Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis (original) (raw)
. Author manuscript; available in PMC: 2020 Aug 13.
Published in final edited form as: N Engl J Med. 2020 Feb 13;382(7):622–631. doi: 10.1056/NEJMoa1803537
Abstract
BACKGROUND
More effective and safer treatments are needed for antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis.
METHODS
We conducted a randomized trial with a 2-by-2 factorial design to evaluate the use of plasma exchange and two regimens of oral glucocorticoids in patients with severe ANCA-associated vasculitis (defined by an estimated glomerular filtration rate of <50 ml per minute per 1.73 m2 of body-surface area or diffuse pulmonary hemorrhage). Patients were randomly assigned to undergo plasma exchange (seven plasma exchanges within 14 days after randomization) or no plasma exchange (control group). Patients were also randomly assigned to follow either a standard-dose regimen or a reduced-dose regimen of oral glucocorticoids. Patients were followed for up to 7 years for the primary composite outcome of death from any cause or end-stage kidney disease (ESKD).
RESULTS
Death from any cause or ESKD occurred in 100 of 352 patients (28.4%) in the plasma-exchange group and in 109 of 352 patients (31.0%) in the control group (hazard ratio, 0.86; 95% confidence interval [CI], 0.65 to 1.13; P = 0.27). The results were similar in subgroup analyses and in analyses of secondary outcomes. We also assessed the noninferiority of a reduced-dose regimen of glucocorticoids to a standard-dose regimen, using a noninferiority margin of 11 percentage points. Death from any cause or ESKD occurred in 92 of 330 patients (27.9%) in the reduced-dose group and in 83 of 325 patients (25.5%) in the standard-dose group (absolute risk difference, 2.3 percentage points; 90% CI, −3.4 to 8.0), which met the criterion for noninferiority. Serious infections at 1 year were less common in the reduced-dose group than in the standard-dose group (incidence rate ratio, 0.69; 95% CI, 0.52 to 0.93), but other secondary outcomes were similar in the two groups.
CONCLUSIONS
Among patients with severe ANCA-associated vasculitis, the use of plasma exchange did not reduce the incidence of death or ESKD. A reduced-dose regimen of glucocorticoids was noninferior to a standard-dose regimen with respect to death or ESKD. (Funded by the U.K. National Institute for Health Research and others; PEXIVAS Current Controlled Trials number, ISRCTN07757494; ClinicalTrials.gov number, NCT00987389.)
End-stage kidney disease (ESKD) and premature death remain common among patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis who present with reduced kidney function or pulmonary hemorrhage.1 Poor outcomes are attributed to a delay in diagnosis and to the use of treatments that have a slow onset of action, incomplete efficacy, and toxic effects.2 More effective, safer therapies are needed.
The rapid removal of ANCAs by means of plasma exchange may reduce organ damage from ANCA-associated vasculitis.3–6 However, the effect of plasma exchange added to immunosuppressive therapy as compared with immunosuppressive therapy alone on clinically important outcomes, such as death and ESKD, is uncertain.7
High-dose glucocorticoids were the first treatments found to be effective in ANCA-associated vasculitis, and they remain a cornerstone of disease management.8,9 However, glucocorticoids have numerous dose-dependent adverse effects, and high-quality data are lacking regarding an effective and relatively safe rate at which glucocorticoid doses can be tapered in patients with ANCA-associated vasculitis.10
We conducted the PEXIVAS trial in patients with severe ANCA-associated vasculitis to compare the efficacy of plasma exchange with no plasma exchange with respect to death or ESKD. The trial also compared a reduced-dose regimen of glucocorticoids with a standard-dose regimen over the first 6 months of the treatment period to determine whether the reduced dose was noninferior to the standard dose with respect to death or ESKD.
Methods
Trial Design
This randomized, controlled trial involving patients with severe, active ANCA-associated vasculitis had a 2-by-2 factorial design, which allowed separate evaluations of initial treatment with plasma exchange as compared with no plasma exchange (with either cyclophosphamide or rituximab administered to all patients) and of two different regimens of oral glucocorticoids. Details of the objectives, design, and methods of the trial have been published previously,11 and the final protocol, amended in 2014, is available with the full text of this article at NEJM.org.
Trial Oversight
The Cambridge University Hospitals NHS Foundation Trust oversaw the trial. No agreements concerning confidentiality of the data were in place between the investigators and the sponsors or the Cambridge University Hospitals NHS Foundation Trust. The Birmingham Clinical Trials Unit was responsible for randomization, maintaining the databases, managing the data, and performing the analyses. The trial was jointly coordinated by the Birmingham Clinical Trials Unit and the Lupus and Vasculitis Clinic, Addenbrooke’s Hospital. Terumo BCT, Fresenius Medical Care Australia, and Baxter Healthcare (Australia) provided in-kind supplies in some countries but had no role in the design or conduct of the trial, the collection or analysis of the data, or the writing of the manuscript. The trial management committee (the first, second, and last authors) designed the trial with assistance from the investigators, prepared the the data and for the fidelity of the trial to the protocol. A data and safety monitoring board reviewed unblinded trial data annually.
Patients
We recruited patients from June 2010 through September 2016. A detailed list of the eligibility criteria is provided in Section S2 in the Supplementary Appendix, available at NEJM.org. In brief, eligible patients were 15 years of age or older, had new or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, a history of a positive test for myeloperoxidase or proteinase 3 antibodies, and kidney involvement (with an estimated glomerular filtration rate of <50 ml per minute per 1.73 m2 of body-surface area) or pulmonary involvement (with diffuse alveolar hemorrhage). Ethics approval was obtained at each site, and patients or their surrogate decision makers provided written informed consent.
Treatments
Patients were randomly assigned with the use of a centralized Internet-based system in a 1:1:1:1 ratio to undergo plasma exchange and follow a standard-dose oral glucocorticoid regimen, to undergo plasma exchange and follow a reduced-dose oral glucocorticoid regimen, to undergo no plasma exchange and follow a standard-dose oral glucocorticoid regimen, or to undergo no plasma exchange and follow a reduced-dose oral glucocorticoid regimen. All patients received induction immunosuppressive therapy with either cyclophosphamide or rituximab. Randomization was performed with the use of a minimization algorithm, with stratification according to age (<60 years vs. ≥60 years), kidney function (serum creatinine level <5.6 mg per deciliter [500 _μ_mol per liter] vs. ≥5.6 mg per deciliter or use of dialysis), ANCA subtype (proteinase 3 vs. myeloperoxidase), severity of pulmonary hemorrhage (no hemorrhage vs. nonsevere hemorrhage vs. severe hemorrhage [defined as an oxygen saturation of ≤85% while the patient was breathing ambient air or the use of mechanical ventilation]), and planned type of induction immunosuppressive therapy (intravenous cyclophosphamide vs. oral cyclophosphamide vs. rituximab). All patients and investigators were aware of the trial-group assignments.
Before randomization, the choice of cyclophosphamide (intravenous or oral) or rituximab was made by the local investigator. With regard to the glucocorticoid regimens, all patients were treated with daily intravenous methylprednisolone for 1 to 3 days, for a maximum cumulative dose of 1 to 3 g, with the dosing decided by the local investigators. All patients then received oral prednisone or prednisolone, with the dose determined on the basis of the patient’s weight (<50 kg, 50 to 75 kg, or >75 kg). The standard-dose regimen was based on modifications of a regimen used in a contemporary international trial and was determined by consensus at a meeting of the investigators.12 Patients in the reduced-dose and standard-dose groups received identical treatments for the first week; at the start of the second week, the dose in the reduced-dose group was reduced by approximately 50%. The dose in the standard-dose group was tapered more gradually, starting in week 3. At 6 months, the cumulative dose of oral glucocorticoids in the reduced-dose group was less than 60% of that in the standard-dose group. After 22 weeks, both groups received 5 mg per day of prednisone or prednisolone until week 52, when local investigators determined subsequent oral glucocorticoid dosing. (All regimens and dosing procedures are described in Sections S3 through S5 and Tables S1 through S3.)
Patients assigned to undergo plasma exchange received 60 ml of albumin replacement per kilogram of body weight by means of centrifugation or filter separation; patients underwent seven treatments within 14 days after randomization. Plasma was allowed as replacement in patients at high risk for bleeding. (Details of the plasma-exchange regimen are provided in Section S6.)
Patients in whom refractory or early relapsing disease developed after randomization were treated with additional pulse glucocorticoids without plasma exchange. After 3 to 6 months of cyclophosphamide treatment, patients received azathioprine to maintain remission until at least week 52, after which time the maintenance therapy was chosen by the local investigator.
Patients were followed from randomization to a common closeout period that ended on July 30, 2017. The protocol originally stipulated a minimum follow-up of 2 years, but this was shortened to the common closeout after inspection of the aggregate data showed that most events occurred less than 12 months after randomization.
Outcomes
The primary outcome was a composite of death from any cause or ESKD, which was defined by 12 or more continuous weeks of renal-replacement therapy (hemodialysis or peritoneal dialysis) or by kidney transplantation. The secondary outcomes were death from any cause, ESKD, sustained remission, serious adverse events, serious infections within 1 year, and health-related quality of life. (Definitions of secondary outcomes are provided in Section S7.) To evaluate sustained remission, we used the Birmingham Vasculitis Activity Score for Granulomatosis with Polyangiitis (BVAS/GPA) to assess disease activity (scores range from 0 to 68, with a score of 0 indicating the absence of disease activity and higher scores indicating increasingly active disease).13 Quality of life was assessed with the use of the 36-Item Short-Form Health Survey (SF-36), version 2 (physical-component and mental-component summary scores), and with the EuroQol-5 Dimensions (EQ-5D) index score and health thermometer score.14,15
Statistical Analysis
For the comparison of plasma exchange with no plasma exchange, we calculated that 164 primary outcome events would provide the trial with 80% power to detect a hazard ratio with plasma exchange of 0.64, at a two-sided alpha level of 0.05. On the basis of data from previous trials, we estimated that 500 patients would need to be enrolled, with a follow-up of 2 to 7 years. In 2014, a review of the rate of death or ESKD among patients enrolled up to that time suggested that a larger sample size of 675 to 725 patients would be needed to obtain 164 events. We therefore planned to enroll 700 patients with a minimum follow-up of 1 year. These calculations assumed no interaction between plasma exchange and no plasma exchange.
The two glucocorticoid regimens were compared with the use of a noninferiority hypothesis for the primary outcome. The noninferiority margin was calculated on the basis of the sample size estimated for the comparison of plasma exchange with no plasma exchange. We calculated that with a sample size of 700 patients, the trial would have more than 80% power to show that the incidence of ESKD or death was no more than 11 percentage points higher with the reduced-dose regimen than with the standard-dose regimen, at a one-sided alpha level of 0.05.
Statistical analyses were performed with SAS software, version 9.3 and higher (SAS Institute), and Stata software, version 14 and higher (Stata-Corp). Analyses for each intervention were adjusted for the other assigned intervention and for each minimization variable. The reference groups for all analyses were the control group (no plasma exchange) and the standard-dose glucocorticoid regimen. Data for patients who were lost to follow-up or who were withdrawn were censored on the last day that the status with respect to the primary outcome was known. Because the statistical analysis plan did not include a provision for correcting for multiplicity, secondary and other outcomes are reported as point estimates and 95% confidence intervals. The widths of the confidence intervals have not been adjusted for multiplicity, so the intervals should not be used to infer definitive treatment effects for secondary outcomes.
For the analysis of plasma exchange, all patients were evaluated according to the assigned group, on the basis of the intention-to-treat principle. The primary composite outcome of death from any cause or ESKD was analyzed with the use of a Cox proportional-hazards model to obtain a hazard ratio and 95% confidence interval.
For the evaluation of the glucocorticoid regimens, our primary objective was to determine whether a reduced-dose regimen was noninferior to the standard-dose regimen with respect to the primary composite outcome. We used the per-protocol population for the primary analysis because an intention-to-treat analysis can increase the risk of falsely showing noninferiority.16 We defined the per-protocol population as patients assigned to the standard-dose group who took at least 70% of the protocol-specified dose in the first 6 months and patients in the reduced-dose group who took no more than 130% of the protocol-specified dose in the first 6 months. The absolute between-group difference in the risk of the primary composite outcome and the corresponding 90% confidence interval were calculated with the use of a binomial model with an identity-link function to compare the absolute risk difference with the noninferiority margin of 11 percentage points. The primary outcome was also analyzed in the two glucocorticoid groups with the use of Cox proportional-hazards models to obtain hazard ratios and 95% confidence intervals for the per-protocol and intention-to-treat populations; analyses for all other outcomes were performed according to the intention-to-treat principle.
For secondary outcomes, death from any cause and ESKD were analyzed separately with the use of Cox proportional-hazards models. The number of patients who had sustained remission and the number of patients with at least one serious adverse event were each compared between groups with the use of a log-binomial regression model to obtain the relative risk. The rates of serious infections in the first year and at the end of the trial were analyzed with the use of negative binomial regression, with an offset for the length of time patients were in the trial included in the model, to obtain incidence rate ratios. Differences in health-related quality of life outcome measures at 1 year were estimated with the use of linear mixed-effects models for repeated measures that included the interaction of time by treatment group, when appropriate.
We performed prespecified subgroup analyses of the primary outcome according to the minimization groups. We conducted the following three prespecified sensitivity analyses of the primary outcome: we limited the analysis to 1 year of follow-up; we assessed the effect of plasma exchange in a per-protocol population that included patients who died within 14 days after randomization, patients assigned to the plasma-exchange group who underwent at least one plasma exchange within 14 days, and patients assigned to the control group who did not undergo plasma exchange within 14 days; and we assessed the two glucocorticoid regimens in the intention-to-treat population. We also performed a post hoc sensitivity analysis to calculate the absolute risk difference in the intention-to-treat population in the comparison of glucocorticoid regimens.
Results
Patients
Our trial included 704 patients at 95 centers in 16 countries; 352 were assigned to undergo plasma exchange and 352 to undergo no plasma exchange; 353 patients were assigned to a reduceddose glucocorticoid regimen, and 351 were assigned to a standard-dose glucocorticoid regimen (Figs. S1 and S2). The median duration of follow-up was 2.9 years. The baseline characteristics are shown in Table 1. A total of 338 patients (96.0%) in the plasma-exchange group and 322 (91.5%) in the control group were included in the per-protocol population; 330 patients (93.5%) in the reduced-dose group and 325 (92.6%) in the standard-dose group were included in the per-protocol population.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Plasma Exchange (N = 352) | No Plasma Exchange (N = 352) | Reduced-Dose Glucocorticoid Regimen (N = 353) | Standard-Dose Glucocorticoid Regimen (N = 351) |
|---|---|---|---|---|
| Age — yr | 62.8±14.4 | 63.5±13.7 | 63.3±14.2 | 63.1±13.9 |
| Female sex — no. (%) | 149 (42.3) | 158 (44.9) | 156 (44.2) | 151 (43.0) |
| History of vasculitis — no. (%) | 35 (9.9) | 28 (8.0) | 34 (9.6) | 29 (8.3) |
| ANCA subtype — no. (%) | ||||
| Proteinase 3 | 143 (40.6) | 143 (40.6) | 143 (40.5) | 143 (40.7) |
| Myeloperoxidase | 209 (59.4) | 209 (59.4) | 210 (59.5) | 208 (59.3) |
| Median C-reactive protein level (IQR) — mg/liter | 50.9 (13.8–122.8) | 42.1 (14.0–97.2) | 44.6 (13.0–117.0) | 45.5 (14.0–98.0) |
| Median hemoglobin level (IQR) — g/liter | 94 (83–105) | 95 (85–105) | 95 (84–105) | 95 (84.5–105) |
| Kidney function | ||||
| Median serum creatinine level (IQR) — _μ_mol/liter | 327 (206–491) | 336 (209–495) | 320 (190–480) | 335 (219–502) |
| Serum creatinine level ≥500 _μ_mol/liter or undergoing dialysis — no. (%) | 101 (28.7) | 104 (29.5) | 102 (28.9) | 103 (29.3) |
| Undergoing dialysis — no. (%) | 66 (18.8) | 74 (21) | 67 (19.0) | 73 (20.8) |
| Severity of pulmonary hemorrhage — no. (%) | ||||
| No hemorrhage | 257 (73.0) | 256 (72.7) | 257 (72.8) | 256 (72.9) |
| Not severe | 64 (18.2) | 66 (18.8) | 65 (18.4) | 65 (18.5) |
| Severe† | 31 (8.8) | 30 (8.5) | 31 (8.8) | 30 (8.5) |
| Organ involvement — no. (%) | ||||
| Cutaneous | 37 (10.5) | 39 (11.1) | 34 (9.6) | 42 (12.0) |
| Mucous membranes or eyes | 30 (8.5) | 36 (10.2) | 30 (8.5) | 36 (10.3) |
| Ear, nose, and throat | 95 (27.0) | 103 (29.3) | 98 (27.8) | 100 (28.5) |
| Cardiovascular | 6 (1.7) | 4 (1.1) | 5 (1.4) | 5 (1.4) |
| Gastrointestinal | 2 (0.6) | 2 (0.6) | 1 (0.3) | 3 (0.9) |
| Pulmonary | 145 (41.2) | 149 (42.3) | 147 (41.6) | 147 (41.9) |
| Kidney | 342 (97.2) | 349 (99.1) | 346 (98.0) | 345 (98.3) |
| Nervous system | 37 (10.5) | 25 (7.1) | 33 (9.3) | 29 (8.3) |
| Other | 61 (17.3) | 59 (16.8) | 59 (16.7) | 61 (17.4) |
| Median BVAS/GPA (IQR)‡ | 9 (7–11) | 9 (7–11) | 9 (7–11) | 9 (7–11) |
| Planned immunosuppressive treatment — no. (%) | ||||
| Intravenous cyclophosphamide | 177 (50.3) | 177 (50.3) | 179 (50.7) | 175 (49.9) |
| Oral cyclophosphamide | 120 (34.1) | 121 (34.4) | 120 (34.0) | 121 (34.5) |
| Rituximab | 55 (15.6) | 54 (15.3) | 54 (15.3) | 55 (15.7) |
OUTCOMES ACCORDING TO PLASMA EXCHANGE
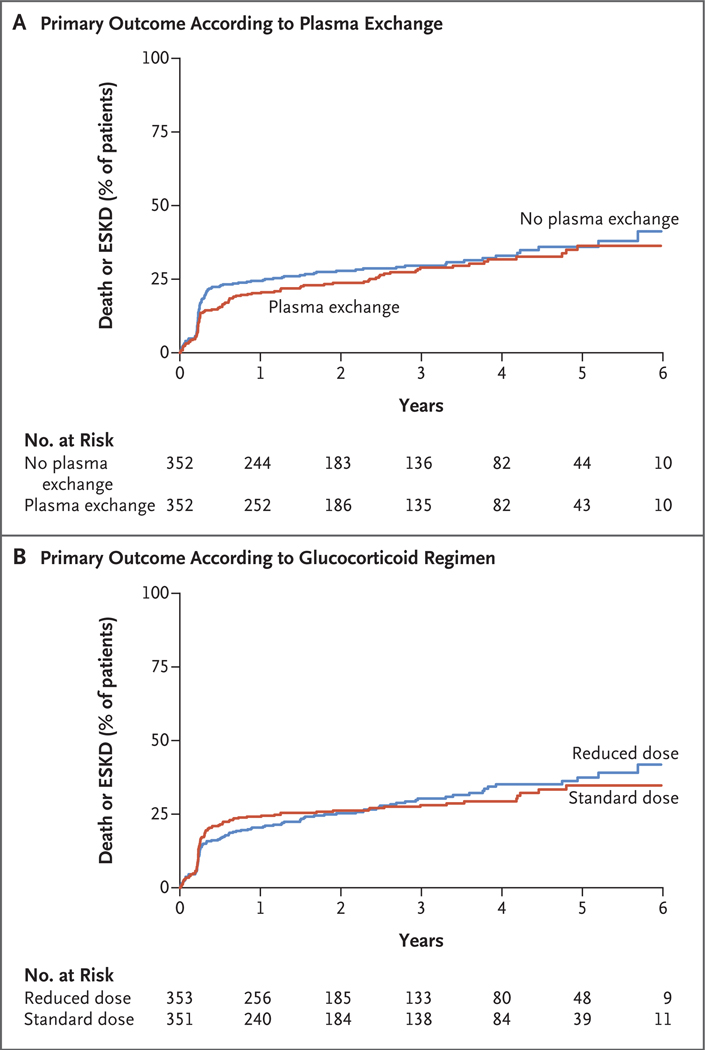
No interaction was detected between the glucocorticoid regimen and plasma-exchange assignments in the analysis of the primary outcome (P = 0.72). The effects of plasma exchange on the primary composite outcome are shown in Table 2 and Figure 1A. Death or ESKD occurred in 100 of 352 patients (28.4%) in the plasma-exchange group and in 109 of 352 (31.0%) in the control group (hazard ratio with plasma exchange, 0.86; 95% confidence interval [CI], 0.65 to 1.13; P = 0.27). The results of sensitivity analyses, including an analysis in which data were censored at 1 year, did not differ substantially from those of the primary analyses (Table 2). There was no evidence of interactions according to subgroup. (Subgroup analyses of the primary outcome are shown in Fig. S3.)
Table 2.
Primary Composite Outcome with Plasma Exchange as Compared with No Plasma Exchange.*
| Analysis | Plasma Exchange | No Plasma Exchange | Hazard Ratio (95% CI) |
|---|---|---|---|
| no. with outcome/total no. (%) | |||
| Primary analysis† | 100/352 (28.4) | 109/352 (31.0) | 0.86 (0.65–1.13) |
| Partially adjusted analysis‡ | 100/352 (28.4) | 109/352 (31.0) | 0.89 (0.68–1.17) |
| Per-protocol analysis | 95/338 (28.1) | 99/322 (30.7) | 0.85 (0.64–1.13) |
| Analysis at 1-year follow-up | 70/352 (19.9) | 85/352 (24.1) | 0.77 (0.56–1.06) |
Figure 1. Kaplan–Meier Curves for the Primary Outcome.
The primary composite outcome was death from any cause or end-stage kidney disease (ESKD). In a trial with a 2-by-2 factorial design, patients with severe antineutrophil cytoplasm antibody–associated vasculitis were assigned to undergo plasma exchange or no plasma exchange (Panel A) and to follow either a reduced-dose regimen or a standard-dose regimen of oral glucocorticoids (Panel B).
Secondary outcomes are summarized in Table 3 and Table S4. There were no significant differences between the plasma-exchange group and the control group in secondary outcomes, including serious adverse events.
Table 3.
Secondary Outcomes.*
| Secondary Outcome | Plasma Exchange vs. No Plasma Exchange | Reduced-Dose vs. Standard-Dose Glucocorticoid Regimen |
|---|---|---|
| effect size (95% CI) | ||
| Death from any cause | 0.87 (0.58–1.29) | 0.78 (0.53–1.17) |
| End-stage kidney disease | 0.81 (0.57–1.13) | 0.96 (0.68–1.34) |
| Sustained remission | 1.01 (0.89–1.15) | 1.04 (0.92–1.19) |
| Serious adverse events | 1.21 (0.96–1.52) | 0.95 (0.75–1.20) |
| Serious infections at 1 year | 1.16 (0.87–1.56) | 0.69 (0.52–0.93) |
OUTCOMES ACCORDING TO GLUCOCORTICOID REGIMENS
The effect of a reduced-dose regimen on the primary outcome is shown in Figure 1B. In the per-protocol population, death or ESKD occurred in 92 of 330 patients (27.9%) in the reduced-dose group and in 83 of 325 (25.5%) in the standard-dose group. The reduced-dose regimen was noninferior to the standard-dose regimen with respect to the primary outcome (absolute risk difference, 2.3 percentage points; 90% CI, −3.4 to 8.0; 95% CI, −4.5 to 9.1). The results were similar in the sensitivity analysis in the intention-to-treat population: death or ESKD occurred in 107 of 353 patients (30.3%) in the reduced-dose group and in 102 of 351 (29.1%) in the standard-dose group (absolute risk difference, taking into account differential follow-up time, 0.01 percentage points; 95% CI, −5.1 to 5.1). An analysis of the primary outcome with the use of survival methods yielded a hazard ratio of 1.04 (95% CI, 0.81 to 1.33) in the per-protocol population, a hazard ratio of 1.00 (95% CI, 0.76 to 1.31) in the intention-to-treat population with the use of all follow-up data, and a hazard ratio of 0.80 (95% CI, 0.58 to 1.10) with data censored at 1 year. There was no evidence of interactions according to subgroup (Fig. S4).
During the period between randomization and 1 year, 142 serious infections occurred in 96 patients (27.2%) in the reduced-dose group and 180 occurred in 116 patients (33.0%) in the standard-dose group (incidence rate ratio, 0.69; 95% CI, 0.52 to 0.93). No other secondary outcomes differed significantly between the two groups (Table 3).
The types and frequencies of serious adverse events were similar in the reduced-dose group and the standard-dose group. (The most common types of serious adverse events are shown in Table S5.) Serious adverse kidney-related events were more common in the reduced-dose group (unadjusted risk ratio, 1.84; 95% CI, 1.18 to 2.87), although the incidence of ESKD did not differ between the groups (hazard ratio, 0.96; 95% CI, 0.68 to 1.34).
Discussion
Our trial in patients with severe ANCA-associated vasculitis showed that plasma exchange did not result in a lower incidence of death or ESKD than no plasma exchange. Reduced exposure to oral glucocorticoids was noninferior to a standard-dose regimen with respect to the risk of death or ESKD and resulted in a lower risk of serious infections in the first year of treatment.
Previous trials have suggested a substantial benefit of plasma exchange in patients with severe kidney disease with respect to reducing the need for dialysis at 12 months.7,17,18 In contrast, our trial did not show large effects. Several factors may have contributed to the difference between our results and those of previous trials. Effect estimates in previous studies may have been confounded by the small number of events19 or by systematic errors from bias resulting from unclear assignment methods.7 Alternatively, improvements in diagnosis, immunosuppression, and supportive care may have reduced the benefits of adjuvant plasma exchange in our trial. Previous studies have also suggested an effect of plasma exchange on ESKD without a benefit with respect to survival, despite the strong association of ESKD with mortality,7 whereas the effects of plasma exchange on ESKD and death were concordant in our trial. This difference among the findings highlights the possibility of chance findings in small trials and the importance of larger trials with longer follow-up.
International guidelines recommend the use of plasma exchange for the treatment of ANCA-associated vasculitis with pulmonary hemorrhage on the basis of observational studies that included fewer than 70 patients.20–24 Our trial targeted the inclusion of patients with pulmonary hemorrhage and enrolled 191 such patients, of whom 61 had severe pulmonary hemorrhage, and the results do not support a treatment effect in patients with pulmonary hemorrhage that differs from that in patients without pulmonary hemorrhage.
Our trial compared two regimens of oral glucocorticoids in ANCA-associated vasculitis. Variations of the two regimens are frequently used.12,25–27 Previous trials that reduced exposure to cyclophosphamide did not show that the reduced exposure lowered infection rates, and some trials showed a higher long-term risk of relapse.12,27–29 Therefore, showing, within the limits of precision that we could estimate, that the reduced-dose regimen decreased the risk of serious infections without increasing the risk of other adverse events represents an important step toward standardizing care.
Our trial had several strengths. It was a large trial involving patients with ANCA-associated vasculitis, and we used an objective, easily ascertained patient-focused outcome. Adherence to the assigned treatments was good, and there were few losses to follow-up. The consistency of the treatment effects across sensitivity analyses and subgroups was also reassuring. In addition, the international enrollment and broad eligibility criteria of the trial enhance the generalizability of the results.
Our trial had several limitations. First, it was an open-label trial, which exposed it to potential bias resulting from crossover, differential use of other therapies, and differential outcome ascertainment.30 However, crossover was infrequent, no other therapies are known to reduce the risk of death or ESKD, and our outcome ascertainment was unlikely to be biased. Second, although the trial was large for a study of a rare disease, some estimates had wide confidence intervals, and differences in outcomes with respect to plasma exchange or the reduced-dose glucocorticoid regimen remain possible. The probability of an undetected benefit must be weighed against the probabilities of additional expenses and inconveniences, as well as harms (such as serious infections) and complications (such as transfusion-related acute lung injury).31
In conclusion, the current trial did not show that the addition of plasma exchange to standard therapy conferred benefits in patients with severe ANCA-associated vasculitis, but it did show that a reduced-dose regimen of oral glucocorticoids was noninferior to a standard-dose regimen.
Supplementary Material
Supplement1
Acknowledgments
Supported by grants from the National Institute for Health Research, United Kingdom (HTA 08/56/04); the Food and Drug Administration (FDA R01 FD003516); the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54 AR0573319); the National Institutes of Health of the U.S. Department of Health and Human Services; the National Health and Medical Research Council, Australia (626939, APP1086192, 631731, and APP1092957); the Canadian Institutes of Health Research (211079 and 159662); the French Ministry of Health, Programme Hospitalier de Recherche Clinique (AOM11142); the Research Committee on Intractable Vasculitides of the Ministry of Health, Labor, and Welfare of Japan; and the Japan Agency for Medical Research and Development (AMED): the Strategic Study Group to Establish the Evidence for Intractable Vasculitis Guideline. Terumo BCT, Terumo BCT Mexico, Fresenius Medical Care Australia, Baxter Healthcare (Australia), and Asahi Kasei Medical provided in-kind plasma-exchange disposables. Dr. Walsh was supported by a New Investigator Award from the Canadian Institutes of Health Research.
Dr. Merkel reports receiving consulting fees from AbbVie, Biogen, CSL Behring, Genzyme, Insmed, Janssen, Kiniska, and Sparrow, grant support and consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, GlaxoSmithKline, and InflaRx, grant support from Kypha, and supplies from Terumo BCT; Dr. Puéchal, receiving study drugs from Roche, lecture fees from Pfizer, honoraria from LFB, and travel support from Sanofi; Dr. Hawley, receiving grant support from Amgen and Shire; Dr. Khalidi, receiving advisory board fees and study drugs from Roche and study drugs from Bristol-Myers Squibb; Dr. Wald, receiving grant support and consulting fees from Baxter; Dr. Pagnoux, receiving grant support, advisory board fees, and fees for serving on a speakers bureau from Roche, fees for serving on an adjudication committee from ChemoCentryx, advisory board fees from GlaxoSmithKline, and fees for serving on a data and safety monitoring board from Sanofi; Dr. Specks, receiving grant support from Genentech, Bristol-Myers Squibb, ChemoCentryx, and GlaxoSmithKline and consulting fees from AstraZeneca and InsMed; Dr. Tesar, receiving advisory board fees from ChemoCentryx, Fresenius Medical Care, Baxter, Amgen, Retrophin, Calliditas, and AbbVie; Dr. Flores-Suárez, receiving lecture fees from Roche Mexico and Nippon Boehringer Ingelheim; Dr. Guillevin, receiving advisory board fees from Lilly, AstraZeneca, and Janssen; and Dr. Jayne, receiving consulting fees from ChemoCentryx, InflaRx, and InsMed.
We thank the patients and their families; the numerous research coordinators and staff at each of the participating centers; Professor Keith Wheatley for his advice on methodology in the development of the trial; Professors Martin Landray and Richard Watts and Dr. Jonathan Emberson for serving on the data and safety monitoring committee; and Professors Andrew Rees, David Scott, and Paul Roderick for serving on the trial steering committee.
Footnotes
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
*
A complete list of the PEXIVAS collaborators is provided in the Supplementary Appendix, available at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
References
- 1.Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011; 70: 488–94. [DOI] [PubMed] [Google Scholar]
- 2.Little MA, Nightingale P, Verburgh CA, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010; 69:1036–43. [DOI] [PubMed] [Google Scholar]
- 3.Xiao H, Heeringa P, Hu P, et al. Anti-neutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002; 110: 955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao H, Heeringa P, Liu Z, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 2005; 167: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little MA, Smyth L, Salama AD, et al. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol 2009; 174: 1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis — a meta-analysis. Rheumatology (Oxford) 2012; 51:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh M, Catapano F, Szpirt W, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis 2011; 57:566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baggenstoss AH, Shick RM, Polley HF. The effect of cortisone on the lesions of periarteritis nodosa. Am J Pathol 1951; 27: 537–59. [PMC free article] [PubMed] [Google Scholar]
- 9.Treatment of polyarteritis nodosa with cortisone: results after three years: report to the Medical Research Council by the Collagen Diseases and Hypersensitivity Panel. Br Med J 1960; 1: 1399–400. [PMC free article] [PubMed] [Google Scholar]
- 10.Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc 1955; 158:459–63. [DOI] [PubMed] [Google Scholar]
- 11.Walsh M, Merkel PA, Peh CA, et al. Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials 2013; 14: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363: 221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum 2001; 44: 912–20. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE. User’s manual for the SF-36v2 health survey. Lincoln, RI: Quality Metric, 2007. [Google Scholar]
- 15.Brooks R EuroQol: the current state of play. Health Policy 1996; 37: 53–72. [DOI] [PubMed] [Google Scholar]
- 16.International Conference on Harmonisation; guidance on statistical principles for clinical trials; availability — FDA: notice. Fed Regist 1998; 63: 49583–98. [PubMed] [Google Scholar]
- 17.Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007; 18: 2180–8. [DOI] [PubMed] [Google Scholar]
- 18.Walters GD, Willis NS, Craig JC. Interventions for renal vasculitis in adults: a systematic review. BMC Nephrol 2010; 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorlund K, Imberger G, Walsh M, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis — a simulation study. PLoS One 2011; 6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapter 13: pauci-immune focal and segmental necrotizing glomerulonephritis. Kidney Int Suppl (2011) 2012; 2: 233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz J, Winters JL, Padmanabhan A, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher 2013; 28:145–284. [DOI] [PubMed] [Google Scholar]
- 22.Klemmer PJ, Chalermskulrat W, Reif MS, Hogan SL, Henke DC, Falk RJ. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis 2003; 42: 1149–53. [DOI] [PubMed] [Google Scholar]
- 23.Lauque D, Cadranel J, Lazor R, et al. Microscopic polyangiitis with alveolar hemorrhage: a study of 29 cases and review of the literature. Medicine (Baltimore) 2000; 79: 222–33. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39: 42–7. [DOI] [PubMed] [Google Scholar]
- 25.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349: 36–44. [DOI] [PubMed] [Google Scholar]
- 26.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA 2010; 304: 2381–8. [DOI] [PubMed] [Google Scholar]
- 27.Jones RB, Tervaert JWC, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–20. [DOI] [PubMed] [Google Scholar]
- 28.Harper L, Morgan MD, Walsh M, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis 2012; 71: 955–60. [DOI] [PubMed] [Google Scholar]
- 29.Walsh M, Faurschou M, Berden A, et al. Long-term follow-up of cyclophosphamide compared with azathioprine for initial maintenance therapy in ANCA-associated vasculitis. Clin J Am Soc Nephrol 2014; 9: 1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackett DL. Commentary: measuring the success of blinding in RCTs: don’t, must, can’t or needn’t? Int J Epidemiol 2007; 36:664–5. [DOI] [PubMed] [Google Scholar]
- 31.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood 2005; 105:2266–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1