Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period (original) (raw)
Abstract
The coronavirus disease 2019 (COVID-19) pandemic is changing the management of many chronic diseases, including that of patients with inflammatory bowel diseases (IBD). In particular, the performance of routine endoscopy is temporarily suspended, and only emergency endoscopy is allowed in many countries where severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread. We highlight different scenarios in which endoscopy should still be performed urgently in patients with IBD, as well as recommendations regarding the use of personal protective equipment. We suggest a pathway for performing safe endoscopy and discuss the potential risks of postponing endoscopy in IBD. Finally, we propose a post-pandemic plan for access to endoscopy.
Introduction
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leading to coronavirus disease 2019 (COVID-19), was declared an official pandemic by WHO on March 11, 2020, at which point infections had been reported in 114 countries.1 As of April 1, 2020, there had been 823 626 cases of COVID-19 reported worldwide.2
COVID-19 is caused by SARS-CoV-2, a RNA virus belonging to the family of Coronaviridae that was first detected in Wuhan, China, in December, 2019. The main symptoms are sore throat, fever, cough, dyspnoea, sputum production, myalgia, fatigue, and headache.3 Diarrhoea is reported as a COVID-19-associated symptom in 2·0–35·6% of patients,4 alongside nausea or vomiting (1·0–17·3%).5 Other atypical symptoms may include loss of sense of smell or taste. The cell entry receptor ACE2 appears to mediate the entry of SARS-CoV-2, and is not only highly expressed in the lung cells, but also throughout the gastrointestinal tract;6 moreover, ACE2 is important in controlling intestinal inflammation, the disruption of which may lead to diarrhoea.7
Given the chronic nature of inflammatory bowel diseases (IBD) and the medications used to treat them, there is concern that patients with IBD may be at increased risk of infection or poorer disease course. This, together with the need to alter health-care utilisation during the pandemic, has led to rationing of endoscopic resources in this patient population. However, there are currently no specific endoscopic recommendations for patients affected by IBD based on direct evidence in the midst of the pandemic. Patients with IBD have been recommended to follow the general public health measures outlined by the WHO.8 Patients with IBD, especially those on systemic corticosteroids, thiopurine, and biologics, are considered to be moderate-to-high risk patients who are susceptible to COVID-19 and its complications. The general strategy expressed by the British Society of Gastroenterology (BSG), the International Organization for the study of Inflammatory Bowel Disease (IOIBD), the European Crohn's and Colitis Organisation (ECCO), and the Crohn's and Colitis Foundation of America (CCFA) is to reduce contact with health-care settings and thus possible exposure to COVID-19: all non-essential endoscopic procedures including colon cancer screening and those for patients with suspected gastrointestinal cancers have been cancelled, as these represent a risk for both patients and health-care personnel in an overstretched health-care environment.9, 10 Only emergency endoscopies are permitted.11
Therefore, before carrying out a diagnostic or therapeutic endoscopic examination in patients with IBD, the risks and benefits of performing the endoscopic procedure should be considered. It is important to ensure that the procedure is necessary and urgent. Candidate patients should be carefully selected, weighing the risks of transmission for patients and health-care professionals, and of making a trained endoscopy team available.
The characteristics of SARS-CoV-2 and its transmission make endoscopy a potential route for infection, and all endoscopies should be considered aerosol-generating procedures. Coughing, gagging, and retching can occur during upper endoscopy while passing flatus and pathogen-containing liquid stools can happen in colonoscopy. A prospective study has demonstrated that endoscopists are exposed to infectious particles during gastrointestinal procedures without recognising being exposed.12 In addition, SARS-CoV-2 may be detectable in stool for several weeks even after clinical recovery,13, 14 although whether stool shedding of viral particles can transmit infection is, at present, unclear.
It is not always clear when an endoscopy should be considered as urgent and needs to be performed in patients with IBD. We considered four different urgent scenarios that could necessitate endoscopy: confirmation of a new diagnosis, especially in a moderate-to-severe scenario when biologics may be chosen as a first-line treatment, given that high-dose corticosteroids might increase the risk of an adverse outcome for COVID-19; a severe acute flare-up in patients with ulcerative colitis; partial bowel obstruction in patients with IBD, which could be secondary to neoplasia or ileocolonic anastomotic stricture; and cholangitis and jaundice in patients with known primary sclerosing cholangitis (PSC) with dominant bile duct stricture. We also propose an endoscopy plan for gradual return to normal service post-pandemic.
In certain urgent situations, such as perianal abscess or fistula, emergency examination under anaesthesia and drainage or seton placement is necessary and the colorectal surgeon will generally perform an on-table flexible sigmoidoscopy to assess the rectum. We have not discussed these situations for an IBD endoscopist. In all other situations, use of non-invasive biomarkers, cross-sectional imaging such as ultrasonography, or video capsule enteroscopy to support wise clinical examination might be able to postpone or replace endoscopic investigations in patients with IBD during the pandemic.
During the pandemic
Scenario 1: new diagnosis of moderate-to-severe IBD
The threshold for doing endoscopy in patients presenting with abdominal pain and altered bowel habit has changed during the pandemic; adherence to first principles is even more important. First, before thinking about a new diagnosis of IBD, we must carefully consider the differential diagnosis and keep in mind the potential gastrointestinal manifestations of COVID-19: fever and respiratory symptoms are the most common presenting features, but these are not the only ones. Of note, the first case of SARS-CoV-2 infection confirmed in the USA reported a 2-day history of nausea and vomiting on admission to hospital and then passed two loose bowel movements.13 In a study from China, 103 (50·5%) of 204 patients presented with gastrointestinal symptoms, including anorexia, diarrhoea, and vomiting.15 If a patient has gastrointestinal symptoms that precede or follow fever, fatigue, and dry cough, a throat swab for SARS-CoV-2 should be performed to rule out infection. Patients with IBD have symptoms that are generally longer than the acute presentation of COVID-19.
After excluding SARS-CoV-2, other gastrointestinal infections should be excluded. Loose stools for more than 4 weeks usually allows discrimination of IBD-associated colitis from most cases of infectious diarrhoea.16 Microbiological analysis of stool samples must be done to exclude common pathogens and Clostridioides difficile.17
In this stressful period, we must not underestimate negative emotions, which can cause symptoms that mimic IBD: in patients with abdominal pain and altered bowel habits we must also assess emotional state (by phone) and rule out irritable bowel syndrome (IBS) clinically and by biomarkers. For differential diagnosis of IBS and to discriminate it from patients with IBD, assessment of clinical and biochemical features is fundamental. Faecal calprotectin should be requested, since it seems to be the most sensitive marker of intestinal inflammation in IBD; a cutoff of 150 μg/g (or alternative recommended thresholds for laboratory-based or point-of-care or home testing kits) discriminates between IBS and IBD.18 The operational characteristics of faecal calprotectin in the scenario of COVID-19 are unclear and the virus may be shed in stool, which should be taken into consideration before requesting this test. Home testing kits are preferable to laboratory testing as laboratory services may be overwhelmed.
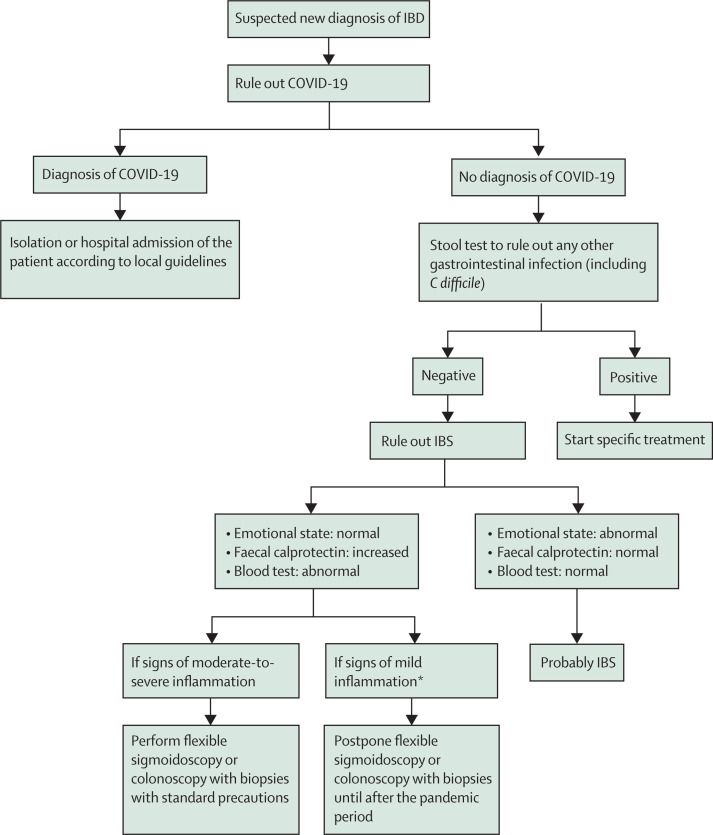
Following these initial investigations, endoscopy is warranted, in the form of either flexible sigmoidoscopy or colonoscopy with biopsies, for patients who continue to have symptoms and for whom there remains a high suspicion of moderate-to-severe IBD. This procedure should be done to establish a diagnosis and to determine severity before embarking on therapy, which might include early introduction of biologics. In addition, in patients with a clinical suspicion of Crohn's disease and who have a normal endoscopy, visualisation of the small intestine is necessary.17 Transabdominal ultrasonography may be useful and is relatively non-invasive. We suggest postponing endoscopic diagnosis for patients who show mild inflammation on blood tests and faecal calprotectin until the COVID-19 situation improves. These suggestions are summarised in the figure 1.
Figure 1.
Proposed flowchart for suspected new diagnosis of IBD
IBD=inflammatory bowel disease. COVID-19=coronavirus disease 2019. IBS=irritable bowel syndrome. *Mild defined as no complications, C-reactive protein not elevated or mildly elevated, haemoglobin >110 g/L, partial Mayo score 2–4 (for ulcerative colitis), Harvey Bradshaw Index 5–7 (for Crohn's disease).
Scenario 2: severe acute flare-up of ulcerative colitis
A severe acute flare-up in patients with ulcerative colitis during this pandemic period poses a substantial challenge. The patient could present with symptoms of severe ulcerative colitis (eg, bloody diarrhoea, abdominal pain, fever, and increased inflammatory biomarkers)19, 20 that could be similar to those of COVID-19. However, rectal bleeding is not common in COVID-19.
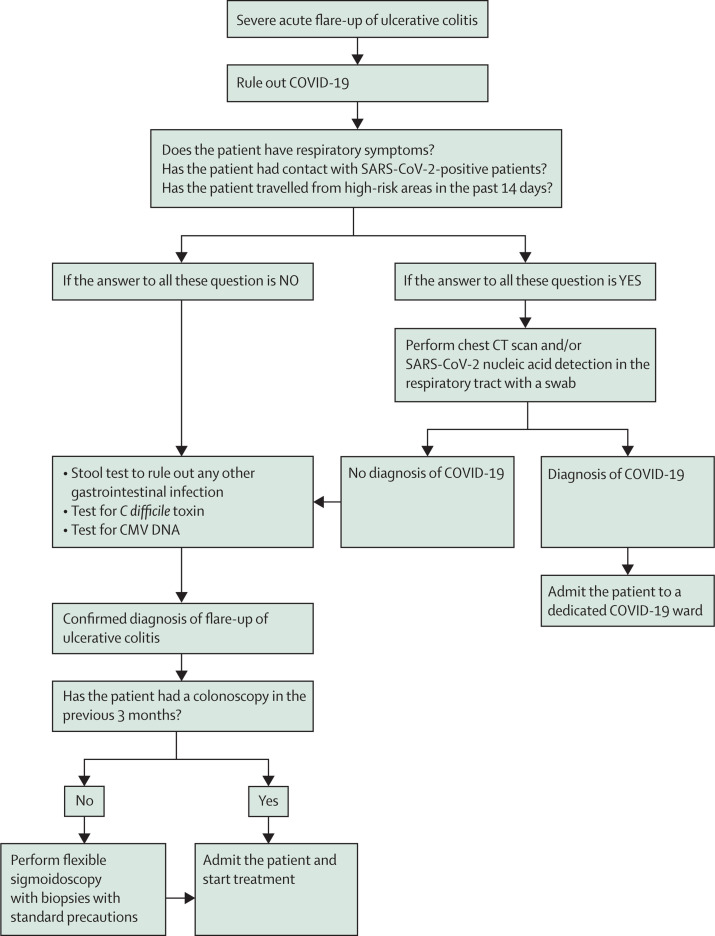
Therefore, the first step is to rule out the symptoms being secondary to or associated with COVID-19. Blood tests could be non-specific because an increase in inflammatory markers such as C-reactive protein and ferritin may occur in either disease, though occasionally ferritin levels can be low in ulcerative colitis. Patients with COVID-19 could have leucopenia (particularly lymphopenia) and thrombocytopenia,21 while patients with a flare-up of ulcerative colitis usually have normal or higher than normal leucocytes and lymphocytes and increased platelet count. If patients also present with respiratory symptoms (eg, cough, dyspnoea), or myalgia or fatigue,3 or they had contact with SARS-CoV-2-positive patients, or have travelled from areas deemed to be of high risk for COVID-19 in the previous 14 days, a chest CT scan, SARS-CoV-2 nucleic acid detection in the respiratory tract with a nasopharyngeal swab,22 or both, should be done to screen for infection. In all cases, standard stool tests should be done for intestinal bacteria (Salmonella, Shigella, Campylobacter, Yersinia, enteropathogenic and enterohaemorrhagic Escherichia coli) and for C difficile toxin, and a blood test for cytomegalovirus DNA should also be done to allow differential diagnosis with other causes of diarrhoea.20
Once the diagnosis of an acute severe flare-up of ulcerative colitis is considered likely and if the patient has not had a colonoscopy in the past 3 months, a flexible sigmoidoscopy (at a minimum) should be done to confirm the clinical suspicion, to define the extent (left sided or more extensive), and to take biopsies to rule out the presence of other infections (eg, cytomegalovirus).23, 24 Even in a pandemic situation, a baseline endoscopy is important to be able to start potentially high-risk therapy with conviction. If a patient has had a recent colonoscopy within the past 3 months that showed moderate-to-severe ulcerative colitis (Mayo endoscopic score 2–3), omission of flexible sigmoidoscopy might be left to the discretion of the gastroenterologist, given the risks presented by endoscopy during the pandemic, and to simply use the baseline panel of investigations described above to rule out infectious causes. One should note that elevated cytomegalovirus DNA titres, following discussion with colleagues in infectious disease, should always mandate flexible sigmoidoscopy.
Patients with a Mayo endoscopy score of 3 and systemic symptoms should be admitted, tested for COVID-19 if this has not already been done; intravenous treatment should be started as soon as possible.20 The use of corticosteroids during the COVID-19 pandemic is controversial.25 Biologics (eg, anti-TNF or anti-integrin agents) should also be considered, once any other infection is ruled out or as a rescue therapy.20, 26 While the choice of best therapy in the pandemic scenario is controversial, biologics may be preferred as a non-steroid option in epidemic regions to avoid adverse outcomes of COVID-19 due to steroid exposure. These suggestions are summarised in figure 2.
Figure 2.
Proposed flowchart for severe acute flare-up of ulcerative colitis
CMV=cytomegalovirus. COVID-19=coronavirus disease 2019.
Scenario 3: Sub-acute obstruction in patients with IBD
IBD is associated with development of multiple complications including sub-acute obstruction. Patients with ulcerative colitis and with colonic Crohn's disease have an increased risk of developing colorectal cancer,19 and so detection of a new colonic stricture is important and should be considered a priority to exclude malignancy.
The American Society for Gastrointestinal Endoscopy (ASGE), the European Society of Gastrointestinal Endoscopy (ESGE), and the BSG consider gastrointestinal obstruction needing urgent decompression or stenting to be among the procedures that should still be done during the pandemic.11 Obstructive strictures in patients with IBD are commonly accompanied by nausea, vomiting, weight loss, and abdominal pain, and require rapid medical, endoscopic, or surgical intervention.27
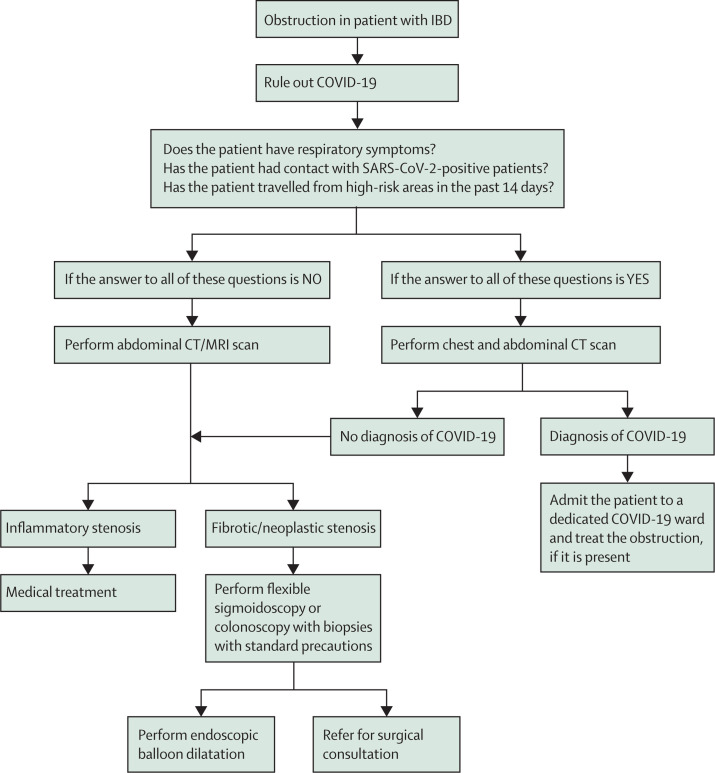
A patient with ulcerative colitis presenting with partial obstructive symptoms is of particular concern since this is highly suggestive of a new colorectal cancer. These patients should be a high priority for colonoscopy with minimal preparation for diagnosis by colonoscopy and biopsies. The operator may consider temporary stenting or balloon dilatation if surgery has to be postponed. However, cross-sectional imaging is more worthwhile in patients with Crohn's disease with partial obstructive symptoms, and endoscopy should be avoided if possible. Nevertheless, in patients known to have post-operative ileocolonic stricture requiring periodic endoscopic balloon dilatation, colonoscopy might be urgently necessary if these patients present with increasing sub-acute obstructive episodes. Medical treatment might alleviate a stricture with a predominantly inflammatory component, but 40% of patients might require urgent surgery for malignancy or benign stricture,28 which could be challenging in the pandemic situation. Patients under programmed sessions of endoscopic dilatations for strictures should not have these sessions deferred, since these patients might otherwise end up being hospitalised.
In patients with strictures at the site of ileocolonic anastomosis, which is characterised by a high rate of recurrence, the role of medical therapy is limited. Surgery has been the main treatment for those with symptomatic ileocolonic anastomosis strictures. However, up to 30% of patients with primary ileocolonic anastomosis could require multiple operations with postoperative morbidity and mortality.29 Evidence increasingly supports endoscopic balloon dilation as a safe and effective alternative to surgery, with the best results for anastomotic strictures rather than de-novo strictures30 and also for those with length of less than 4 cm.31, 32 Therefore, treatment is justified even in this pandemic scenario. These suggestions are outlined in figure 3.
Figure 3.
Proposed flowchart for sub-acute obstruction in patients with IBD
COVID-19=coronavirus disease 2019. IBD=inflammatory bowel disease.
Before starting the diagnostic pathway, risk assessment for COVID-19 should be done 1–2 days before endoscopy (preferably by phone) and also on the day of endoscopy for all partially obstructed patients. Testing for patients for COVID-19 is required, using RT-PCR whenever possible.33 This is important if the partially obstructed patient needs to have limited bowel preparation, since vomiting can be hazardous in an infected patient.
Scenario 4: worsening cholangitis and jaundice in patients with IBD and PSC
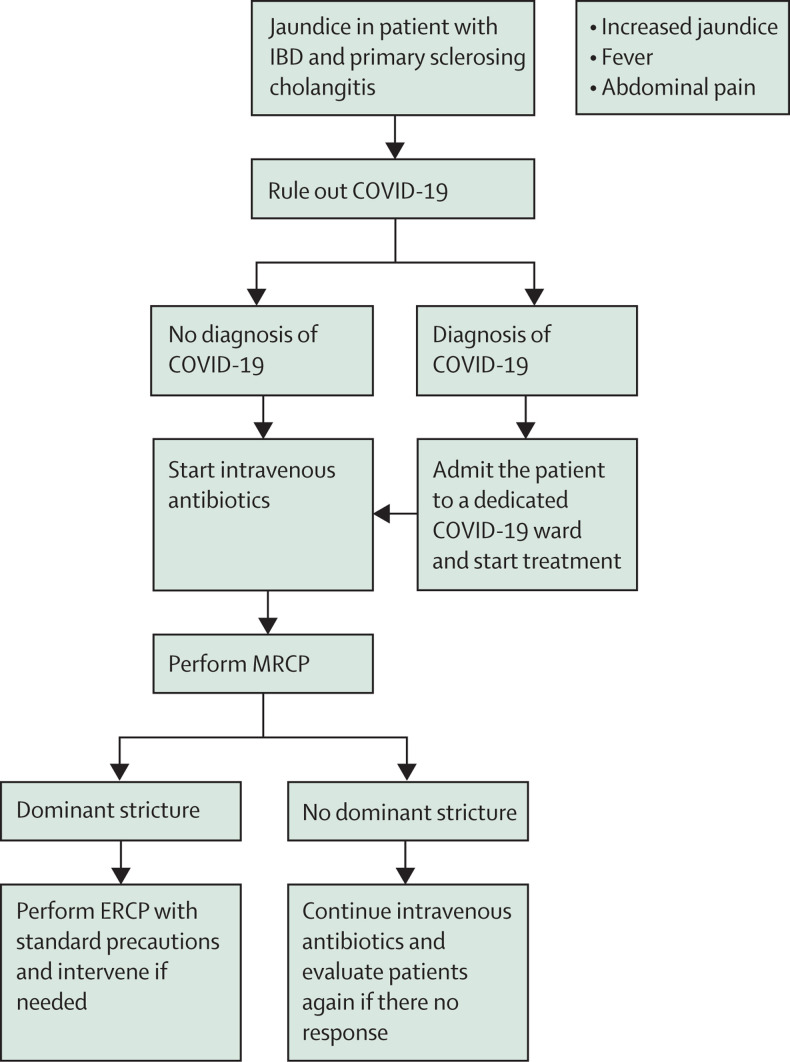
Cholangitis in a patient with PSC associated with IBD is an urgent presentation.34 In this cohort of patients, presentation with increasing jaundice, rising alkaline phosphatase concentrations, and cholangitis with evidence of dominant stricture on magnetic resonance cholangiopancreatography (MRCP) necessitates emergency or urgent endoscopic retrograde cholangiopancreatography (ERCP) and therapeutic intervention. This approach is necessary to relieve the biliary obstruction and obtain brushings for cytology and endobiliary biopsies to rule out cholangiocarcinoma, which can affect further management decisions. This approach is in accordance with the ESGE/European Association for the Study of the Liver guidelines35 and should also apply in the pandemic situation. We suggest ruling out COVID-19 before treatment; nonetheless, in the case of jaundice, the diagnosis of worsening cholangitis is more certain than in the previous scenarios. After imaging (eg, MRCP) to confirm the clinical diagnosis, we suggest performing ERCP with standard precautions for management of a dominant stricture. Planning for such a scenario should involve radiology and endoscopy personnel, as well as intensive care, if necessary. A flowchart for these patients is shown in figure 4.
Figure 4.
Proposed flowchart for cholangitis and jaundice in patients with IBD and PSC
COVID-19=coronavirus disease 2019. ERCP=endoscopic retrograde cholangiopancreatography. IBD=inflammatory bowel disease. MRCP=magnetic resonance cholangiopancreatography. PSC=primary sclerosing cholangitis.
PPE precautions for endoscopy
The presence of SARS-CoV-2 has been demonstrated in the faeces of 53·4% of patients with COVID-19;36 however, whether the viral RNA detected in faecal samples correlates with infectious viral particles is, at present, unclear.37 Nonetheless, there exists the possibility of faecal–oral transmission, although this also remains unproven. In light of these observations, colonoscopy should be considered as requiring protection for health-care staff, particularly the operator.38
ASGE39and ESGE40 have published general guidelines for infection control during endoscopy. Alongside the general precautions for safety of patients, doctors, and nurses, additional measures should be taken to avoid transmission of SARS-CoV-2, in particular, hand hygiene, use of personal protective equipment (PPE), and cleaning of the surfaces in the environment.
ESGE has also updated their guidelines for reprocessing endoscopes and endoscopic accessories using a standardised method, minimising the contact of instruments with the environment and staff. No cases of transmission of hepatitis B or C viruses, and of HIV, have been linked to endoscopy when appropriate cleaning has been performed.40
Safety should be optimised by stringent cleaning of the environment in which endoscopy is to be performed. SARS-CoV-2 is eliminated by common detergents,32, 40 and ultraviolet irradiation and ozone treatment should be used in endoscopy units to clean and sterilise the air, endoscopic equipment, office tables, and walls. Moreover, a chlorine-containing detergent is recommended for everyday floor cleaning.41 ASGE guidelines39 suggest performing endoscopy in a negative pressure room if respiratory infection is suspected, with the next patient not being admitted until at least 30 min have passed. However, this is not always feasible in many hospitals. Repici and colleagues42 suggest that the air in the endoscopy room should be cleaned by opening the windows and waiting at least 1 h before admitting the next patient. Endoscopy lists will have to be very slow.
Attention should also be given to the questioning of patients before the endoscopy, in particular about contact with infected or symptomatic people, whether there is someone in their family with fever, cough, or diarrhoea, or if the patient has travelled in the past 14 days to high-risk areas. Chest CT should be considered case-by-case and it should be performed in suspected cases to rule out COVID-19.43 At present, it might be best to consider all patients at high risk and to take adequate precautions.
The European Centre for Disease Prevention and Control (ECDC) suggest using a filtering face piece (FFP) respiratory class 2 or 3 (FFP2 or FFP3), goggles, or face shield to protect the eyes, together with long-sleeved water-resistant gowns and gloves during aerosol-generating procedures.44 Guidance on how to put on and remove PPE is available online.44 Colonoscopy and upper endoscopy will need to follow the same PPE protocol. We suggest wearing FFP2 or FFP3 and PPE throughout the whole endoscopy, including during interrogation of the patient and writing of the report post-endoscopy (appendix).
Repici and colleagues42 have published suggestions regarding how to perform safe routine endoscopy, stratifying patients into high, intermediate, and low risk of transmitting infection. This classification was initially created for patients with any condition that required endoscopy and at the beginning of the outbreak, when routine endoscopy was allowed; however routine endoscopy has now been discontinued in many hospitals. Therefore, all patients, including those with IBD, should be considered as high-risk patients before undergoing urgent endoscopy. Risk should be determined on the basis of a chest CT scan, SARS-CoV-2 RT-PCR testing of nasopharyngeal swab, or both, to rule out infection, and results should be double-checked. We recommend a careful evaluation of patients before their entry to the endoscopic unit. Negative swab tests for patients with a high index of suspicion for COVID-19 may need to be re-tested before admittance, depending on local guidance.
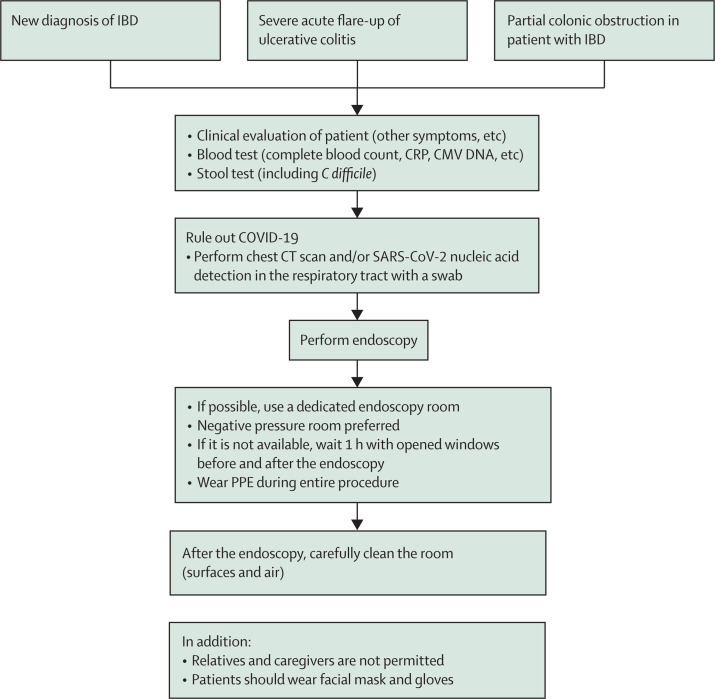
As patients with IBD are often receiving treatment with immunomodulators or biologics, they could be more susceptible to COVID-19; current global registries will provide further information in this regard.9 We suggest that such patients should wear facial mask and gloves and that, where possible, a separate endoscopy room should be used for these vulnerable patients. A flowchart for these patients is shown in figure 5.
Figure 5.
Proposed flowchart for endoscopy in patients with IBD during the COVID-19 pandemic
CMV=cytomegalovirus. COVID-19=coronavirus disease 2019. CRP=C-reactive protein. IBD=inflammatory bowel disease. PPE=personal protective equipment.
Risks in postponing endoscopy in patients with IBD
At present, it is uncertain how long the COVID-19 pandemic will continue, and thus we do not know how long it will be before endoscopic units return to functioning at normal capacity. In the era of treating to target, with the goal of mucosal healing, it may be necessary to re-evaluate and prioritise endoscopic needs post-pandemic. Patients with IBD have a chronic disease with periods of relapse and remission45 requiring endoscopy to monitor not only the efficacy of therapy46 but also to screen for dysplasia and colorectal cancer.47, 48 A prolonged period without endoscopy could have long-term implications for these patients.
Delayed surveillance colonoscopy could increase the risk of high-grade dysplasia and colorectal cancer diagnosis. The ideal system would organise regular surveillance colonoscopy to prevent development of colorectal cancer; however, the precise intervals between surveillance colonoscopies is uncertain in patients with IBD. Longer intervals may increase the probability of developing an advanced colorectal cancer.49 Many countries have established surveillance programmes, with patients starting surveillance with dye chromoendoscopy 8 years after the beginning of symptoms or every year after the diagnosis of PSC.23, 50 After the first colonoscopy, patients undergo further colonoscopies either every year, every 3 years, or every 5 years, depending on their risk.19, 51 A study published in 2019 indicated that after two consecutive negative colonoscopies and in the presence of no other risk factors, there is a low risk of advanced neoplasia and an interval longer than 2 years could be considered.52
One must also consider patients who have already had a diagnosis of low-grade dysplasia or high-grade dysplasia and who are waiting for endoscopic removal or have already had endoscopic removal and who should have the site of the resection checked. Data indicate that the risk of developing high-grade dysplasia or colorectal cancer in the 5 years after initial diagnosis of low-grade dysplasia is 19·5%; 20 patients out of 172 developed colorectal cancer, but only in six patients was it preceded by high-grade dysplasia.53 The risk for these patients with a diagnosis of low-grade dysplasia or high-grade dysplasia could be deemed moderate to high.
Patients with colonic IBD who have recently started therapy using agents such as biologics, JAK inhibitors, or azathioprine, should undergo endoscopy to assess the efficacy of treatment after 6–12 months.20 However, other tools could be used (eg, faecal calprotectin54 or bowel ultrasound17, 19). As such, endoscopic monitoring may not be essential.
A further risk is missed detection of early post-operative recurrence in patients with Crohn's disease after ileocolonic surgery. Ileocolonoscopy to check the anastomosis site should be performed within 6–12 months after the surgery.55 Indirect tools, such as faecal calcprotectin,56 bowel ultrasound, and magnetic resonance enterography, have been shown to be useful in disease monitoring and may help avoid endoscopy.55
In addition, the status of monitoring colonoscopies in clinical trials must be considered in discussion with trial sponsors and research ethics committees; often, these may be deferred or cancelled, but it is important to try to keep patients in clinical trials, as these provide important therapeutic options to patients where the alternatives may be more challenging in the current situation (eg, surgery).
Endoscopy in the post-pandemic period
When the pandemic comes to an end, we will face two major problems: prevention of new outbreaks and long waiting lists due to cancellations of endoscopic procedures in the pandemic period. The number of daily endoscopic examinations will not be able to return immediately to the levels seen pre-pandemic. Sensible plans to ramp up endoscopic examinations will need to be formulated. Selection and stratification of patients according to an order of priority will be necessary—ie, to anticipate endoscopies that cannot be deferred any further and to postpone others, following precise indications.
To prioritise, we could stratify patients according to clinical symptoms, blood test results, non-invasive inflammatory markers, use of biologics, history of recent bowel resection, and previous history of dysplasia or cancer. We propose three different degrees of priority with different time periods of postponement (panel). Collection of data during the post-pandemic period about the consequences of postponed endoscopy in the management of patients is essential.
Panel. Prioritising access to endoscopy for patients with IBD in the post-pandemic period.
1. Priority after pandemic
- •
Patients with mild-to-moderate flare-up confirmed by faecal calprotectin and blood tests (increased C-reactive protein, leucocytosis, anaemia): ileopancolonoscopy or sigmoidoscopy with biopsies to assess disease activity and to exclude cytomegalovirus infection - •
Patients with symptoms of mild sub-acute obstruction or high risk of cancer that have already had an MRI, CT, or ultrasound that confirmed intestinal narrowing - •
Patients with longstanding IBD in surveillance for colorectal cancer if they had dysplasia in the previous colonoscopy, primary sclerosing cholangitis, or a first-degree relative of a patient with colorectal cancer - •
Endoscopic resection in patients with low-grade or high-grade dysplastic colonic lesions detected in a previous colonoscopy - •
New diagnosis of IBD with faecal calprotectin and blood tests suggesting mild-to-moderate inflammation - •
Surveillance to prevent or detect post-operative recurrence within 1 year of surgery if the patient is symptomatic or faecal calprotectin and blood tests worsening - •
Symptomatic patients with moderate pouchitis and altered blood test results (eg, C-reactive protein) - •
Endoscopy after 6 months of biological therapy in symptomatic patients with altered blood tests or faecal calprotectin
2. Postponed with plan to perform endoscopy 3–6 months after pandemic period
- •
Surveillance to prevent or detect post-operative recurrence within 1 year of surgery if the patient has normal faecal calprotectin and blood test results - •
New diagnosis of IBD with faecal calprotectin and blood test results suggesting mild inflammation - •
Endoscopy after 6 months of biological therapy in asymptomatic patients with normal blood test and faecal calprotectin, to check for mucosal healing - •
Mild pouchitis
3. Postponed until after 6 months
- •
Patients in remission, confirmed by the most recent endoscopy and with normal faecal calprotectin and blood test results, to decide whether to continue biologics (since residual mucosal inflammation could lead to relapse soon after discontinuation of biologics) - •
Patients with flare-up not confirmed by faecal calprotectin and blood test results - •
Patients with longstanding IBD in surveillance for colorectal cancer if they had not had dysplasia, stricture, polyps, or histological inflammation in the previous two colonoscopies and in the absence of other risk factors
Conclusion
During the COVID-19 pandemic, it is important that gastroenterology teams continue to offer urgent endoscopic investigations and other relevant investigations to patients with IBD in specific situations—eg, to establish the diagnosis in a new presentation of moderate-to-severe IBD, to assess a patient with ulcerative colitis presenting with features of severe acute colitis, to make a diagnosis when a patient with IBD presents with sub-acute obstruction, and in cases of cholangitis with dominant bile duct stricture in patients with IBD and PSC. In all other situations, endoscopy could be deferred. However, postponed endoscopies may need to be prioritised according to a plan after routine endoscopies become feasible again in the post-pandemic period. All procedures should be performed with stringent safety precautions and PPE, since all endoscopies can generate aerosol droplets and pose a risk of transmission of SARS-CoV-2.
Search strategy and selection criteria
Sources for this paper were identified through searches of PubMed and Medline using the terms “Covid-19, Sars-Cov-2, inflammatory bowel disease, endoscopy” for the introduction and “diagnosis, flare-up, bowel obstruction, primary sclerosing cholangitis (PSC), personal protective equipment, colorectal cancer, dysplasia, mucosal healing, post-operative recurrence, clinical trial” for the scenarios. Of all resulting articles, we selected the latest guidelines of the major gastrointestinal and endoscopy societies and their last recommendations regarding the outbreak of COVID-19 (sometimes including online published suggestions), reviews, and articles for their clinical relevance and alignment to the aim of our paper, as well as relevant references cited in those articles.
This online publication has been corrected. The corrected version first appeared at thelancet.com/gastrohep on February 11, 2021
Acknowledgments
Acknowledgments
MI and SG are supported by the NIHR Birmingham Biomedical Research Centre University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Contributors
MI, SG, and BS drafted the outline and planned this review. MI, SG, RC, and NL drafted the manuscript and figures. RC, NL, RM, SD, GSK, RP, and BS provided critical appraisal of the manuscript. MI, SG, RC, NL, RM, RP, SD, GSK, and BS revised the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
Supplementary appendix
References
- 1.WHO WHO Director-General's opening remarks at the media briefing on COVID-19. 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2.WHO Coronavirus disease 2019 (COVID-19) situation report–72. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Y, Liu P, Shi XL, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-320891. published online March 5. [DOI] [PubMed] [Google Scholar]
- 5.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15047. published online March 25. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020 doi: 10.1101/2020.01.30.927806. published online Jan 31. [DOI] [Google Scholar]
- 7.Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020 doi: 10.1136/gutjnl-2020-320832. published online Feb 26. [DOI] [PubMed] [Google Scholar]
- 8.WHO Q&A on coronaviruses (COVID-19) https://www.who.int/news-room/q-a-detail/q-a-coronaviruses
- 9.Mao R, Liang J, Shen J, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30076-5. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crohn's & Colitis Foundation What IBD patients should know about the 2019 novel coronavirus (COVID-19) https://www.crohnscolitisfoundation.org/what-ibd-patients-should-know-about-2019-novel-coronavirus-covid-19
- 11.British Society of Gastroenterology BSG/JAG statement on bowel screening & endoscopy service provision. https://www.bsg.org.uk/covid-19-advice/bsg-jag-statement-on-bowel-screening-endoscopy-service-provision/
- 12.Johnston ER, Habib-Bein N, Dueker JM, et al. Risk of bacterial exposure to the endoscopist's face during endoscopy. Gastrointest Endosc. 2019;89:818–824. doi: 10.1016/j.gie.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000620. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116:1464–1486. doi: 10.1016/s0016-5085(99)70513-5. [DOI] [PubMed] [Google Scholar]
- 17.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 18.Lozoya Angulo ME, de Las Heras Gómez I, Martinez Villanueva M, Noguera Velasco JA, Avilés Plaza F. Faecal calprotectin, a useful marker in discriminating between inflammatory bowel disease and functional gastrointestinal disorders. Gastroenterol Hepatol. 2017;40:125–131. doi: 10.1016/j.gastrohep.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 20.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 21.Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med. 2020 doi: 10.1056/NEJMc2005203. published online March 27. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y-H, Cai L, Cheng Z-S, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273–284. doi: 10.1093/ecco-jcc/jjy114. [DOI] [PubMed] [Google Scholar]
- 24.British Society of Gastroenterology Endoscopy activity and COVID-19: BSG and JAG guidance – update 22.03.20. https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/
- 25.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J-H, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44:127–144. doi: 10.1111/apt.13670. [DOI] [PubMed] [Google Scholar]
- 27.Rieder F, Latella G, Magro F, et al. European Crohn's and Colitis Organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn's disease. J Crohns Colitis. 2016;10:873–885. doi: 10.1093/ecco-jcc/jjw055. [DOI] [PubMed] [Google Scholar]
- 28.Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn's disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68:1115–1126. doi: 10.1136/gutjnl-2018-318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lian L, Stocchi L, Remzi FH, Shen B. Comparison of endoscopic dilation vs surgery for anastomotic stricture in patients with Crohn's disease following ileocolonic resection. Clin Gastroenterol Hepatol. 2017;15:1226–1231. doi: 10.1016/j.cgh.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Ding NS, Yip WM, Choi CH, et al. Endoscopic dilatation of Crohn's anastomotic strictures is effective in the long term, and escalation of medical therapy improves outcomes in the biologic era. J Crohns Colitis. 2016;10:1172–1178. doi: 10.1093/ecco-jcc/jjw072. [DOI] [PubMed] [Google Scholar]
- 31.Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Endoscopic dilation is an efficacious and safe treatment of intestinal strictures in Crohn's disease. Aliment Pharmacol Ther. 2012;36:151–158. doi: 10.1111/j.1365-2036.2012.05146.x. [DOI] [PubMed] [Google Scholar]
- 32.Thienpont C, D'Hoore A, Vermeire S, et al. Long-term outcome of endoscopic dilatation in patients with Crohn's disease is not affected by disease activity or medical therapy. Gut. 2010;59:320–324. doi: 10.1136/gut.2009.180182. [DOI] [PubMed] [Google Scholar]
- 33.ESGE. ESGENA ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. https://www.esge.com/assets/downloads/pdfs/general/ESGE_ESGENA_Position_Statement_gastrointestinal_endoscopy_COVID_19_pandemic.pdf [DOI] [PMC free article] [PubMed]
- 34.Loftus EV, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aabakken L, Karlsen TH, Albert J, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49:588–608. doi: 10.1055/s-0043-107029. [DOI] [PubMed] [Google Scholar]
- 36.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. published online March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. published online April 1. [DOI] [PubMed] [Google Scholar]
- 38.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. published online March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ASGE Quality Assurance in Endoscopy Committee. Calderwood AH, Day LW, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Beilenhoff U, Biering H, Blum R, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - update 2018. Endoscopy. 2018;50:1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
- 41.Geller C, Varbanov M, Duval RE. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repici A, Maselli R, Colombo M, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.019. published online March 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Zhang X, Liu L, Wang H, Zhao Q. Suggestions for infection prevention and control in digestive endoscopy during current 2019-nCoV pneumonia outbreak in Wuhan, Hubei province, China. Endoscopy. 2020;52:312–314. doi: 10.1055/a-1128-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Centre for Disease Prevention and Control Guidance for wearing and removing personal protective equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19. https://www.ecdc.europa.eu/en/publications-data/guidance-wearing-and-removing-personal-protective-equipment-healthcare-settings
- 45.Stange EF, Travis SPL, Vermeire S, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 47.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 49.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 50.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ten Hove JR, Shah SC, Shaffer SR, et al. Consecutive negative findings on colonoscopy during surveillance predict a low risk of advanced neoplasia in patients with inflammatory bowel disease with long-standing colitis: results of a 15-year multicentre, multinational cohort study. Gut. 2019;68:615–622. doi: 10.1136/gutjnl-2017-315440. [DOI] [PubMed] [Google Scholar]
- 53.Choi CR, Ignjatovic-Wilson A, Askari A, et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol. 2015;110:1461–1471. doi: 10.1038/ajg.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J-F, Chen J-M, Zuo J-H, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–1415. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 55.Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135–149. doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 56.Scarpa M, D'Incà R, Basso D, et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn's disease. Dis Colon Rectum. 2007;50:861–869. doi: 10.1007/s10350-007-0225-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix