Child with radiologically recurrent thalamic tumor (original) (raw)
Clinical History
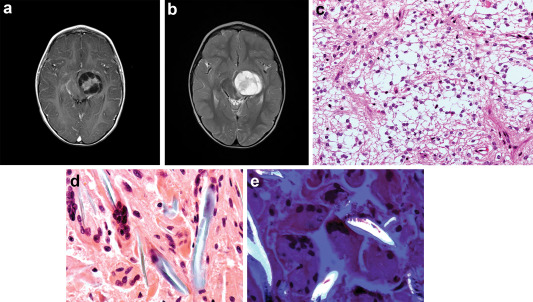
A 3‐year‐old boy initially presented in January 2010, with a 4‐month history of progressive right hemiparesis. Developmentally his motor skills had been somewhat delayed, with walking only at 18 months while dragging his right leg and a left‐hand preference from an early age. Initial MRI revealed a large left thalamic enhancing partly cystic‐solid lesion involving the left posterior limb of the internal capsule and the posterior lentiform, with mass effect on the third ventricle and brainstem (Figure 1a, T2). MRI of the spine was normal.
Figure 1.
He had near total surgical resection with intraoperative neurophysiology monitoring. On immediate postoperative MRI, two small enhancing areas were noted; one adjacent to the left middle cerebral artery (MCA) and one deep within the resection cavity. He had residual right hemiparesis postoperatively and did not require any further treatment other than regular physiotherapy with no improvement in the hemiparesis.
Surveillance scans at 5, 11 and 18 months were stable. At 24 and 27 months, a rim enhancing lesion appeared. Noncontrast CT at 30 months showed a rounded hyperattenuated lesion in the left temporal lobe (Figure 1b). Given the radiological appearances, the presumptive diagnosis was of tumor progression with possible malignant transformation. He was readmitted for a further resection. A preoperative MR raised the possibility that the more superficial sylvian fissure lesion could represent an aneurysm or pseudoaneurysm. CT angiogram showed no enhancement in this lesion. Four‐vessel angiogram was normal.
The patient was admitted for a resection of these lesions during which a solid nodule attached to the M2 of the left MCA segment was removed and labeled Specimen A. The tumor cavity was carefully resected; however, part of the capsule was adherent to the midbrain and this was left behind. This tissue was labeled Specimen B and washings from the CUSA were labeled Specimen C. Postoperative MR scan demonstrated an absent M2 segment of the left MCA and no enhancing areas. The patient was well postoperatively with no worsening of his pre‐existing hemiparesis.
Gross and Microscopic Pathology
Grossly, specimen A was noted to be a solid nodule measuring 1 cm in diameter while specimen B and C were of pale gelatinous material. Specimen B and C histology had fragments of a tumor similar to the original resection (Figure 1c). Sections of specimen A showed abnormal giant cells (Figure 1d) surrounding polarizable material (Figure 1e). What are your diagnoses?
Diagnosis
- Gossypiboma (Textiloma)
- Pilocytic astrocytoma WHO grade 1(residual)
Discussion
Given the histology findings, the operative notes from his first resection were reviewed and there was no report of missing patties or cotton balls. All swabs and patties were accounted for. Hemostasis at the initial surgery had been achieved with the use of FloSeal (Baxter Inc.), a bovine derived hemostatic matrix. The development of this gossypiboma is curious in the absence of gross macroscopic cotton fibers. Possible causes for the foreign body reaction include the hemostatic matrix used at the time of the first surgery or microscopic strands of cotton that may have been retained at the time hemostasis was being achieved.
Surgical hemostatic agents are divided into nonreabsorbable and reabsorbable, with the former group consisting of cotton, muslin and swabs that are removed prior to wound closure 4. Reabsorbable materials are left in intentionally and can cause a foreign body reaction as in the example of Surgicel (Ethicon Inc.), an oxidized cellulose material, commonly used in neurosurgical procedures. Other reabsorbable hemostatic agents in use are: gelatin compounds (Gelfoam‐ Pfizer), collagen compounds (Avitene‐ Bard Davol Inc.) and hemostatic matrix (FloSeal‐ Baxter Inc. or Surgiflo‐ Ethicon 360). Hemostatic matrix agents are thrombin‐gelatin sealants used regularly in both cranial and spinal procedures 1. The gelatin and thrombin components are mixed together at the time of use and are activated, facilitating clotting, when in contact with blood. The median time for degradation for this product is 30 days in comparison to oxidized cellulose and collagen products that have median degradation of 60 days and 90 days, respectively. Complications and adverse effects due to hemostatic matrix are rare. However, in general surgical literature, there have been reports of an inflammatory reaction to this agent causing adhesions that result in small bowel obstruction 2. In the neurosurgical literature, there have been reports of giant cell granulomas with gelatin based hemostatic products.
In rat neurosurgical models, matrix hemostatic sealant along with oxidized cellulose and collagen compounds were shown to have a propensity for inciting a granulomatous inflammatory reaction. Gelatin compounds are not polarizable on light microscopy and the same is true for hemostatic matrix. There are descriptions in the literature of granulomatous reactions to fine cotton fibers causing large intracranial lesions that mimic tumors or abscess. These fibers are derived from cotton pledgets, balls or cotton patties. Using wet cottonoids and copious irrigation reduces the risk of leaving microscopic fibers.
Radiological imaging of gossypiboma can mimic recurrent tumor or abscess in the acute setting. They appear as space occupying lesions and, in some cases, radio‐opaque marker found on swabs is not always apparent 3. In our case, one of the differentials was a traumatic aneurysm as a result of traction from retractors at the first resection. Both angiography and histology ruled this out.
Gossypiboma in neurosurgery is a rare occurrence. Its lack of specific imaging properties means it is easily mistaken for other more commonly occurring intracranial lesions.
References
- 1.Gazzeri R, Galarza M, Neroni M, Alfieri A, Giordano M (2011) Hemostatic matrix sealant in neurosurgery: a clinical and imaging study. Acta Neurochir 153:148–155. [DOI] [PubMed] [Google Scholar]
- 2.lapp B, Santillan A (2011) Small bowel obstruction after FloSeal use. JSLS 15:361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquardt G, Rettig J, Lang J, Seifert V (2001) Retained surgical sponges, a denied neurosurgical reality? Cautionary note. Neurosurg Rev 24:41–43. [DOI] [PubMed] [Google Scholar]
- 4.Ribalta T, McCutcheon I, Neto A, Gupta D, Kumar A, Biddle D_et al_ (2004) Textiloma (Gossypiboma) Mimicking Recurrent Intracranial Tumor. Arch Pathol Lab Med 128:749–758. [DOI] [PubMed] [Google Scholar]