Avocado Consumption, Abdominal Adiposity, and Oral Glucose Tolerance Among Persons with Overweight and Obesity (original) (raw)
ABSTRACT
Background
Although intake of Hass avocado has been cross-sectionally linked to lower abdominal obesity, knowledge of the effects of avocado consumption on abdominal adiposity and glycemic outcomes remains limited.
Objective
The effects of avocado consumption on abdominal adiposity, insulin resistance, oral-glucose-tolerance test (OGTT), and estimated β-cell function were evaluated.
Methods
A total of 105 adults aged 25–45 y (61% female) with BMI ≥25 kg/m2 were randomly assigned to an intervention (N = 53) that received a daily meal with 1 fresh Hass avocado or a control (N = 52) that received an isocaloric meal with similar ingredients without avocado for 12 wk. DXA was used to assess the primary outcomes of abdominal adiposity [visceral adipose tissue (VAT), subcutaneous abdominal adipose tissue (SAAT), and the ratio of VAT to SAAT (VS Ratio)]. Fasted glucose and insulin were used to assess the primary outcomes of insulin resistance (HOMA-IR), and insulin sensitivity (Matsuda index) and β-cell function (Insulinogenic index) were estimated using an OGTT. Changes between groups were compared using an ANCOVA. Secondary analyses were conducted based on sex.
Results
The control group exhibited a greater reduction in SAAT [–54.5 ± 155.8 g (control) compared with 17.4 ± 155.1 g (treatment), P = 0.017] and increase in VS Ratio [0.007 ± 0.047 (control) compared with –0.011 ± 0.044 (treatment), P = 0.024]. Among females, the treatment group exhibited a greater reduction in VAT [1.6 ± 89.8 g (control) compared with –32.9 ± 81.6 g (treatment), P = 0.021] and VS Ratio [0.01 ± 0.05 (control) compared with –0.01 ± 0.03 (treatment), P = 0.001]. Among males, there was no significant difference between groups in changes in abdominal adiposity or glycemic outcomes.
Conclusions
Daily consumption of 1 fresh Hass avocado changed abdominal adiposity distribution among females but did not facilitate improvements in peripheral insulin sensitivity or β-cell function among adults with overweight and obesity.
This study was registered at clinicaltrials.gov as NCT02740439.
Keywords: fiber, monounsaturated fatty acids, abdominal adiposity, insulin, obesity
Introduction
The epidemic of elevated overweight and obesity presents a major public health challenge in the USA, currently affecting ∼70% of the adult population (1). Further, increased abdominal obesity, a hallmark of fat or adiposity-related metabolic dysfunction, impacts over 1 in 3 adults (2). Visceral adipose tissue (VAT) is more closely associated with obesity-related metabolic diseases than subcutaneous abdominal adipose tissue (SAAT) (3–5). Increased VAT has been cross-sectionally and prospectively implicated in impaired glucose tolerance and insulin resistance, and onset of type 2 diabetes, relative to other adipose depots (6, 7). Dietary intervention offers a potentially efficacious solution for abdominal obesity management; however, the influence of diet in reducing VAT is unclear. Weight loss interventions can be effective in reducing VAT and risk of type 2 diabetes (8, 9), but unfortunately inducing and maintaining weight loss is unsuccessful for most individuals (10). Therefore, dietary approaches that promote reduction in VAT and reduction in type 2 diabetes risk, without relying on hypocaloric diets and weight loss, may hold translational potential for individuals with overweight and obesity.
Regular consumption of nutrient-dense whole foods can serve as a nonpharmacological approach to modifying adipose tissue distribution and alleviating the metabolic effects of adiposity. The avocado (Persea americana) is a fruit that is rich in dietary fiber and MUFAs, 2 nutrients that are beneficial for metabolic health (11). One fresh Hass avocado (∼136 g) contains ∼13 g MUFAs, 10 g fiber, and carotenoids and other bioactive components (12). Diets rich in MUFAs and fiber have received considerable attention for their potential to reduce obesity and lower the risk of type 2 diabetes (13–15). Avocado consumers have lower abdominal obesity compared with nonconsumers (11, 16). Further, in a longitudinal study among over 55,000 individuals, habitual consumption of avocados was associated with lower weight gain and reduced risk of having overweight or obesity when assessed 11 y later (17). In a recent 12-wk randomized controlled weight loss study, it was demonstrated that avocados can be consumed as part of a hypocaloric diet weight loss program as well (18). Therefore, avocado consumption is not only a marker of a higher quality diet but can also improve metabolic health and weight status. However, the effects of daily avocado consumption on adipose tissue distribution and related insulin resistance, insulin sensitivity, and pancreatic β-cell function are unclear. The present work involved an investigator-blinded randomized controlled trial to examine the effects of consuming a daily meal with an avocado, relative to an isocaloric meal without the avocado, on abdominal adiposity, insulin resistance, and oral glucose tolerance among young-to-middle-aged adults with overweight or obesity. We hypothesized that participants consuming a daily meal with an avocado would exhibit greater declines in VAT, VS Ratio, insulin resistance, and improvement in glycemic outcomes.
Methods
Participants and study design
A randomized controlled trial design [Persea americana for Total Health (PATH) Study] was undertaken to assess the effects of daily avocado consumption on abdominal adiposity and glycemic measures among adults with overweight or obesity. Data collection procedures were administered at baseline, prior to treatment allocation, and at follow-up 12 wk later. All subjects provided written informed consent prior to study participation. Procedures were administered in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the University of Illinois. The study was registered as a clinical trial on clinicaltrials.gov (NCT02740439). Adults aged between 25 and 45 y with a BMI ≥25 (in kg/m2) were recruited using university e-mail listservs and flyers posted in community buildings and on buses frequented by the public. Data were collected in central Illinois between 2016 and 2018. Participant exclusion criteria included BMI <25, pregnancy or lactation, tobacco use, food allergies and lactose intolerance, latex allergy, prior diagnosis of cognitive, metabolic, and gastrointestinal disease, use of medications that alter normal bowel function and metabolism, and prior malabsorptive or restrictive bariatric surgery within previous 2 y. Given that previously published research on avocado consumption effects on adipose tissue distribution and glucose tolerance were lacking, an a priori power calculation using a moderate effect size (Cohen's d = 0.50), 2-sided α of 0.05, and 80% power, estimated that a sample size of 64 participants/group would be sufficient to address the primary study aims.
Study treatment and control meals
Randomization was completed by a member of the research team (ADMW) who was not involved in data collection or administration of the intervention. Participants randomly assigned to the treatment group consumed 1 meal a day with 175 g (male) and 140 g (female) fresh Hass avocado, whereas the control group consumed an isocaloric meal matched for macronutrient composition without an avocado. A complete description of the meals and ingredient list can be found in Supplemental Table 1. Per serving (50 g), an avocado provides 80 kcal. The majority of the calories in avocados (∼90%) are derived from fats with the greatest proportion accounted for by MUFAs (5 g/serving) and 1 g/serving for polyunsaturated and 1 g/serving for saturated fats. The carbohydrates are predominantly in the form of dietary fiber (4 out of 5 g/serving) whereas proteins are only 1 g/serving. Avocados also provide ≤20 minerals and vitamins. As outlined in Table 1, owing to the macronutrient composition of avocados, the treatment meals were higher in total fiber and lower in saturated fats and higher in MUFAs. Over 90% of the ingredients were identical between the meals but were scaled to meet the desired caloric and macronutrient profiles. The energy content and proportion of macronutrients of meals for females was 20% lower compared with males due to estimated energy needs. The study meals were provided on a 7-d menu cycle designed to be similar to a typical American diet and meet the Acceptable Macronutrient Distribution Ranges (AMDR) set by the Institute of Medicine (∼45% carbohydrates, 35% fat, and 15% protein). Examples of meals included modified versions of Penne Pasta, Cranberry Salad, Spanish Rice, Asian Penne, Turkey Wrap, Spring Bowl, and an Egg Scramble. Participants traveled to the test site twice weekly to pick up meals. Insulated meal coolers, ice packs, and information about food safety procedures were provided. Compliance was assessed using daily logs that participants completed to indicate meal consumption and, for participants in the intervention, avocado consumption. Concealment of allocation was maintained using sequentially numbered containers for meal dispensation. Additionally, staff who prepared and provided participants with the meals were not involved in the data collection procedures.
TABLE 1.
Nutrient comparison between meals with avocado (treatment) and without (control) avocado provided to participants with overweight or obesity1
| Control | Treatment | |||
|---|---|---|---|---|
| Nutrient | Males | Females | Males | Females |
| Energy, kcal | 662 | 530 | 660 | 528 |
| Total fiber, g | 4.0 | 3.2 | 16.0 | 12.8 |
| Soluble fiber, g | 1.0 | 0.8 | 6.0 | 4.8 |
| Pectins, g | 0.0 | 0.0 | 4.0 | 3.2 |
| Insoluble fiber, g | 3.0 | 2.4 | 10.0 | 8.0 |
| Protein, % | 16 | 12.8 | 14 | 11.2 |
| Total fat, % | 39 | 40 | ||
| SFAs, % | 17 | 7 | ||
| MUFAs, % | 10 | 24 | ||
| PUFAs, % | 9 | 5 | ||
| Carbohydrate, % | 45 | 45 |
Dietary assessment
Background diet was monitored prior to and during the final week of the intervention. Participants were asked to record all beverages and foods consumed for ≥7 d and were provided a food diary with detailed instructions given by a trained member of the research staff at the completion of their first laboratory visit. In addition, the record contained written instructions for recording food intake (including how to describe food preparation methods, added fats, brand names, and ingredients of mixed dishes and recipes) and visual aids to estimate serving sizes. Trained staff entered food records into the Nutrition Data Systems-Research Version 2015 [Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN, USA] software under supervision of a registered dietitian (NAK).
Physical activity
Participants were asked to recall their average weekly physical activity during their leisure time over the past month using the Godin Leisure Time Exercise Questionnaire (GLTEQ) (19, 20). The GLTEQ contains 3 open-ended physical activity questions pertaining to the average frequency of mild, moderate, and strenuous physical activities (with examples of each) during free time during a typical week. All participants were asked to refrain from changes in their physical activity levels throughout the intervention.
Anthropometrics and abdominal adiposity assessment
Standing height and weight measurements were completed to calculate BMI with participants wearing lightweight clothing and no shoes. Height and weight were assessed using a stadiometer (model 240; SECA) and a digital scale (WB-300 Plus; Tanita), respectively. Measures were recorded in triplicate and the average value was used in the analyses. Whole body, VAT, and SAAT components were estimated using a Hologic Horizon W densitometer using the standard software (APEX Software version 5.6.0.5; Hologic). These procedures have been previously described in detail (21, 22).
Oral-glucose-tolerance test
At baseline and 12 wk, a standard oral-glucose-tolerance test (OGTT) was administered following a 12-h overnight fast. Participants rested for ≥30 min prior to the insertion of a Teflon catheter into an antecubital vein for repeated blood sampling and remained patent by a 0.9% saline drip. After baseline blood collection, participants ingested 75 g glucose bolus (NOW foods) dissolved in 250 mL of water within 2 min (t = 0 min) (21). Venous blood was collected at the following time points: 0, 15, 30, 45, 60, 90, and 120 min after glucose ingestion into EDTA containing tubes (BD Biosciences). Blood glucose concentration was determined using a biochemical analyzer (YSI 2900 Life Sciences) in duplicate and subsequently centrifuged at 1000 × g for 10 min at 4°C. Aliquots of plasma were frozen and stored at –20°C until analyses. Plasma insulin concentrations were determined using a commercially available ELISA (ALPCO).
Plasma glucose and insulin concentrations were used to determine the Matsuda index and HOMA-IR according to established formulas (23, 24). The Matsuda index was calculated by:
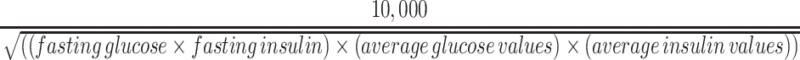 |
(1) |
|---|
HOMA-IR was calculated by:
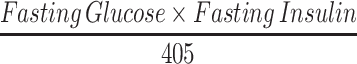 |
(2) |
|---|
Further, the insulinogenic index (IGI) was utilized as a measure of β-cell function and calculated as ratio of insulin concentration at 30 min minus fasting insulin to the difference of glucose at the same time (25).
Statistical analyses
Data from participants who completed the 12-wk intervention (control = 53, treatment = 52) were used to conduct per protocol analyses (see Figure 1). We applied a cutoff of 80% compliance for study meal consumption throughout the trial, for per protocol analyses. Additionally, intent-to-treat analyses were conducted among all participants who were randomly assigned and provided complete data at baseline. Missing values at follow-up were carried forward from baseline values for the intent-to-treat analyses.
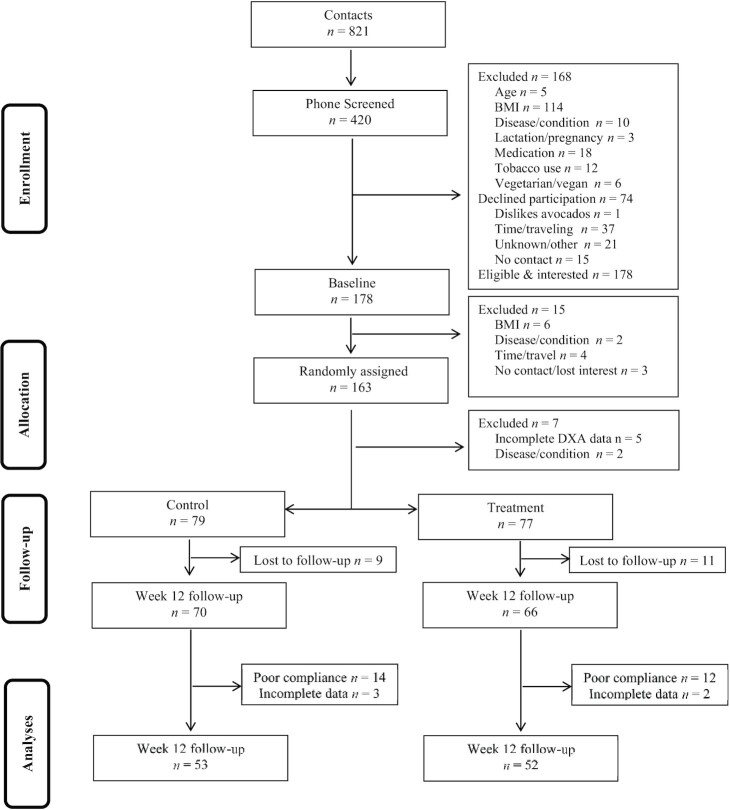
FIGURE 1.
Participant recruitment and inclusion/exclusion information.
Baseline differences in between groups were assessed by independent samples t-test. Primary analyses, i.e., intervention effects on abdominal adiposity (i.e., VAT, SAAT, VS Ratio), insulin resistance (i.e., HOMA-IR), and OGTT (i.e., Matsuda index and IGI) measures were subjected to a univariate ANCOVA whereby change (posttest–baseline) measures were compared between groups, following adjustment of changes in reported energy intake. Critical values were adjusted for false discovery rate (FDR) to act as a check on inflation of Type 1 error (26). In the present analyses, the FDR was set to 0.05, the acceptable level of family-wise error. Secondary analyses separated by sex were conducted since there were significant differences in adiposity variables between females and males. Statistical significance level was set at P <0.05 (2-tailed) and data were analyzed using SPSS version 25 (IBM).
Results
Per protocol analyses
Participants and baseline variables
Participant recruitment and inclusion/exclusion is illustrated in Figure 1. One hundred and fifty-six participants who were randomly assigned provided complete data at baseline and 136 were retained in the study at follow-up. The overall retention rate of the study was 87%. There were no discernable differences in characteristics between participants who dropped out of the study compared with those who remained in the study. Specifically, there were no significant differences in age and baseline adiposity and glycemic variables between participants who dropped out compared with those who remained in the study (all _P_s >0.209).
Following exclusion of participants who either did not provide follow-up data for adiposity variables or those who did not meet the compliance threshold of 80%, 105 participants were included in the final per protocol analyses for adiposity variables of interest. Only 64 participants [34 (control), 30 (treatment)] successfully completed both baseline and follow-up OGTT measures for calculating HOMA-IR, Matsuda index, and IGI.
Participant demographic and anthropometric information is summarized in Table 2. Persons with overweight comprised 41% of the sample whereas 59% had obesity. The majority of the participants were of Caucasian descent (80%) and female (61%). Significant differences in adiposity variables were observed based on sex whereby females had higher SAAT (2383.9 ± 598.5 g compared with 1675.5 ± 749.8 g, P <0.001) whereas males had a higher VS Ratio (0.43 ± 0.14 compared with 0.30 ± 0.09, P <0.001). However, there were no differences in VAT between males and females [711.7 ± 303.7 g (females) compared with 678.0 ± 348.3 g (males), P = 0.601]. There were no significant differences between treatment and control group participants of the same sex.
TABLE 2.
Baseline demographic information and anthropometric characteristics of adults with overweight or obesity participating in the PATH randomized controlled trial1
| All (N = 105) | Control (N = 53) | Treatment (N = 52) | |
|---|---|---|---|
| Age, y | 34.5 ± 5.9 | 34.2 ± 6.0 | 34.9 ± 5.8 |
| Race, n (%) | |||
| White or Caucasian | 83 (80) | 42 (79) | 41 (79) |
| Asian | 11 (11) | 5 (9) | 6 (12) |
| Black or African American | 5 (5) | 3 (6) | 2 (4) |
| American Indian or Alaska Native | 1 (1) | 1 (2) | 0 (0) |
| Other and multiracial | 4 (4) | 2 (4) | 2 (4) |
| Household income, n (%) | |||
| ≤$41,000 | 28 (27) | 14 (26) | 14 (26) |
 41,000– 41,000– 70,000 70,000 |
39 (37) | 21 (40) | 18 (35) |
| ≥$70,000 | 38 (36) | 18 (34) | 20 (39) |
| Height, cm | 171.2 ± 8.9 | 171.1 ± 9.6 | 171.2 ± 8.2 |
| Weight, kg | 95.7 ± 19.4 | 96.9 ± 20.5 | 94.5 ± 18.4 |
| BMI, kg/m2 | 32.6 ± 6.1 | 33.0 ± 6.2 | 32.1 ± 6.0 |
| Overweight (25–29.9) | 43 (41) | 21 (40) | 22 (42) |
| Obesity Class 1 (30–34.9) | 32 (31) | 14 (26) | 18 (35) |
| Obesity Class 2 (35–39.9) | 17 (16) | 8 (15) | 9 (17) |
| Obesity Class 3 (≥40) | 13 (12) | 10 (19) | 3 (6) |
Background diet and physical activity
There were no statistically significant differences in changes in leisure time physical activity score between the treatment and control groups [–3.6 ± 29.9 (control) compared with 11.4 ± 41.6 (treatment), P = 0.59]. However, there was a significant difference between changes in energy consumption between the control and treatment groups [–109.7 ± 549.7 kcal (control) compared with 174.3 ± 568.4 kcal (treatment), P = 0.011]. As the intervention aimed to maintain overall caloric intake throughout the study and between groups, subsequent statistical analyses adjusted for change in energy consumption to account for background changes in energy consumption between groups.
Intervention effects on central adiposity
The baseline values for the adiposity measures are summarized in Table 3 for descriptive purposes. Baseline and follow-up insulin resistance and sensitivity is described in Table 4. The summary of statistical tests contrasting change in adiposity and glycemic outcomes is summarized in Table 5. There was no significant difference between groups in VAT. The control group had a significantly larger reduction in SAAT. There was a significant difference between groups in change in VS Ratio. Examining the results based on sex revealed that, among females, there was a significant difference between groups in changes in VAT, SAAT, and VS Ratio. On the other hand, among males, there was no significant difference between groups in changes in VAT, SAAT, and VS Ratio. Following controlling for FDR, the differences in changes in VS Ratio between groups remained significant. Similarly, the differences in changes in VS Ratio and SAAT among females persisted following controlling for FDR.
TABLE 3.
Baseline and follow-up adiposity of adults with overweight or obesity participating in the PATH randomized controlled trial based on sex and group1
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Control (N = 34) | Treatment (N = 30) | Control (N = 19) | Treatment (N = 22) | |||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Weight, kg | 94.6 ± 18.6 | 94.4 ± 18.3 | 92.4 ± 16.6 | 92.0 ± 16.5 | 101.0 ± 23.5 | 99.9 ± 23.7 | 97.3 ± 20.7 | 97.9 ± 20.9 |
| Fat, % | 44.3 ± 6.2 | 44.0 ± 6.2 | 42.9 ± 4.3 | 42.5 ± 4.6 | 32.3 ± 8.6 | 31.6 ± 8.2 | 31.6 ± 7.6 | 31.9 ± 7.7 |
| VAT, g | 743.9 ± 334.0 | 745.5 ± 324.2 | 675.2 ± 266.1 | 642.3 ± 258.0 | 672.4 ± 409.3 | 618.4 ± 351.4 | 682.8 ± 295.8 | 664.7 ± 272.5 |
| SAAT, g | 2476.6 ± 595.0 | 2415.3 ± 633.8 | 2279.0 ± 594.7 | 2292.6 ± 599.4 | 1701.2 ± 792.4 | 1684.0 ± 867.2 | 1653.4 ± 729.2 | 1675.8 ± 744.2 |
| VS Ratio | 0.30 ± 0.10 | 0.31 ± 0.11 | 0.29 ± 0.08 | 0.28 ± 0.08 | 0.42 ± 0.16 | 0.40 ± 0.17 | 0.44 ± 0.12 | 0.41 ± 0.11 |
TABLE 4.
Baseline and follow-up insulin resistance and sensitivity of adults with overweight or obesity participating in the PATH randomized controlled trial based on sex and group1
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Control (N = 21) | Treatment (N = 16) | Control (N = 13) | Treatment (N = 14) | |||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Glucose, mg/dL | 83.5 ± 12.5 | 82.7 ± 11.5 | 77.5 ± 13.8 | 82.3 ± 11.0 | 80.4 ± 10.2 | 84.3 ± 14.0 | 80.7 ± 11.3 | 87.7 ± 8.7 |
| Insulin, μIU/L | 10.9 ± 6.8 | 11.0 ± 4.8 | 8.3 ± 3.7 | 9.5 ± 5.3 | 9.3 ± 4.7 | 9.7 ± 4.6 | 9.6 ± 5.9 | 11.3 ± 7.3 |
| HOMA-IR | 2.3 ± 1.7 | 2.3 ± 1.2 | 1.6 ± 0.9 | 2.0 ± 1.2 | 2.0 ± 1.3 | 2.0 ± 1.1 | 1.9 ± 1.2 | 2.5 ± 1.9 |
| Matsuda index | 5.4 ± 3.2 | 5.0 ± 2.7 | 6.7 ± 4.4 | 5.6 ± 3.8 | 5.2 ± 2.5 | 4.6 ± 2.4 | 5.5 ± 3.2 | 4.5 ± 2.5 |
| Insulinogenic index | 2.3 ± 2.8 | 2.0 ± 2.6 | 2.0 ± 2.2 | 1.8 ± 1.7 | 2.1 ± 4.1 | 1.2 ± 0.6 | 1.3 ± 1.2 | 1.5 ± 1.5 |
TABLE 5.
Change in adiposity and glycemic outcomes among adults with overweight or obesity participating in the PATH randomized controlled trial based on sex and group1
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Adiposity outcomes | Control (N = 34) | Treatment (N = 30) | P | Control (N = 19) | Treatment (N = 22) | P |
| ΔVAT, g | 1.6 ± 89.8 | –32.9 ± 81.6* | 0.021 | –39.7 ± 99.2 | –18.1 ± 107.1 | 0.500 |
| ΔSAAT, g | –61.2 ± 152.7 | 13.7 ± 133.1* | 0.0212 | –42.4 ± 164.7 | 22.4 ± 184.2 | 0.268 |
| ΔVS Ratio | 0.117 ± 0.047 | –0.015 ± 0.030* | 0.0012 | –0.002 ± 0.046 | –0.007 ± 0.057 | 0.812 |
| Glycemic outcomes | Control (N = 21) | Treatment (N = 16) | P | Control (N = 13) | Treatment (N = 14) | P |
| ΔHOMA-IR | –0.02 ± 0.99 | 0.34 ± 0.89 | 0.377 | 0.10 ± 1.0 | 0.43 ± 0.80 | 0.332 |
| ΔMatsuda index | –0.47 ± 2.57 | –1.12 ± 2.26 | 0.412 | –0.58 ± 2.01 | –0.95 ± 1.82 | 0.615 |
| ΔInsulinogenic index | –0.09 ± 1.49 | –0.26 ± 1.28 | 0.611 | –0.48 ± 1.28 | 0.21 ± 0.93 | 0.121 |
Intervention effects on insulin resistance and sensitivity
The baseline values for the glucose- and insulin-based outcomes are summarized in Table 4 and comparison of changes based on sex and group are summarized in Table 5. There was no difference between groups in changes in HOMA-IR (P = 0.100), Matsuda index (P = 0.285), and the IGI (P = 0.67). Similarly, there were no changes among females or males.
Intent-to-treat analyses
Intervention effects on central adiposity
Intent-to-treat analyses for the adiposity variables were conducted among all participants who were randomly assigned and completed baseline testing in the control [N = 79 (52 females; 27 males)] and treatment [N = 77 (49 females; 28 males)] groups. There was no significant difference between groups in VAT (P = 0.44). The control group had a significantly larger reduction in SAAT (P = 0.012). However, there was a difference between groups in VS Ratio (P = 0.045). Examining the results based on sex revealed that, among females, there was no significant difference between groups in changes in VAT (P = 0.227). However, there were significant differences in SAAT (P = 0.002), and VS Ratio (P = 0.010). On the other hand, among males, there was no significant difference between groups in changes in VAT (P = 0.940), SAAT (P = 0.545), and VS Ratio (P = 0.611). Following controlling for FDR, the significant differences in changes in VS Ratio and SAAT among females persisted.
Intervention effects on insulin resistance and sensitivity
There was a significant difference between groups in changes in HOMA-IR (P = 0.036). However, there was no significant difference between groups in changes in Matsuda index (P = 0.286) and the IGI (P = 0.779). There were no changes among females (all _P_s ≥0.161) or males (all _P_s ≥0.16). Following controlling for FDR, there were no significant differences in changes across glycemic outcomes.
Discussion
The present work determined the effects of daily avocado consumption on abdominal adiposity, insulin resistance, and oral glucose tolerance among adults with overweight and obesity. There were significant differences in VS Ratio between groups, likely due to the greater change in SAAT among control group participants. The greater reduction in SAAT among control group participants was an unexpected result. However, change in SAAT in the control group was not associated with changes in overall energy consumption or change in self-reported leisure time physical activity (data not shown). Therefore, although we do not know the cause of the SAAT change observed in the control group, this change is not related to self-reported changes in overall energy consumption and leisure time physical activity. Analyses based on sex revealed that females in the treatment group exhibited a greater reduction in VS Ratio whereas control group females had a greater reduction in SAAT. However, there were no improvements in HOMA-IR, insulin sensitivity, and β-cell function (i.e., IGI).
Although avocado consumption is a marker of higher diet quality and potentially protects against metabolic risk (11, 16, 27, 28), there has been limited experimental work on adiposity and oral glucose tolerance outcomes. A recent hypocaloric weight loss trial with the inclusion of avocado reported reductions in adiposity and improvements in presumably fasting glucose; however, there were no differences between the treatment and control groups (18). Previous research observed that avocado consumers had higher BMI, waist circumference, and metabolic syndrome risk (16). In a longitudinal study among over 55,000 older adults, it was observed that, among avocado consumers who had healthy weight status at baseline, there was a reduction in the odds of developing overweight or obesity compared with those who did not eat avocado (17). The present work revealed that consuming a daily meal with an avocado improved fat distribution as indicated by a lower VS Ratio among female participants. Relative to other adipose tissue depots, VAT accumulation, surrounding internal organs such as the liver, is associated with type 2 diabetes (6, 29, 30), dyslipidemia (31), inflammation, increased risk of thrombosis (32, 33), and nonalcoholic fatty liver disease (34). Therefore, the decrease in VS Ratio among treatment group participants suggests that avocado intake imparts a beneficial abdominal adiposity profile. However, given that these benefits were not observed among males, the robustness of the treatment effect is limited and additional experimental research is needed to further characterize the effects of daily avocado consumption on fat distribution.
The mechanisms by which avocados may contribute to VAT changes are possibly derived from the higher fiber and MUFA content (16). A whole avocado contains ∼10 g of total fiber, contributing to ≤40% of the recommendations among females or 30% for males. Greater dietary fiber intake is cross-sectionally associated with lower VAT (35, 36) and related to lower gains in VAT (37). Further, independent of caloric restriction, supplemental fiber intake reduces BMI and waist circumference (38). Dietary fiber, specifically insoluble fiber (contributing to 70% of fiber in an avocado), could affect VAT by increasing fecal bulk and shortening transit time (39), and lowering absorption of nutrients and energy (40). Acutely, meals with higher dietary fiber elicit a moderate postprandial blood glucose response, stimulating a greater sensation of satiety in healthy adults (41). It has also been hypothesized that the elevated postprandial glucose and insulin response can affect macronutrient partitioning in a manner that favors adipose tissue accumulation, and that VAT may be more susceptible to the influence of high insulin responses relative to SAAT (42, 43). There is also evidence that the lipid compositional properties of adipose tissue differentially impact whole body and abdominal obesity (44). The degree of obesity and abdominal distribution of body fat have been negatively correlated with the MUFAs and n−3 PUFA contents of adipose tissue. Given that SFAs were higher and MUFAs were lower in perivisceral than in subcutaneous fat, it is possible that consuming diets that substitute SFAs with MUFAs has the potential to shift abdominal adipose distribution. Indeed, dietary fatty acid intake is known to be an important determinant of changes in adipose tissue composition (45).
Regarding potential clinical or biological relevance of our findings, previous work indicates that inducing a 26% reduction in VAT over the course of 12 mo, using a healthy eating and physical activity/exercise program, corresponds to significant improvement in cardiorespiratory fitness, plasma inflammatory markers, lipid profiles, and OGTT (46). The change in VAT tissue among females in the treatment group in the current study was ∼5%; therefore, the change observed in our study was modest. However, considering that the present study was relatively short (3 mo) and did not include an exercise component, the modest change in VAT is not surprising. It is possible that maintaining the treatment regimen over the course of a longer period could have provided the necessary cumulative reduction in VAT to be clinically meaningful. A previous large prospective study observed that annual increases of 3%, 4%, and 3% of VAT, SAAT, and VS Ratio, respectively, were related to an increased risk of diagnosis of diabetes among adults with higher BMI (47). Interestingly, the proportional changes in these measures in the present study were similar in magnitude, albeit the SAAT effects were only observed in the control group. Overall, the changes in abdominal adiposity compartments observed in the present study were modest but could have possible clinical significance if the trajectories for reduction in VAT and VS Ratio were accumulated over a longer period of time. Future longer duration studies are needed to characterize the clinical meaningfulness of these findings.
Interestingly, we observed selective benefits of participating in the intervention for females but not males. This is not entirely surprising since sexual dimorphism in lifestyle interventions targeting obesity have been observed previously (48, 49). Although the explanations for the sexual dimorphism observed in obesity prevalence and differential responses to lifestyle interventions are unclear, recent trends indicate that obesity and extreme obesity prevalence have significantly increased among females, further increasing the need for interventions targeting females. However, additional research that includes equal samples of male participants is necessary to gain a comprehensive understanding of dietary intervention effects. The majority of lifestyle interventions are predominantly comprised of female participants (27% male compared with 73% female) (49), resulting in limited knowledge on dietary implications for adiposity among males. Although the proportions of males (41%) in the present work was greater than most studies, it is possible that we failed to observe significant effects for males due to inadequate sample size of males and/or lower adiposity status of males compared with females at baseline. Given the known differences in adiposity partitioning based on sex, differential effects for adiposity may also have been driven by the fact that the treatment could have contributed to the participants' nutrient recommendations differently. For example, the avocado provided ∼51% of Adequate Intake for fiber for females and ∼42% for males. Future studies are needed to determine the extent to which dosage impacts changes in adiposity distribution between males and females following avocado consumption.
The present work also examined the implications of daily avocado consumption on insulin resistance, OGTT-derived peripheral insulin sensitivity, and β-cell function. Adding approximately half an avocado to a meal has been shown to increase satiety 4–5 h later and reduce postprandial insulin concentrations (50, 51), suggesting that manipulation of meals with avocado could promote both satiety and metabolic benefits. To our knowledge, the present study represents the first randomized controlled study to examine effects of consuming a whole avocado on glucose and insulin over several months among persons with overweight and obesity. We did not observe significant effects of avocado consumption for glucose and insulin outcomes. Given that VAT and insulin sensitivity and resistance are closely linked, this was a surprising finding. However, it is possible that we did not observe benefits for glycemic outcomes due to the modest reductions in VAT among females only. It is also possible that a longer intervention duration is necessary to observe metabolic benefits for insulin sensitivity following avocado consumption. A limitation of the study was that only a subsample of the participants were able to successfully complete the OGTT procedure. This was primarily due to the invasive nature of the procedure and challenges in successful catheter placement among persons with overweight and obesity (52, 53). Therefore, the findings for OGTT should be interpreted with caution. Nevertheless, these findings are consistent with previous work that examined glycemic responses following several weeks of consuming nutrients found in avocados. Lerman-Garber and colleagues observed that a high MUFA diet was associated with a greater decrease in triglycerides; however, there were no benefits for glycemic measures following a test mixed-meal (28). Although our findings did not support our a priori hypothesis regarding glycemic outcomes, this work suggests that daily consumption of a meal including an avocado, a fruit that is rich in fatty acids, does not detrimentally affect insulin resistance or oral glucose tolerance among adults with overweight and obesity. Additional experiments examining the effects of avocado consumption on glucose and insulin responses is needed to further characterize the effects of avocados on insulin resistance and sensitivity.
In conclusion, consumption of a daily meal with an avocado altered abdominal fat distribution over a 12-wk period. However, there were no improvements in insulin sensitivity or β-cell function. Future research is necessary to examine the underlying causes of the differential adiposity-related findings based on sex. Given the increasing prevalence of excess adiposity, there is a vital need to develop and test food-based approaches to reducing adiposity as well as improving metabolic health.
Supplementary Material
nxab187_Supplemental_File
Acknowledgments
We would like to thank Christine Madden for her management of the metabolic kitchen and production of the study meals. We also acknowledge the contributions Corinne Cannavale, Morgan Chojnacki, Melisa Bailey, Joe Beals, Jennifer Kaczmarek, and Andrew Taylor for their assistance in conducting the testing procedures.
The authors’ contributions were as follows—NAK, HDH, NAB, and BHF: conceptualized and obtained funding for the research; CGE, SVT, BAH, and SKB: conducted the research; ADMW: conducted randomization procedures; NAK: analyzed data; all authors: contributed to the interpretation of the results and writing of the manuscript; NAK: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
The research was funded by the Hass Avocado Board. SVT was supported by a fellowship provided by the College of Agricultural, Consumer, and Environmental Sciences. BAH was supported by the Agriculture and Food Research Initiative Competitive Grant no. 2015-68001-23248 from the USDA National Institute of Food and Agriculture to Cooperative Extension.
Author disclosures: The authors report no conflicts of interest.
HDH is a member of The Journal of Nutrition Editorial Board and played no role in the evaluation of the manuscript.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: FDR, false discovery rate; GLTEQ, Godin Leisure Time Exercise Questionnaire; IGI, insulinogenic index; OGTT, oral-glucose-tolerance test; PATH, Persea americana for Total Health; SAAT, subcutaneous abdominal adipose tissue; VAT, visceral adipose tissue.
Contributor Information
Naiman A Khan, Department of Kinesiology and Community Health, University of Illinois, Urbana, IL, USA; Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA.
Caitlyn G Edwards, Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA.
Sharon V Thompson, Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA.
Bridget A Hannon, Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA.
Sarah K Burke, Department of Physical Therapy, University of Florida, Gaineville, FL, USA.
Anne D M Walk, Department of Psychology, Eastern Illinois University, Charleston, IL, USA.
Richard W A Mackenzie, Department of Life Science, Whitelands College, University of Roehampton, London, UK.
Ginger E Reeser, Department of Kinesiology and Community Health, University of Illinois, Urbana, IL, USA.
Barbara H Fiese, Department of Human Development and Family Studies, University of Illinois, Urbana, IL, USA; Family Resiliency Center, University of Illinois, Urbana, IL, USA.
Nicholas A Burd, Department of Kinesiology and Community Health, University of Illinois, Urbana, IL, USA; Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA.
Hannah D Holscher, Department of Kinesiology and Community Health, University of Illinois, Urbana, IL, USA; Division of Nutritional Sciences, University of Illinois, Urbana, IL, USA; Department of Food Science and Human Nutrition, University of Illinois, Urbana, IL, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mainous AG, Tanner RJ, Jo A, Anton SD. Prevalence of prediabetes and abdominal obesity among healthy-weight adults: 18-year trend. Ann Fam Med. 2016;14:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10(4):497–511. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Adrienne Cupples Let al. Abdominal visceral and subcutaneous adipose tissue compartments association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 5.Hwang Y-C, Fujimoto WY, Hayashi T, Kahn SE, Leonetti DL, Boyko EJ. Increased visceral adipose tissue is an independent predictor for future development of atherogenic dyslipidemia. J Clin Endocrinol Metab. 2016;101:678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care. 2003;26:650–5. [DOI] [PubMed] [Google Scholar]
- 7.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, Mcguire DK, Grundy SMet al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes. 2008;32:619–28. [DOI] [PubMed] [Google Scholar]
- 9.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer Xet al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the national weight control Registry. Am J Prev Med. 2014;46:17–23. [DOI] [PubMed] [Google Scholar]
- 11.Dreher ML, Davenport AJ. Hass avocado composition and potential health effects. Crit Rev Food Sci Nutr. 2013;53:738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory . USDA National Nutrient Database for Standard Reference, release 28. Version current: September 2015. [Google Scholar]
- 13.Gillingham LG, Harris-Janz S, Jones PJH. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011;46:209–28. [DOI] [PubMed] [Google Scholar]
- 14.Galvão Cândido F, Xavier Valente F, da Silva LE, Gonçalves Leão Coelho O, Gouveia Peluzio M do C, Gonçalves Alfenas R de C. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: a randomized, double-blinded, placebo-controlled clinical trial. Eur J Nutr. 2018;57:2445–55. [DOI] [PubMed] [Google Scholar]
- 15.Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–6. [DOI] [PubMed] [Google Scholar]
- 16.Fulgoni VL, Dreher M, Davenport AJ. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutr J. 2013;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heskey C, Oda K, Sabaté J, Heskey C, Oda K, Sabaté J. Avocado intake, and longitudinal weight and body mass index changes in an adult cohort. Nutrients. 2019;11:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henning SM, Yang J, Woo SL, Lee R-P, Huang J, Rasmusen A, Carpenter CL, Thames G, Gilbuena I, Tseng C-Het al. Hass avocado inclusion in a weight loss diet supported weight loss and altered gut microbiota: a 12 week randomized parallel-controlled trial. Curr Dev Nutr. 2019;3:nzz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77:359–62. [PubMed] [Google Scholar]
- 20.Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Method. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemiro GM, Skinner SK, Walk AM, Edwards CG, De Lisio M, Holscher HD, Burd NA, Khan NA. Oral glucose tolerance is associated with neuroelectric indices of attention among adults with overweight and obesity. Obesity. 2018;26:1550–7. [DOI] [PubMed] [Google Scholar]
- 22.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20:1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. [DOI] [PubMed] [Google Scholar]
- 24.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, Renn W, Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- 25.Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006;72:298–301. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 27.Wang L, Bordi PL, Fleming JA, Hill AM, Kris-Etherton PM. Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: a randomized, controlled trial. J Am Heart Assoc. 2015;4:e001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerman-Garber I, Ichazo-Cerro S, Zamora-González J, Cardoso-Saldaña G, Posadas-Romero C. Effect of a high-monounsaturated fat diet enriched with avocado in NIDDM patients. Diabetes Care. 1994;17:311–15. [DOI] [PubMed] [Google Scholar]
- 29.Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27:2948–53. [DOI] [PubMed] [Google Scholar]
- 30.Gentile C, Weir T, Cox-York K, Wei Y, Wang D, Reese L, Moran G, Estrada A, Mulligan C, Pagliassotti Met al. The role of visceral and subcutaneous adipose tissue fatty acid composition in liver pathophysiology associated with NAFLD. Adipocyte. 2015;4:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mamo JCL, Watts GF, Barrett PHR, Smith D, James AP, Pal S. Postprandial dyslipidemia in men with visceral obesity: an effect of reduced LDL receptor expression?. Am J Physiol Metab. 2001;281:E626–32. [DOI] [PubMed] [Google Scholar]
- 32.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation. 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes Res. 1995;3:645S–7S. [DOI] [PubMed] [Google Scholar]
- 34.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani D V, Hirschhorn JN, O'Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newby P, Maras J, Bakun P, Muller D, Ferrucci L, Tucker KL. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr. 2007;86:1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JN, Alexander KE, Ventura EE, Toledo-Corral CM, Goran MI. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. Am J Clin Nutr. 2009;90:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, Jacobs DR Jr. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999;282:1539. [DOI] [PubMed] [Google Scholar]
- 38.Thompson SV, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017;106:1514–28. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins DJ, Jenkins AL, Wolever TM, Rao AV, Thompson LU. Fiber and starchy foods: gut function and implications in disease. Am J Gastroenterol. 1986;81:920–30. [PubMed] [Google Scholar]
- 40.Kimm SYS. The role of dietary fiber in the development and treatment of childhood obesity. Pediatrics. 1995;96:1010–14. [PubMed] [Google Scholar]
- 41.Samra RA, Anderson GH. Insoluble cereal fiber reduces appetite and short-term food intake and glycemic response to food consumed 75 min later by healthy men. Am J Clin Nutr. 2007;86:972–9. [DOI] [PubMed] [Google Scholar]
- 42.Du H, van der A DL, van Bakel MME, Slimani N, Forouhi NG, Wareham NJ, Halkjær J, Tjønneland A, Jakobsen MU, Overvad Ket al. Dietary glycaemic index, glycaemic load and subsequent changes of weight and waist circumference in European men and women. Int J Obes. 2009;33:1280–8. [DOI] [PubMed] [Google Scholar]
- 43.Romaguera D, Ängquist L, Du H, Jakobsen MU, Forouhi NG, Halkjær J, Feskens EJM, van der A DL, Masala G, Steffen Aet al. Dietary determinants of changes in waist circumference adjusted for body mass index – a proxy measure of visceral adiposity. PLoS One. 2010;5:e11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garaulet M, Pérez-Llamas F, Pérez-Ayala M, Martínez P, de Medina FS, Tebar FJ, Zamora S. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74:585–91. [DOI] [PubMed] [Google Scholar]
- 45.Hwang J-H, Bluml S, Leaf A, Ross BD. In vivo characterization of fatty acids in human adipose tissue using natural abundance1H decoupled13C MRS at 1.5 T: clinical applications to dietary therapy. NMR Biomed. 2003;16:160–7. [DOI] [PubMed] [Google Scholar]
- 46.Borel A-L, Nazare J-A, Smith J, Alméras N, Tremblay A, Bergeron J, Poirier P, Després J-P. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity. 2012;20:1223–33. [DOI] [PubMed] [Google Scholar]
- 47.Kuwahara K, Honda T, Nakagawa T, Yamamoto S, Hayashi T, Mizoue T. Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci Re. 201;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakicic JM. The effect of physical activity on body weight. Obesity. 2009;17:S34–8. [DOI] [PubMed] [Google Scholar]
- 49.Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity. 2012;20:1234–9. [DOI] [PubMed] [Google Scholar]
- 50.Wien M, Haddad E, Oda K, Sabaté J. A randomized 3×3 crossover study to evaluate the effect of Hass avocado intake on post-ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr J. 2013;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu L, Huang Y, Edirisinghe I, Park E, Burton-Freeman B, Zhu L, Huang Y, Edirisinghe I, Park E, Burton-Freeman B. Using the avocado to test the satiety effects of a fat-fiber combination in place of carbohydrate energy in a breakfast meal in overweight and obese men and women: a randomized clinical trial. Nutrients. 2019;11:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nafiu OO, Burke C, Cowan A, Tutuo N, Maclean S, Tremper KK. Comparing peripheral venous access between obese and normal weight children. Pediatr Anesth. 2010;20:172–6. [DOI] [PubMed] [Google Scholar]
- 53.Sebbane M, Claret PG, Lefebvre S, Mercier G, Rubenovitch J, Jreige R, Eledjam JJ, De La Coussaye JE. Predicting peripheral venous access difficulty in the Emergency Department using body mass index and a clinical evaluation of venous accessibility. J Emerg Med. 2013;44:299–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nxab187_Supplemental_File
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.