ACDC, a global database of amphibian cytochrome-b sequences using reproducible curation for GenBank records (original) (raw)
Abstract
Genetic data are a crucial and exponentially growing resource across all biological sciences, yet curated databases are scarce. The widespread occurrence of sequence and (meta)data errors in public repositories calls for comprehensive improvements of curation protocols leading to robust research and downstream analyses. We collated and curated all available GenBank cytochrome-b sequences for amphibians, a benchmark marker in this globally declining vertebrate clade. The Amphibia’s Curated Database of Cytochrome-b (ACDC) consists of 36,514 sequences representing 2,309 species from 398 genera (median = 2 with 50% interquartile ranges of 1–7 species/genus). We updated the taxonomic identity of >4,800 sequences (ca. 13%) and found 2,359 (6%) conflicting sequences with 84% of the errors originating from taxonomic misidentifications. The database (accessible at 10.6084/m9.figshare.9944759) also includes an R script to replicate our study for other loci and taxonomic groups. We provide recommendations to improve genetic-data quality in public repositories and flag species for which there is a need for taxonomic refinement in the face of increased rate of amphibian extinctions in the Anthropocene.
Subject terms: Taxonomy, Evolutionary biology, Genetic markers, Phylogenetics
| Measurement(s) | DNA • mitochondrial_DNA • cytochrome b |
|---|---|
| Technology Type(s) | digital curation • bioinformatics analysis |
| Sample Characteristic - Organism | Amphibian |
Machine-accessible metadata file describing the reported data: 10.6084/m9.figshare.12587744
Background & Summary
Genetic data repositories are a key research component across scientific disciplines that rely on genetic sequences correctly assigned to a reference taxonomy. Although mistaken identity and composition of sequences within those repositories have long been acknowledged1–5, broad-scale data-quality evaluations remain scarce6–8 and rarely translate into improved databases. Therefore, the uncertainty of genetic data in global platforms such as GenBank3,9,10 represents a paramount obstacle for robust downstream analyses. Critically, quality-screening efforts can resolve misidentification of known, cryptic and undescribed taxa8,11, and inform the definition of reliable taxonomical units for management and biodiversity research12,13.
The widespread sequencing of, and access to, mitochondrial DNA (mtDNA) has boosted taxonomic studies via integrative taxonomy, barcoding, bioprospection, phylogenetics, phylogeography, population and conservation genetics, biogeography, macroecology, and paleoecology14–16. Available mtDNA data outcompetes nuclear DNA data in taxonomic coverage across the ‘Tree of Life’ mainly due to the popularity of 16 S, cytochrome-b (Cytb) and cytochrome oxidase 1 (Cox1) loci, while multiple sequences per species of those loci have proved crucial to define species limits17–19. While Cox1 was proposed as a universal barcode genetic marker20, GenBank’s Cytb records are currently more abundant than Cox1 for all five major vertebrate groups (Table 1).
Table 1.
GenBank records for Cytochrome-b (Cytb) and Cytochrome oxidase subunit I (Cox1) for the main five vertebrate groups.
| Organism | Cytb records | Cox1 records |
|---|---|---|
| Amphibia | 46,116 | 23,675 |
| Aves | 52,136 | 36,573 |
| Fish | 507,149 | 364,802 |
| Mammalia (except humans) | 140,367 | 58,249 |
| Reptilia | 57,998 | 15,471 |
| Total | 803,766 | 498,770 |
Amphibians have the highest rate of newly discovered vertebrate species21 given intense taxonomic efforts11. These ectotherms are however the most threatened vertebrates on Earth22,23, with many species facing extinction owing to emerging and spreading diseases24,25, habitat loss26 and climate change27. Therefore, accurate phylogenetic identification11,28,29 remains critical for future research and conservation actions. Here, we present the Amphibia’s Curated Database of Cytochrome-b sequences (ACDC30, 10.6084/m9.figshare.9944759), a comprehensive and curated database of all amphibian Cytb sequences available in GenBank. We targeted Cytb because it is the most common genetic marker, with the broadest genus- and species-level taxonomic coverage, in the amphibian literature31,32.
We created ACDC30 following a multi-step process implemented in a bioinformatic pipeline combining data retrieval from GenBank, local sequence alignments and quantification of genetic divergences (Fig. 1). On 01 February 2018, we retrieved a total of 39,202 Cytb sequences. Following curation (see Methods), ACDC contains 36,514 unique sequences representing 398 genera and 2,309 species (median = 2 species/genus with 50% interquartile ranges of [1,7]). For 1,363 species and 74 of the 75 amphibian families, there is more than one sequence available (Summary_statistics_ACDC.xlsx30) (median = 7 [3,22] species/family). ACDC represents 29% of the 7,963 currently known amphibian species covering most clades33. Despite the taxonomic accuracy of GenBank records seems to be accurate above the genus level34, our work demonstrates that the problematic issues mostly occur at the species level, and case-by-case assessments of taxonomic identity are necessary.
Fig. 1.
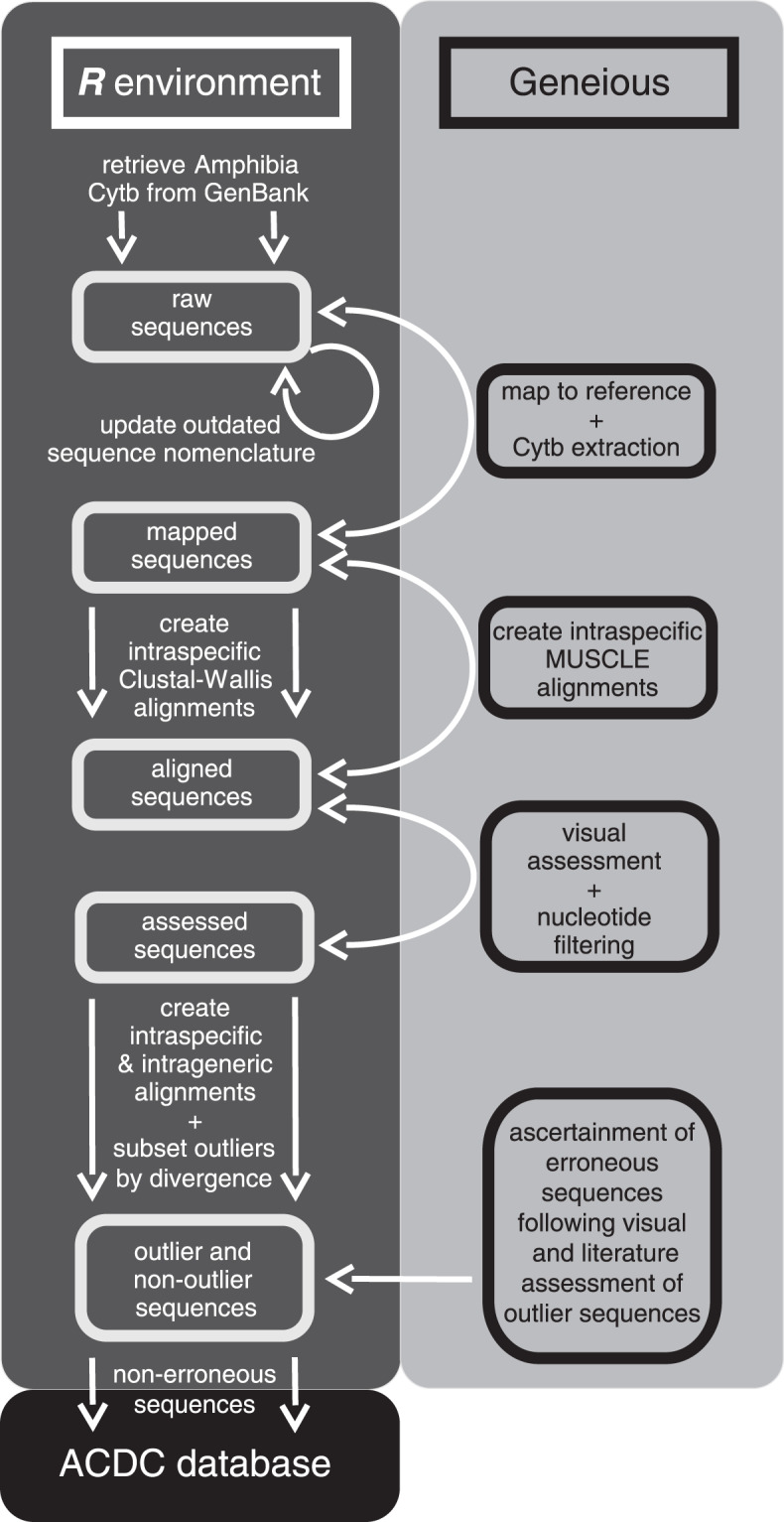
Workflow to collate and curate The Amphibia’s Curated Database of Cytochrome-b sequences (ACDC).
We identified 2,359 conflictive sequences (6% of the collated dataset) from 1,603 Anura, 743 Caudata, and 13 Gymnophiona records. These sequences suffered from wrong taxonomic assignments (>80%), contamination, introgression/hybridization, and submission/ sequencing errors (Fig. 2, Erroneous_sequences.xlsx30) and, as such, they qualify to be tagged as ‘UNVERIFIED’35 in GenBank. We updated the taxonomic identity of ca. 4,800 GenBank records (Taxonomic_corrections.xlsx30), and reverse-complemented reads from >1,000 sequences incorrectly uploaded as backward reads. We provide summary tables listing species/sequences with an uncertain taxonomic assignment (sp./ssp./cf./aff.; Uncertain_taxonomy_to_be_assessed.xlsx30) and potentially belonging to species complexes (Species_notes.xlsx30). These results suggest that several amphibian groups are in need of taxonomic revision. Lastly, we address general recommendations to improve data quality in public genetic repositories (Table 2) and append an R script30 to apply our data-curation protocol to other taxa and loci.
Fig. 2.
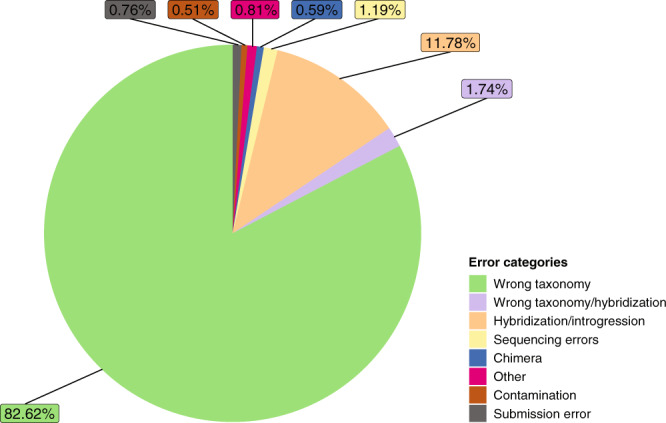
Frequency of error categories in amphibian Cytochrome-b sequences identified from GenBank sequences (01/02/2018). Those errors affect 6% (n = 2,359) of the sequences retrieved. Sequences identified due to incomplete lineage sorting are lumped in ‘Hybridization/Introgression’. Category definitions are explained in Erroneous_sequences.xlsx30.
Table 2.
Recommendations to improve the quality of (meta)data reported in GenBank.
| Recommendation | Audience | |
|---|---|---|
| 1. | Create a GenBank’s default notification system whereby data users can report errors and uncertainties to data owners. | Authors GenBank |
| 2. | Change editing restrictions for GenBank’s ‘DEFINITION’ field allowing authorities to make changes under GenBank personnel’s supervision. GenBank could assign specific taxa to specific experts very much like the assessment of the conservation status of target taxa is assigned to working groups by the International Union for Conservation of Nature. | GenBank |
| 3. | Synchronize GenBank-record identity with manuscript identity, especially cf., aff. and unidentified species (e.g., sp. 1/2/3). GenBank could grant a label of excellence to contribute to data improvement and make it available online for curricular purposes. | Authors GenBank |
| 4. | Before submission to GenBank, users should BLAST their sequences against the GenBank database to detect taxonomic inconsistencies, contamination, and identical sequences already available in GenBank. We recommend that all intrageneric alignments are always visually checked using the range of powerful tools available in commercial and free-source genetic software (e.g., CLC Workbench, Geneious, MEGA). | Authors |
| 5. | GenBank should not remain blasé about accumulating uncertainty, and instead be proactive to resolve taxonomic vagueness as shown in our study (i.e., 1,836 amphibian sequences currently reported as cf./aff./sp./ssp.; see Uncertain_taxonomy_to_be_assessed.xlsx30). Thus, justification of taxonomic assignments above the species level should be part of the data-submission protocol. | GenBank Authors |
| 6. | While improving GenBank reporting etiquette is crucial, how GenBank information is reported in the literature is equally important. Authors should cite in their publications GenBank accession numbers along with full details of each study specimen and sequence (namely sampling locality, specimen identity, assigned phylogenetic clade/lineage/haplotype, and cross-references to published figures/tables). Reporting this information could be enforced as a compulsory requirement for publication by journals and would facilitate data curation in public repositories. | Authors Journal editors |
Ideally, the research community would benefit from future sequencing efforts giving full taxonomic coverage to a selected sample of loci, which could in turn improve our understanding of amphibian biodiversity, evolution, ecology or conservation. mtDNA markers are still the best candidates to implement those efforts, as they are easy to amplify (even in poorly preserved samples), align and curate36. Taxonomic coverage of mtDNA can also be widened as a by-product of full-transcriptome and -genome assemblage, including long-read Next Generation Sequencing. In that respect, the development, integration, and expansion of quality-curated databases like ACDC should promote the generation of novel genomic data covering multiple specimens per species across the amphibian tree of life.
Methods
Workflow
Within the R environment37, on 01/02/2018, we used a key-word string to select and download all amphibian Cytb sequences from the GenBank’s website (www.ncbi.nlm.nih.gov/genbank, National Centre for Biotechnology Information) – see Steps 1–3 in the ACDCv1.0.R script30. We eliminated duplicates using GenBank labels ‘NC’, adjusted the nomenclature of each sequence to conform a genus_species_accession format (e.g., Bufo_bufo_AB123456), and exported all sequences as a single *.fasta file (Step 430). This includes single Cytb sequences, as well as mitochondrial genomes that contain this locus. All these sequences were then mapped against a reference mitochondrial genome (Xenopus tropicalis, AY789013), using the ‘high sensitivity’ option in Geneious® v11.038, and we extracted Cytb nucleotidic sequences (Fig. 1). Then, the nomenclature of all unique taxonomic identities was compared, confirmed and, if applicable, updated (Step 530) against the Amphibian Species of the World Database33.
We exported all mapped Cytb sequences in a *.fasta file from Geneious to the R environment. Therein, we performed ClustalW39 multiple sequence alignments for each species separately using the R package Bioconductor (Step 630). The resulting intraspecific alignments were imported back to Geneious as *.fasta files for batch-alignment through the MUSCLE algorithm (Fig. 1). The former step was mandatory because batch-MUSCLE alignments of multiple sequences (muscle function40 in Bioconductor) does not reorder sequences based on genetic similarity (A.T. Kalinka, pers. comm., 06/08/2018). Within Geneious, we visually resolved nucleotide gaps using the Vertebrate Mitochondrial Code41, and removed sequence ends with ambiguous nucleotides.
Taxonomic assessment and curation
We quantified accuracy on the assignation of sequences to species based on the genetic divergence (%) among sequences within species and genera and the identification of divergence outliers. We implemented three steps to detect sequencing and taxonomic errors based on pairwise-sequence alignments within each genus (Step 7; see Technical Validation). We used ‘uncorrected divergence’ as the genetic distance between every pair of sequences, using the seqinr package42. Firstly, we accepted sequences showing ≤3% divergence within multiple alignments across all sequences of the same species, and subset those with >3% divergence for further examination. Secondly, we also accepted sequences showing >3% divergence within a genus and subset those with ≤3% divergence for further examination. We caution that 3% is a reliable (conservative) divergence threshold for amphibian Cytb43–46 but should be re-estimated for other loci and taxonomical groups. Thirdly, for all potentially erroneous sequences, we assessed taxonomic and geographical veracity against (I) the data-source publication cited in GenBank, (II) the most recent papers dealing the taxon involved, (III) AmphibiaWeb (https://amphibiaweb.org) and (IV) the Amphibian Species of the World Database33 (Fig. 1). References and rationale used to separate erroneous from non-erroneous sequences are given for each sequence (Erroneous_sequences.xlsx30). We removed all erroneous sequences from ACDC and compiled all Amphibia Cytb sequences with uncertain taxonomy (aff./cf./sp./ssp.) (Uncertain_taxonomy_to_be_assessed.xlsx30). Lastly, the curation of genetic data is dependent on the number of available sequences per species and the taxonomic coverage per genus. Therefore, we included summary data for the ACDC database (Summary_statistics_ACDC.xlsx30) to flag species in need of more data and taxonomic resolution in online genetic repositories.
Lastly, our R script includes a routine to assess the Cytb region that maximizes species coverage and number of sequences (Supplementary Files 1 and 2). To do so, we first mapped all ACDC sequences to the Cytb of X. tropicalis (AY789013) using the ‘highest sensitivity’ option in Geneious, then counted non-missing bases for each position (Step 830).
Data Records
The curated database, all files as well as the associated R script are freely available on figshare30. The database consists of two compressed batches of *.fasta files of species with (I) 1 sequence (Species_with_One_Sequence.zip) and (II) > 1 sequences (Species_with_Multiple_Sequences.zip).
Technical Validation
We implemented a three-step sequence of filters to assess Cytb-sequence quality. (I) We retained sequences with complete binominal nomenclature. (II) We mapped all sequences against the Xenopus tropicalis mitochondrial genome (AY789013) and reverse-complemented sequences incorrectly submitted in backward-read format (>1,000). (III) We visually scanned sequence alignments for sequencing errors, whereby non-amino acid gaps (≠3) were filled or replaced by ‘N’ in the absence or presence of diversity at the base in question, respectively.
Supplementary information
Acknowledgements
We are grateful to Angus and Malcolm Young, Brian Johnson, Cliff Williams, and Phill Rudd for their contribution to a productive and relaxing working atmosphere. This work was supported by the Ministerio de Ciencia y Competitividad grant CGL2017-89898-R (AEI/FEDER, EU) grant to DRV.
Author contributions
D.R.V. designed the study. M.P.v.d.B. curated and filtered the data, and wrote the first draft. S.H.P. wrote the R script. All authors contributed to the Data Descriptor and contributed to revisions.
Code availability
The R script used to collate and curate the Amphibia Cytb database is available at figshare (ACDCv1.0.R30).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Matthijs P. van den Burg, Email: thijs.burg@gmail.com
David R. Vieites, Email: vieites@mncn.csic.es
Supplementary information
is available for this paper at 10.1038/s41597-020-00598-9.
References
- 1.Brunak S, Engelbrecht J, Knudsen S. Neural network detects errors in the assignment of mRNA splice sites. Nucleic Acids Res. 1990;18:4797–4801. doi: 10.1093/nar/18.16.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris D. Can you bank on GenBank? Trends Ecol. Evol. 2003;18:317–319. doi: 10.1016/S0169-5347(03)00150-2. [DOI] [Google Scholar]
- 3.Wesche PL, Gaffney DJ, Keightley PD. DNA sequence error rates in Genbank records estimated using the mouse genome as a reference. DNA Seq. 2004;15:362–364. doi: 10.1080/10425170400008972. [DOI] [PubMed] [Google Scholar]
- 4.Buhay JE. “COI-like” Sequences are becoming problematic in molecular systematic and DNA barcoding studies. J. Crustac. Biol. 2009;29:96–110. doi: 10.1651/08-3020.1. [DOI] [Google Scholar]
- 5.Alkan C, Sajjadian S, Eichler EE. Limitations of next-generation genome sequence assembly. Nat. Methods. 2011;8:61–65. doi: 10.1038/nmeth.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machida RJ, Leray M, Ho S-L, Knowlton N. Data Descriptor: Metazoan mitochondrial gene sequence reference dataset for taxonomic assignment of environmental samples. Sci. Data. 2017;4:170027. doi: 10.1038/sdata.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heller P, Casaletto J, Ruiz G, Geller J. Data Descriptor: A database of metazoan cytochrome c oxidase subunit I gene sequences derived from GenBank with CO-ARBitrator. Sci. Data. 2018;5:180156. doi: 10.1038/sdata.2018.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, et al. Detection of potential problematic Cytb gene sequences of fishes in GenBank. Front. Genet. 2018;9:30. doi: 10.3389/fgene.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prada CF, Boore JL. Gene annotation errors are common in the mammalian mitochondrial genomes database. BMC Genomics. 2019;20:73. doi: 10.1186/s12864-019-5447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross HA, Murugan S. Using phylogenetic analyses and reference datasets to validate the species identities of cetacean sequences in GenBank. Mol. Phylogenetics Evol. 2006;40:866–871. doi: 10.1016/j.ympev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Vieites DR, et al. Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc. Natl. Acad. Sci. 2009;16:8267–8272. doi: 10.1073/pnas.0810821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Y-Y, Chen X, Murphy RW. Assessing DNA barcoding as a tool for species identification and data quality control. PLoS ONE. 2013;8:e57125. doi: 10.1371/journal.pone.0057125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morin PA, et al. Applied conservation genetics and the need for quality control and reporting of genetic data used in fisheries and wildlife management. J. Hered. 2010;101:1–10. doi: 10.1093/jhered/esp107. [DOI] [PubMed] [Google Scholar]
- 14.Gershoni M, Templeton AR, Mishmar D. Mitochondrial bioenergetics as a major motive force of speciation. BioEssays. 2009;31:642–650. doi: 10.1002/bies.200800139. [DOI] [PubMed] [Google Scholar]
- 15.Toews DPL, Brelsford A. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 2012;21:3907–3930. doi: 10.1111/j.1365-294X.2012.05664.x. [DOI] [PubMed] [Google Scholar]
- 16.Ballard JWO, Pichaud N. Mitochondrial DNA: More than an evolutionary bystander. Funct. Ecol. 2013;28:218–231. doi: 10.1111/1365-2435.12177. [DOI] [Google Scholar]
- 17.Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Čandek K, Kuntner M. DNA barcoding gap: Reliable species identification over morphological and geographical scales. Mol. Ecol. 2014;15:268–277. doi: 10.1111/1755-0998.12304. [DOI] [PubMed] [Google Scholar]
- 19.Liu, J. et al. Multilocus DNA barcoding – Species Identification with multilocus data. Sci. Rep. 7, 10.1038/s41598-017-16920-2 (2017). [DOI] [PMC free article] [PubMed]
- 20.Herbert PD, Cywinska A, Ball SL, de Waard JR. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köhler J, et al. New amphibians and global conservation: A boost in species discoveries in a highly endangered vertebrate group. BioSience. 2005;55:693–696. doi: 10.1641/0006-3568(2005)055[0693:NAAGCA]2.0.CO;2. [DOI] [Google Scholar]
- 22.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 23.IUCN. The IUCN Red List of Threatened Species. Version 2018-2 (2019).
- 24.Martel A, et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science. 2014;346:630–631. doi: 10.1126/science.1258268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lips KR. Overview of chytrid emergence and impacts on amphibians. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150465. doi: 10.1098/rstb.2015.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cushman SA. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006;128:231–240. doi: 10.1016/j.biocon.2005.09.031. [DOI] [Google Scholar]
- 27.Winter M, et al. Patterns and biases in climate change research on amphibians and reptiles: A systematic review. R. Soc. Open Sci. 2016;3:160158. doi: 10.1098/rsos.160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, et al. Prevalence of cryptic species in morphologically uniform taxa – Fast speciation and evolutionary radiation in Asian frogs. Mol. Phylogenetics Evol. 2018;127:723–731. doi: 10.1016/j.ympev.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Funk WC, Caminer M, Ron SR. High levels of cryptic species diversity uncovered in Amazonian frogs. Proc. R. Soc. Lond. B Biol. Sci. 2011;279:1806–1814. doi: 10.1098/rspb.2011.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Burg MP, Herrando-Pérez S, Vieites DR. 2020. ACDC, a curated database of amphibian cytochrome-b sequences. figshare. [DOI] [PMC free article] [PubMed]
- 31.Grant T, et al. Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae) Bull. Am. Mus. Nat. Hist. 2006;121:1–263. doi: 10.1206/0003-0090(2006)299[1:PSODFA]2.0.CO;2. [DOI] [Google Scholar]
- 32.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenetics Evol. 2011;61:543–583. doi: 10.1016/j.ympev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Frost, D. R. Amphibian Species of the World: an Online Reference, Version 6.0. American Museum of Natural Historyhttp://research.amnh.org/herpetology/amphibia/index.html (2018).
- 34.Layer M, et al. GenBank is a reliable resource for 21st century biodiversity research. Proc. Natl. Acad. Sci. 2019;116:22641–22656. doi: 10.1073/pnas.1911714116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benson DA, et al. GenBank. Nucleic Acids Res. 2012;40:48–53. doi: 10.1093/nar/gkr1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison RG. Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Trends Ecol. Evol. 1989;4:6–11. doi: 10.1016/0169-5347(89)90006-2. [DOI] [PubMed] [Google Scholar]
- 37.R v.3.6.2. (R Core Team, 2018).
- 38.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodenhofer U, Bonatesta E, Horejs-Kainrath C, Hochreiter S. msa: An R package for multiple sequence alignment. Bioinformatics. 2015;31:3997–3999. doi: 10.1093/bioinformatics/btv494. [DOI] [PubMed] [Google Scholar]
- 40.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elzanowski, A. & Ostell, J. The Genetic Codes, https://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi?chapter=tgencodes#SG2 (2019).
- 42.Charif, D. & Lobry, J. R. In Structural approaches to sequence evolution: Molecules, networks, populations Vol. 1 (ed. Bastolla, U. et al) Ch. 10 (Springer Verlag, 2007).
- 43.Vences M, Thomas M, Van Der Meijden A, Chiari Y, Vieites DR. Comparative performance of the 16S rRNA gene in DNA barcoding of amphibians. Front. Zool. 2005;2:5. doi: 10.1186/1742-9994-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vences M, Thomas M, Bonett RM, Vieites DR. Deciphering amphibian diversity through DNA barcoding: chances and challenges. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1859–1868. doi: 10.1098/rstb.2005.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johns GJ, Avise JC. A comparative summary of genetic distances in the vertebrate from the mitochondrial cytochrome b gene. Mol. Biol. Evol. 1998;15:1481–1490. doi: 10.1093/oxfordjournals.molbev.a025875. [DOI] [PubMed] [Google Scholar]
- 46.Smith MA, Poyarkov NA, Jr., Hebert DN. CO1 DNA barcoding amphibians: take the chance, meet the challenge. Mol. Ecol. Resour. 2008;8:235–246. doi: 10.1111/j.1471-8286.2007.01964.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- van den Burg MP, Herrando-Pérez S, Vieites DR. 2020. ACDC, a curated database of amphibian cytochrome-b sequences. figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The R script used to collate and curate the Amphibia Cytb database is available at figshare (ACDCv1.0.R30).