Revised Neuroblastoma Risk Classification System: A Report From the Children's Oncology Group (original) (raw)
PURPOSE
Treatment planning for children with neuroblastoma requires accurate assessment of prognosis. The most recent Children's Oncology Group (COG) risk classification system used tumor stage as defined by the International Neuroblastoma Staging System. Here, we validate a revised classifier using the International Neuroblastoma Risk Group Staging System (INRGSS) and incorporate segmental chromosome aberrations (SCA) as an additional genomic biomarker.
METHODS
Newly diagnosed patients enrolled on the COG neuroblastoma biology study ANBL00B1 between 2007 and 2017 with known age, International Neuroblastoma Staging System, and INRGSS stage were identified (N = 4,832). Tumor MYCN status, ploidy, SCA status (1p and 11q), and International Neuroblastoma Pathology Classification histology were determined centrally. Survival analyses were performed for combinations of prognostic factors used in COG risk classification according to the prior version 1, and to validate a revised algorithm (version 2).
RESULTS
Most patients with locoregional tumors had excellent outcomes except for those with image-defined risk factors (INRGSS L2) with MYCN amplification (5-year event-free survival and overall survival: 76.3% ± 5.8% and 79.9% ± 5.5%, respectively) or patients age ≥ 18 months with L2 MYCN nonamplified tumors with unfavorable International Neuroblastoma Pathology Classification histology (72.7% ± 5.4% and 82.4% ± 4.6%), which includes the majority of L2 patients with SCA. For patients with stage M (metastatic) and MS (metastatic, special) disease, genomic biomarkers affected risk group assignment for those < 12 months (MYCN) or 12-18 months (MYCN, histology, ploidy, and SCA) of age. In a retrospective analysis of patient outcome, the 5-year event-free survival and overall survival using COG version 1 were low-risk: 89.4% ± 1.1% and 97.9% ± 0.5%; intermediate-risk: 86.1% ± 1.3% and 94.9% ± 0.8%; high-risk: 50.8% ± 1.4% and 61.9% ± 1.3%; and using COG version 2 were low-risk: 90.7% ± 1.1% and 97.9% ± 0.5%; intermediate-risk: 85.1% ± 1.4% and 95.8% ± 0.8%; high-risk: 51.2% ± 1.4% and 62.5% ± 1.3%, respectively.
CONCLUSION
A revised 2021 COG neuroblastoma risk classifier (version 2) that uses the INRGSS and incorporates SCAs has been adopted to prospectively define COG clinical trial eligibility and treatment assignment.
INTRODUCTION
The clinical spectrum of neuroblastoma (NB) is reflected by several well-studied clinical and biologic features, which have been used for decades to predict patient outcomes.1-3 Combinations of these prognostic factors are used to risk-stratify patients and inform treatment assignment.3 However, many different NB staging systems and risk classifiers are used worldwide. The International Neuroblastoma Risk Group (INRG) led efforts to develop uniform approaches for staging and pretreatment risk classification,4 providing the impetus for a revised Children's Oncology Group (COG) classifier to harmonize with this effort. The COG Risk Classifier (version 1 [v1]) was established in 20002 (Data Supplement, online only), using the International Neuroblastoma Staging System (INSS) that was developed in 1986.5 The INSS is a postsurgical staging system that uses tumor location with respect to midline structures, lymph node status, and, importantly, extent of upfront surgical resection to determine whether a locoregional tumor is INSS stage 1, 2A, 2B, or 3. Staging includes bone marrow assessment and imaging studies to detect metastases (stage 4 or 4S depending on additional clinical details; Data Supplement).5,6 To create a staging system independent of surgical resection extent, the INRG staging system (INRGSS) was developed in 2005,7 using image-defined risk factors (IDRFs) to categorize locoregional tumors as L1 (IDRF absent) or L2 (IDRF present; Data Supplement). IDRFs identify imaging surrogates of aggressive tumor growth with respect to local structures and have been validated to predict successful primary tumor resection.8,9 Metastatic disease defines INRGSS stage M (metastatic) disease, except in children < 18 months with metastases restricted to skin, liver, and/or limited marrow involvement (stage MS [metastatic, special]; Data Supplement).
CONTEXT
- Key Objective
- Combinations of prognostic clinical and biologic factors have been used to risk-stratify and inform treatment for patients with neuroblastoma for decades; however, schema differ across international cooperative groups. These analyses examined the prognostic impact of clinical risk factors and centrally assessed biomarkers for almost 5,000 Children's Oncology Group (COG) patients treated with modern-era therapy and staged using the International Neuroblastoma Risk Group Staging System (INRGSS).
- Knowledge Generated
- We used these results to develop the revised 2021 COG classifier (version 2) that incorporates INRGSS and biomarkers including segmental chromosome aberrations to classify risk and stratify treatment for future clinical trial design.
- Relevance
- Use of an updated COG classifier will enable more accurate risk stratification and treatment assignment. Furthermore, harmonization with the INRGSS will enable better international collaboration, data sharing, and comparison of clinical trial results and evaluations of novel biomarkers to enable further precision in risk assignment.
The INRG risk classification system uses the INRGSS. The other six criteria in this classifier were the most statistically significant of 35 factors analyzed in 8,800 patients diagnosed between 1990 and 2002 obtained from all major international cooperative groups, including 4,235 COG patients.4 Combinations of these prognostic factors (age, stage, histology, ploidy, MYCN, and 11q status) are used to assign risk,4 facilitating comparison of patient outcomes across clinical trials worldwide. However, the INRG classifier was developed using data from patients decades ago and, therefore, does not account for the impact of more modern treatment on outcomes (eg, high-risk [HR] regimens that include myeloablative consolidation chemotherapy10,11 and immunotherapy regimens12,13), limiting direct comparisons with the COG classifier for validating outcomes for discrete patient subsets. Additionally, many studies have confirmed that segmental chromosome aberrations (SCA; loss or gain of a portion of a chromosome arm) portend unfavorable outcome.14-20 The patient subsets for whom SCAs have prognostic value vary across studies because of heterogeneous populations and therapies,16,19,21-23 emphasizing the importance of validating this genomic biomarker in the context of uniform COG risk classification and treatment approaches.
Here, we analyzed a large cohort of patients with NB enrolled onto the COG biology study, ANBL00B1, to develop a revised classifier. Clinical, biologic, and histologic data assessed centrally (including MYCN status, ploidy, International Neuroblastoma Pathology Classification [INPC] histology, and SCAs at 1p and 11q) were used. Statistical analyses of this cohort provide the framework to assess the impact of these factors on outcomes and develop the revised 2021 COG risk classification system (version 2 [v2]).
METHODS
Patient Cohort
Patients were enrolled on COG ANBL00B1 between October 1, 2007, and January 19, 2017. Eligible patients had newly diagnosed NB, ganglioneuroblastoma, and ganglioneuroma (maturing subtype). Central or local-site IRB approval and written informed consent were obtained. Patients were designated as low-risk (LR),24 intermediate-risk (IR),22,25 or HR using the COG v1 classifier and treated accordingly.10,13,26,27
Risk Biomarkers
Clinical data collected from institutions included age at diagnosis, INSS stage, and IDRF (presence or absence). Pathologically confirmed tumor tissue from local or metastatic sites was tested centrally at the COG Neuroblastoma Reference Laboratory to determine MYCN status (amplified [_MYCN-A_] or nonamplified [_MYCN-NA_]) and ploidy (diploid or hyperdiploid), detected by fluorescence in situ hybridization and flow cytometry, respectively.28 Tumor slides underwent centralized histopathologic assessment of stromal presence, neural differentiation, and mitosis-karyorrhexis index to classify as favorable or unfavorable histology according to INPC criteria.29,30 Based on emerging data for SCA prognostic impact, we tested a subset for 1p and 11q loss of heterozygosity, highly prevalent SCAs, using PCR-based microsatellite marker assays before 201514 and by SNP-array or whole-exome copy-number analysis thereafter (Data Supplement).
Statistical Analyses
Details of the COG statistical approach to risk group classification were published previously.6 Event-free survival (EFS) and overall survival (OS) were previously primary end points; herein, OS is the primary end point. Time to event was calculated from diagnosis until the first occurrence of relapse, progression, secondary malignancy, or death; and time until death for OS. For patients without an event, data were censored at last contact. Survival curves were generated per Kaplan-Meier,31 with EFS and OS values reflecting 5-year estimates with standard errors per Peto.32 In the tables, any denotes all permissible values, including missing or unknown. SAS V9.4 was used for analyses. Data cutoff for analyses was December 31, 2019.
Development of the Risk Classifier
The COG Neuroblastoma Biology Subcommittee met annually (2015-2020) to review outcome data and subgroup analyses and iteratively test proposed risk classifier schemas. Similar to the INRG classification,4 there were some subgroups with small patient numbers and/or incomplete data. Decisions regarding biomarker selection for final COG risk group delineation were based on prognostic strength (size of hazard ratio) in EFS and OS analyses, clinical trial results, published biomarker reports, and knowledge of the treatment strategies used during the study period. In a systematic and objective manner, the 5-year OS and assigned treatment for patients enrolled on trials10,12,13,22,24-27,33 or inferred according to the proportion of patients in v1 classified as LR, IR, or HR were used to assign the v2 risk category for each subgroup.
RESULTS
Patient Characteristics
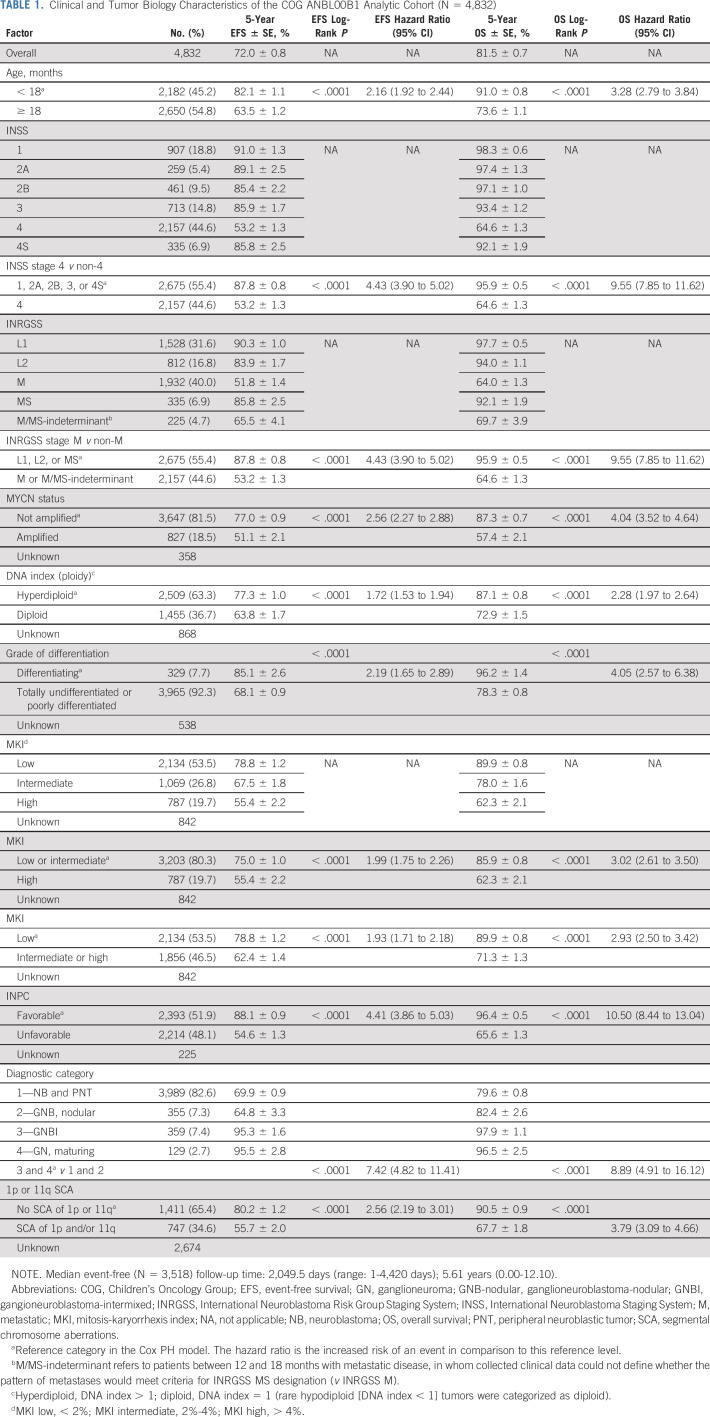
Characteristics and outcomes for 4,832 eligible patients are shown (Table 1, Data Supplement). The 5-year EFS and OS were 72.0% ± 0.8% and 81.5% ± 0.7%, respectively, with a median event-free follow-up of 5.61 years. The distribution of key variables including age, stage, and MYCN status was similar to previous unselected patient cohorts.4,34,35 The distribution by stage was also similar to that of the INRG 1990-2002 cohort used to develop the INRGSS.7 Age, stage, MYCN status, 1p and/or 11q status, INPC category, and individual histologic components (differentiation grade and mitosis-karyorrhexis index) were independently prognostic of EFS and OS. Risk group was assigned on ANBL00B1; 33% of IR and 63% of HR patients were also enrolled on COG therapeutic trial(s).10,12,13,26,27,36
TABLE 1.
Clinical and Tumor Biology Characteristics of the COG ANBL00B1 Analytic Cohort (N = 4,832)
Analysis of Patients With Locoregional Disease.
Stage.
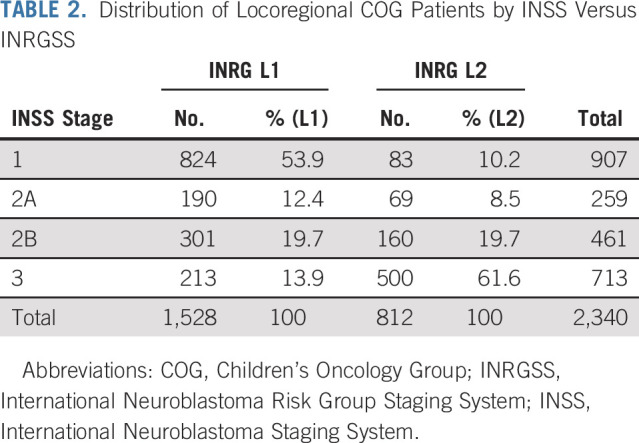
Patients with locoregional tumors had a superior outcome when compared to those with distant metastases (Table 1). To assess the impact of adopting the INRGSS, we determined how patients were mapped from INSS to INRGSS (Table 2), as the staging criteria are based on different features of in situ tumor growth as surrogates for biologic aggressiveness (Data Supplement). Most INSS stage 1, 2A, and 2B tumors were classified as L1 (81%) using the INRGSS criteria, whereas 19% were upstaged to L2. Conversely, 70% of INSS stage 3 tumors were INRGSS stage L2, whereas 30% were downstaged to L1, potentially affecting the assigned risk group for 22% of patients with locoregional tumors.
TABLE 2.
Distribution of Locoregional COG Patients by INSS Versus INRGSS
Pathology category.
The outcome for patients with ganglioneuroblastoma-intermixed and ganglioneuroma-maturing was significantly superior to those with NB and ganglioneuroblastoma-nodular (Table 1). Thus, in v2, ganglioneuroblastoma-intermixed and ganglioneuroma are designated as LR for nonmetastatic (L1 or L2) patients regardless of biomarkers (Data Supplement).
Biology.
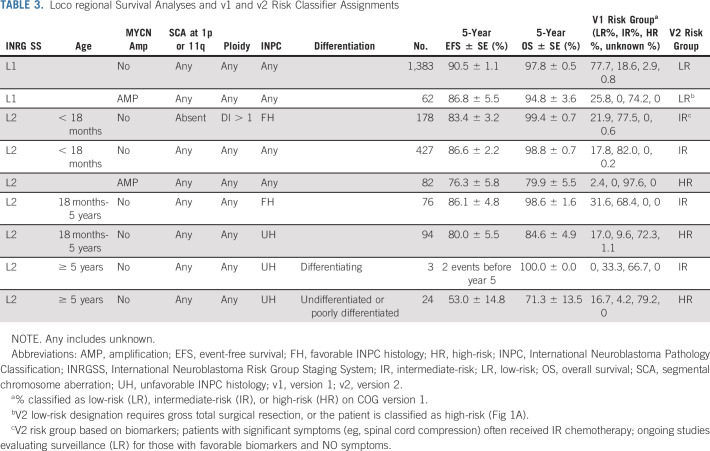
The impact of prognostic factors on outcomes for patients with L1 and L2 tumors was determined to inform the revised classifier (Table 3). All patients with L1 tumors, regardless of age or biomarker status, had EFS and OS of 90.3% ± 1.0% and 97.7% ± 0.5%. Thus, L1 tumors are classified in v2 as LR except rare MYCN-A tumors (4%) that are incompletely resected (macroscopic residual tumor), which are considered HR.
TABLE 3.
Loco regional Survival Analyses and v1 and v2 Risk Classifier Assignments
For the L2 cohort, MYCN status and age were the most significant prognostic factors. The EFS and OS for patients of any age with L2 MYCN-A tumors were 76.3% ± 5.8% and 79.9% ± 5.5%, respectively, the majority of whom received HR therapy. For patients with L2 MYCN-NA tumors and age < 18 months, EFS and OS were 86.6% ± 2.2% and 98.8% ± 0.7% with LR or IR therapy24,25,37 (Table 3). By contrast, survival for patients age ≥ 18 months with L2 _MYCN_-NA tumors was influenced by INPC, ploidy, and SCA status (Table 3, Data Supplement).
The only locoregional patients for whom INPC was used to assign COG v1 risk groups were those with INSS stage 3 disease (Data Supplement). In the context of the INRGSS, INPC was prognostic for most L2 subsets, with the largest impact in patients age ≥ 18 months with MYCN-NA tumors (Table 3, Data Supplement). In this group, those with unfavorable INPC tumors had outcomes inferior to those with favorable or unknown INPC (EFS and OS: 72.7% ± 5.4% and 82.4% ± 4.6% v 88.9% ± 3.6%/97.1% ± 1.9%; N = 129, P = .0004 and P = .0002) despite most having received HR therapy.10,26,36 Thus, these patients, in addition to L2 patients with MYCN-A, are classified HR in v2. L2 patients with SCA 1p and/or 11q (EFS and OS: 67.4% ± 11.1% and 83.3% ± 8.8%) had similar poor outcome but 17 of 19 had unfavorable INPC tumors (Data Supplement). Although a recent study identified subsets of INSS stage 1-3 patients in which individual histologic components identified additional prognostic subgroups,35 we did not find that histologic components could discriminate clinically meaningful subsets of patients age ≥ 18 months with L2 _MYCN_-NA tumors with differing outcomes (Data Supplement).
Analysis of Patients with Metastatic Disease
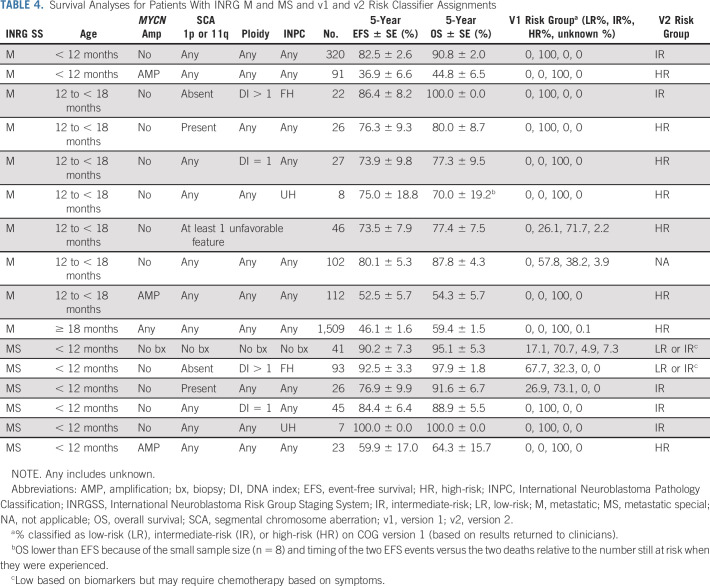
Stage M.
The majority of patients with stage M disease are ≥ 18 months at diagnosis and their outcome remains poor (EFS and OS: 46.1% ± 1.6% and 59.4% ± 1.5%). Within this group, no biomarker is prognostic (Table 4, Data Supplement). Similarly, patients with stage M disease of any age with MYCN-A tumors have poor outcomes (EFS and OS: 44.5% ± 2.3% and 51.0% ± 2.3%). Both groups receive treatment that typically includes one or more myeloablative therapies10,26 and immunotherapy12,13 and remain HR in v2. The prognostic impact of MYCN was particularly notable in infants with stage M disease age < 12 months, as patients with MYCN-NA tumors have superior outcomes in comparison to those with MYCN-A tumors (EFS and OS: 82.5% ± 2.6%/90.8% ± 2.0% v 36.9% ± 6.6% and 44.8% ± 6.5%, P < .0001; Table 4) consistent with earlier studies.38,39 In COG v1, infants with MYCN-NA stage M tumors were categorized as IR and had excellent outcomes despite receiving de-escalated moderate intensity therapy22,25 and remain IR in v2. While infants with stage M disease with ≥ 1 unfavorable biomarker had lower EFS and OS compared to those with all favorable biomarkers (71.9 ± 4.9 and 83.9 ± 4.0 v 91.4 ± 3.0 and 96.1 ± 2.1, P = .0005/P = .0025), because of small numbers and relatively favorable outcomes with IR protocols,24,25,37 this subset remains IR in v2 (Data Supplement).
TABLE 4.
Survival Analyses for Patients With INRG M and MS and v1 and v2 Risk Classifier Assignments
Patients age 12 to < 18 months with INRGSS M disease had outcomes intermediate to those of patients age < 12 and ≥ 18 months. Patients age 12 to < 18 months with INRGSS M tumors and favorable biology (_MYCN-NA_, favorable INPC, and ploidy > 1) were reported to have excellent outcomes,40-42 leading to v1 classification as IR on recent COG trials.22,25 In our cohort, EFS and OS remained excellent for toddlers with all favorable (86.4% ± 8.2% and 100.0% ± 0%) compared to those with ≥ 1 unfavorable feature (INPC unfavorable, ploidy = 1, or SCA present; 73.5% ± 7.9% and 77.4% ± 7.5%) despite the latter patients being classified and treated as HR (Table 4). Thus, toddlers with stage M disease with any unfavorable biomarker are classified as HR in v2.
Stage MS.
Infants with INSS stage 4S disease have more favorable biology and superior outcomes despite receiving less aggressive treatment.22,37,43 The outcome was excellent for our INRG MS cohort (EFS and OS: 85.8% ± 2.5% and 92.1% ± 1.9%) with the exception of those with MYCN-A tumors (EFS and OS: 59.9% ± 17.0% and 64.3% ± 15.7%), the only subgroup previously classified as HR (Table 4, Data Supplement). Risk classification for MS in v2 is discussed in the Data Supplement.
Development of the Revised COG Risk Classifier
Based on the statistical framework previously reported6 and the EFS/OS of patient subsets using INRGSS instead of INSS, with SCA as a prognostic biomarker, the revised classifier takes into consideration intensity of therapy based on assigned risk group (on or according to COG clinical trials10,12,13,22,24-27,33,36) and the probability for successful salvage therapy in the event of tumor progression or recurrence. Specifically, the LR group includes patients with excellent outcomes (OS ≥ 95%)24 with limited or no therapy, the IR group has very good outcomes (OS ≥ 85%-90%)22,25 with moderate-intensity chemotherapy, whereas HR patients have inferior outcomes (OS < 85%)10,26,33 despite intensive, multimodality therapy (Tables 3 and 4).
The algorithm for the COG Neuroblastoma Risk Classifier v2 is summarized in Figure 1 and the Data Supplement. We determined how often risk group assignment differed between v1 and v2. Of 2,466 non-HR patients, only 85 (3.4%) shifted to HR, while reassignments from low to intermediate, or intermediate to low, were more common (Data Supplement). EFS and OS outcomes applying the COG Risk Classifier v1 (LR: 89.4% ± 1.1%/97.9% ± 0.5%; IR: 86.1% ± 1.3% and 94.9% ± 0.8%; HR: 50.8% ± 1.4% and 61.9% ± 1.3%) were similar to those applying v2 (LR: 90.7% ± 1.0% and 97.9% ± 0.5%; IR: 85.1% ± 1.4% and 95.8% ± 0.8%; HR: 51.2% ± 1.4% and 62.5% ± 1.3%; Figs 2A-2D, Data Supplement).
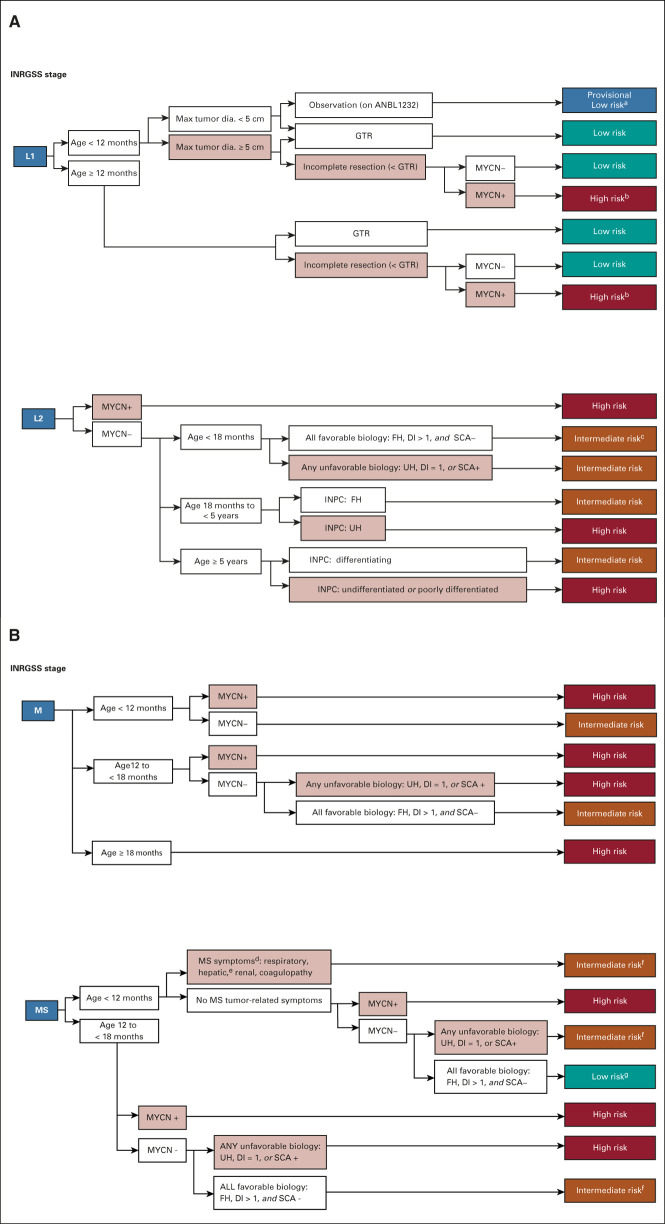
FIG 1.
Risk classifier v2 algorithm for patients with (A) locoregional and (B) metastatic tumors. (A) Patients with locoregional tumors with neuroblastoma and ganglioneuroblastoma (nodular) are classified based on INRG stage (L1 and L2), age, resection, biomarkers (MYCN, ploidy, and SCAs), and INPC. Select patients with all favorable features are eligible for surveillance on the current non–high-risk COG ANBL1232 protocol. L1 or L2 tumors with histopathology diagnostic category of ganglioneuroma or ganglioneuroblastoma-intermixed will be classified as low-risk regardless of biomarkers (and thus are not included in the figure). Ages are broken down by < 18 months, 18 months to < 5 years, and ≥ 5 years based on age categories used by INPC. aIf tumor progresses during observation, biopsy or resect and reclassify with biomarkers (as in COG ANBL1232). bConsider complete resection if feasible. cIf no tumor burden symptoms, consider observation (as in COG ANBL1232). (B) Patients with metastatic tumors are classified by stage (M and MS), age, INPC, and biomarkers. For MS patients, presence or absence of symptoms may influence therapy independent of biomarker status. In previous risk classifiers, missing data were considered as unfavorable. In COG v2, missing data for SCA will not be considered as unfavorable based on the low incidence of SCA in otherwise favorable subsets. dBiopsy contraindicated, defer biopsy until stable (note: biomarker results may modify Risk Class). eHepatomegaly alone is an MS symptom in patients age < 3 months of age (see COG ANBL1232). fResponse-based therapy (as in COG ANBL1232). gMS score–based therapy (as in COG ANBL1232). COG, Children's Oncology Group; DI, DNA index; dia., diameter; FH, favorable histology; GTR, gross-total resection; INPC, International Neuroblastoma Pathology Classifier; INRG, International Neuroblastoma Risk Group; MYCN+, MYCN amplified; MYCN−, MYCN not amplified; SCA, segmental chromosomal aberration; UH, unfavorable histology; v1, version 1; v2, version 2.
FIG 2.
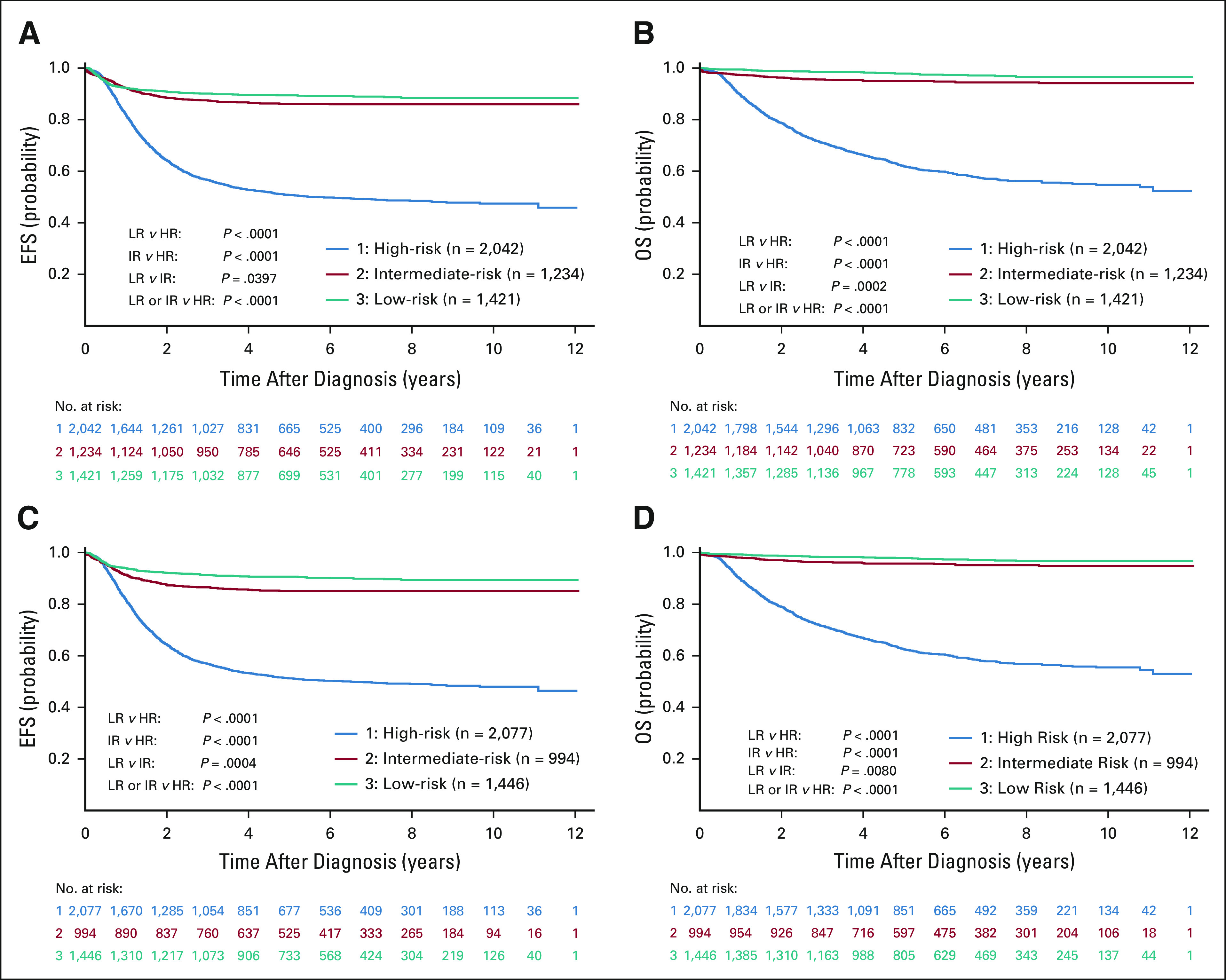
Outcome analyses for risk groups on COGv1 and v2 risk classifiers. (A and B) 5-year EFS and OS for LR, IR, and HR groups according to COG v1. (C and D) 5-year EFS and OS for LR, IR, and HR groups according to COG v2. Log-rank tests were used to compare survival distributions and are shown on the relevant figures and in the Data Supplement. The patient cohorts, eligibility details, and missing data are described in the CONSORT Diagram (Data Supplement). COG, Children's Oncology Group; EFS, event-free survival; HR, high-risk; IR, intermediate-risk; LR, low-risk; OS, overall survival; v1, version 1; v2, version 2.
DISCUSSION
We analyzed outcomes for almost 5,000 patients to develop a revised COG risk classifier that incorporates INRGSS with clinical and biologic prognostic factors, including SCAs. This is the largest NB patient cohort centrally reviewed on a single study (ANBL00B1) used to inform prognostic risk group and determine trial eligibility. As therapy is a variable that markedly affects outcome, it must be taken into consideration when assigning risk group based on outcome analyses. Importantly, our cohort represents patients more recently diagnosed than the cohort used in the INRG risk group analysis4 or the prior COG risk classifier, and is therefore more representative of outcome with modern-era therapies (immunotherapy for HR patients and reduced intensity for non-HR patients). Therapy could be inferred for most patients assuming treatment was provided according to assigned risk group36 and trial enrollments.10,12,13,22,26,27,36
Our analyses indicate that inclusion of 1p and/or 11q SCA can identify patient subsets with inferior outcomes and that SCA status is informative in patients age 12-18 months with stage M _MYCN_-NA and those ≥ 18 months with stage L2 _MYCN_-NA tumors. However, in our cohort, 17 of the 19 patients in the latter group whose tumors had SCA also had unfavorable histology. These patients would be classified as HR based on histology regardless of SCA status. Retrospective studies have repeatedly demonstrated the prognostic strength of SCAs, including 1p and 11q loss of heterozygosity, but also loss of 3p and 4p and gain of 1q, 2p, and 17q, especially in subgroups with otherwise favorable features.15-17,19,23,44 Thus, COG risk classifier v2 defines SCA presence to include ≥ 1 of these seven loci, and toddlers age 12-18 months with stage M, _MYCN_-NA NB with SCAs will be classified as HR. Going forward, we will prospectively determine whether SCA and/or numerical chromosome aberration status alone identifies patients age ≥ 18 months with L2 _MYCN_-NA with inferior outcome independent of INPC.
The original INRG delineation of pretreatment risk groups was based on 5-year EFS: low ≥ 75%, intermediate ≥ 50 to ≤ 75%, and high < 50%.4 To develop the COG risk classifier v2, since outcomes have improved with modern-day therapy, we applied an approach to categorizing patients into new risk groups that is different from that taken during development of the INRG and COG v1 risk classifiers.4,6 The v2 risk group assignments acknowledge treatment received and, unlike the original INRG that used EFS, OS was used in developing v2. OS is increasingly considered a more informative end point that incorporates salvageability, especially for non-HR patients.22 Defining a clinically acceptable range of outcomes, particularly between IR and HR, is challenging. However, this is necessary to assign therapy and determine trial eligibility. It is expected that there will be specific subsets of patients who could be considered to have more favorable HR disease, while others may appear to have outcomes that fall into the lower end of the range for IR patients. Thus, consideration of treatment intensity for specific subsets is critical. In addition, mapping of patients from INSS to INRGSS is not exact, particularly for patients with locoregional disease. As a result, patients may be upstaged and assigned to a higher risk group in v2 compared with v1 or the INRG classifier.6 Similar changes in staging and risk group assignment occurred in the transition from prior staging systems to INSS45 and the COG v1 classifier. Despite all of these potential challenges, the differences in survival for risk groups between v1 and v2 were minimal. More accurate risk assignment with the revised classifier permits groups of patients with predicted poor outcome to be directed toward more intensive therapy, while also reducing intensity and long-term toxicities for patients classified into a lower v2 risk group.
The value of several prognostic markers used in the revised risk classifier is most clearly seen when suboptimal outcomes were identified in small subsets of patients based on multiple other clinical and biologic risk factors. For example, the majority of infants < 12 months with stage M, _MYCN_-_NA_ NB fared well after IR therapy, with EFS and OS > 80% and 90%, respectively. However, those with ≥ 1 unfavorable biomarker (INPC, ploidy, and SCA) had outcomes somewhat inferior compared to those with all favorable features (Data Supplement). International collaborative trials will be necessary to study this and other rare subgroups, including 12 to ≤ 18 month toddlers with stage M, MYCN-NA with ≥ 1 unfavorable biomarker (n = 46) and patients age ≥ 18 months with stage L2, _MYCN_-NA disease with unfavorable histology (n = 121).
Finally, for current-era HR patients with stage M disease age ≥ 18 months, no biomarkers, including _MYCN-_A, are prognostic (Data Supplement).46,47 Recent studies suggest that telomerase maintenance mechanisms48-50 and RAS/MAPK pathway alterations51 may discriminate HR subgroups and/or identify rare IR patients whose disease recurs. However, retrospective evaluation of homogeneously treated populations and, ideally, prospective validation of the prognostic strength of these newer biomarkers and others such as RNA expression signatures and c-MYC52-54 will be required. In addition, integrating response to therapy using imaging,55 tumor-specific transcripts in peripheral blood or bone marrow,56 or liquid biopsies57 may help guide therapeutic decision making. Furthermore, development of nomograms58 and machine learning strategies2 may decrease reliance on binary variables and enable more precise algorithms.
In summary, we have analyzed a large cohort of patients who were uniformly and systematically assigned to risk-based treatment regimens within COG to develop the revised 2021 COG classifier (v2) that incorporates INRGSS and SCA to classify risk and stratify treatment as applicable for clinical trial design. This also aligns with INRG efforts to harmonize data collection and enable international comparisons across patient subsets and trials. COG will collect patient data using the risk criteria included in the 2021 COG v2 and also study additional biomarkers (telomerase maintenance mechanisms and next-generation sequencing data) to determine whether current paradigms can be optimized to attain greater precision in risk assignment.
ACKNOWLEDGMENT
The authors thank pathologists (Drs Samir Kahwash and Miriam Conces) at the Biopathology Center and Nationwide Children's Hospital for review of tumor content for specimens, and local investigators and patients and families for participating in ANBL00B1.
Meredith S. Irwin
Honoraria: Bayer
Arlene Naranjo
Consulting or Advisory Role: Novartis
Susan L. Cohn
Stock and Other Ownership Interests: Merck, Stryker, Amgen, Pfizer, AbbVie, Lilly, Sanofi, Accelerated Medical Diagnostics, Novo Nordisk, Gilead Sciences, United Health Group, Teva
Honoraria: Y-mAbs Therapeutics
Research Funding: United Therapeutics, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/physician/46569/summaryhttps://openpaymentsdata.cms.gov/physician/46569/summary
Wendy B. London
Consulting or Advisory Role: Jubilant Radiopharma, Merck
Research Funding: Agios, Bristol Myers Squibb, Novartis, Aileron Therapeutics, Bluebird Bio
Julie M. Gastier-Foster
Research Funding: Bristol Myers Squibb, Incyte
Jed Nuchtern
Stock and Other Ownership Interests: Insulet Corporation, Lexicon, Intuitive Surgical
John M. Maris
Stock and Other Ownership Interests: Tantigen BIO Inc
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals, Jubilant DraxImage
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs, Neuroblastoma antigens
Rochelle Bagatell
Uncompensated Relationships: Y-mAbs Therapeutics Inc
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part at the Advances in Neuroblastoma Research meeting, Cairns, Australia, June 23, 2016; American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2, 2019.
SUPPORT
U24CA196173 to COG for support of biobank, Chair's Grant U10-CA098543/COG NCTN Network Group Operations Center Grant U10-CA180886, SDC Grant U10-CA098413/COG NCTN Statistics & Data Center Grant U10-CA180899, St Baldrick's Foundation.
AUTHOR CONTRIBUTIONS
Conception and design: Meredith S. Irwin, Arlene Naranjo, Susan L. Cohn, Wendy B. London, Julie M. Gastier-Foster, Jed Nuchtern, John M. Maris, Rochelle Bagatell, Julie R. Park, Michael D. Hogarty
Financial support: Julie M. Gastier-Foster
Administrative support: Julie M. Gastier-Foster
Provision of study materials or patients: Susan L. Cohn, Nilsa C. Ramirez, John M. Maris
Collection and assembly of data: Meredith S. Irwin, Fan F. Zhang, Julie M. Gastier-Foster, Nilsa C. Ramirez, Ruthann Pfau, Elizabeth Wagner, Shahab Asgharzadeh, Hiroyuki Shimada, Rochelle Bagatell, Julie R. Park, Michael D. Hogarty
Data analysis and interpretation: Meredith S. Irwin, Arlene Naranjo, Fan F. Zhang, Susan L. Cohn, Wendy B. London, Julie M. Gastier-Foster, Ruthann Pfau, Shalini Reshmi, Jed Nuchtern, Shahab Asgharzadeh, Rochelle Bagatell, Julie R. Park, Michael D. Hogarty
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Revised Neuroblastoma Risk Classification System: A Report From the Children's Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Meredith S. Irwin
Honoraria: Bayer
Arlene Naranjo
Consulting or Advisory Role: Novartis
Susan L. Cohn
Stock and Other Ownership Interests: Merck, Stryker, Amgen, Pfizer, AbbVie, Lilly, Sanofi, Accelerated Medical Diagnostics, Novo Nordisk, Gilead Sciences, United Health Group, Teva
Honoraria: Y-mAbs Therapeutics
Research Funding: United Therapeutics, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/physician/46569/summaryhttps://openpaymentsdata.cms.gov/physician/46569/summary
Wendy B. London
Consulting or Advisory Role: Jubilant Radiopharma, Merck
Research Funding: Agios, Bristol Myers Squibb, Novartis, Aileron Therapeutics, Bluebird Bio
Julie M. Gastier-Foster
Research Funding: Bristol Myers Squibb, Incyte
Jed Nuchtern
Stock and Other Ownership Interests: Insulet Corporation, Lexicon, Intuitive Surgical
John M. Maris
Stock and Other Ownership Interests: Tantigen BIO Inc
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals, Jubilant DraxImage
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs, Neuroblastoma antigens
Rochelle Bagatell
Uncompensated Relationships: Y-mAbs Therapeutics Inc
No other potential conflicts of interest were reported.
REFERENCES
- 1.Irwin MS, Park JR: Neuroblastoma: Pediatric paradigm for precision medicine. Pediatr Clin North Am 62:225-256, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Liang WH, Federico SA, London WB, et al. : Tailoring therapy for children with neuroblastoma based on risk group classification: Past, present, and future. JCO Clin Cancer Inform 4:895-905, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto NR, Applebaum MA, Volchenboum SL, et al. : Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 33:3008-3017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn SL, Pearson AD, London WB, et al. : The international neuroblastoma risk group (INRG) classification system: An INRG task force report. J Clin Oncol 27:289-297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodeur GM, Pritchard J, Berthold F, et al. : Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466-1477, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Naranjo A, Irwin MS, Hogarty MD, et al. : Statistical framework in support of a revised Children's Oncology Group (COG) neuroblastoma risk classification system. JCO Clin Cancer Inform 2:1-15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monclair T, Brodeur GM, Ambros PF, et al. : The International Neuroblastoma Risk Group (INRG) staging system: An INRG task force report. J Clin Oncol 27:298-303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecchetto G, Mosseri V, De Bernardi B, et al. : Surgical risk factors in primary surgery for localized neuroblastoma: The LNESG1 Study of the European International Society of Pediatric Oncology Neuroblastoma Group. J Clin Oncol 23:8483-8489, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Brisse HJ, McCarville MB, Granata C, et al. : Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the international neuroblastoma risk group project. Radiology 261:243-257, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Park JR, Kreissman SG, London WB, et al. : Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: A randomized clinical trial. JAMA 322:746-755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladenstein R, Pötschger U, Pearson ADJ, et al. : Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol 18:500-514, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Ozkaynak MF, Gilman AL, London WB, et al. : A comprehensive safety trial of chimeric antibody 14.18 with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: Children's Oncology Group study ANBL0931. Front Immunol 9:1355, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AL, Gilman AL, Ozkaynak MF, et al. : Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324-1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attiyeh EF, London WB, Mossé YP, et al. : Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med 353:2243-2253, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Janoueix-Lerosey I, Schleiermacher G, Michels E, et al. : Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol 27:1026-1033, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Schleiermacher G, Janoueix-Lerosey I, Ribeiro A, et al. : Accumulation of segmental alterations determines progression in neuroblastoma. J Clin Oncol 28:3122-3130, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Schleiermacher G, Michon J, Ribeiro A, et al. : Segmental chromosomal alterations lead to a higher risk of relapse in infants with MYCN-non-amplified localised unresectable/disseminated neuroblastoma (a SIOPEN collaborative study). Br J Cancer 105:1940-1948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleiermacher G, Mosseri V, London WB, et al. : Segmental chromosomal alterations have prognostic impact in neuroblastoma: A report from the INRG project. Br J Cancer 107:1418-1422, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defferrari R, Mazzocco K, Ambros IM, et al. : Influence of segmental chromosome abnormalities on survival in children over the age of 12 months with unresectable localised peripheral neuroblastic tumours without MYCN amplification. Br J Cancer 112:290-295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Depuydt P, Boeva V, Hocking TD, et al. : Genomic amplifications and distal 6q loss: Novel markers for poor survival in high-risk neuroblastoma patients. J Natl Cancer Inst 110:1084-1093, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schleiermacher G, Michon J, Huon I, et al. : Chromosomal CGH identifies patients with a higher risk of relapse in neuroblastoma without MYCN amplification. Br J Cancer 97:238-246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twist CJ, Schmidt ML, Naranjo A, et al. : Maintaining outstanding outcomes using response- and biology-based therapy for intermediate-risk neuroblastoma: A report from the Children's Oncology Group study ANBL0531. J Clin Oncol 37:3243-3255, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto N, Mayfield JR, Raca G, et al. : Segmental chromosomal aberrations in localized neuroblastoma can be detected in formalin-fixed paraffin-embedded tissue samples and are associated with recurrence. Pediatr Blood Cancer 63:1019-1023, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strother DR, London WB, Schmidt ML, et al. : Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: Results of Children's Oncology Group study P9641. J Clin Oncol 30:1842-1848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker DL, Schmidt ML, Cohn SL, et al. : Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 363:1313-1323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreissman SG, Seeger RC, Matthay KK, et al. : Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol 14:999-1008, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JR, Scott JR, Stewart CF, et al. : Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: A Children's Oncology Group study. J Clin Oncol 29:4351-4357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambros PF, Ambros IM, Brodeur GM, et al. : International consensus for neuroblastoma molecular diagnostics: Report from the international neuroblastoma risk group (INRG) biology committee. Br J Cancer 100:1471-1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peuchmaur M, d'Amore ESG, Joshi VV, et al. : Revision of the international neuroblastoma Pathology classification: Confirmation of favorable and unfavorable prognostic subsets in ganglioneuroblastoma, nodular. Cancer 98:2274-2281, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Shimada H, Ambros IM, Dehner LP, et al. : The international neuroblastoma pathology classification (the Shimada system). Cancer 86:364-372, 1999 [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P: Non-parametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 32.Peto R, Pike MC, Armitage P, et al. : Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 35:1-39, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granger M, Naranjo A, Bagatell R, et al. : Myeloablative busulfan/melphalan (BuMel) consolidation following induction chemotherapy for patients with newly diagnosed high-risk neuroblastoma: Children’s Oncology Group (COG) Trial ANBL12P1. Transplant Cell Ther 27:490.e1-490.e8, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castleberry RP, Pritchard J, Ambros P, et al. : The International Neuroblastoma Risk Groups (INRG): A preliminary report. Eur J Cancer 33:2113-2116, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Sokol E, Desai AV, Applebaum MA, et al. : Age, diagnostic category, tumor grade, and mitosis-karyorrhexis index are independently prognostic in neuroblastoma: An INRG project. J Clin Oncol 38:1906-1918, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JR, Bagatell R, London WB, et al. : Children's Oncology Group's 2013 blueprint for research: Neuroblastoma. Pediatr Blood Cancer 60:985-993, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Twist CJ, Naranjo A, Schmidt ML, et al. : Defining risk factors for chemotherapeutic intervention in infants with stage 4S neuroblastoma: A report from Children's Oncology Group study ANBL0531. J Clin Oncol 37:115-124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tonini GP, Boni L, Pession A, et al. : MYCN oncogene amplification in neuroblastoma is associated with worse prognosis, except in stage 4s: The Italian experience with 295 children. J Clin Oncol 15:85-93, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt ML, Lukens JN, Seeger RC, et al. : Biologic factors determine prognosis in infants with stage IV neuroblastoma: A prospective children's cancer group study. J Clin Oncol 18:1260-1268, 2000 [DOI] [PubMed] [Google Scholar]
- 40.George RE, London WB, Cohn SL, et al. : Hyperdiploidy plus nonamplified MYCN confers a favorable prognosis in children 12 to 18 months old with disseminated neuroblastoma: A Pediatric Oncology Group study. J Clin Oncol 23:6466-6473, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt ML, Lal A, Seeger RC, et al. : Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: A Children's Cancer Group study. J Clin Oncol 23:6474-6480, 2005 [DOI] [PubMed] [Google Scholar]
- 42.London WB, Castleberry RP, Matthay KK, et al. : Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol 23:6459-6465, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Taggart DR, London WB, Schmidt ML, et al. : Prognostic value of the stage 4S metastatic pattern and tumor biology in patients with metastatic neuroblastoma diagnosed between birth and 18 months of age. J Clin Oncol 29:4358-4364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambros IM, Tonini G-P, Pötschger U, et al. : Age dependency of the prognostic impact of tumor genomics in localized resectable MYCN-nonamplified neuroblastomas. Report from the SIOPEN biology group of the LNESG trials and a COG validation group. J Clin Oncol 38:3685-3697, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castleberry RP, Shuster JJ, Smith EI: The Pediatric Oncology Group experience with the international staging system criteria for neuroblastoma. Member institutions of the Pediatric Oncology Group. J Clin Oncol 12:2378-2381, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Morgenstern DA, Pötschger U, Moreno L, et al. : Risk stratification of high-risk metastatic neuroblastoma: A report from the HR-NBL-1/SIOPEN study. Pediatr Blood Cancer 65:e27363, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Morgenstern DA, Bagatell R, Cohn SL, et al. : The challenge of defining “ultra-high-risk” neuroblastoma. Pediatr Blood Cancer 66:e27556, 2019 [DOI] [PubMed] [Google Scholar]
- 48.Hertwig F, Peifer M, Fischer M: Telomere maintenance is pivotal for high-risk neuroblastoma. Cell Cycle 15:311-312, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peifer M, Hertwig F, Roels F, et al. : Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526:700-704, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koneru B, Lopez G, Farooqi A, et al. : Telomere maintenance mechanisms define clinical outcome in high-risk neuroblastoma. Cancer Res 80:2663-2675, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ackermann S, Cartolano M, Hero B, et al. : A mechanistic classification of clinical phenotypes in neuroblastoma. Science 362:1165-1170, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asgharzadeh S, Pique-Regi R, Sposto R, et al. : Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst 98:1193-1203, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Asgharzadeh S, Salo JA, Ji L, et al. : Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 30:3525-3532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oberthuer A, Hero B, Berthold F, et al. : Prognostic impact of gene expression-based classification for neuroblastoma. J Clin Oncol 28:3506-3515, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Yanik GA, Parisi MT, Naranjo A, et al. : Validation of post-induction Curie scores in high risk neuroblastoma. A Children's Oncology Group (COG) and SIOPEN group report on SIOPEN/HR-NBL1. J Nucl Med 59:502-508, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brownhill SC, Burchill SA: PCR-based amplification of circulating RNAs as prognostic and predictive biomarkers—Focus on neuroblastoma. Pract Lab Med 7:41-44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbou SD, Shulman DS, DuBois SG, et al. : Assessment of circulating tumor DNA in pediatric solid tumors: The promise of liquid biopsies. Pediatr Blood Cancer 66:e27595, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreno L, Guo D, Irwin MS, et al. : A nomogram of clinical and biological factors to predict survival in children with newly diagnosed high risk neuroblastoma: An International Neuroblastoma Risk Group project. Pediatr Blood Cancer 68:e28794, 2021 [DOI] [PubMed] [Google Scholar]