Iron Deficiency in Pregnancy (original) (raw)
. Author manuscript; available in PMC: 2021 Oct 1.
Published in final edited form as: Am J Obstet Gynecol. 2020 Mar 14;223(4):516–524. doi: 10.1016/j.ajog.2020.03.006
Abstract
Iron is essential for the function of all cells through its roles in oxygen delivery, electron transport, and enzymatic activity. Cells with high metabolic rates require more iron and are at greater risk for dysfunction during iron deficiency. Iron requirements during pregnancy increase dramatically as the mother’s blood volume expands and the fetus grows and develops. Thus, pregnancy is a condition of impending or existing iron deficiency, which may be difficult to diagnose because of limitations to commonly utilized biomarkers such as hemoglobin and ferritin concentrations. Iron deficiency is associated with adverse pregnancy outcomes including increased maternal illness, low birth weight, prematurity and intrauterine growth restriction. The rapidly developing fetal brain is at particular risk of ID, which can occur because of maternal ID, hypertension, smoking, or glucose intolerance. Low maternal gestational iron intake is associated with autism, schizophrenia and abnormal brain structure in the offspring. Newborns with iron deficiency have compromised recognition memory, slower speed of processing and poorer bonding that persist in spite of postnatal iron repletion. Preclinical models of fetal iron deficiency confirm that expected iron-dependent processes such as monoamine neurotransmission, neuronal growth and differentiation, myelination and gene expression are all compromised acutely and long-term into adulthood. This review outlines strategies to diagnose and prevent iron deficiency in pregnancy. It describes the neurocognitive and mental health consequences of fetal iron deficiency. It emphasizes that fetal iron is a key nutrient that influences brain development and function across the lifespan.
Keywords: Anemia, ferritin, hemoglobin, preterm birth, fetal growth restriction, brain development, epigenetics, gestational diabetes, hepcidin, metabolism, hippocampus, nutrition neurodevelopmental disorder, iron deficiency, placenta, prematurity, Iron, pregnancy, brain, fetus, mental health, biomarkers
Condensation:
Pregnancy increases the iron requirements for the mother and her fetus. Iron deficiency is associated with adverse pregnancy outcome and compromised offspring neurodevelopment.
Why is iron important in biology and maternal-fetal medicine?
Iron is so fundamental to cellular functions that its uptake by cells is a highly conserved process across species. The mechanism by which iron is taken up from the extracellular environment by plants, yeast and mammals is remarkably similar. Mammalian cells incorporate iron into the porphyrin ring of hemoproteins as well as into enzymes, where the iron moiety is essential for those enzymes’ activities. Examples of the former include hemoglobin, myoglobin and cytochromes. Examples of the latter encompass a wide range of enzymes from hydroxylases that mediate fundamental intermediary cellular metabolism to demethylases that modify the chromatin of DNA and thus gene expression.
Iron sufficiency is essential for oxygen delivery to the maternal-placental-fetal unit to support the increased oxygen consumption demand of pregnancy.1 Maintenance of adequate maternal hemoglobin concentrations supports the oxygen demands of the three components of the unit. Beyond oxygen delivery, iron in cytochromes catalyzes the generation of ATP at a time when the fetal oxygen consumption rate is very high, driven largely by the structural development of fetal organs.2 Of these, the brain is particularly “greedy”, accounting for an astounding 60% of the total fetal oxygen consumption rate.2 This high human fetal brain oxygen consumption rate is due to the structural development of both neurons and glia and far exceeds the brain oxygen consumption rate of other mammalian species.2 The fetal brain develops from a smooth bilobed structure at 24 weeks gestation to a complex sulcated and gyrated structure at term that looks far more like a mature adult brain than like its earlier fetal counterpart.3 The increased surface complexity reflects the massive cellular differentiation of the brain in multiple brain regions and processes that include the hippocampus, the striatum, and the process of myelination.4
Why does pregnancy increase the need for iron?
The onset of pregnancy can be considered a case of impending iron deficiency as evidenced the high rate of the disorder during gestation. In low- and middle-income (LAMI) countries, the rate approaches 80%, while estimated rates in well-resourced countries approach 45% depending on the sensitivity of the markers used to detect it.5,6 The serum concentration of hepcidin, which is regulated by maternal iron status is extremely low during pregnancy1. Since hepcidin is a negative regulator of intestinal iron absorption, a low level indicates a high requirement for iron.1
Pregnancy increases maternal iron demand for three reasons. Maternal plasma and blood volumes are increased during pregnancy.1 Each extra gram of hemoglobin that the mother synthesizes requires an addition 3.46 milligram of elemental iron. In addition, the fetus requires iron for its own metabolic and oxygen delivery needs as well as the loading of its comparatively large endogenous iron stores that will be utilized in the first six months of postnatal life.7,8 The placenta is a highly metabolically active organ with large iron requirements. It has the capacity to store iron in resident reticulo-endothelial cells to provide a buffer against periods of low maternal iron supply.7–9 Overall, pregnancy requires an additional 1 gram of iron, relatively equally divided between the mother and the fetus.
Postnatal iron status during early childhood depends on fetal iron loading
Postnatal iron deficiency is extremely common in infants and toddlers with rates approaching 80% in LAMI countries. In well-resource countries in Europe and in the United States, the rate of iron deficiency in children ranges from 9% to 45%.10,11 Postnatal iron deficiency has been convincingly linked to short and long-term neurodevelopmental abnormalities.12
Until recently, postnatal iron deficiency was thought to be due to a combination of poor dietary iron intake and blood loss (due to intestinal infections). However, a recent large randomized trial of pregnant women in a Chinese population with a moderate rate of iron deficiency demonstrated that postnatal iron deficiency in the offspring was driven to a large degree by the iron status of the neonate, and thus was a function of fetal iron loading.13 Previous studies had shown that neonatal non-anemic iron deficiency, as defined by low cord serum ferritin concentrations, negatively affects neurologic and behavioral functions in the neonatal period and confers long-term risks to neurodevelopment.14–19 Children born with lower fetal iron loading have lower iron stores at 9 months of age and a greater risk of postnatal iron deficiency.20,21 Thus, proper fetal iron loading has taken on greater importance in public health strategies to combat postnatal iron deficiency and its long-term neurodevelopmental risks.
The fetus maintains a relatively constant 75 mg of iron per kilogram of body iron during the last trimester7,22. Fetal iron can be divided into three compartments; red cells, storage iron mostly in the liver, and non-heme, non-storage tissue iron.7 Red cells contain 55 mg of iron per kilogram body weight as hemoglobin and this represents the largest compartment. The amount of fetal iron in hemoglobin can be estimated by assuming 3.46 mg of iron per gram of hemoglobin and a blood volume of 85 ml per kilogram body weight.7 Normal values for hemoglobin concentration in term babies range from 133 to 184 g/L.23 Risks for fetal anemia include acute fetal-maternal or fetal-placental hemorrhage and severe maternal iron deficiency when maternal hemoglobin concentration is less than 90 g/L.24
Iron stores are found mostly in the liver at the end of gestation and represent 12 mg per kilogram body weight.7 Iron stores are proportionately larger in neonates than in older infants, children and adults. The mean cord serum ferritin concentration of the term infant 170 mcg/L and the 5th percentile is 59.13,23 Storage iron can be estimated from the serum ferritin concentration by utilizing nomograms that relate them to liver iron content.25 Negative fetal iron balance can be recognized clinically by a low cord serum ferritin concentration, which indicates loss of fetal iron stores.26 Reduced ferritin concentrations occur in infants born to iron deficient mothers with serum ferritin concentrations <13.4 mcg/L,13 iron sufficient mothers with hypertension during gestation,27 mothers who smoke cigarettes,28 and mothers with glucose intolerance/diabetes mellitus during pregnancy.29,30 Twins can have discordant iron loading where one twin may be profoundly iron deficient.31
The smallest compartment is made up of the non-heme, non-storage tissues, including the brain and the heart, and comprises approximately 8 mg per kilogram body weight.7 No biomarkers index this compartment, yet much of the symptomatology of iron deficiency including fatigue and altered brain function stem from iron deficiency at the tissue level.32,33
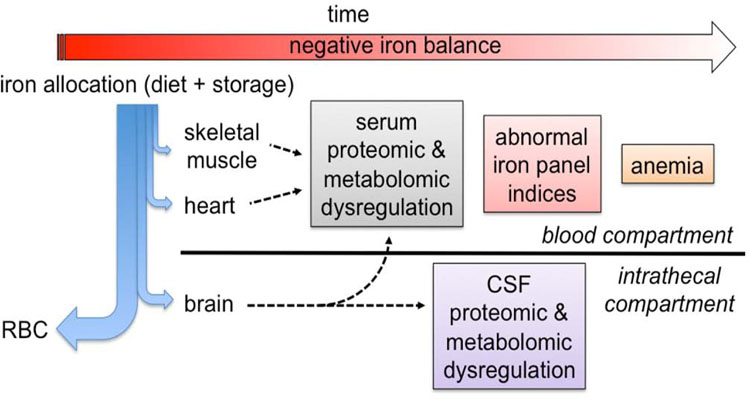
An important concept of fetal iron metabolism is that iron is prioritized to red cells over all other compartments including the brain (Figure 1).34,35 Consequently, anemia represents the end stage of the iron deficiency process and is not a sensitive marker of tissue level iron deficiency including brain iron deficiency.36 Because of the prioritization of fetal iron to the red cells, the iron status of these tissues must be inferred from the serum ferritin.34 Iron status of the fetal and neonatal brain can be assessed from the serum ferritin concentration and the demonstrated relationship between reduced liver iron stores and compromised brain iron status.30,37 Fundamentally, tissue iron is at risk once fetal iron stores have been exhausted.30,34,37 To that end, newborn ferritin concentrations <76 mcg/L (25th percentile) in humans have been associated with abnormal neonatal neurologic function including poorer recognition memory15,17 and slower speed of processing (longer inter-wave latencies) on brainstem evoked responses.16
Figure 1: Iron prioritization during iron deficiency in fetal and neonatal sheep34,35,37 and monkeys.36.
The relative distributional flow of iron is indicated by the thickness of the blue arrows. The red blood cells receive the primary allocation followed sequentially by the brain, the heart and skeletal muscle.34–37 As negative iron balance progresses over time (red arrow), iron-dependent metabolic dysregulation of the skeletal muscle and heart is first noted by alterations in the serum metabolome36. Progressive worsening of iron deficiency subsequently negatively affects brain metabolism at approximately the same time that serum iron panels (eg, ferritin, %TSAT) become abnormal.36 Iron deficiency results in anemia only in the final stage of the process.34–37
The maternal gestational conditions that compromise fetal and neonatal iron status are remarkably common,26 yet routine measurement of iron status in the neonate is not performed. Conversely, the fully iron loaded neonate, who receives the benefit of delayed cord clamping and is growing at the rate described by the WHO growth curves has enough iron to maintain adequate iron delivery to supply the growing tissues and expanding red cell mass for up to 4–6 months.38 Delayed cord clamping improves hemoglobin status at 2 months of age.39
Biomarkers of iron status and diagnosis of ID in pregnant women
Traditionally, the measurement of hemoglobin concentration has been used for patients of all ages to screen for iron status because so much of anemia world-wide is due to iron deficiency. The wide availability, ease of measurement and low cost of hemoglobin measurement are advantages particularly in low resource areas. Normal values for hemoglobin concentration are published and are easily accessed so that the diagnosis of anemia is not problematic.
Nevertheless, there are significant concerns with relying solely on hemoglobin screening. Because iron incorporation into hemoglobin is a highly prioritized process, anemia is the end-stage result of negative iron balance. Thus, hemoglobin concentration lacks the sensitivity to detect early stages of iron deficiency, when there are already physiologic effects at the tissue level. In addition, conditions other than iron deficiency cause anemia, including hemoglobinopathies, hemolysis, and inflammation/chronic disease. Reliance on hemoglobin concentration alone lacks sufficient sensitivity and specificity to diagnose iron deficiency in pregnancy. Iron specific biomarkers, such as serum ferritin, % total iron binding capacity saturation (%TSAT), and hepcidin can be utilized to distinguish iron deficiency anemia from other causes of anemia.
The serum ferritin concentration indexes the body’s storage iron capacity. Ferritin is stored in reticulo-endothelial cells and released to the red cells for hemoglobin synthesis and to the tissues for iron-dependent enzymes and hemoproteins. Both the liver and the placenta have remarkable ability to store iron as ferritin in order to buffer the mother and the fetus from wide swings in iron availability. Ferritin also acts as an intracellular regulator of the labile iron pool by releasing iron when needed via regulated chaperone molecules such as NCOA4.40 Clinically, the finding of low serum ferritin concentration is highly diagnostic for iron deficiency.41 Standards for normal ferritin concentrations in pregnant women are available and easily accessed in practice.
However, elevated or even normal serum ferritin concentrations may be difficult to interpret because ferritin is an acute phase reactant, rising when systemic inflammation is present.42 A high ferritin may represent true total body iron overload or simply a shift of iron into ferritin as a response to inflammation.42 A normal ferritin concentration may mask an iron deficient state if inflammation is present. Current recommendations are to measure a biomarker of inflammation, such as C-reactive protein, if interpretation of serum ferritin in the setting of inflammation or infection.42 Measurement of serum ferritin requires specialized equipment and more blood than measurement of hemoglobin, although the global availability of the test is expanding rapidly. Overall, serum ferritin, if available, should be utilized in conjunction with hemoglobin concentration to more precisely define iron status in pregnancy42.
Other iron biomarkers show promise in refining the diagnosis of iron deficiency in pregnant women. %TSAT is derived from how iron-saturated transferrin, the main iron transport protein, is. Serum iron concentrations decrease early during negative iron balance, thereby decreasing the %TSAT. In stage two iron deficiency, transferrin concentrations increase in order to optimize iron binding capacity for transport, which drives the %TSAT even lower. All of these changes occur before microcytosis or anemia. Measurement of %TSAT has confirmed the tenuous iron status of a large number of pre-anemic women with iron deficiency.6 Reticulocyte hemoglobin concentration also identifies pre-anemic, pre-microcytic iron deficiency and thus can serve as an early biomarker of ID. Its advantage is that most equipment that measures hemoglobin concentration also has the capacity to measure reticulocyte hemoglobin.
In the future, measurement of serum hepcidin concentrations may provide the most information about who should and should not receive iron supplementation. Hepcidin is the master regulator of intestinal iron absorption and iron distribution from the reticulo-endothelial cells.43 Hepcidin is synthesized by the liver in response to iron status and inflammation. Hepcidin is a negative regulator, which means that high concentrations reduce intestinal iron absorption and promote iron sequestration, while low levels increase intestinal iron absorption and iron release from reticulo-endothelial cells. High %TSAT indicating iron sufficiency or high levels of intraleukin-6, a pro-inflammatory cytokine, increase hepcidin synthesis and release. Low %TSAT is found in iron deficiency and suppresses hepcidin. Patients with low hepcidin levels likely require iron and will respond to iron therapy, while those with high hepcidin levels due to iron sufficiency or iron overload do not require additional iron and will not absorb dietary iron.43
The beginning of pregnancy is characterized by very low hepcidin concentrations, indicating a state of negative iron balance, and providing strong evidence that iron needs during pregnancy are high.1 Women with marginal or even adequate iron status in the pre-pregnancy state are thus prone to iron deficiency when they become pregnant.
Iron deficiency and iron deficiency anemia
iron deficiency and iron deficiency anemia are not synonymous. iron deficiency is a state of negative iron balance where iron supply does not meet iron demand, whereas iron deficiency anemia defines the condition when hemoglobin synthesis has been limited by the lack of iron and the person is anemic as a consequence. Because iron is prioritized to red blood cells for hemoglobin synthesis, iron deficiency proceeds through multiple non-anemic stages before anemia ensues. iron deficiency anemia can thus be thought of as the end stage of the iron deficiency process. Iron stores are mobilized during the first stage of iron deficiency, resulting in reduced %TSAT and ferritin concentrations, respectively. This process reflects the body’s attempt to maintain iron delivery to the red cells and non-heme tissues including the brain. However, when placed in direct competition for iron, the red cells will be prioritized over the brain.35–37 Children with pre-anemic iron deficiency demonstrate abnormal brain and behavioral function,44 indicating the likely presence of brain tissue level iron deficiency prior to the appearance of anemia. Genetic models of neuronal specific iron deficiency without anemia confirm that most of the neurocognitive phenotype of iron deficiency anemia is not due to the anemia.33,45
Iron supplementation during pregnancy
The major reasons to maintain an iron sufficient state during pregnancy are to protect the health of the mother, improve pregnancy outcomes and foster fetal development. A recent systematic review demonstrates that iron deficiency in the first and second trimesters is associated with increased maternal morbidity and an increased risk of adverse pregnancy outcomes defined as low birth weight, prematurity or intrauterine growth restriction.46 Randomized controlled trials of iron supplementation of iron deficient pregnant women show that iron therapy reduces the rates of these morbidities.46 Little debate exists as to whether iron deficient pregnant women should have their iron deficiency treated.47,48
Nearly 2 billion of the 7.5 billion people on the planet are iron deficient, making it the most common micronutrient deficiency in the world.5 iron deficiency predominantly affects women, their fetuses and their offspring.5,49 Such stark statistics combined with the evidence that pregnancy induces negative iron balance1 has led governing bodies to consider whether universal supplementation with iron should be policy.5,50 The Office of Dietary Supplements at the National Institutes of Health recently held a workshop to consider the question whether iron sufficient pregnant women should receive iron supplementation.48 The question was considered because many women are not iron deficient and because a small body of emerging research suggests an increased risk of glucose intolerance in iron sufficient pregnant women treated with iron.51 This area deserves further, adequately powered studies and exploration of potential underlying mechanisms (eg, oxidative stress, microbiome alterations) before policy decisions can be made.
The short and long-term effects of gestational iron deficiency on the fetus
Fortunately, during mild maternal iron deficiency, iron is prioritized to the fetus.8,24 However, during moderate and severe ID, the entire maternal-placental-fetal unit becomes iron deficient with significant short and long-term consequences to the fetus. These consequences also occur in fetuses of iron sufficient mothers who nevertheless have gestational conditions that compromise fetal iron delivery. These maternal conditions hypertension, smoking, diabetes mellitus and twinning.26–31 Insufficient fetal iron, whether due to maternal ID or to gestational conditions that compromise maternal fetal iron delivery, results in three risks to the fetus.
The immediate risk is to fetal brain development. Low maternal iron intake in the third trimester results in altered neonatal brain structure including alterations in gray matter that index less complex dendritic architecture.52 Non-anemic iron deficient newborns with cord serum ferritin concentrations <40 mcg/L have compromised recognition memory processing15 as do 2-month old infants with cord serum ferritin concentrations <76 mcg/L.53 The early age at which these abnormalities are detectable strongly implicate abnormal fetal as opposed to postnatal brain development due to iron deficiency. Recognition memory is mediated in large part by the hippocampus, which is a highly metabolic and rapidly developing structure during the late fetal and early neonatal period, making it more vulnerable to the lack of a critical substrate that supports energy metabolism.54
The second risk is to long-term brain development. Fetal/neonatal iron deficiency is associated with long-term neurocognitive dysfunction in spite of spontaneous repletion of iron stores by 9 months of age.18,20 Low neonatal iron status reduces recognition memory performance at 3.5 to 4 years of age even though the infants are no longer iron deficient.18
The third risk is postnatal iron deficiency in infancy and toddlerhood and its attendant neurodevelopmental sequelae. Infants who were iron underloaded as fetuses have poorer iron status at 9 months of age and thus a higher risk of becoming iron deficient.20 iron deficiency during infancy and toddlerhood is associated with slower speed of processing, poorer motor function and increased social dysfunction while iron deficient and significant long-term neuro-morbidities including depression and anxiety as adults.55–58 One reason proper fetal iron loading is important is that human milk is very low in iron. Breast milk may be the only dietary source for the infant in the first 6 postnatal months and thus the infant relies on the iron stored during fetal life to support hemoglobin synthesis and organ development for that period.37,38
The link between fetal or early postnatal iron status and long-term neurocognitive and mental health
Maternal-fetal iron status has been linked to a number of neurocognitive and mental health disorders in the offspring, driven in part by the timing of iron deficiency during pregnancy. Low maternal iron intake at the time of conception is associated with a greater risk of autism in the offspring.59 Low iron intake during the second trimester increases the risk of schizophrenia in the offspring by 30%.60 Infants born with low serum ferritin concentrations, reflecting reduced fetal iron loading, have poorer recognition memory at birth,15 2 months of age,53 and 3.5 years of age.18 They have poorer school performance at 5 to 6 year of age14 and difficulty with planning and attention at 10 years of age.19 Infants with onset of iron deficiency in early postnatal life, likely driven by inadequate fetal iron loading,13 demonstrate slower speed of neural processing at the time of their deficiency.55 The slower speed of neural processing persists beyond the period of iron deficiency and in spite of treatment.61 The infants have a higher risk of cognitive and socio-emotional problems including anxiety and depression in their second decade of life and into young adulthood.58 The resultant loss of education and job potential can be considereable.49 Thus, ensuring fetal nutritional and in particular iron health can be seen as an investment in the next generation and society going forward.
The mechanisms underlying iron deficiency effects on the young brain
Iron is essential for the proper functioning of all cells including those that comprise the brain. The negative effects of developmental iron deficiency on the brain have been well described in pre-clinical models over the past 45 years,12 particularly in the domains of monoamine neurotransmitter metabolism,62,63 neuronal and glial energy metabolism,64,65 myelination66 and gene regulation.67–69 The alterations in the brain induced by iron deficiency are highly consistent with the acute and long-term neurobehavioral deficits that have been described in humans.12
Monoamine neurotransmitters, e.g. dopamine, serotonin, and norepinephrine, are synthesized by the iron containing hydroxylases-tyrosine hydroxylase and tryptophan hydroxylase. In the human, the regulation of these neurotransmitters is highly shaped between mid-gestation and 3 years postnatal age. Research dating from the mid-1970s to the present in preclinical models indicates that early life iron deficiency alters the concentrations of the neurotransmitters, their receptors and their reuptake mechanisms acutely while iron deficient,62,63 and more importantly, long-term into adulthood despite iron repletion.70 These neurochemical changes underlie behavioral changes in the preclinical models and the behaviors are similar to those seen in humans, where anxiety, hesitancy and mood disorders have been documented.12,71
As noted above, the fetal brain is a highly metabolic organ, consuming 60% of the total fetal oxygen consumption rate.2 This high metabolic rate serves the purpose of cellular differentiation and function. Iron is essential in this process through its role in cytochromes in the generation of ATP. Neuronal iron deficiency to the degree found in human newborns reduces oxygen consumption rate by 60%, which in turn results in a more primitive and simple neuronal structure and poorer brain mitochondrial health.65,72 Among the brain regions that are most rapidly developing in the last trimester, the hippocampus is foremost. The hippocampus resides at the center of the recognition memory system that mediates spatial and fact learning. The hippocampus in humans begins to differentiate rapidly at 28 weeks gestation and continues to be highly active in its development until approximately 2 years of age.4 Fetal iron deficiency in preclinical models compromises hippocampal synaptic plasticity and structural gene expression,73,74 metabolism,64 electrophysiologic capacity,75,76 and recognition memory behavior acutely and into adulthood in spite of iron repletion in the neonatal period.33,45 These findings provide biological plausibility for the neurocognitive changes documented in iron deficient newborns.15,17,18
In the human, rapid myelination of the brain begins at 32 weeks gestation and extends into the first two years of postnatal life. Iron containing enzymes are important in the synthesis of fatty acids integral to myelin and early life iron deficiency alters the fatty composition of myelin and results in a hypomyelinated nervous system.66 Hypo- and dysmyelination would result in slower speed of neuronal processing, a finding that is present in iron deficient neonates and infants.16,55,61 Myelin effects persist into adulthood in the preclinical models.68
More recently, iron’s role in fetal and neonatal gene expression has been demonstrated in preclinical models and has been used as a way of demonstrating a mechanism underlying the long-term effects of early life ID. Rodents with fetal iron deficiency modeling the degree of brain iron deficiency seen in humans demonstrate a reduction in the expression of individual synaptic plasticity genes essential for development and function,73,74,77 as well as networks of genes underlying critical neural processes and mental health conditions, including autism, schizophrenia and mood disorders.69 These long-term gene changes are likely epigenetically driven, particularly by alterations to iron containing histone demethylases of the JARID family.78
Based on the understanding of the biology, it should not be surprising that iron deficiency compromises brain acutely in the fetus and newborn. However, it is the long-term effects that are concerning from a societal perspective and thus the etiologies of and potential therapies for the long-term effects are a topic of intense investigation.
The long-term effects can be conceptualized by two basic mechanisms that are not mutually exclusive: the critical period mechanism and the epigenetic modification of chromatin mechanism. The brain is not a homogenous organ; instead, its various areas and processes are distinct entities that develop on different trajectories.4 A period of rapid growth and differentiation often constitutes a critical period,79 wherein lack of substrates necessary for proper development result in permanent structural abnormalities.45,79,80 Such a period has been identified in the fetal/neonatal period for the mouse hippocampus.45 iron deficiency that is not corrected during that period results in long-term structural deficits accompanied by neurocognitive abnormalities.45
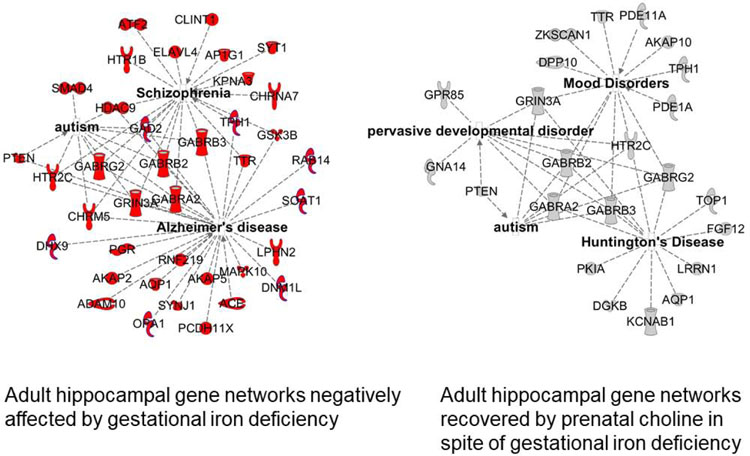
The epigenetic mechanism is rooted in the concept of the Developmental Origins of Adult Health and Disease.81,82 Many regulatory setpoints for genes that control multiple metabolic processes are set in late fetal life, a concept popularized by David Barker and known initially as the “Barker Hypothesis” in the 1990’s.83 The observation that similar principles of “fetal setpoint anticipation” apply to the brain became evident in the early 2000’s with the demonstration that the risk for several adult mental health conditions including schizophrenia are driven in part by fetal conditions.84 Fetal iron status appears to follow this pattern. Low maternal iron intake in the second trimester is associated with a 30% increased risk of schizophrenia.60 Preclinical models demonstrate that a network of genes underlying schizophrenia are activated by fetal iron deficiency.69 These gene networks continue to show abnormal expression in the formerly iron deficiency adult brain, providing biological plausibility to the epidemiologic finding in humans.60,69 The finding that these changes to gene networks are prevented by feeding a diet rich in a methyl donor (choline) support the hypothesis that the long-term effects may be epigenetic in nature (Figure 2).69 A similar line of research demonstrates a link between low first trimester iron intake and the risk of autism in humans,59 with elucidation of the involved gene networks in the preclinical fetal iron deficiency model.69
Figure 2:
Gene networks involved in neurologic and mental health diseases in the adult mouse affected by gestational iron deficiency (left panel) and recovered by prenatal choline supplementation (right panel).69
In summary, the preclinical science across multiple investigative platforms including molecular biology, cellular biology, electrophysiology, structure and behavior have provided a convincing plausibility to the role of fetal iron in brain development and the role of fetal iron deficiency in the short and long-term neurobehavioral abnormalities observed in humans.82
Pre and periconceptional iron and the argument for a life-cycle effect of nutrients
Ongoing research indicates that a woman’s nutritional status heading into pregnancy influences the brain development of the offspring. Peri-conceptional iron and folate iron intake are both related to the subsequent risk of autism in the offspring with lower intakes associated with higher risks.59,85 Undernutrition is not the only problem. Overnutrition in the form of maternal obesity is a risk factor. Elevated pre-pregnancy BMI independent of gestational weight gain increases the risk of mental health and neurodevelopmental disorders in the offspring.86
Conclusions
The information presented in this review shifts the conversation about the role of nutrition in ensuring childhood brain health from solely focusing on postnatal nutrition to focusing on prenatal and even pre-conceptional nutrition. In this way, it argues for policies that promote maternal nutritional health heading into pregnancy, followed by close attention to provision of critical nutrients such as iron during pregnancy. This life-cycle approach to nutrition can be seen as an investment in the mental health of the next generation and an investment in society.
Acknowledgments
The work was supported by grants 5R01 HD-29421, R01 NS-099178, R01 HD-089989, and R01 HD-094809 from the National Institutes of Health.
Abbreviations:
LAMI
Low and Middle Income
%TSAT
%Transferrin Saturation
Glossary of Terms:
C-reactive protein
A protein generated by the liver during conditions of inflammation, such as infection.
Dysmyelination
Abnormal biochemical composition of myelin.
Ferritin
The intracellular storage protein for iron. Measurement of ferritin in the serum or plasma likely comes from ferritin shed from senescent cells and reflects the intracellular storage pool.
Glia
Non-neuronal cells in the brain including oligodendrocytes that make myelin, astrocytes that serve multiple functions including nutrient delivery, and microglia that traffic neurons during development and can generate pro-inflammatory responses.
Hepcidin
A protein generated by the liver in response to iron sufficiency or inflammation that inhibits iron absorption from the intestine and sequesters iron in macrophages.
Labile iron pool
The intracellular iron that is not bound in active enzymes or hemoproteins or stored in ferritin and thus is available for utilization.
Methyl donor
Compounds that through their metabolism can donate a methyl group that can bind to DNA components such as cytosine-guanine islands or histones and thereby alter gene expression. Multiple nutrients such as choline, betaine, folate can act as methyl donors.
Monoamine neurotransmitters
Dopamine, Serotonin and Norepinephrine are the neurotransmitters that are synthesized by iron containing enzymes tyrosine hydroxylase and tryptophan hydroxylase where iron is critical to the enzymatic function and thus the concentration of neurotransmitter produced.
Neurocognitive
Brain functions related to reasoning, learning and memory, and higher functions (eg, multi-tasking, attention, inhibition, working memory).
Neurodevelopment
The process of rapid brain growth and differentiation that takes place in the period of time from conception through early adulthood.
Transferrin Saturation
Transferrin is the major binding protein for iron circulating in the serum. Under normal physiologic conditions, up to two ferric iron molecules are bound to transferrin molecule. The percentage of iron binding sites on transferrin that are occupied by iron is referred to as %Transferrin Saturation (TSAT) and is normally 30–50%.
Oxygen Consumption Rate
The cellular metabolic rate of oxygen in the process of ATP generation as set by the mitochondria.
Synaptic plasticity
The ability of neurons to respond to external influences and thereby modify their anatomic structure and functional output. The term is often used to reflect the flexibility of the functioning brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no conflicts of interest to report.
References:
- 1.Fisher A, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr 2017; 106 (Suppl):1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol 1998; 27 (Suppl):177–209. [DOI] [PubMed] [Google Scholar]
- 3.Cowan MW. The development of the brain. Sci Am 1979; 241:113–133. [PubMed] [Google Scholar]
- 4.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol 2001; 56:5–15. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Available at: www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf2015.
- 6.Auerbach M, Abernathy J, Juul S, Short V, Derman R. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med 2019; 3:1–4. [DOI] [PubMed] [Google Scholar]
- 7.Oski FA. The hematologic aspects of the maternal-fetal relationship In Oski FA, Naiman JL, eds. Hematologic Problems in the Newborn, ed 3, Philadelphia, WB Saunders, 1982: pp 32–33. [Google Scholar]
- 8.Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev 2013; 71:35–51. [DOI] [PubMed] [Google Scholar]
- 9.McArdle HJ, Gambling L, Kennedy C. Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc 2014; 73:9–15. [DOI] [PubMed] [Google Scholar]
- 10.van der Merwe LF, Eussun SR. Iron status of young children in Europe. Am J Clin Nutr 2017; 106 (Suppl.):1663S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia and iron deficiency anemia among young children in the United States. Nutrients. 2016; 8:E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of early iron deficiency in infancy. Nutr Rev 2006; 64:S34–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao Z-Y, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutrition 2012; 142: 2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002; 140:165–70. [DOI] [PubMed] [Google Scholar]
- 15.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, deRegnier R-A. Auditory recognition memory in iron-deficient infants of diabetic mothers. Pediatric Research 2004; 55: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 16.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr 2010; 156:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng F, Mai X, Zhan J, Xu L, Georgieff M, Shao J, Lozoff B. Timing of iron deficiency and recognition memory in infancy. Nutritional Neuroscience, 2020; January 7:1–10. [DOI] [PMC free article] [PubMed]
- 18.Riggins T, Miller NC, Bauer PB, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Developmental Neuropsychology, 2009; 34:762–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabes A, Thomas KM, Langworthy S, Georgieff MK, Nelson CA. Functional and anatomic consequences of diabetic pregnancy on memory in 10-year-old children. J. Dev Beh Pediatr, 2015; 36:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgieff MK, Wewerka SW, Nelson CA, deRegnier R-A. Iron status at 9 months of infants with low iron stores at birth. J Pediatr 2002; 141:405–409. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Guobin X, Zhou M, et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, iron deficiency anemia in a randomized clinical trial in rural china, but iron deficiency remains widespread in mothers and neonates. J. Nutrition, 2015; 145: 1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler EE, O’Donnell AM, Nelson SE, Fomon SJ. Body composition of the reference fetus. Growth 1976; 40:329–41. [PubMed] [Google Scholar]
- 23.Lorenz L, Peter A, Poets CF, Franz AR. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology 2013; 104:194–202. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr 2003; 77:924–30. [DOI] [PubMed] [Google Scholar]
- 25.Saarinen UM, Siimes MA. Serum ferritin in assessment of iron nutrition in healthy infants. Acta Paediatr Scand 1978; 67:745–51. [DOI] [PubMed] [Google Scholar]
- 26.Siddappa AJ, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology 2007; 92: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. Cord transferrin and ferritin levels in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr 1987; 111(2):283–6. [DOI] [PubMed] [Google Scholar]
- 28.Sweet DG, Savage G, Tubman TR, Lappin TR, Halliday HL. Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch Dis Child Fetal Neonatal Ed 2001; 84:F40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: Spectrum and maternal antecedents. J Pediatr 1990; 117:455–461. [DOI] [PubMed] [Google Scholar]
- 30.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr 1992; 121:109–114. [DOI] [PubMed] [Google Scholar]
- 31.Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O’Brien KO. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J Nutrition 2018; 148:1716–1722. [DOI] [PubMed] [Google Scholar]
- 32.Blayney L, Bailey-Wood R, Jacobs A, et al. The effects of iron deficiency on the respiratory function and cytochrome content of rat heart mitochondria, Circ Res 1976; 39:744–8. [DOI] [PubMed] [Google Scholar]
- 33.Carlson ES, Tkac I, Magid R et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutrition 2009; 139(4):672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr, 2017; 106(S): 1588S–1593S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamora TG, Guiang SF III, Georgieff MK, Widness JA. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatric Research, 2016; 79:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao R, Ennis K, Lubach G, Lock E, Georgieff MK, Coe C. Metabolomic analysis of CSF indicates brain metabolic impairment precedes hematological indices of anemia in the iron-deficient infant monkey. Nutritional Neuroscience; 2016; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol; 1992; 262:R485–R491. [DOI] [PubMed] [Google Scholar]
- 38.Lonnerdal B Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr 2017; 106:1681S–87S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grajeda R, Perez-Escamilla R, Dewey KG. Delayed clamping of the umbilical cord improves hematologic status of Guatemalan infants at 2 mo of age. Am J Clin Nutr 1997; 65:425–31. [DOI] [PubMed] [Google Scholar]
- 40.Philpott CC, Jadhav S. The ins and outs of iron: Escorting iron through the mammalian cytosol. Free Rad Biol Med 2019; 133: 112–117. [DOI] [PubMed] [Google Scholar]
- 41.Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 1998; 44:45–51 [PubMed] [Google Scholar]
- 42.Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha SR, Rogers LM, Namaste SM. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr 2017; 106:1626S–1633S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffey R and Ganz T. Iron homeostasis: An anthropocentric perspective. J Biol Chem 2017; 292: 12727–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr 2008; 152:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fretham SJB, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin receptor-1 dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus, 2012; 22: 1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, iron status or iron supplementation. Am J Clin Nutr 2017; 106:1694S–702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgieff MK, Krebs NF, Cusick S. The benefits and risks of iron supplementation in pregnancy and childhood. Annu Rev Nutr 2019; 39:121–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brannon PM, Stover PJ, Taylor CL. Integrating themes, evidence gaps, and research needs identified by workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am J Clin Nutr 2017; 106:1703S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA, International Child Development Steering Group. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007; 369:145–57. [DOI] [PubMed] [Google Scholar]
- 50.Siu AL, US preventative Services Task Force. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: US Preventitive Services Task Force Recommendation Statement. Ann Intern Med 2015; 163:529–36. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Rawal S. Dietary iron intake, iron status and gestational diabetes. Am J Clin Nutr 2017; 106:1672S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monk C, Georgieff MK, Xu D, Hao X, Bansal R, Gustafsson H, Spicer J, Peterson BS. Maternal prenatal iron status and tissue organization in the neonatal brain. Pediatric Research, 2015; 79:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geng F, Mai X, Zhan J, et al. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr 2015; 167:1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgieff MK, Innis S. Controversial nutrients in the perinatal period that potentially affect neurodevelopment: essential fatty acids and iron. Pediatr Res 2005; 57:99R–103R. [DOI] [PubMed] [Google Scholar]
- 55.Roncagliolo M, Garrido M, Walter t, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr 1998; 68: 683–90. [DOI] [PubMed] [Google Scholar]
- 56.Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev 2002; 70:85–101. [DOI] [PubMed] [Google Scholar]
- 57.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 2006; 160:1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lukowski AF, Koss M, Burden MJ, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 2010; 13:54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol 2014; 180:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron and the risk of schizophrenia in offspring. Arch Gen Psychiatry 2008; 65:1136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Algarin C, Peirano P, Garrido M, Pizzaro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 2003; 53:217–23. [DOI] [PubMed] [Google Scholar]
- 62.Youdim MB, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cell Mol Biol 2000; 46:491–500. [PubMed] [Google Scholar]
- 63.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 2006; 171:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutrition 2003; 133:3215–3221. [DOI] [PubMed] [Google Scholar]
- 65.Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier LM, Georgieff MK. Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism and dendrite complexity. Dev Neurosci, 2016; 38:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 2004; 77:681–9. [DOI] [PubMed] [Google Scholar]
- 67.Carlson ES, Stead JDH, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus, 2007; 17: 679–91. [DOI] [PubMed] [Google Scholar]
- 68.Clardy SL, Wanx X, Zhao W, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl 2006; 71:173–96. [DOI] [PubMed] [Google Scholar]
- 69.Tran PV, Kennedy BC, Pisansky MT, Won K-J, Gewirtz JC, Simmons RA, Georgieff MK. Prenatal choline supplementation diminishes early –life iron deficiency induced preprogramming of networks associated with behavioral abnormalities in the adult rat hippocampus. J Nutrition 2016; 146:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unger EL, Hurst AR, Georgieff MK, et al. Behavior and monoamine deficits in pre- and perinatal iron deficiency are not corrected by early postnatal moderate or high iron diet in rats. J Nutrition. 2012; 142: 2040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol 2005; 46:141–53. [DOI] [PubMed] [Google Scholar]
- 72.Bastian T, Von Hohenberg W, Georgieff MK, Lanier L. Chronic energy depletion due to iron deficiency impairs dendritic mitochondrial motility during hippocampal neuron development. J. Neuroscience, 2019; 39:802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran PV, Carlson ES, Fretham SJ, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutrition. 2008; 138(12):2495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci, 2010; 32:238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 2005; 15:1094–1102. [DOI] [PubMed] [Google Scholar]
- 76.Pisansky MT, Wickham RJ, Su J, Fretham S, Yuan L-L, Sun M, Gewirtz JC, Georgieff MK. Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus 2013; 23(10):952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tran PV, Fretham SJB, Carlson ES, Georgieff MK. Long-term reduction of hippocampal BDNF activity following fetal-neonatal iron deficiency in adult rats. Pediatr Res, 2009; 65(5): 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran PV, Kennedy BC, Lien YC, Simmons RA, Georgieff MK. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am J Physiol Integr Comp Phys 2015; 308: R276–R282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hensch TK. Critical period regulation. Annu Rev Neurosci 2004; 27:549–79 [DOI] [PubMed] [Google Scholar]
- 80.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA-1 pyramidal neurons. Dev Neurosci; 2003; 25:412–420. [DOI] [PubMed] [Google Scholar]
- 81.Hanson MA, Poston L, Gluckman PD. DOHaD- the challenge of translating science to policy. J Dev Orig Health Dis 2019; 10:263–267. [DOI] [PubMed] [Google Scholar]
- 82.Barks AK, Hall AM, Tran PV, Georgieff MK. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatric Research 2019; 85:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curran GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation 1996; 94:1310–5. [DOI] [PubMed] [Google Scholar]
- 84.Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry 2010; 68:314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt RJ. Maternal folic acid supplements associated with reduced autism risk in the child. Evid Based Med 2013; 18:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 2015; 9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]