Methamphetamine‐related cardiovascular diseases (original) (raw)
Abstract
Aims
Abuse of crystal methamphetamine (MA) poses a growing problem for health services worldwide. This review summarizes the current literature on the effects of MA on the cardiovascular system.
Methods and results
This article is a presentation of a case report and review of the current literature. In Europe, especially the eastern countries and the eastern states of Germany are affected. MA increases the concentration of catecholamines in the synaptic gap leading to euphoria, alertness, and hunger suppression as well as psychiatric and gastrointestinal complications. MA consumption is associated with hypertension, acute and chronic myocardial toxicity, stroke, coronary artery disease, and sudden cardiac death. Although many aspects of the underlying pathophysiology remain unknown, catecholamine‐mediated pathologies appear to play an important role. The duration of MA consumption is the most important determinant for the prognosis.
Conclusions
Awareness is needed as cardiac complications are important causes of morbidity and mortality in patients with MA consumption. Drug abstinence is the mainstay of therapy, cardiac and other complications should be treated according to the respective guidelines. Incompliance to therapy and frequent relapses are the main challenges for successful treatment. Further research is required to improve the understanding of this rapidly increasing cardiomyopathy.
Keywords: Methamphetamine, Crystal, Cardiomyopathy, Heart failure, Europe
1. Historical context
Methamphetamine (MA) was first synthetized in 1893 by Nagayoshi Nagai in Japan.1 It was sold under the brand name Pervitin in the 1930s and 40s in Germany and was widely used as a stimulant by the German military in World War II. It was initially prescribed to treat obesity, narcolepsy, and hay fever and is still approved for the treatment of obesity and attention deficit hyperactivity disorder.2
2. Prevalence and distribution in Germany and Europe
MA is the most frequently abused amphetamine‐type stimulant in the world. Its expanding market is shown by the increasing need for patient treatment as well as growing numbers of drug seizures worldwide.3 MA seizures are increasing in Europe since the year 2002 with an all‐time high in 2015 especially in Germany, Norway, Turkey, and the Czech Republic.4 Patient surveys in Germany show a higher prevalence of MA abuse in the eastern states, especially the ones situated close to the border with the Czech Republic, e.g. Saxonia (Table 1). Sewage water analyses in European countries also show the highest concentration of MA in Germany, Slovakia, and the Czech Republic (Table 2, Figure 1).
Table 1.
Prevalence of methamphetamine abuse in select German states
| Prevalence of methamphetamine abuse in German states | Lifetime (%) | 12 months (%) | 30 days (%) |
|---|---|---|---|
| Bavaria | 1.1 | 0.4 | 0.0 |
| Hamburg | 0.9 | 0.4 | 0.3 |
| Hesse | 0.7 | 0.0 | 0.0 |
| NRW | 0.3 | 0.2 | 0.0 |
| Thuringia | 1.7 | 0.8 | 0.2 |
| Saxonia | 2.0 | 0.3 | 0.0 |
Table 2.
Wastewater analysis of methamphetamine (MA) in select European cities
| City (country) | MA concentration |
|---|---|
| Chemnitz (Germany) | 240.6 |
| Erfurt (Germany) | 211.2 |
| Budweis (Czech Republic) | 2002 |
| Brno (Czech Republic) | 185.7 |
| Dresden (Germany) | 180.2 |
| Bratislava (Slovakia) | 149.2 |
| Nuremberg (Germany) | 94.8 |
| Oslo (Norway) | 92.5 |
| Magdeburg (Germany) | 85.2 |
| Zurich (Switzerland) | 62 |
| Barcelona (Spain) | 49 |
| Helsinki (Finland) | 46 |
| Vilnius (Lithuania) | 39.7 |
| Munich (Germany) | 7.9 |
| Athens (Greece) | 4 |
| Hamburg (Germany) | 3.6 |
| Porto (Portugal) | 0.5 |
| Paris (France) | Below quant. |
Figure 1.
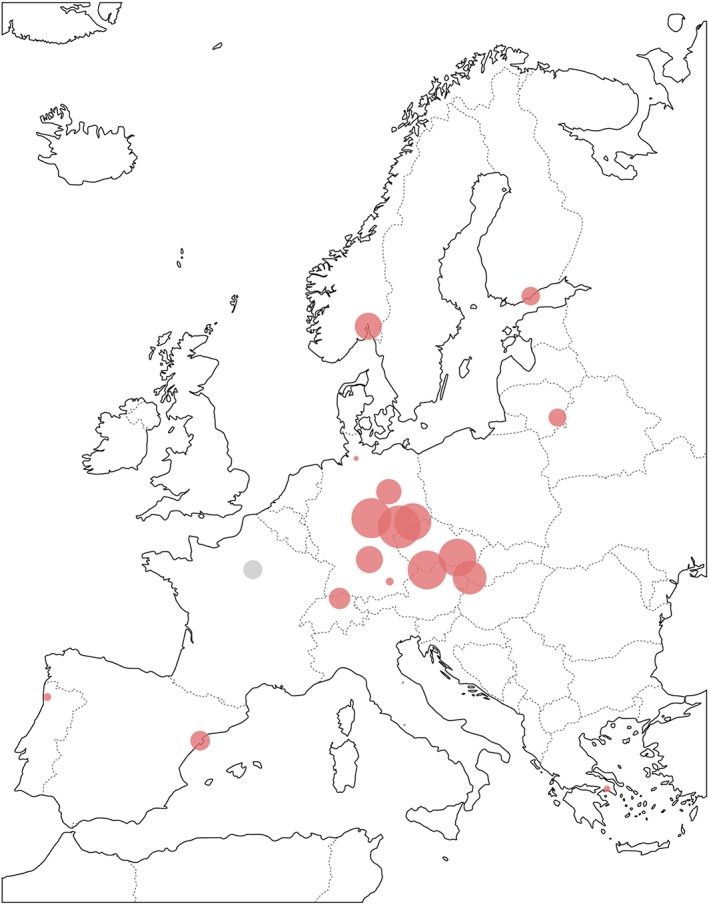
Wastewater analysis of methamphetamine (MA) in select European cities. The size of the dots corresponds to the concentration of MA (see Table 2)
2.1. Pharmacology and pathophysiology
There are multiple routes of MA administration, smoking of crystalline MA being the most popular one. Other routes include nasal inhalation, swallowing, or intravenous injection. Of the two enantiomers, the (+)‐isomer is the biologically more potent and mainly abused one. The weaker (‐)‐isomer is used as a nasal decongestant.7 MA itself does not possess direct sympathomimetic properties but leads to an increase in the concentration of monoamines in the synaptic gap by stimulating the release as well as inhibiting the degradation and reuptake of dopamine, serotonin, norepinephrine, and epinephrine.8 These monoamines, especially dopamine, are responsible for the majority of the desired effects like euphoria, alertness, and hunger suppression.9 The stimulatory effect of MA lasts for hours, compared with minutes in cocaine.10 At the same time, MA causes multiple psychiatric, neurological, cardiac, and gastrointestinal complications.
3. Cardiac complications
3.1. Histological findings
In rat models with long‐term MA administration, histopathogical examinations showed cardiac lesions including atrophy, lysis and necrosis of the myocytes, inflammation, interstitial oedema, fibrosis, and mitochondrial degeneration. These effects were partly reversible after cessation of MA intake.11, 12, 13, 14
A post‐mortem examination of a human heart showed concentric myocardial hypertrophy, extensive myocardial remodelling with perivascular and interstitial fibrosis as well as myocardial scarring due to infarction.15 Independently of the duration of the MA abuse, endomyocardial biopsies show signs of inflammation by increased numbers of T‐lymphocytes and macrophages compared to patients with DCM. There is an increase in perivascular and interstitial fibrosis, the degree of which increases with duration of MA abuse.16 These histological findings show similarities to other forms of toxic myocarditis, e.g. caused by cocaine and catecholamines.17
3.2. Acute cardiovascular toxicity
The acute cardiac effects of MA are caused by its sympathomimetic properties and direct cardiotoxicity. They manifest by an increase in heart rate, systolic blood pressure, and respiratory rate over the course of several hours.18 The cause of direct cardiotoxic effects of MA are still not fully understood, but there is evidence that the formation of free oxygen radicals and nitration of contractile and mitochondrial proteins lead to myocyte degeneration.19 Acute cardiac decompensation with pulmonary oedema due to malignant hypertension has been reported after MA intake.20 Contaminants, which may be intermediaries of MA synthesis or deliberately added impurities to cut the drug, may exert additional toxic effects on their own.21
3.3. Hypertension related complications
Chronic MA abuse can lead to long‐term hypertension and hypertensive cardiomyopathy.22 There also seems to be a correlation between idiopathic pulmonary hypertension and MA consumption.23. MA abuse is the second most common cause for aortic dissection after idiopathic hypertension in the USA.24
3.4. Heart failure
The first autopsy reports linking MA to left ventricular (LV) heart failure and cardiac death were reported in the 1970s.25 The first case reports of MA‐related reversible dilated cardiomyopathy (DCM) have been published in the 1980s.25, 26 A retrospective case series by Wijetunga et al. on patients with a discharge diagnosis of cardiomyopathy and concomitant MA abuse revealed a high percentage (19 of 21 patients) of echocardiographic LV‐dilation LV ejection fraction (EF) reduction.27 Another case series compared echocardiographic features of patients with cardiomyopathy and MA abuse compared with a matched group without MA abuse. Patients with coronary artery and relevant valve disease were excluded. While many characteristics, i.e. gender, age, and obesity were similar, MA abusers had a significantly lower LVEF and more dilated ventricles and atria.28
The pathophysiology leading to heart failure still is not fully understood, but several causes appear to be responsible. For one, the constantly elevated level of catecholamines seems to play a major role in the pathogenesis of MA‐associated DCM. In agreement with this pathology, cases of catecholamine‐induced DCM due to pheochromocytoma are reported, where the LVEF recovered after operative treatment.29, 30, 31 Also, the histological features of MA‐associated cardiomyopathy (MACM) and catecholamine‐induced DCM have been shown to be similar.32
There still is uncertainty about the percentage of patients that develop heart failure or other cardiac pathologies due to MA abuse. A retrospective analysis of patients in an emergency ward showed heart failure in approximately 10% of MA abusers, as determined by an increase in BNP.33 In a case‐control study with 107 cases, MA increased the risk to suffer from cardiomyopathy by a factor of 3.7 in patients under the age of 45 years.34 CYP2D6 is responsible for the first step of MA metabolism. Correlating to this, a small prospective case‐control study showed a, albeit statistically insignificant, trend that individuals with an increased CYP2D6 metabolism were more likely to develop DCM.35 A clinical case of a patient with MA abuse is depicted in Figure 2.
Figure 2.
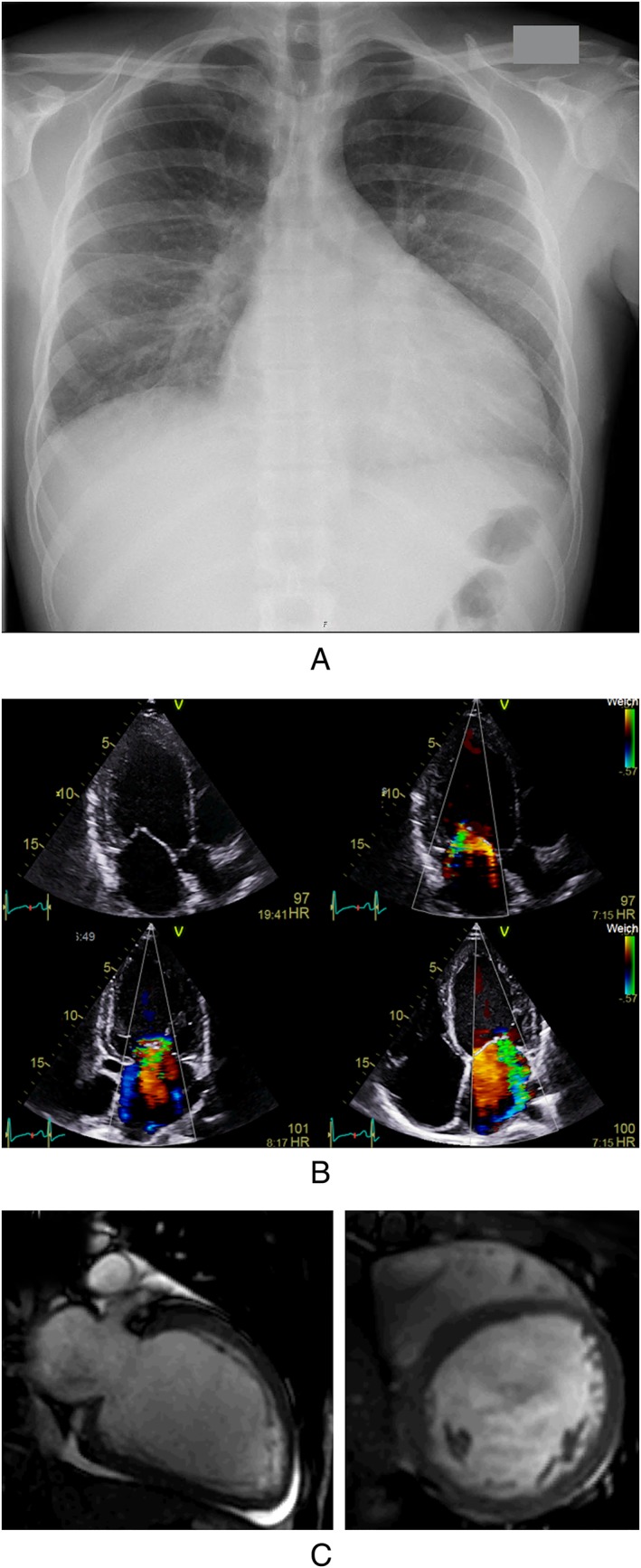
Clinical case report of a patient with frequent MA abuse. A: Chest X‐ray showing cardiomegaly and lung congestion at the time of hospital admission. B: Transthoracic echocardiogram showing LV‐dilatation and moderate to severe secondary mitral regurgitation due to ring dilatation. C: Diastolic 2 chamber and shortaxis views in cardiac MRI showing LV enlargement without signs of myocardial infarction or inflammation and discrete pericardial effusion.
3.5. Takotsubo cardiomyopathy
There have been case reports of reverse takotsubo cardiomyopathy (r‐TTC) with akinesia of the basal areas of the myocardium, in contrast to the more common apical ballooning in TCC. In a study of patients with subarachnoid haemorrhage and TCC, this more uncommon reverse form seemed to correlate with higher levels of catecholamines and younger age.36, 37 Thus, unphysiologically high levels of catecholamines, i.e. after MA consumption, may more often lead to r‐TCC. In a retrospective study by Voskoboinik et al. on patients with MACM, nearly one third of the patients (6/20) presented with an r‐TCC‐like pattern of hypokinesia compared with global hypokinesia. These patients had a shorter duration of MA abuse, lower rate of ventricular fibrosis, and better recovery of LVEF during follow‐up, suggesting that r‐TCC might be an early, reversible form of MACM.38
3.6. Sudden cardiac death
MA abuse leads to multiple abnormal electrocardiogram results, prolongation of the QTc‐interval being the most prominent one. This might explain the higher prevalence of malignant heart rhythm disorders like ventricular tachycardia or torsades de pointes.39, 40 Furthermore, fibrosis is most likely responsible for an increase in shock impedance in patients with an implantable cardioverter‐defibrillator.41
3.7. Coronary artery disease and myocardial infarction
Coronary artery disease is common in MA abusers. Early coronary microcirculation abnormalities and reduced myocardial perfusion can be detected by myocardial contrast echocardiography.42 In an Australian study of 894 MA‐associated deaths, autopsy showed a high prevalence of coronary artery disease (19%), LV‐dilatation (26.3%), LV‐hypertrophy (19%), myocardial scarring (19.8%) despite the young mean age of 37.9 years.43 However, there seems to be a high prevalence of myocardial infarction in the absence of coronary artery disease in MA abusers as well. A patient died of myocardial infarction and subsequent cardiogenic shock after smoking MA. Autopsy revealed diffuse myocardial infarction without coronary artery stenosis.44 In another case report of a young patient, myocardial infarction was attributed to generalized microvascular spasm leading to TIMI‐1 flow in all coronary arteries in the absence of coronary stenosis.45 Spontaneous dissection of multiple coronary arteries leading to myocardial infarction has also been reported.46
3.8. Stroke
MA intake is associated with both haemorrhagic and ischaemic strokes. In a review of case reports and series, 80% of reported strokes in young patients taking MA were haemorrhagic.47 This differs from normal stroke populations, where the majority of strokes are ischaemic, even in young patients.48 Haemorrhagic stroke may be provoked by MA‐associated arterial hypertension.49 Ischaemic stroke on the other hand may be caused by vasoconstriction, vasculitis, or thromboembolism.50, 51 Intracardiac thrombi are reported in up to 33% of MA abusers, which can also lead to coronary occlusions.16, 52 A 10‐year follow‐up of more than 1300 psychiatric patients with MA abuse and propensity score matched controls showed a significant increase in cerebrovascular and cardiovascular disease, mainly haemorrhagic stroke and arrhythmias.40
3.9. Diagnosis
Physical examination may show signs of heart failure. In younger patients presenting with heart failure, drug history should be taken, and in case of doubt, urine analysis to screen for drugs should be performed. In patients with known MA abuse, a 12‐lead electrocardiogram can reveal signs of cardiac damage and QTc‐prolongation. Measurements of BNP can diagnose early stages of heart failure without apparent clinical signs of decompensation. In suspected heart failure, an echocardiography is indicated to assess cardiac function, especially LVEF. 3D speckle tracing imaging markers are useful for early detection of ventricular dysfunction.53 Contrast echocardiography may be helpful in detecting early microvascular changes and myocardial ischaemia.42 Left heart catheterization should be performed to exclude coronary artery disease and, in the case of suspected myocarditis, to obtain myocardial biopsies. Right heart catheterization can help diagnose and differentiate the subtype of pulmonary hypertension. Cardiac MRI (CMR) can further help differentiate between cardiac inflammation and other cardiac diseases (i.e. DCM, amyloidosis, storage diseases, etc.), as well as detecting diffuse interstitial fibrosis and myocardial scars as prognostic markers. A limitation of CMR is the relatively low sensitivity in detecting cardiomyopathic forms of myocarditis.54
3.10. Prognosis
Prognosis is limited by the degree of heart failure due to LVEF reduction, cardiac fibrosis, and complications at the time of diagnosis. Predictors of LVEF recovery are shorter duration of methamphetamine abuse, absence of myocardial fibrosis, smaller left ventricular and left atrial size, and an r‐TCC pattern of initial hypokinesis.16, 38 In contrast, those with evidence of myocardial fibrosis and ventricular enlargement have limited scope for recovery. Interstitial fibrosis as determined by an increase in late gadolinium enhancement in cardiac MRI.55 seems to be an independent prognostic marker. A case report of a patient with severely reduced LVEF but no late gadolinium enhancement showed complete LVEF recovery.56 This has also been observed in a small prospective study of six patients57 and correlates to observations made in idiopathic DCM.58
3.11. Therapy
During acute intoxication with hypertensive crisis, both vasoconstrictive ⍺‐adrenergic and vasodilative β‐adrenergic receptors are activated. Thus, ⍺‐antagonists should be administered prior to β‐antagonists to avoid further increase of blood pressure. Sedation with benzodiazepines can help reduce intrinsic catecholamines. In the long‐term, nonselective beta blockers, i.e. carvedilol, should be administered. Complications, like aortic dissection, pulmonary oedema or stroke should be treated respectively.
Cardiogenic shock may require extracorporeal cardiac and lung support. In cases of persistently reduced LVEF after more than 2 months of complete drug abstinence under optimal medical and device therapy, an assist device (LVAD/BiVAD) or heart transplant should be considered.59 While many acute complications can be treated successfully, strict methamphetamine abstinence is the main challenge of long‐time therapy in MACM. In the early stages of the disease when cardiac fibrosis is still mild, moderate to complete recovery of LVEF can be achieved (Table 3) This also correlates to an improved functional NYHA‐class.16, 60
Table 3.
Echocardiographic findings in methamphetamine_‐_associated cardiomyopathy
| Parameter | Baseline | Continued abuse | Discontinued abuse |
|---|---|---|---|
| LVEF (%) | 19 ± 6 | 21 ± 4 | 43 ± 13 |
| LVEDD (mm) | 67.1 ± 7.4 | 68.2 ± 5.5 | 56.1 ± 6.7 |
Medical therapy for heart failure should be administered according to current guidelines. For patients with severely reduced LVEF, wearable defibrillators, or in case of persistency, the implantation of an implantable cardioverter‐defibrillator should be considered, but compliance is a major obstacle in this patient population.
Cardiac thrombi are common in MACM and often cause thromboembolic complications. If diagnosed, anticoagulation should be established. There is no scientific evidence regarding the optimal duration of therapy. An initial anticoagulant therapy of 6 months is recommended by the European Society of Cardiology in thrombi after myocardial infarction, whereas long‐term anticoagulation should be considered in case of recurrent thrombi. However, it is unclear if this recommendation can be transferred to thrombi in MACM, as the pathology leading to thrombus formation is different. There are no reliable data for the use of nonvitamin K antagonist oral anticoagulants.61
Referral to a medical rehabilitation centre should be recommended to all patients with frequent MA abuse. However, even after a successful initial treatment, relapse numbers remain high. Up to 61% of patients relapse in the first year, another 25% in the following 4 years.62 This emphasizes the importance of frequent outpatient contacts.
4. Conclusions
MA abuse has many deteriorating psychological and somatic effects, cardiac complications being among the major factors for morbidity and mortality. Drug abstinence is the mainstay of therapy, cardiac and other complications should be treated according to the respective guidelines. Incompliance to therapy and frequent relapses are the main challenges for successful treatment.
Conflict of interest
None declared.
Schwarzbach, V. , Lenk, K. , and Laufs, U. (2020) Methamphetamine‐related cardiovascular diseases. ESC Heart Failure, 7: 407–414. 10.1002/ehf2.12572.
References
- 1.Suwaki H. Methamphetamine abuse in Japan. NIDA Res Monogr 1991; 115: 84–98. [PubMed] [Google Scholar]
- 2.Anglin MD, Burke C, Perrochet B, Stamper E, Dawud‐Noursi S. History of the methamphetamine problem. J Psychoactive Drugs 2000; 32: 137–141. [DOI] [PubMed] [Google Scholar]
- 3.Crime UNOoDa . World drug report 2017. 2017.
- 4.EMCDDA EMCfDaDA . European drug report 2017. 2018.
- 5.de Matos H, Atzendorf, Kraus, Piontek . The consumption of new psychoactive substances and methamphetamine—analysis of data from 6 German federal states. Deutsches Ärzteblatt 2018; 115: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EMCDDA . Wastewater analysis and drugs: a European multi‐city study 2018.
- 7.Paratz ED, Cunningham NJ, MacIsaac AI. The cardiac complications of methamphetamines. Heart Lung Circ 2016. Apr; 25: 325–332. [DOI] [PubMed] [Google Scholar]
- 8.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 2007; 47: 681–698. [DOI] [PubMed] [Google Scholar]
- 9.Busto U, Bendayan R, Sellers EM. Clinical pharmacokinetics of non‐opiate abused drugs. Clin Pharmacokinet 1989; 16: 1–26. [DOI] [PubMed] [Google Scholar]
- 10.Cho AK. Ice: a new dosage form of an old drug. Science 1990; 249: 631–634. [DOI] [PubMed] [Google Scholar]
- 11.He SY, Matoba R, Fujitani N, Sodesaki K, Onishi S. Cardiac muscle lesions associated with chronic administration of methamphetamine in rats. Am J Forensic Med Pathol 1996; 17: 155–162. [DOI] [PubMed] [Google Scholar]
- 12.Islam MN, Kuroki H, Hongcheng B, Ogura Y, Kawaguchi N, Onishi S, Wakasugi C. Cardiac lesions and their reversibility after long term administration of methamphetamine. Forensic Sci Int 1995; 75: 29–43. [DOI] [PubMed] [Google Scholar]
- 13.Islam MN, Jesmine K, Kong Sn Molh A, Hasnan J. Histopathological studies of cardiac lesions after long term administration of methamphetamine in high dosage—Part II. Leg Med (Tokyo) 2009; 11: S147–S150. [DOI] [PubMed] [Google Scholar]
- 14.Yi SH, Ren L, Yang TT, Liu L, Wang H, Liu Q. Myocardial lesions after long‐term administration of methamphetamine in rats. Chin Med Sci J 2008; 23: 239–243. [DOI] [PubMed] [Google Scholar]
- 15.Karch SB. The unique histology of methamphetamine cardiomyopathy: a case report. Forensic Sci Int 2011; 212: e1–e4. [DOI] [PubMed] [Google Scholar]
- 16.Schurer S, Klingel K, Sandri M, Majunke N, Besler C, Kandolf R, Lurz P, Luck M, Hertel P, Schuler G, Linke A, Mangner N. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine‐associated cardiomyopathy. JACC Heart Fail 2017; 5: 435–445. [DOI] [PubMed] [Google Scholar]
- 17.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on M, Pericardial D . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648, 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 18.Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P 3rd, Everhart ET, Jones RT. Human pharmacology of the methamphetamine stereoisomers. Clin Pharmacol Ther 2006; 80: 403–420. [DOI] [PubMed] [Google Scholar]
- 19.Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine‐induced left ventricular dysfunction. Cardiovasc Res 2010; 87: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nestor TA, Tamamoto WI, Kam TH, Schultz T. Crystal methamphetamine‐induced acute pulmonary edema: a case report. Hawaii Med J 1989; 48: 457–458 460. [PubMed] [Google Scholar]
- 21.Varner KJ, Hein ND, Ogden BA, Arsenault JR, Carter KM, Soine WH. Chloroephedrine: contaminant of methamphetamine synthesis with cardiovascular activity. Drug Alcohol Depend 2001; 64: 299–307. [DOI] [PubMed] [Google Scholar]
- 22.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 2008; 27: 253–262. [DOI] [PubMed] [Google Scholar]
- 23.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest 2006; 130: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 24.Swalwell CI, Davis GG. Methamphetamine as a risk factor for acute aortic dissection. J Forensic Sci 1999; 44: 23–26. [PubMed] [Google Scholar]
- 25.Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. Can Med Assoc J 1975; 112: 299–304. [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs LJ. Reversible dilated cardiomyopathy induced by methamphetamine. Clin Cardiol 1989; 12: 725–727. [DOI] [PubMed] [Google Scholar]
- 27.Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine‐associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003; 41: 981–986. [DOI] [PubMed] [Google Scholar]
- 28.Ito H, Yeo KK, Wijetunga M, Seto TB, Tay K, Schatz IJ. A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clin Cardiol 2009; 32: E18–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiswell JG, Crago RM. Reversible cardiomyopathy with pheochromocytoma. Trans Am Clin Climatol Assoc 1969; 80: 185–195. [PMC free article] [PubMed] [Google Scholar]
- 30.Elian D, Harpaz D, Sucher E, Kaplinsky E, Motro M, Vered Z. Reversible catecholamine‐induced cardiomyopathy presenting as acute pulmonary edema in a patient with pheochromocytoma. Cardiology 1993; 83: 118–120. [DOI] [PubMed] [Google Scholar]
- 31.Satendra M, de Jesus C, Bordalo e Sa AL, Rosario L, Rocha J, Bicha Castelo H, Correia MJ, Nunes Diogo A. Reversible catecholamine‐induced cardiomyopathy due to pheochromocytoma: case report. Rev Port Cardiol 2014; 33: 177 e171–177 e176. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira VM, Marcelino M, Piechnik SK, Marini C, Karamitsos TD, Ntusi NAB, Francis JM, Robson MD, Arnold JR, Mihai R, Thomas JDJ, Herincs M, Hassan‐Smith ZK, Greiser A, Arlt W, Korbonits M, Karavitaki N, Grossman AB, Wass JAH, Neubauer S. Pheochromocytoma is characterized by catecholamine‐mediated myocarditis, focal and diffuse myocardial fibrosis, and myocardial dysfunction. J Am Coll Cardiol 2016; 67: 2364–2374. [DOI] [PubMed] [Google Scholar]
- 33.Richards JR, Harms BN, Kelly A, Turnipseed SD. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med 2018; 36: 1423–1428. [DOI] [PubMed] [Google Scholar]
- 34.Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, Alimineti K, Kimata C, Schatz IJ. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med 2007; 120: 165–171. [DOI] [PubMed] [Google Scholar]
- 35.Sutter ME, Gaedigk A, Albertson TE, Southard J, Owen KP, Mills LD, Diercks DB. Polymorphisms in CYP2D6 may predict methamphetamine related heart failure. Clin Toxicol (Phila) 2013; 51: 540–544. [DOI] [PubMed] [Google Scholar]
- 36.Kumai T, Inamasu J, Watanabe E, Sugimoto K, Hirose Y. Differences between Takotsubo cardiomyopathy and reverse Takotsubo cardiomyopathy associated with subarachnoid hemorrhage. Int J Cardiol Heart Vasc 2016; 11: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chehab O, Ioannou A, Sawhney A, Rice A, Dubrey S. Reverse Takotsubo cardiomyopathy and cardiogenic shock associated with methamphetamine consumption. J Emerg Med 2017; 53: e81–e83. [DOI] [PubMed] [Google Scholar]
- 38.Voskoboinik A, Ihle JF, Bloom JE, Kaye DM. Methamphetamine‐associated cardiomyopathy: patterns and predictors of recovery. Intern Med J 2016; 46: 723–727. [DOI] [PubMed] [Google Scholar]
- 39.Haning W, Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction 2007; 102: 70–75. [DOI] [PubMed] [Google Scholar]
- 40.Huang MC, Yang SY, Lin SK, Chen KY, Chen YY, Kuo CJ, Hung YN. Risk of cardiovascular diseases and stroke events in methamphetamine users: a 10‐year follow‐up study. J Clin Psychiatry 2016; 77: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 41.Malhotra R, Patel S, Ramchand T, Al NO. Higher defibrillation threshold in methamphetamine cardiomyopathy patients with implantable cardioverter‐defibrillator. Indian Pacing Electrophysiol J 2017; 17: 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng XZ, Shi YY, Chen KQ, Qiao XL, Wang LY. Evaluation of regional myocardial perfusion in methamphetamine abusers using real‐time myocardial contrast echocardiography. Med Ultrason 2019; 21: 56–61. [DOI] [PubMed] [Google Scholar]
- 43.Darke S, Duflou J, Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine‐related death: a national study. Drug Alcohol Depend 2017; 179: 174–179. [DOI] [PubMed] [Google Scholar]
- 44.Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. JAMA 1991; 265: 1152–1154. [PubMed] [Google Scholar]
- 45.Chen JP. Methamphetamine‐associated acute myocardial infarction and cardiogenic shock with normal coronary arteries: refractory global coronary microvascular spasm. J Invasive Cardiol 2007; 19: E89–E92. [PubMed] [Google Scholar]
- 46.Kanwar M, Gill N. Spontaneous multivessel coronary artery dissection. J Invasive Cardiol 2010; 22: E5–E6. [PubMed] [Google Scholar]
- 47.Lappin JM, Darke S, Farrell M. Stroke and methamphetamine use in young adults: a review. J Neurol Neurosurg Psychiatry 2017; 88: 1079–1091. [DOI] [PubMed] [Google Scholar]
- 48.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S. De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 2012; 79: 1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington H, Heller HA, Dawson D, Caplan L, Rumbaugh C. Intracerebral hemorrhage and oral amphetamine. Arch Neurol 1983; 40: 503–507. [DOI] [PubMed] [Google Scholar]
- 50.De Silva DA, Wong MC, Lee MP, Chen CL, Chang HM. Amphetamine‐associated ischemic stroke: clinical presentation and proposed pathogenesis. J Stroke Cerebrovasc Dis 2007; 16: 185–186. [DOI] [PubMed] [Google Scholar]
- 51.Rothrock JF, Rubenstein R, Lyden PD. Ischemic stroke associated with methamphetamine inhalation. Neurology 1988; 38: 589–592. [DOI] [PubMed] [Google Scholar]
- 52.Janardhanan R, Kannan A. Methamphetamine cardiotoxicity: unique presentation with multiple bi‐ventricular thrombi. Am J Med 2016; 129: e3–e4. [DOI] [PubMed] [Google Scholar]
- 53.Zhang LJ, Chen KQ, Shi YY, Qiao XL, Wang LY, Zheng XZ. Findings on 3D speckle tracking echocardiography in asymptomatic methamphetamine abusers. Int J Cardiovasc Imaging 2018; 34: 1589–1593. [DOI] [PubMed] [Google Scholar]
- 54.Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, Carbone I, Catalano C, Fedele F, Frustaci A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy‐proven acute myocarditis. JACC Cardiovasc Imaging 2014; 7: 254–263. [DOI] [PubMed] [Google Scholar]
- 55.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non‐ischaemic cardiomyopathies. Eur Heart J 2005; 26: 1461–1474. [DOI] [PubMed] [Google Scholar]
- 56.Lopez JE, Yeo K, Caputo G, Buonocore M, Schaefer S. Recovery of methamphetamine associated cardiomyopathy predicted by late gadolinium enhanced cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009; 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pujol‐López M, Ortega‐Paz L, Flores‐Umanzor EJ, Perea RJ, Bosch X. Cardiac magnetic resonance as an alternative to endomyocardial biopsy to predict recoverability of left ventricular function in methamphetamine‐associated cardiomyopathy. JACC: Heart Failure 2017; 5: 853–854. [DOI] [PubMed] [Google Scholar]
- 58.Leong DP, Chakrabarty A, Shipp N, Molaee P, Madsen PL, Joerg L, Sullivan T, Worthley SG, De Pasquale CG, Sanders P, Selvanayagam JB. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new‐presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J 2012; 33: 640–648. [DOI] [PubMed] [Google Scholar]
- 59.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed), 2016; 69: 1167. [DOI] [PubMed] [Google Scholar]
- 60.Sliman S, Waalen J, Shaw D. Methamphetamine‐associated congestive heart failure: increasing prevalence and relationship of clinical outcomes to continued use or abstinence. Cardiovasc Toxicol 2016; 16: 381–389. [DOI] [PubMed] [Google Scholar]
- 61.Habash F, Vallurupalli S. Challenges in management of left ventricular thrombus. Ther Adv Cardiovasc Dis 2017; 11: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long‐term perspective on patterns and predictors. Drug Alcohol Depend 2014; 139: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]