The Prevalence of Acute Kidney Injury in Patients Hospitalized With COVID-19 Infection: A Systematic Review and Meta-analysis (original) (raw)
Abstract
Rationale & Objective
Coronavirus disease 2019 (COVID-19) may be associated with high rates of acute kidney injury (AKI) and kidney replacement therapy (KRT), potentially overwhelming health care resources. Our objective was to determine the pooled prevalence of AKI and KRT among hospitalized patients with COVID-19.
Study Design
Systematic review and meta-analysis.
Data Sources
MEDLINE, Embase, the Cochrane Library, and a registry of preprinted studies, published up to October 14, 2020.
Study Selection
Eligible studies reported the prevalence of AKI in hospitalized patients with COVID-19 according to the Kidney Disease: Improving Global Outcomes (KDIGO) definition.
Data Extraction & Synthesis
We extracted data on patient characteristics, the proportion of patients developing AKI and commencing KRT, important clinical outcomes (discharge from hospital, ongoing hospitalization, and death), and risk of bias.
Outcomes & Measures
We calculated the pooled prevalence of AKI and receipt of KRT along with 95% CIs using a random-effects model. We performed subgroup analysis based on admission to an intensive care unit (ICU).
Results
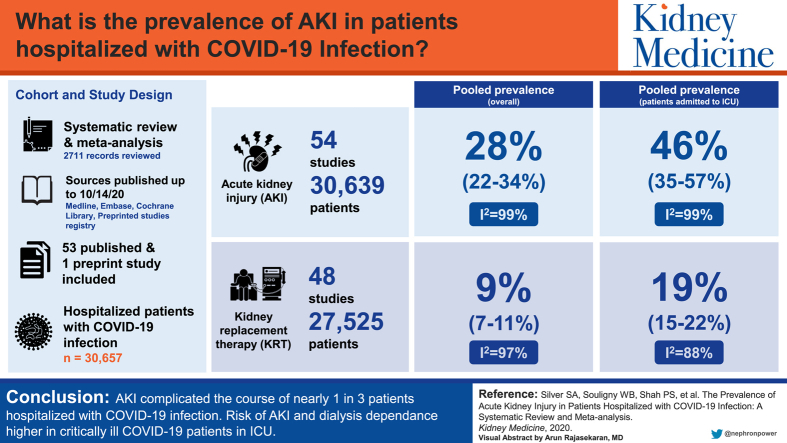
Of 2,711 records reviewed, we included 53 published and 1 preprint study in the analysis, which comprised 30,657 hospitalized patients with COVID-19. Data for AKI were available for 30,639 patients (n = 54 studies), and receipt of KRT, for 27,525 patients (n = 48 studies). The pooled prevalence of AKI was 28% (95% CI, 22%-34%; _I_2 = 99%), and the pooled prevalence of KRT was 9% (95% CI, 7%-11%; _I_2 = 97%). The pooled prevalence of AKI among patients admitted to the ICU was 46% (95% CI, 35%-57%; _I_2 = 99%), and 19% of all ICU patients with COVID-19 (95% CI, 15%-22%; _I_2 = 88%) commenced KRT.
Limitations
There was significant heterogeneity among the included studies, which remained unaccounted for in subgroup analysis.
Conclusions
AKI complicated the course of nearly 1 in 3 patients hospitalized with COVID-19. The risk for AKI was higher in critically ill patients, with a substantial number receiving KRT at rates higher than the general ICU population. Because COVID-19 will be a public health threat for the foreseeable future, these estimates should help guide KRT resource planning.
Index Words: Acute kidney injury, COVID-19, SARS-CoV2, Kidney Replacement therapy, Meta-analysis
Graphical abstract
Plain-Language Summary.
We conducted a meta-analysis and systematic review to determine how common acute kidney injury (AKI) and kidney replacement therapy are among hospitalized patients with coronavirus disease 2019 (COVID-19) infection. We analyzed 54 studies that reported AKI using KDIGO (Kidney Disease: Improving Global Outcomes) stages, comprising 30,657 hospitalized patients with COVID-19. We found that AKI complicated the course of nearly 1 in 3 (28%) patients hospitalized with COVID-19. The risk for AKI was higher in critically ill patients, with a substantial number receiving kidney replacement therapy at rates higher than the non-critically ill population. Because COVID-19 will be a public health threat for the foreseeable future, these estimates should help guide kidney replacement therapy resource planning.
Acute kidney injury (AKI) is a common and serious complication of severe illness. It is associated with higher mortality, prolonged hospital stay, and cardiovascular complications.1,2 In hospitalized patients, AKI is commonly associated with other markers of disease severity such as sepsis,3 hypoxemic respiratory failure leading to mechanical ventilation,4 and hypotension requiring vasopressor support.5 Consequently, it is not surprising that AKI is also a common complication of coronavirus disease 2019 (COVID-19) infection, which in its most severe presentation leads to multisystem critical illness. Recent reports suggest that COVID-19 may also affect the kidney by direct virus-mediated injury, cytokine storm, dysregulation of complement, and hypercoagulability.6
These mechanisms of injury may explain some of the high rates of AKI that have strained nephrology resources. For example, reports from New York City and New Orleans estimate that 20% to 60% of patients with COVID-19 experienced AKI and most patients in an intensive care unit (ICU) received emergent kidney replacement therapy (KRT).7, 8, 9, 10 However, other centers, particularly in China, report much lower rates of AKI and KRT in patients hospitalized with COVID-19.11,12 This wide variation could be due to differences in patient populations, ascertainment of AKI, geographic variation in practice patterns, and study characteristics.
As the COVID-19 pandemic progresses, accurate estimates of AKI associated with COVID-19 will be needed to ensure sufficient KRT resources and that infection control practices are in place to safely care for hospitalized patients. Accordingly, we performed a systematic review and meta-analysis to determine the pooled prevalence of AKI and KRT in patients with COVID-19.
Methods
The review was conducted using Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and reported using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.13,14
Literature Sources and Search
With the direction of a health informatics specialist (see Item S1 for search strategy), we searched Ovid MEDLINE (1946 to October 14, 2020), Embase (1946 to October 14, 2020), and the Cochrane central register of controlled trials (2019 to October 14, 2020). We did not apply language restrictions. We reviewed the bibliographies of identified articles to locate further eligible studies. In addition, we searched the nephrology section of medRxiv (a pre-print repository of medical articles available at medrxiv.org), and the COVID-19 topic section of the website of the American Society of Nephrology for additional studies.
Study Selection
We included studies that reported the prevalence of AKI among hospitalized patients with COVID-19 according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria.15
We excluded case series with fewer than 20 patients, reports on patients younger than 18 years, papers in languages other than English, letters to the editor, commentaries, reviews, and editorials. We also excluded studies with missing data for AKI prevalence after contacting the senior author for clarification. When we suspected multiple reports including the same participants based on the time, location, and authors, we used the most complete publication.
Two reviewers (ZH and SH) individually scanned titles and abstracts for initial selection. We reviewed selected articles in full and independently assessed for confirmation of eligibility. We resolved discrepancies by consensus and involvement of the other authors.
Outcomes
Our 2 outcomes of interest were the prevalence of AKI and KRT initiation among all study patients. We accepted the indication for KRT mentioned in each study because it is difficult to ascertain the reason for KRT from published reports.
Data Extraction and Study Quality Assessment
For each study, we extracted data on study characteristics (location and duration), patient characteristics (age, sex, comorbid conditions including hypertension, cardiovascular disease, chronic kidney disease, diabetes, and baseline serum creatinine value), proportion of patients developing AKI (defined according to KDIGO criteria), proportion of patients receiving KRT, proportion of patients with acute respiratory distress syndrome, proportion of patients requiring ICU admission, and important clinical outcomes (discharge from hospital, ongoing hospitalization, and death).
Because most studies were expected to be case series, we used the National Institutes of Health Quality Assessment Tool for Case Series Studies16 to assess the risk of bias in included studies. This instrument incorporates 9 domains to yield an overall assessment of study quality (good, fair, or poor). We also assessed the quality of studies that were not case series using this tool.
Statistical Analysis
We conducted meta-analyses of proportions using arcsine transformation. We expected clinical and methodological heterogeneity between studies and so calculated pooled proportions and 95% CIs using a random-effects model. We used inverse variance to weigh each study in the pooled analysis. We assessed statistical heterogeneity using _I_2 values. We used a z test to compare differences between subgroups. We assessed for publication bias visually with a funnel plot. We considered P < 0.05 as statistically significant. We performed all analyses with R, version 3.6.1 (R Core team), using the metaprop command from R package meta version 4.13.17
Subgroup Analysis
We planned subgroup analyses across age categories, sex, comorbid conditions (chronic kidney disease, cardiovascular disease, and diabetes), and the specific clinical population (ICU vs non-ICU). We also calculated the pooled prevalence of each KDIGO AKI stage to assess the severity of AKI episodes associated with COVID-19.
Results
Our search strategy yielded 2,711 unique citations (Fig S1a). Of these, we excluded 830 duplicates and 1,645 citations after screening of title and abstract, leaving 236 articles for full-text review. We subsequently excluded 182 studies that did not fulfil our inclusion criteria because they consisted of studies not reporting on AKI as an outcome (N = 66); studies with incomplete data in which the author did not reply to our queries (N = 10); review articles, meta-analyses, and letters to the editor (N = 23); studies that did not use the KDIGO definition of AKI (N = 54); modelling studies (N = 5); studies comprising maintenance dialysis recipients (N = 10); and studies containing duplicate data (N = 14). This yielded 547,8,10, 11, 12,18, 19, 20, 21, 22,23, 24, 25, 26, 27, 28,29, 30, 31, 32 studies that included 1 preprint.26
Risk of Bias Assessment
Overall, most studies were of good methodological quality (Table S1). Studies determined to be of fair quality most commonly did not report the incidence of KRT. There was no evidence of publication bias suggested by visual inspection of the funnel plot (Fig S1b).
Study and Patient Characteristics
Twenty-one reports were from the United States7,8,10,19, 20, 21, 22,24,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44; 17 were from China11,12,23,26,28,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45,46, 47, 48, 49, 50, 51, 52; 12 were from Europe18,27,53, 54, 55, 56, 57, 58, 59, 60, 61, 62; 1 each were from India,63 Bahrain,64 and South Korea25; and 1 report65 included multiple cities from the United States and Europe. The studies included 30,657 hospitalized patients, of whom 12,800 (41.8%) were women. In total, 9,650 patients (n = 40 studies) were admitted to the ICU, 15,728 patients (n = 24 studies) were admitted to a non-ICU setting, and 4,991 (n = 14 studies) patients did not have their hospital setting reported. Mean age of patients ranged between 47 and 71 years. Hypertension and diabetes mellitus were common comorbid conditions. Mean baseline serum creatinine values ranged from 0.67 to 2 mg/dL (Tables 17,8,10, 11, 12,15,18, 19, 20, 21, 22,24, 25, 26, 27, 28,29,30,45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65,66,67 and 2).
Table 1.
Characteristics of Included Studies
| Study | City and Country | Population and Setting | Patient Characteristics | Comorbid Disease | Complications | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age, ya | Female Sex | HTN | CKD | DM | Admission Scr, mg/dLa | AKIb | KRTc | ARDS | Patients Admitted to ICU | Discharged Alive/Still in Hospital | In-Hospital Deaths | |||
| Aggarwal et al63 | New Delhi, India | Patients with COVID-19 and severe acute respiratory illness admitted to Dr Ram Manohar Lohia hospital 4/10/20-4/30/20 | 32 | 54.5 | 41.4% | 34.4% | 0% | 50% | 1.1 | 13 (40.6%) | NR | NR | 37.5% | 1%, D/C; 71%, still hospitalized | 28% |
| Alberici et al18 | Brescia, Italy | Kidney transplant recipients with COVID-19 admitted to Spedali Hospital 2/27/20-3/24/20 | 20 | 59 | 20% | 85% | 100% | 15% | 2 | 6 (30%) | 1 (5%) | 55% | 20% | 15%, D/C; 60%, still hospitalized | 25% |
| Akalin et al19 | New York City, USA | Transplant recipients with COVID-19 admitted or treated as outpatients at Montefiore Medical Center 3/16/20-4/1/20 | 36 (28 admitted) | 60 | 28% | 94% | 100% | 69% | 1.4 | NR | 6 (21%) | NR | NR | 36%, D/C; 43%, still hospitalized | 21% |
| Arentz et al20 | Seattle, USA | Patients with COVID-19 admitted to ICU at Evergreen Hospital 2/20/20-3/5/20 | 21 | 70 | 48% | NR | 47.6% | 33.3% | 1.5 | 4 (19%) | 3 (14%) | 95% | 100% | 47.6%, still hospitalized | 52.4% |
| Argenziano et al21 | New York City, USA | Total patients with COVID-19 admitted to New York Presbyterian Hospital Irving Medical Center 3/11/20-4/6/20 | 1,000 | 63 | 40.4% | 60.1% | 13.7% | 37.2% | NR | 288d (33.9%) | 117 (13.8%)d | 35%d | 23.6% | 69.9%, D/C; 9%, still hospitalized | 21.1% |
| Argenziano et al21 | New York City, USA | ICU Patients with COVID-19 admitted to New York Presbyterian Hospital Irving Medical Center 3/11/20-4/6/20 | 236 | 62 | 33.3% | 66.9% | 11.4% | 42.8% | NR | 184 (78%)d | 83 (35.2%)d | 90%d | NR | NR | NR |
| Argenziano et al21 | New York City, USA | Non-ICU patients with COVID-19 admitted to New York Presbyterian Hospital Irving Medical Center 3/11/20-4/6/20 | 614 | 64 | 42.5% | 59.8% | 16% | 37.8% | NR | 104 (16.9%)d | 34 (5.5%)d | 14%d | NR | NR | NR |
| Argenziano et al21 | New York City, USA | Patients with COVID-19 admitted to ED of New York Presbyterian Hospital Irving Medical Center 3/11/20-4/6/20 | 150 | 55 | 43.3% | 50.7% | 8% | 27% | NR | NR | NR | NR | NR | NR | NR |
| Azoulay et al53 | Paris, France | Patients with COVID-19 admitted to ICU at 4 hospitals 3/11/20-4/6/20 | 379 | 62 | 22.9% | 49.6% | 16.9% | 30.1% | NR | 195 (51.5%) | 74 (19.5%) | NR | 100% | 34%, D/C; 27%, still hospitalized | 39% |
| Bhatraju et al22 | Seattle, USA | Patients with COVID-19 admitted to ICU at 9 hospitals within Seattle region 2/24/20-3/3/20 | 24 | 64 | 38% | NR | 21% | 58% | NR | 6 (25%) | 1 (4%) | 75% | 100% | 21%, D/C; 29%, still hospitalized | 50% |
| Cai et al46 | Shenzhen, China | Patients with COVID-19 admitted to Third People’s Hospital 1/11/20-2/6/20 | 298 | 47.5 | 51.3% | 15.8% | NR | 6% | 0.7 | 17 (5.7%) | 4 (1%) | NR | 10% | 89.9%, D/C; 8.1%, still hospitalized | 1% |
| Caillard et al54 | Various cities, France | Kidney transplant recipients with COVID-19 admitted to hospitals in France 3/4/20-4/21/20 | 243 | 61.6 | 33.3% | 90.1% | 41.3% | 100% | 2 | 106 (43.6%) | 27 (11.1%) | NR | 35% | 82.3%, no. D/C or still hospitalized NR | 17.7% |
| Chan et al7 | New York City, USA | Patients with COVID-19 admitted to Mount Sinai Health System (5 hospitals) 2/23/20-4/15/20 | 3235; (976 ICU) | 66.5 | 42.3% | 36.9% | 10% | 24.7% | 0.9 | Total: 1,406 (43.4%); stage 1: 492 (15.2%); stage 2: 281 (8.5%); stage 3: 633 (19.7%); ICU: 553; non-ICU: 853 | Total: 280 (8.7%); ICU:188; non-ICU: 92 | NR | 25.2% | 61.7%, D/C; 14.5%, still hospitalized | 23.8% |
| Chaudhry et al33 | Detroit, USA | Transplant recipients with COVID-19 admitted to Henry Ford Hospital 3/20/20-4/18/20 | 35 | 62 | 34.3% | 94.3% | 88.6% | 65.7% | NR | 22 (46.8%) | 7 (20%) | 35.3% | 37% | 68.6%, D/C; 8.6%, still hospitalized | 22.8% |
| Cheng et al66 | Wuhan, China | Patients with COVID-19 admitted to Tongji Hospital 1/18/20-2/28/20 | 1392 | 63 | 49% | 36% | 2% | 17% | 0.8 | Total: 99 (7%); stage 1: 42 (3%); stage 2: 22 (2%); stage 3: 35 (3%) | 15 (1%) | NR | 10 | NR | 14% |
| Cravedi et al65 | Multiple cities in USA, Spain, Italy | Kidney transplant recipients with COVID-19 admitted to 12 hospitals in USA, Italy, and Spain 3/2/20-5/15/20 | 144 | 62 | 34.7% | 95.1% | 100% | 52.1% | 1.5 | 74 (52.1%) | NR | NR | NR | 68%, distinction NR | 32% |
| Cummings et al34 | New York City, USA | Patients with COVID-19 admitted to 2 New York Presbyterian hospitals 1/18/20-2/28/20 | 257 | 62 | 33% | 63% | 14% | 36% | 1.5 | 76 (30%)f | 76 (30%)f | NR | NR | 24%, D/C; 37%, still hospitalized | 39% |
| Fava et al55 | Multiple cities, Spain | Kidney transplant recipients with COVID-19 admitted to 5 hospitals in Spain 3/4/20-4/17/20 | 104 | 59.7 | 42.3% | 86.5% | 100% | 30.8% | 1.8 | Total: 47 (45%); stage 1: 30 (29%); stage 2: 7 (6%); stage 3: 10 (10%) | NR | 54.8% | NR | 73.1%, distinction NR | 26.9% |
| Ferguson et al35 | Palo Alto, USA | Patients with COVID-19 admitted to 2 hospitals in Palo Alto (Stanford University Hospital and Valleycare) 3/13/20-4/11/20 | 72; (21 ICU) | 60.4 | 47.2% | 34.7% | 12.5% | 27.8% | 0.9 | Total: 4 (5.6%)f; ICU: 4 (5.6%)f | Total: 4 (5.6%)fICU: 4 (5.6%)f | 18% | 29% | 86.1%, D/C; 5.6%, still hospitalized | 8.3% |
| Fisher et al36 | New York City, USA | Patients with COVID-19 admitted to 3 hospitals in Montefiore Health System 3/1/20-4/26/20 | 3,345 | 64.4 | 46.9% | NR | 12.2% | 27.1% | NR | Total: 1,903 (56.9%); stage 1: 942 (49.5%); stage 2: 387 (20.3%); stage 3: 574 (30.2%) | 164 (4.9%) | NR | 13.1% | 64%, D/C; 12.8%, still hospitalized | 23.2% |
| Fominskliy et al56 | Milan, Italy | Mechanically ventilated patients with COVID-19 admitted to ICU 2/5/20-4/20/20 | 96 | NR | 16.7% | 43.8% | 6.3% | 16.6% | NR | Total: 72 (75%); stage 1: 33 (34.3%); stage 2: 15 (15.6%); stage 3: 24 (25%) | 17 (17.7%) | NR | 100% | NR | 33.3% |
| Guan et al11 | Multiple cities, China | Patients with COVID-19 hospitalized at 552 sites in 30 provinces in China 12/11/19-1/29/20 | 1,099 | 47 | 42% | 15% | 0.7% | 7.4% | NR | 6 (0.5%) | 9 (0.8%)g | 3.4% | 5% | 5%, D/C; 93.6%, still hospitalized | 1.4% |
| Gupta et al37 | Multiple cities, USA | Patients with COVID-19 admitted to ICU 3/4/20-4/11/20 | 3,099 | 62 | 35.4% | 60.3% | 28.9% | 40% | 1.5 | 637 (20.6%)f | 637 (20.6%)f | NR | 100% | NR | NR |
| Goyal et al24 | New York City, USA | Patients with COVID-19 admitted to 2 hospitals in Manhattan (Weill Cornell Medical Center and Lower Manhattan Hospital) 3/3/20-3/27/20 | 393e | 62.2 | 39.4% | 50.1% | 4.6% ESKD | 25.2% | 16% ≥ 1.5 | 18 (4.8%)f | 18 (4.8%)e | NR | NR | 66.2%, D/C; 23.6%, still hospitalized | 10.2% |
| Hirsch et al8 | New York City, USA | Patients with COVID-19 admitted to 13 hospitals in Northwell Medical System 3/1/20-4/5/20 | 5,449; (ICU: 1,395) | 64 | 39.1% | 55.7% | NR | 33% | 1.0 | Total: 1,993 (36.6%); stage 1: 927 (17%); stage 2: 447 (8.2%); stage 3: 619 (11.4%); ICU: 1,060; non-ICU: 993 | 285 (5.2%) | NR | 25.6% | 60.2%, D/C; 16.3%, still hospitalized | 23.5% |
| Hoek et al57 | Multiple cities, Netherlands | Solid organ transplant recipients with COVID-19 admitted to various hospitals in Netherlands 2/27/20-4/30/20 | 23 | 59.3 | 21.7% | 83% | NR | 43% | 2.2 | 1 (4.3%)f | 1 (4.3%)f | NR | NR | NR | 21.7% |
| Hong et al25 | Daegu, South Korea | Patients with COVID-19 admitted to Yeungnam University Medical Center through 3/29/20 | Total: 98; ICU: 13 | 55 | 61.2% | 30.6% | NR | 9.2% | NR | 9 (9.2%); ICU: 8; non-ICU: 1 | 3 (3%); ICU: 3 | 18.4% | 13.2% | 30.6%, D/C; 58.2%, still hospitalized; 6.1%, transferred | 5.1% |
| Huang et al12 | Wuhan, China | Patients with COVID-19 admitted to Jin Yintan Hospital 12/16/19-1/2/20 | Total: 41; (ICU: 13) | 49 | 27% | 15% | NR | 20% | 10% > 1.5 | 3 (7%)f; ICU: 3f | 3 (7%)f; ICU: 3f | 29% | 32% | 68%, D/C; 17%, still hospitalized | 15% |
| Imam et al38 | Detroit, USA | Patients with COVID-19 admitted to 8 hospitals in Beaumont Health system 3/1/20-4/17/20 | 1,305 | 61 | 46.2% | 56.2% | 17.5% | 30.1% | 1.2 | 76 (5.8%) | NR | NR | 26.4% | 78.3%, D/C; 6.4%, still hospitalized | 15.3% |
| Joseph et al58 | Paris, France | Patients with COVID-19 admitted to ICU at Hopital Saint-Louis 3/1/20-6/1/20 0 | 100 | 59 | 30% | 56% | 29% | 30% | 0.7 | Total: 81 (81%); stage 1:44 (44%); stage 2: 10 (10%); stage 3:27 (27%) | 13 (13%) | NR | 100% | 71%, distinction NR | 29% |
| Larsson et al59 | Stockholm, Sweden | Patients with COVID-19 admitted to ICU of Karolinsk University Hospital 3/9/20-4/20/20 | 260 | 59 | 20% | 39.6% | 1.5% | 26.2% | NR | 59 (22.7%)f | 59 (22.7%)f | NR | 100% | 31.3%, D/C; 38.4, still hospitalized | 30.3% |
| Lee et al39 | NYC, USA | Patients with COVID-19 admitted to New York Presbyterian/Weill Cornell Medical center 3/1/20-4/19/20 | 1,002 | 66 | 48% | 60% | 14% | 38% | 0.9 | Total: 294 (29%); stage 1: 182 (18%); stage 2: 29 (3%); stage 3: 83 (8%) | 59 (6%) | NR | 27% | 83%, distinction NR | 17% |
| Lendorf et al60 | Denmark | Patients with COVID-19 admitted to North Zealand Hospital 3/1/20-5/4/20 | 111; (20 ICU) | 68 | 40% | 34% | 7% | 14% | 0.9 | Total: 13 (12%); ICU: 6; non-ICU: 7 | Total: 3 (3%); ICU: 3 | NR | 18% | 81%, D/C; 5%, still hospitalized | 14% |
| Li et al26 | Wuhan and Chongqing, China | Patients with COVID-19 admitted to 4 hospitals in Hubei province and Chongqing (Tongji, Pulmonary, Central and Chongqing Southwest) 1/6/20-2/21/20 | 193 | 57 | 51% | NR | NR | NR | 0.8 | 55 (28%) | 7 (4%) | 28% | NR | 49%, D/C; 34%, still hospitalized | 17% |
| Liu et al47 | Wuhan, China | Patients admitted with COVID-19 pneumonia to Wuhan Infectious Disease Hospital 1/31/20-2/20/20 | 1,190 | 57 | 46.6% | 26.1% | 2.6% | 12.2% | 1 | 51 (4.3%) | NR | 19.2% | NR | 86.8%, distinction NR | 13.2% |
| Mohamed et al10 | New Orleans, USA | Patients with COVID-19 admitted to Ochsner Medical Center 3/1/20-3/31/20 | 575; (ICU: 173) | 65 | 45.7% | 73.7% | 29.9% | 48.9% | 1, de novo AKI; 1.6, prior CKD | Total: 161 (28%); stage 1: 30 (5%); stage 2: 25 (5%); stage 3: 106 (18%); ICU: 105; non-ICU:56 | 89 (15%); ICU: 77; non-ICU: 12 | 30% | 65% | 50%, D/C or still hospitalized (distinction NR) | 50% |
| Mukherjee et al40 | New York City, USA | Patients with COVID-19 admitted to ICU at Bellevue Hospital 3/10/20-4/7/20 | 137 | 59 | 27.2% | 51.1% | 14.6% | 37.2% | 1.1 | 46 (33.6%)f | 46 (33.6%)f | NR | 100% | 29.9%, D/C; 10.2%, still hospitalized | 59.8% |
| Naar et al41 | Boston, USA | Patients with COVID-19 admitted to ICU at Massachusetts General Hospital 3/13/20-4/22/20 | 206 | 60 | 34.9% | NR | 13.1% | 43.2% | NR | 148 (71.8%) | 46 (22.3%) | NR | 100% | NR | NR |
| Naaraayan et al42 | New York City, USA | Patients with COVID-19 admitted to a community hospital 3/12/20-5/13/20 | 370 | 71 | 44.1% | 66.2% | 111.1% | 42.4% | NR | 182 (54.9%) (of 331 eligible) | NR | NR | NR | NR | 41.1% |
| Nowak et al61 | Warsaw, Poland | Patients with COVID-19 admitted to Central Clinical hospital 3/16/20-4/7/20 | 169 | 63.7 | 48.5% | 47.3% | 20.7% | 18.9% | NR | 17 (10.1%) | 1 (0.6%) | 24.3% | 16% | 26.3%, D/C; 45.7%, still hospitalized | 27.2% |
| Okoh et al43 | Newark, USA | Patients with COVID-19 admitted to a quaternary care hospital 3/10/20-4/10/20 | 251 | 62 | 49% | 70% | 18% | 46% | NR | 52 (21%)f | 52 (21%)f | 33% | 33% | 61.3%, distinction NR | 38.6% |
| Pelayo et al67 | Philadelphia, USA | Patients with COVID-19 admitted to a tertiary inner-city hospital | 223 | NR | 48.4% | 80.7% | 17.5% | 46.6% | NR | 110 (49.3%) | 9 (4%) | NR | NR | 80.3%, D/C | 19.7% |
| Portoles et al62 | Madrid, Spain | Patients with COVID-19 admitted to Puerta de Hierro University Hospital 2/25/20-4/24/20 | 1,603 | 64.6 | 40.4% | 35.7% | 9.5% | 15.2% | 1 | 333 (20.8%) | 17 (1.1%) | NR | NR | 87.3%, D/C | 12.3% |
| Qian et al52 | Wenzhou, China | Patients with COVID-19 admitted to First Affiliated Hospital of Wenzhou Medical University 1/28/20-2/16/20 | 37 | 55 | 31.6% | 36.8% | 2.6% | 21.1% | NR | 17 (45.9%) | 3 (8.1%) | 21.6% | NR | 59.1%, D/C; 36.4%, still hospitalized | 2.7% |
| Rubin et al27 | Bordeaux, France | Patients with COVID-19 admitted to 4 ICUs 3/3/20-4/14/20 | 71 | 61 | 23% | 61% | 6% | 30% | 0.8 | Total: 57 (80%); stage I: 20 (35%); stage 2: 20 (35%); stage 3: 17 (30%) | 10 (14%) | NR | 100% | 79%, D/C or still hospitalized (distinction NR) | 21% |
| Shi et al48 | Wuhan, China | Patients admitted with COVID-19 to Renmin Hospital 1/20/20-2/10/20 | 416 | 64 | 50.7% | 30.5% | 3.4% | 14.4% | 0.7 | 8 (1.9%) | 2 (0.4%) | 23.3% | NR | 9.6%, D/C; 79.6%, still hospitalized | 13.7% |
| Suleyman et al44 | Detroit, USA | Patients with COVID-19 admitted to Henry Ford Hospital 3/9/20-3/27/20 | Total: 355; (ICU: 141) | 61.4 | 53.5% | 72.7% | 45.4% | 43.4% | 1.1 | Total: 159 (44.7%); ICU 98; non-ICU: 61 | Total: 25 (5.4%); ICU: 24; non-ICU: 1 | NR | 30.4% | 65%, D/C; 16.4%, still hospitalized | 18.6% |
| Taher et al64 | Manama, Bahrain | Patients with COVID-19 admitted to Salmaniya Medical Complex 4/1/20-5/3/20 | 73 | 54.3 | 39.7% | 42.5% | 8.2% | 45.2% | NR | Total: 29 (39.7%); stage 1: 8 (11%); stage 2: 11 (15.1%); stage 3: 10 (13.6%) | 7 (9.6%) | NR | 31.5% | 82.2%, Distinction not specified | 17.8% |
| Tang et al28 | Wuhan, China | Patients with COVID-19 and ARDS admitted to ICU at Wuhan Pulmonary Hospital 12/24/19-2/7/20 | 73 | 67 | 38.4% | 52.1% | 4.1% | 27.4% | 0.9 | 13 (17.8%) | NR | 100% | 100% | 35.6%, D/C; 35.6%, still in hospital | 28.8% |
| Wang et al49 | Wuhan, China | Patients with COVID-19 admitted to Sino-French branch of Tongji Hospital 3/9/20-3/17/20 | 116 | NR | 46.6% | 40.5% | 6.0% | 17.2% | 0.8 | Total: 12 (10.3%); stage 1: 9 (7.7%); stage 2: 3 (2.6%); stage 3: 0 (0%) | 1 (0.9%) | NR | 16.4% | NR | NR |
| Xia et al50 | Wuhan China | Patients with COVID-19 admitted to ICU of Sino-French branch of Tongji Hospital 2/5/20-3/20/20 | 81 | 66.6 | 33.3% | 53.1% | 3.7% | 23.5% | 0.9 | 41 (50.6%) | 8 (9.9%) | 95.1% | 100% | 25.9%, distinction not specified | 74.1% |
| Xu et al51 | Wuhan, China | Patients with COVID-19 admitted to ICU of 3 hospitals (Wuhan Union Hospital, Jinyitan Hospital, Wuhan Third Hospital) 1/12/20-2/3/20 | 239 | 62.5 | 40.2% | 42.9% | NR | 18.4% | 0.8 | 119 (49.8%) | 12 (5%) | 68.6% | 100% | 61.5%, distinction not specified | 38.4% |
| Yang et al29 | Wuhan, China | Patients admitted with COVID-19 in critical condition to ICU of Jin Yintan Hospital late 12/19-1/26/20 | 52 | 59.7 | 33% | NR | NR | 17% | NR | 15 (29%) | 9 (17%) | 67% | 100% | 15.3%, D/C; 23.1%, still hospitalized | 61.6% |
| Yu et al30 | Wuhan, China | Patients admitted with COVID-19 to 19 ICUs in Wuhan 2/26/20-2/27/20 (cross-sectional study) | 226 | 64 | 38.5% | 42.5% | 1.3% non-ESKD; 2.2% ESKD | 20.8% | 0.7 | Total: 57 (25.2%); stage 1: 23 (10.2%); stage 2: 12 (5.3%); stage 3: 22 (9.7%) | 24 (10.6%) | 71.2% | 100% | NR | NR |
| Zhang et al45 | Wuhan, China | Patients admitted with COVID-19 pneumonia to Zhongnan Hospital 1/2/20-2/10/20 | 221 | 55 | 51.1% | 35.3% | 2.7% | 10% | 0.8 | 10 (4.5%) | 5 (2.3%) | 21.7% | NR | 19%, D/C; 75.6%, still hospitalized | 5.4% |
| Zheng et al31 | Zhejiang, China | Patients admitted to the ICU with COVID-19 at First Affiliated Hospital 1/22/20-3/5/20 | 34 | 66 | 32.4% | 64.7% | 5.9% | 23.5% | 0.9 | 7 (20.6%) | 5 (14.7%) | 97.1% | 100% | 58.8%, D/C; 41.2%, still hospitalized | 0% |
| Zhou et al32 | Wuhan, China | Patients admitted with COVID-19 to 2 hospitals (Jinyitan Hospital and Wuhan Pulmonary Hospital) who were D/C home or died 12/29/19-1/31/20 | 191 | 56 | 38% | 30% | 1% | 19% | 4% > 1.5 | 28 (15%) | 10 (5%) | 31% | 26% | 72%, D/C | 28% |
Table 2.
Summary of AKI Events
| Characteristic | AKI | KRT |
|---|---|---|
| No. of studies | 54 | 49 |
| Pooled prevalence (95% CI) | 28% (22%-34%) | 9% (7%-11%) |
| Kidney events in patients admitted to an ICU | ||
| Pooled prevalence (95% CI) | 46% (35%-57%) | 19% (15%-22%) |
| Kidney events in patients admitted to a non-ICU setting | ||
| Pooled prevalence (95% CI) | 12% (6%-19%) | 1% (0%-3%) |
Outcomes
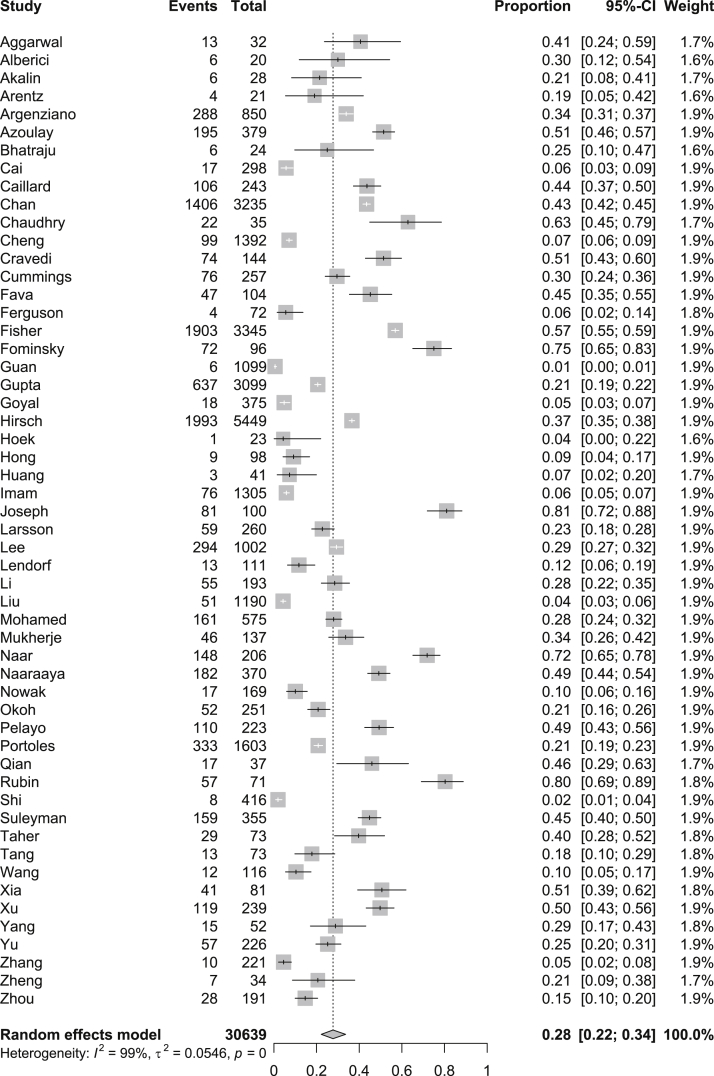
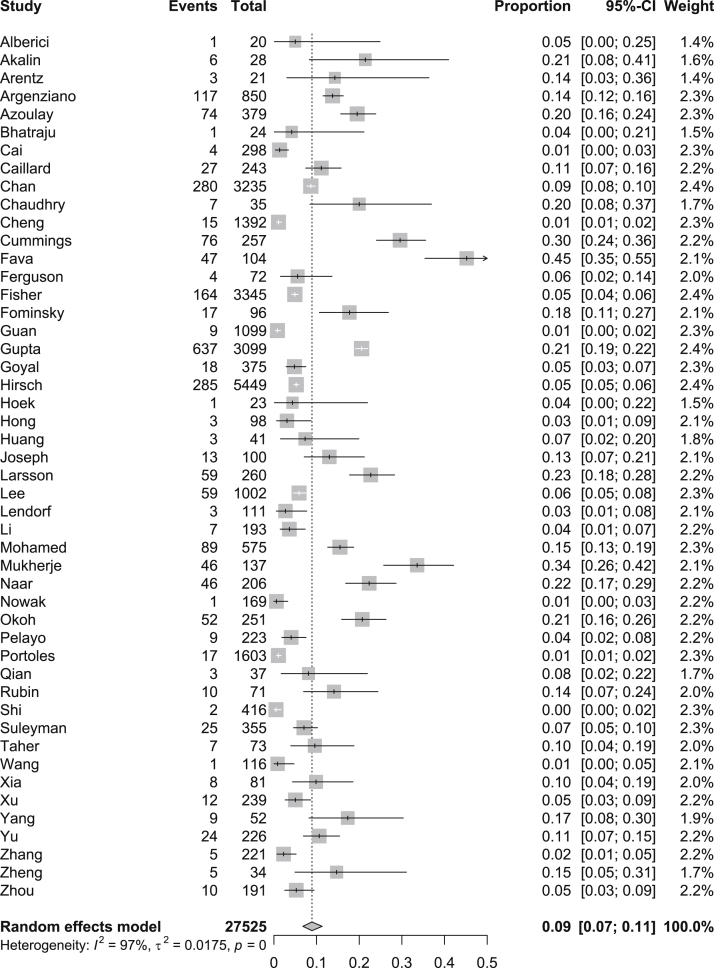
Data for AKI were available for 30,639 patients (n = 54 studies) and data for the receipt of KRT were available for 27,525 patients (n = 48 studies; Table 2). We were able to determine the indication for the initiation of KRT in only 320,24,34 of the 48 studies. For the entire hospitalized population, the pooled prevalence of AKI was 28% (95% CI, 22%-34%; _I_2 = 99%), and the pooled prevalence of receipt of KRT was 9% (95% CI, 7%-11%; _I_2 = 97; Figs 1 and 2).
Figure 1.
Pooled prevalence of acute kidney injury among all patients with coronavirus disease 2019 (COVID-19) using a random-effects model.
Figure 2.
Pooled prevalence of kidney replacement therapy among all patients with coronavirus disease 2019 (COVID-19) using a random-effects model.
Subgroup Analysis
In subgroup analysis, data were available to report outcomes only for patients admitted to an ICU or non-ICU setting, and AKI severity.
ICU Versus Non-ICU Setting
Of the 9,650 patients admitted to an ICU, ascertainment of AKI and receipt of KRT were feasible for only 8,086 and 6,618 patients, respectively (n = 25 studies for AKI; n = 23 studies for KRT). The pooled prevalence of AKI and KRT for patients receiving care in an ICU was 46% (95% CI, 35%-57%; _I_2 = 99%) and 19% (95% CI, 15%-22%; I 2 = 88%), respectively (Fig S2).
Among the 15,728 patients who did not receive care in an ICU, the presence of AKI could be ascertained in 7,799 patients (9 studies), and the receipt of KRT, in 3,745 patients (8 studies). The pooled proportion of AKI and KRT in non-ICU patients was 12% (95% CI, 6%-19%; _I_2 = 98%) and 1% (95% CI, 0%-3%; _I_2 = 88%), respectively (Fig S3). There was a significant difference in risk for AKI and KRT between patients admitted versus those not admitted to the ICU (P < 0.001 for AKI; P < 0.001 for KRT).
AKI Severity
Thirteen studies (n = 6,211 patients) reported on the severity of AKI by KDIGO stage. The pooled prevalence of stage 1 AKI was 44% (95% CI, 38%-50%; _I_2 = 94%), stage 2 AKI was 19% (95% CI, 17%-22%; _I_2 = 76%), and stage 3 AKI was 34% (95% CI, 28%-40%; _I_2 = 94%; Fig S4).
Discussion
In this systematic review and meta-analysis of 54 studies, we found that AKI occurred in ∼30% of patients hospitalized with COVID-19. AKI complicated the course of >45% of patients requiring ICU care, and 1 in 5 patients admitted to the ICU received KRT. Because COVID-19 is expected to remain a public health threat for the foreseeable future and disease surges are anticipated, our data provide important information for clinicians caring for hospitalized patients and administrators who need to marshal KRT resources.
A previous worldwide meta-analysis of 154 studies using KDIGO AKI criteria in patients without COVID-19 reported a pooled incidence rate of 21.6% (95% CI, 19.3%-24.1%), which increased to 31.7% (95% CI, 28.6%-35.0%) in a critical care setting.70 Approximately 10% of patients with AKI received KRT (2% of all patients). In other prospective studies of critically ill patients, between 15% and 30% of patients with AKI received KRT (5%-15% of all patients).69, 70, 71 Therefore, our reported overall AKI prevalence of 28% in hospitalized patients with COVID-19 is consistent with this prior work, but the rate of AKI in critically ill patients is higher. We also identified more use of KRT in COVID-19–associated AKI. These findings are driven primarily by data from the United States and Europe because most of the studies from China reported less overall AKI and use of KRT, which has been described previously in patients without COVID-19.68,69,72 These differences could be explained in part by underrecognition of AKI stemming from differences in the frequency of kidney function measurement, as well as KRT resource limitations and practice pattern variation in the initiation of KRT among different centers.73
These data suggest that COVID-19–associated AKI contributes to a more severe AKI phenotype, and higher rates of KRT should spur further investigation into the mechanisms underlying this complication. Commonly cited hypotheses include a hypercoagulable state and direct viral invasion related to angiotensin-converting enzyme 2 expression on the proximal tubule,6,74,75 supported by small autopsy studies that demonstrated severe proximal tubule injury, peritubular erythrocyte aggregation, glomerular fibrin thrombi, and even collapsing glomerulopathy in a subset of patients.76,77 Notably, pigment casts were present in these reports, suggesting some degree of inflammation and acute tubular necrosis, which is a common cause of AKI in patients with multiorgan failure that is compounded by intravascular volume depletion and mechanical ventilation.78
Our data also give health care providers and administrators estimates of KRT capacity needed in future COVID-19 surges. This planning involves considering human resources (ie, nurses), equipment availability (ie, KRT machines and reverse osmosis devices), disposables (ie, filters, dialysate, and anticoagulation), and protocol development for other acute dialysis modalities (ie, sustained low efficiency dialysis and/or acute peritoneal dialysis) in case continuous kidney replacement therapy resources become overwhelmed.9,79,80 In patients with AKI who survive their COVID-19, there will be increased risks for rehospitalization, recurrent AKI, cardiovascular complications, and death that mostly occur within the first year after hospitalization.81 Current after-care programs for survivors of COVID-19 focus mainly on respiratory and mental health,82 and the high rates of AKI reported here suggest that kidney monitoring should also be incorporated.
The strengths of our systematic review include the use of a comprehensive search strategy that incorporated pre-prints and careful identification of duplicate studies. Some of the studies still temporally overlapped in setting and location, and we included only the most complete reports to avoid double counting. We also ascertained all AKI episodes according to KDIGO criteria.
This study has some limitations. First, there was significant heterogeneity among the included studies that remained unaccounted for in subgroup analysis. Factors that may have contributed to heterogeneity included baseline kidney function ascertainment, hospital setting, and hospital policies. For example, AKI could be underreported in hospitals with less frequent kidney function measurement, and criteria for hospital/ICU admission and KRT initiation are determined locally.
In addition, most studies only reported the presence of AKI based on KDIGO criteria (ie, yes/no) and few provided data on the severity of AKI by KDIGO stage. However, we estimated the point prevalence of each KDIGO AKI stage among studies reporting these data, as well as the use of KRT, which is a marker of severe AKI. Second, detailed information on the subset of patients who received KRT, including modality and prescription used, was limited; therefore, the optimal method of KRT delivery remains an important knowledge gap. Third, data for kidney recovery and mortality were incomplete, with many patients still hospitalized and the follow-up time too short to properly assess these outcomes. Fourth, we may have slightly overestimated KRT rates by virtue of some patients receiving KRT for end-stage kidney disease. Last, although our study included more than 30,000 patients from 54 studies, our estimates could be affected by the exclusion of 130 studies that did not report on AKI or in which KDIGO criteria were not used.
Our systematic review and meta-analysis provides health care providers and administrators with updated and comprehensive information on the epidemiology of AKI and KRT in hospitalized patients with COVID-19. Approximately 50% of critically ill patients may experience AKI, with almost 20% of all critically ill patients receiving KRT. These data identify targets to guide adequate capacity planning in the face of future COVID-19 surges. Given the prominence of AKI and KRT in hospitalized patients with COVID-19, further work is also needed to better characterize COVID-19–associated AKI and kidney-specific treatments. Future reports should provide detailed data on AKI severity, triggers for KRT, pathology (when performed), and information on the KRT prescription (ie, modality, anticoagulation, and ultrafiltration), as well as data for mortality and kidney recovery.
Article Information
Authors’ Full Names and Academic Degrees
Samuel A. Silver, MD, MSc, William Beaubien-Souligny, MD, Prakesh S. Shah, MD, MSc, Shai Harel, MD, MS, Daniel Blum, MD, Teruko Kishibe, MISt, Alejandro Meraz-Munoz, MD, Ron Wald, MDCM, MPH, and Ziv Harel, MD, MSc.
Authors’ Contributions
Study concept and design: RW, ZH; Acquisition, analysis, or interpretation of data: all authors; study supervision: RW, ZH. SAS and WB-S contributed equally. RW and ZH contributed equally. All authors approved the final version of the submitted manuscript. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Dr Silver is supported by a Kidney Research Scientist Core Education and National Training (KRESCENT) Program New Investigator Award (co-funded by the Kidney Foundation of Canada, Canadian Society of Nephrology, and Canadian Institutes of Health Research). Dr Beaubien-Souligny is supported by the Fonds de Recherche du Québec - Santé (FRQS).
Financial Disclosure
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Dr Silver has received speaking fees from Baxter Canada. Dr Wald has received unrestricted research funding and speaker fees from Baxter. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received July 31, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form November 15, 2020.
Footnotes
Complete author and article information provided before references.
Acute kidney injury; COVID-19; SARS-CoV2; Kidney Replacement therapy; Meta-analysis.
Figure S1: Study flow diagram and funnel plot of included studies.
Figure S2: Pooled prevalence of acute kidney injury and kidney replacement therapy among all patients with COVID-19 admitted to an ICU.
Figure S3: Pooled prevalence of acute kidney injury and kidney replacement therapy among all patients with COVID-19 admitted to a non-ICU setting.
Figure S4: Pooled prevalence of AKI among hospitalized patients with COVID-19 by KDIGO stage.
Item S1: Search strategy.
Table S1: Methodological quality of included studies using the National Institutes of Health (NIH) Quality Assessment Tool.
Supplementary Material
Supplementary File (PDF)
Figures S1-S4; Item S1; Table S1
References
- 1.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Legrand M., Rossignol P. Cardiovascular consequences of acute kidney injury. N Engl J Med. 2020;382(23):2238–2247. doi: 10.1056/NEJMra1916393. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw S.M., Uchino S., Bellomo R. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 4.van den Akker J.P.C., Egal M., Groeneveld A.B.J. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care (London, England) 2013;17(3):R98. doi: 10.1186/cc12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito S., Uchino S., Takinami M., Uezono S., Bellomo R. Postoperative blood pressure deficit and acute kidney injury progression in vasopressor-dependent cardiovascular surgery patients. Crit Care. 2016;20(1):74. doi: 10.1186/s13054-016-1253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan L., Chaudhary K., Saha A. Acute kidney injury in hospitalized patients with COVID-19. Pre-print. medRxiv. 2020 doi: 10.1101/2020.05.04.20090944. [DOI] [Google Scholar]
- 8.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldfarb D.S., Benstein J.A., Zhdanova O. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15(6):880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed M.M., Lukitsch I., Torres-Ortiz A.E. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360. 2020;1(7):614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;(2):1–138. [Google Scholar]
- 16.National Institutes of Health (NIH). The National Institutes of Health (NIH) Quality Assessment Tool for Case Series Studies. National Institutes of Health. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed December 17, 2020.
- 17.Balduzzi S., R G., Schwarzer G. Package ‘meta’: how to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argenziano M.G., Bruce S.L., Slater C.L. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong K.S., Lee K.H., Chung J.H. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Wu M, Yao J, et al. Caution on kidney dysfunctions of COVID-19 patients. Pre-print. medRxiv. 2020. 10.1101/2020.02.08.20021212. [DOI]
- 27.Rubin S., Orieux A., Prevel R. Characterisation of acute kidney injury in critically ill patients with severe coronavirus disease-2019 (COVID-19). Pre-print. medRxiv. 2020 doi: 10.1101/2020.05.06.20069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang X., Du R., Wang R. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y., Xu D., Fu S. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y., Sun L.J., Xu M. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. 2020;21(5):378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry Z.S., Williams J.D., Vahia A. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a case-control study. Am J Transplant. 2020;20(11):3051–3060. doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson J., Rosser J.I., Quintero O. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26(8):1679–1685. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher M., Neugarten J., Bellin E. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S., Coca S.G., Chan L. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2020;32(1):161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imam Z., Odish F., Gill I. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.R., Silberzweig J., Akchurin O. Characteristics of acute kidney injury in hospitalized COVID-19 patients in an urban academic medical center. Clin J Am Soc Nephrol. 2020 doi: 10.2215/CJN.07440520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee V., Toth A.T., Fenianos M. Clinical outcomes in critically ill coronavirus disease 2019 patients: a unique New York City public hospital experience. Crit Care Explorations. 2020;2(8):e0188. doi: 10.1097/CCE.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naar L., Langeveld K., El Moheb M. Acute kidney injury in critically-ill patients with COVID-19: a single-center experience of 206 consecutive patients. Ann Surg. 2020;272(4):e280–e281. doi: 10.1097/SLA.0000000000004319. [DOI] [PubMed] [Google Scholar]
- 42.Naaraayan A., Nimkar A., Hasan A. Analysis of male sex as a risk factor in older adults with coronavirus disease 2019: a retrospective cohort study from the New York City metropolitan region. Cureus. 2020;12(8):e9912. doi: 10.7759/cureus.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okoh A.K., Sossou C., Dangayach N.S. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19(1):93. doi: 10.1186/s12939-020-01208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suleyman G., Fadel R.A., Malette K.M. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Network Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang G., Hu C., Luo L. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai Q., Huang D., Ou P. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 47.Liu J., Zhang L., Chen Y. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. doi: 10.1016/j.rmed.2020.106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Wang Z., Zhu Y. Identify the risk factors of COVID-19-related acute kidney injury: a single-center, retrospective cohort study. Front Med. 2020;7:436. doi: 10.3389/fmed.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia P., Wen Y., Duan Y. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. 2020;31(9):2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J., Yang X., Yang L. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care (London, England) 2020;24(1):394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian S.-Z., Hong W.-D., Lingjie M., Chenfeng L., Zhendong F., Pan J.-Y. Clinical characteristics and outcomes of severe and critical patients with 2019 novel coronavirus disease (COVID-19) in Wenzhou: a retrospective study. Front Med. 2020;7:552002. doi: 10.3389/fmed.2020.552002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azoulay E., Fartoukh M., Darmon M. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020;46(9):1714–1722. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caillard S., Anglicheau D., Matignon M. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fava A., Cucchiari D., Montero N. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: a multicentric cohort study. Am J Transplant. 2020;20(11):3030–3041. doi: 10.1111/ajt.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fominskiy E.V., Scandroglio A.M., Monti G. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. 2021;50:102–109. doi: 10.1159/000508657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoek R.A.S., Manintveld O.C., Betjes M.G.H. COVID-19 in solid organ transplant recipients: a single-center experience. Transplant Int. 2020;33(9):1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph A., Zafrani L., Mabrouki A., Azoulay E., Darmon M. Acute kidney injury in patients with SARS-CoV-2 infection. Ann Intensive Care. 2020;10(1):117. doi: 10.1186/s13613-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsson E., Brattstrom O., Agvald-Ohman C. Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol Scand. 2021;65(1):76–81. doi: 10.1111/aas.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lendorf M.E., Boisen M.K., Kristensen P.L. Characteristics and early outcomes of patients hospitalised for Covid-19 in North Zealand, Denmark. Danish Med J. 2020;67(9):1–11. [PubMed] [Google Scholar]
- 61.Nowak B., Szymanski P., Pankowski I. Clinical characteristics and short-term outcomes of patients with coronavirus disease 2019: a retrospective single-center experience of a designated hospital in Poland. Polish Arch Intern Med. 2020;130(5):407–411. doi: 10.20452/pamw.15361. [DOI] [PubMed] [Google Scholar]
- 62.Portoles J., Marques M., Lopez-Sanchez P. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020;35(8):1353–1361. doi: 10.1093/ndt/gfaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aggarwal A., Shrivastava A., Kumar A., Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Assoc Physicians India. 2020;68(7):19–26. [PubMed] [Google Scholar]
- 64.Taher A., Alalwan A.A., Naser N., Alsegai O., Alaradi A. Acute kidney injury in COVID-19 pneumonia: a single-center experience in Bahrain. Cureus. 2020;12(8):e9693. doi: 10.7759/cureus.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cravedi P., Suraj S.M., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20(11):3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng Y., Luo R., Wang X. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(10):1394–1402. doi: 10.2215/CJN.04650420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelayo J., Lo K.B., Bhargav R. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10(4):223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bouchard J., Acharya A., Cerda J. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10(8):1324–1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 71.Prescott G.J., Metcalfe W., Baharani J. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant. 2007;22(9):2513–2519. doi: 10.1093/ndt/gfm264. [DOI] [PubMed] [Google Scholar]
- 72.Yang L., Xing G., Wang L. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386(10002):1465–1471. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 73.Mehta R.L., Burdmann E.A., Cerda J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387(10032):2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 74.Ye M., Wysocki J., William J., Soler M.J., Cokic I., Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17(11):3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 75.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31(8):1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diao B., Wang C., Wang R. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infection. Pre-print. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darmon M., Clec’h C., Adrie C. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9(8):1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the inpatient dialysis unit. Clin J Am Soc Nephrol. 2020;15(5):720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El Shamy O., Patel N., Abdelbaset M.H. Acute start peritoneal dialysis during the COVID-19 pandemic: outcomes and experiences. J Am Soc Nephrol. 2020;31(8):1680–1682. doi: 10.1681/ASN.2020050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silver S.A., Siew E.D. Follow-up care in acute kidney injury: lost in transition. Adv Chronic Kidney Dis. 2017;24(4):246–252. doi: 10.1053/j.ackd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 82.COVID after care. https://www.england.nhs.uk/coronavirus/publication/after-care-needs-of-inpatients-recovering-from-covid-19/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File (PDF)
Figures S1-S4; Item S1; Table S1