they target the same segment of DNA. The PPARs are known to heterodimerize with the retinoid X receptor (RXR) to regulate target gene transcription.
In the liver, PPARα upregulates several enzymes involved in mitochondrial and peroxisomal fatty acid oxidation, microsomal ω-oxidation, and ketogenesis (113), (114), and therefore shifts the hepatic metabolism towards lipid oxidation. The activation of PPARα upregulates the expression of lipoprotein lipase (LPL) while downregulating the hepatic secretion of APOCIII, a LPL inhibitor (115), thereby elevating plasma triglyceride clearance. In vitro and in vivo studies report that PPARα suppresses the secretion of interleukin 1 (IL-1), IL-6 and tumour necrosis factor (TNF), and intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) independent of direct DNA binding (116), (117). These effects were peroxisome proliferator response elements (PPRE)-independent, yet they were hepatoprotective against methionine–choline-deficient diet-induced inflammation and fibrosis, without altering fatty acid oxidation and lipid accumulation (118).
4.4.2. 1. Elafibranor
Elafibranor is a PPAR-alpha/delta (α/δ) agonist, known to regulate lipid and insulin metabolism in NAFLD and NASH (119). A phase 2b, double blind RCT, the GOLDEN study (Europe and USA), compared 80 mg and 120 mg elafibranor daily to placebo in 276 patients with biopsy-proven, non-cirrhotic NASH with NAS ≥3 and ≥1 point, for 52 weeks. NASH was resolved, without the fibrosis worsening in the higher proportion of patients of the 120 mg elafibranor group as compared to the placebo group (19% vs 12%; odds ratio = 2.31; P = .045). However, reversal of NASH was not achieved (119).
An ongoing phase 3 study, RESOLVE-IT (https://clinicaltrials.gov/ct2/show/NCT0270443) includes 2,000 patients with NASH having NAS ≥4, with ≥1 of each component of score, and F1-F3 fibrosis. The trial aims at the histological improvement in NASH, evident by the resolution of NASH without any worsening of fibrosis at 72 weeks, and to evaluate cirrhosis, liver related clinical outcomes, and mortality at 4 years. The results of this trial are due in December 2021.
4.4.2. 2. Saroglitazar
Another dual-PPAR agonist, saroglitazar, has been shown to improve lipid and glucose parameters by predominant PPAR-α and moderate PPAR-γ agonist activity. A phase-2 RCT determined the efficacy of saroglitazar magnesium as compared to placebo in patients with NASH (120). A significant reduction was observed in mean ALT levels from baseline to week 16, with 1 mg (−27.3%), 2 mg (−33.1%) and 4 mg (−44.3%) saroglitazar as compared to placebo (4.1%) (P < 0.001 for all groups). A high proportion of patients showed ≥50% reduction in the mean ALT levels from the baseline to week 16 with 4 mg saroglitazar as compared to the placebo (51.8% versus 3.5%; P < 0.0001). At week 16, a significant proportion of patients showed >30% reduction in the liver fat content with 4 mg saroglitazar as compared to placebo (40.7% vs 8%, P = 0.006).
4.4.3. Thyroid hormone receptor (THR)
Thyroid hormone receptor β (THR-β) is known to be highly expressed in the hepatic cells. It plays a key role in regulation of the metabolic pathways which are known to be damaged in NASH (121). Animal studies have reported a crucial role of THR-β in reducing triglycerides and cholesterol, improving insulin sensitivity, promoting liver regeneration, and reducing apoptosis. Evidences indicate that NASH may be, in part, caused by hepatic hypothyroidism, which is higher in patients with NASH (121), (122).
4.4.3. 1. Resmetirom (MGL-3196)
Encouraged from the earlier preclinical studies Thyroid hormone mimetics are being actively pursued as possible anti-NASH therapy (123). Resmetirom (MGL-3196) is an orally active agonist of THR. It is liver-directed and about 28 times more selective for THR-β versus THR-α than triiodothyronine (124). In NASH, this selectivity for THR-β is believed to provide metabolic benefits of thyroid hormone which are mediated by the liver, while avoiding the unwanted effects of excess thyroid hormone in the heart and bone which are mainly mediated through THR-α (121). In preclinical animal models of NASH, resmetirom and some other thyroid analogues were shown to reduce hepatic triglycerides, hepatic steatosis, lipid peroxidation, inflammatory and fibrosis markers, along with ALT levels (124), (121). A multicentre, randomised, double-blind, placebo-controlled, phase clinical 2 trial based on Resmetirom treatment (ClinicalTrials.gov Identifier**:** NCT03900429) resulted in the significant reduction of hepatic fat in patients with NASH, after 12 weeks and 36 weeks of treatment. The adverse effects of treatment in study groups were mild or moderate, except high occurrence of transient mild diarrhoea and nausea observed in the group treated with Resmetirom (125).
VK2809 is another tissue and receptor subtype selective agonist of THR-β which is specific to the liver tissue and hold promising therapeutic potential. It belongs to the pro-drug family which are cleaved in vivo and release potent thyromimetics. It is believed to improve cholesterol and lipoprotein levels through multiple mechanisms including induction of gene expression of genes associated with lipid metabolism. A phase 2, randomized, double-blind, placebo-controlled, multicenter study has been designed to assess the efficacy, safety, and tolerability of VK2809 in lowering LDL-C and liver fat content when administered in patients with primary hypercholesterolemia and NAFLD for 12 weeks, followed by a 4-week off-drug phase (ClinicalTrials.gov Identifier: NCT02927184). Patients receiving 5mg daily doses of VK2809 demonstrated a statistically significant median reduction of 53.8% in liver fat. Of the patients receiving VK2809, 88% showed hepatic fat reduction ≥30% at 12 weeks. At all doses studied, VK2809 was safe and well tolerated and no adverse effects were reported among patients (126).
4.5. Gastrointestinal hormones (Incretins)
Evidences indicate that peptide hormones from the Langerhans cells of distal small intestine serve as incretins, which trigger the release of insulin by the pancreatic beta cells. These gut derived incretin hormones are potential targets for the treatment of NASH.
4.5.1. Glucagon-like peptide (GLP)-1 agonist
Glucagon-like peptide (GLP)-1 is an intestinal hormone released from the foregut in response to food consumption. GLP-1 exhibits a glucose lowering action by its glucose-dependent ability to stimulate insulin secretion and inhibit glucagon secretion from the pancreas. It also delays gastric emptying and suppresses appetite. These metabolic effects of GLP-1 contribute to its anti-NASH activity (127). Its endogenous form is, however, rapidly degraded in vivo by the enzyme dipeptidyl peptidase-4 (DPP-4). GLP-1 secretion is reported to be impaired in patients with NAFLD and NASH, suggesting potential role of GLP-1 agonists in NAFLD treatment (128). The GLP-1 agonists mimic gastrointestinal endogenous GLP-1 hormone and have increased resistance to DPP-4 (129). They have a beneficial effect on insulin resistance and weight control (130). The presence of GLP-1 receptor in hepatocytes has been reported by several studies; therefore GLP-1 agonists may exert their direct effect on liver. Studies suggest that GLP-1 agonists reduce fat accumulation by activating macroautophagy and chaperone mediated autophagy (131).
A pilot study reports Liraglutide, a GLP-1 agonist, to improve NASH histology upon daily injection (132). Semaglutide is another GLP-1 agonist under study (NCT02970942), having the advantage that it requires weekly dosing only. Therapies for NASH, aimed at using a combination of GLP-1 with glucagon or GLP-2 are in early development stages.
4.6. Cytokines
Cytokines are the cell signaling molecules produced by a variety of cells in the body, including liver cells (133). They are crucial mediators of inflammation related disorders. They include several subfamilies such as including interferons, interleukins (IL), transforming growth factors (TGF), TNF, colony-stimulating factors, and chemokines. Lipid accumulation and inflammation are crucial in NAFLD; therefore, cytokines may play a key role in the pathogenesis of NAFLD by stimulating hepatic inflammation, steatosis, cell apoptosis and necrosis, and by inducing fibrosis. The pro-inflammatory cytokines, IL family and TNFα are reported to control the key features of liver disease.
4.6.1. Tumor necrosis factor-alpha (TNF-α)
TNF-α is a pro-inflammatory cytokine secreted by monocytes/macrophages, neutrophils, and T-cells, and other tissues, such as the adipose tissue, neuronal tissue and endothelium. It is associated with obesity and related insulin resistance (134). Dietary and genetic (ob/ob) mice models of obesity lacking TNFα showed improved insulin sensitivity (135). Clinical investigations show that TNFα expression is increased in obesity and decreases on weight loss (136), (137) indicating its role in obesity and metabolic dysregulation (138). A Spanish study based on 52 obese patients showed that liver disease severity correlated with TNF-α gene expression in adipose and liver tissue (139) Overexpression of TNF-α mRNA was observed in the adipose tissue and liver of NASH patients. An Italian study reported increased prevalence of TNF-α polymorphism in NAFLD patients as compared to the controls (140). Hence, TNFα is proposed as a potential therapeutic target to prevent the consequences of metabolic syndrome.
The anti-TNFα drug, thalidomide helped to improve the hepatic alterations caused by a high fat diet in mice (141). Anti-TNFα antibodies decreased inflammation, necrosis, and fibrosis in an experimental rat model of NASH (142). The neutralization of TNFα by Infliximab showed substantial reduction in steatosis, in ob/ob mice (143). Although animal models of NAFLD show encouraging therapeutic potential of TNF-α inhibition, its role in humans is controversial. Hotamisligil et al (144) reported a relationship between the expression of TNF-α and insulin resistance in NASH. A positive correlation has been reported between circulating TNF-α levels and the degree of liver fibrosis, in patients with NASH (144). Increased expression of TNF-α has been observed in the liver and adipose tissue of NASH patients showing significant fibrosis as compared to those with slight or non-existent fibrosis (145). Hui et al (146) showed increased TNFα levels in steatohepatitis subjects as compared to the controls. However, some studies showed no correlation between insulin resistance and TNFα levels (147), (148).
4.6.2. Transforming growth factor-beta (TGF-β)
Transforming growth factor-beta (TGF-β) is an immunosuppressive and pro-fibrotic cytokine (149). TGF-β1 mediates the activation of stellate cells and plays a key role in hepatic fibrosis (150) (151) (152). Its expression is upregulated in experimental hepatic fibrosis models (153), (154), (155). Furthermore, the TGF-β1 mRNA expression is increased in patients with liver fibrosis (156), (157), (158). Stärkel et al (159) reported that upregulation of TGF-β1 is an early step in progressive fibrotic steatohepatitis. Hasegawa and co-workers showed that the levels of TGF-β1 are increased in NASH patients. Serum TGF-β1 levels may help to distinguish NASH patients from the spectrum of NAFLD (160). Liver fibrosis is believed to be a strong predictor of mortality in patients with NAFLD, therefore, there is growing interest in developing therapeutics to target elements in fibrogenesis. TGF-β is a key pro-fibrogenic cytokine, which may be a promising target to treat fibrosis. High levels of TGF-β, caused by chronic liver damage, result in activation of stellate cells to myofibroblasts and massive hepatocyte cell death contributing to the promotion of liver fibrosis. TGF-β signaling induces Smad-dependent and TAK1-dependent signaling which regulates cell survival, proliferation, fibrosis, and tumorigenesis [161]. Galunisertib (LY2157299) is a promising anti-fibrotic TGF-β inhibitor which inhibits SMAD2 phosphorylation and blocks collagens deposition [162]. Although several approaches to inhibit TGF-β signalling in animal models have yielded promising results, the complex role of TGF- β in the liver in cell proliferation, carcinogenesis and immune modulation is a major problem [163, 164] to developing anti- TGF-β based therapy for NASH.
Cenicriviroc (CVC) is a dual antagonist of chemokine receptor type 2 (CCR2) and type 5 (CCR5), located on hepatic stellate cells and Kupffer cells is being targeted for anti-fibrosis therapy [165]. It blocks overactive inflammatory signalling and disrupts signalling that activates stellate cells thereby targeting both inflammation and fibrogenesis. CVC is orally administered in daily tablets (150 mg) and has a plasma life of 30-40 hours. CENTAUR, a phase 2b, 24-month study included 189 patients with biopsy-proven NASH, with an NAS ≥4 and stage 1-3 fibrosis. The patients were randomized 2:1:1 in three arms as follows: A) continuous administration of 150-mg CVC for 24 months, B) administration of placebo for 12 months followed by an additional 12 months of CVC, and C) administration of placebo for 24 months. The expected primary outcome of ≥2 point decrease in NAFLD activity score without any worsening in fibrosis at 1 year was not achieved. However, the improvement in liver fibrosis without worsening of NASH was achieved in 20% from the treatment group, along with lower levels of interleukin-6, C-reactive protein and fibrogen [166]. Currently, another randomized, double-blind, placebo-controlled, multi-centre phase 3 study, AURORA (https://clinicaltrials.gov/ct2/show/NCT03028740) is evaluating the safety and efficacy of CVC in treatment of fibrosis, in NASH.
4.6.3. Interleukin-11 (IL-11)
Hepatic stellate cells (HSCs) give rise to upto 95% of liver myofibroblasts (167), and are crucial to fibrosis, inflammation and parenchymal dysfunction in NASH (168) (169). They are a major source of proinflammatory myofibroblasts, and therefore, their inhibition or reversal of transformation in the liver, may be a potential therapy for treatment of NASH. Interleukin-11 (IL11) was recently identified as a key factor for pulmonary and cardiovascular fibroblast-to-myofibroblast transformation (170, 171). ERK-dependent IL11 signaling is critical for HSC transformation (154), (155), (172).
Recently, a study by Widjaja et al based on hepatocytes, HSCs, and mouse models, explored the role of IL-11 signaling in NASH pathogenesis (172). They reported that HSCs express high levels of interleukin 11 subunit alpha (IL11RA) and showed a proinflammatory role of IL11 in the liver. The ALT levels reversed from approximately 700 U/L to normal within 3 weeks, upon pharmacologic targeting of IL11 signaling (172). They showed that neutralizing antibodies which block IL11 signaling, caused reduction in fibrosis, steatosis, hepatocyte apoptosis and inflammation, in mice with diet-induced liver steatosis and fibrosis. They also reported that hepatocyte damage is prevented, or reversed, by genetic or antibody-mediated inhibition of IL11. Therefore, targeting IL11 to reverse liver fibrosis may be a beneficial therapy for NASH.
5. Bariatric Surgery
Bariatric surgery or a weight loss surgery may be opted to achieve weight loss in NAFLD/NASH patients. Weight loss through diet and exercise may be difficult to achieve or sustain. Bariatric surgery helps to achieve long-term weight loss. It also helps to improve the metabolic functioning of lipid metabolism and inflammatory pathways associated with pathophysiology of NAFLD (173), (174). Bariatric surgery is currently recommended in patients with BMI ≥40 kg/m2 having no comorbidities, or with a BMI 35 to 39.9 kg/m2 with any serious comorbidity such as T2D, hypertension, NAFLD and NASH (175), (176). A review based on 29 studies showed significant improvement in ALT, aspartate aminotransferase (AST), Gamma-glutamyl transferase (GGT) and histology in patients undergoing bariatric surgery (177). A meta-analysis reported improvement in steatosis, steatohepatitis, and fibrosis following weight loss after bariatric surgery (178). Bariatric surgery is an invasive procedure and is therefore associated with risks such as adjustable gastric banding (AGB), and dietary complications including nutritional deficiencies, postprandial hyperinsulinemic hypoglycaemia, etc. (179), (180), (181), (182), (183). A review by le Roux and Heneghan has outlined the long term complications associated with bariatric surgery (179).
6. Gut Microbiome
The gut microbiome produces substances which help to regulate immunity, nutrition, homeostasis, and several metabolic pathways (184), (185), (186). The gut microbiota interact with liver through the ‘liver-gut axis’, involving specific metabolites such as bile acids (BAs), short-chain fatty acids (SCFAs), and lipopolysaccharides (LPS) (187). Several bacteria are known to be associated with lipid and carbohydrate metabolism. Evidences indicate the effect of gut microbiota on liver function, which contribute to energy metabolism, obesity, T2DM and NAFLD (188), (189). Therefore, gut microbiota may regulate disorders linked to energy metabolism (184), (190), (191) Gut microbiota dysbiosis may be caused by the changes in the environment of the host such as diet, alcohol intake, genetic factors, and antibiotics (192). Gut microbiota dysbiosis plays a pivotal role in the pathogenesis of metabolic disorders including NAFLD (193)
Studies based on animal models suggest that probiotics, prebiotics and synbiotics have a modulatory effect on gut microbiota. The oral administration of probiotics is shown to improve abnormal lipid metabolism and dysbiosis of gut microbiota (194). Supplementation of prebiotics and synbiotics improved hypercholesterolemia-associated hepatic changes in rats by regulating genes involved in β-oxidation and lipogenesis, including PPAR-α, carnitine palmitoyltransferase 1 (CPT-1), sterol regulatory element-binding protein 1c (SREBP-1c), fatty acid synthase (FAS) and malic enzyme (ME) (195). Improvement in hepatic inflammation and insulin resistance was observed in rats treated with synbiotics. Also, a reduction in the amount of Gram-negative Enterobacteriales and Escherichia coli was seen in the colonic mucosa.
Fecal microbiota transplantation (FMT) is a method for transplantation of fecal bacteria from a healthy donor to re-populate gut microbiota in patients for the treatment of several diseases (196), (197), (198). FMT is being widely explored for the treatment of NAFLD/NASH. Intrahepatic lipid accumulation, insulin resistance and pro-inflammatory cytokines were improved in mouse models, by FMT (199), (200). In a RCT based on patients with metabolic syndrome, increase in insulin sensitivity was observed after 6 weeks of receiving gut microbiota from healthy Caucasian males (201). FMT may also be developed as a potential therapeutic method for NASH.
7. Summary
While there is no FDA-approved medication for NAFLD/NASH, dietary and lifestyle intervention is the mainstay of treatment. Several medications are in pipeline of therapy of NASH, holding promise for a successful therapy in future. As of now, the first line drugs such as pioglitazone and vitamin E remain the strategy for disease management in patients.
Acknowledgements
This work was supported by the ICMR (59/05/2019/ONLINE/BMS/TRM), SERB (SRG/2019/000398), and Wellcome Trust/DBT India Alliance Fellowship (IA/I/16/2/502691) awarded to Sinha RA.
Abbreviations
NASH
non-alcoholic steatohepatitis
NAFLD
non-alcoholic fatty liver disease
HCC
hepatocellular carcinoma
CR
caloric restriction
CHO
carbohydrates
HDL
high density lipoprotein
ALT
alanine transaminase
PUFA
polyunsaturated fatty acids
TRF
time-restricted feeding
HFD
high-fat diet
RCT
randomized controlled trial
TZD
thiazolidinediones
LPL
lipoprotein lipase
FFA
free fatty acid
PPAR
peroxisome proliferator-activated receptor
GLUT-4
glucose transporter type-4
T2D
type 2 diabetes
MPC
mitochondrial pyruvate carrier
NF-kB
nuclear factor kappa B
SCD
stearoyl-CoA desaturase
CVD
cardiovascular disease
FXR
farnesoid X receptor
OCA
obeticholic acid
TNF
tumour necrosis factor
VCAM1
vascular cell adhesion molecule 1
IL-1
interleukin 1
ICAM1
intercellular adhesion molecule 1
THR
thyroid hormone receptor
GLP-1
glucagon-like peptide
DPP-4
dipeptidyl peptidase-4
TGF
transforming growth factors
HSCs
Hepatic stellate cells
IL-11
Interleukin-11
AGB
adjustable gastric banding
Bas
bile acids
SCFAs
short-chain fatty acids
CPT-1
carnitine palmitoyltransferase 1
SREBP-1c
sterol regulatory element-binding protein 1c
FAS
fatty acid synthase
ME
malic enzyme
FMT
Fecal microbiota transplantation
References
- 1.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36(3):317–24. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 4.Raza S, Rajak S, Anjum B, Sinha RA. Molecular links between non-alcoholic fatty liver disease and hepatocellular carcinoma. Hepatoma Res. 2019;5:42. doi: 10.20517/2394-5079.2019.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindenmeyer CC, McCullough AJ. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis. 2018;22(1):11–21. doi: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13(4):196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152(5):1090–1099 e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14(1):124–31 e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthiah MD, Sanyal AJ. Current management of non-alcoholic steatohepatitis. Liver Int. 2020;40(1):89–95. doi: 10.1111/liv.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond) 2008;115(5):141–50. doi: 10.1042/CS20070402. [DOI] [PubMed] [Google Scholar]
- 14.Mahady SE, George J. Exercise and diet in the management of nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1172–82. doi: 10.1016/j.metabol.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, Owen N, C. AusDiab Steering Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30(3):516–22. doi: 10.2337/dc06-1996. [DOI] [PubMed] [Google Scholar]
- 16.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 17.Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30(3):683–8. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 18.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50(1):68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 19.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I, Blendis L, Halpern Z, Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48(6):1791–8. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 20.Newton JL, Jones DE, Henderson E, Kane L, Wilton K, Burt AD, Day CP. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57(6):807–13. doi: 10.1136/gut.2007.139303. [DOI] [PubMed] [Google Scholar]
- 21.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29(11):2518–27. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD002968.pub2. CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB, N. C. R. Group Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106(3):460–8. doi: 10.1038/ajg.2010.488. quiz 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53. doi: 10.1016/S0140-673611)60749-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, G. Lancet Physical Activity Series Working Physical Activity Series Working: Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29. doi: 10.1016/S0140-673612)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157–66. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Fealy CE, Haus JM, Solomon TP, Pagadala M, Flask CA, McCullough AJ, Kirwan JP. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J Appl Physiol (1985) 2012;113(1):1–6. doi: 10.1152/japplphysiol.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21(3):134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludwig DS, Ebbeling CB. The Carbohydrate-Insulin Model of Obesity: Beyond Calories In, Calories Out. JAMA Intern Med. 2018;178(8):1098–1103. doi: 10.1001/jamainternmed.2018.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong T, Guo M, Zhang P, Sun G, Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: A meta-analysis. PLoS ONE. 2020;15(1):e0225348. doi: 10.1371/journal.pone.0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. 2007;30(5):1075–80. doi: 10.2337/dc06-2169. [DOI] [PubMed] [Google Scholar]
- 33.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 34.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525–31. doi: 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 35.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–9. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 36.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, Nonalcoholic N. Steatohepatitis Clinical Research: Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–71. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storlien JH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237(4817):885–8. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 38.Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, Shimano H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38(6):1529–39. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 39.Levy JR, Clore JN, Stevens W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology. 2004;39(3):608–16. doi: 10.1002/hep.20093. [DOI] [PubMed] [Google Scholar]
- 40.Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology. 2016;63:2032–2043. doi: 10.1002/hep.28392. [DOI] [PubMed] [Google Scholar]
- 41.Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, Martinez ME, Villasenor A, Sears DD, Marinac CR, Gallo LC. Intermittent Fasting and Human Metabolic Health. J Acad Nutr Diet. 2015;115(8):1203–12. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, Chen RQ, Chen ZY. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):219. doi: 10.1186/s12876-019-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung H, Chou W, Sears DD, Patterson RE, Webster NJ, Ellies LG. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65(12):1743–1754. doi: 10.1016/j.metabol.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upadhyay A, Anjum B, Godbole NM, Rajak S, Shukla P, Tiwari S, Sinha RA, Godbole MM. Time-restricted feeding reduces high-fat diet associated placental inflammation and limits adverse effects on fetal organ development. Biochem Biophys Res Commun. 2019;514(2):415–421. doi: 10.1016/j.bbrc.2019.04.154. [DOI] [PubMed] [Google Scholar]
- 45.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–84. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, A. American Gastroenterological, D. American Association for the Study of Liver and G. American College of The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306–17. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2008;14(1):22–8. doi: 10.3748/wjg.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T 3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16(8):4128–36. doi: 10.1128/mcb.16.8.4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fajas L, Fruchart JC, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998;10(2):165–73. doi: 10.1016/s0955-0674(98)80138-5. [DOI] [PubMed] [Google Scholar]
- 51.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10(9):1096–107. doi: 10.1101/gad.10.9.1.096. [DOI] [PubMed] [Google Scholar]
- 52.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15(19):5336–48. [PMC free article] [PubMed] [Google Scholar]
- 53.Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem. 1999;274(7):3970–7. doi: 10.1074/jbc.274.7.3.970. [DOI] [PubMed] [Google Scholar]
- 54.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10):2968–74. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 55.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman JB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314(2):580–5. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 57.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277(28):25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 58.Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122(7):1924–40. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 59.Leclercq IA, Sempoux C, Starkel P, Horsmans Y. Limited therapeutic efficacy of pioglitazone on progression of hepatic fibrosis in rats. Gut. 2006;55(7):1020–9. doi: 10.1136/gut.2005.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119(2):466–78. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 61.Betteridge DJ. Effects of pioglitazone on lipid and lipoprotein metabolism. Diabetes Obes Metab. 2007;9(5):640–7. doi: 10.1111/j.1463-1326.2007.00715.x. [DOI] [PubMed] [Google Scholar]
- 62.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 63.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180(1):32–9. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55(7):1953–62. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137(1):25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 66.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colca JR, McDonald WG, Adams WJ. MSDC-0602K, a metabolic modulator directed at the core pathology of nonalcoholic steatohepatitis. Expert Opin Investig Drugs. 2018;27(7):631–636. doi: 10.1080/13543784.2018.1494153. [DOI] [PubMed] [Google Scholar]
- 69.Soden JS, Devereaux MW, Haas JE, Gumpricht E, Dahl R, Gralla J, Traber MG, Sokol RJ. Subcutaneous vitamin E ameliorates liver injury in an in vivo model of steatocholestasis. Hepatology. 2007;46(2):485–95. doi: 10.1002/hep.21690. [DOI] [PubMed] [Google Scholar]
- 70.Sokol RJ, McKim JM, Jr, Goff MC, Ruyle SZ, Devereaux MW, Han D, Packer L, Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114(1):164–74. doi: 10.1016/s0016-5085(98)70644-4. [DOI] [PubMed] [Google Scholar]
- 71.Azzi A, Gysin R, Kempna P, Munteanu A, Negis Y, Villacorta L, Visarius T, Zingg JM. Vitamin E mediates cell signaling and regulation of gene expression. Ann N Y Acad Sci. 2004;1031:86–95. doi: 10.1196/annals.1331.009. [DOI] [PubMed] [Google Scholar]
- 72.Morante M, Sandoval J, Gomez-Cabrera MC, Rodriguez JL, Pallardo FV, Vina JR, Torres L, Barber T. Vitamin E deficiency induces liver nuclear factor-kappaB DNA-binding activity and changes in related genes. Free Radic Res. 2005;39(10):1127–38. doi: 10.1080/10715760500193820. [DOI] [PubMed] [Google Scholar]
- 73.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–57. doi: 10.1001/jama.297.8.8.42. [DOI] [PubMed] [Google Scholar]
- 75.Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341 doi: 10.1136/bmj.c5702. c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, 3rd, Larrick J, Kunkel SL. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155(3):1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 78.Bhat VB, Madyastha KM. Antioxidant and radical scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochem Biophys Res Commun. 2001;288(5):1212–7. doi: 10.1006/bbrc.2001.5922. [DOI] [PubMed] [Google Scholar]
- 79.Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41(4):592–8. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 80.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54(5):1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–23. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 83.Lawitz EJ, Coste A, Poordad F, Alkhouri N, Loo N, McColgan BJ, Tarrant JM, Nguyen T, Han L, Chung C, Ray AS, et al. Acetyl-CoA Carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients With Nonalcoholic Steatohepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(12):1983–1991.e3. doi: 10.1016/j.cgh.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 84.Stiede K, Miao W, Blanchette HS, Beysen C, Harriman G, Harwood HJJR, Kelley H, Kapeller R, Schmalbach T, Westlin WF. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: A randomized, double-blind, crossover study. Hepatology. 2017;66(2):324–334. doi: 10.1002/hep.29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hodson L, Fielding BA. Stearoyl-CoA desaturase: rogue or innocent bystander? Prog Lipid Res. 2013;52(1):15–42. doi: 10.1016/j.plipres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Ntambi JM. The regulation of stearoyl-CoA desaturase (SCD) Prog Lipid Res. 1995;34(2):139–50. doi: 10.1016/0163-7827(94)00010-j. [DOI] [PubMed] [Google Scholar]
- 87.Miyazaki M, Flowers JT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6(6):484–96. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 88.Issandou M, Bouillot A, Brusq JM, Forest MC, Grillot D, Guillard R, Martin S, Michiels C, Sulpice T, Daugan A. Pharmacological inhibition of stearoyl-CoA desaturase 1 improves insulin sensitivity in insulin-resistant rat models. Eur J Pharmacol. 2009;618(1–3):28–36. doi: 10.1016/j.ejphar.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Walle P, Takkunen M, Mannisto V, Vaittinen M, Lankinen M, Karja V, Kakela P, Agren J, Tiainen M, Schwab U, Kuusisto J, et al. Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism. 2016;65(5):655–666. doi: 10.1016/j.metabol.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, Oren R, F. Group The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2085–91 e1. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 91.Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47(1):135–41. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 92.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD004816.pub5. CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lonardo A, Ballestri S, Targher G, Loria P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9(5):629–50. doi: 10.1586/17474124.2015.965143. [DOI] [PubMed] [Google Scholar]
- 94.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, I. Pravastatin in Chronic Liver Disease Study Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453–63. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 95.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP, G. S. C. Group Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 96.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15(5):665–74. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jhaveri MA, Kowdley KV. New developments in the treatment of primary biliary cholangitis—Role of obeticholic acid. Ther Clin Risk Manag. 2017;13:1053–1060. doi: 10.2147/TCRM.S113052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cariou B. The farnesoid X receptor (FXR) as a new target in non-alcoholic steatohepatitis. Diabetes Metab. 2008;34(6 Pt 2):685–691. doi: 10.1016/S1262-3636(08)74605-6. [DOI] [PubMed] [Google Scholar]
- 99.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 100.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328(1):116–22. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314(2):584–95. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- 102.Ding L, Pang S, Sun Y, Tian Y, Yu L, Dang N. Coordinated Actions of FXR and LXR in Metabolism: From Pathogenesis to Pharmacological Targets for Type 2 Diabetes. Int J Endocrinol. 2014;2014 doi: 10.1155/2014/751859. 751859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roda A, Pellicciari R, Gioiello A, Neri F, Camborata C, Passeri D, De Franco F, Spinozzi S, Colliva C, Adorini L, Montagnani M, et al. Semisynthetic bile acid FXR and TGR5 agonists: physicochemical properties, pharmacokinetics, and metabolism in the rat. J Pharmacol Exp Ther. 2014;350(1):56–68. doi: 10.1124/jpet.114.214650. [DOI] [PubMed] [Google Scholar]
- 104.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47(18):4559–69. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 105.Jhaveri MA, Kowdley KV. New developments in the treatment of primary biliary cholangitis - role of obeticholic acid. Ther Clin Risk Manag. 2017;13:1053–1060. doi: 10.2147/TCRM.S113052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 107.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78(4):617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, Goodman Z, Bedossa P, MacConell L, Shringarpure R, Shah A, et al. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials. 2019;84 doi: 10.1016/j.cct.2019.06.017. 105803. [DOI] [PubMed] [Google Scholar]
- 110.Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, Mutnick D, Bursulaya B, Schmeits J, Wu X, Bao D, et al. Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH) J Med Chem. 2017;60(24):9960–9973. doi: 10.1021/acs.jmedchem.7b00907. [DOI] [PubMed] [Google Scholar]
- 111.Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, Loomba R, Ratziu V, Kochuparampil J, Fischer L, Vaidyanathan S, et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp Clin Trials. 2020;88 doi: 10.1016/j.cct.2019.105889. 105889. [DOI] [PubMed] [Google Scholar]
- 112.Lamichane S, Dahal Lamichane B, Kwon SM. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int J Mol Sci. 2018;19(4) doi: 10.3390/ijms19040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim H, Mendez R, Zheng Z, Chang L, Cai J, Zhang R, Zhang K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor alpha to regulate metabolic hormone FGF21. Endocrinology. 2014;155(3):769–82. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Velkov T. Interactions between Human Liver Fatty Acid Binding Protein and Peroxisome Proliferator Activated Receptor Selective Drugs. PPAR Res. 2013;2013 doi: 10.1155/2013/938401. 938401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH. PPAR{alpha} mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am J Physiol Endocrinol Metab. 2007;292(2):E421–34. doi: 10.1152/ajpendo.00157.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mansouri RM, Bauge E, Staels B, Gervois P. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-alpha control of the acute-phase response. Endocrinology. 2008;149(6):3215–23. doi: 10.1210/en.2007-1339. [DOI] [PubMed] [Google Scholar]
- 117.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274(45):32048–54. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 118.Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536(1–2):182–91. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 119.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5):1147–1159 e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 120.Cardoso AC, de Figueiredo-Mendes C, Villela-Nogueira CA, Sanyal AJ. New drugs for non-alcoholic steatohepatitis. Liver Int. 2020;40(1):96–101. doi: 10.1111/liv.14354. [DOI] [PubMed] [Google Scholar]
- 121.Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid. 2019;29(9):1173–1191. doi: 10.1089/thy.2018.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bohinc BN, Michelotti G, Xie G, Pang H, Suzuki A, Guy CD, Piercy D, Kruger L, Swiderska-Syn M, Machado M, Pereira T, et al. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology. 2014;155(11):4591–601. doi: 10.1210/en.2014-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sinha RA, Bruinstroop E, Singh BK, Yen PM. Thyroid hormones and thyromimetics: A new approach to NASH? Hepatology. 2020 doi: 10.1002/hep.31204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kelly MJ, Pietranico-Cole S, Larigan JD, Haynes NE, Reynolds CH, Scott N, Vermeulen J, Dvorozniak M, Conde-Knape K, Huang KS, So SS, et al. Discovery of 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro(1,2,4)triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57(10):3912–23. doi: 10.1021/jm4019299. [DOI] [PubMed] [Google Scholar]
- 125.Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 126.Loomba R, Neutel J, Mohseni R, Bernard D, Severance R, Dao M, Saini S, Margaritescu C, Homer K, Tran B, Mancini M, et al. VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat with both low and high doses in patients with non-alcoholic fatty liver disease: a phase 2 randomized, placebo-controlled trial. J Hepatol. 2019;70:e150–151. doi: 10.1016/S0618-8278(19)30266-X. [DOI] [Google Scholar]
- 127.Jinnouchi H, Sugiyama S, Yoshida A, Hieshima K, Kurinami N, Suzuki T, Miyamoto F, Kajiwara K, Matsui K, Jinnouchi T. Liraglutide, a glucagon-like peptide-1 analog, increased insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp examination in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Res. 2015;2015 doi: 10.1155/2015/706416. 706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernsmeier CAC, Meyer-Gerspach LS, Blaser Jeker L, Steinert RE, Heim MH, Beglinger C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS One. 2014;9(1):e87488. doi: 10.1371/journal.pone.0087488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 130.Dhir G, Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med. 2018;66(1):7–10. doi: 10.1136/jim-2017-000554. [DOI] [PubMed] [Google Scholar]
- 131.Wang XC, Gusdon AM, Liu H, Qu S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J Gastroenterol. 2014;20(40):14821–30. doi: 10.3748/wjg.v20.i40.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, L. t. team. Abouda G, Aldersley MA, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 133.Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–43. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 134.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 135.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 136.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59(9):1259–64. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 137.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83(8):2907–10. doi: 10.1210/jcem.83.8.5.026. [DOI] [PubMed] [Google Scholar]
- 138.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95(5):2111–9. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Van Hoof M, Rahier J, Horsmans Y. Tamoxifen-induced steatohepatitis. Ann Intern Med. 1996;124(9):855–6. doi: 10.7326/0003-4819-124-9-199605010-00015. [DOI] [PubMed] [Google Scholar]
- 140.Valenti L, Fracanzani AL, Dongiovanni P, Santorelli G, Branchi A, Taioli E, Fiorelli G, Fargion S. Tumor necrosis factor alpha promoter polymorphisms and insulin resistance in nonalcoholic fatty liver disease. Gastroenterology. 2002;122(2):274–80. doi: 10.1053/gast.2002.31065. [DOI] [PubMed] [Google Scholar]
- 141.Pinto Lde F, Compri CM, Fornari JV, Bartchewsky W, Cintra DE, Trevisan M, Carvalho Pde O, Ribeiro ML, Velloso LA, Saad MJ, Pedrazzoli J, Jr, et al. The immunosuppressant drug, thalidomide, improves hepatic alterations induced by a high-fat diet in mice. Liver Int. 2010;30(4):603–10. doi: 10.1111/j.1478-3231.2009.02200.x. [DOI] [PubMed] [Google Scholar]
- 142.Koca SS, Bahcecioglu IH, Poyrazoglu OK, Ozercan IH, Sahin K, Ustundag B. The treatment with antibody of TNF-alpha reduces the inflammation, necrosis and fibrosis in the non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Inflammation. 2008;31(2):91–8. doi: 10.1007/s10753-007-9053-z. [DOI] [PubMed] [Google Scholar]
- 143.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 144.Lesmana CR, Hasan I, Budihusodo U, Gani RA, Krisnuhoni E, Akbar N, Lesmana LA. Diagnostic value of a group of biochemical markers of liver fibrosis in patients with non-alcoholic steatohepatitis. J Dig Dis. 2009;10(3):201–6. doi: 10.1111/j.1751-2980.2009.00386.x. [DOI] [PubMed] [Google Scholar]
- 145.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34(6):1158–63. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 146.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40(1):46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 147.Muller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, Giani G, Illig T, Thorand B, Kolb H. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia. 2002;45(6):805–12. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- 148.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148(5):535–42. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 149.Douglas HE. TGF-ss in wound healing: a review. J Wound Care. 2010;19(9):403–6. doi: 10.12968/jowc.2010.19.9.7.8235. [DOI] [PubMed] [Google Scholar]
- 150.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19(2):129–40. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 151.Shek FW, Benyon RC. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur J Gastroenterol Hepatol. 2004;16(2):123–6. doi: 10.1097/00042737-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 152.Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34(5):859–67. doi: 10.1053/jhep.2001.28457. [DOI] [PubMed] [Google Scholar]
- 153.De Bleser PJ, Niki T, Rogiers V, Geerts A. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol. 1997;26(4):886–93. doi: 10.1016/s0168-8278(97)80257-7. [DOI] [PubMed] [Google Scholar]
- 154.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation n vivo. J Hepatol. 1999;30(1):77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 155.Czaja MJ, Weiner FR, Flanders KC, Giambrone MA, Wind R, Biempica L, Zern MA. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108(6):2477–82. doi: 10.1083/jcb.108.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Annoni G, Weiner FR, Zern MA. Increased transforming growth factor-beta 1 gene expression in human liver disease. J Hepatol. 1992;14(2–3):259–64. doi: 10.1016/0168-8278(92)90168-o. [DOI] [PubMed] [Google Scholar]
- 157.Castilla A, Prieto J, Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N Engl J Med. 1991;324(14):933–40. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- 158.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139(6):1221–9. [PMC free article] [PubMed] [Google Scholar]
- 159.Starkel P, Sempoux C, Leclercq I, Herin M, Deby C, Desager JP, Horsmans Y. Oxidative stress, KLF6 and transforming growth factor-beta up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol. 2003;39(4):538–46. doi: 10.1016/s0168-8278(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 160.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15(10):1667–72. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 161.Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, ten Dijke P. TGF-β signalling and liver disease. FEBS J. 2016;283:2219–2232. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 162.Luangmonkong T, Suriguga S, Bigaeva E, Boersema M, Oosterhuis DK, de Jong P, Schuppan DH, Mutsaers AM, Olinga P. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br J Pharmacol. 2017;174(18):3107–3117. doi: 10.1111/bph.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 164.Fabregat I, Fernando J, Mainez J, Sancho P. TGF-beta signaling in cancer treatment. Curr Pharm Des. 2014;20(17):2934–2947. doi: 10.2174/13816128113199990591. [DOI] [PubMed] [Google Scholar]
- 165.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, et al. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS One. 2016 Jun 27;11(6):e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356–365. doi: 10.1016/j.cct.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 167.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275(4):2247–50. doi: 10.1074/jbc.275.4.2.247. [DOI] [PubMed] [Google Scholar]
- 169.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, Ng B, Patone G, Chow K, Khin E, Tan J, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552(7683):110–115. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ng B, Dong J, D'Agostino G, Viswanathan S, Widjaja AA, Lim WW, Ko NSJ, Tan J, Chothani SP, Huang B, Xie C, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med. 2019;11(511) doi: 10.1126/scitranslmed.aaw1237. [DOI] [PubMed] [Google Scholar]
- 172.Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D'Agostino GA, Ng B, Lim WW, Tan J, Paleja BS, Tripathi M, et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology. 2019;157(3):777–792 e14. doi: 10.1053/j.gastro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 173.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130(6):1564–72. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 174.Viana EC, Araujo-Dasilio KL, Miguel GP, Bressan J, Lemos EM, Moyses MR, de Abreu GR, de Azevedo JL, Carvalho PS, Passos-Bueno MR, Errera FI, et al. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-alpha. Prospective clinical trial. Obes Surg. 2013;23(8):1252–61. doi: 10.1007/s11695-013-0894-2. [DOI] [PubMed] [Google Scholar]
- 175.Gastrointestinal surgery for severe obesity. NIH consensus development conference, March 25-7,1991. Nutrition. 1996;12(6):397–404. [PubMed] [Google Scholar]
- 176.Burguera B, Agusti A, Arner P, Baltasar A, Barbe F, Barcelo A, Breton I, Cabanes T, Casanueva JF, Couce JE, Dieguez C, et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J Endocrinol Invest. 2007;30(10):844–52. doi: 10.1007/bf03349226. [DOI] [PubMed] [Google Scholar]
- 177.Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: a Systematic Review of Liver Biochemistry and Histology. Obes Surg. 2015;25(12):2280–9. doi: 10.1007/s11695-015-1691-x. [DOI] [PubMed] [Google Scholar]
- 178.Chavez-Tapia JC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;1 doi: 10.1002/14651858.CD007340.pub2. CD007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.le Roux CW, Heneghan HM. Bariatric Surgery for Obesity. Med Clin North Am. 2018;102(1):165–182. doi: 10.1016/j.mcna.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 180.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25(11–12):1150–6. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 181.Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017;13(5):727–741. doi: 10.1016/j.soard.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 182.Hammer HF. Medical complications of bariatric surgery: focus on malabsorption and dumping syndrome. Dig Dis. 2012;30(2):182–6. doi: 10.1159/000336681. [DOI] [PubMed] [Google Scholar]
- 183.Marsk R, Jonas E, Rasmussen F, Naslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia. 2010;53(11):2307–11. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 184.Li X, Li C. Analysis of changes in intestinal flora and intravascular inflammation and coronary heart disease in obese patients. Exp Ther Med. 2018;15(5):4538–4542. doi: 10.3892/etm.2018.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li CY, Dempsey JL, Wang D, Lee S, Weigel KM, Fei Q, Bhatt JK, Prasad B, Raftery D, Gu H, Cui JY. PBDEs Altered Gut Microbiome and Bile Acid Homeostasis in Male C57BL/6 Mice. Drug Metab Dispos. 2018;46(8):1226–1240. doi: 10.1124/dmd.118.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zununi Vahed S, Moghaddas Sani H, Rahbar Saadat Y, Barzegari A, Omidi Y. Type 1 diabetes: Through the lens of human genome and metagenome interplay. Biomed Pharmacother. 2018;104:332–342. doi: 10.1016/j.biopha.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 187.Park JS, Seo JH, Youn HS. Gut microbiota and clinical disease: obesity and nonalcoholic Fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16(1):22–7. doi: 10.5223/pghn.2013.16.1.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qin J, Li R, Raes J, Arumugam M, Burgdorf JS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende JR, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, Marti-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 190.Baddini Feitoza A, Fernandes Pereira A, Ferreira da Costa N, Goncalves Ribeiro B. Conjugated linoleic acid (CLA): effect modulation of body composition and lipid profile. Nutr Hosp. 2009;24(4):422–8. [PubMed] [Google Scholar]
- 191.Zhang C, Bjorkman A, Cai K, Liu G, Wang C, Li Y, Xia H, Sun L, Kristiansen K, Wang J, Han J, et al. Impact of a 3-Months Vegetarian Diet on the Gut Microbiota and Immune Repertoire. Front Immunol. 2018;9:908. doi: 10.3389/fimmu.2018.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 193.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 194.Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. The relationship between non-alcoholic fatty liver disease and small intestinal bacterial overgrowth among overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2017;30(11):1161–1168. doi: 10.1515/jpem-2017-0252. [DOI] [PubMed] [Google Scholar]
- 195.Alves CC, Waitzberg DL, de Andrade LS, Dos Santos Aguiar L, Reis JB, Guanabara CC, Junior OA, Ribeiro DA, Sala P. Prebiotic and Synbiotic Modifications of Beta Oxidation and Lipogenic Gene Expression after Experimental Hypercholesterolemia in Rat Liver. Front Microbiol. 2017;8:2010. doi: 10.3389/fmicb.2017.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bakker GJ, Nieuwdorp M. Fecal Microbiota Transplantation: Therapeutic Potential for a Multitude of Diseases beyond Clostridium difficile. Microbiol Spectr. 2017;5(4) doi: 10.1128/microbiolspec.BAD-0008-2017. [DOI] [PubMed] [Google Scholar]
- 197.de PF, Frissen Groot MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes. 2017;8(3):253–267. doi: 10.1080/19490976.2017.1293224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. 2018;47(2):192–202. doi: 10.1111/apt.14397. [DOI] [PubMed] [Google Scholar]
- 199.Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7(1):1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–94. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 201.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte JS, Bartelsman JF, Dallinga-Thie JM, Ackermans JT, Serlie JJ, Oozeer R, Derrien M, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6 e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]