PBX and MEIS as Non-DNA-Binding Partners in Trimeric Complexes with HOX Proteins (original) (raw)
Abstract
HOX, PBX, and MEIS transcription factors bind DNA through a homeodomain. PBX proteins bind DNA cooperatively as heterodimers with MEIS family members and also with HOX proteins from paralog groups 1 to 10. MEIS proteins cooperatively bind DNA with ABD-B class HOX proteins of groups 9 and 10. Here, we examine aspects of dimeric and higher-order interactions between these three homeodomain classes. The most significant results can be summarized as follows. (i) Most of PBX N terminal to the homeodomain is required for efficient cooperative binding with HOXD4 and HOXD9. (ii) MEIS and PBX proteins form higher-order complexes on a heterodimeric binding site. (iii) Although MEIS does not cooperatively bind DNA with ANTP class HOX proteins, it does form a trimer as a non-DNA-binding partner with DNA-bound PBX-HOXD4. (iv) The N terminus of HOXD4 negatively regulates trimer formation. (v) MEIS forms a similar trimer with DNA-bound PBX-HOXD9. (vi) A related trimer (where MEIS is a non-DNA-binding partner) is formed on a transcriptional promoter within the cell. (vii) We observe an additional trimer class involving non-DNA-bound PBX and DNA-bound MEIS-HOXD9 or MEIS-HOXD10 heterodimers that is enhanced by mutation of the PBX homeodomain. (viii) In this latter trimer, PBX is likely to contact both MEIS and HOXD9/D10. (ix) The stability of DNA binding by all trimers is enhanced relative to the heterodimers. These findings suggest novel functions for PBX and MEIS in modulating the function of DNA-bound MEIS-HOX and PBX-HOX heterodimers, respectively.
Ablation and misexpression of individual Hox genes have confirmed their importance in patterning the anteroposterior axis of the animal embryo (19, 35). Thirty-nine Hox genes have thus far been identified in the mammalian genome (35). They are organized into four clusters, A to D, with related genes occupying similar positions in each of the four clusters (29). These related genes fall into 13 paralogous groups. The products of groups 1 to 8 are more similar to the fly HOX protein Antennapedia (ANTP class), while 9 to 13 are related to the fly Abdominal-B (ABD-B class).
Hox gene products function as sequence-specific DNA-binding transcription factors, as evidenced by their ability to regulate natural and artificial promoters in cell culture (18, 48, 49, 53, 61, 63) and in the animal embryo (3, 12, 21, 33, 45, 47, 51, 64).
Homeodomain-DNA interactions are facilitated through residues in the flexible N-terminal arm and the recognition helix in the homeodomain (20), with the majority of HOX proteins binding a TAAT core motif. Asparagine 51 in the recognition helix plays a key role in the specificity of target site recognition, both in monomeric and in heterodimeric complexes (7, 8, 25, 41, 46, 62). The size of the HOX family and their relatively poor discrimination in target site recognition suggest that they may interact with cofactors.
Recently, it has been shown that the affinity and specificity of DNA binding by HOX proteins is indeed augmented by cofactor interactions. HOX cofactors in mammals include PBX (15, 32, 39, 44, 47) and MEIS (37, 57), members of the TALE family (9) of homeodomain proteins. The Drosophila homologs of mammalian PBX and MEIS are Extradenticle (EXD) (50) and Homothorax (HTH) (52), respectively. In mammals, while the majority of HOX proteins interact with PBX (HOX paralogs 1 to 10) (13), only the group 9 and 10 ABD-B class HOX proteins complex with MEIS (57).
We and others have shown that a conserved motif present N terminal to the homeodomain of HOX proteins (11, 15, 23, 27, 39, 43, 44, 54, 55) and residues in the homeodomain of PBX (14, 15, 22, 31, 46) contact each other within the PBX-HOX cooperative complex. These results have been confirmed and extended through the resolution of the crystal structure of the cooperative complex (41, 46). Systematic deletions carried out in HOXA9 and MEIS proteins have mapped amino acids (aa) 1 to 61 in HOXA9 and a region C terminal to the homeodomain of MEIS as responsible for mediating HOX-MEIS interactions (57).
In addition to their interaction with HOX proteins, MEIS and PBX form stable heterodimers that cooperatively bind DNA (16). The MEIS-related protein PREP1 interacts with PBX in a similar fashion (6). This interaction requires a portion of the first 89 aa in PBX and conserved N-terminal regions of MEIS or PREP. The chimeric oncoprotein E2A-PBX (24, 40) lacks the first 89 residues of PBX1 and is unable to interact with MEIS (16).
Recently, a number of groups have reported on the formation of trimeric complexes involving proteins of the HOX, PBX, and MEIS extended families. In each case, a DNA-bound PBX-HOX (or HOX-like) heterodimer tethers a member of the MEIS/PREP family via protein-protein interactions. Thus, PREP1 associates with DNA-bound HOXB1 and PBX, thereby modulating transcriptional activity (6). Mutation of the PREP1 homeodomain actually improves formation of the triple complex, strongly suggesting that DNA binding by PREP1 is not required. MEIS is tethered to a DNA-bound heterodimer of the HOX-related protein PDX1 with PBX, again apparently as a non-DNA-binding partner (59). Last, a DNA-bound PBX-HOXA9 heterodimer can form a trimer with MEIS on a site that does not accommodate MEIS binding (58). Importantly, the latter study has shown that the PBX-HOX-MEIS trimer can be found in the cell as a stable complex in the absence of DNA binding (58). These observations suggest additional and potentially widespread interactions between these homeodomain-containing proteins that are likely to play key roles in their activities as transcriptional regulators.
In this study, we examine dimeric and higher-order interactions between PBX, HOX, and MEIS family members and describe two trimer classes, one of which has not been previously reported. Our results broaden the understanding of mechanisms by which HOX, PBX, and MEIS proteins interact to regulate target gene expression.
MATERIALS AND METHODS
Plasmid construction.
SP6-driven expression vectors for full-length and deletion derivatives of HOXD4, PBX1A, MEIS1A, and MEIS-VP16 used for in vitro translation and transfection were generated by subcloning the relevant coding sequences into pCS2+ as described below. pCS2MEIS was produced by subcloning a _Bam_HI-_Xho_I fragment containing the entire Meis1a coding region in these same sites in pCS2+. pCS2MEIS-VP16 encoded the VP16 acidic activation domain fused to the N terminus of MEIS1A. To generate this vector, the C-terminal 79 codons of the VP16 open reading frame were amplified by PCR, introducing _Bam_HI and _Pst_I at the 5′ and 3′ ends, respectively. An ATG was also introduced at the 5′ end of the amplified product. A _Pst_I adapter was cloned at the _Sph_I site at the 5′ end of the Meis1a coding region to accommodate an in-frame fusion to the _Bam_HI-_Pst_I VP16 product. The resulting fusion protein is missing the first 30 aa and is not compromised for cooperative interactions. pCS2PBX(N51S) was generated by substituting an _Nco_I-_Stu_I fragment of E2A-PBX1(N51S) into the PBX1A coding region. pCS2MEIS(N51S) was produced by site-directed mutagenesis using Quick Change (Stratagene). To create a vector for MEIS(N51S)-VP16, a _Cla_I-_Xho_I fragment from pCS2MEIS(N51S) was subcloned in the same sites in pCS2MEIS-VP16. An _Xho_I-_Eco_RI fragment encompassing the coding sequence of PBX1A was blunted with Klenow enzyme and cloned into the _Stu_I site in pCS2+, generating pCS2PBX1A for transcription from the SP6 promoter. Sequences encoding PBX(89-430) (Fig. 1B) were amplified by PCR with a 5′ oligonucleotide containing an _Nde_I site and an in-frame start codon and a 3′ oligonucleotide containing a _Bam_HI site. This was cloned into the pET-16b vector (Novagen) in the T7 orientation. Sequences encoding PBX(233-430) were cloned as a PCR product in pPCRScript SK(+). Subsequently a _Bam_HI-_Sac_I fragment was cloned into the _Bam_HI-_Stu_I sites in pCS2+. The internal deletions of PBX1A (Fig. 1B) were generated in the M13mp19 phagemid by site-directed mutagenesis using the Sculptor mutagenesis kit (Amersham Corp.). The deletion mutants were then amplified by PCR and cloned unidirectionally into _Eco_RI-_Xho_I sites in pCS2+ vector. pCS2D4 was generated by subcloning a _Bam_HI-_Xba_I fragment containing the coding sequence of Hoxd4 into the same site in pCS2+. Using the Sculptor mutagenesis kit (Amersham), the asparagine 51 codon in the box of Hoxd4 was converted to a serine codon by using Hoxd4 cloned in the M13mp19 phagemid. Subsequently a _Pst_I-_Xba_I fragment containing this N51S mutation was substituted for the wild-type sequence in pCS2D4 to give pCS2D4(N51S). All mutations generated by site-directed mutagenesis or PCR were confirmed by restriction analysis and sequencing. The construction of vectors for the N-terminally histidine tagged HOXD4 and T7-driven HOXD9 and HOXD10 is described elsewhere (44, 54). The luciferase reporter construct pML is described elsewhere (44, 48). pML(2XPBX · MEIS) was constructed as described for pML(5×HOX · PBX) (44).
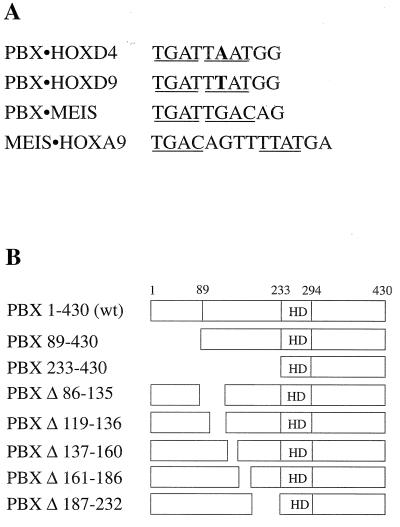
FIG. 1.
(A) Nucleotide sequences of the target sites used in this study. Position 6 is bold in both the PBX-HOXD4 and PBX-HOXD9 target sites. Core recognition elements for homeodomain binding are underlined. (B) Schematic representation of wild-type (wt) PBX1A and the different N-terminal and internal deletions used in this study. The homeodomain (HD) in PBX1A is comprised of aa 233 to 295.
In vitro transcription and translation of expression vectors.
HOXD4, PBX, MEIS, PBX(N51S), MEIS(N51S), MEIS-VP16, and MEIS(N51S)-VP16 were produced by an SP6 TnT coupled in vitro transcription-translation kit (Promega). HOXD9 and HOXD10 were produced from a similar kit, using T7 polymerase in the coupled reaction. A reaction containing [35S]methionine was performed in parallel to control for the differences in the translation efficiency. Quantitation was as described previously (43).
Protein purification.
N-terminally truncated HOXD4 was expressed as an N-terminal histidine-tagged fusion protein and purified as described elsewhere (54). The purity and concentration of the protein were estimated as described earlier (54). Fab fragments of monoclonal antibody (MAb) 10D11 were prepared by first digesting the immunoglobulin G’s with papain (Gibco, Toronto, Ontario, Canada) as described previously (2) to yield monovalent fragments (Fabs). After inactivation of papain, Fabs were repurified with protein G-Sepharose (Upstate Biotechnology, Lake Placid, N.Y.). Intermediate and final products were characterized to >98% purity (data not shown). No immunoglobulin G was detected in Fab preparations.
EMSA and measurement of dissociation rates.
Electrophoretic mobility shift assay (EMSA) and dissociation rate experiments were performed as described previously (43). Labeled DNA probes containing PBX-HOX, PBX-MEIS, or MEIS-HOX consensus sites (Fig. 1A) were used in this study. A PBX-HOX consensus site (Fig. 1A) containing A or T at position 6 was used as the cold competitor in dissociation rate experiments with HOXD4 or HOXD10, respectively. Antibodies against PBX1A and VP16 were obtained commercially (Santa Cruz). Quantification of the labeled DNA-bound monomer, dimer, and trimer complexes at various time points and estimation of the half-lives were carried out as described previously (43). Pore exclusion limit electrophoresis was performed as described elsewhere (17) except that Tris-borate-EDTA running buffer was replaced with recirculated Tris-acetate-EDTA as for all other EMSA experiments. The figures showing the EMSA data were produced electronically in Freehand 7.0.2 for Macintosh. Autoradiographs were scanned as reflective grayscale images, using a Umax 1260 scanner and the autodensity function. The resulting images were saved as PICT files in Adobe Photoshop 4.0 for Macintosh and then placed in Freehand for labeling. Other than uniform size changes, the images were unmodified.
Transfections and luciferase assays.
Transient transfection was performed in HEK293 cells as described previously (44). HEK293 cells were cultured in alpha minimal essential medium supplemented with 10% fetal calf serum and antibiotics (Sigma). Luciferase reporter assays were performed as detailed elsewhere (49).
RESULTS
Cooperative interactions between heterodimers involving PBX, HOX, and MEIS show dependence on DNA-binding activity of both partners.
HOX, PBX, and MEIS homeodomain proteins have been shown to bind DNA cooperatively in all heterodimeric combinations. More recent studies have also demonstrated higher-order trimeric complexes in which MEIS is tethered to a DNA-bound PBX-HOX (or HOX-like) heterodimer. In this report, we investigate two types of trimeric complexes formed with HOX, PBX, and MEIS proteins. To understand the interactions required for trimer formation, we have further characterized dimer interactions with respect to DNA-binding and functional protein domains.
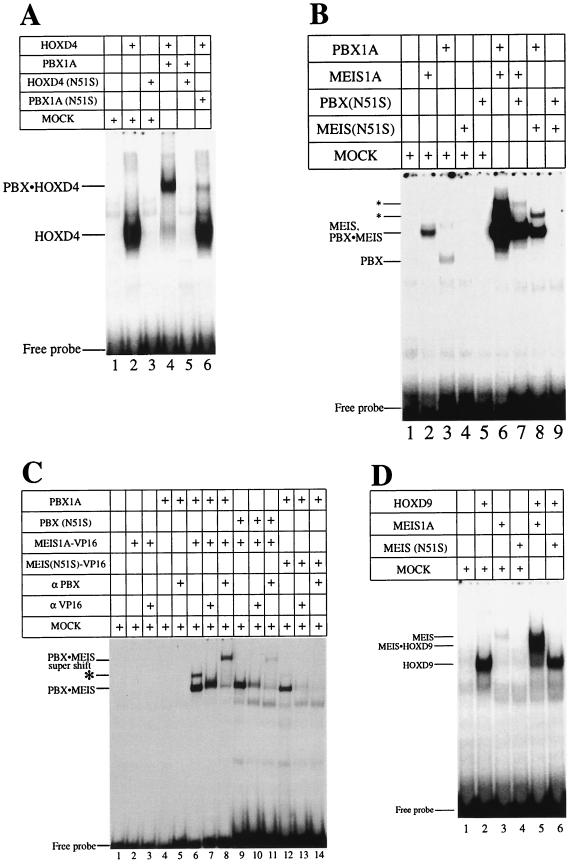
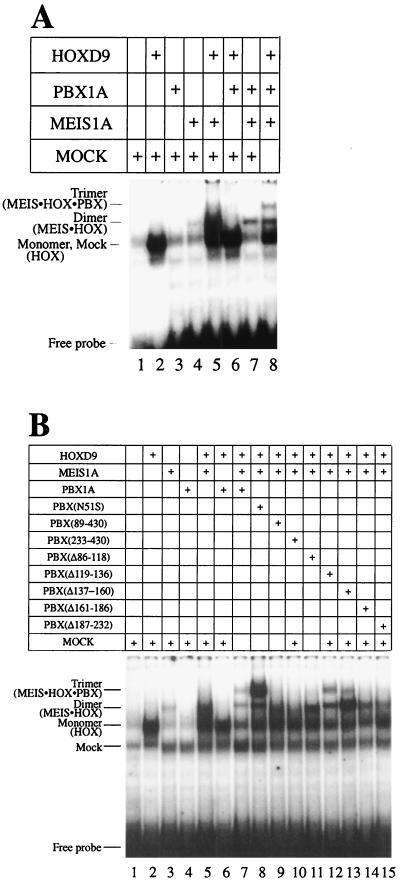
Mutation of asparagine 51 in the homeodomain severely decreases the affinity of DNA-binding (60). Conversion of asparagine 51 to serine (N51S mutation) in the homeodomain of the ANTP class protein HOXD4 led to the complete loss of detectable DNA binding as a monomer (Fig. 2A, lane 3) and as a heterodimer in the presence of PBX1A (Fig. 2A, lane 5). A similar mutation in the homeodomain of PBX1A severely impaired the formation of a heterodimeric complex with wild-type HOXD4, although complex formation was not abrogated (Fig. 2A, lane 6). Thus, both PBX1A and HOXD4 make important contributions to DNA binding by the heterodimer. Likewise, PBX1A critically contributes to cooperative DNA binding with the ABD-B class protein HOXD9 since the N51S mutation in PBX1A abolished heterodimer formation (Fig. 3B, lanes 4 and 5).
FIG. 2.
DNA-binding activity of both partners contributes to cooperative heterodimer formation in EMSA. Wild-type or N51S mutants of HOXD4, PBX1A, and MEIS1A were tested for the ability to form cooperative heterodimers. (A) PBX-HOXD4 interaction. Full-length HOXD4 bound as a monomer (lane 2) and a cooperative heterodimer with PBX1A (lane 4) on a PBX-HOX consensus site (Fig. 1A). Mutating asparagine 51 in the homeodomain of HOXD4 resulted in the loss of monomeric DNA binding and heterodimeric DNA binding with PBX1A (lanes 3 and 5). Lane 6 shows that a similar mutation in the homeodomain of PBX resulted in the formation of a reduced cooperative complex. (B) PBX-MEIS interaction. MEIS and PBX bound weakly as monomers (lanes 2 and 3) to a PBX-MEIS site (Fig. 1A) but together formed a strong cooperative complex (lane 6). Mutating asparagine 51 in the homeodomain of MEIS or PBX resulted in the loss of monomer binding activity (lanes 4 and 5) and a significant reduction in the formation of the DNA-bound heterodimeric complex (lanes 7 and 8). Incubating MEIS(N51S) with PBX(N51S) results in the complete loss of the cooperative complex (lane 9). Asterisks denote two higher-order complexes of PBX and MEIS. (C) Dimeric and higher-order complexes that form on a PBX-MEIS binding site (lane 6) contain both PBX1A and a MEIS-VP16 fusion protein, as shown by the use of N51S mutants (lanes 9 and 12) and antibodies to PBX1A (lanes 8, 11, and 14) and VP16 (lanes 7, 10, and 13). Note that lanes 9 to 14 were exposed longer than lanes 1 to 8. The asterisk denotes the faster of the two higher-order complexes also seen in panel B. The slowest species is also visible with a longer exposure. (D) DNA binding by MEIS is indispensable for the formation of a MEIS-HOXD9 cooperative complex. HOXD9 bound well as a monomer and a cooperative dimer with MEIS1A to a MEIS-HOX site (lanes 2 and 5). Poor MEIS binding (lane 3) was lost upon mutating asparagine 51 to serine (lane 4). Incubating MEIS(N51S) in the presence of HOXD9 also resulted in the loss of the cooperative heterodimer. Mock lysate was used to normalize the levels of lysate in the reactions where one of the translated proteins was missing.
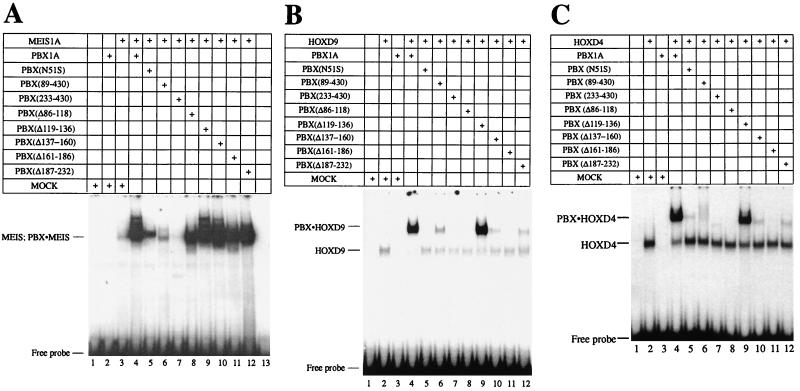
FIG. 3.
Mapping domains of PBX important for cooperative interactions. (A) Interaction of PBX1A with MEIS1A. Deletions encompassing region 1–232 of PBX1A (Fig. 1B) were assayed for the ability to interact with MEIS1A on a PBX-MEIS site in EMSA. The majority of the PBX1A-MEIS1A complex was abolished in the presence of PBX(89–430) (lane 6). Deleting the entire region N terminal to the PBX homeodomain [PBX(233-430)] resulted in the complete loss of the cooperative complex (lane 7). A series of deletions covering aa 86 to 232 did not reveal changes in the formation of the cooperative complex (lanes 8 to 12). Lane 1 served as a mock control. (B) Interaction of PBX1A with HOXD9. EMSA was performed as for panel A except that HOXD9 was used in the place of MEIS1A. PBX1A and deleted derivatives (Fig. 1B) were analyzed for the ability to form a cooperative complex with HOXD9 on a T6 probe (Fig. 1A). Except PBX(Δ119-136) (lane 9), all deletions of PBX had a profound impact on the formation of a cooperative complex (lanes 5 to 11). Lane 1 served as a mock control; lanes 2 and 4 show the DNA-bound HOXD9 monomer and PBX-HOXD9 cooperative complex, respectively. (C) As for panel B but with HOXD4 replacing HOXD9.
It has been previously shown (16) that PBX and MEIS proteins cooperatively bind an appropriate site on DNA (Fig. 1A). Unexpectedly, incubation of wild-type PBX1A and MEIS1A proteins gave rise to at least three retarded species in EMSA (Fig. 2B, lane 6), suggesting that the fastest-migrating species is the heterodimer, while the two slower-mobility bands are higher-order complexes. This was confirmed by pore exclusion limit electrophoresis (data not shown). The use of N51S mutants of either PBX1A or MEIS1A reduced the levels of all three complexes, indicating that all species are heteromeric (Fig. 2B, lanes 7 to 9).
To further dissect these complexes, we made use of a MEIS1A-VP16 fusion protein bearing either a wild-type or N51S homeodomain. Fusion to VP16 allowed us to dissect the complex components with appropriate antibodies. All three species were again formed between PBX1A and MEIS1A-VP16, albeit at reduced levels perhaps due to modification of the MEIS N terminus (Fig. 2C, lane 6). Similar to what was seen previously, the use of N51S mutants of PBX1A or MEIS1A-VP16 reduced the amounts of all three complexes (Fig. 2C, lanes 9 and 12). Importantly, inclusion of antibodies against either PBX1A or VP16 also reduced the formation of all species, confirming the heteromeric nature of these complexes (Fig. 2C, lanes 7, 8, 10, 11, 13, and 14).
Presumably, the fastest-migrating complex is a simple PBX1A-MEIS1A heterodimer. Significant amounts of this complex were formed even when one of the partners contained the N51S mutation. Conversely, DNA-binding activity by these proteins in isolation is extremely poor, even for the wild-type versions. One interpretation consistent with these results is that cooperativity in DNA binding by PBX-MEIS heterodimers is not simply a consequence of the stable association between these two proteins, but that this association promotes greatly increased DNA-binding activity by both partners. This is clearly the case in PBX-HOX interactions, where the HOX YPWM motif stabilizes the tertiary architecture of the extended PBX homeodomain (41, 46).
The interaction between ABD-B-class HOX proteins and MEIS has been well characterized (57). To investigate the importance of DNA binding by the MEIS partner in this complex, we used MEIS1A(N51S) in the presence of HOXD9 in EMSA. We find that DNA binding by MEIS1A is absolutely required for the formation of a cooperative complex with HOXD9 (Fig. 2D, lanes 5 and 6). Thus, DNA binding by the partners chosen for study is a key aspect of heterodimer formation under our conditions and is entirely consistent with the results of other studies.
Mapping N-terminal regions in PBX required for the formation of a heterodimer with MEIS or HOX.
The above results corroborate a role for PBX and MEIS in stabilizing their partners on DNA through protein-protein interaction. It was shown earlier that the region spanning aa 1 to 89 (region 1–89) in PBX harbors a MEIS interaction motif (16, 26). Deletion of this region severely decreased, but did not abolish, the formation of a cooperative complex (Fig. 3A, lane 6), as noted previously (26). Deleting the entire region N terminal to the homeodomain (aa 1 to 232) resulted in a complete loss of the cooperative complex in the presence of MEIS (Fig. 3A, lane 7). This suggested that additional sequences required for interaction with MEIS lay between aa 89 and 232. Internal deletions of the region (Fig. 1B) were created and used in EMSA, but they did not reveal striking differences in the formation of a cooperative complex (Fig. 3A; compare lanes 8 to 12 to lane 4). This confirms that the key motif for interaction with MEIS lies within residues 1 to 89 of PBX. That internal deletions did not impair the ability of region 1–89 to interact with MEIS suggests an absence of a strict spacing requirement for this function and indicates a degree of flexibility in the domains involved in this contact.
PBX can also dimerize with HOX partners. Clearly one important site of interaction is the PBX homeodomain that is contacted by the HOX YPWM motif. We looked for other regions of PBX required for interaction with HOX partners through the use of truncated PBX derivatives (Fig. 1B) in EMSA. Interestingly, region 1–89 of PBX, which mediates PBX-MEIS interaction, is also important for interaction with HOXD9 (Fig. 3B; compare lane 6 to lane 4) and HOXD4 (3C). This demonstrates that aa 1 to 89 of PBX could harbor an independent or overlapping binding site for MEIS and HOX proteins. However, we note that the absence of residues 1 to 89 did not prevent PBX from forming a strong complex with HOXA1 (data not shown), HOXB7 (16), and HOXA5 (26), and so these results may not be broadly applicable to all HOX proteins.
In addition, five internal deletions spanning aa 90 to 232 of PBX (Fig. 1B) reveal that all but a single region are required for efficient cooperative complex formation with HOXD9 and HOXD4 in steady-state EMSA (Fig. 3B and C, lanes 8 to 12). Additionally, these mutations decreased the stability of complex formation at least 20-fold (data not shown). The only exception is a stretch of alanines between residues 119 and 136 (lane 9) that is not conserved in Ceh-20, the homolog of PBX in Caenorhabditis elegans (9). This alanine-rich region has been reported to inhibit PBX-HOX interactions (42). Because deletion of residues 119 to 136 does not impinge on heterodimer formation, the detrimental 86-to-135 deletion is unlikely to disrupt spacing requirements between N- and C-terminal regions. Residues 119 to 136 divide two highly conserved domains called PBC-A and PBX-B in the PBC family of proteins containing PBX, EXD, and Ceh-20 (10). Our results suggest that both of these conserved domains are important for heterodimer formation with HOX partners.
MEIS1A can act as a non-DNA-binding partner in trimers with PBX1A-HOXD4 or PBX1A-HOXD9.
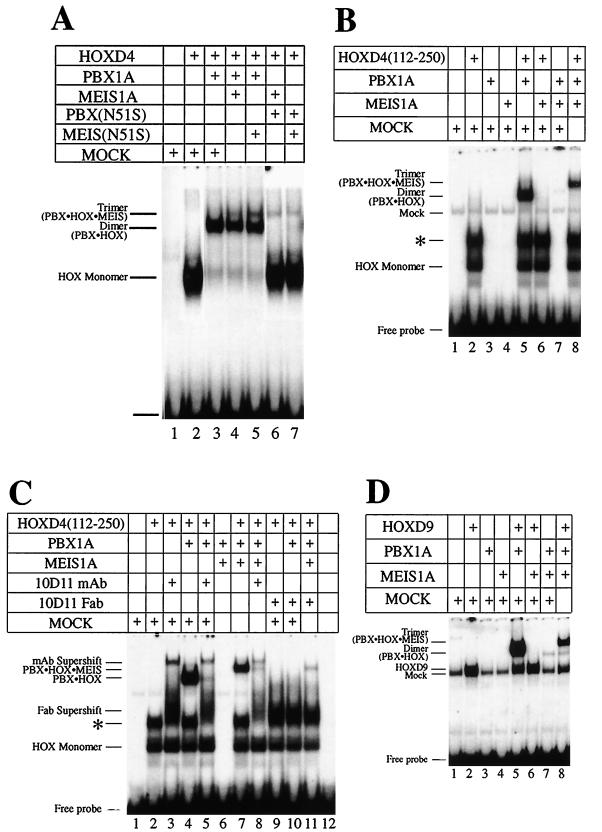
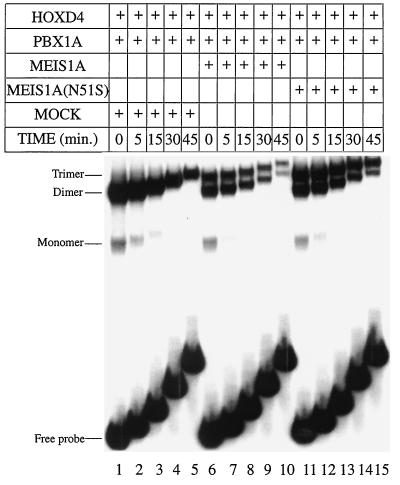
Only HOX proteins of groups 9 and 10 cooperatively bind DNA with MEIS (57), while groups 1 to 10 bind with PBX (13, 56). Because PBX can also cooperate with MEIS (16, 57), we reasoned that PBX could bridge MEIS and ANTP HOX partners in a trimeric complex. Additionally, the MEIS-like protein PREP1 forms a trimeric complex with PBX and HOXB1, which is not an ABD-B class HOX protein (5). This led us to investigate whether MEIS could form a trimer with DNA-bound PBX-HOXD4. A PBX-HOX binding site with A at position 6 (Fig. 1A) was used as the target DNA. Addition of MEIS1A to HOXD4 and PBX1A led to a novel band that migrated slower than the PBX1A-HOXD4 heterodimeric complex (Fig. 4A, lane 3 versus 4), suggestive of a trimeric complex. We have confirmed the presence of all three proteins in this complex with antibodies to PBX1A and MEIS1A (data not shown) as well as HOXD4 (see below).
FIG. 4.
Formation of a trimeric complex by MEIS(N51S). (A) MEIS1A is the non-DNA-binding component in the trimer with PBX-HOXD4 as seen in EMSA. Full-length HOXD4 binds an A6 site (Fig. 1A) as a monomer (lane 2) and as a cooperative dimer with PBX (lane 3). Addition of MEIS1A or MEIS(N51S) leads to the formation of an additional low-mobility complex (lanes 4 and 5). Loss of the cooperative dimer and retention of the presumptive trimer were observed when PBX(N51S) was used in the presence of MEIS1A and HOXD4 or of MEIS(N51S) and HOXD4 (lanes 6 and 7). (B) Deletion of aa 1 to 111 from HOXD4 reveals a negative effect of this region on the formation of the trimer. Deleting the first 111 aa resulted in a more robust trimeric complex: all of the heterodimer is converted to presumptive heterotrimer in lane 8 (compare to lane 4 in Fig. 4A). Also compare lanes 4 and 7 in Fig. 1C. (C) A MAb against the HOXD4 YPWM prevents the formation of the trimeric complex in EMSA. Addition of MAb 10D11 (54) or the respective Fab fragments led to the loss of the dimeric and trimeric complexes (compare lane 5 to lane 4, lane 8 to lane 7, and lanes 10 and 11 to lanes 4 and 7). (D) MEIS1A can be a non-DNA-binding component in a trimer with PBX1A-HOXD9. Addition of MEIS1A to PBX1A and HOXD9 led to the formation of a low-mobility band in EMSA (compare lane 8 to lane 5). On the site used here, no or little DNA-binding activity was observed for MEIS1A as a monomer (lane 4) or in combination with HOXD9 (lane 6) or PBX1A (lane 7), suggesting that DNA binding by MEIS1A is not involved in the formation of the presumptive trimer. ∗, an apparent dimer of HOXD4 that we have shown to be very unstable (data not shown). A 32P-labeled T6 target site (Fig. 1A) was used as a probe.
MEIS does not bind the A6 site as a monomer (Fig. 4B, lane 4) or as a dimer with either HOX or PBX (Fig. 4B, lanes 6 and 7), indicating that MEIS is a non-DNA-binding component in the trimeric complex. Supporting this idea, use of the DNA-binding-impaired MEIS1A(N51S) in conjunction with wild-type and N51S versions of PBX1A did not reduce formation of the trimer (Fig. 4A; compare lanes 4 and 5 and lanes 6 and 7). These results are in stark contrast to the debilitating effect of MEIS(N51S) on heterodimer formation with PBX1A or HOXD9 and confirm the non-DNA-binding role of MEIS1A in the formation of a trimer with DNA-bound PBX1A-HOXD4.
We showed above that the DNA-binding activity of PBX1A in a cooperative complex with HOXD4 is required for optimal heterodimer formation. We therefore examined the formation of the presumptive trimer in the presence of PBX(N51S). Unlike its effect on heterodimer formation with a HOX partner only, PBX(N51S) did not markedly reduce trimer formation with wild-type MEIS and HOX (Fig. 4A, lanes 4 and 6), indicating that the presence of wild-type MEIS1A enhances complex formation.
In the above experiment, the trimer was less abundant than the PBX1A-HOXD4 heterodimer (Fig. 4A). When an N-terminally truncated form of HOXD4 [HOXD4(112-250)] was used, there was no effect on heterodimer formation with PBX1A (Fig. 4B, lane 5). However, upon further addition of MEIS1A, we observed greatly enhanced formation of the slower-mobility complex at the expense of the heterodimer (Fig. 4B, lane 5 versus lane 8). Thus, residues 1 to 111 of HOXD4 exert a negative influence on formation of the trimer. This result also demonstrates that HOXD4 is a component of the trimer.
Cooperative interactions between PBX and ANTP class HOX proteins are dependent on the YPWM motif in the HOX partner (34). To confirm the presence of HOXD4 in the trimeric complex, we made use of the HOXD4 anti-YPWM MAb 10D11 (54) and its respective Fab fragments. With either reagent, dimeric (Fig. 4C, lanes 4, 5, and 10) and trimeric (Fig. 4C, lanes 7, 8, and 11) complexes were abrogated. HOXD4 is thus a component of the trimer.
ABD-B HOX proteins dimerize with PBX cooperatively on a PBX-HOX consensus site with T at position 6 (Fig. 1). HOXD9 and MEIS1A do not form a complex on this site (Fig. 4D, lane 6), while MEIS1A and PBX1A form a very poor complex (Fig. 4D, lane 7). The robust PBX1A-HOXD9 heterodimer that forms on this site (Fig. 4D, lane 5) is shifted to a slower-mobility complex upon addition of MEIS1A, indicative of trimer formation (Fig. 4D, lane 8). MEIS1A can therefore participate in presumptive trimer formation with HOX partners of both the ANTP and ABD-B classes.
MEIS stabilizes the trimeric complex through protein contacts.
We next compared the relative stability of the dimeric and trimeric complexes. For this purpose, wild-type and N51S versions of MEIS were used. As seen earlier (54), the half-life of a PBX1A-HOXD4 dimer was determined to be 14 min (Fig. 5, lanes 1 to 5). In the presence of MEIS1A, the presumptive trimeric complex had a longer half-life (25 min) than the dimer measured in the same reaction (17 min) (Fig. 5, lanes 6 to 10). Moreover, trimers containing MEIS1A(N51S) were more stable than complexes with the wild-type protein (Fig. 5, lanes 11 to 15), with half-lives of 35 and 19 min, respectively. These data suggest that trimer formation may be favored over the dimer in the cell.
FIG. 5.
MEIS1A and MEIS(N51S) can stabilize a trimeric complex as a non-DNA-binding partner. Dissociation rates were determined on an A6 labeled probe in the presence of a 100-fold excess of A6 cold competitor for the following complexes: PBX1A-HOXD4 (lanes 1 to 5), PBX1A-HOXD4 and MEIS1A-PBX1A-HOXD4 (lanes 6 to 10), and PBX1A-HOXD4 and MEIS(N51S)-PBX1A-HOXD4 (lanes 11 to 15).
PBX is a non-DNA-binding partner with DNA-bound MEIS-HOX complexes.
MEIS and PBX proteins are related by sequence both within and outside of the homeodomain. Given that MEIS can act as a non-DNA-binding partner in a trimer with DNA-bound HOX-PBX, we hypothesized that PBX could likewise interact with DNA bound MEIS-HOX. To explore the above possibility, we performed EMSA with HOXD9 and MEIS1A in the presence of PBX1A, using a labeled consensus binding site for MEIS-HOX heterodimers (Fig. 1A). The addition of PBX to a reaction containing HOXD9 and MEIS led to the formation of a slower-migrating band indicative of a trimeric complex (Fig. 6A, lanes 5 and 7).
FIG. 6.
PBX1A is the non-DNA-binding partner in a trimeric complex with MEIS1A and HOXD9. (A) PBX1A forms a trimeric complex with MEIS1A and HOXD9 in EMSA. Addition of PBX1A to MEIS1A and HOXD9 results in the decrease of monomer (lane 2) and dimer (lane 5) bands and the formation of a new low-mobility band (lane 8). (B) PBX1A is the non-DNA-binding partner in the trimeric complex and contacts both MEIS1A and HOXD9. This is revealed by the improved trimer formation with PBX(N51S) (lane 8) and reduced trimer formation with most N-terminal mutants of PBX1A (lanes 9 to 15 and Fig. 1B). A MEIS-HOX consensus binding site (Fig. 1A) (57) was used as probe in both panels.
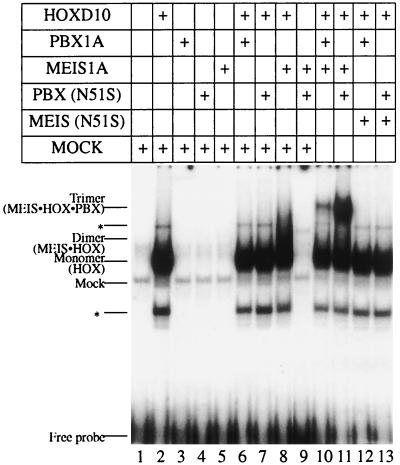
PBX does not bind as a monomer or a cooperative dimer with HOX on this site (Fig. 6A, lanes 3 and 6). This suggests that PBX is a non-DNA-binding component in a trimeric complex. However, since PBX and MEIS form a faint dimer on this probe (Fig. 6A, lane 7), it is possible that HOXD9 is the non-DNA-binding partner in the presumptive trimer. To assess whether DNA binding by PBX is required for the formation of the slower-mobility complex, we used PBX1A(N51S) in the presence of HOXD9 and MEIS1A. Surprisingly, this led to the formation of a more robust trimeric complex (Fig. 6B, lane 8) and confirmed that PBX plays a non-DNA-binding role in this process. A similar result was obtained with another ABD-B HOX protein, HOXD10 (Fig. 7, lanes 10 and 11). These findings additionally suggest that PBX1A(N51S) adopts an altered conformation that permits more efficient interaction with one or both partners in the DNA-bound dimer.
FIG. 7.
PBX1A can form a trimeric complex with MEIS1A and HOXD10 as a non-DNA-binding partner in EMSA. PBX1A or PBX(N51S) forms a trimeric complex with MEIS1A and HOXD10 on a MEIS-HOX binding site (lanes 10 and 11). ∗, minor band resulting from the in vitro translation of HOXD10.
Next, we asked whether DNA binding by MEIS1A is required for formation of the trimeric complex. The MEIS(N51S) mutant was used in EMSA with HOXD10 and either PBX1A or PBX1A(N51S). As seen in Fig. 7A, DNA binding by MEIS1A is essential for trimer formation (lanes 12 and 13), just as it is in heterodimeric DNA binding with ABD-B class HOX proteins of paralogous groups 9 and 10.
PBX contacts both partners in a DNA-bound MEIS-HOX complex and stabilizes binding.
PBX can dimerize with either MEIS or HOX proteins on the appropriate site on DNA. PBX1A is a non-DNA-binding protein in the presumptive trimeric complex based on DNA-bound MEIS-HOXD9 heterodimer. The ability of PBX to interact with either of these proteins to form heterodimers raised the possibility that PBX could make simultaneous contacts with both partners in the trimeric complex. To address this issue, we used N-terminal deletions of PBX, some of which selectively abolish dimer interactions with HOXD9 or MEIS1A. Residues N terminal to aa 89 of PBX1A are required for dimeric interaction with both HOXD9 and MEIS1A, whereas residues C terminal to aa 89 are dispensable for interaction with MEIS1A but required for interaction with HOXD9 (Fig. 3A and B). The results shown in Fig. 6B show that amino acids both N terminal and C terminal to aa 89 of PBX are required for formation of the trimeric complex with DNA-bound MEIS1A-HOXD9. We suggest that C-terminal domains between aa 89 and 232 are important for trimer formation because they contact HOXD9 just as they do in the dimer. While residues 1 to 89 of PBX1A are important for dimer interaction with both HOXD9 and MEIS1A, region 1–89 is the only strong binding site for MEIS on this protein. It seems likely therefore that this region of PBX continues to contact MEIS in the trimer. This does not exclude the possibility that region 1–89 contacts both partners in the trimer. In summary, we provide evidence that PBX1A contacts both HOXD9 and MEIS1A as a nonbinding partner in a trimeric complex. Moreover, contact with only one of the DNA-bound partners is not sufficient for the formation of the trimeric complex.
We next explored the role of PBX1A in the trimeric complex through dissociation rate experiments. HOXD10 bound to a T6 site (Fig. 1A) has a half-life of 6 min. The heterodimeric complex formed in the presence of MEIS dissociated with a half-life of 9 to 10 min. Formation of the trimer by coincubation with PBX further increased the half-life to 13 min. While these effects are modest, they suggest that increased stability could favor trimer formation in vivo.
MEIS can form a trimeric complex in vivo as a non-DNA-binding component.
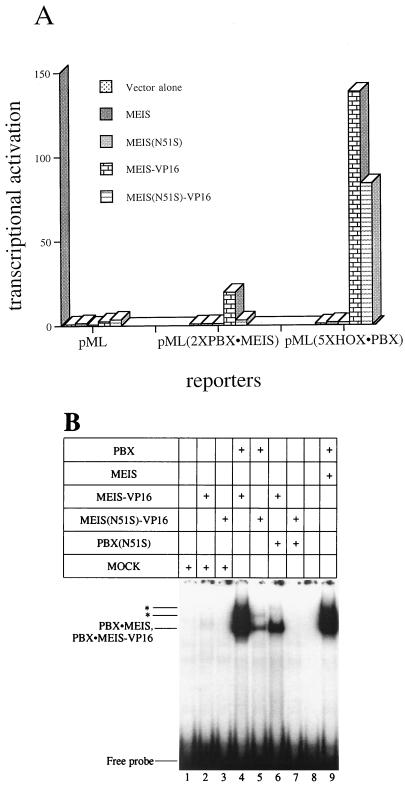
Formation of a trimeric complex in the cell was assessed with a transcriptional assay in transfections of HEK293 cells. A MEIS-VP16 fusion protein was used to provide a strong transcriptional readout since wild-type HOX, PBX, and MEIS proteins do not activate transcription from multimerized binding sites (reference 58 and our unpublished observations). Three promoters driving luciferase expression were tested. The first promoter in vector pML carries only the adenovirus major late promoter and therefore lacks binding sites for all of the complexes under study. The second, pML(2×PBX · MEIS), carries two PBX-MEIS binding sites. Last, pML(5×HOX · PBX) carries five copies of a PBX-HOX cooperative binding site (44). Importantly, MEIS does not bind this site, either alone or in heterodimers with HOX and PBX partners. Therefore, the only way for MEIS-VP16 to activate expression of this reporter is if it is tethered by protein interactions to the PBX-HOX heterodimers which do form at the promoter.
As shown in Fig. 8A, MEIS-VP16 strongly activated transcription of pML(5×HOX · PBX), despite its inability to bind the sites linked to this promoter. To further exclude a role for DNA binding in the activation of this reporter by MEIS-VP16, we used an N51S derivative. MEIS(N51S)-VP16 also robustly activated transcription of the pML(5×HOX · PBX) reporter (Fig. 8A). By contrast, MEIS(N51S)-VP16 only poorly activated transcription through the promoter in pML(2×PBX · MEIS), which requires DNA binding by a MEIS protein. As expected, none of the expressed proteins activated transcription of the minimal pML vector.
FIG. 8.
MEIS(N51S) can form a trimeric complex with HOX and PBX in vivo. (A) Effects of transfected expression vectors for MEIS1A, MEIS(N51S), MEIS-VP16, or MEIS(N51S)-VP16 on luciferase reporters containing no specific binding sites (pML) or binding sites for PBX-MEIS [pML(2×PBX · MEIS)] or HOX-PBX [pML(5×HOX · PBX)]. Results were reproducible, and data from one experiment are presented. Values are expressed as fold activation over transfection of the respective reporter plasmids alone. (B) Characterization of MEIS-VP16 and MEIS(N51S)-VP16 fusion proteins in EMSA. MEIS-VP16 or MEIS(N51S)-VP16 used for panel A were able to form complexes with PBX1A to the same degree as their wild-type counterparts (compare lanes 4, 5, and 9). ∗, higher-order PBX-MEIS complex also seen in Fig. 2B.
This experiment relies on the presence of PBX and HOX proteins in the host 293 cells. These cells express all three PBX genes (36) and are expected to express a number of HOX genes as well. However, to ensure that an appropriate HOX partner was available for trimer formation, we repeated the experiment with a cotransfected vector for HOXD4. Highly similar results were obtained (data not shown).
The integrity of the VP16 fusion proteins was confirmed by EMSA. MEIS-VP16 formed strong cooperative complexes with PBX1A on a PBX-MEIS binding site (Fig. 8B, lane 4) that was reduced when MEIS(N51S)-VP16 was used (Fig. 8B, lane 5).
Thus, compromising the DNA-binding activity of MEIS-VP16 strongly reduces transcriptional activation from a promoter in which DNA binding is required as a heterodimer with PBX but not through a promoter in which MEIS-VP16 is expected to interact as a non-DNA-binding partner of bound PBX-HOX heterodimers. These results strongly argue for the formation of trimeric homeoprotein complexes in the cell. Further, while MEIS is unable to bind DNA directly with ANTP class HOX proteins, our results suggest that MEIS can nonetheless modulate the function of the ANTP class via protein-protein interactions.
DISCUSSION
We have examined protein domains and DNA-binding activities required for the formation of heterodimeric and heterotrimeric complexes on DNA. First, we show that the DNA-binding activities of HOX, PBX, and MEIS proteins make important contributions to complex formation in all heterodimeric permutations. Second, region 1–89 in PBX, besides mediating interactions with MEIS, is important for interactions with the ANTP class HOXD4 and ABD-B group HOXD9 proteins. Third, PBX and MEIS can act as non-DNA-binding partners in the formation of heterotrimeric complexes with HOX proteins. Evidence supporting in vivo trimer formation was obtained using a MEIS(N51S)-VP16 fusion protein to activate transcription of a luciferase reporter bearing a PBX-HOX consensus site. Last, we map a domain in the N terminus of HOXD4 that exerts a negative effect on the formation of the trimeric complex.
Cooperative DNA binding with a compromised homeodomain.
Asparagine 51 in the recognition helix of the homeodomain plays an important role in protein-DNA interactions. Via its side chain, it establishes a hydrogen bond with a specific base in the major groove (7, 8, 25, 41, 46, 62). In agreement with the structural data and the results of others, we observe loss of monomer binding to target DNA by HOX, PBX, or MEIS when Asp 51 in the homeodomain is mutated to serine. A reduction in heterodimeric complex formation was also noted with all N51S mutants. The retention of some DNA binding by compromised PBX-MEIS heterodimers may be in part explicable by the strong interactions between these proteins even in the absence of DNA (16). However, this cannot be the explanation for residual binding by PBX-HOXD4 dimers whose interactions off of DNA are expected to be much weaker. This shows that site-specific DNA binding by the PBX partner is not a strict requirement for cooperative interactions with HOX proteins and that less specific interactions are enough to achieve a degree of cooperativity. This can also be inferred from the finding that naturally occurring binding sites for PBX-HOX heterodimers deviate from the consensus and do not bind the heterodimer with optimal affinity (21, 43, 47). This situation is reminiscent of the reduced requirement for specific DNA binding by Matα2 in heterodimers with Mata1, both of which are homeodomain proteins (60). We note that specific DNA binding by a trimer composed of PBX1A(N51S), MEIS1A(N51S), and HOXD4 must be mediated largely by HOXD4 (Fig. 2A). Likewise, interaction of DFD with a monomeric binding site (45) may be stabilized by trimer formation with EXD and HTH.
Cooperativity with HOXD9 and HOXD4 is dependent on the MEIS interaction domain of PBX.
Structure-function analysis has demonstrated that a region comprising aa 1 to 89 in PBX is important in mediating interactions with MEIS (57). Here we show that this same region is required for interaction with ANTP (HOXD4) and ABD-B (HOXD9) group proteins. It must be noted, however, that we have not consistently observed decreased complex formation between PBX(89-430) and HOXA1, nor has this been observed by other groups with HOXB7 (16) and HOXA5 (26). Thus, region 1–89 of PBX may play a limited and specific role in its interactions with a subset of HOX partners.
E2A-PBX is a chimeric oncogene (24) encoding a fusion of the transcriptional activation domain of E2A with residues 89 to 430 of PBX1. Due to the absence of the PBX N terminus, this oncoprotein does not interact with MEIS (16). However, E2A-PBX retains the ability to form cooperative complexes on DNA with ANTP class HOX proteins (14, 26) despite our demonstration that the missing PBX N terminus is required for this interaction. This paradox would be resolved if the E2A portion functionally compensates for the missing residues.
We have also mapped two additional domains that are important in mediating PBX-HOXD9 interactions, namely, residues 86 to 118 and 137 to 232. Three smaller deletions within the latter region abrogate the formation of PBX-HOXD4 and PBX-HOXD9 cooperative complexes. These results implicate most of the region N terminal to the PBX1A homeodomain in cooperative interactions with HOX partners. Additional functions for portions of the PBX N terminus include interaction with MEIS (16) and PREP (6), transcriptional repression (30), and the control of subcellular localization (1, 4, 52a). This is consistent with the high conservation of this stretch within and across species (9, 10).
Higher-order complexes of PBX and MEIS.
We have used N51S mutants and specific antibodies to show that PBX1A and MEIS1A form higher-order complexes on an appropriate binding site, in addition to the dimeric complexes already described (16, 26). We have shown that DNA binding is required for the formation of all complexes, but this is not surprising given that a DNA-bound PBX-MEIS heterodimer is likely to form the scaffold for the two higher-order complexes. While the functional domains and biological significance of these structures remain to be explored, it seems reasonable to speculate that the N termini of both proteins are required for their formation, as they are for the heterodimer. While it is unlikely that the short oligonucleotides used here could accommodate binding by all members of the higher-order complex, naturally occurring enhancer elements may well facilitate these interactions.
Two trimeric complexes of HOX, PBX, and MEIS.
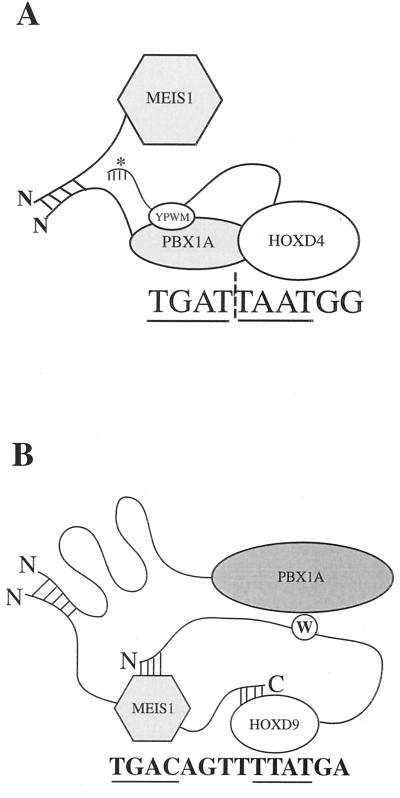
In the first trimer category described here, MEIS is a non-DNA-binding partner with DNA-bound PBX-HOX containing either the ANTP class HOXD4 protein or the ABD-B class HOXD9 or HOXD10 protein. The latter trimer may be less surprising, given the known ability of MEIS to bind DNA cooperatively with ABD-B members of groups 9 and 10 (57). More surprising, despite the inability of MEIS to interact with ANTP HOX proteins like HOXD4, it can nonetheless form a trimer with a DNA-bound PBX-HOXD4 heterodimer (Fig. 4A). We suggest that because PBX can contact both MEIS and HOXD4, it thereby bridges the other two proteins in the trimer (Fig. 9A).
FIG. 9.
Model summarizing the formation of trimeric complexes by HOX cofactors MEIS and PBX as non-DNA-binding partners. (A) MEIS as the non-DNA-binding partner. PBX and HOX bind cooperatively to a consensus heterodimer binding site. MEIS and PBX interact through their N termini. ∗, deletion of the N terminus of HOXD4 results in a more robust trimeric complex. The significance of this effect is discussed in the text. (B) PBX as the non-DNA-binding partner. MEIS and HOXD9 or HOXD10 cooperatively bind a consensus MEIS-HOX binding site. We propose that PBX and MEIS interact via their N termini and that the PBX homeodomain contacts the tryptophan motif in HOXD9. The N terminus of PBX is looped to suggest the presence of intramolecular interactions important for the formation of the PBX-HOXD9 dimer and MEIS-HOXD9-PBX trimer. The importance of the N51S mutation in PBX and MEIS (not shown) is addressed in the Discussion. The core binding sites in both panels are underlined.
This trimer form has also been described in recent reports from other groups. MEIS is a non-DNA-binding partner in trimers with PBX-PDX1 (59) and PBX-HOXA9 (58). Similarly, the MEIS-related protein PREP1 has been shown to be a non-DNA-bound partner in a trimeric complex with PBX-HOXB1 (5).
The second trimer category has not been previously described. Here it is PBX that is the non-DNA-binding partner in a trimer with MEIS-HOXD9 or MEIS-HOXD10. In this trimer, PBX is likely to make simultaneous contacts with both DNA-bound MEIS and HOX proteins for the following reasons. PBX and HOX proteins do not make stable associations off of DNA, yet PBX is a non-DNA-bound partner in the trimer. Therefore, one contact is likely to be with MEIS, which makes stable contacts with residues 1 to 89 of PBX even off of DNA (16). In addition, three internal deletions of PBX which disrupt heterodimer formation with HOXD9, but not MEIS, also abolish trimer formation. The simplest explanation of the data is that these contacts are retained in the trimer and are required. However, one PBX deletion mutant [PBX(Δ137-60)] that was required for heterodimerization with HOXD9 only mildly affected trimer formation. This may be because conformational changes provoked by trimer assembly render this region dispensable.
We observe a striking increase in trimer levels with the PBX1A(N51S) mutant. A more modest increase was also observed for MEIS1A(N51S) in the first trimer type. Likewise, deleting the entire homeodomain of PREP1 strongly enhances the formation of a PREP-PBX-HOX trimer (5). Together, these results suggest that intramolecular interactions involving N51 of the TALE class homeodomain affect conformation and availability for trimer formation. A similar enhancement in the formation of a MEIS-PBX-HOX trimer was observed when N-terminal residues 1 to 111 were deleted in HOXD4 (Fig. 4B). Together, these results point to an inhibitory action of the TALE class homeodomain and the HOX N terminus on trimer formation. These may provide a basis for the modulation of trimer levels through posttranslational modification.
PBX, MEIS, and HOX proteins have been shown to synergize for the production of leukemia in mice (28, 38). Our results suggest that one way this may be accomplished is through effects on target gene regulation mediated by the trimeric complexes described here. A role in the stabilization of PBX-HOX and MEIS-HOX complexes seems likely from our study but should not be the only role for these trimers. The presence of a third homeodomain protein should modify interactions between the trimeric complex and other enhancer factors or the basal transcriptional machinery. Additionally, it seems likely that the specificity of target gene regulation would be increased through interaction with promoter/enhancer elements that mediate binding by all three members of the trimeric complex. Naturally occurring regulatory sites may provide the opportunity for DNA binding by all three components of the heterotrimers described here, thereby greatly increasing the affinity and specificity of DNA binding.
ACKNOWLEDGMENTS
We thank A. Buchberg for a gift of the Meis1a cDNA, H. Huang for the MEIS1A antibody, M. Kamps for a gift of the E2A-PBX1(N51S) cDNA, S. Malliartchouk for advice on Fab fragment production, M. Saleh for subcloning the Meis1a coding sequence into the pCS2+ expression vector, A. Tremblay for help with antibodies, H. Ball and J. Licht for advice on pore exclusion limit electrophoresis, and members of the lab for helpful discussions.
K.S. is a recipient of an Internal Studentship of the Faculty of Medicine, McGill University. M.S.F. is a Chercheur-Boursier of the Fonds de la Recherche en Santé du Québec. This work was funded by grants to M.S.F. from the Medical Research Council of Canada and the Cancer Research Society, Inc.
REFERENCES
- 1.Abu-Shaar M, Ryoo H D, Mann R S. Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 1999;13:935–945. doi: 10.1101/gad.13.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew S M, Titus J A. Fragmentation of immunoglobulin G. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1992. [Google Scholar]
- 3.Bergson C, McGinnis W. An autoregulatory enhancer element of the Drosophila homeotic gene Deformed. EMBO J. 1990;9:4287–4297. doi: 10.1002/j.1460-2075.1990.tb07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthelsen J, Kilstrup-Nielsen C, Blasi C, Mavilio F, Zappavigna V. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 1999;13:946–953. doi: 10.1101/gad.13.8.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthelsen J, Zappavigna V, Ferretti E, Mavilio F, Blasi F. The novel homeoprotein Prep1 modulates Pbx-Hox protein cooperativity. EMBO J. 1998;17:1434–1445. doi: 10.1093/emboj/17.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthelsen J, Zappavigna V, Mavilio F, Blasi F. Prep1, a novel functional partner of Pbx proteins. EMBO J. 1998;17:1423–1433. doi: 10.1093/emboj/17.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billeter M, Güntert P, Luginbühl P, Wüthrich K. Hydration and DNA recognition by homeodomains. Cell. 1996;85:1057–1065. doi: 10.1016/s0092-8674(00)81306-9. [DOI] [PubMed] [Google Scholar]
- 8.Billeter M, Qian Y Q, Otting G, Müller M, Gehring W, Wüthrich K. Determination of the nuclear magnetic resonance solution structure of an Antennapedia homeodomain-DNA complex. J Mol Biol. 1993;234:1084–1097. doi: 10.1006/jmbi.1993.1661. [DOI] [PubMed] [Google Scholar]
- 9.Bürglin T. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bürglin T R, Ruvkin G. A new motif in PBX genes. Nat Genet. 1992;1:319–320. doi: 10.1038/ng0892-319. [DOI] [PubMed] [Google Scholar]
- 11.Chan S, Mann R. A structural model for a homeotic protein-extradenticle-DNA complex accounts for the choice of HOX protein in the heterodimer. Proc Natl Acad Sci USA. 1996;93:5223–5228. doi: 10.1073/pnas.93.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan S K, Ryoo H D, Gould A, Krumlauf R, Mann R S. Switching the in vivo specificity of a minimal Hox-responsive element. Development. 1997;124:2007–2014. doi: 10.1242/dev.124.10.2007. [DOI] [PubMed] [Google Scholar]
- 13.Chang C-P, Brocchieri L, Shen W-F, Largman C, Cleary M L. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol. 1996;16:1734–1745. doi: 10.1128/mcb.16.4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C-P, de Vivo I, Cleary M L. The Hox cooperativity motif of the chimeric oncoprotein E2a-Pbx1 is necessary and sufficient for oncogenesis. Mol Cell Biol. 1997;17:81–88. doi: 10.1128/mcb.17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang C-P, Shen W-F, Rozenfeld S, Lawrence H J, Largman C, Cleary M. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 16.Chang C P, Jacobs Y, Nakamura T, Jenkins N A, Copeland N G, Cleary M L. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol Cell Biol. 1997;17:5679–5687. doi: 10.1128/mcb.17.10.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clos J, Westwood J T, Becker P B, Wilson S, Lambert K, Wu C. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell. 1990;63:1085–1097. doi: 10.1016/0092-8674(90)90511-c. [DOI] [PubMed] [Google Scholar]
- 18.Di Rocco G, Mavilio F, Zappavigna V. Functional dissection of a transcriptionally active, target-specific Hox-Pbx complex. EMBO J. 1997;16:3644–3654. doi: 10.1093/emboj/16.12.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favier B, Dollé P. Developmental functions of mammalian Hox Genes. Mol Hum Reprod. 1997;3:115–131. doi: 10.1093/molehr/3.2.115. [DOI] [PubMed] [Google Scholar]
- 20.Gehring W J, Qian Y Q, Billeter M, Furukubo-Tokunaga K, Schier A F, Resendez-Perez D, Wüthrich K. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 21.Gould A, Morrison A, Sproat G, White R A H, Krumlauf R. Positive cross-regulation and enhancer sharing—two mechanisms for specifying overlapping Hox expression patterns. Genes Dev. 1997;11:900–913. doi: 10.1101/gad.11.7.900. [DOI] [PubMed] [Google Scholar]
- 22.Green N C, Rambaldi I, Teakles J, Featherstone M S. A conserved C-terminal domain in PBX increases DNA binding by the PBX homeodomain and is not a primary site of contact for the YPWM motif of HOXA1. J Biol Chem. 1998;273:13273–13279. doi: 10.1074/jbc.273.21.13273. [DOI] [PubMed] [Google Scholar]
- 23.Johnson F B, Parker E, Krasnow M A. extradenticle protein is a selective cofactor for the Drosophila homeotics: role of the homeodomain and YPWM amino acid motif in the interaction. Proc Natl Acad Sci USA. 1995;92:739–743. doi: 10.1073/pnas.92.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamps M P, Murre C, Sun X-H, Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990;60:547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- 25.Kissinger S R, Liu B, Martin-Blanco E, Kornberg T B, Pabo C O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 Å resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990;63:579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- 26.Knoepfler P S, Calvo K R, Chen H, Antonarakis S E, Kamps M P. Meis1 and pKnox1 bind DNA cooperatively with Pbx1 utilizing an interaction surface disrupted in oncoprotein E2a-Pbx1. Proc Natl Acad Sci USA. 1997;94:14553–14558. doi: 10.1073/pnas.94.26.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoepfler P S, Kamps M P. The pentapeptide motif of Hox proteins is required for cooperative DNA binding with Pbx1, physically contacts Pbx1, and enhances DNA binding by Pbx1. Mol Cell Biol. 1995;15:5811–5819. doi: 10.1128/mcb.15.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg A M, Sauvageau G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 1998;17:3714–3725. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 30.Lu Q, Kamps M P. Selective repression of transcriptional activators by pbx1 does not require the homeodomain. Proc Natl Acad Sci USA. 1996;93:470–474. doi: 10.1073/pnas.93.1.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Q, Kamps M P. Structural determinants within Pbx1 that mediate cooperative DNA binding with pentapeptide-containing Hox proteins: proposal for a model of a Pbx1-Hox DNA complex. Mol Cell Biol. 1996;16:1632–1640. doi: 10.1128/mcb.16.4.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q, Knoepfler P S, Scheele J, Wright D D, Kamps M P. Both Pbx1 and E2A-PBX1 bind the DNA motif ATCAATCAA cooperatively with the products of multiple murine Hox genes, some of which are themselves oncogenes. Mol Cell Biol. 1995;15:3786–3795. doi: 10.1128/mcb.15.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maconochie M K, Nonchev S, Studer M, Chan S-K, Pöpperl H, Sham M H, Mann R S, Krumlauf R. Cross-regulation in the mouse HoxB complex: the expression of Hoxb2 in rhombomere 4 is regulated by Hoxb1. Genes Dev. 1997;11:1885–1895. doi: 10.1101/gad.11.14.1885. [DOI] [PubMed] [Google Scholar]
- 34.Mann R S, Chan S-K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 35.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 36.Monica K, Galili N, Nourse J, Saltman D, Cleary M L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol. 1993;11:6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moskow J J, Bullrich F, Huebner K, Daar I O, Buchberg A M. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol Cell Biol. 1995;15:5434–5443. doi: 10.1128/mcb.15.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura T, Largaespada D A, Shaughnessy J D, Jr, Jenkins N A, Copeland N G. Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukemias. Nat Genet. 1996;12:149–153. doi: 10.1038/ng0296-149. [DOI] [PubMed] [Google Scholar]
- 39.Neuteboom S T C, Peltenburg L T C, van Dijk M A, Murre C. The hexapeptide LFPWMR in Hoxb-8 is required for cooperative DNA binding with Pbx1 and Pbx2 proteins. Proc Natl Acad Sci USA. 1995;92:9166–9170. doi: 10.1073/pnas.92.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nourse J, Mellentin J D, Galili N, Wilkinson J, Starbridge E, Smith S D, Cleary M L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990;60:535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- 41.Passner J M, Ryoo H D, Shen L, Mann R S, Aggarwal A K. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 42.Peltenburg L T C, Murre C. Specific residues in the Pbx homeodomain differentially modulate the DNA-binding activity of Hox and Engrailed proteins. Development. 1997;124:1089–1098. doi: 10.1242/dev.124.5.1089. [DOI] [PubMed] [Google Scholar]
- 43.Phelan M L, Featherstone M S. Distinct HOX N-terminal arm residues are responsible for specificity of DNA recognition by HOX monomers and HOX · PBX heterodimers. J Biol Chem. 1997;272:8635–8643. doi: 10.1074/jbc.272.13.8635. [DOI] [PubMed] [Google Scholar]
- 44.Phelan M L, Rambaldi I, Featherstone M S. Cooperative interactions between HOX and PBX proteins mediated by a conserved peptide motif. Mol Cell Biol. 1995;15:3989–3997. doi: 10.1128/mcb.15.8.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinsonneault J, Florence B, Vaessin H, McGinnis W. A model for extradenticle function as a switch that changes HOX proteins from repressors to activators. EMBO J. 1997;16:2032–2042. doi: 10.1093/emboj/16.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piper D E, Batchelor A H, Chang C P, Cleary M L, Wolberger C. Structure of a HoxB1-Pbx1 heterodimer bound to DNA: role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell. 1999;96:587–597. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 47.Pöpperl H, Bienz M, Studer M, Chan S-K, Aparacio S, Brenner S, Mann R, Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent on exd/pbx. Cell. 1995;81:1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- 48.Pöpperl H, Featherstone M S. An autoregulatory element of the murine Hox-4.2 gene. EMBO J. 1992;11:3673–3680. doi: 10.1002/j.1460-2075.1992.tb05452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rambaldi I, Nagy Kovàcs E, Featherstone M S. A proline-rich transcriptional activation domain in murine HOXD-4 (HOX-4.2) Nucleic Acids Res. 1994;22:376–382. doi: 10.1093/nar/22.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rauskolb C, Peifer M, Wieschaus E. extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell. 1993;74:1101–1112. doi: 10.1016/0092-8674(93)90731-5. [DOI] [PubMed] [Google Scholar]
- 51.Regulski M, Dessain S, McGinnis N, McGinnis W. High-affinity binding sites for the Deformed protein are required for the function of an autoregulatory enhancer of the Deformed gene. Genes Dev. 1991;5:278–286. doi: 10.1101/gad.5.2.278. [DOI] [PubMed] [Google Scholar]
- 52.Rieckhof G E, Casares F, Ryoo H D, Abu-Shaar M, Mann R S. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 52a.Saleh, M., and M. S. Featherstone. Unpublished data.
- 53.Schnabel C A, Abate-Shen C. Repression by HoxA7 is mediated by the homeodomain and the modulatory action of its N-terminal-arm residues. Mol Cell Biol. 1996;16:2678–2688. doi: 10.1128/mcb.16.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shanmugam K, Featherstone M S, Saragovi H U. Residues flanking the HOX YPWM motif contribute to cooperative interactions with PBX. J Biol Chem. 1997;272:19081–19087. doi: 10.1074/jbc.272.30.19081. [DOI] [PubMed] [Google Scholar]
- 55.Shen W, Chang C, Rozenfeld S, Sauvageau G, Humphries R, Lu M, Lawerence H, Cleary M, Largman C. Hox homeodomain proteins exhibit selective complex stabilities with Pbx and DNA. Nucleic Acids Res. 1996;24:898–906. doi: 10.1093/nar/24.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen W-F, Rozenfeld S, Lawrence H J, Largman C. The Abd-B-like Hox homeodomain proteins can be subdivided by the ability to form complexes with Pbx1a on a novel DNA target. J Biol Chem. 1997;272:8198–8206. doi: 10.1074/jbc.272.13.8198. [DOI] [PubMed] [Google Scholar]
- 57.Shen W F, Montgomery J C, Rozenfeld S, Moskow J J, Lawrence H J, Buchberg A M, Largman C. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol. 1997;17:6448–6458. doi: 10.1128/mcb.17.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen W F, Rozenfeld S, Kwong A, Kömüves L G, Lawrence H J, Largman C. HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol Cell Biol. 1999;19:3051–3061. doi: 10.1128/mcb.19.4.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swift G H, Liu Y, Rose S D, Bischof L J, Steelman S, Buchberg A M, Wright C V, MacDonald R J. An endocrine-exocrine switch in the activity of the pancreatic homeodomain protein PDX1 through formation of a trimeric complex with PBX1b and MRG1 (MEIS2) Mol Cell Biol. 1998;18:5109–5120. doi: 10.1128/mcb.18.9.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vershon A K, Jin Y, Johnson A D. A homeo domain protein lacking specific side chains of helix 3 can still bind DNA and direct transcriptional repression. Genes Dev. 1995;9:182–192. doi: 10.1101/gad.9.2.182. [DOI] [PubMed] [Google Scholar]
- 61.Viganò M A, Di Rocco G, Zappavigna V, Mavilio F. Definition of the transcriptional activation domains of three human HOX proteins depends on the DNA-binding context. Mol Cell Biol. 1998;18:6201–6212. doi: 10.1128/mcb.18.11.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolberger C, Vershon A K, Liu B, Johnson A D, Pabo C O. Crystal structure of a MATα2 homeodomain-operator complex suggests a general model for homeodomain-DNA interactions. Cell. 1991;67:517–528. doi: 10.1016/0092-8674(91)90526-5. [DOI] [PubMed] [Google Scholar]
- 63.Zappavigna V, Falciola L, Citterich M H, Mavilio F, Bianchi M E. HMG1 interacts with HOX proteins and enhances their DNA binding and transcriptional activation. EMBO J. 1996;15:4981–4991. [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng C, Pinsonneault J, Gellon G, McGinnis N, McGinnis W. Deformed protein binding sites and cofactor binding sites are required for the function of a small segment-specific regulatory element in Drosophila embryos. EMBO J. 1994;13:2362–2377. doi: 10.1002/j.1460-2075.1994.tb06520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]