Results of Surgical Treatment of Patients with Malignant Eccrine Poroma (original) (raw)
Abstract
Objectives:
Malignant eccrine poroma is a rare cutaneous malignancy. This study was a review of a series of patients with malignant eccrine poroma who underwent surgical treatment conducted in order to evaluate the management techniques and outcomes of treatment modalities.
Methods:
All cases of surgically excised malignant eccrine poroma performed in a single clinic between 2012 and 2018 were included in the study. The details of patient age, gender, anatomical location of the tumor, histopathological features, and treatment modalities were analyzed.
Results:
The average tumor size was 2.53 cm (range: 0.3-7 cm). The average tumor thickness was 3.06 mm (range: 2.5-4 mm). The mean clean tumor margin after the first excision was 1.28 mm and the mean tumor margin after the second excision was 8.83 mm. No recurrence or distant metastasis was detected in any of the patients during the follow-up period.
Conclusion:
Unlike frequent skin cancers, rare skin cancers, like malignant eccrine poroma, don’t have definite treatment algorithms constituted from randomized trials. The findings of patient series are very useful to guide physicians in these cases.
Keywords: Eccrine porocarcinoma, malignant eccrine poroma, skin cancer
Malignant eccrine poroma was first described by Pinkus and Mehregan in 1963 as a skin lesion combining the features of eccrine poroma and Paget’s dermatosis.[1] It is a rare cutaneous malignancy which originates from the intraepidermal sections of the eccrine glands. It is most common in the lower extremities. It has also been reported in other locations, such as the scalp, face, and upper extremities.[2–4] It can progress from the benign lesion of eccrine poroma. The term eccrine porocarcinoma has been used synonomously with malignant eccrine poroma.
Wide local excision and Mohs micrographic surgery are most frequent treatment modalities used in treatment. Excision of the regional lymph node is recommended in cases with radiologically or clinically proven lymph node metastasis. Metastatic malignant eccrine poroma has been reported in rare cases.[5] In cases of metastatic disease, the prognosis is poor. Although chemotherapy and radiotherapy is usually recommended in metastatic cases, there are currently no definitive treatment algorithms.[6]
The aim of this study was to review a series of patients with malignant eccrine poroma to evaluate the surgical management techniques, histopathological features, and outcomes of treatment modalities applied.
Methods
The data of patients with malignant eccrine poroma who underwent surgery in a single clinic between 2012 and 2018 were reviewed retrospectively and included in the study. The ethical guidelines of the 1975 Declaration of Helsinki were observed in the conduct of this study, and informed consent was obtained from all of the patients. The patients were classified according to age, gender, anatomical localization of the tumor, tumor histopathology, treatment, and survival and recurrence rate. Only pathologically proven cases of malignant eccrine porocarcinoma were included. All of the patients were hospitalized and treated with appropriate respect. The patients’ files were evaluated for tumor management and the methods used, including excision to achieve a tumor-free surgical margin and reconstruction of soft tissue defects, as well as monitoring for tumor recurrence, metastatic spread, and survival. All of the lesions were surgically excised, and in cases of patients who were appropriate candidates for sentinel lymph node dissection or elective lymph node dissection, the dissection was performed simultaneously. All excised material was sent for histopathological verification of the diagnosis. The follow-up data were prepared based on control visits and the medical records of the plastic surgery, dermatology, oncology, and the pathology clinics.
A descriptive analysis was performed using GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA, USA.) (Table 1).
Table 1.
Cohort demographics for malignant eccrine poroma
| Mean age, years | 62.1±23.4 |
|---|---|
| Female | 58 |
| Male | 63.8 |
| Age range, years | 30-86 |
| Female | 30-86 |
| Male | 38-86 |
| Male: Female ratio | 1:0.4 |
Results
Seven patients operated on for malignant eccrine poroma were included in the study. All of the patients presented at the clinic with a suspicious skin lesion (Fig. 1). Total excision of the lesion was planned following a dermatology consultation. The lesions were excised and sent for histopathological examination. The mean age of the patients was 62 years (range: 30-86 years). The mean follow-up period was 36 months. The patients were evaluated according to age, gender, anatomical localization of the tumor, treatment modality, survival and recurrence rate (Tables 1, 2). The lesions were located on the scalp in 14% (n=1) of the patients, the face in 42% (n=3), the inguinal region in 14% (n=1), and the back in 28% (n=2). The average tumor size was 2.53 cm (range: 0.3-7 cm). The average tumor thickness was 3.06 mm (range: 2.5-4 mm). In all, 42% (n=3) of the patients required a single-stage excision, while 57% (n=4) required 2 or more excisions for a clear tumor margin (Figs. 2, 3). The mean tumor margin after a single excision was 1.28 mm and the mean tumor margin after the second excision was 8.83 mm. Dermal invasion was present with no involvement of the subcutaneous fat in 57% (n=4) of the patients. There was no perineural or angiolymphatic invasion in any of the members of the study group. Ductal differentiation and intracytoplasmic lumina were highlighted with carcinoembryonic antigen immunohistochemistry and the luminal border of the duct was diastase resistant and periodic acid-Schiff-positive results were noted. Ki-67 staining revealed marked proliferative activity. Positronemission tomography scan and ultrasound imaging of the lymph nodes were performed and there were no signs of metastatic lesions in any of the patients. Sentinel lymph node biopsy (SLNB) was performed for 2 patients following re-excision with a primary tumor depth of more than 3 mm and a high mitotic count. A clear margin was confirmed after the re-excision and no tumor infiltration was found in the sentinel lymph nodes. In 1 patient with a large primary tumor with a diameter of 4.5 cm and a family history, complete excision of the regional lymph nodes was performed. No recurrence or distant metastasis was detected in any of the patients during the follow-up period.
Figure 1.
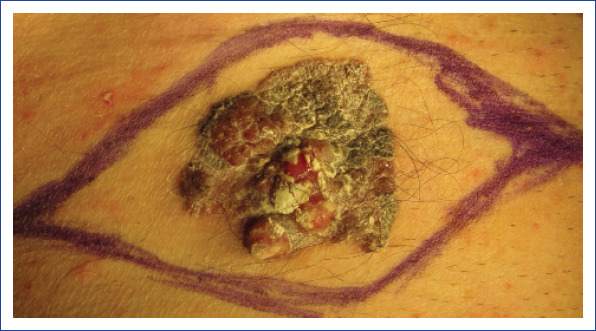
Preoperative photograph of a large primary lesion of malignant eccrine poroma in the inguinal lesion.
Table 2.
Descriptive analysis of the results
| Localization | |
|---|---|
| Scalp | 14% (n=1) |
| Face | 42% (n=3) |
| Inguinal | 14% (n=1) |
| Back | 28% (n=2) |
| Histopathological features | |
| Mitosis | 85% (n=6) |
| Atypia | 85% (n=6) |
| Necrosis | 14% (n=1) |
| Dermal invasion | 57% (n=4) |
| Angiolymphatic infiltration | 0% (n=0) |
| Perineural infiltration | 0% (n=0) |
| Tumor size | |
| Mean | 2.53 cm |
| Range | 0.3–7 cm |
| Tumor thickness | |
| Mean | 3.06 mm |
| Range | 2.5–4 mm |
| Number of excision performed for adequate surgical margin | |
| Single stage excision | 42% (n=3) |
| Double stage excision | 57% (n=4) |
| Tumor margin | |
| Mean margin after first excision | 1.28 mm |
| Mean margin after second excision | 8.83 mm |
| Lymph node management | |
| Sentinel lymph node biopsy | 28% (n=2) |
| Complete excision of regional lymph nodes | 14% (n=1) |
Figure 2.
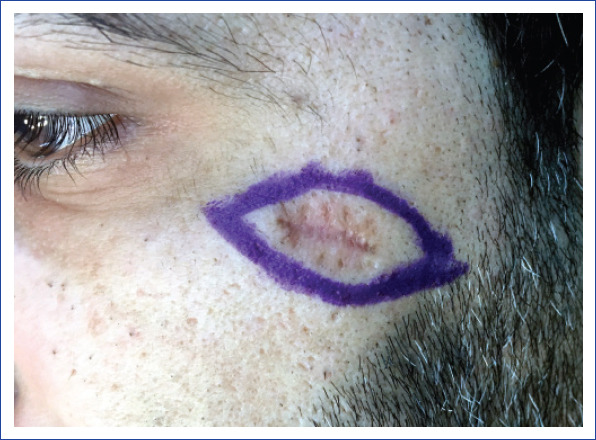
Preoperative surgical planning of re-excision in a patient with malignant eccrine poroma in the facial region.
Figure 3.
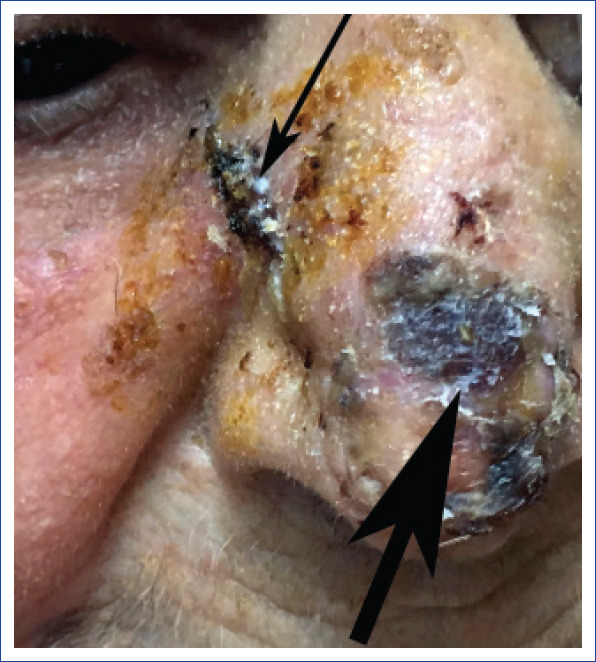
Preoperative view of a patient with malignant eccrine poroma prior to a re-excision in the nasal region. (Large arrow indicates area of malignant eccrine poroma and small arrow indicates an area of adjacent squamous cell carcinoma).
Discussion
The management of malignant eccrine poroma is a challenge for physicians. Since it’s a rare tumor, there are no definite treatment algorithms constituted from randomized trials.[7] Lymph node dissection is usually recommended in cases of nodal involvement. Even in cases of clear margins after wide excision, lymph node metastasis has been reported in up to 20% of cases.[8, 9] However, in our series we did not encounter any lymph node metastasis following re-excision with 8.8 mm of clean tumor margin. In cases with radiological evidence of lymph node metastasis, complete lymph node excision is the preferred method of treatment. SLNB has been suggested by some authors for cases in which no radiological or clinical evidence for nodal involvement is found. Robson et al.[10] suggested that SLNB could be performed for tumors with a depth of more than 7 mm. However, the exact role of SLNB remains unclear. It has been argued that even an aggressive case might have a negative SLNB result.[11] We performed SLNB in patients with a tumor depth of more than 3 mm, which was above the average tumor depth, and with a high mitotic count. Considering the unreliable results of SLNB, the absence of a standard treatment algorithm, high recurrence rates, and the poor prognosis in cases of distant metastasis, we argue that primary tumors with a large diameter of more than 4 cm and tumor depth of more than 7 mm could be considered for prophylactic lymph node dissection in selected cases with high risk, for example, in patients with a family history of skin cancer.
The clinical appearance of the lesion may be nodular, infiltrative, ulcerated, or polypoid. Previous studies have demonstrated an association between ulceration, multinodularity, and local recurrence, and metastasis has been seen.[12] Seborrheic keratosis, dermal nevus, basal cell carcinoma, squamous cell carcinoma, amelanotic melanoma, and eccrine poroma should be included in the differential diagnosis.[13] Mohs micrographic surgery (MMS) has been proposed to lower the local recurrence in malignant eccrine poroma. Xu et al. has shown that MMS was superior to wide local excision in terms of local recurrence and distant metastasis rates.[14–16] In our center, none of the cases were treated with MMS, but considering the amount of reexcision required to achieve adequate tumor margin, the use of MMS could be justified.
Treatment of malignant eccrine poroma is challenging for both surgeons and oncologists. Standard guidelines are not available because of its rarity. Case series are important in terms of guiding physicians in the absence of these guidelines. Cooperative studies involving several treatment centers are critical in these kinds of rare tumors. A combination of this information based on a large number of cases could provide the foundation that will lead to better decision-making.
Disclosures
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept – M.S.; Design – M.S.; Supervision – M.S., S.K.Y.; Materials – M.S.; Data collection &/or processing – M.S.; Analysis and/or interpretation – M.S., S.K.Y.; Literature search – S.K.Y.; Writing – S.K.Y.; Critical review – S.K.Y.
References
- 1.Pinkus H, Mehregan AH. Epidermotropic Eccrine Carcinoma. A Case Combining Features Of Eccrine Poroma And Paget's Dermatosis. Arch Dermatol. 1963;88:597–606. doi: 10.1001/archderm.1963.01590230105015. [DOI] [PubMed] [Google Scholar]
- 2.Raheemullah A, Allamaneni S, Weber S, Singh R. Eccrine Porocarcinoma Presenting as a Hand Cyst. J Hand Surg Am. 2016;41:e425–e427. doi: 10.1016/j.jhsa.2016.07.112. [DOI] [PubMed] [Google Scholar]
- 3.Masamatti SS, Narasimha A, Bhat A, Chowdappa V. Eccrine Porocarcinoma of the Scalp:A Rare Case Report with Review of Literature. J Clin Diagn Res. 2016;10:ED15–6. doi: 10.7860/JCDR/2016/16083.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asghar AH, Mahmood H, Faheem M, Rizvi S, Khan KA, Irfan J. Porocarcinoma: a rare sweat gland malignancy. J Coll Physicians Surg Pak. 2009;19:389–90. [PubMed] [Google Scholar]
- 5.Barzi AS, Ruggeri S, Recchia F, Bertoldi I. Malignant metastatic eccrine poroma. Proposal for a new therapeutic protocol. Dermatol Surg. 1997;23:267–72. [PubMed] [Google Scholar]
- 6.Mandaliya H, Nordman I. Metastatic Eccrine Porocarcinoma:A Rare Case of Successful Treatment. Case Rep Oncol. 2016;9:454–6. doi: 10.1159/000448073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawaya JL, Khachemoune A. Poroma:a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053–61. doi: 10.1111/ijd.12448. [DOI] [PubMed] [Google Scholar]
- 8.Snow SN, Reizner GT. Eccrine porocarcinoma of the face. J Am Acad Dermatol. 1992;27:306–11. doi: 10.1016/0190-9622(92)70187-k. [DOI] [PubMed] [Google Scholar]
- 9.Marone U, Caracò C, Anniciello AM, Di Monta G, Chiofalo MG, Di Cecilia ML, et al. Metastatic eccrine porocarcinoma:report of a case and review of the literature. World J Surg Oncol. 2011;9:32. doi: 10.1186/1477-7819-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, et al. Eccrine porocarcinoma (malignant eccrine poroma):a clinicopathologic study of 69 cases. m J Surg Pathol. 2001;25:710–20. doi: 10.1097/00000478-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Sahn RE, Lang PG. Sentinel lymph node biopsy for high-risk nonmelanoma skin cancers. Dermatol Surg. 2007;33:786–92. doi: 10.1111/j.1524-4725.2007.33171.x. [DOI] [PubMed] [Google Scholar]
- 12.Luz Mde A, Ogata DC, Montenegro MF, Biasi LJ, Ribeiro LC. Eccrine porocarcinoma (malignant eccrine poroma):a series of eight challenging cases. Clinics (Sao Paulo) 2010;65:739–42. doi: 10.1590/S1807-59322010000700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma:clinical and pathological studies of 12 cases. J Dermatol. 2007;34:516–22. doi: 10.1111/j.1346-8138.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 14.Xu YG, Aylward J, Longley BJ, Hinshaw MA, Snow SN. Eccrine Porocarcinoma Treated by Mohs Micrographic Surgery:Over 6-Year Follow-up of 12 Cases and Literature Review. Dermatol Surg. 2015;41:685–92. doi: 10.1097/DSS.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 15.Wittenberg GP, Robertson DB, Solomon AR, Washington CV. Eccrine porocarcinoma treated with Mohs micrographic surgery:A report of five cases. Dermatol Surg. 1999;25:911–3. doi: 10.1046/j.1524-4725.1999.99121.x. [DOI] [PubMed] [Google Scholar]
- 16.D'Ambrosia RA, Ward H, Parry E. Eccrine porocarcinoma of the eyelid treated with Mohs micrographic surgery. Dermatol Surg. 2004;30:570–1. doi: 10.1111/j.1524-4725.2004.30181.x. [DOI] [PubMed] [Google Scholar]