Potential Role of Lycopene in the Prevention of Postmenopausal Bone Loss: Evidence from Molecular to Clinical Studies (original) (raw)
Abstract
Osteoporosis is a metabolic bone disease characterized by reduced bone mineral density, which affects the quality of life of the aging population. Furthermore, disruption of bone microarchitecture and the alteration of non-collagenous protein in bones lead to higher fracture risk. This is most common in postmenopausal women. Certain medications are being used for the treatment of osteoporosis; however, these may be accompanied by undesirable side effects. Phytochemicals from fruits and vegetables are a source of micronutrients for the maintenance of bone health. Among them, lycopene has recently been shown to have a potential protective effect against bone loss. Lycopene is a lipid-soluble carotenoid that exists in both all-trans and _cis_-configurations in nature. Tomato and tomato products are rich sources of lycopene. Several human epidemiological studies, supplemented by in vivo and in vitro studies, have shown decreased bone loss following the consumption of lycopene/tomato. However, there are still limited studies that have evaluated the effect of lycopene on the prevention of bone loss in postmenopausal women. Therefore, the aim of this review is to summarize the relevant literature on the potential impact of lycopene on postmenopausal bone loss with molecular and clinical evidence, including an overview of bone biology and the pathophysiology of osteoporosis.
Keywords: bone, osteoporosis, lycopene, tomato, postmenopause
1. Introduction
Osteoporosis is an age-related health problem which reduces the quality of life. It is estimated that over 200 million people suffer from osteoporosis worldwide [1]. Approximately 30% of postmenopausal women in Europe and the United States are affected by osteoporosis [2]. Based on the projected estimations, there will be a 32% increase in the incidence of osteoporosis and low bone mass in older adults (aged ≥ 50) by 2030 [3]. Moreover, the cost of treatments for osteoporosis is rapidly increasing annually as the total number of fractures increases. Developing countries especially may face a huge economic burden due to more fragility fractures occurring with higher life expectancies and a growing elderly population [4,5]. The projected estimate of the increase in the incidence of hip fractures in women and men worldwide is 240% and 310%, respectively, by 2050.
The most prominent types of fractures occur at the sites of the hip, spine vertebrae, and distal forearm [4]. Complications of osteoporosis, such as hip fractures, lead to a four-times-higher mortality rate in the adult population worldwide [6]. In general, one in three women (50 years or older) may have osteoporotic fractures [7]. Osteoporosis can be categorized into two types: primary osteoporosis and secondary osteoporosis [8]. Primary osteoporosis can be further divided into postmenopausal osteoporosis (type 1) and senile osteoporosis (type 2) [9]. Postmenopausal osteoporosis is primarily caused by estrogen deficiency, which occurs in women after menopause, while senile osteoporosis gradually develops with aging (>70 years old) in both sexes [10]. Secondary osteoporosis generally has a definable etiology such as malnutrition, chronic disease, endocrine dysfunction, medication side effects, or metastatic or hematological malignancy [8].
Diets rich in phytochemicals, particularly carotenoids from fruits and vegetables, have been found to be effective for the maintenance of bone mineral status [11,12]. Among them, the potential protective effects of lycopene against bone loss have been recently documented [13,14]. Lycopene is an acyclic carotenoid, containing 11 conjugated double bonds in the all-_trans_-isomeric form or various _cis_-configurations [15]. Compared to all-trans, _cis_-isomers are considered to be more bioavailable because they are highly soluble in lipid micelles, readily taken up by intestinal cells, less likely to crystalize, and they also preferentially bind to chylomicrons [16]. Thus, _cis_-isomers are more easily transported within cells, across the plasma membrane, and into the tissue matrix [16,17].
Tomatoes and tomato-based products are rich sources of lycopene and represent more than 80% of human dietary sources containing lycopene. Depending on the variety of tomato, the average lycopene content lies between 0.7–20 mg/100 g fresh weight [18]. However, dietary sources of lycopene are primarily found in the all-_trans_-isomeric form. For example, red tomatoes contain almost 90% of their total lycopene in the all-_trans_-form [15], yet _cis_-lycopene concentrations in body tissues are higher than those of other lycopene isomers [18,19]. This could be due to isomerization occurring during food processing or post consumption during digestion and absorption. Although lycopene has beneficial effects on human health, there is a gap between dietary intake and the amount available for biological action in the body. Therefore, various food processing techniques (e.g., heat treatment) are used to improve the bio-accessibility of all-_trans_-lycopene. These processing techniques can disrupt the cell wall and release lycopene out of the cells. Additionally, since lycopene is fat-soluble, the incorporation of fat into the meal or red tomato product increases the bioavailability of lycopene. There are other natural sources of more bioavailable lycopene, such as orange heirloom tomatoes that contain >90% of lycopene in the _cis_-isomeric form [20,21]. In these tomatoes, the carotenoid isomerase enzyme, which converts _cis_-lycopene to all-trans, is in a nonfunctional form; thus, they retain considerably higher amounts of _cis_-isomers and may be a better source of dietary lycopene for humans.
There is an emerging interest among researchers to study lycopene for the prevention of postmenopausal osteoporosis and to explore ways to improve lycopene bioavailability from natural sources such as tomatoes. The purpose of this review is to discuss the literature regarding the impact of lycopene on bone metabolism, including an investigation into natural sources of more bioavailable lycopene.
2. Bone Biology, Modelling, and Remodeling
Bone is a specialized connective tissue that is responsible for the framework of the body. Primarily, the skeleton provides support for the body and assists its movements. Bones also act as a major mineral reservoir, carrying 99% of calcium, 85% of phosphorus, and 65% of magnesium body stores, and they are the main repository of growth factors and cytokines [8]. Moreover, bone plays an important role in acid-base balance and hormonal functions related to phosphate metabolism, blood glucose, and fat deposition in the body [8]. Bone also helps in the detoxification of heavy metals and other waste materials by removing them from blood circulation [8,22,23]. Bone generally comprises three components: organic matrix, inorganic salts, and water. Approximately 90% of the organic matrix consists of collagenous protein, non-collagenous protein, and growth factors. The inorganic matrix contains mainly calcium and phosphorus in the form of hydroxyapatite crystals [24]. Depending on the degree of porosity, bones are categorized as cortical bone (compact) or trabecular bone (cancellous). Cortical bone is denser, while trabecular bone is more porous. The porosity of cortical bone is approximately 3–5% and may increase with age [23,25]. Cortical bones represent 80% of the adult skeleton. Trabecular bone is more metabolically active than cortical bone. This difference occurs due to the higher surface area of trabecular bone compared to cortical bone [23].
Bone tissue contains four different cell types: osteoblasts, osteoclasts, osteocytes, and bone lining cells. Osteoblasts originate from mesenchymal stem cells and are responsible for bone formation [26]. Osteoclasts are derived from mononuclear hematopoietic stem cells and are responsible for bone resorption [27]. The majority of mature osteoblasts may undergo apoptosis, while a minority re-differentiate into osteocytes or lining cells [28]. Approximately 90–95% of bone cells are osteocytes, which have a long life span of nearly 25 years [24,29]. Bone marrow, found in the bone cavity, consists of two types of cells: hematopoietic and stromal. Hematopoietic stem cells produce osteoclasts, immune cells, platelets, and red blood cells, while mesenchymal stem cells produce osteoblasts, cartilage, and adipocytes [27,29].
Bone tissue undergoes two major physiological processes: modeling and remodeling. Bone modeling is characterized by a change in the shape of the bone as a result of physiological influences or mechanical forces [30]. For example, bone modeling widens the bones with aging [8]. Moreover, bone modeling is upregulated in hypoparathyroidism, chronic kidney disease (CKD), and medical treatments containing anabolic agents [31]. During bone modeling, the changes in the shape of bones are regulated by independent actions of osteoblasts and osteoclasts [32].
In contrast, bone remodeling occurs throughout life and is responsible for the removal of older bone and its replacement with new bone structure [8,33,34]. Bone remodeling involves a sequence of cellular activities that occur within a specialized multicellular unit [35]. The bone multicellular unit is predominantly comprised of osteoclasts, osteoblasts, and osteocytes [24]. There are 3–4 million basic multicellular units (BMU) produced each year, and approximately one million among them actively participate in the bone remodeling process [36]. Bone remodeling has four major stages: activation, resorption, reversal, and formation [35]. In the first step, osteoblastic stromal cells or lining cells are activated via lining cell retraction and endosteal membrane digestion by collagenase. Following this, osteoclasts initiate bone resorption by dissolving the mineral matrix [8,35]. At the end of the resorption phase, mononuclear cells such as monocytes, osteocytes, and preosteoblasts are found in resorption cavities [8]. During the reversal phase, a cement lining rich in mucopolysaccharides is deposited between old and new bone, as well as signaling molecules that can activate osteoblast precursors. Therefore, the reversal phase is considered a transitional phase between resorption and formation of new bone [35]. Lastly, a new organic matrix is produced by osteoblasts, which eventually mineralizes into new bone [32]. The resorption and reversal phases last for 2 weeks and 4–5 weeks, respectively. The formation phase is the longest and lasts approximately 4–6 months until the new bone is completely formed [30,37,38].
Approximately 90% of cortical bone is calcified; thus, it contains a low surface area to volume ratio. This leads to a slower rate of remodeling in cortical bone compared to trabecular bone [23]. Approximately 25% of the body’s trabecular bone is remodeled each year compared to only 2.5% of cortical bone [23]. Bone remodeling is regulated by various systemic and local factors. Genetics, mechanical factors, vascular factors, nutrition, and hormones are considered systemic regulators, while growth factors, matrix proteins, and cytokines act as local regulators [8,24,35,37,39]. At menopause, bone remodeling increases and continues at a higher rate for 5–10 years due to the decrease in levels of estrogen [31].
3. Postmenopausal Osteoporosis: A Silent Disease
Postmenopausal osteoporosis is a common metabolic disease among older women (≥50 years). It is characterized by reduced bone mineral density along with disruption of bone microarchitecture and alteration of non-collagenous protein in bone, which together lead to higher fracture risk [8]. This disease reduces women’s overall quality of life by significantly increasing their rates of morbidity, disability, and mortality. Osteoporotic bone tends to be more fragile and easily fractured [40]. In general, one in eight people will experience a second fracture within a year after their first osteoporotic fracture [41].
Bone mineral density (BMD), bone mineral content (BMC), and the quantity and quality of bone are the distinguishing factors between people with osteoporotic versus healthy bones [42]. The risk of osteoporosis is primarily evaluated through bone mineral density, which is predominantly measured by dual-energy X-ray absorptiometry (DEXA) scanning [38]. Other methods include magnetic resonance imaging (MRI), ultrasound, and microcomputed tomography [42,43]. Bone density is a quantitative measure, but its limitation is that it does not measure bone quality [44]. Therefore, measurements of bone turnover markers as proxies for bone formation and bone resorption are used to evaluate the quality of bone in tandem with DEXA scanning [38].
For the diagnosis of osteoporosis, BMD values are converted into a T-score [2]. The T-score is calculated by dividing the difference between a female patient’s BMD and the mean BMD of young, healthy women by the standard deviation of the reference population [2]. A T-score between −1 and +1 SD (standard deviation) is considered to be normal BMD. A T-score between −1 and −2.5 SD is categorized as osteopenia. A T-score of −2.5 SD is considered to be osteoporotic [27,40,43,45,46]. An imbalance between bone resorption and formation initially leads to osteopenia, which is characterized by low mineralization and is likely to further progress to osteoporosis [47]. The most common pharmacological treatments currently being used for osteopenia and osteoporosis are bisphosphonates, denosumab, anabolic agents, and hormone replacement therapy [41]. Most of these treatments have been shown to increase bone mass by up to 10% over 3–5 years [48]. Postmenopausal women are advised to take these medications with calcium and vitamin D supplements to increase their effectiveness [49].
3.1. Risk Factors of Postmenopausal Osteoporosis
Risk factors for osteoporosis can be primarily categorized as modifiable and nonmodifiable (Table 1). Heredity is the major nonmodifiable factor of osteoporosis, and children of parents with osteoporosis and fractures are themselves more prone to develop osteoporosis [50]. Osteoporosis is a polygenic disease that involves several genes. In rare instances, osteoporosis can be inherited due to mutations in single genes. Mutations of two genes of type 1 collagen (COL1A1 and COL1A2) are responsible for the dominant osteoporotic disease called “osteogenesis imperfecta”, which is characterized by low bone mass and increased bone fragility [51,52,53,54]. Osteoporosis inheritance has also been linked with inactivating mutations in the aromatase (CYP19A1) and estrogen receptor alpha genes (ERα) [55,56].
Table 1.
Risk factors of postmenopausal osteoporosis.
| Fixed Risk Factors | Modifiable Risk Factors |
|---|---|
| Menopause age [57] | Inadequate calcium and vitamin D intake [58,59] |
| Menopause and hysterectomy [57,60] | Alcohol consumption [61] |
| Estrogen deficiency and amenorrhea [62] | Cigarette smoking [63] |
| Family history of osteoporosis [64,65] | Low body mass index (<20 kg/m2) [66] |
| Previous fractures [67] | Eating disorders [68] |
| Height loss (>0.5 cm per year) [69] | Inadequate physical exercise [70,71] |
| Ethnicity (Caucasian and Asian population are at high risk) [72] | Frequent falls [73] |
Estrogen deficiency is the primary risk factor of postmenopausal osteoporosis; other contributors besides the genetics mentioned above include modifiable factors such as nutrition, certain medications, and lifestyle (Table 1) [10,74]. Bone cells (osteoblast, osteoclast, and osteocytes) contain estrogen receptors on their surface [75]. Stimulation of estrogen receptors, particularly on osteoblasts, may inhibit the activation of osteoclasts and thus reduce bone resorption and protect bones from osteoporosis [76]. Oxidative-stress-generating factors such as poor nutrition, low antioxidants in the body, smoking, alcohol intake, and excessive caffeine intake can be modified through lifestyle changes [6,40]. For example, a diet low in calcium may induce secretion of parathyroid hormone, which activates osteoclasts and bone resorption [76].
Interestingly, some cross-sectional studies have found an inverse relationship between sleep duration and BMD in elderly women [77,78]. It has been hypothesized that a shorter waketime reduces the daily mechanical loading that induces bone remodeling, and thus reduces BMD [78]. Lower melatonin levels due to reduced light exposure may lead to fewer interactions between estrogen and its receptors and, thus, negatively impact BMD [79]. However, other studies report either positive or null relationships between sleep duration and BMD in postmenopausal women [80,81,82,83]. The conflicting evidence in this area may be due to confounding study participant factors that are not uniformly controlled for across the studies, such as age, body composition, diet, and nighttime-only sleep duration versus inclusion of daytime naps.
3.2. Pathophysiology of Postmenopausal Osteoporosis
The occurrence of postmenopausal osteoporosis is dependent mainly on body estrogen levels. Estrogen regulates bone turnover either by directly interfering with osteocytes and osteoclasts or by regulating T-lymphocyte function and the formation of osteoblasts [84]. Estrogen has both skeletal and non-skeletal functions, and, due to the former deficiencies, can cause bone-related diseases (Figure 1). Women are at higher risk 3–5 years after the onset of menopause [85]. The mechanism by which estrogen deficiency causes postmenopausal osteoporosis is complex. Estrogen can influence bone remodeling through inhibiting cell differentiation and increasing osteoclast apoptosis [85].
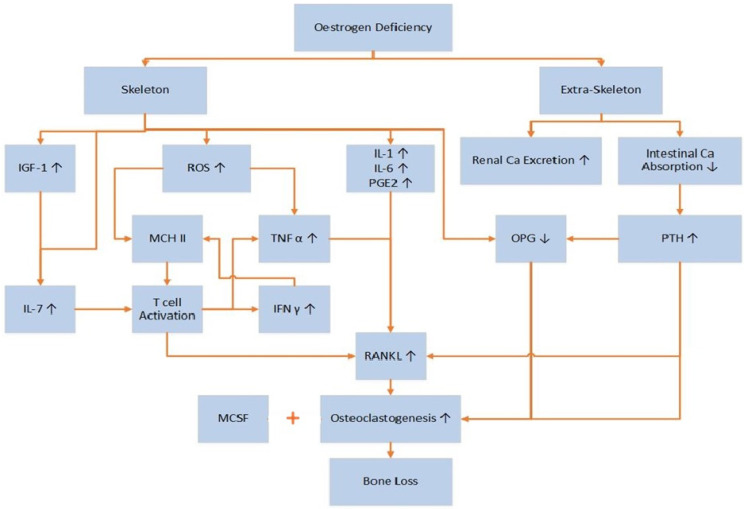
Figure 1.
Occurrence of bone loss through estrogen deficiency (reference from [10,86]). Estrogen deficiency increases the production of IL-7 directly and via increased production of IGF-1. IL-7 activates T-cells to produce IFN-γ and TNF-α. Reactive oxygen species (ROS), along with IFN-γ, upregulate MHC II, located in antigen-presenting cells that may further activate T-cells. Activated T-cells produce RANKL and TNF-α. Other cytokines, IL-1, IL-6, and PGE2, also increase the production of RANKL. Decreased osteoprotegerin (OPG) due to insufficient estrogen directly influences osteoclastogenesis. Beyond the skeletal activities, estrogen deficiency may increase renal calcium excretion while decreasing intestinal calcium absorption. This stimulates the parathyroid glands to produce PTH, which can reduce the production of OPG and increase the production of RANKL and, therefore, increase bone resorption. All these actions together are involved in postmenopausal bone loss.
Molecular markers of bone metabolism are widely used to assess bone-related disorders. These markers include enzymes and nonenzymatic peptides produced by bones. Bone formation or resorption markers correlate with the metabolic phase in which they are produced [38]. Short-term estrogen deficiency (3 weeks) is associated with low levels of bone formation markers in early postmenopausal women [87]. Interestingly, studies have shown that long-term estrogen deficiency increased both bone resorption and bone formation markers in postmenopausal women, suggestive of enhanced bone turnover with increased net bone loss [88,89,90,91]. Estrogen deficiency increases renal calcium excretion while decreasing intestinal calcium absorption [10], and the resultant fall in calcium levels can activate various bone resorption mechanisms that include PTH, osteocalcin, OPG, and the RANK/RANKL system [91,92,93,94,95]. These bone resorption markers are, therefore, found in the blood in higher concentrations in osteoporosis. Conversely, osteocalcin, which is secreted by osteoclasts, directly binds calcium and enables bone mineralization by increasing hydroxyapatite absorption and, thus, is a marker of bone formation [96]. However, insufficient calcium and phosphorous stores in osteoporotic women reduce hydroxyapatite crystal formation, leaving more osteocalcin free to circulate in the blood [93,94]. The molecular mechanisms responsible for these complex changes are not yet fully elucidated [88].
As mentioned earlier, oxidative stress can be coupled with osteoporosis. Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and their neutralization by antioxidants. Reactive oxygen species are formed as a result of cellular respiration, enzymatic activities in mitochondria, and cellular responses to cytokines induced by external stimuli [76]. Reactive oxygen species include both highly reactive oxygen-containing molecules and free radicals such as hydroxyl (OH), superoxide (O2−), and hydrogen peroxides (H2O2) [40]. Free radicals can oxidize lipids and proteins, thus causing cell damage and altered function [97,98]. Reactive oxygen species suppress differentiation and proliferation of osteoblasts and are significantly involved in osteoclast differentiation and bone resorption [99,100]. Menopause increases oxidative stress; thus, the oxidized microenvironment produced by ROS plays a major role in causing postmenopausal osteoporosis [85,99,101]. Antioxidants are directly involved in the scavenging process of ROS. A lack of antioxidants may increase proinflammatory cytokines, especially tumor necrosis factor (TNF-α), and thereby induce bone loss [98].
There are two cytokines primarily responsible for osteoclastogenesis: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL), which are produced by bone marrow stromal cells and osteoblasts during bone remodeling [31]. The RANK (receptor activator of nuclear factor kappa B), RANKL, and OPG system has been identified as a primary regulator of the bone remodeling process. Osteoprotegerin (OPG), which is produced by osteoblasts, is considered to be a decoy receptor for RANKL. RANKL binds to its receptor RANK on osteoclast precursors in the presence of M-CSF. Upon this, osteoclast precursors differentiate and combine to form multinucleated osteoclasts, which can start bone resorption [85]. Due to insufficient estrogen levels in postmenopausal women, OPG is downregulated and RANKL activity upregulated, thereby increasing osteoclastogenesis [10].
4. Carotenoid Lycopene: Chemistry and its Isomers
To date, more than 700 carotenoids [102] have been identified, of which 40–50 are present in the human diet in fruits and vegetables [103]. However, only 20 have been found in human tissues or blood [104,105]. There are two classes of carotenoids: nonoxygenated carotenoids and oxygenated carotenoids. Nonoxygenated carotenoids are unsaturated hydrocarbons such as lycopene, α-carotene, β-carotene, γ-carotene, and ζ-carotene, whereas oxygenated carotenoids are the xanthophylls [18].
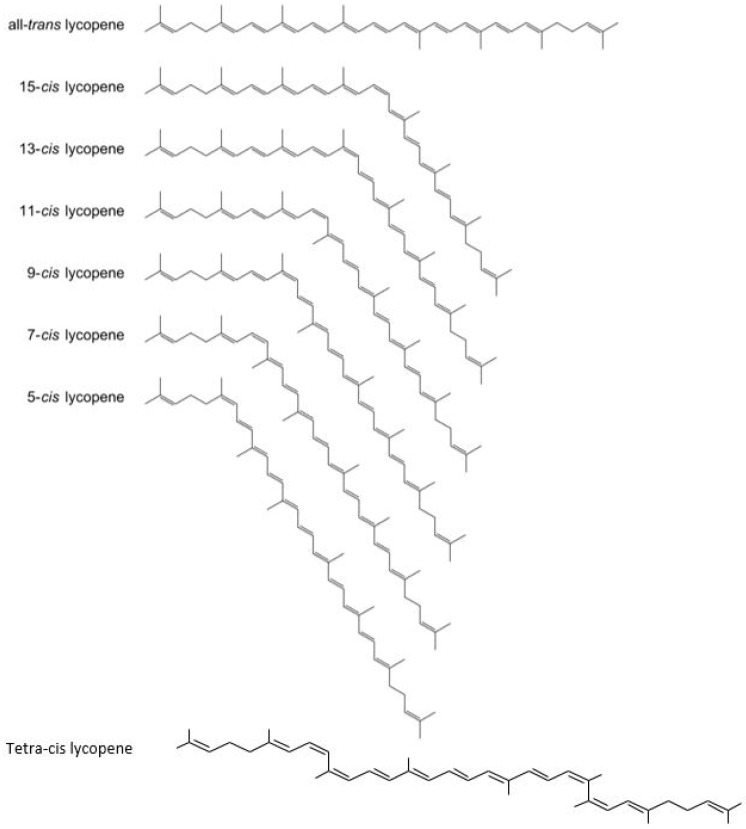
The major carotenoids found in the human body and human diet are β-carotene, α-carotene, α-cryptoxanthin, lutein, zeaxanthin, and lycopene [106,107]. Carotenoids are localized within chloroplasts and chromoplasts in plant cells. In chloroplasts, the carotenoids are found in association with proteins, whereas chromoplasts contain a crystalline form of carotenoids [108]. Lycopene, a member of the carotenoid pigment family, is responsible for the specific red color in many fruits and vegetables, best typified by fresh tomatoes and tomato products [107]. It is a lipid-soluble antioxidant produced by plants and some microorganisms. Unlike β-carotene, lycopene does not contain a terminal β-ionone ring and thus does not have provitamin A activity. Lycopene is an acyclic carotenoid containing 11 conjugated double-bonds in its all-_trans_-isomeric form or in various _cis_-configurations (Figure 2) [15]. Having 11 conjugated double-bonds, lycopene is theoretically assumed to have 2048 possible _cis–trans_-conformations in nature, but only a few have been identified so far. Due to its molecular structure, only certain _cis_-isomers exist, predominately 5-cis, 9-cis, 13-cis, and 15-cis. The most stable isomeric form is 5-cis, followed by all-trans, 9-cis, 13-cis, 15-cis, 7-cis, and, finally, 11-cis as the least stable [104].
Figure 2.
All-_trans_-lycopene and geometrical isomers.
Phytochemicals from fruits and vegetables are reported to aid in the maintenance of bone metabolism. In particular, carotenoids such as α-carotene, β-carotene, canthaxanthin, and lycopene have demonstrated beneficial effects on skeletal health; there is a clear positive association between lycopene intake and reduced bone loss in humans [109]. Recently, an inverse relationship between hip fracture risk and consumption of carotenoids from fruits and vegetables was reported in men aged 45–74 [110]. Lycopene may suppress the formation of preosteoclasts from osteoprogenitor cells, thereby disrupting the osteoclast formation pathway [5].
Lycopene Bioavailability, Absorption, and Metabolism
The bioavailability of ingested lycopene is dependent on the dose of lycopene consumed, linkages between molecules in the food matrix, incorporation of fats, level of dietary fiber, interactions of lycopene with other carotenoids, and genetic factors [18,111]. Moreover, the bioavailability of lycopene differs depending on the isomeric form. In fact, _cis_-isomers of lycopene are estimated to be 8.5 times more bioavailable than all-trans lycopene [16]. The higher bioavailability of _cis_-isomers compared to all-trans is mostly due to the former’s increased solubility in mixed micelles [112]. Compared to all-trans, _cis_-isomers are less likely to crystalize, highly soluble in oil, preferentially micellarized, readily taken up by intestinal cells, and easily transported within cells as well as across plasma and tissue matrices [16,17]. It is suggested that due to the polar nature and kinked forms of _cis_-isomers, they are less prone to crystallize [18,113]. Moreover, their preferential solubility is likely due to the smaller chain length of _cis_-isomers based on the bending of their structures, which may not be found in _trans_-lycopene isomers [114]. Lastly, thermal processing and mixing with oil can further increase the bioavailability of _cis_-lycopene present in tomatoes [18].
Interestingly, there are some natural sources, such as orange heirloom tomatoes, that contain more bioavailable _cis_-lycopene [15,20]. Almost 90% of the lycopene in orange heirloom tomatoes is in the _cis_-form compared to 90% of the all-_trans_-isomeric form of lycopene in red tomatoes [15]. This is due to a mutation known as tangerine in the carotenoid _cis-trans_-isomerase gene [115]. The _cis_-form gives a deep orange color to these tomatoes [20,116]. As the tangerine mutant lacks the ability to convert poly-_cis_-lycopene into all-trans, orange heirloom tomatoes predominantly accumulate tetra-_cis_-lycopene along with other precursors of lycopene such as phytoene, phytofluene, ζ-carotene, and neurosporene [20].
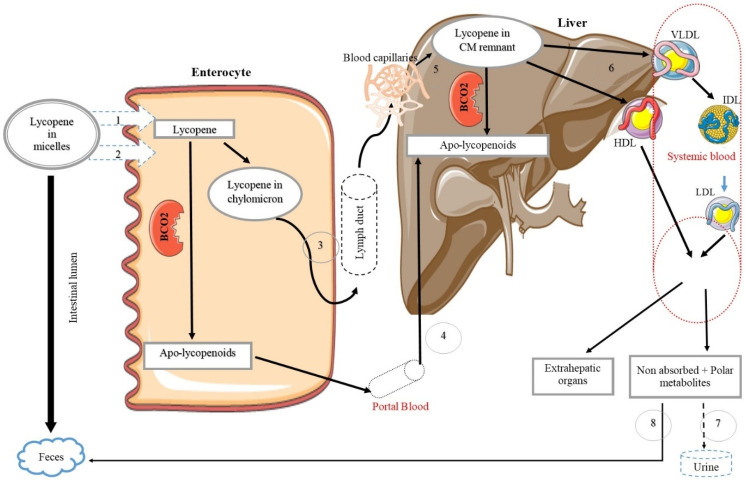
Being lipid-soluble, lycopene is absorbed into the body following the same pathway as fats. The foremost step of lycopene absorption is the breakdown of the food matrix and the release of carotenoids into the gastrointestinal lumen. Mechanical alteration of the food structure by cooking and other methods of food processing may improve carotenoid release from the food matrix [117]. Lycopene then enters intestinal mucosal cells through the formation of bile acid micelles [118]. However, bile production depends on the amount of fat present in the diet; therefore, it is necessary to incorporate fat with the lycopene-containing food in order to increase its solubility [119]. In general, lycopene absorption from dietary sources ranges from 10–30% [104], and, according to previous studies, a minimum 5–10 g of fat in a meal is required to ensure better absorption of carotenoids [120]. Once lycopene enters the enterocytes, it is either cleaved by β-carotene−9′,10′-oxygenase (BCO2) to produce lycopenoids or incorporated into chylomicrons and secreted into lymphatic and blood circulation [117,120]. Absorbed lycopene can either accumulate in the liver or be packed into VLDL and HDL and thereby re-enter the blood. Via blood circulation, lycopene is deposited in extrahepatic organs, mainly the adrenal glands, adipose tissue, prostate, and testes [121]. Non-absorbed lycopene and the excess metabolic products are excreted from the body in urine and feces (Figure 3).
Figure 3.
Simplified diagram of lycopene metabolism in the body (reference from [117,119,120,133,134,135,136]). Lycopene enters the enterocytes by active (1) and passive (2) transporters. There, it is packed in chylomicrons or converted to apo-lycopenoids by BCO2. Then, the chylomicrons or apo-lycopenoids are transferred to the liver via the lymphatic (3) and the portal venous (4) systems. Chylomicron remnants (CM) pass to the blood capillaries and are then absorbed by the liver via receptor-mediated endocytosis (5). Lycopene is packaged in very low-density lipoproteins (VLDL) and high-density lipoproteins (HDL) by the liver and released to the systemic circulation (6). Lycopene travels to the extrahepatic organs through the systemic blood and is available there for its biological action. Polar metabolites are excreted in the urine by the kidneys (7), and non-absorbed lycopene is excreted through biliary excretion in feces (8).
Lycopene concentrations in body tissues are higher than those of other carotenoids [18,19,119]. Half of the carotenoids in human serum are lycopene, and, among them, _cis_-isomers account for 58–73% of total lycopene in serum [16,122,123]. In the human body, lycopene is present at 1 nmol/g in adipose tissues and is found in higher concentrations of up to 20 nmol/g in testes, adrenal, and prostate glands [124]. Interestingly, neither lycopene nor other carotenoids have been found in brain stem tissues [125]. In addition, lycopene appears to have a long half-life; a recent study indicated that lycopene and its metabolites could be detected in the skin of humans up to 40 days after consumption [126].
The distribution of lycopene isomers is similar between plasma and tissues. Regardless of the isomeric forms of lycopene consumed, plasma and other tissues contain more _cis_-isomers, mainly the 5-_cis_-form [119,122]. Lycopene metabolites, or lycopenoids, can be the products of lycopene metabolism and oxidation. Kopec et al., were the first to identify the series of lycopenoids present in human blood, but they are found in plasma in only negligible amounts [127]. Similarly, lycopene metabolites can be found in some foods, but at 1000-fold lower concentrations than that of lycopene itself [127]. Some studies suggest that metabolites of lycopene may play a role in the biological activities of lycopene. However, only a limited number of studies have investigated the role of lycopene metabolites in vivo [128,129,130,131,132].
5. Evidence of the Effect of Lycopene on Bone Health
Recently, the effect of lycopene on bone health has received additional attention from researchers [137]. The beneficial effects of lycopene on bone health have been studied using animal models, cell cultures, and epidemiological/clinical studies, as described below and in Table 2, Table 3 and Table 4, in the context of postmenopausal osteoporosis.
Table 2.
The effect of lycopene on postmenopausal bone loss based on human trials.
| Author and Year | Cohort | Lycopene Formulation and Study Duration | Outcome |
|---|---|---|---|
| Russo et al. (2020) [143] | Postmenopausal women(n = 39)Age: 63 ± 7 years | 3.9 mg/day as tomato sauce3 months | Patients who consumed tomato sauce did not show a significant loss of BMD compared to control group |
| Mackinnon et al. (2011) [141] | Postmenopausal women(n = 60)Age: 50–60 years | 30 mg/day (regular tomato juice),70 mg/day (lycopene-rich tomato juice),30 mg/day (Lyc-O-Mato capsules)4 months | Lycopene intervention in capsule or juice form supplying at least 30 mg/day led to decreased oxidative stress and bone resorption markers |
| Mackinnon (2010) [144] | Postmenopausal women(n = 45)Age: 55 years | 43.33 mg/day supplementation4 months | Lycopene supplemented group showed significantly lower levels of bone resorption marker (NTx) |
| Mackinnon et al. (2011) [140] | Postmenopausal women(n = 23)Age: 50–60 years | Lycopene intake at baseline and after one month of lycopene restriction was 3.5 mg/d and 0.13 mg/d, respectively (using 7-day dietary records) | Bone resorption marker (NTx) was increased after a month of lycopene restrictionEndogenous antioxidant enzymes (SOD and catalase) were decreased after a month of lycopene restriction |
| Rao et al. (2007) [145] | Postmenopausal women(n = 33)Age: 50–60 years | Lycopene intake categorized into four groups as ranged from 1.76 to 7.35 mg/day (using 7-day dietary records) | Serum NTx values dose-dependently decreasedPostmenopausal women who consumed 7.35 mg lycopene/day had lower serum NTx compared to the other three groupsNo difference in bone formation markers |
Table 3.
The effects of lycopene on postmenopausal bone loss based on rodent trials.
| Author and Year | Animal Strain | Lycopene Dose and Study Duration | Outcome |
|---|---|---|---|
| Oliveira et al. (2019) [152] | Female Wistar rats | 10 mg/kg BW/day4 weeks pre-OVX and 8 weeks post-OVX | Decreased bone loss in femur epiphysis in the OVX + lycopene group compared to the OVX control group |
| Li et al. (2018) [153] | Female Sprague-Dawley rats | 50 mg/kg BW/day12 weeks | Higher bone volume and trabecular thickness with low trabecular spaces in the OVX + lycopene group compared to the OVX control groupIncreased bone contact and bone area around the implant were in the lycopene-treated group compared to controls |
| Ardawi et al. (2016) [99] | Female Wistar rats | 15, 30, 45 mg/kg BW per day12 weeks | Lycopene treatment dose-dependently enhanced BMD and BMC at the lumbar spine and humerus compared to OVX control groupLycopene (30 and 45 mg/kg BW) increased bone formation markers (serum-OC and serum PINP) while bone resorption markers (serum-CTX-1 and urine-DPD) were decreased |
| Iimura et al. (2015) [14] | Female Sprague-Dawley6-week-old | 0, 50, 100, 200 mg lycopene/kg diet/day9 weeks | Lycopene (100 mg/kg) increased lumbar spine BMD and femoral-breaking force compared to OVX control groupBone resorption markers were low in all lycopene-treated groups |
| Iimura et al. (2014) [13] | Female Sprague–Dawley6-week-old | 0, 50, 100 mg/kg diet lycopene9 weeks | Lycopene (100 mg/kg) increased BMD of the lumbar spine and the tibial proximal metaphysis compared to OVX control group |
| Liang et al. (2012) [151] | Female Wistar rats8-week-old | 20, 30, 40 mg/kg BW/day8 weeks | Lycopene (30 and 40 mg/kg BW) dose-dependently increased BMD and BMC in OVX rats compared to OVX control group |
Table 4.
The effects of lycopene on bone cells (osteoblasts and osteoclasts).
| Author and Year | Cell Line | Lycopene Concentration | Outcome |
|---|---|---|---|
| Russo et al. (2020) [143] | Human osteoblast-like cell line Saos-2 | 5 and 10 μM | Lycopene suppressed RANKL expression indicating the reduction of bone resorptionLycopene reduced the stimulatory effect of ALP within 24 h indicating possible role in mineralization |
| Oliveira et al. (2019) [152] | Osteoblastic cells from femur medullary canals of ovariectomized female rats | 1 μM | Lycopene upregulated the genes associated with bone metabolism of osteoblastic cells within 3–10 days |
| Costa-Rodrigues et al. (2018) [156] | Osteoblastic cells (human mesenchymal stem cells bone-marrow-derived) Osteoclastic cells (human peripheral blood mononuclear cells) | 5 nM−50 μM | Lycopene (≥500 nM) increased osteoblastic cell proliferation and differentiationLycopene (≥500 nM) significantly decreased osteoclast differentiation |
| Marcotorchino et al. (2012) [159] | RAW 264.7 cells | 0.5, 1, 2 μM | Lycopene dose-dependently reduced the lipopolysaccharides (LPS) mediated activation of inflammatory cytokine (TNF-α) produced by macrophages |
| Feng et al. (2010) [160] | RAW 264.7 cells | 1–10 μM | Lycopene dose-dependently inhibited the increase of nitric oxide production and the secretion of IL-6 when RAW cells were stimulated by LPS |
| Stefano et al. (2007) [161] | RAW 264.7 cells | 5, 10, 20 μM | Lycopene (20 μM) significantly inhibited the ROS accumulated due to addition of gliadinLycopene (20 μM) significantly inhibited increase in nitric oxide synthase levels |
| Rao et al. (2003) [34] | Osteoclast were generated from bone marrow cells | 0.01, 0.1, 1, 10 μM | Lycopene (10 μM) significantly inhibited PTH stimulated resorption by osteoclasts |
5.1. Epidemiological and Clinical Studies
Human epidemiological studies have investigated the specific effects of tomato/lycopene on bone health, and the majority have shown a positive correlation between tomato/lycopene consumption and the prevention of bone loss (Table 2). The Framingham Osteoporosis study evaluated associations between total and isolated carotenoids with BMD in older adults (~75 years old) [138]. An inverse relationship between lycopene levels and four-year bone loss in the lumbar spine in older women (~75 years old) was observed [138], and a follow-up study reported a protective effect of lycopene against hip fractures [139]. Mackinnon et al. reported a notable increase in a clinically relevant bone resorption marker, the crosslinked N-telopeptide of type 1 bone biomarker (NTx), as well as oxidative stress markers in postmenopausal women after one-month restriction of lycopene in the diet [140]. This also led to a drastic reduction in serum lycopene along with other carotenoids such as α-carotene, β-carotene, lutein, and zeaxanthin [140]. Similarly, 30 mg/d lycopene supplementation in postmenopausal women in either juice or capsule form for four months decreased serum NTx level [141]. An epidemiological study in premenopausal women, which evaluated the effect of dietary carotenoids on bone mineral status, showed a positive correlation between lycopene intake and total body BMC and BMD [12]. Another study revealed lower levels of serum lycopene in postmenopausal women with osteoporosis compared to non-osteoporotic women [142].
5.2. Animal Trials
Ovariectomy (OVX) is the most widely used surgical technique for the induction of osteoporosis in rodents and other animal models to mimic the hormonal and skeletal status of postmenopausal women [146]. Along with ovariectomy, most trials incorporate “sham”-operated animals as controls, which undergo surgery without the ovaries being removed [42]. Female rats are considered an excellent animal model for postmenopausal osteoporosis, but the age of the animals and site selection for harvesting the bones must be defined with care. Other experimental protocols related to bone loss have used interventions such as hormonal, dietary, and immobilization in rats, which had more variable effects on the rate of bone loss [147]. Ovariectomized rats fed a low calcium diet demonstrated rapid bone loss, supporting the use of this model as a gold standard for the evaluation of drugs for the treatment of osteoporosis [148,149]. Higher serum levels of osteocalcin, a biomarker of bone turnover, were measured in ovariectomized rats fed a low calcium diet compared to sham-operated rats [38,149]. Ovariectomized rats are considered most suitable for the evaluation of preventative agents for postmenopausal osteoporosis, although not for evaluation of treatment of osteoporosis over a long period of time [42,150]. This is because the rate of bone turnover in OVX rats become similar to their sham counterparts in studies >12 months, and also because the exact parameters for expected increases in the skeletal size of trabecular and cortical bone in the rat with long term OVX are not yet established [150].
Lycopene has been shown in multiple studies to dose-dependently increase BMD in ovariectomized rats [151]. Similarly, lycopene reduced bone fragility in ovariectomized rats and improved femoral bone energy, as assessed using a mechanical breaking test [14]. More recently, lycopene supplementation in ovariectomized rats was found to significantly alter levels of biomarkers of bone turnover in blood and urine, reducing bone resorption and increasing osteoblast activity. Simultaneously, lycopene treatment increased the enzyme action of glutathione peroxidase, catalase, and superoxide dismutase, and downregulated oxidative stress [99]. Taken together, these animal studies suggest that lycopene has bone-protective benefits (Table 3).
5.3. Bone Cell Culture Studies
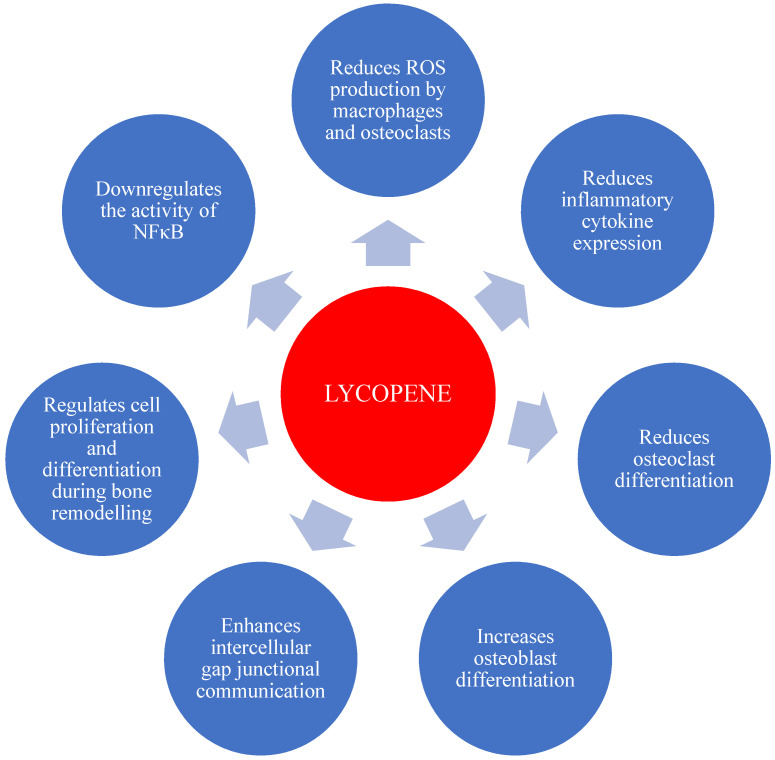
Multiple in vitro studies have demonstrated that lycopene and other carotenoids directly affect both osteoclasts and osteoblasts (Table 4). Ishimi and coworkers reported that osteoclast-like cell formation induced by 1α, 25(OH) 2 D3 (Calcitriol), IL-1β, and parathyroid hormone was inhibited by retinoic acid and carotenoids, including β -carotene, canthaxanthin, and lycopene, with retinoic acid being most effective [154]. Park et al., also found retinoic acid to have the highest activity in inducing differentiation of the osteoblastic cell line MC3T3-E1, with retinol, β -carotene, lycopene, and canthaxanthin showing lesser but similar effectiveness [155]. Lycopene inhibited osteoclast formation and bone resorption by rat bone marrow cells in a model of parathyroid hormone-induced osteoclastogenesis [34]. More recently, human osteoclast and osteoblast precursor cells, treated with 500 nM lycopene for 21 days, demonstrated reduced osteoclastogenesis while increasing osteoblastogenesis [156]. Lack of nuclear factor kappa B (NFκB) may lead to a reduction in osteoclast precursors differentiating into mature osteoclasts, thereby reducing bone loss. Interestingly, some studies suggest that derivatives of carotenoids can downregulate the activity of NFκB activity, a regulator of cytokine expression [8,157,158], suggesting NFκB modulation is a candidate pathway for lycopene’s protective effects against osteoporosis. Taken together, evidence from in vitro studies suggest that lycopene has beneficial effects on bone health via molecular mechanisms that are summarized in Figure 4.
Figure 4.
Schematic representation of the potential effects of lycopene on bone cells (reference from [13,14,99].
6. Conclusions
Postmenopausal bone loss is a public health issue in the aging population. Medications can prevent and treat bone loss in some patients but may have negative side effects. Phytochemicals present in fruits and vegetables play a major role in noncommunicable disease management. As summarized in this review, lycopene has a protective effect against bone loss; this has been demonstrated in in-vitro studies, in animal models of osteoporosis, and human clinical studies. The epidemiological and clinical studies discussed in this review have demonstrated that lycopene intake (≥30 mg/day) is effective in reducing bone resorption markers in postmenopausal women [140,141]. Tomatoes are a major source of dietary lycopene; however, due to the relatively low bioavailability of all-_trans_-lycopene isomers found in red tomatoes, several techniques are used to increase bioavailability, such as adding oils or making cooked sauces and pastes. Other sources of more bioavailable lycopene can be found in nature. For example, orange heirloom tomatoes contain >90% of lycopene in a _cis_-isomeric form, which is estimated to be 8.5 times more bioavailable than all-_trans_-lycopene [16]. Further studies are warranted to compare the relative benefits of these tomato varieties and lycopene isomers in protecting against bone loss.
Acknowledgments
The figures were made using freely available resources: Smart Servier medical art (https://smart.servier.com/) and Creative Commons resources (https://creativecommons.org/licenses/by-nc-sa/3.0/).
Abbreviations
| CKD | Chronic kidney disease |
|---|---|
| BMU | Basic multicellular unit |
| BMD | Bone mineral density |
| BMC | Bone mineral content |
| SD | Standard deviation |
| DEXA | Dual-energy X-ray absorptiometry |
| MRI | Magnetic resonance imaging |
| ROS | Reactive oxygen species |
| OH | Hydroxyl |
| O2− | Superoxide |
| H2O2 | Hydrogen peroxides |
| TNF | Tumor necrosis factor |
| OPG | Osteoprotegerin |
| M-CSF | Macrophage colony-stimulating factor |
| RANK | Receptor Activator of Nuclear Factor Kappa B |
| RANKL | Receptor Activator of Nuclear Factor Kappa B Ligand |
| IL | Interleukin |
| IGF | Insulin-like growth factor |
| IFN | Interferon |
| MHC | Major histocompatibility complex |
| PGE | Prostaglandin E2 |
| PTH | Parathyroid hormone |
| BCO2 | Β-carotene-9′,10′-oxygenase |
| VLDL | Very low-density lipoprotein |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| IDL | Intermediate density lipoprotein |
| CM | Chylomicron |
| NTx | N-telopeptide of type 1 bone biomarker |
| SOD | Superoxide dismutase |
| OVX | Ovariectomy |
| CTX-1 | Carboxy terminal crosslinked telopeptides of type 1 collagen |
| DPD | Deoxypyridinoline |
| PINP | Procollagen type 1 N-terminal propeptide |
| NFκB | Nuclear factor kappa B |
| COL1A | Collagen type 1 alpha |
| CYP19A1 | Aromatase genes |
| ERα | Estrogen receptor alpha genes |
Author Contributions
The manuscript was conceptualized and written through the contribution of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Heritage Fruit Crop Research Trust (HFCRT) Wanganui, New Zealand (PR40335) and U.S.W. is supported by the Massey doctoral scholarship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Cooper C., Campion G., Melton L.J., III Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 2.Szulc P., Bouxsein M.L. Overview of osteoporosis: Epidemiology and clinical management. Vertebr. Fract. Initiat. Resour. Doc. 2011;1:1–65. [Google Scholar]
- 3.Wright N.C., Looker A.C., Saag K.G., Curtis J.R., Delzell E.S., Randall S., Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauley J.A., Chalhoub D., Kassem A.M., Fuleihan G.E.-H. Geographic and ethnic disparities in osteoporotic fractures. Nat. Rev. Endocrinol. 2014;10:338. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- 5.Pandey M.K., Gupta S.C., Karelia D., Gilhooley P.J., Shakibaei M., Aggarwal B.B. Dietary nutraceuticals as backbone for bone health. Biotechnol. Adv. 2018;36:1633–1648. doi: 10.1016/j.biotechadv.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Rao L., Rao A. Oxidative stress and antioxidants in the risk of osteoporosis—Role of phytochemical antioxidants lycopene and polyphenol-containing nutritional supplements. In: Venketeshwer Rao L.R., editor. Phytochemicals—Isolation, Characterisation and Role in Human Health. InTech Open Access Publisher; Rijeka, Croatia: 2015. pp. 247–260. [Google Scholar]
- 7.International Osteoporosis Foundation Facts and Statistics. [(accessed on 5 May 2020)]; Available online: https://www.iofbonehealth.org/facts-statistics.
- 8.Kini U., Nandeesh B. Radionuclide and Hybrid Bone Imaging. Springer; Berlin/Heidelberg, Germany: 2012. Physiology of bone formation, remodeling, and metabolism; pp. 29–57. [Google Scholar]
- 9.Duque G., Troen B.R. Understanding the mechanisms of senile osteoporosis: New facts for a major geriatric syndrome. J. Am. Geriatr. Soc. 2008;56:935–941. doi: 10.1111/j.1532-5415.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 10.Sipos W., Pietschmann P., Rauner M., Kerschan-Schindl K., Patsch J. Pathophysiology of osteoporosis. Wien. Med. Wochenschr. 2009;159:230–234. doi: 10.1007/s10354-009-0647-y. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura M., Nakamura M., Ogawa K., Ikoma Y., Ando F., Shimokata H., Yano M. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: Findings from post-menopausal Japanese female subjects. Osteoporos. Int. 2011;22:143–152. doi: 10.1007/s00198-010-1239-9. [DOI] [PubMed] [Google Scholar]
- 12.Wattanapenpaiboon N., Lukito W., Wahlqvist M.L., Strauss B.J. Dietary carotenoid intake as a predictor of bone mineral density. Asia Pac. J. Clin. Nutr. 2003;12:467–473. [PubMed] [Google Scholar]
- 13.Iimura Y., Agata U., Takeda S., Kobayashi Y., Yoshida S., Ezawa I., Omi N. Lycopene intake facilitates the increase of bone mineral density in growing female rats. J. Nutr. Sci. Vitaminol. 2014;60:101–107. doi: 10.3177/jnsv.60.101. [DOI] [PubMed] [Google Scholar]
- 14.Iimura Y., Agata U., Takeda S., Kobayashi Y., Yoshida S., Ezawa I., Omi N. The protective effect of lycopene intake on bone loss in ovariectomized rats. J. Bone Miner. Metab. 2015;33:270–278. doi: 10.1007/s00774-014-0596-4. [DOI] [PubMed] [Google Scholar]
- 15.Burri B.J., Chapman M.H., Neidlinger T.R., Seo J.S., Ishida B.K. Tangerine tomatoes increase total and tetra-cis-lycopene isomer concentrations more than red tomatoes in healthy adult humans. Int. J. Food Sci. Nutr. 2009;60:1–16. doi: 10.1080/09637480701782084. [DOI] [PubMed] [Google Scholar]
- 16.Cooperstone J.L., Ralston R.A., Riedl K.M., Haufe T.C., Schweiggert R.M., King S.A., Timmers C.D., Francis D.M., Lesinski G.B., Clinton S.K. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015;59:658–669. doi: 10.1002/mnfr.201400658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida B., Roberts J., Chapman M., Burri B. Processing tangerine tomatoes: Effects on lycopene-isomer concentrations and profile. J. Food Sci. 2007;72:307–312. doi: 10.1111/j.1750-3841.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 18.Shi J., Maguer M.L. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000;40:1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- 19.Rao A., Waseem Z., Agarwal S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int. 1998;31:737–741. doi: 10.1016/S0963-9969(99)00053-8. [DOI] [Google Scholar]
- 20.Cooperstone J.L., Francis D.M., Schwartz S.J. Thermal processing differentially affects lycopene and other carotenoids in cis-lycopene containing, tangerine tomatoes. Food Chem. 2016;210:466–472. doi: 10.1016/j.foodchem.2016.04.078. [DOI] [PubMed] [Google Scholar]
- 21.Unlu N.Z., Bohn T., Francis D., Clinton S.K., Schwartz S.J. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-β-carotene varieties of tomatoes. J. Agric. Food Chem. 2007;55:1597–1603. doi: 10.1021/jf062337b. [DOI] [PubMed] [Google Scholar]
- 22.Morgan K.T. Nutritional determinants of bone health. J. Nutr. Elder. 2008;27:3–27. doi: 10.1080/01639360802059670. [DOI] [PubMed] [Google Scholar]
- 23.Bartl R., Frisch B. Osteoporosis: Diagnosis, Prevention, Therapy. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2009. pp. 7–22. [Google Scholar]
- 24.Florencio-Silva R., Sasso G.R.d.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed Res. Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burr D.B., Akkus O. Chapter 1—Bone Morphology and Organization. In: Burr D.B., Allen M.R., editors. Basic and Applied Bone Biology. Academic Press; San Diego, CA, USA: 2014. pp. 3–25. [Google Scholar]
- 26.Alves R. Ph.D. Thesis. Erasmus University; Rotterdam, The Netherlands: 2012. Osteoblast Differentiation and Bone: Relevant proteins, regulatory processes and the vascular connection. [Google Scholar]
- 27.McCormick R.K. Osteoporosis: Integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern. Med. Rev. 2007;12:113. [PubMed] [Google Scholar]
- 28.Jilka R.L., Weinstein R.S., Bellido T., Parfitt A.M., Manolagas S.C. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J. Bone Miner. Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 29.Horcajada M.-N., Offord E. Naturally plant-derived compounds: Role in bone anabolism. Curr. Mol. Pharmacol. 2012;5:205–218. doi: 10.2174/1874467211205020205. [DOI] [PubMed] [Google Scholar]
- 30.Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3:131–139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iñiguez-Ariza N.M., Clarke B.L. Bone biology, signaling pathways, and therapeutic targets for osteoporosis. Maturitas. 2015;82:245–255. doi: 10.1016/j.maturitas.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Langdahl B., Ferrari S., Dempster D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016;8:225–235. doi: 10.1177/1759720X16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron R., Hesse E. Update on bone anabolics in osteoporosis treatment: Rationale, current status, and perspectives. J. Clin. Endocrinol. Metab. 2012;97:311–325. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao L.G., Krishnadev N., Banasikowska K., Rao A.V. Lycopene 1—Effect on osteoclasts: Lycopene inhibits basal and parathyroid hormone-stimulated osteoclast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures. J. Med. Food. 2003;6:69–78. doi: 10.1089/109662003322233459. [DOI] [PubMed] [Google Scholar]
- 35.Dempster D.W., Raisz L.G. Bone physiology: Bone cells, modeling, and remodeling. In: Holick M., Nieves J., editors. Nutrition and Bone Health. Humana Press; New York, NY, USA: 2015. pp. 37–56. [Google Scholar]
- 36.Manolagas S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 37.Hadjidakis D.J., Androulakis I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 38.Shetty S., Kapoor N., Bondu J.D., Thomas N., Paul T.V. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2016;20:846. doi: 10.4103/2230-8210.192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui J.A., Partridge N.C. Physiological bone remodeling: Systemic regulation and growth factor involvement. Physiology. 2016;31:233–245. doi: 10.1152/physiol.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao L., Rao A. Oxidative stress and antioxidants in the risk of osteoporosis—Role of the antioxidants lycopene and polyphenols. In: Valdés-Flores M., editor. Topics in Osteoporosis. Volume 5. InTech Open Access Publisher; Rijeka, Croatia: 2013. pp. 117–144. [Google Scholar]
- 41.Ferdous H., Afsana F., Qureshi N.K., Rouf R.S.B. Osteoporosis: A Review. BIRDEM Med. J. 2016;5:30–36. doi: 10.3329/birdem.v5i1.28371. [DOI] [Google Scholar]
- 42.Kruger M.C., Morel P.C. Experimental control for the ovariectomized rat model: Use of sham versus nonmanipulated animal. J. Appl. Anim. Welf. Sci. 2016;19:73–80. doi: 10.1080/10888705.2015.1107727. [DOI] [PubMed] [Google Scholar]
- 43.Annapoorna N., Rao G.V., Reddy N., Rambabu P., Rao K.S. An increased risk of osteoporosis during acquired immunodeficiency syndrome. Int. J. Med. Sci. 2004;1:152. doi: 10.7150/ijms.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siris E., Boonen S., Mitchell P., Bilezikian J., Silverman S. What’s in a name? What constitutes the clinical diagnosis of osteoporosis? Osteoporos. Int. 2012;23:2093–2097. doi: 10.1007/s00198-012-1991-0. [DOI] [PubMed] [Google Scholar]
- 45.The National Institute of Health Bone Mass Measurement: What the Numbers Mean. [(accessed on 5 May 2020)]; Available online: https://www.bones.nih.gov/health-info/bone/bone-health/bone-mass-measure.
- 46.Adler R.A. Update on osteoporosis in men. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:759–772. doi: 10.1016/j.beem.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Feng X., McDonald J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nieves J.W., Cosman F. Nutrition and Bone Health. Springer; Berlin/Heidelberg, Germany: 2015. Impact of Nutrition on Medications for Osteoporosis; pp. 681–686. [Google Scholar]
- 49.International Osteoporosis Foundation Treatment. [(accessed on 2 August 2020)]; Available online: https://www.osteoporosis.foundation/health-professionals/treatment.
- 50.Ferrari S., Karasik D. Gene–Diet Interactions on Bone. In: Holick M., Nieves J., editors. Nutrition and Bone Health. Humana Press; New York, NY, USA: 2015. pp. 21–36. [Google Scholar]
- 51.Ralston S.H. Osteoporosis as an hereditary disease. Clin. Rev. Bone Miner. Metab. 2010;8:68–76. doi: 10.1007/s12018-010-9073-3. [DOI] [Google Scholar]
- 52.Spotila L.D., Constantinou C.D., Sereda L., Ganguly A., Riggs B.L., Prockop D.J. Mutation in a gene for type I procollagen (COL1A2) in a woman with postmenopausal osteoporosis: Evidence for phenotypic and genotypic overlap with mild osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA. 1991;88:5423–5427. doi: 10.1073/pnas.88.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prockop D.J., Colige A., Helminen H., Khillan J., Pereira R., Vandenberg P. Mutations in type 1 procollagen that cause osteogenesis imperfecta: Effects of the mutations on the assembly of collagen into fibrils, the basis of phenotypic variations, and potential antisense therapies. J. Bone Miner. Res. 1993;8:489–492. doi: 10.1002/jbmr.5650081311. [DOI] [PubMed] [Google Scholar]
- 54.Marini J.C., Forlino A., Cabral W.A., Barnes A.M., San Antonio J.D., Milgrom S., Hyland J.C., Körkkö J., Prockop D.J., De Paepe A. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morishima A., Grumbach M.M., Simpson E.R., Fisher C., Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 56.Smith E.P., Boyd J., Frank G.R., Takahashi H., Cohen R.M., Specker B., Williams T.C., Lubahn D.B., Korach K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan S.D., Lehman A., Nathan N.K., Thomson C.A., Howard B.V. Age of Menopause and Fracture Risk in Post-Menopausal Women Randomized to Calcium+ Vitamin D, Hormone Therapy, or the combination: Results from the Women’s Health Initiative Clinical Trials. Menopause. 2017;24:371. doi: 10.1097/GME.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dawson-Hughes B., Heaney R.P., Holick M.F., Lips P., Meunier P.J., Vieth R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 59.Joint FAO/WHO Committee on Food Additives (JECFA) WHO Technical Repport Series. Volume 68 World Helath Organization; Geneva, Switserland: 2007. Evaluation of certain food additives and contaminants: Sixty-eighth report of the Joint FAO/WHO Expert Committee on Food Additives. [Google Scholar]
- 60.Melton L.J., III, Achenbach S.J., Gebhart J.B., Babalola E.O., Atkinson E.J., Bharucha A.E. Influence of hysterectomy on long-term fracture risk. Fertil. Steril. 2007;88:156–162. doi: 10.1016/j.fertnstert.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanis J.A., Johansson H., Johnell O., Oden A., De Laet C., Eisman J.A., Pols H., Tenenhouse A. Alcohol intake as a risk factor for fracture. Osteoporos. Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 62.Fazeli P.K., Klibanski A. Effects of anorexia nervosa on bone metabolism. Endocr. Rev. 2018;39:895–910. doi: 10.1210/er.2018-00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanis J.A., Johnell O., Odén A., Johansson H., De Laet C., Eisman J., Fujiwara S., Kroger H., McCloskey E., Mellstrom D. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 64.Kanis J., Johansson H., Odén A., Johnell O., De Laet C., Eisman J., McCloskey E., Mellstrom D., Melton III L., Pols H. A family history of fracture and fracture risk: A meta-analysis. Bone. 2004;35:1029–1037. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 65.Seeman E., Hopper J.L., Bach L.A., Cooper M.E., Parkinson E., McKay J., Jerums G. Reduced bone mass in daughters of women with osteoporosis. N. Engl. J. Med. 1989;320:554–558. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- 66.De Laet C., Kanis J., Odén A., Johanson H., Johnell O., Delmas P., Eisman J., Kroger H., Fujiwara S., Garnero P. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 67.Kanis J., Johnell O., De Laet C., Johansson H., Odén A., Delmas P., Eisman J., Fujiwara S., Garnero P., Kroger H. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 68.Misra M., Golden N.H., Katzman D.K. State of the art systematic review of bone disease in anorexia nervosa. Int. J. Eat. Disord. 2016;49:276–292. doi: 10.1002/eat.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moayyeri A., Luben R.N., Bingham S.A., Welch A.A., Wareham N.J., Khaw K.T. Measured height loss predicts fractures in middle-aged and older men and women: The EPIC-Norfolk prospective population study. J. Bone Miner. Res. 2008;23:425–432. doi: 10.1359/jbmr.071106. [DOI] [PubMed] [Google Scholar]
- 70.Bass S.L., Saxon L., Daly R., Turner C.H., Robling A.G., Seeman E., Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A study in tennis players. J. Bone Miner. Res. 2002;17:2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 71.Kemmler W., Lauber D., Weineck J., Hensen J., Kalender W., Engelke K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: Results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS) Arch. Intern. Med. 2004;164:1084–1091. doi: 10.1001/archinte.164.10.1084. [DOI] [PubMed] [Google Scholar]
- 72.International Osteoporosis Foundation Fixed Risk. [(accessed on 25 August 2020)]; Available online: https://www.osteoporosis.foundation/health-professionals/about-osteoporosis/risk-factors/fixed-risks.
- 73.Tinetti M.E. Preventing falls in elderly persons. N. Engl. J. Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 74.International Osteoporosis Foundation About Osteoporosis. [(accessed on 24 July 2020)]; Available online: https://www.osteoporosis.foundation/health-professionals/about-osteoporosis.
- 75.Becker C. Pathophysiology and clinical manifestations of osteoporosis. Clin. Cornerstone. 2006;8:19–27. doi: 10.1016/S1098-3597(06)80062-1. [DOI] [PubMed] [Google Scholar]
- 76.Jahanian E., Karimifar M., Rafieian-Kopaei M. Antioxidants as a novel way to alleviate the adverse effects of oxidative stress in osteoporosis. J. Parathyr. Dis. 2016;4:61. [Google Scholar]
- 77.Kim N., Choi H.-R., Kim S.-W., Kim B.-S., Won C.-W., Kim S.-Y. Association between bone mineral density and sleep duration in the Korean elderly population. Korean J. Fam. Med. 2014;35:90. doi: 10.4082/kjfm.2014.35.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi D., Takahashi O., Deshpande G.A., Shimbo T., Fukui T. Association between osteoporosis and sleep duration in healthy middle-aged and elderly adults: A large-scale, cross-sectional study in Japan. Sleep Breath. 2012;16:579–583. doi: 10.1007/s11325-011-0545-6. [DOI] [PubMed] [Google Scholar]
- 79.Hill S.M., Spriggs L.L., Simon M.A., Muraoka H., Blask D.E. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Lett. 1992;64:249–256. doi: 10.1016/0304-3835(92)90050-6. [DOI] [PubMed] [Google Scholar]
- 80.Ochs-Balcom H.M., Hovey K.M., Andrews C., Cauley J.A., Hale L., Li W., Bea J.W., Sarto G.E., Stefanick M.L., Stone K.L. Short sleep is associated with low bone mineral density and osteoporosis in the Women’s Health Initiative. J. Bone Miner. Res. 2020;35:261–268. doi: 10.1002/jbmr.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu X., Zhao X., Lu H., Jiang F., Ma X., Zhu S. Association between sleep duration and bone mineral density in Chinese women. Bone. 2011;49:1062–1066. doi: 10.1016/j.bone.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Wang K., Wu Y., Yang Y., Chen J., Zhang D., Hu Y., Liu Z., Xu J., Shen Q., Zhang N. The associations of bedtime, nocturnal, and daytime sleep duration with bone mineral density in pre-and post-menopausal women. Endocrine. 2015;49:538–548. doi: 10.1007/s12020-014-0493-6. [DOI] [PubMed] [Google Scholar]
- 83.Niu J., Sahni S., Liao S., Tucker K.L., Dawson-Hughes B., Gao X. Association between sleep duration, insomnia symptoms and bone mineral density in older Boston Puerto Rican adults. PLoS ONE. 2015;10:e0132342. doi: 10.1371/journal.pone.0132342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khosla S., Oursler M.J., Monroe D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012;23:576–581. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okman-Kilic T. Estrogen deficiency and osteoporosis. In: Dionyssiotis Y., editor. Advances in Osteoporosis. InTech Open Access Publisher; Rijeka, Croatia: 2015. pp. 7–17. [Google Scholar]
- 86.Weitzmann M.N., Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Charatcharoenwitthaya N., Khosla S., Atkinson E.J., McCready L.K., Riggs B.L. Effect of blockade of TNF-α and interleukin-1 action on bone resorption in early postmenopausal women. J. Bone Miner. Res. 2007;22:724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 88.Garnero P., Sornay-Rendu E., Chapuy M.C., Delmas P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996;11:337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 89.Dresner-Pollak R., Parker R.A., Poku M., Thompson J., Seibel M.J., Greenspan S.L. Biochemical Markers of Bone Turnover Reflect Femoral Bone Loss in Elderly Women. Calcif. Tissue Int. 1996;59:328–333. doi: 10.1007/s002239900135. [DOI] [PubMed] [Google Scholar]
- 90.Wu X.-Y., Li H.-L., Xie H., Luo X.-H., Peng Y.-Q., Yuan L.-Q., Sheng Z.-F., Dai R.-C., Wu X.-P., Liao E.-Y. Age-related bone turnover markers and osteoporotic risk in native Chinese women. BMC Endocr. Disord. 2014;14:8. doi: 10.1186/1472-6823-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iki M., Akiba T., Matsumoto T., Nishino H., Kagamimori S., Kagawa Y., Yoneshima H., Group J.S. Reference database of biochemical markers of bone turnover for the Japanese female population. Japanese Population-based Osteoporosis (JPOS) Study. Osteoporos. Int. 2004;15:981–991. doi: 10.1007/s00198-004-1634-1. [DOI] [PubMed] [Google Scholar]
- 92.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S.-i., Tomoyasu A., Yano K., Goto M., Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jagtap V.R., Ganu J.V., Nagane N.S. BMD and serum intact osteocalcin in postmenopausal osteoporosis women. Indian J. Clin. Biochem. 2011;26:70–73. doi: 10.1007/s12291-010-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalaiselvi V., Prabhu K., Mani Ramesh V.V. The association of serum osteocalcin with the bone mineral density in postmenopausal women. J. Clin. Diagn. Res. 2013;7:814. doi: 10.7860/JCDR/2013/5370.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tariq S., Tariq S., Lone K.P., Khaliq S. Alkaline phosphatase is a predictor of Bone Mineral Density in postmenopausal females. Pak. J. Med. Sci. 2019;35:749. doi: 10.12669/pjms.35.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zoch M.L., Clemens T.L., Riddle R.C. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wauquier F., Leotoing L., Coxam V., Guicheux J., Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Domazetovic V., Marcucci G., Iantomasi T., Brandi M.L., Vincenzini M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone. Metab. 2017;14:209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ardawi M.-S.M., Badawoud M.H., Hassan S.M., Rouzi A.A., Ardawi J.M., AlNosani N.M., Qari M.H., Mousa S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone. 2016;83:127–140. doi: 10.1016/j.bone.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 100.Callaway D.A., Jiang J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015;33:359–370. doi: 10.1007/s00774-015-0656-4. [DOI] [PubMed] [Google Scholar]
- 101.Mendoza C.C.C., Zamarripa C.A.J. In: Menopause Induces Oxidative Stress in Oxidative Stress and Chronic Degenerative Diseases-A Role for Antioxidants. Morales-González J.A., editor. InTech Open Access Publisher; Rijeka, Croatia: 2013. pp. 289–307. [Google Scholar]
- 102.Britton G., Liaaen-Jensen S., Pfander H. Carotenoids: Handbook. Birkhäuser; Basel, Switzerland: 2012. [Google Scholar]
- 103.Khachik F. Distribution and metabolism of dietary carotenoids in humans as a criterion for development of nutritional supplements. Pure Appl. Chem. 2006;78:1551–1557. doi: 10.1351/pac200678081551. [DOI] [Google Scholar]
- 104.Rao A.V., Rao L.G. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 105.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bacanli M., BaŞAran N., BaŞAran A.A. Lycopene: Is it Beneficial to Human Health as an Antioxidant? Turk. J. Pharm. Sci. 2017;14:311–318. doi: 10.4274/tjps.43043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Viuda-Martos M., Sanchez-Zapata E., Sayas-Barberá E., Sendra E., Pérez-Álvarez J., Fernández-López J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Crit. Rev. Food Sci. Nutr. 2014;54:1032–1049. doi: 10.1080/10408398.2011.623799. [DOI] [PubMed] [Google Scholar]
- 108.Schieber A., Carle R. Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci. Technol. 2005;16:416–422. doi: 10.1016/j.tifs.2005.03.018. [DOI] [Google Scholar]
- 109.Hunter D.C., Skinner M.A., Lister C.E. Impact of phytochemicals on maintaining bone and joint health. Nutrition. 2008;24:390–392. doi: 10.1016/j.nut.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 110.Dai Z., Wang R., Ang L.W., Low Y.L., Yuan J.M., Koh W.P. Protective effects of dietary carotenoids on risk of hip fracture in men: The Singapore Chinese Health Study. J. Bone Miner. Res. 2014;29:408–417. doi: 10.1002/jbmr.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castenmiller J.J., West C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998;18:19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- 112.Unlu N.Z., Bohn T., Francis D.M., Nagaraja H.N., Clinton S.K., Schwartz S.J. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br. J. Nutr. 2007;98:140–146. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 113.Meléndez-Martínez A.J., Paulino M., Stinco C.M., Mapelli-Brahm P., Wang X.-D. Study of the time-course of cis/trans (Z/E) isomerization of lycopene, phytoene, and phytofluene from tomato. J. Agric. Food Chem. 2014;62:12399–12406. doi: 10.1021/jf5041965. [DOI] [PubMed] [Google Scholar]
- 114.Boileau A.C., Merchen N.R., Wasson K., Atkinson C.A., Erdman J.W., Jr. Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. Nutr. J. 1999;129:1176–1181. doi: 10.1093/jn/129.6.1176. [DOI] [PubMed] [Google Scholar]
- 115.Kachanovsky D.E., Filler S., Isaacson T., Hirschberg J. Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proc. Natl. Acad. Sci. USA. 2012;109:19021–19026. doi: 10.1073/pnas.1214808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Englert G., Brown B.O., Moss G.P., Weedon B.C., Britton G., Goodwin T.W., Simpson K.L., Williams R.J. Prolycopene, a tetra-cis-carotene with two hindered cis-double bonds. J. Chem. Soc. Chem. Comm. 1979;12:545–547. doi: 10.1039/c39790000545. [DOI] [Google Scholar]
- 117.Wang X.-D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012;96:1214–1222. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furr H.C., Clark R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997;8:364–377. doi: 10.1016/S0955-2863(97)00060-0. [DOI] [Google Scholar]
- 119.Boileau T.W.-M., Boileau A.C., Erdman J.W., Jr. Bioavailability of all-trans and cis-Isomers of Lycopene. Exp. Biol. Med. 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 120.Anese M., Mirolo G., Fabbro A., Lippe G. Lycopene bioaccessibility and bioavailability from processed foods. J. Sci. Ind. Res. 2013;72:543–547. [Google Scholar]
- 121.Bramley P.M. Is lycopene beneficial to human health? Phytochemistry. 2000;54:233–236. doi: 10.1016/S0031-9422(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 122.Schierle J., Bretzel W., Bühler I., Faccin N., Hess D., Steiner K., Schüep W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997;59:459–465. doi: 10.1016/S0308-8146(96)00177-X. [DOI] [Google Scholar]
- 123.Marković K., Hruškar M., Vahčić N. Lycopene content of tomato products and their contribution to the lycopene intake of Croatians. Nutr. Res. 2006;26:556–560. doi: 10.1016/j.nutres.2006.09.010. [DOI] [Google Scholar]
- 124.Stahl W., Sies H. Lycopene: A biologically important carotenoid for humans? Arch. Biochem. Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 125.Stahl W., Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. Nutr. J. 1992;122:2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- 126.Ross A.B., Vuong L.T., Ruckle J., Synal H.A., Schulze-Koenig T., Wertz K., Ruembeli R., Liberman R.G., Skipper P.L., Tannenbaum S.R. Lycopene bioavailability and metabolism in humans: An accelerator mass spectrometry study. Am. J. Clin. Nutr. 2011;93:1263–1273. doi: 10.3945/ajcn.110.008375. [DOI] [PubMed] [Google Scholar]
- 127.Kopec R.E., Riedl K.M., Harrison E.H., Curley Jr R.W., Hruszkewycz D.P., Clinton S.K., Schwartz S.J. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 2010;58:3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sicilia T., Bub A., Rechkemmer G., Kraemer K., Hoppe P.P., Kulling S.E. Novel lycopene metabolites are detectable in plasma of preruminant calves after lycopene supplementation. Nutr. J. 2005;135:2616–2621. doi: 10.1093/jn/135.11.2616. [DOI] [PubMed] [Google Scholar]
- 129.Cichon M.J., Moran N.E., Riedl K.M., Schwartz S.J., Clinton S.K. Identification of an Epoxide Metabolite of Lycopene in Human Plasma Using 13C-Labeling and QTOF-MS. Metabolites. 2018;8:24. doi: 10.3390/metabo8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gajic M., Zaripheh S., Sun F., Erdman J.W., Jr. Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. Nutr. J. 2006;136:1552–1557. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 131.Chung J., Koo K., Lian F., Hu K.Q., Ernst H., Wang X.-D. Apo-10′-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. Nutr. J. 2012;142:405–410. doi: 10.3945/jn.111.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ip B.C., Hu K.-Q., Liu C., Smith D.E., Obin M.S., Ausman L.M., Wang X.-D. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet–promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev. Res. 2013;6:1304–1316. doi: 10.1158/1940-6207.CAPR-13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Olson J.A. Absorption, transport and metabolism of carotenoids in humans. Pure Appl. Chem. 1994;66:1011–1016. doi: 10.1351/pac199466051011. [DOI] [Google Scholar]
- 134.Parker R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10:542–551. doi: 10.1096/fasebj.10.5.8621054. [DOI] [PubMed] [Google Scholar]
- 135.Papachristodoulou D., Snape A., Elliott W.H., Elliott D.C. Biochemistry and Molecular Biology. Oxford University Press; Oxford, UK: 2014. pp. 163–190. [Google Scholar]
- 136.Wang X.-D., Krinsky N.I., Marini R.P., Tang G., Yu J., Hurley R., Fox J.G., Russell R.M. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: A model for human beta-carotene metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 1992;263:480–486. doi: 10.1152/ajpgi.1992.263.4.G480. [DOI] [PubMed] [Google Scholar]
- 137.Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 138.Sahni S., Hannan M.T., Blumberg J., Cupples L.A., Kiel D.P., Tucker K.L. Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2009;89:416–424. doi: 10.3945/ajcn.2008.26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sahni S., Hannan M.T., Blumberg J., Cupples L.A., Kiel D.P., Tucker K.L. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: A 17-year follow-up from the Framingham Osteoporosis Study. J. Bone Miner. Res. 2009;24:1086–1094. doi: 10.1359/jbmr.090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mackinnon E., Venket Rao A., Rao L. Dietary restriction of lycopene for a period of one month resulted in significantly increased biomarkers of oxidative stress and bone resorption in postmenopausal women. J. Nutr. Health Aging. 2011;15:133–138. doi: 10.1007/s12603-011-0026-4. [DOI] [PubMed] [Google Scholar]
- 141.Mackinnon E., Rao A., Josse R., Rao L. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type 1 collagen in postmenopausal women. Osteoporos. Int. 2011;22:1091–1101. doi: 10.1007/s00198-010-1308-0. [DOI] [PubMed] [Google Scholar]
- 142.Yang, Zhang, Penniston, Binkley, Tanumihardjo Serum carotenoid concentrations in postmenopausal women from the United States with and without osteoporosis. Int. J. Vitam. Nutr. Res. 2008;78:105–111. doi: 10.1024/0300-9831.78.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Russo C., Ferro Y., Maurotti S., Salvati M.A., Mazza E., Pujia R., Terracciano R., Maggisano G., Mare R., Giannini S. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Transl. Med. 2020;18:43. doi: 10.1186/s12967-020-02238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mackinnon E.S. Ph.D. Thesis. University of Toronto; Ontario, Canada: 2010. The Role of the Carotenoid Lycopene as an Antioxidant to Decrease Osteoporosis Risk in Women: Clinical and In Vitro Studies. [Google Scholar]
- 145.Rao L., Mackinnon E., Josse R., Murray T., Strauss A., Rao A. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos. Int. 2007;18:109–115. doi: 10.1007/s00198-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 146.Calciolari E., Donos N., Mardas N. Osteoporotic animal models of bone healing: Advantages and pitfalls. J. Invest. Surg. 2017;30:342–350. doi: 10.1080/08941939.2016.1241840. [DOI] [PubMed] [Google Scholar]
- 147.Lelovas P.P., Xanthos T.T., Thoma S.E., Lyritis G.P., Dontas I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- 148.Ozsahin E.T., Çam B., Dere F., Kurkcu M., Evruke C., Soames R., Oguz O. The effect of alendronate sodium on trabecular bone structure in an osteoporotic rat model. Turk. J. Phys. Med. Rehabil. 2017;63:165–174. doi: 10.5606/tftrd.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gao X., Ma W., Dong H., Yong Z., Su R. Establishing a rapid animal model of osteoporosis with ovariectomy plus low calcium diet in rats. Int. J. Clin. Exp. Pathol. 2014;7:5123. [PMC free article] [PubMed] [Google Scholar]
- 150.Thompson D., Simmons H., Pirie C., Ke H. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17:125–133. doi: 10.1016/8756-3282(95)00285-L. [DOI] [PubMed] [Google Scholar]
- 151.Liang H., Yu F., Tong Z., Zeng W. Lycopene effects on serum mineral elements and bone strength in rats. Molecules. 2012;17:7093–7102. doi: 10.3390/molecules17067093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oliveira G.R., Vargas-Sanchez P.K., Fernandes R.R., Ricoldi M.S.T., Semeghini M.S., Pitol D.L., de Sousa L.G., Siessere S., Bombonato-Prado K.F. Lycopene influences osteoblast functional activity and prevents femur bone loss in female rats submitted to an experimental model of osteoporosis. J. Bone Miner. Metab. 2019;37:658–667. doi: 10.1007/s00774-018-0970-8. [DOI] [PubMed] [Google Scholar]
- 153.Li X., Xue W., Cao Y., Long Y., Xie M. Effect of lycopene on titanium implant osseointegration in ovariectomized rats. J. Orthop. Surg. Res. 2018;13:237. doi: 10.1186/s13018-018-0944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ishimi Y., Ohmura M., Wang X., Yamaguchi M., Ikegami S. Inhibition by carotenoids and retinoic acid of osteoclast-like cell formation induced by bone-resorbing agents in vitro. J. Clin. Biochem. Nutr. 1999;27:113–122. doi: 10.3164/jcbn.27.113. [DOI] [Google Scholar]
- 155.Park C.-K., Ishimi Y., Ohmura M., Yamaguchi M., Ikegami S. Vitamin A and carotenoids stimulate differentiation of mouse osteoblastic cells. J. Nutr. Sci. Vitaminol. 1997;43:281–296. doi: 10.3177/jnsv.43.281. [DOI] [PubMed] [Google Scholar]
- 156.Costa-Rodrigues J., Fernandes M.H., Pinho O., Monteiro P.R.R. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene. J. Nutr. Biochem. 2018;57:26–34. doi: 10.1016/j.jnutbio.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 157.Boyce B.F., Yao Z., Xing L. Functions of NF-κB in Bone. Ann. N. Y. Acad. Sci. 2010;1192:367. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Linnewiel-Hermoni K., Motro Y., Miller Y., Levy J., Sharoni Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic. Biol. Med. 2014;75:105–120. doi: 10.1016/j.freeradbiomed.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 159.Marcotorchino J., Romier B., Gouranton E., Riollet C., Gleize B., Malezet-Desmoulins C., Landrier J.F. Lycopene attenuates LPS-induced TNF-α secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol. Nutr. Food Res. 2012;56:725–732. doi: 10.1002/mnfr.201100623. [DOI] [PubMed] [Google Scholar]
- 160.Feng D., Ling W.-H., Duan R.-D. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-κB in macrophages. Inflamm. Res. 2010;59:115–121. doi: 10.1007/s00011-009-0077-8. [DOI] [PubMed] [Google Scholar]
- 161.De Stefano D., Maiuri M.C., Simeon V., Grassia G., Soscia A., Cinelli M.P., Carnuccio R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-γ. Eur. J. Pharmacol. 2007;566:192–199. doi: 10.1016/j.ejphar.2007.03.051. [DOI] [PubMed] [Google Scholar]