Empagliflozin in Patients with Chronic Kidney Disease (original) (raw)
. Author manuscript; available in PMC: 2023 Jan 12.
Published in final edited form as: N Engl J Med. 2022 Nov 4;388(2):117–127. doi: 10.1056/NEJMoa2204233
Abstract
Background
This study, the EMPA-KIDNEY trial, was designed to assess the effects of empagliflozin in a broad range of patients with chronic kidney disease (CKD) at risk of progression.
Methods
We randomly assigned 6609 participants to empagliflozin (10mg once daily) versus matching placebo. Eligibility required an estimated glomerular filtration rate (eGFR) of ≥20 to <45 ml/minute/1.73m2; or ≥45 to <90 ml/minute/1.73m2 with a urinary albumin-to-creatinine ratio (ACR) of ≥200 mg/g. The primary outcome was a composite of kidney disease progression (end-stage kidney disease, a sustained eGFR <10 ml/minute/1.73m2, a sustained decline in eGFR of ≥40%, or a renal death) or death from cardiovascular causes.
Results
During a median of 2.0 years follow-up, a primary outcome event occurred in 432 of 3304 patients (13.1%) in the empagliflozin group and in 558 of 3305 patients (16.9%) in the placebo group (hazard ratio, 0.72; 95% CI 0.64 to 0.82; P<0.001), with consistent results in those with or without diabetes and across the range of eGFR studied. There were fewer hospitalizations from any cause in the empagliflozin group (0.86; 0.78 to 0.95, P=0.003), but no statistically significant effect on hospitalization for heart failure or cardiovascular death (4.0% vs 4.6%), or death from any cause (4.5% vs 5.1%). The rates of serious adverse events were broadly similar in the two groups.
Conclusions
Empagliflozin reduced the risk of the composite outcome of kidney disease progression or cardiovascular death in a wide range of patients at risk of CKD progression. (Funding:Boehringer Ingelheim, Eli Lilly and others; Clinicaltrials.gov:NCT03594110, EuDRACT: 2017-002971-24).
Chronic kidney disease (CKD) is often progressive, with decreased glomerular filtration rate (GFR) and the presence of albuminuria representing key risk factors for subsequently developing kidney failure.1 Slowing CKD progression and avoiding the need for dialysis or a kidney transplant is highly desirable due to the impact of such procedures on quality of life, cardiovascular morbidity and mortality, and the substantial costs of kidney replacement therapy.2
In patients with diabetic kidney disease with increased levels of albuminuria, large placebo-controlled trials have shown that renin-angiotensin system (RAS) inhibitors,3–5 sodium-glucose cotransporter 2 (SGLT2) inhibitors,6,7 and the non-steroidal mineralocorticoid receptor antagonist finerenone8,9 all reduced the risk of progression to kidney failure. There is geographic variation, but globally the majority of people with CKD have low levels of albuminuria (i.e., a urinary albumin-to-creatinine ratio [ACR] less than 300 milligrams per gram [mg/g]) and do not have diabetes.10,11 Therefore, studying a wide range of patients with CKD has particular public health importance. A prespecified subgroup analysis from a trial of the SGLT2 inhibitor dapagliflozin in patients with CKD and a urinary ACR of at least 200 mg/g, found that kidney benefits extended to patients without diabetes, but there was limited information from patients with an estimated GFR (eGFR) below 30 ml per minute per 1.73 m2 or on how these benefits might vary among the wider range of patients with CKD.7,12,13
This multicenter international randomized parallel group double-blind placebo-controlled clinical trial of EMPAgliflozin once daily to assess cardio-renal outcomes in patients with chronic KIDNEY disease (EMPA-KIDNEY) was designed to assess the effects of SGLT2 inhibition with empagliflozin on kidney disease progression, cardiovascular disease and safety in a wide range of patients with CKD, and aimed to include large numbers of patients without diabetes, patients with an eGFR) less than 30 ml per minute per 1.73 m2 of body-surface area, and patients with low levels of proteinuria, as measured by urinary ACR.14
Methods
Trial Design and Oversight
Details of rationale of the present study and trial design have been reported previously.14,15 Our study was designed and led by a Steering Committee that included representatives from the central coordinating office at the University of Oxford, each recruiting region, the sponsor (Boehringer Ingelheim), and other clinical and statistical experts. An independent Data Safety Monitoring Board (DSMB, known as the Data Monitoring Committee) was responsible for regular review of unblinded data to ensure participant safety, and for a Protocol-defined formal interim analysis for efficacy. The Protocol and the Data Analysis Plan (DAP) are available with the full text of this article at NEJM.org and at empakidney.org. The trial was conducted at 241 centers in eight countries. Regulatory authorities and ethics committees for each center approved the trial.
Participants
Adults with a race-adjusted CKD-EPI16 eGFR of at least 20 but less than 45 ml per minute per 1.73 m2 of body-surface area (irrespective of level of albuminuria); or an eGFR of at least 45 but less than 90 ml per minute per 1.73 m2 with a urinary ACR of at least 200 mg/g at the screening visit were eligible provided they were prescribed a clinically appropriate dose of single-agent RAS-inhibitor. Patients could also be included if an investigator judged that such treatment was either not tolerated or not indicated. Patients with or without diabetes were eligible. Those with polycystic kidney disease or a kidney transplant were excluded. Full details of eligibility criteria are provided in the Protocol (see Supplementary material available at NEJM.org). All participants provided written informed consent.
Trial Procedures
All eligible participants entered a pre-randomization run-in phase and were provided with a 15-week supply of once daily placebo tablets. During this time, local investigators reviewed screening data, assessed current RAS-inhibitor use, and approved potential participants for later randomization. Throughout the trial, clinical responsibility for participants remained with their local doctors.
After completing at least 6 weeks of run-in, willing participants had central samples of blood and urine collected for central analysis and storage, and were randomly allocated to receive empagliflozin (10 mg once daily) or matching placebo using minimized randomization with a 10% stochastic element.17 At follow-up visits, participants provided information on their kidney status (i.e., any dialysis treatment or receipt of a kidney transplant), adherence to study treatment (with reasons for stopping) and details of concomitant medication. They were also asked in a structured interview about any serious adverse events (and Protocol-specified non-serious adverse events), underwent clinical measurements of blood pressure and weight, and had blood collected for local safety assessments of creatinine, liver function and potassium. Blood samples and, at selected visits, urine samples were sent to the central laboratory for efficacy analyses and archiving. Adaptations due to coronavirus-19 and assay methods are provided in the Supplementary Appendix.
Outcomes
The prespecified primary outcome was the first occurrence of the composite outcome of kidney disease progression or cardiovascular death. Kidney disease progression included end-stage kidney disease (ESKD), defined as commencing maintenance dialysis or receipt of a kidney transplant; a sustained decline in eGFR to less than 10 ml per minute per 1.73 m2; a sustained decline in eGFR of at least 40% from baseline; or renal death. The term ‘sustained’ was defined as either as measured at two consecutive scheduled study follow-up visits at least 30 days apart, or as measured at the final follow-up visit or the last scheduled visit before death (or withdrawal of consent or loss to follow-up). Central laboratory serum creatinine measurements were used to estimate GFR, with local laboratory creatinine measurements used when central results were missing. The prespecified key secondary outcomes were hospitalization for heart failure or cardiovascular death; all-cause hospitalizations (first and subsequent, combined); and death from any cause. The other secondary outcomes were the components of the primary outcome: kidney disease progression; death from cardiovascular causes; and ESKD or death from cardiovascular causes. Details of the tertiary, safety and laboratory assessments and planned exploratory assessments are in the Data Analysis Plan in the Protocol, available at NEJM.org. Key subgroup analyses of the primary outcome were prespecified to be by diabetes status, eGFR, and urinary ACR at baseline. All deaths, potential hospitalizations for heart failure, myocardial infarction, stroke, liver injury, ketoacidosis, lower limb amputation, acute kidney injury and serious genital infections were subject to adjudication by blinded clinicians using prespecified definitions and source documents collected from sites. Clinical outcome definitions are provided in the Supplementary Appendix.
Statistical Analysis
Follow-up was planned until at least 1070 participants had experienced a first primary outcome, in order to provide 90% power at two-sided P=0.05 to detect an 18% relative reduction in risk.14 The Protocol specified that a single formal interim analysis for efficacy should be conducted when 150 participants had experienced a first ESKD event. Based on the number of primary outcomes at the time (n=624), the two conditions for recommending an early stop for efficacy were prespecified as a hazard ratio for the primary outcome and the other secondary outcome of ESKD or death from cardiovascular causes of <0.778, with two-sided P values of <0.0017 and <0.05, respectively (see Protocol for details).
All analyses were performed according to the intention-to-treat principle and included data from all randomized participants including information collected between the formal interim analysis and final follow-up visits.18–20 A Cox proportional hazards regression model adjusted for baseline variables specified in the minimization algorithm (age, sex, prior diabetes, eGFR, urinary ACR, and region) was used to estimate the hazard ratio and 95% confidence intervals (CI) for empagliflozin versus placebo for time-to-event analyses.21 Key secondary outcomes were prespecified to be adjusted for multiple testing using the Hochberg “step-up” procedure with a family-wise error rate of 0.029. For the outcome of first and subsequent all-cause hospitalizations, a semi-parametric joint frailty model was used.22 Effects of empagliflozin on the tertiary and exploratory outcomes based on annual rate of change in eGFR were assessed with shared parameter models.23 Further statistical details are provided in supplementary statistical methods and the pre-specified DAP at NEJM.org. The original full database is held and analyses performed by the Nuffield Department of Population Health at the University of Oxford using SAS software, version 9.4 (SAS Institute). The Steering Committee was responsible for manuscript writing and the decision to publish.
Results
Recruitment and Follow-Up
From February 2019 to April 2021, 8544 potential participants attended a screening visit from which 8184 (96%) entered the pre-randomization run-in and 6609 were randomized (Supplementary Appendix, Fig. S1). At randomization, mean age was 63.8 years, 33% of participants were women and 54% did not have diabetes (Table 1), and broadly representative of patients with CKD at risk of progression (Table S1). Mean±standard deviation eGFR was 37.3±14.5 ml per minute per 1.73 m2 and 35% had an eGFR less than 30 ml per minute per 1.73 m2. Median urinary ACR was 329 mg/g, and 48% had a urinary ACR below 300 mg/g (Tables 1 and S2).
Table 1. Characteristics of Participants at Randomization.
| Empagliflozin(N=3304) | Placebo(N=3305) | |||
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age at randomization (years) | ||||
| Mean (SD) | 63.9 | (13.9) | 63.8 | (13.9) |
| Sex | ||||
| Female | 1097 | (33%) | 1095 | (33%) |
| Race (all regions) | ||||
| White | 1939 | (59%) | 1920 | (58%) |
| Black | 128 | (4%) | 134 | (4%) |
| Asian | 1194 | (36%) | 1199 | (36%) |
| Mixed | 14 | (<1%) | 7 | (<1%) |
| Other‡ | 29 | (1%) | 45 | (1%) |
| PRIOR DISEASE | ||||
| Prior diabetes | ||||
| Yes | 1525 | (46%) | 1515 | (46%) |
| No | 1779 | (54%) | 1790 | (54%) |
| Prior diabetes type | ||||
| Type 1 | 34 | (1%) | 34 | (1%) |
| Type 2 | 1470 | (44%) | 1466 | (44%) |
| Other/unknown | 21 | (1%) | 15 | (0.0%) |
| History of cardiovascular disease* | ||||
| Yes | 861 | (26%) | 904 | (27%) |
| No | 2443 | (74%) | 2401 | (73%) |
| CLINICAL MEASUREMENTS | ||||
| Blood pressure (mmHg) | ||||
| Mean systolic (SD) | 136.4 | (18.1) | 136.7 | (18.4) |
| Mean diastolic (SD) | 78.1 | (11.7) | 78.1 | (11.9) |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 29.7 | (6.7) | 29.8 | (6.8) |
| LABORATORY MEASUREMENTS | ||||
| Estimated GFR (ml per minute per 1.73 m2)† | ||||
| Mean (SD) | 37.4 | (14.5) | 37.3 | (14.4) |
| <30 | 1131 | (34%) | 1151 | (35%) |
| ≥30 <45 | 1467 | (44%) | 1461 | (44%) |
| ≥45 | 706 | (21%) | 693 | (21%) |
| Urinary ACR (mg/g)† | ||||
| Geometric mean (95% CI) | 219 | (205-234) | 226 | (211-242) |
| Median (Q1-Q3) | 331 | (46-1061) | 327 | (54-1074) |
| <30 | 665 | (20%) | 663 | (20%) |
| ≥30 ≤300 | 927 | (28%) | 937 | (28%) |
| >300 | 1712 | (52%) | 1705 | (52%) |
| NT-proBNP (ng/L) | ||||
| Median (IQR) | 162 | (70-421) | 159 | (68-417) |
| CONCOMITANT MEDICATION USE | ||||
| RAS-inhibitor | 2831 | (86%) | 2797 | (85%) |
| Any diuretic | 1362 | (41%) | 1453 | (44%) |
| Any lipid-lowering medication | 2190 | (66%) | 2188 | (66%) |
| CAUSE OF KIDNEY DISEASE | ||||
| Diabetic kidney disease | 1032 | (31%) | 1025 | (31%) |
| Hypertensive/renovascular disease | 706 | (21%) | 739 | (22%) |
| Glomerular disease | 853 | (26%) | 816 | (25%) |
| Other | 387 | (12%) | 421 | (13%) |
| Unknown | 326 | (10%) | 304 | (9%) |
On March 07 2022, the independent DSMB reported that based on 624 first primary outcomes, both conditions for stopping early for efficacy were met at the formal interim analysis. Follow-up was completed on July 05 2022, at which time median follow-up was 2.0 years (interquartile range, 1.5 to 2.4 years). In all, 6552 participants (99.1%) were alive and completed final follow-up or had died during follow-up. Vital status was missing for 18 (0.3%) participants, and 39 participants (0.6%) withdrew consent (Fig. S1). All eligible events were adjudicated.
At 12 months of follow-up (the approximate midpoint), 2909 [89.6%] of the empagliflozin group and 2924 [90.3%] of the placebo group reported taking most (i.e. >80%) of their study treatment. By final follow-up, study treatment was discontinued by 557 (16.9%) surviving participants allocated empagliflozin, and by 640 (19.4%) allocated placebo. This included 18 (0.5%) participants in the empagliflozin group and 31 (0.9%) in the placebo group who started treatment with an open-label SGLT2 inhibitor. Table S3 provides details of the reasons for discontinuation.
Primary and Secondary Outcomes
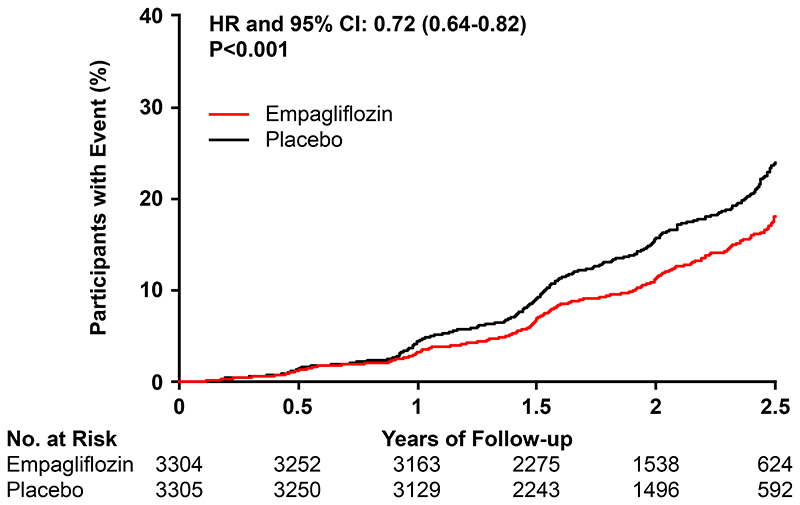
The primary outcome of kidney disease progression or death from cardiovascular causes occurred in 432 participants (13.1%) in the empagliflozin group and 558 participants (16.9%) in the placebo group (hazard ratio, 0.72; 95% CI 0.64 to 0.82; P<0.001) (Fig. 1).
Figure 1.
The primary outcome of kidney disease progression or death from cardiovascular causes occurred in 432 participants (13.1%) in the empagliflozin group and 558 participants (16.9%) in the placebo group. This represented 42 fewer primary outcomes per 1000 patients treated for 2 years.
After controlling the family-wise error rate for the three key secondary outcomes, there were significantly fewer first and subsequent hospitalizations from any cause in the empagliflozin group (24.8 versus 29.2 hospitalizations per 100 patient years: hazard ratio, 0.86; 95% CI 0.78 to 0.95, P=0.003) (Tables 2 and S4). There was no statistically significant effect on the composite of hospitalization for heart failure or death from cardiovascular causes (hazard ratio, 0.84; 95% CI 0.67 to 1.07; P=0.15), or on death from any cause (hazard ratio, 0.87; 95% CI 0.70 to 1.08; P=0.21) (Table 2 and Fig. S2).
Table 2. Primary, Secondary, Tertiary and Safety Outcomes.
| Empagliflozin(N=3304) | Placebo(N=3305) | Hazard ratio(95% CI) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Events per 100 person-years | N (%) | Events per 100 person-years | |||||
| Primary outcome: Kidney disease progression or death from cardiovascular causes | 432 | (13.1%) | 6.85 | 558 | (16.9%) | 8.96 | 0.72 (0.64-0.82) | <0.001 |
| Key secondary outcomes | ||||||||
| Hospitalization for heart failure or death from cardiovascular causes | 131 | (4.0%) | 2.04 | 152 | (4.6%) | 2.37 | 0.84 (0.67-1.07) | 0.15 |
| All-cause hospitalization¶ | 24.8 | 29.2 | 0.86 (0.78-0.95) | 0.003 | ||||
| Death from any cause | 148 | (4.5%) | 2.28 | 167 | (5.1%) | 2.58 | 0.87 (0.70-1.08) | 0.21 |
| Other secondary outcomes | ||||||||
| Kidney disease progression | 384 | (11.6%) | 6.09 | 504 | (15.2%) | 8.09 | 0.71 (0.62-0.81) | |
| Death from cardiovascular causes | 59 | (1.8%) | 0.91 | 69 | (2.1%) | 1.06 | 0.84 (0.60-1.19) | |
| End-stage kidney disease or death from cardiovascular causes | 163 | (4.9%) | 2.54 | 217 | (6.6%) | 3.40 | 0.73 (0.59-0.89) | |
| Safety outcomes | ||||||||
| Serious urinary tract infection | 52 | (1.6%) | 0.81 | 54 | (1.6%) | 0.84 | 0.94 (0.64-1.37) | |
| Serious genital infection | 1 | (<0.1%) | 0.02 | 1 | (<0.1%) | 0.02 | ||
| Serious hyperkalemia | 92 | (2.8%) | 1.44 | 109 | (3.3%) | 1.72 | 0.83 (0.63-1.09) | |
| Serious acute kidney injury | 107 | (3.2%) | 1.67 | 135 | (4.1%) | 2.11 | 0.78 (0.60-1.00) | |
| Serious dehydration | 30 | (0.9%) | 0.46 | 24 | (0.7%) | 0.37 | 1.25 (0.73-2.14) | |
| Liver injury | 13 | (0.4%) | 0.20 | 12 | (0.4%) | 0.19 | 1.09 (0.50-2.38) | |
| Ketoacidosis§ | 6 | (0.2%) | 0.09 | 1 | (0.0%) | 0.02 | ||
| Lower limb amputation | 28 | (0.8%) | 0.43 | 19 | (0.6%) | 0.29 | 1.43 (0.80-2.57) | |
| Bone fracture | 133 | (4.0%) | 2.09 | 123 | (3.7%) | 1.93 | 1.08 (0.84-1.38) | |
| Severe hypoglycemia# | 77 | (2.3%) | 1.20 | 77 | (2.3%) | 1.21 | 1.00 (0.73-1.37) | |
| Symptomatic dehydration* | 83 | (2.5%) | 1.30 | 76 | (2.3%) | 1.19 | 1.10 (0.81-1.51) |
The hazard ratios for the comparison of empagliflozin with placebo on kidney disease progression and for death from cardiovascular causes separately were 0.71 (95% CI 0.62 to 0.81) and 0.84 (95% CI 0.60 to 1.19), respectively (Fig. S3). For the composite of ESKD or death from cardiovascular causes, the hazard ratio was 0.73 (95% CI 0.59 to 0.89) (Tables 2 and S5).
Tertiary and Exploratory Outcomes
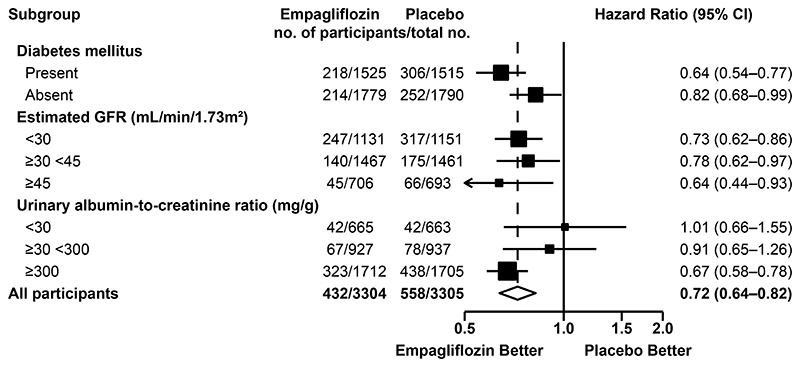
The effect of empagliflozin on the primary outcome was generally consistent across the prespecified subgroups. In particular, the benefits were consistent in patients with or without diabetes and regardless of eGFR at randomization. There was some evidence that the proportional risk reduction may be larger in those with higher urinary ACR (Figs. 2 and S4). Results were similar in prespecified exploratory subgroup analyses of the kidney disease progression outcome (Fig. S5).
Figure 2.
The primary outcome of kidney disease progression or death from cardiovascular causes occurred in 432 participants (13.1%) in the empagliflozin group and 558 participants (16.9%) in the placebo group. This represented 42 fewer primary outcomes per 1000 patients treated for 2 years.
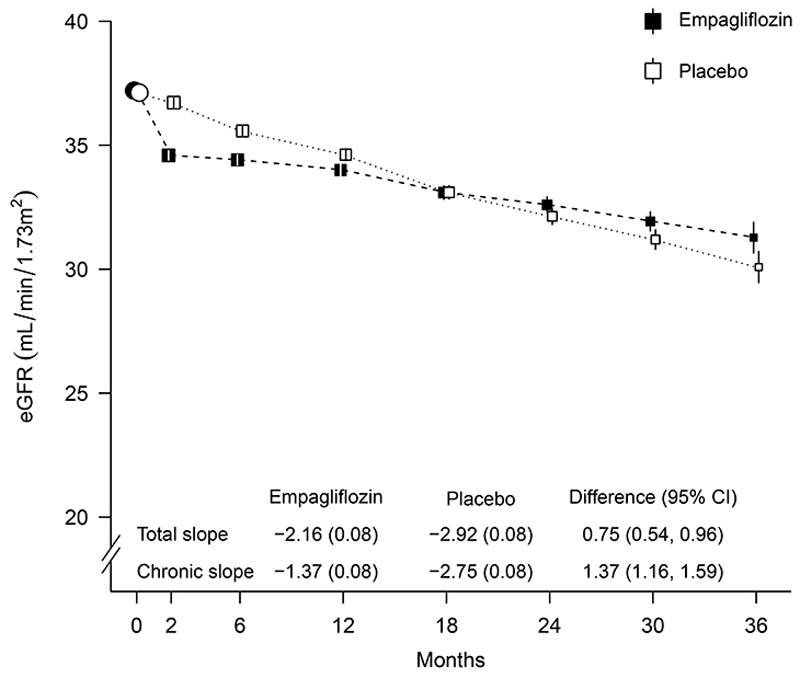
There was an acute drop in eGFR on commencing study treatment, followed by a slowing of the rate of annual decline. Overall, the between-group difference in total slope was 0.75 (95% CI 0.54 to 0.96) ml per minute per 1.73 m2 per year. For chronic slopes (i.e. from 2 months to final follow-up), there was a between-group difference of 1.37 (95% CI 1.16 to 1.59) ml per minute per 1.73 m2 per year (Figs. 3 and S6). Prespecified exploratory analyses by subgroups showed the rate of decline in the chronic slope was slower in the empgalfilozin group in all the key subgroups, including patients with low urinary ACR. Between group differences in rate of eGFR decline were larger in the subgroups of participant with faster rate of annual decline (i.e. patients with diabetes, higher eGFR, or higher baseline urinary ACR) (Fig. S7).
Figure 3. Effect of allocation to empagliflozin on estimated glomerular filtration rate.
Shown are forest plots of the hazard ratios for the primary outcome according to key prespecified baseline subgroups (with the diamond representing the overall result). Hazard ratios, confidence intervals, and P values were estimated with the use of Cox proportional-hazards regression models, adjusted for age, sex, prior diabetes, estimated glomerular filtration rate (GFR), urinary albumin-to-creatinine ratio (ACR) and region. Tests for heterogeneity or trend in the hazard ratio for subgroups were estimated through the inclusion of relevant interaction terms.
Error bars presented are 95% confidence intervals.
There were no significant effects of empagliflozin on any specific cause of death (Figure S3), major cardiovascular events (hazard ratio, 0.93 95% CI 0.76-1.12), self-reported episodes of gout, or development of new-onset diabetes (Tables S5 and S6).
Safety Outcomes and Adverse Events
Ketoacidosis occurred in 6 patients in the empagliflozin group versus 1 patient in the placebo group (0.09 versus 0.02 per 100 patient-years). Lower limb amputations occurred in 28 patients in the empagliflozin group and 19 in the placebo group (0.43 versus 0.29 per 100 patient-years). The incidence of serious urinary tract infections, hyperkalemia, acute kidney injuries, serious or symptomatic dehydrations, liver injuries, and bone fractures were broadly similar in each group (Tables 2 and S7). There was no apparent evidence that empagliflozin increased the incidence of serious adverse events overall, or in any particular MedDRA system organ class (Table S8).
Clinical Measurements and Laboratory Assessments
There were reductions in weighted-average differences [standard error, SE] in mean body weight (-0.9 [0.1] kg) and blood pressure (systolic -2.6 [0.3] mmHg; diastolic -0.5 [0.2] mmHg), but no significant effect on glycated hemoglobin (Table S9). The geometric mean urinary ACR was reduced by 19% (95% CI 15% to 23%). Table S10 provides details of the observed increases in hematocrit and hemoglobin, and the absence of clinically relevant differences in blood calcium, phosphate, or sodium measured in a subset of participants at 18 months.
Discussion
In this population of patients with a wide range of causes of CKD, GFR and levels of albuminuria, empagliflozin safely reduced the risk of the primary outcome of kidney disease progression or death from cardiovascular causes by about 28%. Treatment was effective irrespective of whether patients had diabetes, and across a broad range of eGFR down to around 20 ml per minute per 1.73 m2. Risk of hospitalization for any cause was also reduced by 14%.
The effect of SGLT2 inhibition on kidney disease progression or cardiovascular death seen in the present trial is quantitatively similar to that seen in two other large placebo-controlled trials in CKD populations.6,7 The CREDENCE trial of canagliflozin required all participants to have type 2 diabetes and a urinary ACR of at least 300 mg/g, and excluded patients with an eGFR of less than 30 ml per minute per 1.73 m2.6 The DAPA-CKD trial of dapagliflozin required participants to have a urinary ACR of 200 mg/g and an eGFR of 25 to 75 ml per minute per 1.73 m2. It included 1398 participants without diabetes and 624 participants with an eGFR below 30 ml per minute per 1.73 m2.7 EMPA-KIDNEY adds substantially to the existing evidence by demonstrating consistent benefits among 3569 (54%) participants without diabetes and, separately, among 2282 (35%) participants with an eGFR below 30 ml per minute per 1.73 m2. Despite recruiting 3192 (48%) participants with a urinary ACR below 300 mg/g, there was a limited number of primary outcomes in these types of patient as their CKD was progressing at a slower rate than participants with a urinary ACR of at least 300 mg/g. Prespecified exploratory analyses of the annual rate of change in eGFR - an accepted surrogate for kidney disease progression24 – showed empagliflozin slowed the rate of chronic eGFR decline in patients with a urinary ACR below 300 mg/g at baseline (including those with urinary ACR <30 mg/g).
Key trial strengths are its large size and broad eligibility criteria, the high level of adherence to study treatment, and the almost complete follow-up of all participants.
The trial has certain limitations, including the lower-than-expected cardiovascular event rate, which reduced statistical power to assess the secondary or tertiary cardiovascular outcomes. Nevertheless, the hazard ratios for cardiovascular outcomes are consistent with the totality of the evidence: a meta-analysis of the other CKD trials indicated that SGLT2 inhibitors lower risk of cardiovascular death by 16% (0.84; 95% CI 0.73 to 0.97) and the composite of hospitalization for heart failure or cardiovascular death by 27% (0.73; 95% CI 0.65 to 0.82).13
In summary, in a broad range of patients with CKD, including large numbers without diabetes, with an eGFR below 30 ml per minute per 1.73 m2, and with low urinary ACR, we found that empagliflozin reduced the risk of kidney disease progression or death from cardiovascular causes in a broad range of patients with CKD at risk of progression.
Supplementary Material
Supplementary materials
Acknowledgements
We thank the participants, the local site staff, regional coordinating centre staff, and all members of EMPA-KIDNEY committees.
Funding
EMPA-KIDNEY is sponsored by Boehringer Ingelheim, with grant funding provided to the University of Oxford from Boehringer Ingelheim and Eli Lilly. EMPA-KIDNEY is coordinated by the MRC Population Health Research Unit at the University of Oxford, which is part of the Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health. Funding is provided to CTSU by the UK Medical Research Council (MRC) (Ref: MC_UU_00017/3), the British Heart Foundation, NIHR Biomedical Research Council, and Health Data Research (UK). WGH was funded by an MRC–Kidney Research UK Professor David Kerr Clinician Scientist Award (MR/R007764/1).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Rights Retention Statement
For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY ND) licence to any Author Accepted manuscript version arising.
Contributor Information
Christoph Wanner, University Clinic of Würzburg, Germany
Jennifer B. Green, Duke Clinical Research Institute, Durham, North Carolina, US
Sibylle J. Hauske, Boehringer Ingelheim International; Vth Department of Medicine, University Medical Center Mannheim, University of Heidelberg, Mannheim, Germany
David Preiss, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, UK; Medical Research Council Population Health Research Unit at the University of Oxford
Michael Hill, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, UK; Medical Research Council Population Health Research Unit at the University of Oxford
Susanne Brenner, University Clinic of Würzburg, Germany
Alfred K. Cheung, University of Utah, Salt Lake City, US
Zhi-Hong Liu, National Clinical Research Center of Kidney Diseases, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China
Jing Li, Fuwai Hospital, Chinese academy of Medical Sciences, National Center for Cardiovascular Diseases, Beijing, China
Takashi Kadowaki, The University of Tokyo School of Medicine/Toranomon Hospital
Masaomi Nangaku, The University of Tokyo School of Medicine, Tokyo, Japan
Adeera Levin, University of British Columbia, Vancouver, Canada
David Cherney, University of Toronto, Canada
Aldo P. Maggioni, ANMCO Research Center, Florence, Italy
Roberto Pontremoli, Università degli Studi and IRCCS Ospedale Policlinico San Martino di Genova, Italy
Rajat Deo, University of Pennsylvania Perelman School of Medicine, Philadelphia, US
Shinya Goto, Tokai University School of Medicine, Isehara, Japan
Xavier Rossello, Hospital Universitario Son Espases, Health Research Institute of the Balearic Islands (IdISBa), Universitat Illes Balears (UIB), Palma de Mallorca, Islas Baleares, Spain
Katherine R. Tuttle, Providence Health Care and University of Washington, US
Dominik Steubl, Boehringer Ingelheim International; Department of Nephrology, Hospital Rechts der Isar, Technical University of Munich, Germany
Michaela Petrini, Boehringer Ingelheim Pharmaceuticals, Inc
Dan Massey, Elderbrook Solutions GmbH on behalf of Boehringer Ingelheim Pharma GmbH & Co.KG
Jens Eilbracht, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, UK; Medical Research Council Population Health Research Unit at the University of Oxford; Boehringer Ingelheim International
Martina Brueckmann, Boehringer Ingelheim International; First Department of Medicine, Faculty of Medicine Mannheim, University of Heidelberg, Mannheim, Germany
Data Sharing Statement
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org
References
- 1.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–73. doi: 10.1016/s0140-6736(12)61350-6. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. doi: 10.1056/NEJMoa011303. (In eng) [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62. doi: 10.1056/nejm199311113292004. (In eng) [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 7.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 8.Bakris GL, Agarwal R, Anker SD, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 10.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–80.:S0140-6736(11)60178-5. doi: 10.1016/S0140-6736(11)60178-5. [pii] (In eng) [DOI] [PubMed] [Google Scholar]
- 12.Wheeler DC, Stefansson BV, Jongs N, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31. doi: 10.1016/S2213-8587(20)30369-7. [DOI] [PubMed] [Google Scholar]
- 13.Staplin N, Roddick AJ, Emberson J, et al. Net effects of sodium-glucose co-transporter-2 inhibition in different patient groups: a meta-analysis of large placebo-controlled randomized trials. EClinicalMedicine. 2021;41:101163. doi: 10.1016/j.eclinm.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EMPA-KIDNEY Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022;37(7):1317–1329. doi: 10.1093/ndt/gfac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749–761. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. 50/9/604 [pii] (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 18.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. British Journal of Cancer. 1976;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. British Journal of Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peto R, Peto J. Asymptotically efficient invariant test procedures. J Roy Stat Soc. 1972;135(2):185–207. [Google Scholar]
- 21.Cox DR. Regression Models and Life-Tables. J R Stat Soc B. 1972;34(2):187. In English) (>://A1972N572600003) [Google Scholar]
- 22.Rogers JK, Yaroshinsky A, Pocock SJ, Stokar D, Pogoda J. Analysis of recurrent events with an associated informative dropout time: Application of the joint frailty model. Statistics in Medicine. 2016;35(13):2195–2205. doi: 10.1002/sim.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Statistics in Medicine. 2006;25(1):143–163. doi: 10.1002/sim.2249. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials
Data Availability Statement
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org