Barrett’s oesophagus and oesophageal cancer following oesophageal atresia repair: a systematic review (original) (raw)
Abstract
Background
Concern exists that patients born with oesophageal atresia (OA) may be at high risk for Barrett’s oesophagus (BO), a known malignant precursor to the development of oesophageal adenocarcinoma. Screening endoscopy has a role in early BO identification but is not universal in this population. This study aimed to determine prevalence of BO after OA repair surgery, to quantify the magnitude of this association and inform the need for screening and surveillance.
Methods
A systematic review, undertaken according to PRISMA guidelines, was preregistered on PROSPERO (CRD42017081001). PubMed and EMBASE were interrogated using a standardized search strategy on 31 July 2020. Included papers, published in English, reported either: one or more patients with either BO (gastric/intestinal metaplasia) or oesophageal cancer in patients born with OA; or long-term (greater than 2 years) follow-up after OA surgery with or without endoscopic screening or surveillance.
Results
Some 134 studies were identified, including 19 case reports or series and 115 single- or multi-centre cohort studies. There were 13 cases of oesophageal cancer (9 squamous cell carcinoma, 4 adenocarcinoma) with a mean age at diagnosis of 40.5 (range 20–47) years. From 6282 patients under long-term follow-up, 317 patients with BO were reported. Overall prevalence of BO was 5.0 (95 per cent c.i. 4.5 to 5.6) per cent, with a mean age at detection of 13.8 years (range 8 months to 56 years). Prevalence of BO in series reporting endoscopic screening or surveillance was 12.8 (95 per cent c.i. 11.3 to 14.5) per cent.
Conclusion
Despite a limited number of cancers, the prevalence of BO in patients born with OA is relatively high. While limited by the quality of available evidence, this review suggests endoscopic screening and surveillance may be warranted, but uncertainties remain over the design and effectiveness of any putative programme.
There are concerns that patients born with oesophageal atresia are at high risk for Barrett’s oesophagus, a known malignant precursor. This systematic review aimed to determine the prevalence of Barrett's oesophagus in patients born with oesophageal atresia under long-term follow-up to quantify the magnitude of the association and inform the need for screening. The prevalence of Barrett’s oesophagus is relatively high in this population, at 5 per cent, and suggests endoscopic screening may be warranted.
Introduction
A number of reports have described oesophageal adenocarcinoma and squamous cell carcinoma (SCC) arising in adult survivors of surgery for oesophageal atresia (OA)1–6. The development of gastric and intestinal metaplasia in the oesophagus during childhood, adolescence or early adulthood has been widely documented7–16. These observations lead to the question of how these patients should be followed up to permit prompt detection of premalignant oesophageal mucosal changes. Currently, there is little consensus on either requirement for, or timing of, endoscopic screening or surveillance in patients born with OA.
Gastro-oesophageal reflux (GOR) is common following OA repair. The aetiology is probably contributed to by impaired oesophageal motility as well as disruption of the inherent antireflux mechanisms as a consequence of mobilization required to achieve an oesophageal anastomosis. The oesophageal mucosa may then be subjected to repeated exposure to refluxate that precipitates metaplasia. An international consensus statement has defined paediatric Barrett’s oesophagus (BO) as oesophageal metaplasia that is intestinal metaplasia positive or negative17.
Replacement of normal squamous epithelium in the distal oesophagus with columnar epithelium, as consequence of GOR, encompasses at least three different epithelial patterns. These are an intestinal type, usually harbouring mucous and goblet cells, as well as gastric fundus and cardiac types. Current evidence suggests that intestinal metaplasia represents the highest risk for subsequent dysplasia culminating in adenocarcinoma18. Controversy exists regarding the degree of malignant potential attributable to gastric metaplasia19.
BO is frequently occult and poorly correlated with the presence of reflux symptoms. One study reported no association between presence of symptoms of GOR in patients aged 15–19 years with and without histological evidence of BO7. Symptoms alone cannot be used to identify BO.
Whilst BO is well recognized following OA repair, the scale of the problem and associated morbidity has not been quantified beyond a handful of studies13,20–22. Without this evidence it is difficult to determine whether endoscopic screening and surveillance are indicated.
The primary aim of this review was to determine the prevalence of BO and oesophageal cancer in children, adolescents and adults born with OA to determine whether endoscopic screening and surveillance might be indicated. The secondary aim was to assimilate data to inform the design of any such surveillance programme in this population.
Methods
This review was performed in accordance with the PRISMA guidelines for systematic reviews and according to a defined protocol registered with PROSPERO (York University, York, UK) prior to commencing the review (registration number: CRD42017081001)23,24.
Search strategy
The search strategy was deliberately broad in order to be comprehensive and included studies reporting BO and/or oesophageal cancer in patients with repaired OA, in addition to those documenting long-term follow-up of patients born with OA. Several types of article were included to ensure that the search was systematic and that the findings would be as robust as possible. In addition to focusing on articles reporting outcomes of patients with OA, articles reporting cohorts of children having antireflux procedures or upper gastrointestinal endoscopy were also examined since these may have included patients born with OA. Searches were performed on 31 July 2020 using both the PubMed and Embase databases. In all databases, adjacency operators and truncation symbols were used in text word searches, when appropriate, to capture variations in phrasing and expression of terms. All synonymous terms were combined first using the Boolean ‘OR’. The three distinct concepts related to intervention, population and study design were combined with the Boolean ‘AND’. No language or date restrictions were applied. The detailed search strategy for each database used is included in Fig. S1, supplementary material. As well as using these databases, references in systematic reviews and randomized controlled trials, found in the search, were also included.
Study inclusion criteria
Articles that met one or both of the following criteria were included: any study that reported at least one patient with BO or oesophageal cancer who had undergone either OA repair or oesophageal replacement having been born with OA; or any study that reported long-term follow-up (defined as minimum 2 years) of patients following OA repair or oesophageal replacement regardless of whether they included BO or oesophageal cancer, and regardless of the use of endoscopic screening (a single endoscopy) or surveillance (a programme of sequential endoscopies).
All study types were eligible for inclusion, including cohort studies and systematic reviews, with or without meta-analysis, and case reports. For the purposes of the search, a wide definition of BO was used that included any definition used by source article authors, including both gastric and intestinal metaplasia and heterotopic gastric mucosa.
Study exclusion criteria
Studies were excluded if the patients had only an H-type tracheo-oesophageal fistula without OA. Studies were also excluded if they were abstracts only from conference presentations or published in non-English language. Where multiple reports from the same centre or authors were identified that resulted in duplication of cases or patient cohorts, either the first reporting study or the largest, in terms of patient numbers, was included.
Article selection
Two reviewers independently assessed each title and abstract of all identified citations. Full-text articles were obtained if either reviewer considered the citation potentially relevant with a low threshold for retrieval. Full texts of selected studies were then reviewed critically to assess eligibility. Reasons for exclusion of studies were recorded. The final set of studies included in the systematic review was determined by consensus. The online resource Rayyan was used to assist with article screening and selection25. A priori it was decided not to use any risk of bias assessment tool and, as it was anticipated that all studies would probably be observational in nature, no study would be excluded based on methodology alone.
Data extraction
Data were extracted independently, reviewed to ensure accuracy and entered into an electronic database recording paper title and author, study type, number of patients, length of follow-up, detail of endoscopic screening and/or surveillance and number of patients with BO/oesophageal cancer.
Outcomes
The following outcomes were selected a priori: the number of patients with oesophageal cancer born with OA; the overall prevalence of BO and oesophageal cancer in patients born with OA; and the prevalence of BO and oesophageal cancer in patients born with OA who had undergone endoscopic screening or surveillance.
Further relevant clinical details of any patient with oesophageal cancer born with OA (such as age at diagnosis, type and site of cancer, detection method and outcome) were recorded if available, as were details of endoscopic screening or surveillance programmes and clinical details of patients with BO identified at endoscopy. For the purposes of reporting in this review, intestinal metaplasia was defined as metaplastic change alongside the presence of goblet cells and gastric metaplasia defined as metaplastic change without goblet cells.
Statistical analysis
Data were entered and stored in an Excel (Microsoft, Redmond, Washington, USA) spreadsheet, descriptive analysis of data was undertaken using SPSS version 25 (IBM, Armonk, New York, USA). Data are reported as mean, median and range. The overall prevalence of BO and oesophageal cancer in patients born with OA was calculated by dividing the number of individuals with either BO or oesophageal cancer reported among the total population of OA patients by the total number of patients. The prevalence amongst the population who had undergone endoscopic screening or surveillance was calculated in a similar way, but limiting denominator population to those who had undergone one or more endoscopies.
Results
Characteristics of included studies
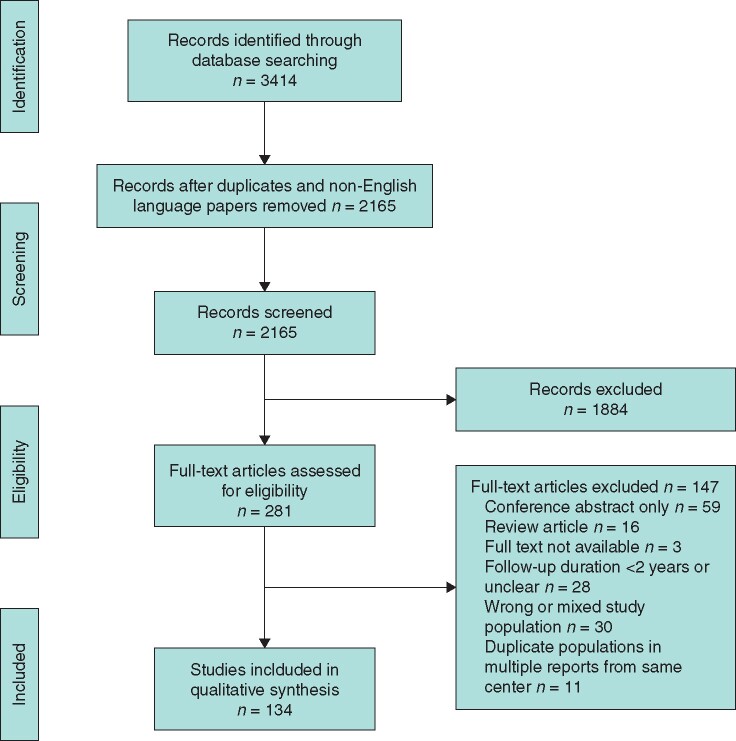
A total of 134 articles met the inclusion criteria. Details of excluded articles are shown in Fig. 1 including unavailability (3), conference abstract only (59), review article (16) and those which did not meet the inclusion criteria (58) involving short or unclear follow-up duration, wrong or mixed study population or disease process (such as oesophageal replacement in which OA and non-OA populations could not be separated). There were no cases of BO nor oesophageal carcinoma in these excluded studies. Populations published in multiple reports from the same centre were also excluded (11 populations)26–36.
Fig. 1.
PRISMA diagram
The 134 articles were published between 1972 and 2020 and included 10 case reports and nine case series, reporting one or more cases of BO or oesophageal cancer in OA patients, and 115 either single- or multi-centre cohort studies, documenting long-term follow-up of OA patients with or without endoscopic screening or surveillance. These involved a total of 6282 OA patients with long-term follow-up (greater than 2 years) following either primary repair and/or oesophageal replacement. This total population figure was used as the denominator for the subsequent calculation of BO and oesophageal cancer prevalence. Median individual study population size was 87 (range 42–870) patients. The 6282 OA patients comprised both those who were documented to have undergone endoscopy during follow-up, including 1727 who had endoscopic screening or surveillance, and those who had not.
Oesophageal cancer
There were 13 patients with oesophageal cancer identified in seven cohort studies and case reports from four centres in three countries (Table 1). Median age at diagnosis of oesophageal cancer was 40.5 (range 20–47) years; four were adenocarcinomas and nine SCCs. Five tumours were detected in the mid/distal oesophagus, three were adjacent to the site of the oesophageal anastomosis and two were in interposed segments replacing oesophagus (skin and colon)1,2,5,6,37. Three patients, two with adenocarcinoma and one with SCC, also had endoscopic evidence of BO1,2,5. There was one patient, with BO and low-grade dysplasia, in whom SCC was detected at surveillance endoscopy5.
Table 1.
Reported cases of oesophageal malignancy following oesophageal atresia repair or replacement
| Author | Setting and study type | No of patients | Age at diagnosis (years) | Malignancy type, site and grade | Clinical details | Outcome |
|---|---|---|---|---|---|---|
| LaQuaglia et al. 198737 | Case report (USA) | 1 | 45 | Squamous cell carcinoma Proximal oesophagus/skin tube T4N0M0 | F, Gross type C Antethoracic skin tube conduit Non-smoker, no ETOH | Resection and colonic interposition Local proximal recurrence: re-resection and local radiotherapy |
| Adzick et al. 19893 | Case report (USA) | 1 | 20 | Adenocarcinoma Distal oesophagus/GOJ, T2N0M0 | F, Gross type C Non-smoker, no ETOH No evidence of Barrett’s/oesophagitis | Oesophagogastrectomy and colonic interposition Alive at 1 year – no recurrence |
| Deurloo et al. 20016 | Case report (Netherlands) | 1 | 38 | Squamous cell carcinoma Mid-oesophageal (2 cm distal to previous anastomosis) T3N1M0 | M, Gross type C Anastomotic stricture resection 18 mo Occasional smoker, 4 units ETOH/day | Neo-adjuvant chemotherapy Subtotal oesophagectomy and gastric tube interposition Postoperative radiotherapy Alive at 2 years – no recurrence |
| Alfaro et al. 20052 | Case report (USA) | 1 | 46 | Adenocarcinoma (Barrett’s and high-grade dysplasia) Mid-oesophagus Moderately invasive | F, primary repair | Neoadjuvant chemoradiotherapy Oesophagectomy and gastric transposition Alive at 2 months |
| Pultrum et al. 20051 | Case report (Netherlands) | 1 | 22 | Adenocarcinoma (and Barrett’s) At site of anastomosis T3N1M1 – moderate to highly differentiated | F, Gross type C Nissen fundoplication for GORD Endoscopic surveillance – no Barrett’s | Palliative radiotherapy and intraluminal stenting Died |
| Jayasekera et al. 20125 | Case series (Australia) | 4 | 44, 46, 46, 44 | Squamous cell carcinoma At site of anastomosis, T3N0M0 Mid/distal oesophagus (and associated sub-carinal mass) TXN2M0 SCC in situ, mid/distal oesophagus Mediastinal mass eroding through ribs and sternum | F, Gross type C Primary repair Heavy smoker 4 years (15–19 yo), non-smoker 25 years, no ETOH F, Gross type C Primary repair Non-smoker and no ETOH M, Gross type C 2× anastomotic stricture resection Smoker (20 pack years), 10g ETOH/week Barrett’s and low-grade dysplasia (annual surveillance for 10 years) M, Gross type C Repair of recurrent fistula and resection of stricture | Oesophagectomy, no chemoradiotherapy Recurrent local and metastatic disease 4 years later – died Chemoradiotherapy – ongoing at time of publication Unsuccessful endoscopic resection, ongoing chemoradiotherapy |
| Vergouwe et al. 20184 | Case series (Netherlands) | 4 | 36, 42, 45, 47 | Squamous cell carcinoma Distal oesophagus (25–32 cm) pT1bN0M0 Proximal oesophagus, with invasion of surrounding structures (trachea) T4N2M0 3 cm distal to anastomosis, pT2N0M0 Adenocarcinoma in colonic interposition pT2N1M0, moderately differentiated | F, Gross type A Primary repair (Livaditis elongation) Non-smoker and no ETOH M, Gross type A Delayed primary repair VACTERL Smoker, moderate ETOH M, Gross type C Primary repair Heavy smoker (27 pack years) and ETOH M, Gross type C Gastrostomy and oesophagostomy Colonic interposition (7 mo) VACTERL Smoker, minimal ETOH | Subtotal oesophagectomy, gastrectomy, colon interposition – metastatic disease at 12 months Chemotherapy (tumour unresectable) – alive at 6 years, no recurrence Oesophagectomy and gastric tube reconstruction. Further tumour in native cervical oesophagus 15 years later – died Chemotherapy, resection and gastric tube pull-up. Alive at 1 year |
At last recorded follow-up, five patients were alive, having completed treatment, five patients were receiving ongoing treatment and three had died (Table 1).
The overall prevalence of oesophageal cancer in OA patients under long-term follow-up was 0.002 per cent (13 of 6282 patients) with a prevalence of 0.06 per cent (1 of 1727) in the cohort who had undergone either endoscopic screening or surveillance.
Barrett’s oesophagus
Some 317 patients with BO were reported in 48 cohort studies and case reports from 30 centres in 18 countries7–10,12–16,20–22,38–73, representing all reported patients with BO under long-term follow-up for OA.
Of these, intestinal metaplasia was identified in 54 patients, gastric metaplasia in 227, low-grade dysplasia in one, heterotopic gastric mucosa in three patients and type of metaplasia unspecified in 38.
The overall prevalence of BO in OA patients under long-term follow-up was 5.0 (95 per cent c.i. 4.5 to 5.6) per cent (317 of 6282 patients) (Fig. S2, supplementary material). The mean age at detection of BO was 13.8 years, median 16 years (range 8 months to 56 years).
Endoscopic screening and surveillance
There were 1727 patients who underwent one or more endoscopies with or without biopsies during OA follow-up. The 24 studies in which either endoscopic screening or surveillance were undertaken are summarized in Table 2 7–10,12–15,20–22,49,51,56–60,68,73–77. They report endoscopies performed in defined OA populations with known numbers.
Table 2.
Studies reporting endoscopic screening following oesophageal atresia repair or replacement
| Author | Setting and study type | Population and age (range) | Intervention | Outcomes |
|---|---|---|---|---|
| Ure et al. 199574 | Single centre, prospective cohort | Long gap OA with colonic interposition 1963–1971 (n = 9) Mean 24 (22–27) years | UGIE + biopsies (n = 3) | 0 cases of metaplasia or malignancy (0%) |
| Somppi et al. 199815 | Single centre, prospective cohort | OA repair/replacement 1963–1993 (n = 51) | UGIE + biopsies (n = 41) | 2 gastric metaplasia (4.9%)Mean 12.6 (3.5–30) years |
| Khan et al. 199875 | Single centre, retrospective cohort | Colonic interposition for oesophageal replacement 1974–1993 (n = 25 of which OA n = 23) | UGIE + biopsies (n = 13) | 0 cases of metaplasia (0%)(5–15 years) |
| Krug et al. 199914 | Single centre, prospective cohort | OA repair 1971–1978 (n = 39) (18–26 years) | UGIE + biopsies (n = 34) | 2 intestinal metaplasia (5.8%) |
| Deurloo et al. 200312 | Single centre, prospective cohort | OA repair 1947–1972 (n = 38) Median 34 (28–45) years | UGIE + biopsies (n = 21) | 1 intestinal metaplasia (4.8%) |
| Deurloo et al. 200513 | Single centre, prospective cohort | OA repair 1973–1985 (n = 92) Median 17 (10–26) years | UGIE + biopsies (n = 40) | 3 gastric metaplasia (7.5%) |
| Holschneider et al. 200749 | Single centre, retrospective cohort | Fundoplications 1993–2005 (n = 160 of which OA n = 87) Median 4.3 years (1 mo to 10 years) | UGIE + biopsies (n = 40) | 1 intestinal metaplasia (2.5%) |
| Taylor et al. 200751 | Single centre, prospective cohort | OA repair before 1982 reviewed in clinic 2000–2003 (n = 132) Mean 33 (22–48) years | UGIE + biopsies (n = 62) | 7 intestinal metaplasia (11.3%) of which 3 had concurrent low-grade dysplasia1 squamous cell carcinoma* |
| Castilloux et al. 201020 | Single centre, prospective cohort | OA repair and >2 years old (or <2 years old and indication for UGIE) 2005–2008 (n = 45) Median 7.3 years (5 mo to 17 years) | UGIE + biopsies | 16 gastric metaplasia (35.6%)Median 9.8 (3.4–13.2) years |
| Sistonen et al. 201010 | Single centre, prospective cohort | OA repair 1947–1985 (n = 98) Mean 36 (21–57) years | UGIE + biopsies | 15 gastric metaplasia, 6 intestinal metaplasia (20.7%) |
| Burjonrappa et al. 20119 | Single centre, retrospective cohort | OA repair 1990–2009 (n = 51) Mean 6.6 years (7 mo to 19 years) | UGIE + biopsies (n = 38) | 11 gastric metaplasia, 1 intestinal metaplasia (31.6%)Mean 13 years |
| Pedersen et al. 20138 | Single centre, prospective cohort | OA repair 1993–2005 (n = 59) Median 10.2 (5–15) years | UGIE + biopsies (n = 56) | 1 intestinal metaplasia (1.8%) |
| Huynh-Trudeau et al. 201556 | Single centre, prospective cohort | OA repair/interposition with dysphagia (n = 41) Mean 25 (18–44) years | UGIE + biopsies (n = 32) | 6 gastric metaplasia, 4 intestinal metaplasia (31.3%) |
| Koziarkiewicz et al. 201557 | Single centre, prospective cohort | OA repair 1990–2005 (n = 30) Median 13.7 (7–17) years | UGIE + biopsies (n = 12) | 2 intestinal metaplasia (16.7%) |
| Reismann et al. 201576 | Single centre, retrospective cohort | Long gap OA treated with gastric transposition 1999–2012 (n = 9) Mean 6.2 (1.4–10.2) yeears | UGIE +/- biopsies (n = 8) | 0 cases of metaplasia or malignancy (0%) |
| Cartabuke et al. 201658 | Single centre, retrospective cohort | OA repair/replacement 2011–2014 (n = 43) Median 8 (i.q.r. 3–20) years | UGIE + biopsies (n = 31) | 2 patients Barrett’s oesophagus (type not specified) (6.5%) |
| Gatzinsky et al. 201659 | Single centre, prospective cohort | OA repair 1968–1983 (n = 29) Median 31 (25–40) years | UGIE + biopsies (n = 24) | 2 intestinal metaplasia (8.3%) |
| Iwańczak et al. 201660 | Single centre, retrospective cohort | Thoracoscopic OA +/-TOF repair (n = 22) Mean 47 (16–79) months | UGIE +/- biopsies (n = 11) | 1 gastric metaplasia (9.1%) |
| Koivusalo et al. 201621 | Single centre, retrospective cohort | Treated OA 1980–2014 (n = 211) | UGIE + biopsies (n = 209) | 31 gastric metaplasia, 4 intestinal metaplasia (16.7%)Median 22 (16–32) years |
| Schneider et al. 20167 | Multicentre, prospective cohort | Primary OA repair (n = 120) Mean 16.5 (15–19) years | UGIE + biopsies (n = 120) | 50 gastric metaplasia, 1 intestinal metaplasia (42.5%) |
| Hsieh et al. 201722 | Multicentre, retrospective cohort | OA followed up in specialist clinic (n = 541) | UGIE + biopsies | 7 intestinal metaplasia (1.3%)Median 10 (2–17.2) years |
| Vergouwe et al. 201868 | Single centre, prospective cohort | OA patients 1948–1999 (n = 151) Median 25.4 (16.8–68.6) years | UGIE + biopsies (n = 151) | 26 gastric metaplasia, 10 intestinal metaplasia (23.8%) |
| Youn et al. 201877 | Single centre prospective cohort | Gastric tube interposition (n = 25 with OA) Median 12 (3–18) years | UGIE + biopsies (n = 20) | 0 cases of metaplasia (0%)Median 15.4 (9–18) years |
| Petit et al. 201973 | Single centre prospective cohort | OA patients 2005–2014 (n = 77) Median 4.9 (3.6–8) years | UGIE + biopsies (n = 73) | 9 gastric metaplasia (12.3%)Median 2 years (1–3) |
Twenty studies reported results of endoscopic screening; a single endoscopy to assess for BO, which was undertaken at mean age of 20 years (median 16 years (range 16 months to 57 years))7,8,10,12–15,20,49,51,56–60,68,74–77. While many of these studies, reporting screening endoscopies, suggested a requirement for further surveillance when BO was identified, few subsequently outlined their proposed surveillance regimen68.
Two studies reported the results of a combination of screening and surveillance endoscopies, but did not report the age range at which these were undertaken22,73. Two studies reported endoscopic surveillance in paediatric populations9,21. The first reported results from 3-yearly surveillance endoscopies from the age of 3 years until transition to adult care9. Additional ‘off-schedule’ endoscopies were undertaken in children with severe reflux in whom surgical intervention was under consideration9. In the second study, surveillance endoscopies were undertaken at 1, 3, 5, 10, 15 and over 15 years until the age of 1721.
There were 221 patients with BO (intestinal metaplasia, 49; gastric metaplasia, 170; metaplasia type unspecified, 2). The prevalence of BO in the cohort who had undergone endoscopic screening or surveillance was 12.8 (95 per cent c.i. 11.3 to 14.5) per cent (221 of 1727 patients) (range per series 0–42.5 per cent)7–10,12–15,20–22,49,51,56–60,68,73–77. Intestinal metaplasia was detected at a mean age of 38.5 years (median 38.5 (range 2-56) years) and gastric metaplasia at a mean age of 9.5 years (median 16.5 (range 2–56) years).
In those detected before the age of 16 years, identified by paediatric endoscopies, of the 49 patients with intestinal metaplasia, 11 were 15 years or younger and 38 were older than 15 years. Among those with gastric metaplasia, 60 patients were 15 years or younger and 101 were older than 15 years.
From studies reporting endoscopic surveillance, in six patients gastric metaplasia preceded intestinal metaplasia on sequential endoscopies, with gastric metaplasia occurring 1–5 years prior21,22. While there were two reported cases of resolution of BO (1 gastric and 1 intestinal) either spontaneously or following anti-reflux treatment, the majority of cases of BO persisted9,47,66. Gastric and intestinal metaplasia were present concurrently at screening endoscopy in four patients22. Three patients had intestinal metaplasia associated with low-grade dysplastic changes at screening endoscopy51. A single oesophageal cancer (SCC) was reported in the population who had undergone endoscopic surveillance5,51.
Discussion
This systematic review identified a notable global prevalence of BO in this population, highest in those who had undergone endoscopic screening. Oesophageal cancer following OA repair or replacement remained rare, however, with just 13 patients reported, the majority of whom had SCC not adenocarcinoma. Only a single cancer (an SCC) was picked up by endoscopic surveillance.
The present review should be considered in the context of increasing concern that patients born with OA are at increased risk for developing oesophageal cancer5,48,68,78. Although the absolute number of cases of oesophageal cancer identified was relatively low, the likelihood of under-reporting seems considerable. The majority of studies reported follow-up in the paediatric period, in patients aged 15 years or younger, whereas all cancer diagnoses have occurred in adulthood with a mean age at diagnosis of 40 years. As there are no population-based cohort studies of patients born with OA being followed into adult life, it is not possible to define with certainty the true prevalence of oesophageal cancer in this population. The closest estimate is a population-based study from Finland of 272 patients born with OA with median 35 years of follow-up. No patients with oesophageal cancer were identified11. With a background incidence of oesophageal cancer in Finland at the time of 4.3 per 100 000 they were only able to exclude a prevalence of oesophageal cancer in patients born with OA of greater than 500 times that of the background population. Of note, patients in the present analysis developed oesophageal cancer at a younger age (median 40.5 years) than the general population, where the median age at diagnosis is around 64 years79.
BO is a recognized precursor to oesophageal adenocarcinoma, implying that endoscopic screening and surveillance of at-risk individuals, such as those with OA, might identify premalignant change and permit early interventions80. Based on the present review, an overall prevalence of BO in patients born with OA appears to be about 5 per cent in a mixed screened and unscreened population, rising to around 13 per cent in the screening and surveillance cohort. This is notably higher than the background prevalence of BO in both adult and paediatric populations, reported at 1.3–1.6 per cent and 0.002 per cent respectively81–83.
Despite this high prevalence, no patient under endoscopic surveillance progressed to adenocarcinoma. However, the majority of studies included in the review report cases of BO identified from screening rather than surveillance endoscopies. Although prevalence rates from screening suggest that endoscopic surveillance may be justified, it is unclear to what extent it would be either clinically beneficial or cost-effective.
A range of screening and surveillance programmes was identified in the present review. The youngest patient identified with BO (gastric metaplasia) was aged 8 months42. Intestinal metaplasia has been reported in a patient as young as 2 years22. In the present study, one in five cases of intestinal metaplasia and one third of gastric metaplasia cases, detected by endoscopic screening or surveillance, were in children aged 15 years or less. This may be taken to suggest that screening should start during childhood and, indeed, some authors have advocated that screening should commence during the teenage years or early 20s7,9,48. The optimal frequency of surveillance in this population also remains unclear. ESPGHAN guidance recommends three surveillance endoscopies during childhood in asymptomatic patients with treated OA: after stopping anti-reflux therapy, before the age of 10 years and a further endoscopy on transition to adult care84. Current adult guidelines recommend surveillance endoscopies every 2–5 years, depending upon the length and type of BO, with more frequent surveillance advised when dysplastic changes are present19,85.
In line with guidelines, the present review included both gastric and intestinal metaplastic change in the definition of BO19. This may explain why the prevalence of BO was as high as 43 per cent in one study7. Intestinal metaplasia is generally considered to be the significant risk factor for malignancy, specifically adenocarcinoma86, although the relative risks associated with gastric metaplasia, columnar epithelium without goblet cells, remains a subject of controversy18,87. The lack of documented progression of BO to oesophageal cancer in patients born with OA in the present review means the importance of either gastric or intestinal epithelial metaplasia in this population cannot be evaluated.
A notable observation in the present review was the preponderance of SCC rather than adenocarcinoma. The absence of a recognizable precursor lesion for SCC suggests that endoscopic surveillance based on BO would be ineffective. Until there is a sufficient number of high-quality studies with follow-up over a long time period, no firm conclusions can be drawn.
Despite the present study being limited by the quality of existing available evidence, the broad approach to identifying patients at risk and wide study inclusion criteria have proved informative. Few studies documented prospective endoscopic screening and surveillance programmes and this limits the ability to make comparisons between different screening or surveillance programmes. In view of the numbers involved, international collaborative studies should be undertaken to identify the optimal screening and surveillance programmes in this population and assess their clinical benefit and cost effectiveness.
Supplementary Material
zrab069_Supplementary_Data
Acknowledgements
The authors wish to acknowledge the RCSEng Clinical Research Initiative who provided educational support for this review. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Contributor Information
L Tullie, Department of Paediatric Surgery and Urology, Southampton Children’s Hospital, Southampton, UK; National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, University College London Great Ormond Street Institute for Child Health, London, UK; Stem Cell and Cancer Biology Laboratory, The Francis Crick Institute, London, UK.
A Kelay, Department of Paediatric Surgery and Urology, Southampton Children’s Hospital, Southampton, UK.
G S Bethell, Department of Paediatric Surgery and Urology, Southampton Children’s Hospital, Southampton, UK; University Surgery Unit, Faculty of Medicine, University of Southampton, Southampton, UK.
C Major, Department of Paediatric Surgery and Urology, Southampton Children’s Hospital, Southampton, UK.
N J Hall, Department of Paediatric Surgery and Urology, Southampton Children’s Hospital, Southampton, UK; University Surgery Unit, Faculty of Medicine, University of Southampton, Southampton, UK.
Funding
L.T. is funded by the NIHR Great Ormond Street Hospital Biomedical Research Centre and G.B. is funded by the NIHR Academic Clinical Fellow programme.
Disclosure. The authors declare no conflicts of interest.
Supplementary material
Supplementary material is available at BJS Open online.
References
- 1.Pultrum BB, Bijleveld CM, de Langen ZJ, Plukker JTM. Development of an adenocarcinoma of the esophagus 22 years after primary repair of a congenital atresia. J Pediatr Surg 2005;40:e1–e4 [DOI] [PubMed] [Google Scholar]
- 2.Alfaro L, Bermas H, Fenoglio M, Parker R, Janik JS. Are patients who have had a tracheoesophageal fistula repair during infancy at risk for esophageal adenocarcinoma during adulthood? J Pediatr Surg 2005;40:719–720 [DOI] [PubMed] [Google Scholar]
- 3.Adzick NS, Fisher JH, Winter HS, Sandler RH, Hendren WH. Esophageal adenocarcinoma 20 years after esophageal atresia repair. J Pediatr Surg 1989;24:741–744 [DOI] [PubMed] [Google Scholar]
- 4.Vergouwe FW, Gottrand M, Wijnhoven BP, Ijsselstijn H, Piessen G, Bruno MJ et al. Four cancer cases after esophageal atresia repair: time to start screening the upper gastrointestinal tract. World J Gastroenterol 2018;24:1056–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayasekera CS, Desmond PV, Holmes JA, Kitson M, Taylor ACF. Cluster of 4 cases of esophageal squamous cell cancer developing in adults with surgically corrected esophageal atresia – time for screening to start. J Pediatr Surg 2012;47:646–651 [DOI] [PubMed] [Google Scholar]
- 6.Deurloo JA, van Lanschot JJB, Drillenburg P, Aronson DC. Esophageal squamous cell carcinoma 38 years after primary repair of esophageal atresia. J Pediatr Surg 2001;36:629–630 [DOI] [PubMed] [Google Scholar]
- 7.Schneider A, Gottrand F, Bellaiche M, Becmeur F, Lachaux A, Bridoux-Henno L et al. Prevalence of Barrett esophagus in adolescents and young adults with esophageal atresia. Ann Surg 2016;264:1004–1008 [DOI] [PubMed] [Google Scholar]
- 8.Pedersen RN, Markøw S, Kruse-Andersen S, Qvist N, Hansen TP, Gerke O et al. Esophageal atresia: gastroesophageal functional follow-up in 5–15 year old children. J Pediatr Surg 2013;48:2487–2495 [DOI] [PubMed] [Google Scholar]
- 9.Burjonrappa SC, Youssef S, St-Vil D. What is the incidence of Barrett's and gastric metaplasia in esophageal atresia/tracheoesophageal fistula (EA/TEF) patients? Eur J Pediatr Surg 2011;21:25–29 [DOI] [PubMed] [Google Scholar]
- 10.Sistonen SJ, Koivusalo A, Nieminen U, Lindahl H, Lohi J, Kero M et al. Esophageal morbidity and function in adults with repaired esophageal atresia with tracheoesophageal fistula. Ann Surg 2010;251:1167–1173 [DOI] [PubMed] [Google Scholar]
- 11.Sistonen SJ, Koivusalo A, Lindahl H, Pukkala E, Rintala RJ, Pakarinen MP. Cancer after repair of esophageal atresia: population-based long-term follow-up. J Pediatr Surg 2008;43:602–605 [DOI] [PubMed] [Google Scholar]
- 12.Deurloo JA, Ekkelkamp S, Bartelsman JFWM, Ten Kate FJW, Schoorl M, Heij HA et al. Gastroesophageal reflux: prevalence in adults older than 28 years after correction of esophageal atresia. Ann Surg 2003;238:686–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deurloo JA, Ekkelkamp S, Taminiau JAJM, Kneepkens CMF, ten Kate FWJ, Bartelsman JFWM et al. Esophagitis and Barrett esophagus after correction of esophageal atresia. J Pediatr Surg 2005;40:1227–1231 [DOI] [PubMed] [Google Scholar]
- 14.Krug E, Bergmeijer JHLJ, Dees J, de Krijger R, Mooi WJ, Hazebroek FWJ. Gastroesophageal reflux and Barrett’s esophagus in adults born with esophageal atresia. Am J Gastroenterol 1999;94:2825–2828 [DOI] [PubMed] [Google Scholar]
- 15.Somppi E, Tammela O, Ruuska T, Rahnasto J, Laitinen J, Turjanmaa V et al. Outcome of patients operated on for esophageal atresia: 30 years' experience. J Pediatr Surg 1998;33:1341–1346 [DOI] [PubMed] [Google Scholar]
- 16.Lindahl H, Rintala R, Sariola H. Chronic esophagitis and gastric metaplasia are frequent late complications of esophageal atresia. J Pediatr Surg 1993;28:1178–1180 [DOI] [PubMed] [Google Scholar]
- 17.Sherman PM, Hassall E, Fagundes-Neto U, Gold BD, Kato S, Koletzko S et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol 2009;104:1278–1295 [DOI] [PubMed] [Google Scholar]
- 18.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011;103:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P et al. ; British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7–42 [DOI] [PubMed] [Google Scholar]
- 20.Castilloux J, Bouron-Dal Soglio D, Faure C. Endoscopic assessment of children with esophageal atresia: lack of relationship of esophagitis and esophageal metaplasia to symptomatology. Can J Gastroenterol 2010;24:312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koivusalo AI, Pakarinen MP, Lindahl HG, Rintala RJ. Endoscopic surveillance after repair of oesophageal atresia: longitudinal study in 209 patients. J Pediatr Gastroenterol Nutr 2016;62:562–566 [DOI] [PubMed] [Google Scholar]
- 22.Hsieh H, Frenette A, Michaud L, Krishnan U, Dal-Soglio DB, Gottrand F et al. Intestinal metaplasia of the esophagus in children with esophageal atresia. J Pediatr Gastroenterol Nutr 2017;65:e1–e4 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tullie L, Kelay A, Bethell G, Major C, Hall N. A systematic review of the prevalence and age at presentation of Barrett's oesophagus and oesophageal cancer in patients born with oesophageal atresia. PROSPERO, 2017
- 25.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers NA. Oesophageal atresia with distal tracheo-oesophageal fistula – a long term follow-up. Prog Pediatr Surg 1977;10:5–17 [PubMed] [Google Scholar]
- 27.Lindahl H, Louhimo I, Virkola K. 30-year follow-up of the original Sulamaa (end-to-side) operation for oesophageal atresia. Z Kinderchir 1983;38:152–154 [DOI] [PubMed] [Google Scholar]
- 28.Lindahl H, Louhimo I, Virkola K. Colon interposition or gastric tube? Follow-up study of colon–esophagus and gastric tube–esophagus patients. J Pediatr Surg 1983;18:58–63 [DOI] [PubMed] [Google Scholar]
- 29.Lindahl H. Long-term prognosis of successfully operated oesophageal atresia – with aspects on physical and psychological development. Z Kinderchir 1984;39:6–10 [DOI] [PubMed] [Google Scholar]
- 30.Koivusalo A, Pakarinen MP, Rintala RJ. The cumulative incidence of significant gastrooesophageal reflux in patients with oesophageal atresia with a distal fistula – a systematic clinical, pH-metric, and endoscopic follow-up study. J Pediatr Surg 2007;42:370–374 [DOI] [PubMed] [Google Scholar]
- 31.Deurloo JA, Klinkenberg EC, Ekkelkamp S, Heij HA, Aronson DC. Adults with corrected oesophageal atresia: is oesophageal function associated with complaints and/or quality of life? Pediatr Surg Int 2008;24:537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koivusalo AI, Pakarinen MP, Rintala RJ. Modern outcomes of oesophageal atresia: single centre experience over the last twenty years. J Pediatr Surg 2013;48:297–303 [DOI] [PubMed] [Google Scholar]
- 33.Presse N, Taillefer J, Maynard S, Bouin M. Insufficient body weight of adults born with esophageal atresia. J Pediatr Gastroenterol Nutr 2016;62:469–473 [DOI] [PubMed] [Google Scholar]
- 34.Jonsson L, Freiberg LG, Gatzinsky V, Kotz K, Sillen U, Abrahammson K. Treatment and follow-up of patients with long-gap esophageal atresia: 15 years’ of experience from the Western region of Sweden. Eur J Pediatr Surg 2016;26:150–159 [DOI] [PubMed] [Google Scholar]
- 35.Koivusalo AI, Rintala RJ, Pakarinen MP. Outcomes of fundoplication in oesophageal atresia associated gastrooesophageal reflux disease. J Pediatr Surg 2018;53:230–233 [DOI] [PubMed] [Google Scholar]
- 36.Righini Grunder F, Petit L-M, Ezri J, Jantchou P, Aspirot A, Laberge S et al. Should proton pump inhibitors be systematically prescribed in patients with esophageal atresia after surgical repair? J Pediatr Gastroenterol Nutr 2019;69:45–51 [DOI] [PubMed] [Google Scholar]
- 37.LaQuaglia MP, Gray M, Schuster SR. Esophageal atresia and ante-thoracic skin tube esophageal conduits: squamous cell carcinoma in the conduit 44 years following surgery. J Pediatr Surg 1987;22:44–47 [DOI] [PubMed] [Google Scholar]
- 38.Shamberger RC, Eraklis AJ, Kozakewich HPW, Hendren WH. Fate of the distal esophageal remnant following esophageal replacement. J Pediatric Surg 1988;23:1210–1214 [DOI] [PubMed] [Google Scholar]
- 39.Lindahl H, Rintala R, Louhimo I. Failure of the Nissen fundoplication to control gastroesophageal reflux in esophageal atresia patients. J Pediatr Surg 1989;24:985–987 [DOI] [PubMed] [Google Scholar]
- 40.Lindahl H, Rintala R, Sariola H, Louhimo I. Cervical Barrett's esophagus: a common complication of gastric tube reconstruction. J Pediatr Surg 1990;25:446–448 [DOI] [PubMed] [Google Scholar]
- 41.Cheu HW, Grosfeld JL, Heifetz SA, Fitzgerald J, Rescorla F, West K. Persistence of Barrett's esophagus in children after antireflux surgery: influence on follow-up care. J Pediatr Surg 1992;27:260–266 [DOI] [PubMed] [Google Scholar]
- 42.Othersen HB Jr, Ocampo RJ, Parker EF, Smith CD, Tagge EP. Barrett's esophagus in children. Diagnosis and management. Ann Surg 1993;217:676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovar JA, Gorostiaga L, Echeverry J, Torrado J, Eizaguirre I, Garay I. Barrett's oesophagus in children and adolescents. Pediatr Surg Int 1993;8:389–394 [Google Scholar]
- 44.Schier F, Korn S, Michel E. Experiences of a parent support group with the long-term consequences of esophageal atresia. J Pediatr Surg 2001;36:605–610 [DOI] [PubMed] [Google Scholar]
- 45.De la Hunt MN, Jackson CR, Wright C. Heterotopic gastric mucosa in the upper esophagus after repair of atresia. J Pediatr Surg 2002;37:14–15 [DOI] [PubMed] [Google Scholar]
- 46.Kawahara H, Imura K, Yagi M, Kubota A, Okada A. Collis-Nissen procedure in patients with esophageal atresia: long-term evaluation. World J Surg 2002;26:1222–1227 [DOI] [PubMed] [Google Scholar]
- 47.Schalamon J, Lindahl H, Saarikoski H, Rintala RJ. Endoscopic follow-up in esophageal atresia – for how long is it necessary? J Pediatr Surg 2003;38:702–704 [DOI] [PubMed] [Google Scholar]
- 48.Little DC, Rescorla FJ, Grosfeld JL, West KW, Scherer LR, Engum SA. Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg 2003;38:852–856 [DOI] [PubMed] [Google Scholar]
- 49.Holschneider P, Dübbers M, Engelskirchen R, Trompelt J, Holschneider A. Results of the operative treatment of gastroesophageal reflux in childhood with particular focus on patients with esophageal atresia. Eur J Pediatr Surg 2007;17:163–175 [DOI] [PubMed] [Google Scholar]
- 50.Sri Paran T, Decaluwe D, Corbally M, Puri P. Long-term results of delayed primary anastomosis for pure oesophageal atresia: a 27-year follow up. Pediatr Surg Int 2007;23:647–651 [DOI] [PubMed] [Google Scholar]
- 51.Taylor ACF, Breen KJ, Auldist A, Catto-Smith A, Clarnette T, Crameri J et al. Gastroesophageal reflux and related pathology in adults who were born with esophageal atresia: a long-term follow-up study. Clin Gastroenterol Hepatol 2007;5:702–706 [DOI] [PubMed] [Google Scholar]
- 52.Holland AJA, Ron O, Pierro A, Drake D, Curry JI, Kiely EM et al. Surgical outcomes of esophageal atresia without fistula for 24 years at a single institution. J Pediatr Surg 2009;44:1928–1932 [DOI] [PubMed] [Google Scholar]
- 53.Burjonrappa S, Thiboutot E, Castilloux J, St-Vil D. Type A esophageal atresia: a critical review of management strategies at a single center. J Pediatr Surg 2010;45:865–871 [DOI] [PubMed] [Google Scholar]
- 54.Tran S, Misra S, Bittner JG, Pipkin W, Hatley R, Howell CG. Heterotopic gastric mucosa of the upper esophagus following repair of esophageal atresia with tracheoesophageal fistula. J Pediatr Surg 2011;46:e37–e39 [DOI] [PubMed] [Google Scholar]
- 55.Gottrand M, Michaud L, Guimber D, Coopman S, Sfeir R, Bonnevalle M et al. Barrett esophagus and esophagojejunal anastomotic stenosis as complications of esophagogastric disconnection in children with esophageal atresia. J Pediatr Gastroenterol Nutr 2013;57:93–95 [DOI] [PubMed] [Google Scholar]
- 56.Huynh-Trudeau V, Maynard S, Terzic T, Soucy G, Bouin M. Dysphagia among adult patients who underwent surgery for esophageal atresia at birth. Can J Gastroenterol Hepatol 2015;29:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koziarkiewicz M, Taczalska A, Jasiñska-Jaskula I, Grochulska-Cerska H, Piaseczna-Piotrowska A. Long-term complications of congenital esophageal atresia – single institution experience. Indian Pediatr 2015;52:499–501 [DOI] [PubMed] [Google Scholar]
- 58.Cartabuke RH, Lopez R, Thota PN. Long-term esophageal and respiratory outcomes in children with esophageal atresia and tracheoesophageal fistula. Gastroenterol Rep (Oxf) 2016;4:310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gatzinsky V, Andersson O, Eriksson A, Jönsson L, Abrahamsson K, Sillén U. Added value of pH multichannel intraluminal impedance in adults operated for esophageal atresia. Eur J Pediatr Surg 2016;26:172–179 [DOI] [PubMed] [Google Scholar]
- 60.Iwańczak B, Kosmowska-Miśków A, Kofla-Dłubacz A, Palczewski M, Grabiński M, Pawłowska K et al. Assessment of clinical symptoms and multichannel intraluminal impedance and pH monitoring in children after thoracoscopic repair of esophageal atresia and distal tracheoesophageal fistula. Adv Clin Exp Med 2016;25:917–922 [DOI] [PubMed] [Google Scholar]
- 61.Ostlie D, Leys C, Struckmeyer S, Parker M, Nichol P, Acher C. Long-term outcomes of patients with tracheoesophageal fistula/esophageal atresia: survey results from tracheoesophageal fistula/esophageal atresia online communities. Eur J Pediatr Surg 2016;26:476–480 [DOI] [PubMed] [Google Scholar]
- 62.Ho T, Sistonen S, Koivusalo A, Pakarinen M, Rintala R, Stenman U-H et al. No tissue expression of KRAS or BRAF mutations in 61 adult patients treated for esophageal atresia in early childhood. Eur J Pediatr Surg 2018;28:413–419 [DOI] [PubMed] [Google Scholar]
- 63.Koivusalo AI, Pakarinen MP. Outcome of surgery for pediatric gastroesophageal reflux: clinical and endoscopic follow-up after 300 fundoplications in 279 consecutive patients. Scand J Surg 2018;107:68–75 [DOI] [PubMed] [Google Scholar]
- 64.Wanaguru D, Langusch C, Krishnan U, Varjavandi V, Jiwane A, Adams S et al. Is fundoplication required after the Foker procedure for long gap esophageal atresia? J Pediatr Surg 2017;52:1117–1120 [DOI] [PubMed] [Google Scholar]
- 65.Friedmacher F, Kroneis B, Huber-Zeyringer A, Schober P, Till H, Sauer H et al. Postoperative complications and functional outcome after esophageal atresia repair: results from longitudinal single-center follow-up. J Gastrointest Surg 2017;21:927–935 [DOI] [PubMed] [Google Scholar]
- 66.Koivusalo AI, Sistonen SJ, Lindahl HG, Rintala RJ, Pakarinen MP. Long-term outcomes of oesophageal atresia without or with proximal tracheooesophageal fistula – Gross types A and B. J Pediatr Surg 2017;52:1571–1575 [DOI] [PubMed] [Google Scholar]
- 67.Tan L-Z, Gifford AJ, Clarkson CM, Henry GM, Krishnan U. Barrett's esophagus and eosinophilic esophagitis in a young pediatric patient with esophageal atresia. J Pediatr Surg Case Rep 2015;3:272–275 [Google Scholar]
- 68.Vergouwe FWT, Ijsselstijn H, Biermann K, Erler NS, Wijnen RMH, Bruno MJ et al. High prevalence of Barrett’s esophagus and esophageal squamous cell carcinoma after repair of esophageal atresia. Clin Gastroenterol Hepatol 2018;16:513–521.e6 [DOI] [PubMed] [Google Scholar]
- 69.Harrison LR, Kenwright D, Stringer MD. Esophageal heterotopic gastric mucosa in esophageal atresia. J Pediatr Surg Case Rep 2018;32:23–26 [Google Scholar]
- 70.Svetanoff WJ, Smithers CJ, Jennings R. Weighted abdominal traction for assistance in abdominal closure. J Pediatr Surg Case Rep 2018;29:59–62 [Google Scholar]
- 71.Courbette O, Omari T, Aspirot A, Faure C. Characterization of esophageal motility in children with operated esophageal atresia using high-resolution impedance manometry and pressure flow analysis. J Pediatr Gastroenterol Nutr 2020;71:304–309 [DOI] [PubMed] [Google Scholar]
- 72.Putra J, Arva NC, Tan SY, Melin-Aldana H, Bass LM, Mitchell PD et al. Barrett esophagus and intestinal metaplasia of the gastroesophageal junction in children: a clinicopathologic study. J Pediatr Gastroenterol Nutr 2020;70:562–567 [DOI] [PubMed] [Google Scholar]
- 73.Petit LM, Righini-Grunder F, Ezri J, Jantchou P, Aspirot A, Soglio DD et al. Prevalence and predictive factors of histopathological complications in children with esophageal atresia. Eur J Pediatr Surg 2019;29:510–515 [DOI] [PubMed] [Google Scholar]
- 74.Ure B, Slany E, Eypasch E, Gharib M, Holschneider A, Troidl H. Long-term functional results and quality of life after colon interposition for long-gap oesophageal atresia. Eur J Pediatr Surg 1995;5:206–210 [DOI] [PubMed] [Google Scholar]
- 75.Khan AR, Stiff G, Mohammed AR, Alwafi A, Ress BI, Lari J. Esophageal replacement with colon in children. Pediatr Surg Int 1998;13:79–83 [DOI] [PubMed] [Google Scholar]
- 76.Reismann M, Granholm T, Ehrén H. Partial gastric pull-up in the treatment of patients with long-gap esophageal atresia. World J Pediatr 2015;11:267–271 [DOI] [PubMed] [Google Scholar]
- 77.Youn JK, Park T, Kim S-H, Han J-W, Jang H-J, Oh C et al. Prospective evaluation of clinical outcomes and quality of life after gastric tube interposition as esophageal reconstruction in children. Medicine (Baltimore) 2018;97:e13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daltveit DS, Klungsøyr K, Engeland A, Ekbom A, Gissler M, Glimelius I et al. Cancer risk in individuals with major birth defects: large Nordic population based case-control study among children, adolescents, and adults. BMJ 2020;371:m4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tullie LGC, Sohn H-M, Zylstra J, Mattsson F, Griffin N, Sharma N et al. A role for tumor volume assessment in resectable esophageal cancer. Ann Surg Oncol 2016;23:3063–3070 [DOI] [PubMed] [Google Scholar]
- 80.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002;122:26–33 [DOI] [PubMed] [Google Scholar]
- 81.Ronkainen J, Aro P, Storskrubb T, Johansson S-E, Lind T, Bolling-Sternevald E et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 2005;129:1825–1831 [DOI] [PubMed] [Google Scholar]
- 82.Zagari RM, Fuccio L, Wallander M-A, Johansson S, Fiocca R, Casanova S et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut 2008;57:1354–1359 [DOI] [PubMed] [Google Scholar]
- 83.Cohen MC, Ashok D, Gell M, Bishop J, Walker J, Thomson M et al. Pediatric columnar lined esophagus vs Barrett's esophagus: is it the time for a consensus definition. Pediatr Dev Pathol 2009;12:116–126 [DOI] [PubMed] [Google Scholar]
- 84.Krishnan U, Mousa H, Dall'Oglio L, Homaira N, Rosen R, Faure C et al. ESPGHAN-NASPGHAN guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with esophageal atresia-tracheoesophageal fistula. J Pediatr Gastroenterol Nutr 2016;63:550–570 [DOI] [PubMed] [Google Scholar]
- 85.Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016;111:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 2014;371:836–845 [DOI] [PubMed] [Google Scholar]
- 87.Westerhoff M, Hovan L, Lee C, Hart J. Effects of dropping the requirement for goblet cells from the diagnosis of Barrett's esophagus. Clin Gastroenterol Hepatol 2012;10:1232–1236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
zrab069_Supplementary_Data