Extracellular Vesicles and Their Roles in Cancer Progression (original) (raw)
. Author manuscript; available in PMC: 2021 Mar 30.
Abstract
Extracellular vesicles (EVs) produced by cancer cells function as a unique form of intercellular communication that can promote cell growth and survival, help shape the tumor microenvironment, and increase invasive and metastatic activity. There are two major classes of EVs, microvesicles (MVs) and exosomes, and they differ in how they are formed. MVs are generated by the outward budding and fission of the plasma membrane. On the other hand, exosomes are derived as multivesicular bodies (MVBs) fuse with the plasma membrane and release their contents. What makes EVs especially interesting is how they mediate their effects. Both MVs and exosomes have been shown to contain a wide-variety of bioactive cargo, including cell surface, cytosolic, and nuclear proteins, as well as RNA transcripts, micro-RNAs (miRNAs), and even fragments of DNA. EVs, and their associated cargo, can be transferred to other cancer cells, as well as to normal cell types, causing the recipient cells to undergo phenotypic changes that promote different aspects of cancer progression. These findings, combined with those demonstrating that the amounts and contents of EVs produced by cancer cells can vary depending on their cell of origin, stage of development, or response to therapies, have raised the exciting possibility that EVs can be used for diagnostic purposes. Moreover, the pharmaceutical community is aggressively pursuing the use of EVs as a potential drug delivery platform. Here, in this chapter, we will highlight what is currently known about how EVs are generated, how they impact cancer progression, and the different ways they are being exploited for clinical applications.
Keywords: Microvesicles, Extracellular vesicles, Intercellular communication, Exosomes, Tumor microenvironment, Multivesicular bodies, Therapy deliver system
1. EVs: A Brief Introduction and History
Cells respond in specific ways to signals that they receive from their environment. The classic and perhaps still the best-known mechanism is the growth factor signaling paradigm. Soluble factors released by cells, such as epidermal growth factor (EGF), can bind to their cognate receptors, that is, the EGF receptor, expressed on the surfaces of other cells, resulting in their activation. These receptors then stimulate the activation of several intracellular signaling pathways that ultimately induce changes in gene expression. In the case of the EGF receptor, these changes in gene expression typically result in increased cell growth, survival, and migration [1]. In addition to soluble factors, cells can also communicate with other cells through the formation and release of multiple types of non-classical secretory vesicles, generally referred to as EVs. There are two major classes of EVs, MVs and exosomes, and they are produced via distinct mechanisms [2–5]. MVs are formed as the result of the direct outward budding and fission of the plasma membrane, while exosomes are produced as some of the MVBs containing intraluminal vesicles (ILVs) in the endolysosomal degradation pathway which are trafficked to the cell surface. The MVBs then fuse with the plasma membrane and release their contents, now termed exosomes, into the extracellular environment. Both classes of EVs have been shown to contain various types of proteins including cell surface receptors, cytosolic signaling proteins, transcriptions factors, metabolic enzymes, extracellular matrix proteins, and RNA binding proteins. In addition to proteins, EVs also contain RNA transcripts, microRNAs (miRNAs), and fragments of genomic DNA [6].
The first published manuscript describing EVs dates back to a study carried out by the British scientist Peter Wolf in 1967. The main focus of this study was to understand the process of blood coagulation. In one set of the experiments, blood samples were ultracentrifuged and a fraction containing lipid-rich particles with coagulant properties was isolated and referred to as “platelet dust” [7]. By most accounts, platelet dust is considered likely to be the first identification of EVs, although the study lacked any information regarding how they were formed or the exact nature of their contents. It was not until more than 15 years later that two independent studies, one by Harding et al., and the other by Pan et al., directly characterized at least one of the major classes of EVs, namely exosomes [8, 9]. In these studies, the transferrin receptor was shown to associate with vesicles of about 50–120 nm in diameter that were being actively secreted by reticulocytes as they were undergoing their maturation into red blood cells [8, 9]. Using electron microscopy (EM), Harding et al. captured images of maturing reticulocytes that showed these transferrin receptor-positive vesicles originated from the fusion of MVBs containing ILVs with the plasma membrane. The ILVs released into the extracellular space were coined “exosomes” [8], a term that is still used today to describe EVs that are derived from the endolysosomal pathway. These landmark studies represented some of the earliest findings describing important mechanistic insights into how at least one class of EVs (i.e., exosomes) was formed and released by cells.
Determining why cells generate EVs has been another primary focus of research in the EV field. One of the most influential studies showing how important a role EVs play in promoting cancer progression was performed in 2008 by Al-Nedawi et al. [10]. They discovered that highly aggressive forms of brain tumor cells produced large quantities of EVs. When these vesicles were isolated and used to treat other brain cancer cells, the recipient cells became more aggressive and grew faster, compared to untreated control cells [10, 11], highlighting for the first time that cancer cells generate EVs to communicate with other cancer cells.
The findings mentioned above, in many ways, laid the groundwork for what we know today as the EV field. There have been several subsequent studies which have helped further determine the mechanisms that regulate the biogenesis of MVs and exosomes. They have also led to many remarkable advances in our understanding of EVs and how they mediate an ever-increasing number of physiological and pathological processes, especially cancer. This has resulted in cancer researchers and pharmaceutical companies devoting a substantial amount of time and resources to determine how this form of intercellular communication impacts different aspects of cancer progression, with the hope that this information will potentially lead to new approaches to treat the disease. There is also a growing momentum toward using EVs as diagnostic and prognostic indicators of cancer, as well as a therapy deliver system. Each of these topics will be discussed.
2. EV Isolation and Classification
There are two major classes of EVs and they can be distinguished from one another based on how they are formed (i.e., their biogenesis) and their relative sizes. One of these classes of EVs is most commonly referred to as MVs, but they are also sometimes given other names, including large EVs, shedding vesicles, and when specifically generated by cancer cells, oncosomes. MVs typically range in size from approximately 200 to 800 nm in diameter [2], but MVs as large as 8.0–10 μm have been reported [12, 13]. The formation of this type of vesicle is initiated as discrete microdomains along the plasma membrane enriched in lipid rafts, cholesterol, and phosphatidylserine begin to extend, or bud, outward [3, 5]. MVs then continue to protrude from the surface of the cell until the neck of the vesicle becomes small enough to undergo a fission event, which results in the release of the MV from the cell. The second major class of EVs is exosomes. These 30–150 nm vesicles are generated as MVBs in the endolysosomal degradation pathway undergo a maturation process where the limiting membranes of the MVBs go through a series of invagination and budding events, resulting in the formation of smaller vesicles, referred to as ILVs, in the lumen of the MVB. While most MVBs are trafficked to the lysosome and degraded, some of them escape this fate and are rerouted to the cell surface. At this point, the MVBs fuse with the plasma membrane and release exosomes into the extracellular space. This class of EVs is also sometimes referred to as small EVs or microparticles. It is also worth mentioning here that the lack of a standardized nomenclature in the EV field has often caused some confusion and even has cast doubt as to the validity of the entire EV field. This confusion and doubt is sometimes confounded by the fact that there are several examples in the literature where a specific class of EV is said to have been isolated and studied, but is referred to by the wrong name. Thus, efforts such as the one put forth by the International Society of Extracellular Vesicles (ISEV) to standardize EV nomenclature and isolation procedures are much needed [14].
EVs are present in a wide-variety of biological fluids, ranging from blood and cerebral fluid to the conditioned medium collected from cultures of cells. To isolate the different classes of EVs from such biological fluids, two analogous approaches that take advantage of filtration and/or differential centrifugation are commonly used (Fig. 1, left side). To describe how these approaches work, we will use the conditioned medium collected from cells as the starting material. The medium is first centrifuged at a low speed (300 × g) to remove floating cells and debris. The supernatant is then subjected to filtration using a membrane with a 220 nm pore size, where EVs larger than 220 nm in diameter are retained by the membrane and can be resuspended using an appropriate buffer or solution. Alternatively, the partially clarified medium can be centrifuged at 10,000 × g. The EVs retained by the filter, or pelleted following centrifugation, are considered to be the MV fraction. The filtered conditioned medium, or the resulting supernatant from the 10,000 × g spin, is then subjected to ultracentrifugation at 120,000 × g to pellet the exosomes.
Fig 1.
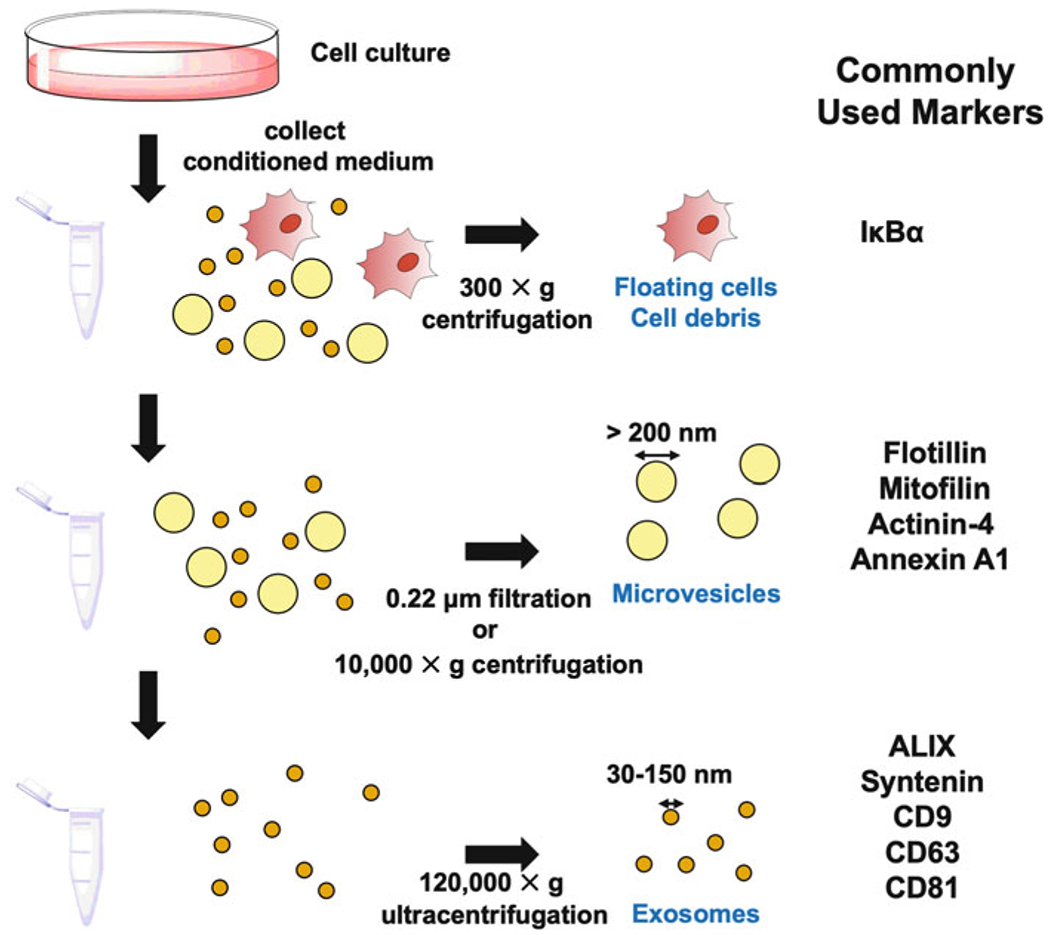
Common EV isolation approaches and markers. Biological liquids, that is, the conditioned medium collected from cells in culture are subjected to a low-speed centrifugation (300 × g) to remove any intact floating cells and cell debris (top panel). Microvesicles can be purified by either filtering the conditioned medium through a 0.22 μm pore size filter, or by centrifugation at 10,000 × g (middle panel). The flow through or supernatant is then subjected to ultracentrifugation (120,000 × g) to pellet exosomes (bottom panel). The cells, microvesicles, and exosomes can be probed for the proteins listed under Commonly Used Markers
It is critical to demonstrate that the different EV fractions collected are indeed enriched with either exosomes or MVs, and this is often achieved through the detection of specific marker proteins that are associated with EVs (Fig. 1, right side). For example, apoptosis-linked gene 2-interacting protein X (ALIX), syntenin-1, CD9, CD63, and CD81 are considered exosomal markers, while mitofilin, actinin-4, annexin A1, and flotillin have all been used to identify MVs [6, 15]. There are also proteins that have been shown to be enriched in the EVs derived from various types of cancer or transformed cells. Two good examples include the immune regulator, programmed cell death ligand 1 (PD-L1), and the nonreceptor tyrosine kinase, focal adhesion kinase (FAK). Specifically, PD-L1 can be readily detected in the exosomes derived from patients with highly aggressive forms of melanoma and brain tumors but not in the exosomes from healthy individuals [16–19]. Similarly, we showed that FAK was highly enriched in the MVs produced by fibroblasts ectopically expressing an oncogenic form of diffuse B-cell lymphoma (Dbl) [20], suggesting that the incorporation of cargo into EVs is not random, but rather the result of a selective process.
While the conventional approaches used to isolate EVs have provided valuable insights into their contents and function, it is also becoming increasingly clear that the MV and exosome fractions do not contain a homogeneous population of vesicles. Rather, it is likely that these fractions contain multiple subclasses of exosomes or MVs, as well as other biological material that copurifies with EVs. For example, in one study, the exosomal fractions that were initially isolated from cancer cell conditioned medium using differential centrifugation were then further resolved based on their densities and hydrodynamic properties using asymmetric-flow field-flow fractionation (AF4). Analysis of the resulting fractions revealed the existence of not only two different sized exosomes, that is, large and small exosomes, but also a vesicle-like structure with a diameter smaller than 40 nm, which was referred to as exomeres [21]. Moreover, experiments were recently performed where the exosomes produced by cells were collected and then subjected to iodixanol gradient ultracentrifugation. The results of these experiments showed that classical exosomes could be resolved from a unique “non-vesicle” fraction that lacked membrane-enclosed structures, and could not be disrupted by the addition of detergent [6, 21]. Conventional MV preparations may similarly contain other types of vesicles. For example, it has been suggested that MV preparations may also contain apoptotic bodies that form as dying cells bleb [3, 6, 14, 15, 21].
3. Mechanisms of EV Formation and Release
3.1. MV Biogenesis
MVs are generated through the direct budding and fission of the plasma membrane (Fig. 2). Most types of cells are capable of forming and releasing MVs, but highly aggressive forms of cancer cells tend to produce more MVs, compared to lower grade cancer cells, or their respective normal cell counterparts [22]. Certain signaling proteins that are known to contribute to cancer progression can stimulate the production of MVs. Such is the case for a mutant form of the EGF receptor, referred to as the EGF receptor variant type III (EGFRvIII). Analysis of glioma cells ectopically expressing EGFRvIII by transmission electron microscopy (TEM) showed that significantly more MVs could be detected budding from their surfaces, compared to the parental cell line [10, 11]. These results were consistent with other findings that showed that treating different cancer cell lines, such as HeLa cervical carcinoma cells, with EGF led to increased MV formation and release [22, 23]. Our group then went on to discover the signaling pathway that was responsible for mediating this effect. Specifically, we showed that EGF-stimulation of cells resulted in the sequential activation of the small GTP binding protein RHOA, Rho-associated coiled coil containing kinase (ROCK), and Lim kinase (LIMK) [24]. LIMK then, in turn, phosphorylated cofilin and inactivated its filamentous (F)-actin severing activity, allowing for the assembly and buildup of actin filaments that was necessary for the budding of MVs from the surfaces of cells [24, 25].
Fig 2.
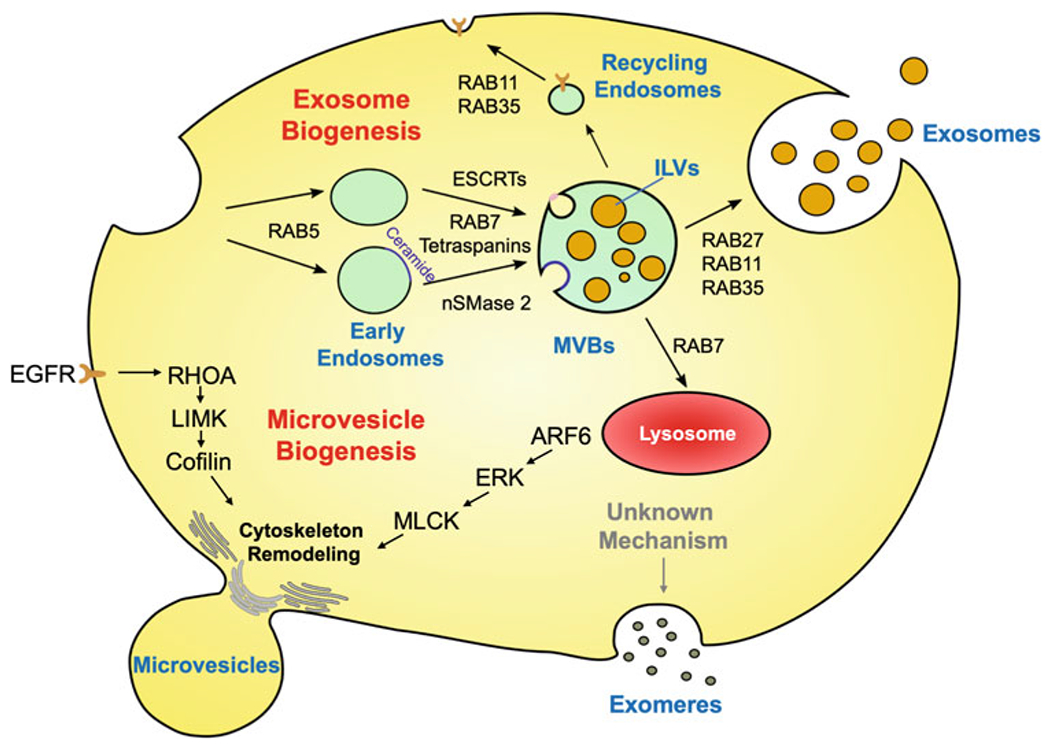
Scheme depicting the biogenesis of different classes of EVs. Exosomes originate from the endolysosomal pathway. The plasma membrane invaginates to form early endosomes in a RAB5-dependent manner. The endosomes are further trafficked and processed by ESCRTs, RAB7, tetraspanins, and nSMase 2 to generate multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). The MVBs are either trafficked by RAB7 to lysosomes and degraded, or directed to the plasma membrane by RAB27, RAB11, or RAB35. The MVBs then fuse with the plasma membrane and their contents are released. Microvesicles are formed as an outcome of an outward budding and fission from the plasma membrane. This process is mediated by RHOA or ARF6 signaling events that regulate cytoskeletal remodeling. Exomeres and nonvesicle fractions are considered as unique forms of non-membrane enclosed structures that are copurified with exosomes. The mechanisms underlying their formation are still unknown
Another signaling protein that was identified as a critical regulator of MV biogenesis, and specifically for MV release, is ADP-ribosylation factor 6 (ARF6). ARF6 is another small GTP binding protein, and member of the RAS superfamily. ARF6 is best known for its ability to mediate cytoskeletal remodeling and plasma membrane and intracellular vesicle trafficking. ARF6 activity is frequently found to be elevated in invasive/aggressive forms of melanoma and breast cancer cells, and inhibiting ARF6 activities in many of these same cells blocks their aggressive phenotypes [26–28]. It was shown that cancer cells ectopically expressing an activated form of ARF6, ARF6Q67L, generated at least three fold more MVs, compared to control cells [29]. This effect was found to be due to the ability of ARF6Q67L to strongly activate the extracellular signal regulated kinase (ERK), a member of the MAP kinase family. Treating cells expressing ARF6Q67L with U0126 to inhibit ERK activity, resulted in the formation of large MVs along the plasma membrane of the cells. This finding suggests that ARF6, working through ERK, regulates the fission or pinching off of MVs that are budding from the surfaces of cells. Indeed, it was subsequently shown that ERK activity promoted MV release via regulation of myosin light chain kinase (MLCK) and myosin light chain (MLC) phosphorylation. The activation of this pathway causes contraction of the cytoskeleton at the neck of MVs, resulting in their fission [29].
In addition to actin-cytoskeletal remodeling, the lipid composition of the plasma membrane also plays an important role in MV formation and release [30]. Normally, the lipid-bilayer that makes up the surfaces of cells contains a variety of lipids, with at least some of them being differentially distributed between the inner and outer leaflets of the plasma membrane. For example, aminophospholipids are highly enriched in the inner leaflet, while the outer leaflet contains more phospholipids. The asymmetric distribution of different lipids in the plasma membrane are typically maintained by lipid translocases. However, under conditions where cytosolic calcium levels are increased, lipid translocases become inhibited, and lipid scramblases are activated. This results in the loss of lipid asymmetry and promotes the curvature of the plasma membrane that is required for MV budding [5, 31, 32].
Several additional cellular processes are known to influence the biogenesis of MVs in cancer cells. Among the most important of these factors is the metabolic reprogramming that cancer and transformed cells undergo. Cancer cells are frequently characterized by increased glucose consumption. However, their glycolytic pathway is uncoupled from the tricarboxylic acid (TCA) cycle and energy production [33]. Thus, cancer cells need to compensate for this limitation by using alternative mechanisms to input into the TCA cycle. One of the most common of these mechanisms is by increasing glutamine metabolism [34]. The first step in this process is converting glutamine to glutamate by the mitochondrial enzyme glutaminase. Interestingly, it was discovered that inhibiting glutaminase activity in MDA-MB-231 breast cancer cells using 968, or BPTES, strongly inhibited their ability to form and release MVs [35]. Yet another factor that impacts the generation of MVs is the glycosaminoglycan hyaluronic acid (HA). In a study carried out by Rilla et al., Madin-Darby Canine Kidney (MDCK) cells ectopically expressing hyaluronan synthase 3 (HAS3) were engineered to generate a cell line that expressed large amounts of HA. One of the unique characteristics of these cells was their ability to form more MVs [36].
It is clear, and somewhat expected, that signaling mechanisms that control actin-cytoskeletal and lipid remodeling are important regulators of MV formation and release. However, many important questions regarding how cells generate MV remain to be answered. Among these questions are the following: How does the altered metabolism of cancer cells promote MV formation? Are there additional signaling proteins and cellular processes that are essential for generating MVs? Finally, can strategies to block MV formation and/or release be developed and used as a potential treatment for cancer?
3.2. Exosome Biogenesis
Exosomes originate from MVBs in the endolysosomal pathway (Fig. 2). The mechanisms underlying MVB formation are well-established and involve a family of proteins, referred to as endosomal sorting complex required for transport (ESCRT) proteins [37, 38]. The ESCRTs are further divided into four classes: ESCRT-0, -I, -II, and -III, with each member of this family playing a distinct role in promoting MVB formation. ESCRT-0, -I, and -II work together to recruit ubiquitinated proteins to the surfaces of MVBs, at which point ESCRT-III binds to ESCRT-II to promote membrane invagination and fission, forming ILVs [4, 5, 37, 38]. The MVBs containing ubiquitinated cargo are then typically transported to the lysosomes, where they fuse with this organelle and their contents are degraded. However, some MVBs are not trafficked to the lysosome but are instead redirected to the cell surface. These MVBs fuse with the plasma membrane and release their ILVs, now termed exosomes, into the extracellular environment.
Given that exosomes are derived from the endolysosomal pathway, it was not unexpected that small interfering RNA (siRNA) screens, like the one performed by Colombo et al., identified several ESCRT proteins, as well as other endolysosomal machinery, as being important for exosome biogenesis [39]. For example, it was shown that depleting cells of the ESCRT-0 proteins hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) and signal transducing adaptor molecule 1 (STAM1), as well as the ESCRT-I protein tumor susceptibility gene 101 (TSG101), profoundly inhibited the production of exosomes. On the other hand, the screen also identified proteins that had the opposite effect. Such was the case for the ESCRT-III protein vacuolar protein sorting 4 homolog B (VSP4B). Depleting cells of this protein significantly enhanced the number of exosomes released by the cells [39]. However, another study showed that the binding of the ESCRT accessory protein ALIX to another component of the ESCRT-III complex, CHMP4, was critical for the release of exosomes [40], so it is unclear why VSP4B negatively regulates exosome biogenesis.
Exosomes can also be formed in an ESCRT-independent manner. This was shown in a study where human epithelial type 2 (HEp-2) cells depleted of several critical components of the ESCRT complex, including HRS, TSG101, VPS22, and VPS24, were still able to form MVBs, and likely produce exosomes [41]. One ESCRT-independent mechanism of MVB formation that has been identified involves the sphingolipid ceramide. Treating Oli-neu murine oligodendrocytes cells with GW4869 to inhibit neutral sphingomyelinase 2 (nSMase2) activity and prevent the conversion of sphingomyelin to ceramide, led to a reduction in MVB formation and the number of exosomes released by the cells [42, 43]. The way that ceramide is thought to promote exosome biogenesis has to do with its ability to spontaneously assemble into unique microdomains in the membranes of MVBs. Due to its small polar head group, the generation of high local concentrations of ceramide along the surfaces of MVBs causes inward membrane curvatures and leads to the formation of ILVs, and ultimately exosomes [44–46].
Another family of proteins that influences exosome biogenesis are the tetraspanins. These membrane spanning proteins are best known for the roles they play in regulating endocytic trafficking, although several members of this family, such as CD9, CD63, CD81, and CD82 are also known to localize to MVBs and to be enriched in exosomes [3–5]. The relevance of tetraspanins in exosome biogenesis could be appreciated from experiments in which CD63 was genetically deleted from HEK293 cells, where the cells were shown to produce fewer exosomes, compared to wild-type control cells [47]. Moreover, CD63 also appears to be important for sorting certain proteins into exosomes. This was shown in studies that were investigating how the Epstein-Barr virus latent membrane protein 1 (LMP1) becomes incorporated into the exosomes isolated from HEK293 cells that are ectopically expressing the protein. Under conditions where CD63 expression was knocked-out by shRNA in these same cells, the amount of LMP1 detected in the exosomes generated by these cells was shown to be greatly reduced, suggesting that the presence of LMP1 in exosomes was dependent on CD63 [48, 49]. Other members of the tetraspanin family have been similarly shown to help recruit proteins into exosomes. For example, the exosomes from HEK293 cells ectopically expressing the tetraspanins CD9 or CD82 were found to be highly enriched in the cell adhesion protein, β-catenin [50].
The RAB family of small GTP binding proteins is comprised of approximately 70 members, and they are generally regarded as key regulators of intracellular vesicle trafficking processes [51, 52]. Certain RABs have been shown to have important consequences in MVB formation and trafficking, as well as in the release of exosomes [51, 52]. One such member of this family, RAB5, promotes the formation of early endosomes. When endocytosis occurs and early endosomes are formed, RAB5 activates the phosphatidylinositol 3-kinase VPS34 to generate phosphatidylinositol-3-phosphate (PI3P). Endosomal membranes enriched with PI3P then recruit the RAB5 effector, early endosome antigen 1 (EEA1), to promote the fusion of endocytic vesicles, an early step in the formation of MVBs [51]. It was shown that cells ectopically expressing a dominant-active form of RAB5, RAB5Q79L, produced fewer exosomes, presumably because MVB formation/trafficking was disrupted [42]. Similarly, as endocytic vesicles continue to mature into late endosomes and MVBs, the RAB5 associated with these vesicles is replaced by RAB7, raising the possibility that RAB7 may also be important for the production of exosomes [51]. Indeed, it was subsequently shown that MCF7 breast cancer cells depleted of RAB7 also were impaired in their ability to release exosomes [40].
RAB11, RAB27, and RAB35 have all been suggested to mediate the docking of MVBs to the cell surface. Knocking-down the expression of any of these RAB family members using siRNAs in cells consistently showed two unique phenotypes. First, the cells lacking RAB11, RAB27, or RAB35 expression all began to accumulate MVBs along the inner surface of their plasma membrane, as detected by fluorescent microscopy. Second, the ability of these cells to release exosomes into the extracellular space was reduced [4, 5, 52–54]. However, importantly, the exosomes that were still produced by cells depleted of RAB27 were found not to be depleted of protein content. These findings suggest that RAB11, RAB27, and RAB35 are all important for the docking of MVBs with the plasma membrane. Under conditions where their functions are blocked, MVBs build up along the surfaces of cells because they cannot efficiently dock and fuse with the plasma membrane to generate exosomes [55–58].
Collectively, the findings described above further reinforce the idea that inhibiting several different steps in the endolysosomal pathway can have important consequences on exosome biogenesis. This also seems to be the case when the end point of this pathway, that is, the lysosome, is inhibited. When cells are treated with either Bafilomycin A1 or chloroquine to inhibit lysosomal activity, there is a correspondingly strong increase in the number of exosomes released by the cells [59–61]. This effect on exosome production can be attributed to lysosomal inhibition causing the MVBs that would normally fuse with lysosomes and be degraded to instead be rerouted to the plasma membrane and their contents released as exosomes.
There may be mechanisms by which cells can alter their lysosomal activity to promote exosome generation. In support of this idea, our laboratory recently discovered that reducing the expression and activation levels of the NAD+-dependent protein acylase, sirtuin 1 (SIRT1), in breast cancer cells results in the release of more exosomes [61]. Moreover, these exosomes were shown to contain protein cargo that was heavily ubiquitinated. Since the ubiquitination of proteins is a conventional lysosomal sorting signal [62], we suspected that the increased numbers of exosomes containing ubiquitinated cargo being secreted by cells depleted of SIRT1 was due to lysosomal dysfunction. Indeed, we subsequently showed that the lysosomes in cells with reduced levels of SIRT1 had poorly functioning proton pumps and were unable to maintain the acidic environment required for proper protein degradation [61]. As a result, the MVBs containing ubiquitinated cargo that would have normally been degraded instead gave rise to exosomes.
Like MVs, there are several additional cellular processes that have been identified as important regulators of exosome biogenesis. One good example is the post-translational modification of proteins that are mediated by interferon-stimulated gene 15 (ISG15). The enzymatic reaction carried-out by IGS15 on its substrates is referred to as ISGylation, and it is analogous to the process by which ubiquitin is added to proteins. The ISGylation of proteins serves as a way to modify their activities, and in particular this modification has been shown to regulate protein degradation [63]. A study performed by Villarroya-Beltri et al. showed that TSG101, a component of the ESCRT family of proteins, could be ISGylated, an effect that resulted in more MVBs being trafficked to lysosomes and degraded. Since exosomes are derived from MVBs, this increase in MVB degradation led to a corresponding decrease in exosome production [60].
4. EVs and Cancer Progression
4.1. How EVs Mediate Cancer Cell Phenotypes
Although it is now widely accepted that virtually all types of cancer cells are capable of generating EVs that promote various aspects of cancer progression (Fig. 3), this was far from the case a little more than a decade ago. At that time, EVs were considered to be nothing more than a cellular artifact. However, this all changed with the publication of a pair of studies in 2008 that focused on determining how a highly oncogenic form of the EGF receptor, EGFRvIII, promoted brain tumor progression [10, 11]. In one of the studies, Al-Nedawi et al. generated U373 glioma cells ectopically expressing the EGFRvIII, and noticed by electron microscopy that the cells had what appeared to be a large number of vesicles decorating their surfaces [10]. Analysis of the conditioned medium collected from these cells confirmed that EVs were being actively released by the cells. However, these findings were made all the more exciting when it was discovered that the EGFRvIII was a major protein cargo in the EVs. When these EVs were used to treat parental U373 glioma cells, it was shown that the mutant receptor could be “horizontally” transferred to the recipient cells. This transfer event resulted in the activation of the cell growth and survival proteins, AKT and ERK, and enhanced the ability of the parental glioma cells to grow when cultured under anchorage independent (i.e., soft agar) conditions [10]. The second study published by Skog et al. showed that tumor derived EVs could be isolated from the serum collected from glioblastoma patients. Analysis of their contents revealed that they contained the EGFRvIII, as well as the transcript that encodes for the mutant receptor [11]. These findings showed that EVs can influence the behavior of cancer cells.
Fig 3.
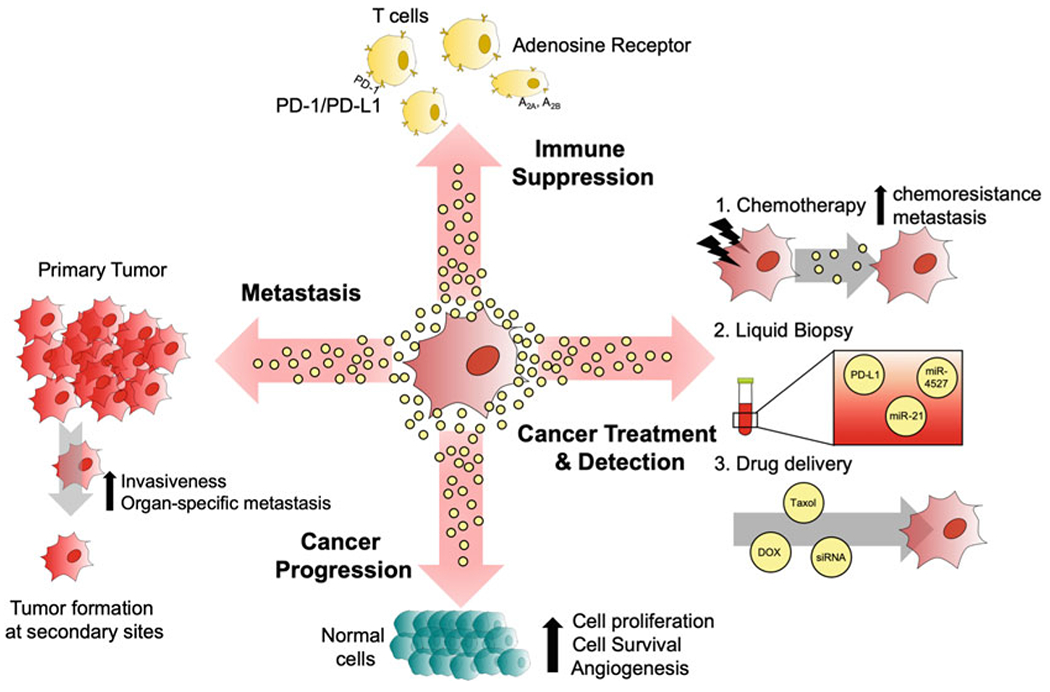
Illustration showing some of the major roles and potential clinical applications for cancer cell-derived EVs. The EVs generated by cancer cells have the ability to suppress the immune system of the host by activating PD-1/PD-L1 or adenosine receptor signaling pathways. EVs have also been shown to promote several other aspects of cancer progression, including cell growth, survival, and angiogenesis through a variety of mechanisms. EVs also play an important role in metastasis, as they have been shown to increase the rates of organ-specific metastatic spread and the overall invasiveness of tumors. Clinically, it has been shown that cancer cells treated with chemotherapy generate EVs that can promote chemoresistance. The contents of EVs can be used for diagnostic purposes, and EVs can serve as therapeutic vehicles to deliver drugs or siRNA into cancer cells
Not long after these ground-breaking studies were published, many more findings linking the generation of EVs to cancer progression were reported. For example, our laboratory showed that the MVs generated by the aggressive breast cancer MDA-MB-231 cell line, and the U87-MG glioma cell line, were capable of causing nontransformed cells to acquire several of the phenotypes of a transformed cell [20, 22]. Specifically, treating nontransformed fibroblasts, or normal mammary epithelial cells (NMECs), with MVs from MDA-MB-231 or U87-MG cells promoted their ability to survive and grow in soft agar [22]. The MVs generated by the cancer cells were also found to be capable of causing fibroblasts to grow as tumors when injected subcutaneously into the flanks of mice. We then identified the protein cross-linking enzyme tissue transglutaminase (tTG) and the extracellular matrix (ECM) protein fibronectin as being important for mediating this effect. We showed that tTG could cross-link the fibronectin associated with the MVs, creating a unique form of this extracellular matrix protein that allowed the vesicles to dock onto recipient fibroblasts, strongly activate FAK and ERK, and promote cellular transformation [22]. More recently, it was reported that the MVs generated by mouse embryonic fibroblasts (MEFs) ectopically expressing an oncogenic form of Dbl were highly enriched with activated FAK [20]. Treating naïve fibroblasts with these MVs promoted FAK-dependent signaling activities in the recipient cells, such that they grew efficiently in soft agar [20].
The EVs generated by cancer and transformed cells have consistently been shown to promote the survival of other cancer cells, as well as normal cells [20, 22, 61, 64, 65]. One way that this effect is likely to be important is in the context of an expanding tumor, where cancer cells remain viable despite being exposed to an environment that is highly stressful with limiting amounts of nutrients and oxygen. Thus, the generation and transfer of EVs between cancer cells may serve as a survival mechanism that helps ensure the growth of the tumor. Consistent with this idea, it has been shown that the EVs from cancer cells can protect recipient cells from nutrient deprivation-induced cell death [20, 22]. However, EVs may also promote chemoresistance [64, 65]. Chemotherapies are a first line treatment for cancer patients, and one of the most commonly used drugs in this regard is paclitaxel (PTX) [66]. Although PTX is often initially effective and reduces tumor burden, the cancer cells often become resistant to the therapy. While many hypotheses have been proposed to explain the development of chemoresistance, several recent studies have suggested that cancer cells use EVs to mediate these effects. For example, our group showed that treating the MDA-MB-231 breast cancer cells with PTX caused them to generate more exosomes, compared to untreated control cells. The treatment also caused the exosomes to become highly enriched in survivin, a protein known for promoting cell survival [64]. We then went on to show that treating SKBR3 breast cancer cells with exosomes isolated from PTX-treated MDA-MB-231 cells caused them to become far less sensitive to PTX treatment. However, depleting the expression of survivin from the donor MDA-MB-231 cells, which resulted in the loss of survivin from the exosomes produced by the transfectants, eliminated this protective effect [64].
4.2. EVs and Tumor Angiogenesis
Another way that EVs contribute to cancer progression is by promoting tumor angiogenesis. Tumors can grow to approximately 1–3 mm in size before they require more oxygen and nutrients to sustain their growth [67]. To overcome this limitation, cancer cells actively begin to secrete factors that recruit endothelial cells to the tumor to promote the formation of new blood vessels (i.e., angiogenesis). Some of the most extensively studied angiogenic factors released by cells are a family of highly related soluble growth factors, referred to as vascular endothelial growth factors (VEGFs) [68]. Secreted forms of VEGF bind the VEGF receptor expressed in endothelial cells and cause them to migrate toward the tumor and grow, resulting in the formation of new blood vasculature. However, it has been suggested that the EVs produced by cancer cells can also mediate this process [69, 70]. One example came from work performed by Al-Nedawi et al. [69]. Specifically, it was shown that the EVs generated by A431 epidermoid carcinoma cells contained the EGF receptor. When these EVs were used to treat cultures of human umbilical vein endothelial cells (HUVECs), the EGF receptor was transferred to the recipient cells where it stimulated signaling events that increased the production of VEGF and stimulated blood vessel formation [69].
In another study by our laboratory, we found that EVs, and in particular MVs, released by cancer cells stimulated angiogenesis [70]. We first showed that MVs released from MDA-MB-231 breast cancer cells could potently activate the VEGF receptor and promote angiogenesis to a greater extent than an equivalent amount of recombinant VEGF. We then found that this effect was insensitive to an inhibitory antibody that targets VEGF, referred to as bevacizumab (Avastin®). The underlying mechanism of this resistance had to do with a specific isoform of VEGF that was expressed along the surfaces of the MVs, where it was cross-linked by the enzyme tTG into a higher oligomeric form. This unique form of VEGF associated with the MVs in such a way that it could still potently activate VEGF receptors and stimulate blood vessel formation but was incapable of being recognized by the inhibitory antibody [70].
5. EVs and Metastasis
Metastasis is the leading cause of death among all cancer patients. It is a complicated process that involves cancer cells within the primary tumor being able to escape their local environment and enter the circulation, a process referred to as intravasation. The circulating cancer cells then need to survive the hostile conditions encountered in the blood stream until the time that they eventually exit the circulation, or extravasate. Only a small percentage of cancer cells can successfully complete this process and go on to “seed” a secondary site and form a new tumor. It is also worth mentioning that the ability of cancer cells to establish tumors at secondary sites is not random. Rather, it is known that certain types of cancer cells preferentially metastasize to specific organs. For example, breast cancers tend to spread to the lung, liver, bone, or brain, while prostate cancers most often metastasize to the bone [71, 72].
The first step of metastasis involves the cells of the primary tumor breaking free from their local environment. For this to occur, the cells must undergo an epithelial-to-mesenchymal transition (EMT) which involves the downregulation of α-, β-, γ-catenin and E-cadherin, and the increased expression of N-cadherin, fibronectin, and vimentin [73]. Consequently, cells that undergo these changes gain the ability to migrate and invade into the normal tissue that surrounds a tumor. It has been recently suggested that EVs produced by cancer cells can help promote EMT. For example, it was shown that when Chinese hamster ovary (CHO) cells were treated with EVs released by the highly aggressive U87-MG glioblastoma cell line, they began to migrate more rapidly, compared to cultures of untreated cells [74]. The findings from another study showed that treating urothelial cells with EVs isolated from bladder cancer cells caused a reduction in the expression levels of E-cadherin and β-catenin, two of the changes that are known to occur in cells that have undergone EMT. The authors then went on to determine whether treating urothelial cells with cancer cell-derived EVs affected their ability to migrate. Using time-lapse microscopy imaging, it was shown that EVs significantly increased the migration of the urothelial cells, suggesting that EVs could potentially mediate one of the important aspects regarding cell invasion and metastasis, namely, cell migration [75].
The EVs produced by aggressive cancer cells have also been shown to be capable of breaking down and remodeling the extracellular matrix, which is important for creating a path for cancer cells in the primary tumor to migrate through and intravasate into the blood stream. Several ectopeptidases known to be involved in tumor microenvironment remodeling/degradation have been detected in EVs produced by cancer cells, including matrix metal-loproteases (MMPs), a disintegrin and metalloproteinases (ADAMs), and ADAMs with thrombospondin motifs (ADAMTS). A good example highlighting this fact came from a study where proteomic analyses were performed on the exosomes isolated from MDCK cells versus MDCK cells that were ectopically expressing an oncogenic form of H-Ras. The results of the screen showed that MMP-1, MMP-14, MMP-10, ADAM-10, and ADAMTS1 were all enriched in the exosomes isolated from transformed cells, relative to the exosomes from control MDCK cells [76]. Other proteins known to degrade different components of the extracellular matrix have also been identified in the EVs generated by various types of cancer cells, including the active forms of membrane type 1 MMP (MT1-MMP, also known as MMP-14), MMP-2, MMP-9, as well as extracellular matrix metalloproteinase inducer (EMMPRIN) [13, 29, 77–80]. These findings highlight how EVs not only can reprogram cancer cells to enhance their ability to migrate but also reshape the extracellular matrix, such that the cancer cells have a better chance of escaping their local environment and entering the blood stream.
There is evidence that EVs can potentially contribute to the later stages of metastasis as well, particularly when circulating tumor cells extravasate the blood stream and settle at a secondary site [57, 81]. As mentioned earlier, metastasis is not a random process. Indeed, the “seed-and-soil” hypothesis was originally proposed by Stephen Paget in 1889 when he observed that certain types of cancer cells (i.e., the seeds) preferentially metastasize to specific organs (i.e., the soil) [82]. Although the mechanisms that mediate organotropism are not clear, some recent findings suggest EVs may play an important role in this process. For example, it was shown that high levels of the miR-200 family of microRNAs could be detected in the EVs released by 4T1E cells, a breast cancer cell line which preferentially metastasizes to the lungs, but not in the EVs produced by the nonmetastatic 4TO7 breast cancer cell line [83]. Since miR-200s are frequently upregulated in metastatic breast cancers [84], it was hypothesized that exosomes containing miR-200s could potentially help promote metastatic spread by promoting the formation of the premetastatic niche, and thus prepare the future site of colonization (in this case, the lungs) for the arrival of cancer cells. This idea was tested by performing tumor colonization assays in mice. In these experiments, the exosomes isolated from the metastatic 4T1E breast cancer cells were introduced into the blood stream of mice, followed by injection of the nonmetastatic 4TO7 breast cancer cells. Several weeks later, the extent to which the circulating tumor cells was able to colonize the lungs was determined. Injection of 4TO7 cells alone into the circulation of mice resulted in few lung metastases, as expected. However, when the same experiment was carried-out on mice that had been treated with the exosomes derived from the metastatic 4T1E cells, the number of 4TO7 cells that colonized the lungs significantly increased. To determine whether this effect was due to the large amount of miR-200s that associated with the EVs produced by 4T1E cells, the authors ectopically expressed miR-200c in the nonmetastatic 4TO7 cells, and then isolated their EVs. Not only was it shown that the ectopically expressed miR-200c could be detected in the EVs generated by the transfected cells, but the authors also used these EVs in another tumor colonization assay. This time when the mice were treated with the EVs isolated from 4TO7 cells ectopically overexpressing miR-200c, the authors found that the parental 4TO7 cells again gained the ability to efficiently colonize the lungs [83]. Although it is still unclear exactly how EVs enriched in miR-200s promote lung metastases, it is possible that the EVs accumulate in the lungs to help prepare the tissue for the arrival of the cancer cells, making it more likely that the circulating cancer cells will successfully colonize the lungs. It is also worth mentioning that the miR-200 family of microRNAs have been detected in plasma samples collected from patients with highly aggressive and potentially metastatic tumors [85]. Although it was not directly shown in this study whether miR-200s were associated with EVs, these findings serve as further evidence that EVs may be important for promoting metastasis.
Proteins associated with EVs have also been shown to promote organ-specific metastatic spread. Perhaps the best example of this involves integrins, a family of cell adhesion proteins. There are several different types of integrins, and each one binds to specific extracellular matrix proteins. Hoshino et al. discovered that metastatic cancer cells generate exosomes that express distinct sets of integrins, allowing them to accumulate at specific future sites of metastasis [81]. To demonstrate this, the authors took advantage of two different clones of the MDA-MB-231 breast cancer cell line, namely 4715-LuT and 1833-BoT, which were derived based on their ability to preferentially metastasize to the lung and bone, respectively. The exosomes released by each of these cell types were isolated and then used in tumor colonization assays. The mice were first treated with exosomes from the 4715-LuT cells (i.e., the cells that metastasize to the lung), or phosphate-buffered saline (PBS), followed by injection into the circulation of the animals with either 4715-LuT or 1833-BoT cells. As expected, the 4715-LuT cells preferentially colonized the lungs, and the number of metastatic lesions detected in this tissue increased significantly in the mice that had also been treated with exosomes. While this finding was interesting and suggested that exosomes can increase the rates of metastasis, the real surprise came when it was shown that the injection of exosomes isolated from the 4715-LuT cells was sufficient to cause the 1833-BoT cells, which normally metastasize to the bone, to instead colonize the lung. It was then determined that the specific integrins that associated with these exosomes were critical for mediating this effect. Specifically, the authors showed that integrin β4 was distinctly expressed in the exosomes generated by the 4715-LuT cells. Since integrin β4 is known to bind to the extracellular matrix protein laminin, which is highly expressed in the lungs, the idea was that exosomes expressing integrin β4 would preferentially accumulate in the lungs and help form the premetastatic niche. The authors went on to investigate this possibility by knocking-down the expression of integrin β4 in 4715-LuT cells, which resulted in the loss of the expression of this integrin from the exosomes generated by these cells. When these exosomes were used in another tumor colonization assay, it was determined that they lost their ability to promote lung metastasis [81], underscoring how the protein composition of EVs can potentially promote tumor-specific metastasis.
6. EVs and Cancer-Mediated Immunosuppression
A tumor is typically not recognized and destroyed by the innate and adaptive immune systems of the patient. Indeed, it is now appreciated that the cancer cells within a tumor are actively generating a microenvironment that is immunosuppressive [86–88]. One of the best understood mechanisms of cancer-mediated immunosuppression is through the increased expression of programmed death-ligand 1 (PD-L1) on the surfaces of cancer cells. PD-L1 serves as the ligand for programmed death 1 (PD-1), a cell surface receptor expressed by various types of immune cells, and in particular, by CD8+ T cells (i.e., cytotoxic T cells). Thus, as immune cells that are actively surveilling the tumor microenvironment engage cancer cells, the PD-L1 on the surfaces of cancer cells binds and activates PD-1 expressed on immune cells. The activation of PD-1 promotes signaling events that inhibit the growth and function of immune cells. This mechanism of cancer-mediated immune evasion has been attracting a good deal of attention from the pharmaceutical industry and has led to the generation of several FDA approved inhibitors of the PD-1/PD-L1 pathway, including nivolumab (OPDIVO®, Bristol-Myers Squibb), pembrolizumab (KEY-TRUDA®, Merck), atezolizumab (TECENTRIQ®, Genentech), cemiplimab-rwlc (Libtayo®, Regeneron and Sanofi), and avelumab (BEVENCIO®, Pfizer and EMS Serono) [86–91]. By using these drugs and interfering with the ability of cancer cells to activate PD-1 signaling in immune cells, the function of immune cells is maintained, enabling them to efficiently target and destroy cancer cells.
Interestingly, it was shown that PD-L1 could be readily detected in exosomes isolated from a variety of cancer cells types, including melanoma, prostate cancer, colorectal cancer, head and neck cancer, and glioblastoma [16, 17, 19, 92]. When these PD-L1 positive exosomes were used to treat to cultures of CD8+ T cells, they were shown to strongly suppress their growth and immune activity [16, 17, 19]. Moreover, when cancer cell-derived EVs containing PD-L1 were introduced into the circulation of mice via tail vein injections, the levels of total CD8+ T cells detected in the animals were found to be significantly reduced. However, if the mice were administered exosomes carrying PD-L1 and inhibitory anti-PD-L1/PD-1 antibodies, the reduction in CD8+ T cells could be partially restored [16, 17]. Collectively, these findings suggested that cancer cells release exosomes expressing PD-L1 as a potential mechanism to suppress the immune response within the local tumor microenvironment, as well as systemically. Moreover, this also raises the interesting possibility that these exosomes can provide and additional layer of protection to the tumor, by engaging and inactivating immune cells before they have an opportunity to reach the cancer cells.
Another way that exosomes produced by cancer cells may mediate immunosuppression is by influencing the levels of extracellular adenosine. Typically, when extracellular adenosine binds to the A2A or A2B adenosine receptor subtypes expressed in immune cells, it activates signaling proteins that stimulate the biosynthesis of cyclic AMP (cAMP) [93, 94]. The buildup of cAMP in immune cells has been shown to inhibit their activation [95–97]. As a result, the level of extracellular adenosine needs to be tightly controlled for a proper immune response to occur. Two ectonucleotidases, CD39 and CD73, have been identified as important regulators of extracellular adenosine levels. CD39 catalyzes the hydrolysis of ATP to AMP, while CD73 dephosphorylates AMP to adenosine. Both CD39 and CD73 are commonly expressed on the surfaces of immune cells, as a way to fine tune the immune response. However, many types of cancers cells have been shown to upregulate the expression CD39 and CD73 [98, 99]. Thus, cancer cells can suppress immune cell activity by converting extracellular ATP to adenosine. The increase in extracellular adenosine within the tumor microenvironment would then activate A2A or A2B receptor signaling in surveilling immune cells, resulting in their inactivation [95, 96, 100].
Interestingly, the exosomes isolated from certain types of cancer cells were also found to express CD39 and CD73 [101]. When these exosomes were isolated and then combined with ATP, the CD39 and CD73 associated with the exosomes efficiently converted the ATP to adenosine [95, 96, 101]. Clayton et al. then went on to show that treating T cells with this reaction mixture was sufficient to block their growth and activation [101]. The findings connecting EVs to cancer-mediated immunosuppression are also potentially important in the context of treating cancer patients with PD-L1/PD-1 therapies. While these therapies have shown promising results in a subset of patients in clinical trials, it has been reported that as many as 50–90% of patients with various forms of cancer do not respond to the treatment [91]. One interesting idea to consider regarding PD-L1/PD-1 therapy resistance is whether exosomes generated by cancer cells could be responsible for mediating this effect. If determined to be true, then combining PD-L1/PD-1 therapies with drugs that inhibit exosome and MV biogenesis could be an effective strategy to overcome this problem.
7. EVs and Their Potential Uses in the Clinics
There is a growing appreciation that EVs can be used in different ways for clinical applications (Fig. 3). For example, the amounts and contents of EVs released by cancer cells can change depending on their cell of origin, stage of development, and response to treatments, making them potentially important sources of diagnostic information [16, 64, 102–105]. Moreover, since EVs function as satellites of communication and can efficiently mediate the transfer of information to recipient cells, they are also being pursued as a novel platform for drug delivery [106, 107]. In this section, we will highlight the potential clinical applications of EVs.
7.1. EVs and Liquid Biopsies
Biopsies are one of the major approaches used by oncologists to identify the type and stage of a patient’s cancer. Conventional biopsies involve the use of a needle or sharp instrument to obtain a small portion of the tumor. However, these procedures are often invasive, are painful, and carry the risk of infection. Moreover, biopsies are often performed only after there is a strong suspicion that the patient has cancer and thus serve to confirm the diagnosis.
Recently, liquid biopsies have been proposed as a noninvasive alternative approach to conventional biopsies. The idea behind liquid biopsies is to analyze the plasma, serum, or other types of biofluids collected from patients for the presence of specific proteins or nucleic acids that are associated with disease, in this case cancer. Since liquid biopsy samples are relatively easy to obtain, many of the problems encountered when conducting conventional biopsies are eliminated. Moreover, liquid biopsies can be performed as part of routine patient exams, increasing the likelihood that individuals with cancer can be diagnosed at earlier stages of the disease cancer.
Since the EVs generated by cancer cells can be found in the circulation, there have been efforts to isolate them and analyze their contents as a form of liquid biopsy [16, 102, 103]. One good example of how EVs might potentially be used in this regard can be seen in a study that was identifying differentially expressed cargo in exosomes released by melanoma cells. In this study, the major immune response regulator PD-L1 was determined to be significantly enriched in exosomes from metastatic melanoma cells, compared to exosomes from less aggressive cells. These findings were then extended to show that exosomes isolated from blood samples taken from patients with metastatic melanoma were similarly enriched in PD-L1 [16], suggesting that the presence of PD-L1 in exosomes could potentially be used for diagnostic purposes.
Many different microRNAs that are expressed in exosomes have also been proposed as potential cancer biomarkers. For example, Akers et al. showed that miR-21 was highly expressed in exosomes isolated from cerebrospinal fluid taken from glioblastoma patients, but was absent in exosomes collected from healthy individuals [102]. Likewise, Dejima et al. monitored the expression levels of miR-21 and miR-4527 in EVs isolated from blood samples taken from non–small cell lung cancers (NSCLC) patients who had undergone tumor resection. The authors found that patients with higher levels of miR-21 and miR-4527 in their EVs had a significantly worse prognosis [103].
7.2. EVs as a Drug Delivery System
The efficient delivery of a drug can significantly increase its efficacy and specificity. Various types of materials have been engineered and used for drug delivery approaches, including silica-based nanoparticles, hydrogels, and synthetic liposomes [108]. While these approaches have yielded some success, a number of limitations have also been encountered. For example, the use of nanoparticles and hydrogels to deliver therapeutic agents has frequently caused cytotoxicity and inflammation, and synthetic liposomes tend to be inefficiently taken up by cells [108]. However, the use of EVs as an approach to deliver therapies could potentially circumvent many of these issues. This is largely because EVs are produced by cells, making them inherently biocompatible [109]. Moreover, the composition of EV surface proteins can potentially be modified to more efficiently target them to certain tissues [106].
One of the best examples of how EVs are being used as a therapeutic vehicle comes from work performed by Raghu Kalluri (MD Anderson Cancer Center) and colleagues. Pancreatic cancer is a highly aggressive disease, and the current treatment options available to patients are ineffective [110]. The Kalluri laboratory set out to develop a new approach to treat pancreatic cancer using exosomes. Specifically, they took advantage of the fact that the vast majority of pancreatic tumors have a mutated form of K-Ras [111, 112]. They designed siRNAs that specifically targeted the mutant protein and then used electroporation to introduce them into exosomes isolated from fibroblasts [106]. The exosomes loaded with K-Ras siRNAs were administered to mouse models of K-Ras-driven pancreatic cancer. While the control animals formed aggressive tumors and died within a short period of time, the mice that received the experimental therapy showed tumor regression and lived significantly longer.
8. Concluding Remarks
The growth of the EV field over the past decade has been exponential, and we have learned a great deal about the roles these vesicles play in different aspects of cancer progression (Fig. 3). Many of the exciting discoveries made involving EVs have now added credibility to a cell biological phenomenon that was once considered to be an artifact. However, as is often the case with any young and rapidly developing research field, new questions arise that need to be addressed. In the case of EVs, one of the important questions is how many different types of vesicles are produced by cells? This is a challenging research endeavor that will require the use of rigorous isolation approaches and biochemical characterizations to define each of the subclasses of MVs and exosomes. Moreover, it will be important to determine the mechanisms that control the loading of specific proteins and nucleic acid species into EVs, and to understand how these mechanisms are altered in cancer or transformed cells. Addressing these questions will likely be a major focus of the EV field over the next several years. Finally, a pressing question that will need to be answered concerns whether EVs can be used as diagnostic tools, or for the delivery of therapies. However, with more than 60 clinical trials related to EVs and cancer underway, it seems likely that the answer to this question is within reach.
References
- 1.Wilson KJ, Mill C, Lambert S et al. (2012) EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling. Growth Factors 30:107–116. 10.3109/08977194.2011.649918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latifkar A, Hur YH, Sanchez JC et al. (2019) New insights into extracellular vesicle biogenesis and function. J Cell Sci 132:jcs222406. 10.1242/jcs.222406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas SLN, Breakefield XO, Weaver AM (2017) Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 27:172–188. 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75:193–208. 10.1007/s00018-017-2595-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 6.Jeppesen DK, Fenix AM, Franklin JL et al. (2019) Reassessment of exosome composition. Cell 177:428–445.e18. 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288. 10.1111/j.1365-2141.1967.tb08741.x [DOI] [PubMed] [Google Scholar]
- 8.Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339. 10.1083/jcb.97.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967–978. 10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- 10.Al-Nedawi K, Meehan B, Micallef J et al. (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10:619–624. 10.1038/ncb1725 [DOI] [PubMed] [Google Scholar]
- 11.Skog J, Würdinger T, van Rijn S et al. (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minciacchi VR, Freeman MR, Di Vizio D (2015) Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 40:41–51. 10.1016/j.semcdb.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Vizio D, Morello M, Dudley AC et al. (2012) Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 181:1573–1584. 10.1016/j.ajpath.2012.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Théry C, Witwer KW, Aikawa E et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowal J, Arras G, Colombo M et al. (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci 113:E968–E977. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Huang AC, Zhang W et al. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560:382–386. 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poggio M, Hu T, Pai C et al. (2019) Suppression of exosomal PD-L1 induces systemic antitumor immunity and memory. Cell 177:414–427.e13. 10.1016/j.cell.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodoraki M, Yerneni SS, Hoffmann TK et al. (2018) Clinical significance of PD-L1 þ exosomes in plasma of head and neck cancer patients. Clin Cancer Res 24:896–906. 10.1158/1078-0432.CCR-17-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricklefs FL, Alayo Q, Krenzlin H et al. (2018) Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 4:eaar2766. 10.1126/sciadv.aar2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreger BT, Dougherty AL, Greene KS et al. (2016) Microvesicle cargo and function changes upon induction of cellular transformation. J Biol Chem 291:19774–19785. 10.1074/jbc.M116.725705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Freitas D, Kim HS et al. (2018) Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20:332–343. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonyak MA, Li B, Boroughs LK et al. (2011) Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A 108:4852–4857. 10.1073/pnas.1017667108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Vizio D, Kim J, Hager MH et al. (2009) Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 69:5601–5609. 10.1158/0008-5472.CAN-08-3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Antonyak MA, Zhang J, Cerione RA (2012) RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31:4740–4749. 10.1038/onc.2011.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada A, Nishida E, Mizuno K et al. (2002) Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393:809–812. 10.1038/31735 [DOI] [PubMed] [Google Scholar]
- 26.Tague SE, Muralidharan V, D’Souza-Schorey C (2004) ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci 101:9671–9676. 10.1073/pnas.0403531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto S, Onodera Y, Hashimoto A et al. (2004) Requirement for Arf6 in breast cancer invasive activities. Proc Natl Acad Sci 101:6647–6652. 10.1073/pnas.0401753101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchesin V, Castro-Castro A, Lodillinsky C et al. (2015) ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J Cell Biol 211:339–358. 10.1083/jcb.201506002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muralidharan-Chari V, Clancy J, Plou C et al. (2009) ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19:1875–1885. 10.1016/j.cub.2009.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123:1603–1611. 10.1242/jcs.064386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tricarico C, Clancy J, D’Souza-Schorey C (2017) Biology and biogenesis of shed micro-vesicles. Small GTPases 8:220–232. 10.1080/21541248.2016.1215283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccin A, Murphy WG, Smith OP (2007) Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21:157–171. 10.1016/j.blre.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 33.Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707. 10.1016/j.cell.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 34.Lukey MJ, Katt WP, Cerione RA (2017) Targeting amino acid metabolism for cancer therapy. Drug Discov Today 22:796–804. 10.1016/j.drudis.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson KF, Erickson JW, Antonyak MA et al. (2013) Rho GTPases and their roles in cancer metabolism. Trends Mol Med 19:74–82. 10.1109/TMI.2012.2196707.Separate [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rilla K, Pasonen-Seppänen S, Deen AJ et al. (2013) Hyaluronan production enhances shedding of plasma membrane-derived microvesicles. Exp Cell Res 319:2006–2018. 10.1016/j.yexcr.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 37.Henne WM, Stenmark H, Emr SD (2013) Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol 5:a016766–a016766. 10.1101/cshperspect.a016766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christ L, Raiborg C, Wenzel EM et al. (2017) Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem Sci 42:42–56. 10.1016/j.tibs.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 39.Colombo M, Moita C, van Niel G et al. (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126:5553–5565. 10.1242/jcs.128868 [DOI] [PubMed] [Google Scholar]
- 40.Baietti MF, Zhang Z, Mortier E et al. (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14:677–685. 10.1038/ncb2502 [DOI] [PubMed] [Google Scholar]
- 41.Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10:925–937. 10.1111/j.1600-0854.2009.00920.x [DOI] [PubMed] [Google Scholar]
- 42.Trajkovic K, Hsu C, Chiantia S et al. (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247. 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- 43.Kosaka N, Iguchi H, Yoshioka Y et al. (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452. 10.1074/jbc.M110.107821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulbins E, Kolesnick R (2003) Raft ceramide in molecular medicine. Oncogene 22:7070–7077. 10.1038/sj.onc.1207146 [DOI] [PubMed] [Google Scholar]
- 45.Goñi FM, Alonso A (2009) Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 1788:169–177. 10.1016/j.bbamem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 46.Holopainen JM, Subramanian M, Kinnunen PKJ (1998) Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry 37:17562–17570. 10.1021/bi980915e [DOI] [PubMed] [Google Scholar]
- 47.Hurwitz SN, Conlon MM, Rider MA et al. (2016) Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J Extracell Vesicles 5:31295. 10.3402/jev.v5.31295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verweij FJ, Van Eijndhoven MAJ, Hopmans ES et al. (2011) LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J 30:2115–2129. 10.1038/emboj.2011.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurwitz SN, Nkosi D, Conlon MM et al. (2017) CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling. J Virol 91:1–19. 10.1128/JVI.02251-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chairoungdua A, Smith DL, Pochard P et al. (2010) Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 190:1079–1091. 10.1083/jcb.201002049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langemeyer L, Fröhlich F, Ungermann C (2018) Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol 28:957–970. 10.1016/j.tcb.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 52.Blanc L, Vidal M (2018) New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 9:95–106. 10.1080/21541248.2016.1264352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu C, Morohashi Y, Yoshimura SI et al. (2010) Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 189:223–232. 10.1083/jcb.200911018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savina A, Fader CM, Damiani MT, Colombo MI (2005) Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6:131–143. 10.1111/j.1600-0854.2004.00257.x [DOI] [PubMed] [Google Scholar]
- 55.Ostrowski M, Carmo NB, Krumeich S et al. (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12:19–30. 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- 56.Zhang F, Li R, Yang Y et al. (2019) Specific decrease in B-cell-derived extracellular vesicles enhances post-chemotherapeutic CD8+ T cell responses. Immunity 50:738–750.e7. 10.1016/j.immuni.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 57.Peinado H, Alečović M, Lavotshkin S et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18:883–891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoshino D, Kirkbride KC, Costello K et al. (2013) Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5:1159–1168. 10.1016/j.celrep.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edgar JR, Manna PT, Nishimura S et al. (2016) Tetherin is an exosomal tether. Elife 5:1–19. 10.7554/eLife.17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villarroya-Beltri C, Baixauli F, Mittelbrunn M et al. (2016) ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun 7. 10.1038/ncomms13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latifkar A, Ling L, Hingorani A et al. (2019) Loss of sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev Cell 49:393–408.e7. 10.1016/j.devcel.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Husnjak K, Dikic I (2012) Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem 81:291–322. 10.1146/annurev-biochem-051810-094654 [DOI] [PubMed] [Google Scholar]
- 63.Albert M, Bécares M, Falqui M et al. (2018) ISG15, a small molecule with huge implications: regulation of mitochondrial homeostasis. Viruses 10:629. 10.3390/v10110629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreger BT, Johansen ER, Cerione RA, Antonyak MA (2016) The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers (Basel) 8. 10.3390/cancers8120111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crow J, Atay S, Banskota S et al. (2017) Exosomes as mediators of platinum resistance in ovarian cancer. Oncotarget 8:11917–11936. 10.18632/oncotarget.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marupudi NI, Han JE, Li KW et al. (2007) Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf 6:609–621. 10.1517/14740338.6.5.609 [DOI] [PubMed] [Google Scholar]
- 67.Folkman J, Long DM, Becker FF (1963) Growth and metastasis of tumor in organ culture. Cancer 16:453–467. [DOI] [PubMed] [Google Scholar]
- 68.Simons M, Gordon E, Claesson-Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17:611–625. 10.1038/nrm.2016.87 [DOI] [PubMed] [Google Scholar]
- 69.Al-Nedawi K, Meehan B, Kerbel RS et al. (2009) Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci 106:3794–3799. 10.1073/pnas.0804543106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng Q, Zhang C, Lum D et al. (2017) A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun 8:14450. 10.1038/ncomms14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obenauf AC, Massagué J (2015) Surviving at a distance: organ-specific metastasis. Trends Cancer 1:76–91. 10.1016/j.trecan.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695. 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 73.Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition find the latest version: review series the basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. 10.1172/JCI39104.1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christianson HC, Svensson KJ, van Kuppevelt TH et al. (2013) Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci 110:17380–17385. 10.1073/pnas.1304266110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franzen CA, Blackwell RH, Todorovic V et al. (2015) Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis 4:e163–e110. 10.1038/oncsis.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tauro BJ, Mathias RA, Greening DW et al. (2013) Oncogenic H-Ras reprograms Madin-Darby Canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics 12:2148–2159. 10.1074/mcp.M112.027086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimoda M, Khokha R (2013) Proteolytic factors in exosomes. Proteomics 13:1624–1636. 10.1002/pmic.201200458 [DOI] [PubMed] [Google Scholar]
- 78.Sidhu SS, Mengistab AT, Tauscher AN et al. (2004) The microvesicle as a vehicle for EMMPRIN in tumor–stromal interactions. Oncogene 23:956–963. 10.1038/sj.onc.1207070 [DOI] [PubMed] [Google Scholar]
- 79.Bobrie A, Krumeich S, Reyal F et al. (2012) Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 72:4920–4930. 10.1158/0008-5472.CAN-12-0925 [DOI] [PubMed] [Google Scholar]
- 80.Hakulinen J, Sankkila L, Sugiyama N et al. (2008) Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem 105:1211–1218. 10.1002/jcb.21923 [DOI] [PubMed] [Google Scholar]
- 81.Hoshino A, Costa-Silva B, Shen T-L et al. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573. 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- 83.Le MTN, Hamar P, Guo C et al. (2014) miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 124:5109–5128. 10.1172/JCI75695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gravgaard KH, Lyng MB, Laenkholm AV et al. (2012) The miRNA-200 family and miRNA-9 exhibit differential expression in primary versus corresponding metastatic tissue in breast cancer. Breast Cancer Res Treat 134:207–217. 10.1007/s10549-012-1969-9 [DOI] [PubMed] [Google Scholar]
- 85.Madhavan D, Zucknick M, Wallwiener M et al. (2012) Circulating miRNAs as surrogate markers for circulating tumor cells and prognostic markers in metastatic breast cancer. Clin Cancer Res 18:5972–5982. 10.1158/1078-0432.CCR-12-1407 [DOI] [PubMed] [Google Scholar]
- 86.Dunn GP, Bruce AT, Ikeda H et al. (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998. 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 87.Mittal D, Gubin MM, Schreiber RD, Smyth MJ (2014) New insights into cancer immunoediting and its three component phaseselimination, equilibrium and escape. Curr Opin Immunol 27:16–25. 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating the role of immunity in cancer suppression and promotion. Science 331:78. [DOI] [PubMed] [Google Scholar]
- 89.Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461. 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alsaab HO, Sau S, Alzhrani R et al. (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 8:1–15. 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Q, Li T, Yue W (2018) Drug response to PD-1/PD-L1 blockade: based on biomarkers. Onco Targets Ther 11:4673–4683. 10.2147/OTT.S168313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Theodoraki M-N, Yerneni SS, Hoffmann TK et al. (2018) Clinical significance of PD-L1 + exosomes in plasma of head and neck cancer patients. Clin Cancer Res 24:896–905. 10.1158/1078-0432.CCR-17-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Calker D, Müller M, Hamprecht B (1979) Adenosine regulates via two different types of receptors, the accumulation of cyclic amp in cultured brain cells. J Neurochem 33:999–1005. 10.1111/j.1471-4159.1979.tb05236.x [DOI] [PubMed] [Google Scholar]
- 94.Huang S, Apasov S, Koshiba M, Sitkovsky M (1997) Role of A2a extracellular adenosine receptor-mediated signaling in adenosinemediated inhibition of T-cell activation and expansion. Blood 90:1600–1610 [PubMed] [Google Scholar]
- 95.Vijayan D, Young A, Teng MWLL, Smyth MJ (2017) Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 17:709–724. 10.1038/nrc.2017.86 [DOI] [PubMed] [Google Scholar]
- 96.Allard B, Beavis PA, Darcy PK, Stagg J (2016) Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol 29:7–16. 10.1016/j.coph.2016.04..001 [DOI] [PubMed] [Google Scholar]
- 97.Ohta A, Sitkovsky M (2001) Role of Gprotein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414:916–920. 10.1038/414916a [DOI] [PubMed] [Google Scholar]
- 98.Bastid J, Regairaz A, Bonnefoy N et al. (2015) Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res 3:254–265. 10.1158/2326-6066.cir-14-0018 [DOI] [PubMed] [Google Scholar]
- 99.Turcotte M, Spring K, Pommey S et al. (2015) CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res 75:4494–4503. 10.1158/0008-5472.CAN-14-3569 [DOI] [PubMed] [Google Scholar]
- 100.Ohta A, Gorelik E, Prasad SJ et al. (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci 103:13132–13137. 10.1073/pnas.0605251103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clayton A, Al-Taei S, Webber J et al. (2011) Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 187:676–683. 10.4049/jimmunol.1003884 [DOI] [PubMed] [Google Scholar]
- 102.Akers JC, Ramakrishnan V, Kim R et al. (2013) miR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One 8:1–13. 10.1371/journal.pone.0078115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dejima H, Iinuma H, Kanaoka R et al. (2017) Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol Lett 13:1256–1263. 10.3892/ol.2017.5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allenson K, Castillo J, San Lucas FA et al. (2017) High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol 28:741–747. 10.1093/annonc/mdx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keklikoglou l, Cianciaruso C, Güç E et al. (2019) Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol 21:190–202. 10.1038/s41556-018-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamerkar S, Lebleu VS, Sugimoto H et al. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546:498–503. 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang T, Martin P, Fogarty B et al. (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm Res 32:2003–2014. 10.1007/s11095-014-1593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tibbitt MW, Dahlman JE, Langer R (2016) Emerging frontiers in drug delivery. J Am Chem Soc 138:704–717. 10.1021/jacs.5b09974 [DOI] [PubMed] [Google Scholar]
- 109.Johnsen KB, Gudbergsson JM, Skov MN et al. (2014) A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta Rev Cancer 1846:75–87. 10.1016/j.bbcan.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 110.Warsame R, Grothey A (2012) Treatment options for advanced pancreatic cancer: a review. Expert Rev Anticancer Ther 12:1327–1336. 10.1586/era.12.115 [DOI] [PubMed] [Google Scholar]
- 111.Lanfredini S, Thapa A, O’Neill E (2019) RAS in pancreatic cancer. Biochem Soc Trans 47:961–972. 10.1042/BST20170521 [DOI] [PubMed] [Google Scholar]
- 112.Stephen AG, Esposito D, Bagni RG, McCormick F (2014) Dragging ras back in the ring. Cancer Cell 25:272–281. 10.1016/j.ccr.2014.02.017 [DOI] [PubMed] [Google Scholar]