History of the Plague: An Ancient Pandemic for the Age of COVID-19 (original) (raw)
Abstract
During the fourteenth century, the bubonic plague or Black Death killed more than one third of Europe or 25 million people. Those afflicted died quickly and horribly from an unseen menace, spiking high fevers with suppurative buboes (swellings). Its causative agent is Yersinia pestis, creating recurrent plague cycles from the Bronze Age into modern-day California and Mongolia. Plague remains endemic in Madagascar, Congo, and Peru. This history of medicine review highlights plague events across the centuries. Transmission is by fleas carried on rats, although new theories include via human body lice and infected grain. We discuss symptomatology and treatment options. Pneumonic plague can be weaponized for bioterrorism, highlighting the importance of understanding its clinical syndromes. Carriers of recessive familial Mediterranean fever (FMF) mutations have natural immunity against Y. pestis. During the Black Death, Jews were blamed for the bubonic plague, perhaps because Jews carried FMF mutations and died at lower plague rates than Christians. Blaming minorities for epidemics echoes across history into our current coronavirus pandemic and provides insightful lessons for managing and improving its outcomes.
Keywords: Bioterrorism, Black Death, Bubonic plague, COVID-19, Evolutionary adaptation, Familial Mediterranean fever (FMF), Pneumonic plague, Pyrin, Yersinia pestis
Clinical Significance.
- •
The Black Death or bubonic plague killed more than 25 million people in fourteenth-century Europe. - •
Yersinia pestis (the plague bacteria) can be easily weaponized as a bioterrorism agent. - •
Early plague treatment is curative, but its symptomatology can be nonspecific. Modern outbreaks still regularly occur. The plague existed in the ancient world and has killed more than 200 million across centuries. - •
Familial Mediterranean fever carriers have plague immunity, which is an important evolutionary adaptation.
Alt-text: Unlabelled box
Introduction
Killing more than 25 million people or at least one third of Europe's population during the fourteenth century, the Black Death or bubonic plague was one of mankind's worst pandemics, invoking direct comparisons to our current coronavirus “modern plague.”1, 2, 3 An ancient disease, its bacterial agent (Yersinia pestis) still causes periodic outbreaks and remains endemic in some parts of the world.4, 5, 6 Additionally, because it could be weaponized for world bioterrorism, understanding its clinical syndromes, epidemiology, and treatment options remains critical for medical practitioners.5 , 6 Finally, recent molecular discoveries linking recessive familial Mediterranean fever mutations to plague immunity have revolutionized how scientists and historians alike view this novel evolutionary adaptation.7 , 8 This history of medicine article sheds light onto the plague and provides insights that can help us manage the COVID-19 epidemic.
History of Plague Epidemics
The plague has afflicted humanity for thousands of years.1, 2, 3 Molecular studies identified the presence of the Y. pestis plague DNA genome in 2 Bronze Age skeletons dated at roughly 3800 years old.9 In the biblical book 1 Samuel from approximately 1000 BCE, the Philistines experience an outbreak of tumors associated with rodents, which might have been bubonic plague.3 Scholars identify 3 plague pandemics.10 , 11 The first pandemic or Justinian plague probably came from India and reached Constantinople in 541-542 CE. At least 18 waves of plague spread across the Mediterranean basin into distant areas like Persia and Ireland from 541 to 750 CE.10 , 11
The second pandemic or Black Death arrived in Messina in Sicily, probably from Central Asia via Genoese ships carrying flea-laden rats in October 1347, which initiated a wave of plague infections that rapidly spread across most of Europe like a relentless wildfire.10, 11, 12 In Europe, plague-stricken citizens were often dead within a week of contracting the illness. Ultimately, at least one third of the European population (more than 25 million people) died between 1347 and 1352 from the Black Death.10, 11, 12 The plague spread to France and Spain in 1348 and then to Germany, Switzerland, and Austria. It decimated London in 1349 and reached Scandinavia and northern England by 1350.10, 11, 12 The plague died out by the century's end, but outbreaks resurfaced and spread throughout Europe over the next 400 years. In 1656-1657, two thirds of the population in Naples and Genoa died from the disease. In 1665-1666, London lost about one quarter of its citizens to plague, about 100,000, and the same number died in Vienna in 1679.3 , 13 , 14 Moscow recorded more than 100,000 plague deaths during 1770-1771.3 , 13 , 14
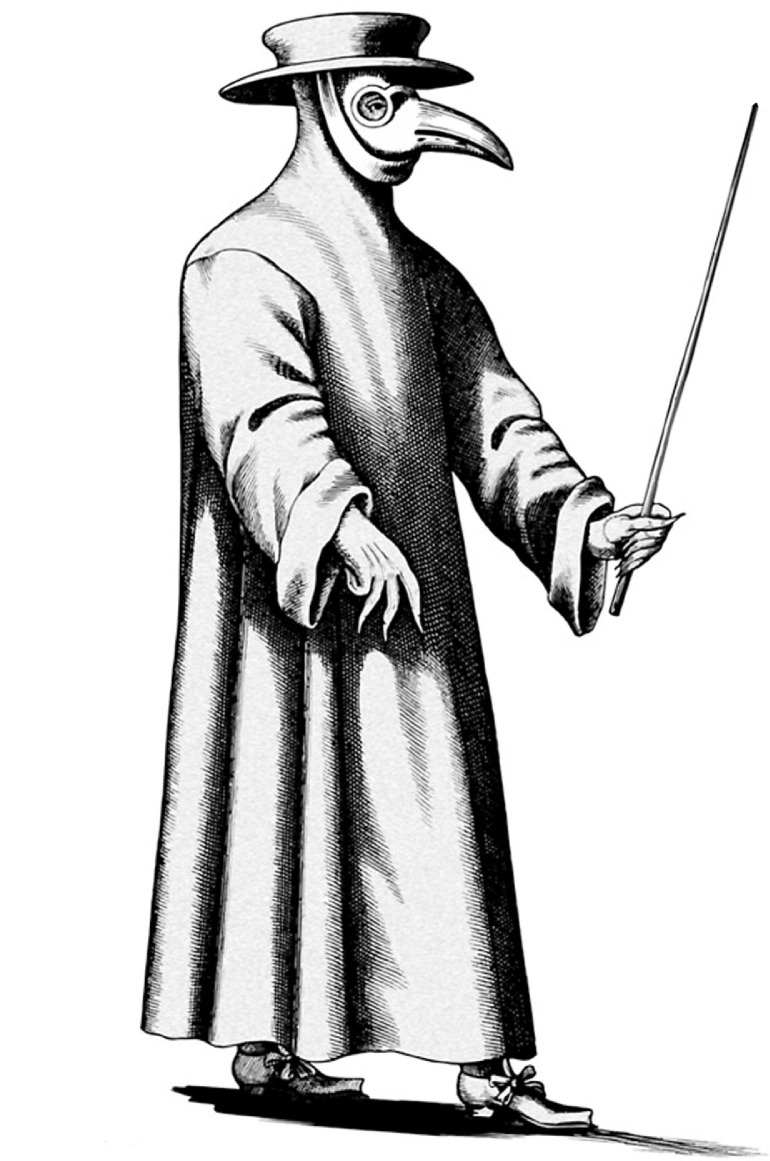
The fourteenth-century bubonic plague transformed European society and economies, leading to severe labor shortages in farming and skilled crafts.1, 2, 3 The geopolitical impact included a decline in power and international status of the Italian states.15 During the Black Death, European Christians blamed their Jewish neighbors for the plague, claiming Jews were poisoning the wells. These beliefs led to massacres and violence.2 , 16 At least 235 Jewish communities experienced mass persecution and destruction during this period, often preemptively in a futile effort at plague containment.16 The ancient physicians Hippocrates (c. 460-c. 370 BCE) and Galen (129-c. 210 CE) promoted the miasma theory, or poisoned air, to explain disease transmission, which Medieval Europeans believed caused the Black Death.11 , 17 People of that period thought warm baths permitted plague miasma to enter humans’ pores, so public baths were closed. Victims’ clothes and possessions were thought contaminated and were burned, and cats were killed as possible transmission agents. So-called “plague doctors” wore protective clothing with a long cape, mask, and a bill-like portion over the mouth and nose containing aromatic substances (partly to block out the putrid smell of decaying corpses), perhaps an early version of the modern hazmat suit10 (Figure 1).
Figure 1.
Costume of the plague doctor. The plague doctor wore a black hat, beaked white mask, which contained aromatic substances to block out the smell of decaying bodies, and a waxed gown. The rod or pointer kept afflicted patients away. The earliest version of a protective hazmat suit. Courtesy National Library of Medicine.
The third plague pandemic began in Yunnan Province in southwest China around 1855, where outbreaks had occurred since 1772, and spread to Taiwan.10 , 11 , 18 It hit Canton in 1894, where it caused 70,000 deaths, and then appeared in Hong Kong. Ships carried it to Japan, India, Australia, and North and South America between 1910 and 1920.10 , 11 , 18 An estimated 12 million people died from the plague in India between 1898 and 1918.19 Rats from merchant ships brought the plague to Chinatown in San Francisco in 1900.20 Although few European cases of the plague were reported after 1950, isolated outbreaks still occur worldwide.4 , 20 It is estimated that more than 200 million people have died from the plague throughout human history.10
Plague Microbiology
Y. pestis is an aerobic, gram-negative coccobacillus in the family Enterobacteriaceae.21, 22, 23 Genetic DNA analysis shows that it diverged from its enteric pathogenic relative, Yersinia pseudotuberculosis, up to 6000 years ago.24 After incubation for 24 to 48 hours in blood or on MacConkey agar at 37°C, small bacterial colonies can be identified.21, 22, 23 Its primary vector for transmission is the Xenopsylla cheopis flea, although roughly 80 species of fleas can carry it. During the Black Death, the flea was transported by the black rat or Rattus.21, 22, 23 A controversial new theory argues that ectoparasites such as human fleas and lice also spread the disease during the second plague pandemic.25 Fleas can also survive in infected clothing or grain.11 , 19, 20, 21 The bacteria multiply in infected rodents (more than 280 mammalian species can serve as carriers) and block the fleas’ alimentary canal, causing the fleas to regurgitate the Y. pestis bacteria into its animal host.11 , 19, 20, 21 The bacterium is named for the Pasteur Institute physician Alexandre Yersin, who provided the first, most accurate description of its causative agent in 1894 during the Hong Kong outbreak. However, the Japanese physician Shibasaburo Kitasato was an independent coinvestigator whose bacterial plates were unfortunately contaminated and led to erroneous observations.19 In 1898, Dr. Paul-Louis Simond in Karachi showed that fleas from infected rats could transmit the disease to healthy rats, and Ricardo Jorge in 1927 reported that wild rodents serve as a plague reservoir.10
Clinical Presentation, Treatment, and Prophylaxis
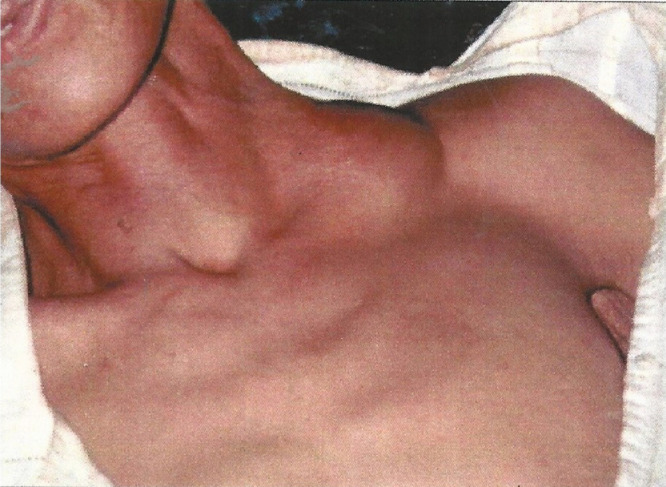
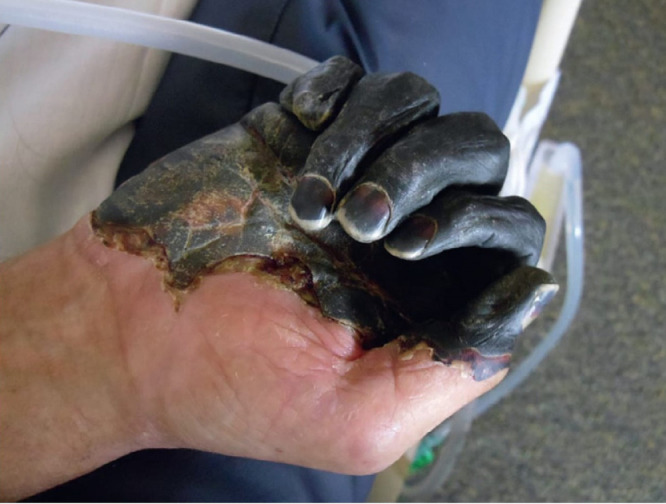
There are 3 major clinical forms of the plague.10 , 21, 22, 23 In the most common bubonic subtype, infected persons develop sudden onset of high fevers (>39.4°C), terrible pains in their limbs and abdomen, and headaches generally between 3 and 7 days after exposure. The bacteria reproduce rapidly in lymph nodes located closest to the flea bites, leading to painful swellings (“buboes”) in the groin, cervical, or axillary lymph nodes, which can enlarge to the size of an egg (or up to 10 cm) (Figure 2)12. About 60% of untreated victims die within 1 week of exposure as the pus-filled buboes suppurate and the patient succumbs to overwhelming infection.10 , 21, 22, 23 During the time of the Black Death, it must have been truly terrifying to witness otherwise healthy individuals cut down rapidly by a seemingly invisible demon. The rarer septicemic plague form (10%-15% of cases) occurs when the bacteria multiply in the blood, often triggering disseminated intravascular coagulation and gangrene of the extremities, ears, or nose10 , 21, 22, 23 (Figure 3). Finally, the infrequent, fulminant pneumonic plague syndrome represents the only form with human-to-human transmission as inhalation of aerosolized droplets (much like coronavirus transmission) from infected patients or even cats leads rapidly to hemoptysis and death. Because this clinical subtype is specifically aerosolized, pneumonic plague could be used for potential bioterrorist attacks.5 , 6 , 26 Its initially nonspecific, flu-like symptoms include sudden onset of high fevers and dyspnea within 4 days of plague exposure, progressing quickly to a purulent, frothy, or ultimately bloody cough.21, 22, 23 Chest X-ray for primary pneumonic plague may show lobar pneumonia, which spreads rapidly throughout the lungs. The blood-tinged sputum is highly infectious.21, 22, 23 The latter 2 clinical subtypes are invariably fatal without treatment.
Figure 2.
Buboes (swellings). Cervical buboes in a patient with bubonic plague from Madagascar. From Prentice MB, Rahalison L. Plague. Lancet. 2007;369:1196-1207. doi: 10.1016/S0140-6736(07)60566-2. Copyright Elsevier 2007.
Figure 3.
Gangrene from plague sepsis. A man from Oregon developed bubonic plague after being bitten by an infected cat, leading to sepsis and acral amputation. Courtesy Centers for Disease Control and Prevention.
Plague transmission is generally from infected fleas by rodent vectors or, rarely, in clothing or grain but may also occur through ingesting contaminated animals, physical contact with infected victims, or direct inhalation of infectious respiratory droplets.21, 22, 23 Early recognition and treatment with streptomycin (or gentamycin) or a combination of doxycycline, ciprofloxacin, and chloramphenicol can cure the bubonic plague.21, 22, 23 One study compared the plague fatality rate in the United States from 1900-1942 (before antibiotics were available) at 66% compared with cases after 1942 and the advent of antibiotic treatments with a death rate of only 13%.20 Prompt identification of plague infections and the introduction of appropriate antibiotics will generally lead to a full recovery, but because its initial symptoms may include a nonspecific fever and often no clear exposure to infected animals or fleas can be identified, diagnosis may be delayed, leading to death. The gold standard for diagnosis is isolation of the bacteria from tissue or body fluids, which should only be done in a biosafety level 3 laboratory, although confirmatory serologic testing for antibodies to the F1 antigen may also be performed.21, 22, 23 Empiric chemoprophylaxis with oral doxycycline or ciprofloxacin for 7 days is recommended for family members or others in close contact to victims of plague.21, 22, 23 , 27 There was a whole-cell, formalin-killed vaccine, but it was discontinued because it was only protective against bubonic plague. Efforts continue to produce a vaccine effective against the rare pneumonic plague subtype, which potentially could be used for biowarfare.5 , 6
Modern Plague Outbreaks
More than just a historical oddity, plague outbreaks continue to surface and cause occasional deaths throughout the world.4 , 20 Plague reservoirs exist in animal hosts, including wild squirrels, rats, prairie dogs, marmots, gophers, and other rodents; cats can become infected and transmit Y. pestis via aerosolized droplets.10 , 21, 22, 23 An outbreak hit Los Angeles in 1924, killing 30 people, when a man contracted the disease and died after handling a dead rat. A Catholic priest administering last rites to victims and mourners attending associated funerals all also died of pneumonic plague.28 The “telluric hypothesis” proposes that plague bacteria can survive in soil and not simply on rodents, which may explain why plague foci persist despite aggressive efforts to eradicate its hosts.11 One recent analysis reported that Madagascar, Congo, and Peru remain the most plague-endemic countries.5 Indeed, between 2010 and 2015, there were 3248 cases and 584 plague deaths worldwide, with the majority (75%) being in Madagascar.5 Plague eruptions can disrupt production in modern economies, just as it did in the Middle Ages. In 2005, 130 men working in a diamond mine in Congo contracted plague, causing 57 deaths. Similarly, 162 workers were sickened in 2006 at a gold mine in Congo, leading to 45 deaths and temporarily shutting down these operations.23 The World Health Organization (WHO) has deemed plague to be a reemerging disease since the 1990s.5 Two unrelated teens contracted plague in separate incidents in August 2015 while visiting Yosemite National Park in California, apparently from infected squirrels, although local bears also demonstrated antibodies against Y. pestis.29 Indeed, a healthy 15-year old boy died in July 2020 from plague in Mongolia after eating an infected marmot (similar to a large ground squirrel), and Mongolia has had almost 600 cases of marmot plague since 1928, with an associated mortality rate of 74%.5 , 30
Systematic attempts to destroy plague reservoirs largely failed. For decades until 1991, the Union of Soviet Socialist Republics (USSR) launched an impressive, aggressive plague-eradication program. Poisons were placed manually into thousands of rodent burrows, pesticides like DDT were widely deployed to kill plague hosts, and potential mammalian carriers were destroyed.26 Although such laborious efforts decreased cases, the plague was never fully eliminated. The potential toxicity to humans and the native ecosystem from insecticides promoted a shift toward vector control (not eradication) and epidemiological sampling to monitor the presence of Y. pestis in local rodent populations.4, 5, 6 , 26 Current programs balance ongoing surveillance among plague vectors with protecting the natural environment as a multipronged approach toward plague containment.4, 5, 6 , 26
Plague and Bioterrorism
The Centers for Disease Control and Prevention (CDC) classifies Y. pestis as a Category A (tier 1) biologic agent for potential bioterrorism.5 It can be released and spread easily, which creates a major public health hazard and could lead to quarantines and potentially widespread economic devastation.5 , 6 Pneumonic plague leads to death rapidly without prompt recognition and treatment. Its initial nonspecific symptomatology of flu-like illness coupled with a mistaken perception that plague is simply an obscure, dormant disease make it an ideal weapon for biowarfare.5 , 6 Indeed, Tatars leveraged its lethality in 1346 by catapulting plague-ridden corpses into the Genoese-controlled seaport of Caffa, in one of the first uses of biological agents to wage war.3 The Imperial Japanese Unit 731 during World War II developed and deployed biological weapons in Manchuria and China. On October 27, 1940, Japanese warplanes dropped plague-contaminated rice and fleas into Chuhsien, China, which led to an outbreak of pneumonic plague.3 , 5 , 6 The World Health Organization estimates that if only 50 kg of Y. pestis were released in aerosolized form over a major city, the deadly pneumonic plague subtype could cause widespread devastation and death. The bacteria remain viable for up to 1 hour at a distance of up to 10 km from the drop point.5 , 6 Because a main goal of bioterrorism would be to incite fear among its population, plague is an ideal biological tool because its victims die quickly in a horrific fashion (with hemoptysis, respiratory failure, high fevers, and the like).
Familial Mediterranean Fever and Y. Pestis
Molecular advances have linked familial Mediterranean fever (FMF) gene mutations to plague immunity.7 , 31, 32, 33, 34 FMF is a rare, recessive disease mostly seen in people of Arab, Armenian, Jewish, or Turkish ancestry. Symptoms of FMF include abdominal pain, arthritis, and fevers lasting 12-72 hours, although those affected are usually completely normal between spells.32 , 33 Pyrin is its gene protein product, from the Greek word for “fever.” As an extremely important and versatile immune regulator, pyrin fights infection and cancer. When bacteria attack a cell, the immune system is activated. Pyrin is one of the major players in this immune system cascade and plays a crucial role in mounting and maintaining human defense systems against pathogens. Pyrin activates caspase-1, an enzyme that facilitates programmed cell death, and participates in IL-1β processing for fever production.8 , 31, 32, 33 Y. pestis reduces production of IL-1β and IL-18, blocking the immune system from mounting a robust immune response.8 , 31, 32, 33 The bacteria run unchecked as natural defenses are shut off.
Patients who carry the FMF mutation have a “gain-of-function” in the pyrin gene, as its activity is always “on.” Y. pestis shuts off pyrin in subjects who lack the mutation, which increases susceptibility to plague infections.7 , 8 , 31, 32, 33, 34 Like sickle cell trait and resistance to malaria, those harboring the FMF mutation have plague immunity, as an important example of an evolutionary adaptation.35 Up to 20%-40% of Israeli Jews in some studies may carry a recessive mutation in the FMF gene.36, 37, 38 This mutation is found throughout the Middle East, but during the Black Death, Jews were the only large European community with Middle Eastern origins. We hypothesize that the presence of the FMF mutation would have allowed fourteenth-century Jews to survive plague at higher rates than their non-Jewish neighbors, which may have led European Christians to blame Jews for spreading the plague.2 , 16 , 17 It is unknown if FMF carriers possess resistance to other infections, including to coronavirus, which may warrant further investigation.
Conclusions
Plague represents a reemerging infectious disease with potential use for bioterrorism.5 , 6 From prehistory to the modern era, Y. pestis has killed millions of people. Outbreaks of worldwide plague foci in both developed and underdeveloped countries continue to occur.4, 5, 6 Although modern medicine has greatly improved therapies and limited its spread, many clinical practitioners remain unfamiliar with its symptomatology, thus preventing timely recognition and treatment.10 , 11 , 20, 21, 22, 23 Advanced technology has demonstrated the selective genetic benefit of protective FMF mutations, which likely provided plague immunity for many medieval Jews and perhaps contributed to violence against them during this period.7 , 8 , 16
Our historical examination of plague provides important contemporary parallels with the COVID-19 pandemic. Both the coronavirus and the fourteenth-century Black Death pandemics likely originated in Asia.3 , 39 During the Black Death, minority groups (Jews) were persecuted for supposedly spreading the disease. In a similar fashion echoing across centuries of history, Asians and other minorities have been blamed for spreading COVID-19, as one group marginalizes another amid a sea of anxiety, fear, and irrational hatred.16 , 40 Reminiscent of the treatment of Jews during the plague, there have been acts of ethnic and racial hostility directed at Asians and immigrants based on the false belief that these individuals, because of their ethnicity, are responsible for the pandemic.16 , 40 Studying the genetic, medical, and social science aspects of plague pandemics can lead us to greater understanding of the interplay among history, humanity, and science.
Footnotes
Funding: None.
Conflicts of Interest: None.
Authorship: Both authors had access to the data and a role in writing this manuscript.
References
- 1.Benedictow OJ. Boydell Press; Woodbridge, UK: 2004. The Black Death 1346-1353. The Complete History; pp. 3–67. [Google Scholar]
- 2.Kelly J. HarperCollins; New York, NY: 2006. The Great Mortality. An Intimate History of the Black Death, the Most Devastating Plague of All Time; pp. 138–141. 1-77. [Google Scholar]
- 3.Zietz BP, Dunkelberg H. The history of the plague and the research on the causative agent Yersinia pestis. Int J Hyg Environ Health. 2004;207(2):165–178. doi: 10.1078/1438-4639-00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L, Yang G, Zhang Z, et al. Reemergence of human plague in Yunnan, China in 2016. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari I, Grier G, Byers M. Deliberate release: plague-a review. J Biosaf Biosecur. 2020;2(1):10–22. doi: 10.1016/j.jobb.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayanan N, Lacy CR, Cruz JE, et al. Disaster preparedness: biological threats and treatment options. Pharmacotherapy. 2018;38(2):217–234. doi: 10.1002/phar.2068. [DOI] [PubMed] [Google Scholar]
- 7.Patin E. Plague as a cause for familial Mediterranean fever. Nat Immunol. 2020;21(8):833–834. doi: 10.1038/s41590-020-0724-3. [DOI] [PubMed] [Google Scholar]
- 8.Ratner D, Orning MP, Proulx MK, et al. The Yersinia pestis effector YopM inhibits pyrin inflammasome activation. PLoS Pathog. 2016;12(12) doi: 10.1371/journal.ppat.1006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyrou MA, Tukhbatova RI, Wang CC, et al. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nat Commun. 2018;9(1):2234. doi: 10.1038/s41467-018-04550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riedel S. Plague: from natural disease to bioterrorism. BUMC Proc. 2005;18(2):116–124. doi: 10.1080/08998280.2005.11928049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bramanti B, Stenseth NC, Walløe L, et al. Plague: a disease which changed the path of human civilization. Adv Exp Med Biol. 2016;918:1–26. doi: 10.1007/978-94-024-0890-4-1. [DOI] [PubMed] [Google Scholar]
- 12.Prentice MB, Rahalison L. Plague. Lancet. 2007;369:1196–1207. doi: 10.1016/S0140-6736(07)60566-2. [DOI] [PubMed] [Google Scholar]
- 13.Cohn SK, Alfani G. Households and plague in early modern Italy. J Interdisc Hist. 2007;38:177–205. doi: 10.1162/jinh.2007.38.2.177. [DOI] [Google Scholar]
- 14.Cohn SK. Oxford University Press; New York, NY: 2002. The Black Death Transformed. Disease and Culture in Early Renaissance Europe; pp. 7–40. [Google Scholar]
- 15.Alfani G, Percoco M. Plague and long-term development: the lasting effects of the 1629-1630 epidemic on the Italian cities. Econ History Rev. 2019;72(4):1175–1201. doi: 10.1111/ehr.12652. [DOI] [Google Scholar]
- 16.Colet C, Santiveri MI, Xavier J, et al. The Black Death and its consequences for the Jewish community in Tarrega: Lessons from history and archeology. Medieval Globe. 2014;1(1):63–96. [Google Scholar]
- 17.Cohn SK. Plague violence and abandonment from the Black Death to the early modern period. Annales de démographie historique. 2017;134(2):39–61. doi: 10.3917/adh.134.0039. [DOI] [Google Scholar]
- 18.Bramanti B, Dean KR, Walløe L, et al. The third plague pandemic in Europe. Proc Biol Sci. 2019;286(1901) doi: 10.1098/rspb.2018.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry RD, Fetherston JD. Yersinia pestis-etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35–66. doi: 10.1128/CMR.10.1.35-66.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kugeler KJ, Staples JE, Hinckley AF, et al. Epidemiology of human plague in the United States, 1900-2012. Emerg Infect Dis. 2015;21(1):16–22. doi: 10.3201/eid2101.140564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mead PS. In: Principles and Practice of Infectious Diseases. Bennett JE, Dolin R, Blaser MJ, editors. Elsevier Publishing; London, UK: 2019. Plague (Yersinia pestis). pp. 2779–2787. eds. [Google Scholar]
- 22.Yang R. Plague: recognition, treatment, and prevention. J Clin Microbiol. 2018;56(1) doi: 10.1128/JCM.01519-17. e01519-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler T. Review article: plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg. 2013;89(4):788–793. doi: 10.4269/ajtmh.13-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demeure CE, Dussurget O, Fiol GM, et al. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019;20(5):357–370. doi: 10.1038/s41435-019-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean KR, Krauer F, Walløe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115(6):1304–1309. doi: 10.1073/pnas.1715640115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SD, Atshabar B, Schmid BV, et al. Living with plague: lessons from the Soviet Union's antiplague system. Proc Natl Acad Sci U S A. 2019;116(19):9155–9163. doi: 10.1073/pnas.1817339116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfred-Cato S, Cooley KM, Fleck-Derderian S, et al. Treatment of human plague: a systematic review of published aggregate data on antimicrobial efficacy, 1939-2019. Clin Infect Dis. 2020;70:S11–S19. doi: 10.1093/cid/ciz1230. [DOI] [PubMed] [Google Scholar]
- 28.Viseltear AJ. The pneumonic plague epidemic of 1924 in Los Angeles. Yale J Biol Med. 1974;47(1):40–54. [PMC free article] [PubMed] [Google Scholar]
- 29.Danforth M, Novak M, Petersen J, et al. Investigation of and response to 2 plague cases, Yosemite National Park, California, USA, 2015. Emerg Infect Dis. 2016;22(12):2045–2053. doi: 10.3201/eid2212.160560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guy J, Gansukh B. Teenage boy dies from bubonic plague after eating marmot. CNN. July 15, 2020. Available at: https://buffalonews.com/news/national/teenage-boy-in-mongolia-dies-from-bubonic-plague-after-eating-marmot/article\_b39d55e0-40dc-5f07-a1ad-6e45be233085.html. Accessed July 15, 2020.
- 31.Malik HS, Bliska JB. The pyrin inflammasome and the Yersinia effector interaction. Immunol Rev. 2020;297:96–107. doi: 10.1111/imr.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Özen S, Batu ED, Demir S. Familial Mediterranean fever: recent developments in pathogenesis and new recommendations for management. Front Immunol. 2017;8:253. doi: 10.3389/fimmu.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migita K, Asano T, Sato S, et al. Familial Mediterranean fever: overview of pathogenesis, clinical features, and management. Immunol Med. 2018;41(2):55–61. doi: 10.1080/13497413.2018.1481579. [DOI] [PubMed] [Google Scholar]
- 34.Park YH, Remmers EF, Lee W, et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat Immunol. 2020;21:857–867. doi: 10.1038/s41590-020-0705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:1–22. doi: 10.1038/nrdp.2018.10. 18010. [DOI] [PubMed] [Google Scholar]
- 36.Gershoni-Baruch R, Shinawi M, Leah K, et al. Familial Mediterranean fever: prevalence, penetrance, and genetic drift. Eur J Hum Genet. 2001;9(8):634–637. doi: 10.1038/sj.ejhg. [DOI] [PubMed] [Google Scholar]
- 37.Aksentijevich I, Torosyan Y, Samuels J, et al. Mutation and haplotype studies of familial Mediterranean fever reveal new ancestral relationships and evidence for a high carrier frequency with reduced penetrance in the Ashkenazi Jewish population. Am J Hum Genet. 1999;64(4):949–962. doi: 10.1086/302327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoffman N, Magal N, Shohat T, et al. Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups. Eur J Hum Genet. 2000;8:307–310. doi: 10.1038/sj.ejhg.5200446. [DOI] [PubMed] [Google Scholar]
- 39.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26(7):842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jan T. Asian American doctors and nurses are fighting racism and the coronavirus. Washington Post. May 19, 2020 https://www.washingtonpost.com/business/2020/05/19/asian-american-discrimination/ Available at: Accessed on June 1, 2020. [Google Scholar]