Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine (original) (raw)
. Author manuscript; available in PMC: 2021 Jul 1.
Published in final edited form as: Nat Rev Drug Discov. 2020 Jun 17;19(7):480–494. doi: 10.1038/s41573-020-0070-z
Abstract
The Hippo pathway is an evolutionarily conserved signalling pathway with key roles in organ development, epithelial homeostasis, tissue regeneration, wound healing and immune modulation. Many of these roles are mediated by the transcriptional effectors YAP and TAZ, which direct gene expression via control of the TEAD family of transcription factors. Dysregulated Hippo pathway and YAP/TAZ–TEAD activity is associated with various diseases, most notably cancer, making this pathway an attractive target for therapeutic intervention. This Review highlights the key findings from studies of Hippo pathway signalling across biological processes and diseases, and discusses new strategies and therapeutic implications of targeting this pathway.
The Hippo pathway was first discovered and delineated in Drosophila, in which tumour-suppressive roles for the pathway were identified, as mutations in genes encoding key effectors resulted in tissue overgrowth1–8. Conservation of the pathway has guided studies that have described its dysregulation in various human cancers and other diseases, as well as essential functions in development and regeneration. Accordingly, interest in understanding the molecular functions, the regulation and the therapeutic targeting of this pathway has come to the forefront of research across numerous fields.
The Hippo pathway consists of a network of signals that culminate to direct the function of the transcriptional regulators YAP and TAZ (orthologues of Yorkie in Drosophila) (Fig. 1). These paralogous factors (hereafter described together as YAP/TAZ) share conserved structural domains and regulatory mechanisms, and when localized in the nucleus direct the activity of a range of transcription factors. The best-characterized transcription factors regulated by YAP/TAZ are the TEAD family, which includes four members in mammals (TEAD1–TEAD4) and a single orthologue in flies, known as Scalloped. Nuclear YAP/TAZ direct gene expression programmes that promote cell proliferation and survival signals, and control cell fate decisions. YAP/TAZ binding to the TEAD transcription factors has been linked to most, if not all, YAP/TAZ-directed biological functions, including pro-tumorigenic phenotypes that arise from dysregulated Hippo pathway signalling. Recent efforts have therefore been devoted to disrupting interactions between these factors with the hope that such a strategy may offer therapeutic benefits.
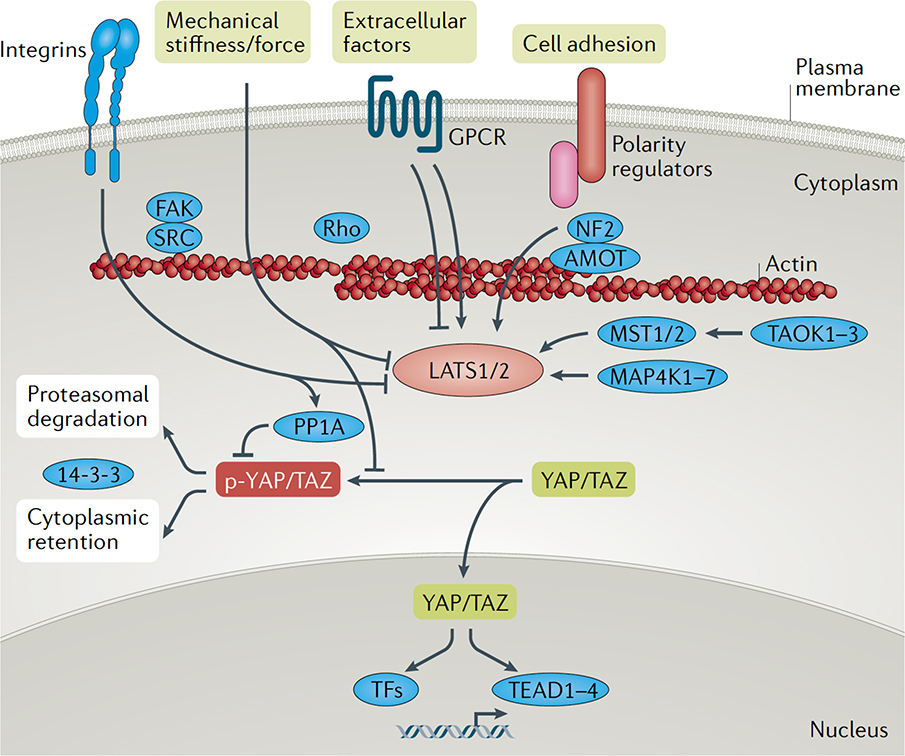
Fig 1. Key signals regulating YAP/TAZ activity.
YAP and TAZ activity is regulated by the LATS1 and LATS2 kinases, which phosphorylate YAP/TAZ on conserved residues. Phosphorylated YAP/TAZ (p-YAP/TAZ) associate with 14-3-3 proteins and are retained in the cytoplasm and targeted for degradation via the proteasome, consequently leading to low nuclear levels. Various upstream effectors of the LATS1 and LATS2 kinases have been identified, including the MST, MAP4K and TAOK families of kinases, which phosphorylate and activate LATS1/2. Cell polarity and adhesion regulators facilitate altered actin dynamics and Hippo pathway effector association to promote LATS1/2-mediated regulation of YAP/TAZ. G protein-coupled receptors (GPCRs), mechanical cues and signals transduced by the extracellular matrix and matrix-binding integrins - such as those transduced by FAK and SRC - can inactivate LATS1/2 or induce the dephosphorylation of YAP/TAZ via PP1A activation, collectively leading to hypo-phosphorylated YAP/TAZ. Hypo-phosphorylated YAP and TAZ accumulate in the nucleus, where they can bind to various transcription factors (TFs), most notably the TEAD family, to direct gene expression changes that control a range of biological events.
YAP/TAZ are predominantly regulated by phosphorylation, which promotes cytoplasmic localization and, therefore, inhibition of YAP/TAZ transcriptional activity. Two members of the NDR family of kinases, LATS1 and LATS2 (hereafter described as LATS1/2) and their single homologue in flies, known as Warts, are major regulators of YAP/TAZ. They phosphorylate YAP/TAZ and lead to their destabilization and restriction from accessing the nucleus9. Numerous upstream signals direct YAP/TAZ activity, including those activated by the extracellular matrix (ECM), mechanical forces, cell adhesion, cell polarity, mitogens, tyrosine kinase receptors, G protein-coupled receptors (GPCRs) and alterations in cellular metabolism9. Many of these signals are relayed via LATS1/2, although LATS1/2-independent regulation has also been reported10. Thus, complex networks of intrinsic and extrinsic signals have the potential to direct the localization and activity of YAP/TAZ. Understanding these cues in different cells, tissues and diseases has become the focus of many recent studies. This Review summarizes our current understanding of Hippo–YAP/TAZ signalling in diseases such as cancer and outlines the key roles for the pathway in tissue and organ development, homeostasis, tissue regeneration and immune modulation. We discuss current strategies that are being developed to manipulate or block Hippo pathway signalling, with the hope that such strategies may offer promise for disease therapy and/or directions of tissue regeneration.
Dysregulated Hippo signalling in cancer
Advances in next-generation sequencing approaches have afforded unprecedented amounts of genomic information on cancers. Although there are no classical hotspot mutations in Hippo pathway effectors, there is emerging evidence of a large number of cancers with aberrant levels of YAP/TAZ and gene expression associated with dysregulated Hippo pathway activity (also reviewed elsewhere11). Below, we outline what is known about dysregulated YAP/TAZ in cancers and the potential paths for this Hippo pathway dysregulation.
Elevated YAP/TAZ levels and activity in cancer.
Analyses of various tumours and cancer cell lines indicate a prominent role for YAP/TAZ in cancer (Table 1). Data from The Cancer Genome Atlas for over 9,000 tumours show that the Hippo pathway is one of the eight signalling pathways that are frequently altered in human cancer12. Notably, the YAP1 and WWTR1 genes, encoding YAP and TAZ, respectively, are amplified in ~14% of head and neck squamous cell carcinomas, ~16% of lung squamous carcinomas, ~17% of cervical squamous cell carcinomas and ~15% of oesophageal squamous cell carcinomas12. Increased levels of YAP/TAZ and TEAD protein expression in the nucleus correlate with poor prognosis and increased therapeutic resistance in various cancers arising from thoracic, gastric, genitourinary, gynaecological, skin, bone and brain tissues11. Some of these prognostic outcomes relate to increased YAP/TAZ levels that result from gene amplification, but other mechanisms that account for elevated YAP/TAZ levels remain largely unknown. Some aberrant YAP/TAZ levels can be explained by the intersection of Hippo pathway signalling with the activation of pro-tumorigenic signalling pathways. For example, YAP nuclear localization and activation are induced by phosphoinositide 3-kinase (PI3K) and 3-phosphoinositide-dependent protein kinase 1 (PDK1), which concomitantly inhibit the Hippo pathway and lead to poor prognosis in patients13,14. Altered mechanotransduction or signalling through pathways such as Wnt, transforming growth factor-β (TGFβ), MAP kinase, GPCRs or metabolic networks12 likely promotes aberrant YAP/TAZ activity in different contexts.
Table 1.
WWTR1 and YAP1 association with cancer
| Gene association | Hippo pathway effector expression | Supportive studies |
|---|---|---|
| Breast carcinoma | ||
| Hypermethylation of the promoter region of LATS1 (reF.166) | High expression of nuclear TAZ in high-grade breast carcinomas and triple-negative breast cancer167,168 | Overexpression of TAZ in low-expressing non-tumorigenic mammary or breast cancer cells induces transformation, tumorigenicity and migratory activity169; loss of TAZ in isolated breast cancer stem cells impairs metastatic colonization and chemoresistance169 |
| Cervical cancer | ||
| WWTR1 is amplified in ∼10% of cervical cancers; YAP1 is amplified in ∼12% of cervical cancers170 | YAP levels are elevated in cervical squamous cell carcinomas171; LATS1 was downregulated in 45% (36/80) of cervical cancers172 | High levels of YAP are observed in mouse models induced by transgenic expression of HPV proteins E6/E7 (reF.173); YAP knockdown impairs growth and YAP overexpression promotes growth of subcutaneous cervical squamous cell carcinoma xenografts in mice173 |
| Colorectal cancer | ||
| Hypermethylation of STK3, WWC1 and TAOK2 (reF.174) | YAP expression and nuclear levels are high in colorectal cancer samples175; YAP-regulated gene expression is associated with poor prognosis176 | Hyperactivation of YAP results in widespread early-onset intestinal polyp formation following dextran sodium sulfate treatment in mice64; deletion of MST1/2 leads to undifferentiated cell expansion in the small and large intestine of mice177 |
| Oesophageal carcinoma | ||
| YAP1 (11q22.1) is amplified in oesophageal carcinomas178 | High nuclear YAP is a predictor of worse prognosis and is associated with therapy resistance179 | YAP overexpression confers cancer stem cell properties and resistance to chemotherapy to benign oesophageal cells, and enhances subcutaneous tumour formation by the oesophageal adenocarcinoma cell line SKGT-4 (reFs180,181) |
| Head and neck cancer | ||
| Mutations in FAT1 in ∼20% of cases, amplification of WWTR1 in ∼14% of cases and amplification of YAP1 in ∼10% of cases | Elevated nuclear staining of YAP or TAZ correlates with tumour recurrence, resistance to radiotherapy, resistance to immunotherapy and poor outcome182,183; YAP is a potential biomarker for resistance to cetuximab183 | YAP/TAZ knockdown impairs primary tumour growth and metastasis formation after orthotopic transplantation184 |
| Hepatocellular carcinoma | ||
| Amplification of human chromosome 11q22 (reF.185) | Elevated YAP and TAZ levels186 | Amplification at mouse chromosome 9qA1 in a mouse model initiated from progenitor cells; Yap1 amplification and deletion of Mst1/2, Nf2 and Sav1 all lead to hepatocellular carcinoma76,187–189; YAP inhibition restores hepatocyte differentiation in advanced hepatocellular carcinoma; genetic and pharmacological disruption of YAP–TEAD–YAP activity prevents hepatocellular carcinoma139 |
| Malignant mesothelioma | ||
| NF2 alteration (32% of tumours); loss or mutation of LATS1 (21%), LATS2 (7%), MST1 (16%), MST2 (2%) and SAV1 (14%)190,191 | Elevated YAP levels in about 70% of mesotheliomas192 | YAP knockdown impairs the growth of mesothelioma cell lines193 |
| Meningioma | ||
| Associated with NF2 mutations194 | Nuclear expression of YAP in 92% of _NF2_-mutant meningiomas195 | YAP overexpression confers on immortalized human arachnoid cells the ability to form meningiomas195 |
| Neuroblastoma | ||
| Mutations of PTPN14 (negative regulator of YAP) identified at relapse196 | Elevated TAZ mRNA expression is prognostic of poor outcome | TAZ knockout impaired the growth of subcutaneous xenografts of the cell line SK-N-AS197; pharmacological inhibition of YAP/TAZ with verteporfin inhibited metastasis in mice198 |
| Lung carcinomas | ||
| Mutations in FAT1 in ∼18.5% of lung carcinomas; WWTR1 is amplified in ∼27% of lung squamous cell carcinomas199 | Elevated YAP levels in tissues obtained from patients with acquired EGFR inhibitor resistance54; TAZ levels are a prognostic factor in non-small-cell lung cancer progression200,201 | Tankyrase inhibitor sensitizes lung cancer cells to EGFR inhibition via stabilizing angiomotins and inhibiting YAP signalling202 |
| Ovarian cancer | ||
| WWTR1 is amplified in ∼14% of ovarian carcinomas203 | Elevated nuclear YAP expression and YAP/TAZ target gene expression correlate with poor prognosis204–206 | YAP overexpression confers on fallopian tube epithelial secretory cells the ability to form tumours207 |
| Undifferentiated pleomorphic sarcomas | ||
| Loss of NF2 (6%), SAV1 (8%) or LATS2 (16%)208 | High nuclear YAP staining in human samples | Elevated nuclear YAP staining in sarcomas arising by targeted activation of oncogenic KRAS and loss of p53 in fibroblasts; YAP knockdown impairs the growth of subcutaneous xenografts of primary mouse UPS cell lines209 |
YAP/TAZ are also implicated in metastatic progression - the leading cause of cancer-related death - which involves several events that participate in enhancing the migratory potential of aggressive cancers. Early studies indicated that ectopic expression of YAP has potent pro-metastatic activity, particularly nuclear localized mutants of YAP15. This activity relies on TEAD binding, suggesting that disruption of this interaction may offer therapeutic potential in aggressive cancers. YAP promotes a metabolic shift in cancers that metastasize to lymph nodes by inducing the expression of genes that promote fatty acid oxidation16. Deletion of Yap1 or pharmacological inhibition of fatty acid oxidation suppressed lymph node metastasis in mice. Metabolic vulnerability of cancer cells with elevated nuclear YAP/TAZ levels may therefore offer potential therapeutic potential in treating metastatic disease. Further supporting this idea are observations that metastatic triple-negative breast cancer cells with high YAP/TAZ levels are distinctly sensitive to inhibitors of glutamine transaminases, such as aminooxyacetate (AOA), which results from YAP/TAZ-induced expression of transaminases, such as GOT117.
Disruption of upstream YAP/TAZ regulators. The mechanisms contributing to altered YAP/TAZ activity in human cancers seem to be diverse. Although not frequent or conserved across many cancers, loss-of-function mutations in negative regulators of YAP/TAZ or activating mutations that have the potential to promote nuclear YAP/TAZ activity have been observed. Consistent with this, the presence of nuclear YAP is strongly associated with mutations in NF2 (encoding Merlin) in tumours that arise from the nervous system, such as schwannomas, meningiomas and ependymomas11. Mutations in NF2 and LATS2 have also been observed in malignant mesothelioma12,18,19. However, whether aberrant YAP/TAZ activity in these cancers contributes to disease phenotypes has not yet been revealed. Interestingly, activating mutations in genes coding for guanine nucleotide-binding proteins, G(q) subunit α (GNAQ) and GNA11 - which are downstream of GPCRs and found in more than 80% of uveal melanomas - drive uncontrolled nuclear YAP activation, which directly promotes tumorigenesis20, 21. These identifiable alterations may offer an opportunity to predict responsiveness to therapeutics targeting YAP/TAZ activity.
Gene fusions in cancer related to the Hippo pathway.
Gene fusions are structural chromosomal aberrations that can often involve the exchange of regulatory regions of genes, and can act as strong drivers of tumorigenesis. Core effectors of Hippo signalling can contain such anomalies. For example, a fusion between the genes WWTR1 and CAMTA1 (encoding calmodulinbinding transcription activator 1) accounts for more than 90% of a type of vascular cancer called epithelioid haemangioendotheliomas22. In this type of tumour, the encoded TAZ–CAMTA1 fusion protein has similar transcriptional activity and tumorigenic functions to TAZ. The TAZ portion of the fusion protein retains an amino-terminal region that includes LATS phosphorylation sites as well as the TEAD binding domain, but has replaced the carboxy-terminal transactivation domain with a C-terminal domain from CAMTA122. Interestingly, TEAD4 is required for the transforming activity of TAZ–CAMTA1, indicating that the TEAD binding interface is conserved23. Therefore, patients with epithelioid haemangioendothelioma may benefit from potential therapeutics that target TAZ–TEAD binding. Fusion of the YAP1 and TFE3 (encoding transcription factor E3) genes has been observed in other vascular cancers24, and additional gene fusions involving WWTR1, NF2, LATS1 and LATS2 have been found in lung cancer25. Thus, it seems that genetic alterations that activate YAP/TAZ function may be enriched in some cancers and additional study of these diseases will offer a greater understanding of YAP/TAZ function in cancer.
Role of the Hippo pathway in resistance to targeted therapy.
Targeted therapies are an attractive approach to selectively treat cancer with mutations in key oncogenic drivers. However, intrinsic and acquired resistance to targeted therapies is common and presents a barrier to the efficacy of such treatments. Elevated YAP/TAZ levels contribute to resistance to chemotherapy as well as to therapies that target activating mutations in EGFR, RAS and BRAF26. For instance, in EGFR-mutant lung cancer cells, YAP activation induces the expression and activation of the tyrosine kinase receptor AXL, which mediates intrinsic and acquired resistance, although the prevalence of AXL-driven resistance in patients is largely unknown27, 28. YAP is also a key driver in enabling compensation and bypassing of KRAS dependency in pancreatic and colon cancer models29, 30. In pancreatic ductal adenocarcinoma, YAP–TEAD complexes direct cell cycle progression, DNA replication and a shift to a mesenchymal phenotype to compensate for the loss of oncogenic KRAS function29. In colon and lung cancers, the interaction between YAP and transcription factor FOS induces an epithelial–mesenchymal transition programme to bypass KRAS dependence. YAP1 amplification was also found to bypass inhibition of RAF and MEK in lung, skin, colorectal and thyroid cancers by promoting survival by upregulating the anti-apoptotic gene BCLX (also known as Bcl-2-like protein 1, BCL2L1)31. Interestingly, YAP and TAZ were among the top candidates from a large-scale functional genetic study that aimed to identify mechanisms of resistance to ALK inhibition in ALK-addicted lung cancer32. In most of these cases, a combinatorial regimen of YAP/TEAD inhibition and targeted therapy against these other pathways would be expected to overcome intrinsic and acquired resistance. Thus, elucidating the mechanism of YAP and TAZ activation during drug therapy resistance is of significant interest when considering targeted therapies in cancer 33,34.
The Hippo pathway in immune modulation
Hippo pathway components have prominent roles in immune regulation. The best studied is MST1 (encoded by STK4), as early studies showed that mutations in STK4 can lead to immunodeficiency in both human and mouse35–37. MST1 positively participates in T cell development, adhesion and migration as well as key dendritic cell functions38–42. Although studies do support a role for YAP/TAZ in T cell biology and innate immunity, MST1 seems to modulate immune functions independently of canonical Hippo pathway YAP/TAZ regulation.
Early evidence for the role of MST1 and MTS2 (encoded by STK3) in immune responses came from the study of regulator for cell adhesion and polarization enriched in lymphoid tissue (RAPL; also known as Ras association domain family member 5, RASSF5), which is required for immune cell trafficking. RASSF5 interacts with and stimulates MST activity43. MST1 overexpression in the mouse pro-B lymphocyte cell line BAF stimulated cell adhesion, and MST1 knockdown inhibited cell polarization and adhesion in vitro. Germline STK4 knockout mice display a significant reduction of naive T cells - but not of effector or memory T cells - in circulation, supporting a role for MST1 in the maintenance of circulating naive T lymphocytes44. In this context, loss of MST1 did not dramatically affect the phosphorylation of LATS1/2 or YAP in T cells44, suggesting that the essential MST1 functions in T cells may be independent of conventional Hippo pathway signalling. Subsequent studies showed that deletion of STK3 and STK4 does not affect T cell development, but dramatically reduces the number of circulating CD4+ and CD8+ T cells45. This is likely owing to a severely compromised T cell ingress from lymph nodes, revealing a specific role for MST in lymphocyte trafficking and migration45.
Systematic deletion of the Hippo pathway components in dendritic cells - which are essential for antigen presentation to T cells and serve as messengers between the innate and adaptive immune response - has shown that MST1/2 are selectively required for CD8+, but not CD8−, dendritic cells to orchestrate CD8+ T cell-mediated immune response in vivo41. Mechanistically, deletion of STK3 and STK4 compromises mitochondrial function and bioenergetics, and MST1/2-deficient dendritic cells have low interleukin 12 (IL-12) production and defects in crosstalk with non-canonical nuclear factor NF-κB signalling41. Notably, deletion of LATS1/2 or YAP1/WWTR1 did not produce similar phenotypes41, supporting the observation that conventional Hippo pathway signalling mediated by MST1/2 is not involved in the regulation of dendritic cells. Homozygous mutations in STK4 can, extremely rarely, cause immunodeficiency in patients35,36. These patients are characterized by a progressive loss of circulating naive T cells, impaired survival of T cells in vitro, increased viral and bacterial infections and autoimmune defects 35,36. These observations establish a pathological significance of MST1 in the human immune system, particularly in maintaining normal T cell functions.
Both YAP and TAZ have also been identified as key effectors of T cell biology. TAZ was shown to promote CD4+ helper T (TH) cell differentiation towards the TH17 cell fate, which are key adaptive immune cells that produce IL-17. Interestingly, the role for TAZ in promoting TH17 cells is described as independent of the TEAD transcription factors46. YAP levels are increased upon T cell activation, having immunosuppressive functions that blunt CD4+ and CD8+ T cell activating signals and TH cell differentiation47. YAP has also been identified as being required for proper CD4+CD25+ regulatory T (Treg) cell function48. T cell-specific deletion of YAP1 results in increased antitumour T cell responses, which include an increase in T cell tumour infiltration and local activation47, as well as in dysfunctional Treg cells that are unable to suppress antitumour immunity48. Activation of CD8+ T cells induces expression of several Hippo pathway effectors, including MOB1A, LATS1 and TEAD1/249. It was proposed that cell–cell contact upon clonal expansion of activated T cells leads to Hippo activation and YAP inhibition, offering a potential mechanism for coupling CD8+ T cell terminal differentiation with clonal expansion.
Interestingly, YAP/TAZ have also been implicated in innate immunity50, 51. Cytosolic RNA and DNA sensing and signalling is strongly enhanced under Hippo activating conditions, in which YAP/TAZ are inactive in the cytoplasm51. YAP/TAZ can bind and directly inhibit TBK1 kinase, which is activated and has a key role in innate response51. YAP/TAZ deletion or inactivation by overexpression of Hippo pathway kinases relieved their inhibition of TBK1 to boost innate immune response and increase antiviral response51. The function of YAP in TBK1 regulation is independent of its TEAD-dependent transcription co-activator activity. The interferon regulatory factor IRF3 is a key transcription factor in immune response by inducing cytokine expression. YAP inhibits IRF3 by blocking its dimerization and nuclear translocation in response to viral infection50. Inhibitor of NF-κB kinase subunit ε (IKKε), which is the closest TBK1 paralogue with similar functions and is also activated by infection, phosphorylates YAP and promotes its lysosomal degradation50. Collectively, these studies establish an inhibitory function of YAP/TAZ in innate immune response, but future studies are needed to clarify their mechanisms of action.
Hippo pathway in cancer immunity and interplay with the immune system.
Besides directly acting in immune cells as described in the above section, alteration of the Hippo pathway in cancer cells can impact the interplay between cancer cells and the host immune system. In a mouse model of liver cancer, high YAP activity in cancer-initiating cells promotes the recruitment of type II macrophages, which are immunosuppressive, to inhibit the host immune response and likely contribute to protection of cancer cells from immune surveillance52. This is mediated by YAP-TEAD-dependent expression of cytokines to recruit macrophages. In a mouse model of prostate cancer driven by loss of Pten and Smad, high YAP activity in tumour cells stimulated the recruitment of myeloid-derived suppressor cells to inhibit host immune response53. At the molecular level, YAP–TEAD induced expression of cytokines, such as C-X-C motif chemokine 5 (CXCL5), to recruit myeloid-derived suppressor cells in the tumour microenvironment, thus allowing immune tolerance and tumour progression53. These studies indicate that high YAP activity in cancer cells contributes to the immunosuppressive niche.
YAP and TAZ can both stimulate the expression of programmed cell death 1 ligand (PDL1)54–56, a ligand for the PD1 receptor, which creates an immunosuppressive environment. TAZ promotes the expression of PDL1 in lung and breast cancer cells55, 57, whereas YAP promotes the expression of PDL1 in melanomas and head and neck squamous cell carcinoma cells56,58. Therefore, targeting YAP/TAZ may offer an attractive approach to reduce PDL1 levels and, thus, enhance the effect of immunotherapy using neutralizing antibodies against PDL1 and/or PD1. Deletion of Yap1 in T cells using a CD4-cre murine model increased antitumour immune responses, as Yap1 knockout mice could not support growth of the syngenic B16 melanoma xenograft47,48. Notably, combination of YAP inhibition using verteporfin, which disrupts YAP nuclear activity, with an antibody against PD1 led to synergistic reduction of tumour growth, supporting the notion that YAP inhibition promotes the efficacy of immunotherapies48. An immunosuppressive function of YAP has been observed in other cell types59. Conditional deletion of Yap1/Wwtr1 in mouse epicardium reduced the expression of interferon-γ (IFNγ), an important regulator of Treg cells. Consequently, reduced recruitment of Treg cells was observed, which led to profound post-myocardial infarction, pericardial inflammation and myocardial fibrosis.
The function of the Hippo pathway in cancer immunity is rather complex. Several syngeneic mouse xenograft model experiments indicate that LATS1/2 exhibits strong inhibitory activity towards cancer cell immunity60. LATS1/2 deletion in cancer cells blocked xenograft tumour formation in immune-competent syngeneic mice, which interestingly was a phenotype not observed in immunodeficient mice59. Further characterization showed that LATS1/2 deletion caused stronger immunogenic exosome secretion and type I interferon response, leading to tumour suppression. YAP/TAZ activation seems to partially contribute to the increased cancer cell immunity in the LATS1/2 knockout cells, indicating that LATS1/2 autonomously suppresses cancer cell immunity.
The data from the xenograft study apparently contradict the data from LATS1/2 genetically engineered mouse models, which collectively support that loss of LATS1/2 promotes tumorigenesis. However, such differences could be attributed to experimental settings. In xenograft tumour model experiments, the mouse hosts receive many tumour cells at once, whereas tumours in genetically engineered mouse models presumably originate from a single or few cancer-initiating cells. High YAP activity in one cancer-initiating cell may not induce a strong immune response in the genetically engineered mouse models, but may confer a growth advantage to such a cancer-initiating cell. By contrast, many cancer cells with high levels of YAP in the xenograft model may be sufficient to induce a strong immune response, leading to elimination of tumour cells by the host immune system. Future studies are therefore needed to clarify the context of Hippo pathway signalling in immune cells and the interplay between cancer and immune surveillance.
Tissue regeneration and repair
Multicellular organisms have the ability to replace damaged or injured tissues or organs, which in some cases can include complete regeneration. Some examples of this complete regeneration include accurate regeneration of complete tail fins in fish, replacement of an amputated limb in frogs and regeneration of the liver following partial hepatectomy in humans. Hippo pathway signalling has emerged as central to such regeneration, playing key parts in stem cell proliferation, survival and specification, ultimately determining organ patterning and size.
Liver regeneration.
Early insight into the involvement of Hippo pathway signalling in regeneration came from observations in Drosophila intestines, which showed that the YAP/TAZ homologue Yorkie is critical for intestinal stem cell expansion following injury61–63. Studies in mammalian animal models, such as mice, revealed similar importance for YAP/TAZ in promoting the regenerative capacity of intestinal stem cells64–68, with recent single-cell RNA-sequencing studies highlighting YAP as a central factor in the regeneration of distinct intestinal stem cell populations69,70. A prime example of the importance of Hippo pathway signalling in regeneration is the involvement in liver recovery following partial hepatectomy. YAP/TAZ activity is essential for liver regeneration following damage, and increased levels and activity of nuclear YAP are observed in the liver following partial hepatectomy in rat models71,72. Conditional deletion of Yap1 and Wwtr1 in mice results in defective regeneration following partial hepatectomy73. Similarly, deletion of Yap1 compromises the regenerative response of hepatocytes following cholestatic liver injury74. Interestingly, transient ectopic expression of YAP75 or deletion of upstream Hippo pathway effectors, such as Stk3, Stk4 76–78, Nf2 79,80 or Sav1 76 (encoding WW45, a regulator of MST1/2 expression that restricts proliferation and promotes apoptosis), drives strong pro-proliferative signals that result in liver overgrowth. Ectopic expression of YAP can also drive hepatocyte plasticity, as hepatocytes convert into a hepatic progenitor state that enables ex vivo expansion and transplantation of such cells into animals. Remarkably, these cells have the ability to revert back to hepatocytes if YAP levels are brought back to basal levels81. Such a strategy is effective in liver repopulation following partial hepatectomy in mice82. Similarly, transient depletion of MST1/2 kinases facilitates regeneration in aged mice following partial hepatectomy83. Given that deletion of Yap1/Wwtr1 in the liver of mice seems to have no overt consequences on homeostasis84, manipulating YAP/TAZ levels in the liver may prove to be an effective strategy for regeneration. However, given the pro-tumorigenic roles of YAP/TAZ in the liver and other organs, such an approach would require caution.
Lung regeneration.
YAP is also involved in the regeneration of the lung following pneumonectomy. Pneumonectomy in mice results in elevated levels of YAP in expanding alveolar type II cells, a cell population that has stem cell potential85, and conditional deletion of Yap1 and/or Wwtr1 in this cell population results in defective lung regeneration in response to injury86–88. In the context of the severe lung injury of pneumonectomy, YAP activation is mediated by mechanical tension-regulated signals that are relayed by the GTPase CDC4286. Thus, controlled regulation of YAP/TAZ activity in alveolar type II cells may offer a mechanism for distal lung damage. YAP also contributes to the maintenance of basal stem cells of the airway epithelium, which has an important function in regenerating damaged airway tissue. Ectopic expression of YAP is sufficient to drive basal airway cell expansion 89–91, raising the possibility that controlled activation of YAP could provide a strategy for airway epithelium regeneration.
Wound healing.
Roles for YAP/TAZ in basal stem cell control are apparent in epidermal development and skin wound repair. Nuclear YAP and TAZ localization is observed in the basal layer of skin and these nuclear levels are elevated upon wound healing92. Ectopic expression of an activated YAP mutant or dysregulation of upstream regulators of YAP localization - such as the cell adhesion regulator α-catenin93,94 - results in the expansion of epidermal basal cells, which resembles an uncontrolled epidermal damage response. Skin-specific deletion of both Yap1 and Wwtr1 in adult mice severely impairs regeneration after wounding 92,93,95–97. YAP/TAZ activity is essential in skin homeostasis as deletion of Yap1/Wwtr1 slows proliferation of basal layer cells and leads to hair loss and tissue damage92. Thus, strategies directed at inhibiting YAP/TAZ activity would have to take this important homeostatic role into account.
Cardiac regeneration.
Cardiomyopathies make up a group of heart muscle diseases that result in heart failure that is linked to the loss of cardiomyocytes. The maintenance and regeneration of cardiomyocytes have been linked to YAP/TAZ activity. For example, ectopic expression of YAP in the heart is sufficient to stimulate the proliferation of postnatal cardiomyocytes and cardiac regeneration in mice, and improve contractility after myocardial infarction 98–100. Conversely, cardiacspecific deletion of Yap1 represses mouse neonatal heart regeneration 98–100. Interestingly, recent screening efforts have identified TT-10 (C11H10FN3OS2) as an activator of YAP that promotes cardiomyocyte proliferation and improves cardiac function after myocardial infarction in mice101,102. Mechanistically, TT-10 inhibits the phosphorylation of GSK3β, which leads to the activation of Wnt/β-catenin signalling and nuclear accumulation of YAP101,102. Microenvironmental changes, tensional forces and actin remodelling have been linked to the activation of YAP in regenerating cardiomyocytes 103–105, and therefore offer research directions for heart regeneration strategies.
Other tissue regeneration.
Broad functions for YAP/TAZ have been described in a number of contexts of tissue regeneration, including essential roles in repairing damaged mammalian corneal epithelium106, Schwann cells107, neuromuscular junctions108, skeletal development and bone repair109. YAP/TAZ are also involved in the homeostatic turnover of tissues in the mammalian tooth110. Notably, the regeneration of amputated limbs in animal models with high regenerative capacity relies on YAP/TAZ activity. For example, Xenopus limb bud and tail regeneration requires proper Yap1 activity111, 112, and the control of tissue growth in regenerating zebrafish fins relies on the activation of YAP113. Planarian flatworms, which have a tremendous regenerative capacity, also rely on YAP for the proper growth and patterning of stem cell populations following damage114. Several conserved themes are evident from studies of YAP/TAZ in regeneration, which include essential roles for YAP/TAZ in somatic stem cell regulation, alterations in the microenvironment and responses to mechanical and tensional forces in damaged tissues. The relationship between YAP/TAZ and altered mechanical forces is of particular interest, as the opportunity for therapeutic intervention that can either activate the regenerative programme or inhibit aberrant wound healing responses may be accessible from gaining better knowledge into these cues. Interestingly, the activity of YAP/TAZ does not seem to be essential for development of some tissues. For example, YAP is dispensable in the homeostasis of the intestinal epithelium and liver hepatocytes under normal physiological conditions. Therefore, better understanding of the signals that control the regenerative capacity of YAP/TAZ versus the developmental roles for YAP/TAZ may hold promise for regenerative therapies.
YAP/TAZ in fibrotic disease
A hallmark of fibrotic disease is the excessive deposition of the ECM, including cross-linked collagen fibres, which results in the stiffening of tissues and eventual dysfunction of affected organs. YAP/TAZ act as effectors of mechanical cues, which has led to the investigation of these factors in a range of fibrotic diseases. ECM stiffening promotes the nuclear activity of YAP/TAZ in cancer-associated fibroblasts, and fibroblasts of the liver115,116, kidney117, lung118 and skin119, as nuclear YAP/TAZ promote fibrotic cellular phenotypes, such as myofibroblast differentiation and increased matrix remodelling potential. Several genes that encode key secreted factors implicated in fibrosis are direct YAP/TAZ targets in various cell models in vitro, most of which are also targets of the TEAD family of transcription factors. These genes include well-characterized pro-fibrotic factors, such as connective tissue growth factor (CTGF), plasminogen activator inhibitor 1 (PAI-1) and the lysyl oxidase (LOX) family of collagen cross-linking enzymes118,120. Therapeutic strategies that target these YAP/TAZ-regulated pro-fibrotic factors are being explored for fibrotic diseases, including a monoclonal antibody (FG-3019 from FibroGen) that interferes with CTGF function121, small-molecule inhibitors of PAI-1 (such as TM5275)122 and inhibitors of LOX and LOX-like proteins (such as the non-selective LOX inhibitor β-aminoproprionitrile123 and the specific LOXL2 blocking antibody AB0023124.
Several lines of evidence support YAP/TAZ as contributors to fibrotic disease in vivo. These include reports of elevated YAP/TAZ levels and transcriptional activity in fibroblasts as well as in alveolar and respiratory epithelium of patients with idiopathic pulmonary fibrosis118,125. Elevated TAZ levels correlate with local tissue stiffness in idiopathic pulmonary fibrosis, supporting the idea that increased mechanical forces may drive YAP/TAZ activity. Increased nuclear YAP has also been observed in patients with primary sclerosing cholangitis and primary biliary cirrhosis, which are chronic fibrotic disorders of liver injury74. Ectopic expression of YAP or TAZ in immortalized mouse fibroblasts that have been adoptively transferred, and that accumulate in mouse lungs, is sufficient to drive pro-fibrotic phenotypes118. Expression of YAP or TAZ in the duct cells of the liver drives fibrosis progression that parallels fibrosis in non-alcoholic fatty liver disease126–128. Renal fibrosis induced in mice by unilateral ureteral obstruction exhibits elevated YAP levels129. Interestingly, unilateral ureteral obstruction-induced myofibroblast accumulation, matrix deposition and fibrosis can be suppressed following deletion of Yap1/Wwtr1 in Gli1+ cells129, which are recruited mesenchymal cells thought to function as progenitors in the kidney130. Furthermore, mice lacking one allele of Wwtr1 are more resistant to bleomycin-induced fibrosis131. Likewise, knockdown or pharmacological inhibition of YAP/TAZ delays wound healing96 and prevents TGFβ-induced renal fibrosis117. Collectively, these studies suggest that targeting aberrant YAP/TAZ activity in fibrotic diseases may hold promise for therapy.
YAP/TAZ converge with pro-fibrotic signalling pathways, such as TGFβ signalling. Indeed, YAP/TAZ are essential for pro-fibrotic cues induced by TGFβ in a stiff microenvironment117, 118, 132. As with YAP/TAZ activity, TGFβ-induced signalling is increased by matrix stiffness and the synergistic activation of these signals in fibroblasts strongly promotes myofibroblast activation, matrix production and fibroblast proliferation and contractility. Depletion of YAP/TAZ blocks TGFβ-induced myofibroblast transformation and production of the ECM, whereas ectopic expression of activated YAP/TAZ promotes these events. YAP/TAZ bind and directly regulate TGFβ-activated SMAD transcription factors 133, 134, offering a mechanism for pro-fibrotic signalling cross-talk. However, YAP/TAZ can induce pro-fibrotic effects in response to matrix stiffness in the absence of exogenous TGFβ, suggesting that they also play a broader part in fibrosis 115,118. Consistent with this idea, nuclear YAP/TAZ activity correlates with increased phosphorylated PTEN, phospho-S6 ribosomal protein (p-S6) and total S6 in fibrotic lungs 125, all of which suggest increased PI3K–AKT–mTOR signalling in cells with activated YAP/TAZ. As such, elevated nuclear YAP/TAZ levels may function to establish and coordinate a fibrogenic signalling network that is necessary for relaying and responding to microenvironment signals135. Targeting this dysregulated network or the mechanical/matrix signals that drive elevated nuclear YAP/TAZ activity would therefore have strong therapeutic potential. One recent such strategy that has shown promise is activation of the Gα-coupled dopamine receptor D1 (DRD1) through selective agonists, which results in selective inhibition of YAP/TAZ in lung and liver mesenchymal cells136. DRD1 activation reversed ECM stiffening in vitro and tissue fibrosis in animal models, highlighting a potentially interesting therapeutic avenue for selectively targeting YAP/TAZ activity in fibrotic disease.
Targeting the Hippo pathway
Given that several Hippo pathway members are bona fide tumour suppressors and aberrant YAP/TAZ activity is implicated in a range of cancers and other diseases, targeting this pathway offers potential opportunity for therapy. Blocking the activity of the YAP/TAZ–TEAD transcriptional complex is one potential approach. Indeed, recent in vivo tumour studies with preliminary compounds that disrupt YAP/TAZ-TEAD interactions suggest that this approach may be tenable. Inhibiting proteins encoded by YAP/TAZ-activated genes have also shown promise for therapy121, 137, 138, and thus understanding these gene products in specific disease contexts may offer translational opportunity. Furthermore, given the roles of the Hippo pathway in regeneration and wound repair, harnessing the ability to conditionally alter Hippo pathway activity with therapeutics may also prove successful in the clinic. Below, we highlight current avenues for therapeutic intervention (Fig 2), some of which are showing promise with initial studies.
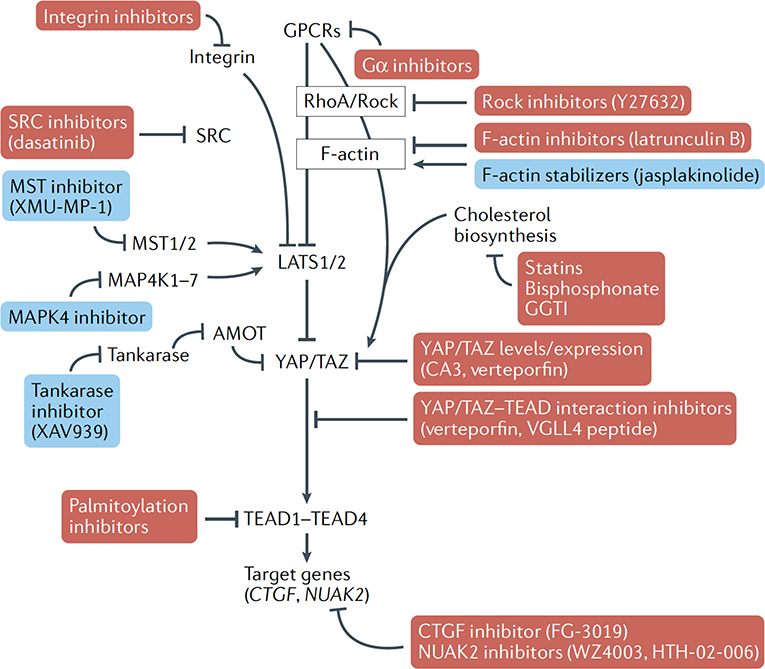
Fig. 2. Proposed strategies and small molecules (direct and indirect) that could promote or inhibit YAP/TAZ activity.
Opportunities exist for small molecules for biological intervention of key YAP/TAZ-regulatory signals that may offer targeted medical promise. These include various targets that would result in repression of YAP/TAZ nuclear activity (shown in red boxes), such as inhibitors of repressors of LATS1/2 activity, inhibitors of YAP/TAZ interacting transcription factors (such as the TEAD family) or inhibitors of important downstream target genes that drive aberrant biological processes. Activation of YAP/TAZ may also be desirable, such as in the context of medical regenerative strategies, and may be achieved by repressing the activity of effectors that promote YAP/TAZ cytoplasmic retention (shown in blue boxes). CTGF, connective tissue growth factor; GPCR, G protein-coupled receptor.
Inhibition of YAP/TAZ–TEAD-dependent transcription.
Initial efforts to identify inhibitors of YAP/TAZ–TEAD activity relied on screening for inhibitors of TEAD-regulated transcriptional reporters. The first such inhibitor, verteporfin (porphyrin compound), was identified from a small library of FDA-approved compounds, and was reported to block the interaction between YAP and TEAD and suppress tumour growth in mice 139. Several reports have since indicated that verteporfin has the ability to block YAP-TEAD activity in various contexts, but this inhibition may not be specific to only inhibiting YAP-TEAD complexes as verteporfin has also been reported to have proteotoxic effects140–142. These caveats, along with poor pharmacokinetics, suggest that this compound may not translate well to the clinic. However, identification of verteporfin as an inhibitor of YAP-TEAD activity has initiated a wealth of screening efforts, with other less well tested compounds also emerging. As with verteporfin, the compounds CA3 and C19 have been identified in transcriptional reporter screens as inhibitors of YAP–TEAD activity143,144, with CA3 exhibiting in vivo efficacy. In silico modelling of compound libraries led to the identification of CPD3.1, which disrupts YAP association with TEADs and represses TEAD transcriptional activity, cell proliferation and cell migration145. Similarly, MYF-01–37 was recently identified as a covalent binder of TEADs that disrupts binding with YAP/TAZ. MYF-01–37 was shown to be effective in targeting EGFR-mutant non-small-cell lung cancer cells when used in combination with EGFR and MEK inhibitors146. Details of additional compounds proposed to target YAP/TEAD activities in various cancers by impacting their interaction, activity or localization have been reviewed elsewhere147,148. Although these compounds provide useful research reagents to investigate pathway biology, their target specificity and selectivity remain to be determined. Therefore, whether these screening methods are useful for targeted therapy remains to be confirmed.
Rational design for targeting the YAP/TAZ–TEAD interface.
Very simplistically, the TEAD transcription factors have a DNA-binding domain (DBD) and a YAP-binding domain (YBD). The DBD, as the name suggests, is responsible for DNA binding specificity and harbours a helix–turn–helix homeodomain fold. The YBD has a β-sandwich fold with four helices. Structurally, the interaction between the YBD in TEADs and the TEAD-binding domain in YAP comprises three highly conserved interfaces, with the third interface being the major energetic determinant of high-affinity binding141–143 (Fig. 3a). Crystal structures of YAP–TEAD complexes reveal clear surface pockets at the second and third interfaces that might enable the rational design of TEAD inhibitors. Notably, residues within the TEAD pocket that associate with YAP at interface 3 are 100% identical across all TEAD family members, suggesting that targeting this interface would offer possible pan-TEAD ligands, which could prove useful across many different contexts. Indeed, such efforts to target the YAP-TEAD interface have yielded promising peptides and small molecules that provide proof of concept and could serve as valuable research tools to further validate the strategy144. The in vivo impact of inhibitors binding interface 3, however, remains to be determined. Furthermore, given that none of the interfaces is more accessible than the others, whether inhibiting a specific TEAD interface would be more efficacious or provide more specificity remains to be seen. Other key challenges ahead include ensuring permeability, specificity, efficacy and, ultimately, safety for identified compounds, while providing a sufficient therapeutic window. Further, whereas most efforts have focused on disrupting the YAP-TEAD interaction, it will be important to keep in mind other known TEAD-interacting proteins, such as the transcription cofactor vestigial-like protein family members (VGLL1–VGLL4, which are also transcriptional co-activators of TEADs) and p160145. VGLL1 and VGLL4 bind TEADs at interfaces 1 and 2, but not at interface 3. How an inhibitor of YAP binding would impact interactions with other TEAD co-activators and how this could translate to phenotypic consequences are all areas of active investigation across various groups.
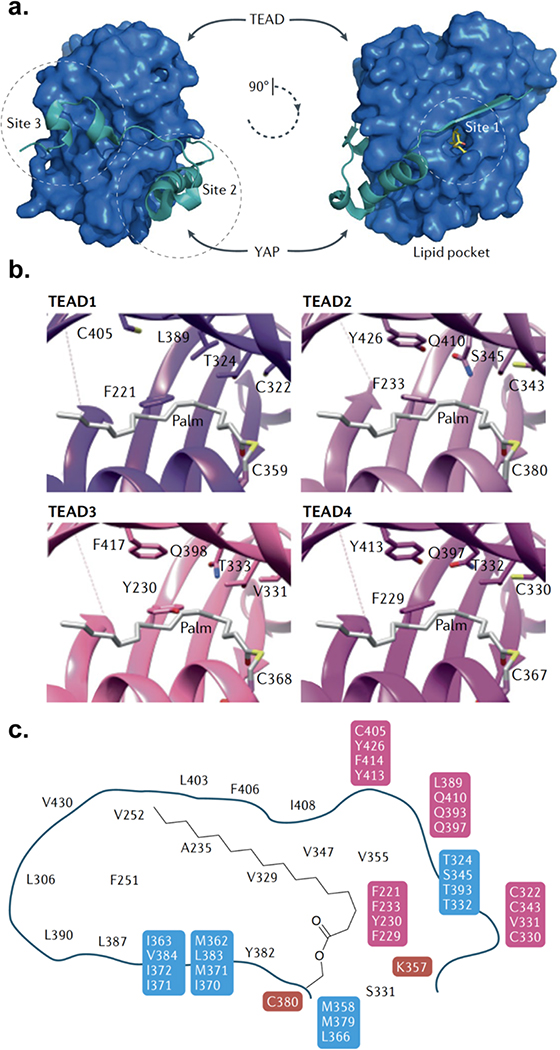
Fig. 3. Structural biology of the TEAD–YAP interface and the TEAD lipid pocket.
(a) TEAD proteins contain a highly conserved carboxy-terminal YAP/TAZ binding domain (YBD). The binding interface between the TEAD YBD (blue) and the TEAD-binding peptide of YAP (teal) comprises three highly conserved interfaces (sites 1, 2 and 3) that contribute to high-affinity binding. TEADs also contain a central hydrophobic lipid pocket (shown at the right) with an Spalmitoyl cysteine (yellow sticks) modification at a universally conserved cysteine. (b) Snapshots of the lipid pockets of all four TEADs highlight the difficulty in developing pan-TEAD lipid pocket ligands as well as providing a rubric for the design of isoform-specific ligands. Residues shown are lipid pocket residues that are nonconserved in at least one of the four human TEAD paralogues. (c) Schematic representation of the human TEAD lipid pocket. Residues in black as well as those highlighted in red are invariant among human TEADs (residues in red are directly important for lipidation). Those highlighted in blue are non-identical in at least one human TEAD paralogue but still similar. Residues highlighted in pink are non-identical and non-similar in at least one paralogue.
Post-translational palmitoylation of TEADs at a conserved cysteine extends the palmitoyl group into the hydrophobic cavity in TEADs 149,150. Unlike other palmitoylated proteins, TEAD palmitoylation does not impact its trafficking and cellular localization, and its influence on YAP/TAZ binding is controversial. Although the regulation and functional relevance of TEAD palmitoylation remains to be elucidated, it might maintain TEAD folding and stability149, 150. Palmitoylation impacts TEAD stability, which has made functional evaluation of the mutants lacking the conserved cysteine technically challenging, as this renders them inherently unstable. Compounds that bind in this pocket could provide valuable insights into the functional consequences and therapeutic potential of targeting this site. It is worth highlighting that this hydrophobic pocket, although 80% conserved across the four TEADs, is not identical and the key residues involved have been highlighted in Fig. 3b,c. This is in contrast to the site 3 pocket on TEADs responsible for the YAP–TEAD interaction, which is 100% identical across all TEADs. Specifically, structural analyses of the residues in the pocket revealed a Tyr230 in TEAD3 relative to a Phe residue in other TEADs, which results in steric hindrance and altered hydrophobicity of the lipid pocket. This observation suggests that any small molecules targeting this lipid pocket could potentially bind TEAD3 differently, thereby making identification of pan-TEAD ligands a potential challenge for this targeting strategy. It also raises the question of whether all TEADs need to be effectively inhibited and whether certain cancers are more dependent on a specific TEAD over others. Flufenamate drugs, which are non-steroidal anti-inflammatory drugs, bind in the TEAD lipid pocket and displace palmitoylation without inhibiting the YAP–TEAD interaction. Treatment with these compounds decreased tumour cell migration and proliferation moderately, thereby providing a promising proof of concept for this strategy as well151. Recently, flufenamic acid derivatives comprising chloromethylketone moieties that bind to the conserved cysteine in the lipid pocket have also been shown to inhibit YAP-TEAD interaction, transcriptional activity and glioblastoma growth in vitro152. For additional reference, the structures and details of compounds targeting the Hippo pathway have been recently reviewed elsewhere 34 (Fig. 4).
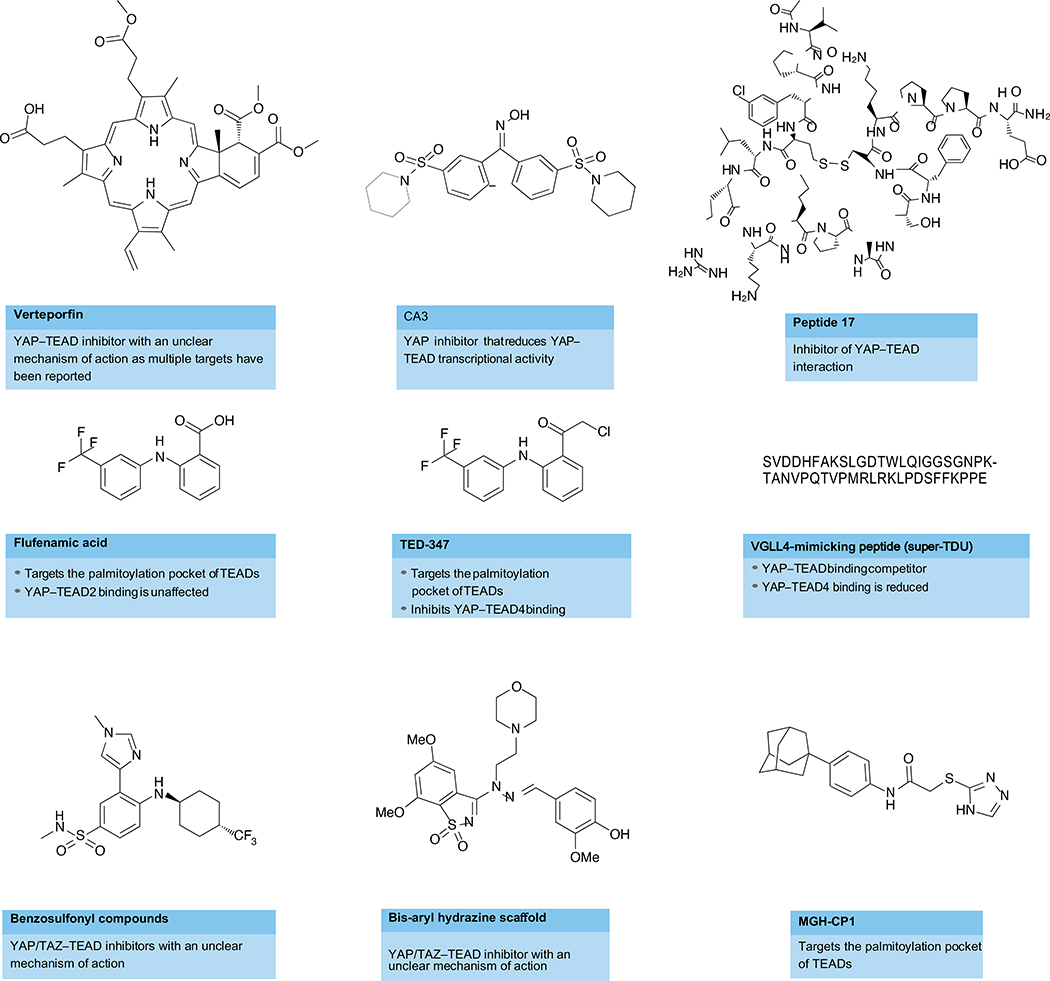
Fig. 4. Representative compounds targeting the Hippo pathway.
Key potentially druggable sites in the protein–protein interaction between YAP/TAZ and TEAD have been identified, as well as a highly conserved palmitoylation pocket in TEADs. Some small molecules that target these interactions are shown, such as Peptide 17 210,211. Although compounds such as verteporfin or CA3 successfully inhibit TEAD transcriptional activity and have shown antitumour activity in vivo, the target specificity and selectivity of these compounds remain to be confirmed. Other approaches have focused on disrupting the interaction between TEAD and other transcriptional co-activators, such as vestigial-like protein family members (VGLL1-VGLL4) with VGLL4-mimmicking peptides, such as ‘super-TDU’. Finally, compounds such as flufenamic acid and TED-347, which target the lipid pocket at the core of all four TEADs and inhibit palmitoylation, have shown antitumour activity in vitro. TDU, Tondu.
VGLL4 peptide mimetics.
In addition to YAP and TAZ, VGLL1-VGLL4 interact with TEADs via their Tondu (TDU) domains153–155. VGLL1-VGLL3 may be transcriptional co-activators of TEADs, but VGLL4 has been identified as a tumour suppressor downregulated in gastric cancer156. Mechanistically, VGLL4 competes with YAP for binding to TEADs via a TDU domain and thereby functions as a YAP antagonist153–155. Although the mechanistic details of how VGLL4 is dysregulated in cancer remain to be elucidated, a rationally designed ‘super-TDU’ peptide targeted the YAP–TEAD interaction and inhibited growth of gastric cancer cells in vitro and tumour growth in vivo in mice156 (Fig. 4). The therapeutic path ahead for these specific peptides remains challenging, but this study provides yet another promising proof of concept for targeting YAP-TEAD interactions in cancers. Future studies will address whether this approach would be broadly applicable across cancers or is specific to gastric cancer.
Altering localization and stability of YAP/TAZ–TEADs.
The nuclear–cytoplasmic shuttling of YAP and TAZ has been known for some time and is critically important for their regulation157. As transcription co-activators, YAP/TAZ have to be located in the nucleus for their activity. Therefore, blocking YAP/TAZ nuclear localization could be an approach to inhibit YAP/TAZ activity and curtail tumour growth. Indeed, compounds that target a range of upstream YAP/TAZ effectors have been reported. For example, stearoyl-CoA-desaturase 1 (SCD1) has been reported to promote nuclear YAP/TAZ activity in lung cancer cells, and pharmacological inhibition of SCD1 can reduce YAP/TAZ activity158. YAP/TAZ stability is also a potential targeting mechanism, as YAP/TAZ degradation is controlled via multiple mechanisms. For example, YAP/TAZ degradation may be stimulated by activating specific ubiquitin–proteasome pathways or by inhibiting deubiquitination signals. Interestingly, a recent study identified p38 mitogen-activated protein kinase-dependent nuclear–cytoplasmic shuttling of TEADs in response to cellular stress159. TEADs also have a highly conserved bipartite nuclear localization signal160. TEAD4 has a short splicing form, TEAD4-S - which retains the YAP interaction domain but lacks the DBD161 - that localizes in both the nucleus and the cytoplasm, and may function as a dominant negative. Given that expression of nuclear TEADs is increased and leads to poor prognosis across multiple cancers, blocking the nuclear entry of TEADs or promoting cytoplasmic shuttling and degradation could be a viable strategy that warrants further investigation.
Targeting core kinases in the pathway.
As discussed in this Review and elsewhere162, extensive studies have highlighted a key role of the Hippo pathway in tissue repair and regeneration. Specifically, LATS1/2 and MST1/2 inhibitors might be useful in the context of wound healing and regeneration. Recently, pharmacological modulation of MST1/2 kinase activity with XMU-MP-1 demonstrated significant improvement in tissue repair and regeneration; XMU-MP-1 was identified through a high-throughput screening effort as an ATP-competitive inhibitor of both MST1 and MST2, and improved repair and regeneration in multiple mouse models of both acute and chronic liver injury and colitis163. Importantly, to address safety concerns when targeting a tumour suppressor, the study showed that treating control mice with XMU-MP-1 daily for up to 2 months did not induce tumours. This could suggest that short-term inhibition of these kinases is a possible therapeutic approach, in contrast to what could be concluded by Stk3/4 knockout mouse phenotypes across multiple tissues, which cause hepatocellular carcinoma, colonic adenoma or disrupted lung structures and neonatal lethality 78,164,165. It is worth noting that unlike small molecules that target the kinase activity in adult mice and are reversible, and generally incomplete, genetic deletion in mouse models causes complete inactivation and is not reversible; thus, the latter likely produces stronger effects.
Recent studies identified a surprising, yet promising, role of LATS1/2 for immunotherapy approaches in Hippo-independent cancers60. This study has suggested the value of developing LATS1/2 kinase inhibitors to enhance cancer cell immunogenicity. From a drug discovery standpoint, targeting the pathway using kinase inhibitors is certainly more straightforward and promising than targeting transcription factors and protein-protein interactions. However, the key concern is the wealth of evidence that LATS1/2 kinases have important tumour suppressor roles in a range of tissues - as well as a potential function in suppressing fibrosis - making safety a key liability for such inhibitors. Future studies will address whether this strategy could be safe by enabling intermittent dosing of these compounds, what determines selectivity between normal and damaged tissues, and whether there is a therapeutic window in which these kinases can be inhibited to promote tissue repair without the long-term liability of promoting tumour growth. Undoubtedly, these studies provide valuable research reagents, starting points for medicinal chemistry efforts and, more importantly, proof of concept that targeting the upstream kinases in the Hippo pathway could provide a great path ahead in the field of repair and regeneration. Further chemical optimization of these inhibitors would be required to completely evaluate this strategy.
Future perspectives
Completion of The Cancer Genome Atlas has revealed frequent alterations of the Hippo pathway - such as activation of YAP/TAZ - in human cancer, thus suggesting the components of the Hippo pathway as targets for cancer treatment. Indeed, numerous companies are developing compounds targeting the Hippo pathway for cancer and other clinical indications. The most obvious molecular targets would be the YAP/TAZ transcription co-activators and the TEAD transcription factors, and the interaction between them. Verteporfin and VGLL4 mimetic peptides can inhibit YAP/TAZ-dependent transcription as well as suppress tumour growth in mouse models, thus validating the proof of concept of targeting this transcription module for cancer treatment. However, therapeutically targeting transcription factors or protein–protein interactions is traditionally challenging. Moreover, using systemic inhibition of YAP/TAZ warrants caution as it may produce severe side effects and toxicity. YAP/TAZ and TEAD might be appealing targets for treatment of fibrotic diseases, which are common pathological complications in the lung, heart, liver and kidney, although additional studies are required to prove these claims.
TEAD might represent a targetable transcription factor owing to its unique biochemical and structural properties. Palmitoylation seems to be important for TEAD function, protein stability and YAP interaction. Therefore, an exciting possibility is to inhibit TEAD palmitoylation to block the YAP–TEAD-dependent transcription. The palmitate moiety on TEAD is buried in a deep hydrophobic pocket inside the YBD of TEAD, which offers an attractive conformation to be inhibited by small molecules.
YAP/TAZ promotes wound healing and tissue regeneration, and, under physiological conditions, YAP/TAZ activity is restricted mainly by the LATS1/2 kinases. Protein kinases are readily druggable and the pharmaceutical industry has a plethora of successful experience in developing protein kinase inhibitor therapy. Therefore, LATS1/2 and their upstream activators MST1/2 kinases are attractive targets for wound healing and tissue regeneration. It is particularly noteworthy that high YAP activity can promote the proliferation of cardiomyocytes, which have low regeneration potential. Thus, LATS inhibitors might be valuable to promote cardiac regeneration. The concern of LATS1/2 inhibition in inducing cancer does exist; however, short-term inhibition for regeneration purposes may not cause tumour development, which could take a long time.
The scientific knowledge gained in the past decade from basic research on the emerging Hippo pathway has revealed the exciting potential of targeting the pathway components for various clinical indications. One major hurdle is that the YAP/TAZ transcription module is not readily druggable. Another key limiting factor is our incomplete understanding of the physiological functions of the Hippo pathway. However, with future advancement in fundamental biology of the Hippo pathway and new technologies, we anticipate with high enthusiasm that targeting the Hippo pathway will lead to fruitful therapeutic treatment.
Acknowledgements
K. L.G. is supported by grants from NIH (CA196878, CA217642, GM51586). X.V. is supported by grants from NIH (R01HL124392 and R21HD094012) and the American Cancer Society (130257-RSG-17-138-01-CSM). The authors acknowledge P. Cotelle for Fig. 3c,d, C. Noland for Fig. 3a and P. Calses for Fig. 4.
Footnotes
Competing interests
K.-L.G. is a co-founder and has an equity interest in Vivace Therapeutics, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. A.D. is an employee of Genentech and shareholder at Roche.
References
- 1.Jia J, Zhang W, Wang B, Trinko R & Jiang J The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17, 2514–2519 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Justice RW, Zilian O, Woods DF, Noll M & Bryant PJ The Drosophila tumor suppressor gene Warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Kango-Singh M et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Pantalacci S, Tapon N & Leopold P The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921–927 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Tapon N et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Udan RS, Kango-Singh M, Nolo R, Tao C & Halder G Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914–920 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Huang J, Dong J & Pan D hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with Salvador and Warts. Cell 114, 445–456 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Xu T, Wang W, Zhang S, Stewart RA & Yu W Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Meng Z, Moroishi T & Guan KL Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont S et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Zanconato F, Cordenonsi M & Piccolo S YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Vega F et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell 173, 321–337.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan R, Kim NG & Gumbiner BM Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl Acad. Sci. USA 110, 2569–2574 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Escudero R et al. Overexpression of PIK3CA in head and neck squamous cell carcinoma is associated with poor outcome and activation of the YAP pathway. Oral. Oncol. 79, 55–63 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Lamar JM et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl Acad. Sci. USA 109, E2441–2450 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CK et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 363, 644–649 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Yang CS et al. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 19, e43577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi AB et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl Acad. Sci. USA 92, 10854–10858 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami H et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 71, 873–883 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Feng X et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 25, 831–845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu FX et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25, 822–830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanas MR et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci. Transl. Med. 3, 98ra82 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Tanas MR et al. Mechanism of action of a WWTR1(TAZ)–CAMTA1 fusion oncoprotein. Oncogene 35, 929–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonescu CR et al. Novel YAP1–TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 52, 775–784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhanasekaran SM et al. Transcriptome metaanalysis of lung cancer reveals recurrent aberrations in NRG1 and Hippo pathway genes. Nat. Commun. 5, 5893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gujral TS & Kirschner MW Hippo pathway mediates resistance to cytotoxic drugs. Proc. Natl Acad. Sci. USA 114, E3729–E3738 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiso E et al. YAP-dependent AXL overexpression mediates resistance to EGFR inhibitors in NSCLC. Neoplasia 19, 1012–1021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JE et al. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem. Biophys. Res. Commun. 474, 154–160 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Kapoor A et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 179, 1239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao DD et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171–184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin L et al. The Hippo effector YAP promotes resistance to RAF-and MEK-targeted cancer therapies. Nat. Genet. 47, 250–256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson FH et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 27, 397–408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen CDK & Yi C YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer 5, 283–296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calses PC, Crawford JJ, Lill JR & Dey A Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends Cancer 5, 297–307 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Nehme NT et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119, 3458–3468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdollahpour H et al. The phenotype of human STK4 deficiency. Blood 119, 3450–3457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du X et al. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J. Immunol. 192, 1525–1535 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Tang F et al. The kinases NDR1/2 act downstream of the Hippo homolog MST1 to mediate both egress of thymocytes from the thymus and lymphocyte motility. Sci. Signal. 8, ra100 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Dang TS et al. Defective leukocyte adhesion and chemotaxis contributes to combined immunodeficiency in humans with autosomal recessive MST1 deficiency. J. Clin. Immunol. 36, 117–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X et al. Mst1 kinase regulates the actin-bundling protein L-plastin to promote T cell migration. J. Immunol. 197, 1683–1691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du X et al. Hippo/Mst signalling couples metabolic state and immune function of CD8α+ dendritic cells. Nature 558, 141–145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C et al. Dendritic cell MST1 inhibits TH17 differentiation. Nat. Commun. 8, 14275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katagiri K, Imamura M & Kinashi T Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol. 7, 919–928 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Zhou D et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc. Natl Acad. Sci. USA 105, 20321–20326 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mou F et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J. Exp. Med. 209, 741–759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng J et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat. Immunol. 18, 800–812 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Stampouloglou E et al. Yap suppresses T-cell function and infiltration in the tumor microenvironment. PLoS Biol. 18, e3000591 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni X et al. YAP is essential for Treg-mediated suppression of antitumor immunity. Cancer Discov. 8, 1026–1043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaventhiran JE et al. Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell. Proc. Natl Acad. Sci. USA 109, E2223–E2229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S et al. YAP antagonizes innate antiviral immunity and is targeted for lysosomal degradation through IKKε-mediated phosphorylation. Nat. Immunol. 18, 733–743 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q et al. Hippo signalling governs cytosolic nucleic acid sensing through YAP/TAZ-mediated TBK1 blockade. Nat. Cell Biol. 19, 362–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo X et al. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 31, 247–259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang G et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 6, 80–95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee BS et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem. Biophys. Res. Commun. 491, 493–499 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Janse van Rensburg HJ et al. The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. 78, 1457–1470 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Kim MH et al. YAP-induced PD-L1 expression drives immune evasion in BRAFi-resistant melanoma. Cancer Immunol. Res. 6, 255–266 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Feng J et al. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 36, 5829–5839 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Miao J et al. YAP regulates PD-L1 expression in human NSCLC cells. Oncotarget 8, 114576–114587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramjee V et al. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J. Clin. Invest. 127, 899–911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moroishi T et al. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell 167, 1525–1539.e17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karpowicz P, Perez J & Perrimon N The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137, 4135–4145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaw RL et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137, 4147–4158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staley BK & Irvine KD Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr. Biol. 20, 1580–1587 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai J et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383–2388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barry ER et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493, 106–110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y & Wrana JL Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Yui S et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22, 35–49.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azzolin L et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Serra D et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 569, 66–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ayyaz A et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Grijalva JL et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G196–G204 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Wang C et al. Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol. Med. Rep. 5, 410–414 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Lu L, Finegold MJ & Johnson RL Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp. Mol. Med. 50, e423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bai H et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 56, 1097–1107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camargo FD et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Lu L et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl Acad. Sci. USA 107, 1437–1442 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varelas X et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev. Cell 18, 579–591 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Zhou D et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benhamouche S et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 24, 1718–1730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang N et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yimlamai D et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yovchev M et al. Experimental model for successful liver cell therapy by Lenti TTR-YapERT2 transduced hepatocytes with tamoxifen control of Yap subcellular location. Sci. Rep. 6, 19275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loforese G et al. Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol. Med. 9, 46–60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lodge EJ et al. Homeostatic and tumourigenic activity of SOX2+ pituitary stem cells is controlled by the LATS/YAP/TAZ cascade. eLife 10.7554/eLife.43996 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hogan BL et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Z et al. MAPK-mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep. 16, 1810–1819 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Sun T et al. TAZ is required for lung alveolar epithelial cell differentiation after injury. JCI Insight 10.1172/jci.insight.128674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LaCanna R et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Invest. 130, 2107–2122 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szymaniak AD, Mahoney JE, Cardoso WV & Varelas X Crumbs3-mediated polarity directs airway epithelial cell fate through the Hippo pathway effector Yap. Dev. Cell 34, 283–296 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahoney JE, Mori M, Szymaniak AD, Varelas X & Cardoso WV The Hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 30, 137–150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao R et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell 30, 151–165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elbediwy A et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 143, 1674–1687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schlegelmilch K et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silvis MR et al. α-Catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 4, ra33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beverdam A et al. Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J. Invest. Dermatol. 133, 1497–1505 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Lee MJ, Byun MR, Furutani-Seiki M, Hong JH & Jung HS YAP and TAZ regulate skin wound healing. J. Invest. Dermatol. 134, 518–525 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Zhang H, Pasolli HA & Fuchs E Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl Acad. Sci. USA 108, 2270–2275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Gise A et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl Acad. Sci. USA 109, 2394–2399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xin M et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. USA 110, 13839–13844 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin Z et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 115, 354–363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ito M et al. Characterization of a small molecule that promotes cell cycle activation of human induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell Cardiol. 128, 90–95 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Hara H et al. Discovery of a small molecule to increase cardiomyocytes and protect the heart after ischemic injury. JACC Basic. Transl. Sci. 3, 639–653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bassat E et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morikawa Y et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci. Signal. 8, ra41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vite A, Zhang C, Yi R, Emms S & Radice GL α-Catenin-dependent cytoskeletal tension controls Yap activity in the heart. Development 145, dev149823 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nowell CS et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 18, 168–180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mindos T et al. Merlin controls the repair capacity of Schwann cells after injury by regulating Hippo/YAP activity. J. Cell Biol. 216, 495–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao K et al. Muscle Yap is a regulator of neuromuscular junction formation and regeneration. J. Neurosci. 37, 3465–3477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng Y et al. Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep. 14, 2224–2237 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Hu JK et al. An FAK–YAP–mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell 21, 91–106.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayashi S, Tamura K & Yokoyama H Yap1, transcription regulator in the Hippo signaling pathway, is required for Xenopus limb bud regeneration. Dev. Biol. 388, 57–67 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Hayashi S et al. Transcriptional regulators in the Hippo signaling pathway control organ growth in Xenopus tadpole tail regeneration. Dev. Biol. 396, 31–41 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Mateus R et al. Control of tissue growth by Yap relies on cell density and F-actin in zebrafish fin regeneration. Development 142, 2752–2763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin AY & Pearson BJ Planarian yorkie/YAP functions to integrate adult stem cell proliferation, organ homeostasis and maintenance of axial patterning. Development 141, 1197–1208 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Caliari SR et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci. Rep. 6, 21387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mannaerts I et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 63, 679–688 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Szeto SG et al. YAP/TAZ are mechanoregulators of TGF-β–Smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 27, 3117–3128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu F et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol 308, L344–L357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piersma B et al. YAP1 Is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am. J. Pathol. 185, 3326–3337 (2015). [DOI] [PubMed] [Google Scholar]
- 120.Zhao B et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aikawa T, Gunn J, Spong SM, Klaus SJ & Korc M Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol. Cancer Ther. 5, 1108–1116 (2006). [DOI] [PubMed] [Google Scholar]
- 122.Huang WT, Vayalil PK, Miyata T, Hagood J & Liu RM Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am. J. Respir. Cell Mol. Biol. 46, 87–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ledwozyw A The effect of β-aminopropionitrile on bleomycin-induced lung injury in rats. Acta Physiol. Hung. 83, 91–99 (1995). [PubMed] [Google Scholar]
- 124.Barry-Hamilton V et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Gokey JJ et al. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight 10.1172/jci.insight.98738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Machado MV et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J. Hepatol. 63, 962–970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen P et al. Pathogenesis of non-alcoholic fatty liver disease mediated by YAP. Hepatol. Int. 12, 26–36 (2018). [DOI] [PubMed] [Google Scholar]
- 128.Wang X et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 24, 848–862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liang M et al. Yap/Taz deletion in Gli+ cell-derived myofibroblasts attenuates fibrosis. J. Am. Soc. Nephrol. 28, 3278–3290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kramann R et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitani A et al. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am. J. Respir. Crit. Care Med. 180, 326–338 (2009). [DOI] [PubMed] [Google Scholar]
- 132.Toyama T et al. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J. Invest. Dermatol. 138, 78–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Varelas X et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10, 837–848 (2008). [DOI] [PubMed] [Google Scholar]
- 134.Hiemer SE, Szymaniak AD & Varelas X The transcriptional regulators TAZ and YAP direct transforming growth factor β-induced tumorigenic phenotypes in breast cancer cells. J. Biol. Chem. 289, 13461–13474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chan MW, Hinz B & McCulloch CA Mechanical induction of gene expression in connective tissue cells. Methods Cell Biol. 98, 178–205 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Haak AJ et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci. Transl. Med. 11, eaau6296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gill MK et al. A feed forward loop enforces YAP/TAZ signaling during tumorigenesis. Nat. Commun. 9, 3510 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lagares D et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med. 9, eaal3765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu-Chittenden Y et al. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dasari VR et al. Verteporfin exhibits YAP-independent anti-proliferative and cytotoxic effects in endometrial cancer cells. Oncotarget 8, 28628–28640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang C et al. Verteporfin inhibits YAP function through up-regulating 14–3-3σ sequestering YAP in the cytoplasm. Am. J. Cancer Res. 6, 27–37 (2016). [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang H et al. Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci. Signal. 8, ra98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Song S et al. A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol. Cancer Ther. 17, 443–454 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Basu D et al. Identification, mechanism of action, and antitumor activity of a small molecule inhibitor of Hippo, TGF-β, and Wnt signaling pathways. Mol. Cancer Ther. 13, 1457–1467 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Smith SA et al. Antiproliferative and antimigratory effects of a novel YAP–TEAD interaction inhibitor identified using in silico molecular docking. J. Med. Chem. 62, 1291–1305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kurppa KJ et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell 37, 104–122.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Santucci M et al. The Hippo pathway and YAP/TAZ-TEAD protein–protein interaction as targets for regenerative medicine and cancer treatment. J. Med. Chem. 58, 4857–4873 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Gibault F, Sturbaut M, Bailly F, Melnyk P & Cotelle P Targeting transcriptional enhanced associate domains (TEADs). J. Med. Chem. 61, 5057–5072 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Noland CL et al. Palmitoylation of TEAD transcription factors is required for their stability and function in Hippo pathway signaling. Structure 24, 179–186 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Chan P et al. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat. Chem. Biol. 12, 282–289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pobbati AV et al. Targeting the central pocket in human transcription factor TEAD as a potential cancer therapeutic strategy. Structure 23, 2076–2086 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bum-Erdene K et al. Small-molecule covalent modification of conserved cysteine leads to allosteric inhibition of the TEAD·Yap protein–protein interaction. Cell Chem. Biol. 26, 378–389.e13 (2019). [DOI] [PubMed] [Google Scholar]
- 153.Maeda T, Chapman DL & Stewart AF Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J. Biol. Chem. 277, 48889–48898 (2002). [DOI] [PubMed] [Google Scholar]
- 154.Pobbati AV, Chan SW, Lee I, Song H & Hong W Structural and functional similarity between the VGLL1–TEAD and the YAP–TEAD complexes. Structure 20, 1135–1140 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Vaudin P, Delanoue R, Davidson I, Silber J & Zider A TONDU (TDU), a novel human protein related to the product of vestigial (Vg) gene of Drosophila melanogaster interacts with vertebrate TEF factors and substitutes for Vg function in wing formation. Development 126, 4807–4816 (1999). [DOI] [PubMed] [Google Scholar]
- 156.Jiao S et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25, 166–180 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Zhao B et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Noto A et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene 36, 4573–4584 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Lin KC et al. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat. Cell Biol. 19, 996–1002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Magico AC & Bell JB Identification of a classical bipartite nuclear localization signal in the Drosophila TEA/ATTS protein scalloped. PLoS ONE 6, e21431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qi Y et al. A splicing isoform of TEAD4 attenuates the Hippo–YAP signalling to inhibit tumour proliferation. Nat. Commun. 7, ncomms11840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fu V, Plouffe SW & Guan KL The Hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 49, 99–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fan F et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med. 8, 352ra108 (2016). [DOI] [PubMed] [Google Scholar]
- 164.Zhou D et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl Acad. Sci. USA 108, E1312–E1320 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin C, Yao E & Chuang PT A conserved MST1/2–YAP axis mediates Hippo signaling during lung growth. Dev. Biol. 403, 101–113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takahashi Y et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin. Cancer Res. 11, 1380–1385 (2005). [DOI] [PubMed] [Google Scholar]
- 167.Cordenonsi M et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011). [DOI] [PubMed] [Google Scholar]
- 168.Diaz-Martin J et al. Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer. Endocr. Relat. Cancer 22, 443–454 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Chan SW et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 68, 2592–2598 (2008). [DOI] [PubMed] [Google Scholar]
- 170.Cancer Genome Atlas Research Network. et al. Integrated genomic and molecular characterization of cervical cancer. Nature 543, 378–384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu T et al. Clinical significance of Yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int. J. Gynecol. Cancer 23, 735–742 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Deng J et al. LATS1 suppresses proliferation and invasion of cervical cancer. Mol. Med. Rep. 15, 1654–1660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.He C et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol. Med. 7, 1426–1449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Y et al. Comprehensive molecular characterization of the Hippo signaling pathway in cancer. Cell Rep. 25, 1304–1317.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Y, Xie C, Li Q, Xu K & Wang E Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biol. 34, 2169–2174 (2013). [DOI] [PubMed] [Google Scholar]
- 176.Lee KW et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin. Cancer Res. 21, 357–364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Imajo M, Ebisuya M & Nishida E Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 17, 7–19 (2015). [DOI] [PubMed] [Google Scholar]
- 178.Cancer Genome Atlas Research Network. et al. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muramatsu T et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis 32, 389–398 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Song S et al. The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin. Cancer Res. 21, 2580–2590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song S et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 74, 4170–4182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ge L et al. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PLoS ONE 6, e27529 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eun YG et al. Clinical significance of YAP1 activation in head and neck squamous cell carcinoma. Oncotarget 8, 111130–111143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hiemer SE et al. A YAP/TAZ-regulated molecular signature is associated with oral squamous cell carcinoma. Mol. Cancer Res. 13, 959–968 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zender L et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Han SX et al. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J. Immunol. Res. 2014, 261365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Song H et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl Acad. Sci. USA 107, 1431–1436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee KP et al. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl Acad. Sci. USA 107, 8248–8253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fitamant J et al. YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep. 10, 1692–1707 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bueno R et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 48, 407–416 (2016). [DOI] [PubMed] [Google Scholar]
- 191.Hmeljak J et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 8, 1548–1565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sekido Y Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol. Int. 61, 331–344 (2011). [DOI] [PubMed] [Google Scholar]
- 193.Mizuno T et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene 31, 5117–5122 (2012). [DOI] [PubMed] [Google Scholar]
- 194.Tanahashi K et al. Activation of Yes-associated protein in low-grade meningiomas is regulated by merlin, cell density, and extracellular matrix stiffness. J. Neuropathol. Exp. Neurol. 74, 704–709 (2015). [DOI] [PubMed] [Google Scholar]
- 195.Baia GS et al. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol. Cancer Res. 10, 904–913 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schramm A et al. Mutational dynamics between primary and relapse neuroblastomas. Nat. Genet. 47, 872–877 (2015). [DOI] [PubMed] [Google Scholar]
- 197.Wang M et al. Transcriptional co-activator TAZ sustains proliferation and tumorigenicity of neuroblastoma by targeting CTGF and PDGF-β. Oncotarget 6, 9517–9530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seong BK et al. A metastatic mouse model identifies genes that regulate neuroblastoma metastasis. Cancer Res. 77, 696–706 (2017). [DOI] [PubMed] [Google Scholar]
- 199.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Malik SA, Khan MS, Dar M, Hussain MU & Mudassar S TAZ is an independent prognostic factor in non-small cell lung carcinoma: elucidation at protein level. Cancer Biomark. 18, 389–395 (2017). [DOI] [PubMed] [Google Scholar]
- 201.Xie M et al. Prognostic significance of TAZ expression in resected non-small cell lung cancer. J. Thorac. Oncol. 7, 799–807 (2012). [DOI] [PubMed] [Google Scholar]
- 202.Wang H et al. Tankyrase inhibitor sensitizes lung cancer cells to endothelial growth factor receptor (EGFR) inhibition via stabilizing angiomotins and inhibiting YAP signaling. J. Biol. Chem. 291, 15256–15266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Berger AC et al. A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell 33, 690–705.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jeong W et al. Activation of YAP1 is associated with poor prognosis and response to taxanes in ovarian cancer. Anticancer Res. 34, 811–817 (2014). [PMC free article] [PubMed] [Google Scholar]
- 205.Xia Y et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS ONE 9, e91770 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang X et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 30, 2810–2822 (2011). [DOI] [PubMed] [Google Scholar]
- 207.Hua G et al. YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells. Oncogene 35, 2247–2265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Merritt NM et al. A comprehensive evaluation of Hippo pathway silencing in sarcomas. Oncotarget 9, 31620–31636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Eisinger-Mathason TS et al. Deregulation of the Hippo pathway in soft-tissue sarcoma promotes FOXM1 expression and tumorigenesis. Proc. Natl Acad. Sci. USA 112, E3402–E3411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang Z et al. Structure-based design and synthesis of potent cyclic peptides inhibiting the YAP–TEAD protein–protein interaction. ACS Med. Chem. Lett. 5, 993–998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Konradi AW, Lin TT-LT Benzosulfonyl compoundshttps://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019040380 (2018).