The evolution of the synapsid tusk: insights from dicynodont therapsid tusk histology (original) (raw)
Abstract
The mammalian tusk is a unique and extreme morphotype among modern vertebrate dentitions. Tusks—defined here as ever-growing incisors or canines composed of dentine—evolved independently multiple times within mammals yet have not evolved in other extant vertebrates. This suggests that there is a feature specific to mammals that facilitates the evolution of this specialized dentition. To investigate what may underpin the evolution of tusks, we histologically sampled the tusks of dicynodont therapsids: the earliest iteration of tusk evolution and the only non-mammalian synapsid clade to have acquired such a dentition. We studied the tissue composition, attachment tissues, development and replacement in 10 dicynodont taxa and show multiple developmental pathways for the adult dentitions of dicynodont tusks and tusk-like caniniforms. In a phylogenetic context, these developmental pathways reveal an evolutionary scenario for the acquisition of an ever-growing tusk—an event that occurred convergently, but only in derived members of our sample. We propose that the evolution of an ever-growing dentition, such as a tusk, is predicated on the evolution of significantly reduced tooth replacement and a permanent soft-tissue attachment. Both of these features are fixed in the dentitions of crown-group mammals, which helps to explain why tusks are restricted to this clade among extant vertebrates.
Keywords: Synapsida, tusk, histology, Dicynodontia, Permian, Triassic
1. Introduction
The mammalian dentition is considered an epitome of evolutionary specialization, and the remarkable diversity of modern mammalian lineages is often attributed to the sophistication of their feeding system [1–8]. The ancestral dentition of mammals has long been considered the most derived among vertebrates [9–11] with a combination of important features such as crown morphology specialization (heterodonty), prismatic enamel, precise tooth-to-tooth occlusion, a single replacement event (diphyodonty), and a soft-tissue attachment between the teeth and the jaw (gomphosis) [12]. From this stereotyped ancestral condition, mammals have evolved numerous morphological novelties unparalleled in other vertebrate groups, including sabre-teeth, ever-growing teeth, highly complex occlusal surfaces and tusks [12].
Tusks, in particular, have evolved convergently in multiple lineages of mammals and can be found today in an array of artiodactyls, pinnipeds, dugongs, rock hyraxes and, perhaps most famously, elephants [12]. Tusks are found in mammals from small size classes (e.g. hyraxes) to the largest size classes (e.g. elephants) and can function in defense, competition, burrowing, sexual selection and even assist with locomotion, as in the walrus [13]. In addition to the modern diversity of tusks, the fossil record reveals even further disparity, especially among extinct proboscideans like Deinotherium, Zygolophodon and the ‘shovel-tusked’ gomphotheres [12,14]. Despite tusks being a feature of some of the most iconic modern and fossil mammals, both the definition of a tusk and the evolutionary steps that led to the development of this dental morphology remain ambiguous. To address both issues, we used the densely sampled fossil record of tusked dicynodont therapsids as a model system to examine tusk evolution. Analysing histological and micro-computed tomography (µ-CT) data, we propose four steps in the evolution of dicynodont tusks that can more broadly shed light on the acquisition of mammalian tusks.
2. Background
(a) . What is a tusk?
Here, we define a tusk based on gross morphology, continuous tissue deposition and eruption, tissue composition, and tooth attachment tissues. In using multiple characteristics to differentiate a tusk from other dental structures, it is possible to distinguish between dentitions that may have anatomical similarities, but are not developmentally homologous. The term ‘tusk’ has been widely used to describe any incisor or canine that extends out from the oral cavity [13]. Further, the term is also used in a range of dentitions where there is an enlarged tooth position but the crown does not extend out from the oral cavity such as the palatal ‘tusks’ of some extinct amphibians or the canine-like ‘tusks’ in some heterodontosaurid dinosaurs (e.g. [15,16]). However, a key developmental characteristic for a true tusk is continuous growth, allowing it to be distinguished from enlarged teeth that are morphologically similar but have determinate growth and eruption. Although vestigial deciduous teeth can be replaced early in ontogeny (e.g. the tush in elephants), a wide-open pulp cavity indicating active and continuous dentine deposition and eruption is a feature of the permanent tusk dentition [13]. Further distinguishing a tusk from other tooth types is its tissue composition. Due to the geometric constraints of producing both enamel and dentine continuously [17,18], ever-growing teeth will contain enamel, but the tissue will not cover the entirety of the dentine core as in other dentitions. In tusks, an enamel cap is present initially at eruption but is eventually worn away [12,13,19]. The result is a mature histology consisting mainly of dentine with cementum coating the root and occasionally at the point of new eruption prior to any wear [13,19]. Importantly, it is this adult tissue composition that distinguishes a tusk from ever-growing teeth such as rodent incisors or hypselodont molars where both enamel and dentine are continuously deposited [17,18,20,21]. Finally, the mode of attachment for a tusk must be ligamentous (i.e. a gomphosis) to accommodate the sustained movement of the tusk occlusally throughout life. Fusion of the tooth to the jawbone (i.e. ankylosis) precludes continuous tissue deposition and eruption. Therefore, although this mode of attachment can be found in many tooth types, it is a requirement for an ever-growing dentition. In summary, a tusk is defined and can be identified by having a combination of features: (i) a portion of the crown that extends out from the oral cavity, (ii) continuous growth and eruption, necessitating (iii) a gomphosis throughout the lifespan of the tooth and (iv) dentine as the sole tissue forming the crown.
(b) . The evolution of the mammalian tusk
Tusks, as we have defined above, are notably restricted to mammals among modern vertebrate groups. That this anatomical novelty convergently evolved multiple times within Mammalia is peculiar and stands in stark contrast to its absence in other vertebrate dentitions. Thus, the evolutionary history of tusked dentitions can provide insight into how mammals acquired tusks and why this dental innovation is unique to mammals among modern vertebrates.
The most refined evolutionary scenario explaining tusk evolution exists for proboscideans, as the fossil record sheds light on the history of tusk morphology. Early proboscideans had enlarged incisors that retained the plesiomorphic condition of being entirely capped with enamel [22]. In more crownward proboscidean taxa, this enamel was restricted to a longitudinal band [14], suggesting a developmental shift towards continuous growth similar to modern rodent incisors [17,21]. Finally, multiple species of derived proboscideans are documented as independently losing enamel, resulting in the dentine-only tusk recognized in modern Elephas and Loxodonta [14]. This trend towards enamel reduction and continuous growth in the tusks of proboscideans represents one evolutionary pathway; yet it remains unclear the extent to which this model may be broadly applied to other mammalian tusks (e.g. [23]).
(c) . Tusked dicynodont therapsids
The first tusks are described in a far older clade of non-mammalian synapsids, the Dicynodontia, which diverged in the Roadian or Wordian Stage of the Permian Period (ca 270 Ma) and persisted until the Late Triassic (ca 201 Ma; e.g. [24]). The term tusk is often used to describe the enlarged, paired maxillary caniniform teeth that distinguish dicynodont anomodonts from their non-dicynodont anomodont relatives (although it is noteworthy that some early anomodonts possessed exceptional dentitions in their own right, including early instances of tooth-to-tooth occlusion and sabre-toothed canines [25–27]).
Interestingly, previous descriptions of tusk development and tissue organization suggest that not all dicynodont tusks were constructed uniformly. Owen [28] provided the first description of dicynodont tusk structure and stated that they were composed primarily of dentine, with very thin layers of enamel and cementum, as well as an open pulp cavity. Subsequent authors largely regarded dicynodont tusks as enamel-less (e.g. [29–33]) and ever-growing (e.g. [29,34–38]), although there are a few reports of tusk replacement in some early dicynodonts (e.g. [29,39,40]). Camp & Welles [29] suggested only Triassic dicynodonts acquired ever-growing tusks whereas earlier Permian-aged tusks were not ever-growing. Finally, the tissues of attachment in dicynodonts have been reported as an ankylosis as in Diictodon [41] and a permanent gomphosis as in Lystrosaurus and some other members of Dicynodontoidea [38,41].
Dicynodont tusks are the earliest iteration of this morphological novelty, evolving well before the definitive mammalian patterns of tooth attachment and replacement were established. Thus, they provide a unique evolutionary perspective on the acquisition of this tooth morphotype. Here, we present histological samples from a broad phylogenetic and temporal range of dicynodont taxa, with the goal of documenting tissue composition, attachment, root shape (which may be used as a proxy for active/inactive tissue deposition) and replacement. Using these data, we propose an evolutionary scenario to describe how dicynodonts acquired their tusks, compare this scenario with that known for proboscidean tusks and comment on how the evolution of the earliest synapsid tusks can shed light on the evolution of this morphological extreme in modern mammals.
3. Methods
(a) . Specimen selection
Thin sections of nine dicynodont specimens were made available from the National Museum of Tanzania (NMT), National Heritage Conservation Commission (NHCC) of Zambia, the University of Washington Burke Museum (UWBM) and Iziko: South African Museum (SAM). This material was supplemented with older nitrocellulose peels of three specimens housed at the Field Museum of Natural History that were serially sectioned by Olson [42], as well as X-ray µ-CT of specimens from the NHCC, NMT, FMNH, Evolutionary Studies Institute (BP) and National Museum, Bloemfontein (NMQR). Electronic supplementary material, table S1 summarizes the specimens, taxonomy and methods of examination
(b) . Histology
Histological thin sections were made following standard thin-sectioning practices established by Lamm [43]. Specimens were embedded in Castolite AC polyester resin, cut into sections (2 mm thick) using a Buehler Isomet low-speed saw and ground at gradually decreasing grit and polished on a Buehler Ecomet Grinder. Using a Nikon Eclipse POL 100 polarized microscope, thin sections were examined in plain and cross-polarized light. Measurements were taken using NIS Elements software. The procedure used to produce the nitrocellulose peels is described in Olson [42] with additional methodological description in Olsen and Whitmore [44].
(c) . Micro-computed tomography
Specimens of Pristerodon (NHCC LB231); Brachyprosopus (FMNH UR 2513); Compsodon (NHCC LB14); Endothiodon (NMT RB162, NHCC LB648) were scanned using the GE Phoenix V|tome|x S240 scanner at the University of Chicago's PaleoCT facility and specimens of Eodicynodon (BP/1/6230 and NMQR 2978) were scanned at the Evolutionary Studies Institute with a Nikon Metrology XTH 225/320 LC. See electronic supplementary material, table S1 for specific scanning parameters for each specimen.
(d) . Parsimony, sequence, R
The histological details described below inform the assignment of a developmental pathway character state. Each taxon studied here was assigned one of four proposed developmental pathways. Ancestral state reconstructions were generated using four recent phylogenetic hypotheses [45–48] and maximum parsimony in R, and these reconstructions were run with a sequential transition cost (see Discussion for details on rationale).
4. Descriptions
(a) . Tissue composition
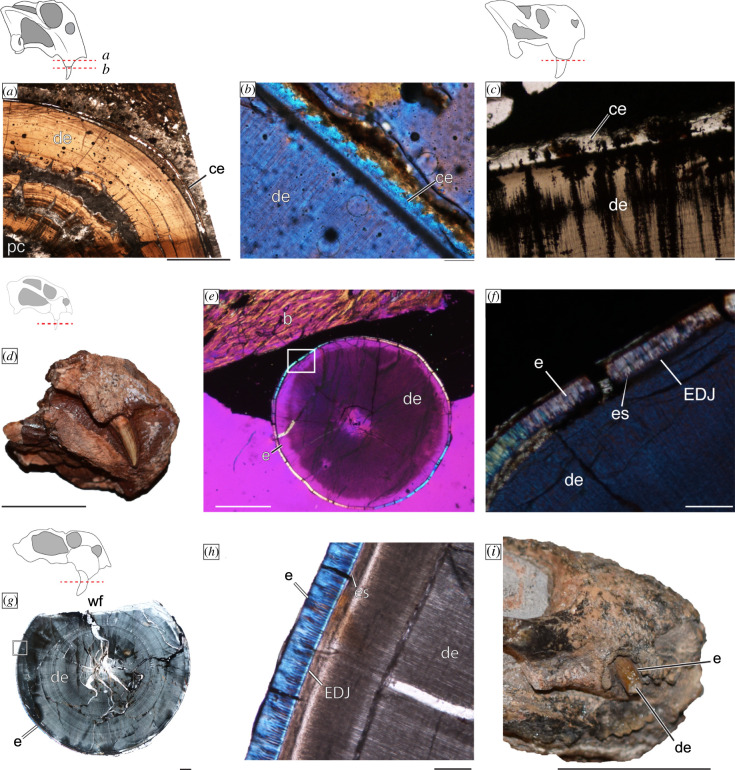
All of the sampled tusks were mainly composed of thick dentine walls whose roots extended deep into the maxilla. The occlusal ends of some specimens contained no evidence of enamel (Dicynodontoides, Dolichuranus and Lystrosaurus), and in those specimens, cementum can be observed inside and just crownward of the alveoli (figure 1a–c). Importantly, Diictodon, and at least some of the indeterminate dicynodontoids, have a thin (less than 40 µm), but distinct capping layer of enamel (figure 1d_–_h). Enamel can be identified by a clear enamel–dentine junction (EDJ), dentine tubules that extend across the EDJ to form enamel spindles, and the birefringent nature of the tissue under polarized light (figure 1e,f,g,h). The medium size of the Diictodon specimen (basal skull length ca 10 cm) and an unworn tusk surface (figure 1d) raise the possibility that the tusk was recently erupted. The enamel found in the unidentified Dicynodontoidea material likely represents adult tissues given the large sizes of these specimens (basal skull length range: 20–40 cm) as well as evidence of lingual wear facets (figure 1g). This suggests that even in adulthood these tusks had an enamel cap that was asymmetrically worn.
Figure 1.
Tissue composition of dicynodont tusks. Cementum is present in Lystrosaurus in (a) the root of UWBM 118 028 and (b) at eruption in UWBM 118 026 under polarized light with a lambda filter as well as in (c) Dolichuranus (NMT RB891). Enamel is found on newly erupted tusks of Diictodon (NHCC LB828) evident in gross morphology (d) as well as under polarized light with a lambda filter (e,f). Enamel is also present on sections through a caniniform of Dicynodontoidea indet. (NHCC LB832) whose tusks contain wear facets (g,h). Finally, in (i), a specimen of Pristerodon (NHCC LB773), with a tusk apparently composed of enamel and dentine (specimen not thin-sectioned). Scale bars a = 2 mm; b, c, f, h = 100 µm; d, i = 1 cm; e = 0.5 cm; f, g = 1000 µm. Abbreviations: b, bone; ce, cementum; de, dentine; e, enamel; es, enamel spindles; or, open root; ps, periodontal space; wf, wear facet. (Online version in colour.)
The μ-CT and acetate peel data were not of sufficiently high resolution to identify capping tissues for Eodicynodon, Endothiodon, Brachyprosopus or Compsodon. Although the acetate peel and μ-CT data for Pristerodon did not contain erupted tissues, an adult specimen of Pristerodon (NHCC LB773) preserves a tusk with a thin and distinct outer layer from the dentine core that appears most similar to enamel (figure 1i). Thus, we tentatively recognize enamel capping tissue in Pristerodon.
(b) . Attachment tissues
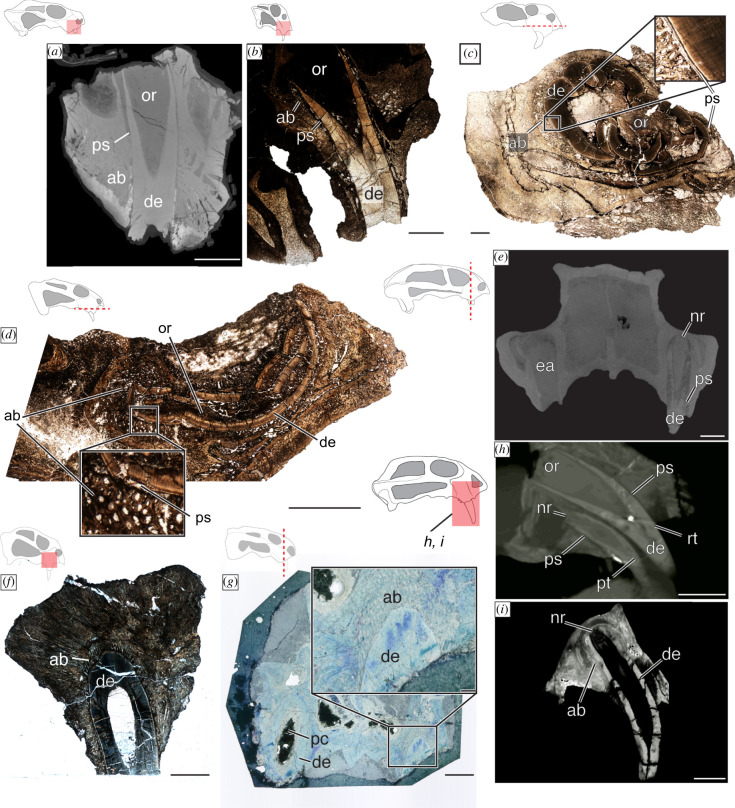
Generally, the attachment was ligamentous in the derived dicynodont taxa examined here. This is evident in the periodontal space that separates the hard tissues of the teeth from the surrounding alveolar bone (figure 2a_–_e; electronic supplementary material, figure S1A–D). Our Dolichuranus sample included only erupted tusk material (i.e. no root) and thus lacks information about attachment. However, a thin layer of cementum is present and preserves faint Sharpey's fibres, indicating that a ligament once anchored into this tissue (figure 1c). Therefore, we tentatively assign a gomphosis to Dolichuranus, in addition to the stronger evidence of a permanent gomphosis in Pristerodon, Endothiodon, Dicynodontoides, Compsodon and some dicynodontoids, and confirm the gomphosis previously described in Lystrosaurus [38,41]. Eodicynodon, Diictodon and Brachyprosopus are the only taxa in our sample that had ankylosed caniniform positions (figure 2e,f,g,i) revealing that these were not ever-growing teeth. Consistent with previous reports [41], we found attachment in Diictodon to be variable, with NHCC LB837 corroborating previous descriptions of an ankylosed dentition (figure 2g), whereas NHCC LB828 (electronic supplementary material, figure S1E) and FMNH PR 1772 (electronic supplementary material, figure S1F) present a gomphosis. A similar discordance was observed in Eodicynodon, where BP/1/6230 preserves a distinct periodontal space around both an old tooth and its replacement (figure 2h), whereas NMQR 2978, a larger and presumably adult specimen, had both tusks ankylosed to the jaw (figure 2i).
Figure 2.
Development, replacement and attachment histology of dicynodont tusks. Open roots with a periodontal space were found in (a) Pristerodon (NHCC LB231), (b) Lystrosaurus (SAM-PK-K011604) and (c) Dicynodontoidea indet. (NHCC LB836) although there is a pathological deformation in this specimen and (d) Dicynodontoides (NMT RB248). It is important to note that an additional specimen of Pristerodon suggests (a) represents a newly erupted tooth (see electronic supplementary material, figure S1). (e) Micro-CT imagery of Compsodon (NHCC LB14) reveals an empty alveolus and a retained tusk with a periodontal space that suggests a gomphosis tooth attachment. However, the root system narrows apically. An ankylosed tooth attachment is found in (f) Diictodon (NHCC LB837) and (g) an acetate peel of Brachyprosopus (FMNH PR 1770). Micro-CT imagery of a smaller specimen (BP/1/6230) (h) and larger specimen (NMQR 2978) (i) of Eodicynodon reveal evidence of replacement (h) and the adult tissues of functionally mature teeth (i). Scale bars a_–_g, I = 5000 µm; h, I = 1000 µm. Abbreviations: ab: alveolar bone; de: dentine; ea; empty alveolus; nr: narrow root; or: open root; pc, pulp cavity; ps: periodontal space; pt: primary tooth; rt: replacement tooth. (Online version in colour.)
(c) . Pulp cavity geometry
Specimens of Eodicynodon, Diictodon, Pristerodon, Dicynodontoides, Lystrosaurus and some indeterminate dicynodontoids have open roots forming a conical pulp cavity (figure 2a_–_d,h; electronic supplementary material, figure S1E). However similar to attachment tissues, this open-root system was not consistently observed in Diictodon or Eodicynodon. In Diictodon, NHCC LB828 has an open root in a gomphosis (electronic supplementary material, figure S1E), but narrowing of the pulp cavity is evident in the acetate peel of FMNH PR 1772 (electronic supplementary material, figure S1F), and NHCC LB837 preserves a narrow root apex, ankylosed to the jaw (figure 2f). In the smaller specimen of Eodicynodon, BP/1/6230, the open root is evident in a new replacement tooth, whereas the pulp cavity of the tooth being replaced shows narrowing at the root apex (figure 2h). Further, in the larger specimen, NMQR 2978, a narrowing of the pulp cavity is evident in both tusks. Thus, we propose that the open-root system observed in some specimens of Diictodon and Eodicynodon represents a transient ontogenetic stage of the tooth, rather than evidence of continuous dentine deposition in those taxa.
In addition to Diictodon and Eodicynodon, a closed root was present in Brachyprosopus (figure 2g; electronic supplementary material, figure S1A), Endothiodon (electronic supplementary material, figure S1D) and Compsodon (figure 2e). This indicates that these five taxa had determinate dentine deposition whereas there are histological indicators of active and continuous dentine deposition in Pristerodon, Dicynodontoides, Lystrosaurus and the indeterminate dicynodontoids.
(d) . Tusk replacement
In the sample included here, only a single specimen of a juvenile/subadult Eodicynodon (BP/1/6230) preserves evidence of replacement at the caniniform position (figure 2h). The right caniniform position has two teeth, a smaller caniniform (crown and root length = 10.8 mm) with a narrowing pulp cavity and a much larger caniniform (crown and root length = 18.5 mm) that have merged bony crypts and are both in a gomphosis (figure 2h). There is no evidence of erosion of the juvenile tooth, and it likely could have been simultaneously functional with its larger replacement. Interestingly, the Compsodon specimen has an empty left alveolus that could be interpreted as a replacement (figure 2e); however, there is no additional histological evidence of replacement (e.g. bony crypts, replacement tissues). Further, the right caniniform position contains a tooth with a narrowing pulp cavity that is suspended in a gomphosis. This suggests that the ‘adult’ teeth of Compsodon were in a permanent gomphosis, making them susceptible to post-mortem loss [49,50] rather than capturing a replacement event.
5. Discussion
(a) . Significance of ontogeny for caniniform characteristics
The Eodicynodon, Diictodon and Pristerodon specimens included here demonstrate how character states related to the enlarged caniniforms/tusks of dicynodonts change through ontogeny. Our interpretation of these changes follows LeBlanc et al.'s [41] general model of the ontogeny of tooth attachment in non-mammalian synapsids. In Diictodon, the lack of wear on the enamel and open root in the dentition of NHCC LB828 (electronic supplementary material, figure S1E) likely represents an early stage of development in which a gomphosis was retained. FMNH PR 1772 represents an intermediate stage of tusk ontogeny, and NHCC LB837 represents the latest stage of development where a gomphosis was replaced by an ankylosis and the pulp cavity narrowed apically (figure 2f). Alternatively, these specimens could represent variation or species-level differences among Diictodon, but we lack sufficient anatomical evidence in support of these alternatives, and recent studies failed to find evidence for multiple species [32,51].
Variation in the character states observed in the enlarged caniniforms of Pristerodon may also represent ontogenetic differences. The ligamentous attachment and pulp cavity of Pristerodon suggest an ever-growing dentition (figure 2a), but the enamel apparent in NHCC LB231 would not be compatible with this developmental style (figure 1i). Similar to Diictodon, it is possible that our sample of Pristerodon represents recently erupted teeth or differences between species or individuals. Patterns of intraspecific variation have been studied in some detail in Pristerodon and generally support the presence of a single species [39,52,53], but ontogenetic variation in the taxon has not received as much attention as in Diictodon. However, the ontogeny of Pristerodon is a topic that could be investigated further given the relatively high abundance of Pristerodon specimens [54].
The double-tusked morphology of Eodicynodon (BP/1/6230) provides further evidence that these changes in characters are influenced by ontogeny. BP/1/6230 is a juvenile specimen in comparison to the adult-sized NMQR 2978 and differences in attachment and pulp cavity shape relate to the ontogeny not only of the specimens but also the caniniform. The younger specimen has evidence of replacement that occurred before the functional caniniform ankylosed to the jaw; therefore, both teeth are at an ‘earlier’ developmental stage in a gomphosis. In the adult, there is no evidence of replacement and the caniniform is ankylosed to the jaw. From these observations, the caniniform of Eodicynodon appears similar to the plesiomorphic synapsid pattern of tooth ontogeny although replacement may have become less frequent in adulthood.
Importantly, Eodicynodon BP/1/6230 represents the only juvenile specimen in our sample, as well as the only one to present evidence of replacement. The dearth of replacement in morphologically adult dicynodonts reinforces the importance of considering the ontogeny of the individual in addition to the ontogeny of the caniniform dentition. There are sparse previous reports of ‘double-tusked’ dicynodonts, including the Eodicynodon specimen studied here [55], Emydops [36] and Kannemeyeria [35], but these are often interpreted as pathologies rather than evidence of replacement. ‘Double tusk’ morphology may well be pathological; it is a developmental anomaly known in modern tusked mammals (e.g. elephants; [56]). There are also additional reports of specimens in which eruption of the tusks is occurring in Diictodon [51,57,58], an indeterminate pylaecephalid (BP/1/2831; [39]) and possibly in Kembawacela [46], but in some of these cases, it is uncertain whether this is evidence of tooth replacement or the eruption of the first set of tusks. Although the juvenile Eodicynodon shows evidence of replacement, other Eodicynodon specimens show no further evidence of replacement, as would be expected if this tooth position was replaced repeatedly. This is uncommon for non-mammalian synapsids, which frequently show histological or morphological evidence of polyphyodonty (e.g. [41,50,59,60]) except for taxa with similarly exaggerated caniniform morphology (e.g. gorgonopsians [60]). Interestingly, rare evidence of replacement is restricted to the caniniform position of dicynodonts: in dicynodont taxa that have retained ‘postcanine’ dentitions, previous work has noted frequent replacement, including in Pristerodon FMNH PR 1771 [59] and some endothiodonts [61]. This indicates that dicynodont caniniforms/tusks were either monophyodont (i.e. never replaced) or had dramatically reduced replacement akin to diphyodonty, where a single generation of ‘baby teeth’ was replaced by a larger adult dentition.
(b) . Patterns of evolution in dicynodont ‘tusks’
Although dicynodont caniniforms appear morphologically similar, not all of them were tusks as defined here. It is only through detailed histological and µ-CT analyses that this diversity of form is apparent. Taken together, these data capture evidence for multiple strategies in the development of the dicynodont caniniform (figure 3). These strategies deviate from the ancestral synapsid condition (i.e. ankylosed, frequent polyphyodonty; Developmental Pathway 1) and include the reduction of caniniform tooth replacement with a retained ankylosed attachment (Developmental Pathway 2) that was directly observed in our ontogenetic sample of Eodicynodon; the reduced replacement, loss of an ankylosed state of attachment and the retention of a permanent gomphosis (Developmental Pathway 3); and the acquisition of an ever-growing tusk retained in a permanent gomphosis that was not replaced and is solely composed of dentine (Developmental Pathway 4). These pathways are largely distinct from one another as a result of differences in periodontal tissue development and the activity of the dental lamina. Tooth phenotypes and development are remarkably plastic (e.g. [62–64]), yet for attachment, convergent acquisition of a permanent gomphosis is rampant in vertebrate evolution, whereas ‘reversals’ to a plesiomorphic state are rarely observed in extant or fossil datasets [41,65]. Further, for replacement, there are suggestions that a diphyodont-level of replacement acts as an evolutionary rachet, prohibiting a reversal to polyphyodonty from a diphyodont or monophyodont state [66]. As such, we hypothesize that the evolution of more derived developmental pathways is best viewed as an irreversible sequence of events, where the independent acquisition of a subsequent developmental pathway is more likely than any reversion to a previous one (figure 3).
Figure 3.
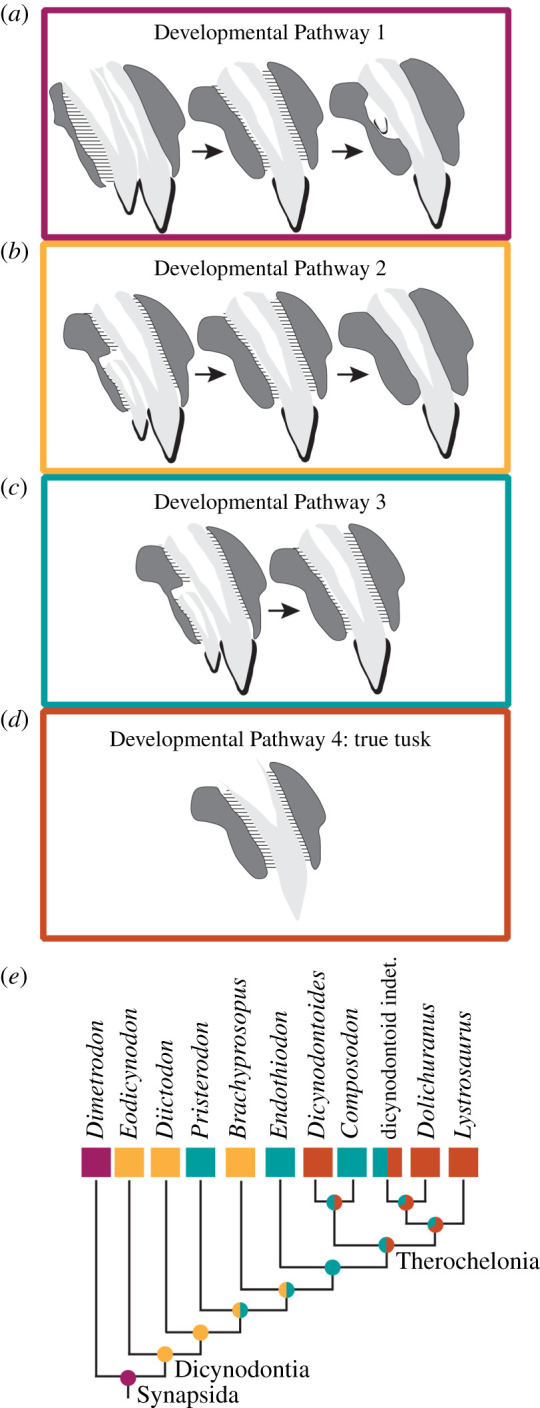
Proposed developmental pathways for dicynodont tusk evolution are described in our sample. (a) Developmental Pathway 1 represents the plesiomorphic synapsid tooth condition with frequent replacement, ankylosed attachment and a complete enamel covering. (b) Developmental Pathway 2 is similar to Developmental Pathway 1 except that replacement events are significantly less frequent. (c) Developmental Pathway 3 is an enamel-covered caniniform that has its ankylosed attachment replaced with a permanent gomphosis, but maintains a reduced frequency of replacement. (d) Developmental Pathway 4 is that of a true tusk where enamel is not present, the tusk is suspended in a permanent gomphosis and dentine is continuously deposited and erupted. (e) In a phylogenetic context, these developmental pathways reveal multiple independent acquisitions of a permanent gomphosis and an ever-growing tusk. Importantly, reduced replacement is a synapomorphy that arises early in dicynodont evolution. Phylogenetic tree adapted from Angielczyk & Kammerer [47] with alternative phylogenies discussed in the electronic supplementary material, Materials. (Online version in colour.)
We further consider these developmental pathways in a phylogenetic context with the sample of dicynodonts studied here. There are multiple hypothesized phylogenetic arrangements for the taxa included in this study (see electronic supplementary material, figure S2), although most recent phylogenies [67–70] support a topology similar to that proposed by Angielczyk & Kammerer [47] (figure 3). In all phylogenetic arrangements, the caniniform position in dicynodonts is ancestrally more tooth-like with reduced replacement from the polyphyodont condition plesiomorphic for synapsids (figure 3; electronic supplementary material, figure S2). In an irreversible model of evolution for these developmental pathways, the acquisition of a gomphosis occurs multiple times, consistent with trends in other synapsids [41]. In our sample, a gomphosis is acquired in Pristerodon and at the node uniting Endothiodontia and Therochelonia (figure 3), although it is also likely that increased sampling in this phylogenetic interval would reveal additional independent acquisitions of a gomphosis, especially considering that the ‘postcanine’ dentitions of some anomodont taxa stemward of Diictodon acquired a permanent gomphosis [61]. A gomphosis in the caniniform position became ancestral in the unnamed clade that encompasses Endothiodontia + Therochelonia, and we predict that all members of this clade would have a soft-tissue attachment strategy (figure 3). Within the derived clade of Therochelonia, under an irreversible model, there were at least four independent acquisitions of an ever-growing tusk with continuous eruption (i.e. Developmental Pathway 4), including in Dicynodontoides, in some dicynodontoids, in Dolichuranus and in Lystrosaurus (figure 3). Although Dicynodontoides is middle-to-late Permian in age, these findings generally support Camp & Welles' [29] previous proposal of ever-growing dentitions arising later in dicynodont evolutionary history, and our data reveal it likely evolved convergently. In conclusion, our survey of dicynodont caniniform histology and development reveals that the clade's eponymous ‘tusk’ varies surprisingly in its anatomical composition. As such, we urge caution in using the term ‘tusk’ to describe the dentitions of dicynodonts based on gross anatomy alone. We suggest that ‘tusk’ be exclusive to clades that have evidence of a true, ever-growing and erupting tusk, and that ‘caniniform’ be used to describe an enlarged tooth with determinate growth and in cases where the details of tooth attachment style and ontogeny are uncertain.
(c) . Evolution of ever-growing tusks
The evolutionary scenario evident in this earliest acquisition of an ever-growing tusk can shed light on the distribution of ever-growing dentitions in modern vertebrates. Although our data incompletely sample dicynodont diversity and evolutionary history, they do provide a testable hypothesis about the evolution of tusks in the clade. The major distinctions between the suite of features found in different dicynodont caniniforms studied here relate to attachment and replacement, two features that are often considered functionally tied to one another [41,59,60,71]. Significantly reducing the frequency of replacement from the ancestral synapsid state (Developmental Pathway 2) would have increased selective pressure to build a more durable tooth. As such, the acquisition of a permanent gomphosis would have facilitated such functional durability as well as movement within the jaw during growth. The result is a caniniform that may appear morphologically similar to a tusk but retains determinate growth, similar to other teeth (Developmental Pathway 3). In turn, a combination of reduced replacement and a permanent gomphosis facilitated the acquisition of an ever-growing dentition (Developmental Pathway 4).
The evolutionary sequence of developmental pathways observed in dicynodont history in many ways mirrors that for proboscideans, but with some important differences. Similar to proboscidean tusks, in dicynodonts, there is a loss of enamel that relates to the constraints of a continuously growing dentition. Importantly, however, there are no recorded dicynodont tusks that have an enamel band akin to that observed as an evolutionary intermediate in proboscideans [14]. This suggests that either proboscideans acquired two independent methods of continuous dental tissue deposition—one that produced an ever-growing tooth with enamel and another that resulted in a tusk—or that we currently lack representative histological sampling of dicynodonts from a stage in tusk evolution characterized by the presence of an enamel band. In either case, dicynodonts and proboscideans independently evolved tusks multiple times and did so in derived members of their respective clades [14].
The evolution of ever-growing tusks in dicynodonts can shed light more broadly on the distribution of ever-growing dentitions in modern vertebrates. This study, coupled with comparisons to proboscidean tusk evolution, suggests that the acquisition of reduced replacement and a permanent gomphosis may provide the foundation upon which ever-growing dentitions, and specifically tusks, can evolve. Ever-growing tusks in dicynodonts were only acquired once replacement was significantly reduced (possibly in a diphyodont condition) and a permanent gomphosis became ubiquitous. This may explain why in modern groups, mammals—who are the most diverse diphyodont and monophyodont vertebrates with a gomphosis—have uniquely and repeatedly acquired tusks. Finally, our study highlights that millions of years before the evolution of the first mammals, dicynodonts acquired tusks and likely did so through similar evolutionary pathways and possibly in response to similar selective pressures.
Supplementary Material
Acknowledgements
We thank S. Olroyd, J. Lungmus, L. Lichty and D. Begun for scanning of specimens as well as J. Benoit and A. Duhamel for access to scanned material. K. Abrams and A. Stroup assisted with specimen preparation and curation, and L. Marilao and M. Rich assisted with photography of specimens. Many specimens were collected as a part of TZAM fieldwork and we thank the NHCC, NMT, and field teams for specimen collection. Publishing was made possible by a grant from the Wetmore Colles fund. Finally, we thank handling editor D. Costa, A. LeBlanc and K. Brink for their helpful feedback that greatly improved this manuscript.
Ethics
We had permission for all of the specimens to be included in this study by the host institutions and affiliated curators.
Data accessibility
All data included in this study are histological images and are figured. Per standard practice, individual, high-resolution images are deposited on MorphoBank (project no. P4097) and are publicly available at http://morphobank.org/permalink/?P4097.
Authors' contributions
M.W.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing-original draft, writing-review and editing; K.D.A.: conceptualization, data curation, funding acquisition, supervision, visualization, writing-original draft, writing-review and editing; B.R.P.: conceptualization, funding acquisition, writing-original draft, writing-review and editing; C.S.: conceptualization, data curation, funding acquisition, resources, supervision, writing-original draft, writing-review and editing
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests
Funding
This study was funded by National Geographic 158R-18 (to B.R.P.); NSF DEB-1701383 (to M.R.W.); NSF EAR-1337569 (to K.D.A.) and NSF EAR-1337291 (to C.A.S.).
References
- 1.Crompton AW, Jenkins FA. 1968. Molar occlusion in Late Triassic mammals. Biol. Rev. Camb. Phil. Soc. 43, 427-458. ( 10.1111/j.1469-185X.1968.tb00966.x) [DOI] [PubMed] [Google Scholar]
- 2.Crompton AAW, Parker P. 1978. Structures that distinguish them from reptiles: evolution of the mammalian masticatory apparatus. Am. Sci. 66, 192-201. [PubMed] [Google Scholar]
- 3.Hopson JA. 1969. The origin and adaptive radiation of mammal-like reptiles and nontherian mammals. Ann. NY Acad. Sci. 167, 199-216. ( 10.1111/j.1749-6632.1969.tb20445.x) [DOI] [Google Scholar]
- 4.Luo Z, Kielan-jaworowska Z, Cifelli RL. 2004. Evolution of dental replacement in mammals. Bull. Carnegie Mus. Nat. Hist. 9058, 159-175. ( 10.2992/0145-9058(2004)36[159:EODRIM]2.0.CO;2) [DOI] [Google Scholar]
- 5.Luo ZX, Gatesy SM, Jenkins FA, Amaral WW, Shubin NH. 2015. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proc. Natl Acad. Sci. USA 112, E7101-E7109. ( 10.1073/pnas.1519387112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo ZX. 2007. Transformation and diversification in early mammal evolution. Nature 450, 1011-1019. ( 10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- 7.Schultz JA, Martin T. 2014. Function of pretribosphenic and tribosphenic mammalian molars inferred from 3D animation. Naturwissenschaften 101, 771-781. ( 10.1007/s00114-014-1214-y) [DOI] [PubMed] [Google Scholar]
- 8.Grossnickle DM. 2017. The evolutionary origin of jaw yaw in mammals. Sci. Rep. 7, 45094. ( 10.1038/srep45094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley HG. 1888. On the nature and limits of reptilian character in mammalian teeth. Proc. R. Soc. Lond. 44, 129-141. ( 10.1098/rspl.1888.0011) [DOI] [Google Scholar]
- 10.Smith HM. 1958. Evolutionary lines in tooth attachment and replacement in reptiles: their possible significance in mammalian dentition. Trans. Kansas Acad. Sci. 61, 216-225. ( 10.2307/3626649) [DOI] [Google Scholar]
- 11.Gaengler P, Metzler E. 1992. The periodontal differentiation in the phylogeny of teeth—an overview. J. Periodontal Res. 27, 214-225. ( 10.1111/j.1600-0765.1992.tb01671.x) [DOI] [PubMed] [Google Scholar]
- 12.Ungar PS. 2010. Mammal teeth: origin, evolution, and diversity. Baltimore, ML: The Johns Hopkins University Press. [Google Scholar]
- 13.Sperber GH. 1976. Tusks. Dent J. 42, 257-268. [PubMed] [Google Scholar]
- 14.Shoshani J, Tassy P. 1996. The proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Thulborn RA. 1974. A new heterodontosaurid dinosaur (Reptilia: Ornithischia) from the Upper Triassic Red Beds of Lesotho. Zool. J. Linn. Soc. 55, 151-175. ( 10.1111/j.1096-3642.1974.tb01591.x) [DOI] [Google Scholar]
- 16.Steyer JS, Damiani R, Sidor CA, O'Keefe FR, Larsson HCE, Maga A, Ide O. 2006. The vertebrate fauna of the Upper Permian of Niger. IV. Nigerpeton ricqlesi (Temnospondyli: Cochleosauridae), and the edopoid colonization of Gondwana. J. Vertebr. Paleontol. 26, 18-28. ( 10.1671/0272-4634(2006)26[18:TVFOTU]2.0.CO;2) [DOI] [Google Scholar]
- 17.Tummers M, Thesleff I. 2008. Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns. Evol. Dev. 10, 187-195. ( 10.1111/j.1525-142X.2008.00226.x) [DOI] [PubMed] [Google Scholar]
- 18.Koenigswald W. 2011. Diversity of hypsodont teeth in mammalian dentitions—construction and classification. Palaeontogr. Abteilung a-Palaozoologie-Stratigraphie 294, 63-94. ( 10.1127/pala/294/2011/63) [DOI] [Google Scholar]
- 19.Shoshani J. 1996. Skeletal and other basic anatomical features of elephants. In The proboscidea: evolution and palaeoecology of elephants and their relatives (eds Shoshani J, Tassy P), pp. 9-20. Oxford, UK: Oxford University Press. [Google Scholar]
- 20.Melo TP, Ribeiro AM, Martinelli AG, Soares MB. 2019. Early evidence of molariform hypsodonty in a Triassic stem-mammal. Nat. Commun. 10, 2841. ( 10.1038/s41467-019-10719-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tummers M, Thesleff I. 2003. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development 130, 1049-1057. ( 10.1242/dev.00332) [DOI] [PubMed] [Google Scholar]
- 22.Tassy P. 1996. Who is who among the Proboscidea? In The proboscidea: evolution and palaeoecology of elephants and their Relatives2 (eds Shoshani J, Tassy P), pp. 38-48. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Biewer JN, Velez-Juarbe J, Parham JF. 2020. Insights on the dental evolution of walruses based on new fossil specimens from California. J. Vertebr. Paleontol. 40, e1833896. ( 10.1080/02724634.2020.1833896) [DOI] [Google Scholar]
- 24.Angielczyk KD, Kammerer CF. 2018. Non-mammalian synapsids: the deep roots of the mammalian family tree. In Handbook of zoology: mammalia: mammalian evolution, diversity and systematics (eds Zachos FE, Asher RJ), pp. 117-198. Berlin, Germany: De Gruyter. [Google Scholar]
- 25.Cisneros JC, Abdala F, Rubidge BS, Dentzien-Dias PC, De Oliveira Bueno A. 2011. Dental occlusion in a 260-million-year-old therapsid with saber canines from the permian of Brazil. Science 331, 1603-1605. ( 10.1126/science.1200305) [DOI] [PubMed] [Google Scholar]
- 26.Cisneros JC, Abdala F, Jashashvili T, Bueno ADO, Dentzien-dias P, Cisneros JC. 2015. Tiarajudens eccentricus and Anomocephalus africanus, two bizarre anomodonts (Synapsida, Therapsida) with dental occlusion from the Permian of Gondwana. R. Soc. Open Sci. 2, 1500900. ( 10.1098/rsos.150090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rybczynski N, Reisz RR. 2001. Earliest evidence for efficient oral processing in a terrestrial herbivore. Nature 411, 684-687. ( 10.1038/35079567) [DOI] [PubMed] [Google Scholar]
- 28.Owen R. 1845. Odontography, Or, a treatise on the comparative anatomy of the teeth, their physiological relations, mode of development, and microscopic structure, in the vertebrate animals. London, UK: Hippolyte Bailiére. [PMC free article] [PubMed] [Google Scholar]
- 29.Camp CL, Welles SP. 1956. Triassic dicynodont reptiles. Part I. The North American genus Placerias. Mem. Univ. Calif. 13, 255-304. [Google Scholar]
- 30.Poole DFG. 1956. The structure of the teeth of some mammal-like reptiles. J. Microsc. Sci. 97, 303-312. ( 10.1242/jcs.s3-97.38.303) [DOI] [Google Scholar]
- 31.King GM. 1981. The functional anatomy of a Permian dicynodont. Phil. Trans. R. Soc. Lond. B 291, 243-322. ( 10.1098/rstb.1981.0001) [DOI] [Google Scholar]
- 32.Sullivan G, Reisz RR, Smith RMH. 2002. The Permian mammal-like herbivore Diictodon, the oldest known example of sexually dimorphic armament. Proc. R. Soc. B 270, 173-178. ( 10.1098/rspb.2002.2189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angielczyk KD, Huertas S, Smith RMH, Tabor NJ, Sidor CA, Steyer JS, Tsuji LA, Gostling NJ. 2014. New dicynodonts (Therapsida, Anomodontia) and updated tetrapod stratigraphy of the Permian Ruhuhu Formation (Songea Group, Ruhuhu Basin) of southern Tanzania. J. Vertebr. Paleontol. 34, 1408-1426. ( 10.1080/02724634.2014.880448) [DOI] [Google Scholar]
- 34.Broom R. 1923. On the structure of the skull in the carnivorous dinocephalian reptiles. Proc. Zool. Soc. 44, 661-684. ( 10.1111/j.1096-3642.1923.tb02203.x) [DOI] [Google Scholar]
- 35.Camp CL. 1956. Triassic dicynodont reptiles. Part II. Triassic dicynodonts compared. Mem. Univ. Calif. 13, 305-348. [Google Scholar]
- 36.Frobisch J, Reisz RR. 2008. A new species of Emydops (Synapsida, Anomodontia) and a discussion of dental variability and pathology in dicynodonts. J. Vertebr. Paleontol. 28, 770-787. ( 10.1671/0272-4634(2008)28[770:ANSOES]2.0.CO;2) [DOI] [Google Scholar]
- 37.Rozefelds AC, Warren A, Whitfield A, Bull S. 2011. New evidence of large Permo-Triassic dicynodonts (Synapsida) from Australia. J. Vertebr. Paleontol. 31, 1158-1162. ( 10.1080/02724634.2011.595858) [DOI] [Google Scholar]
- 38.Whitney MR, Tse Y, Sidor CA. 2019. Histological evidence of trauma in tusks of southern African dicynodonts. Palaeontol. Afr. 53, 75-80. [Google Scholar]
- 39.Toerien M. 1953. The evolution of the palate in South African Anomodontia and its classificatory significance. PhD thesis, University of Witwatersrand, Johannesburg, South Africa. [Google Scholar]
- 40.Fong RK, Leblanc ARH, Sidor CA, Pittman M, LaFlamme M. 2018. Dental histology of the dicynodont Lystrosaurus. In 78th Ann. Meet. Societ of Vertegrate Paleontology, Albuquerque, NM, 17–20 October, p. 128. See https://vertpaleo.org/wp-content/uploads/2021/03/SVP-2018-program-book-V4-FINAL-with-covers-9-24-18.pdf. [Google Scholar]
- 41.LeBlanc ARH, Brink KS, Whitney MR, Abdala F, Reisz RR. 2018. Dental ontogeny in extinct synapsids reveals a complex evolutionary history of the mammalian tooth attachment system. Proc. R. Soc. B 285, 20181792. ( 10.1098/rspb.2018.1792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson EC. 1944. Origin of mammals based upon cranial morphology of the therapsid suborders. Geol. Soc. Am. Spec. Pap. 55, 1-136. ( 10.1130/SPE55) [DOI] [Google Scholar]
- 43.Lamm E-T. 2013. Preparation and sectioning of specimens. In Bone histology of fossil tetrapods (eds Padian K, Lamm E-T), pp. 55-160. Berkley, Germany: University of California Press. [Google Scholar]
- 44.Olsen FR, Whitmore FC. 1944. Machine for serial sectioning of fossils. J. Paleontol. 18, 210-215. [Google Scholar]
- 45.Angielczyk KD, Rubidge BS, Day MO, Lin F. 2016. A reevaluation of Brachyprosopus broomi and Chelydontops altidentalis, dicynodonts (Therapsida, Anomodontia) from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin, South Africa. J. Vertebr. Paleontol. 36, e1078342. ( 10.1080/02724634.2016.1078342) [DOI] [Google Scholar]
- 46.Angielczyk KD, Benoit J, Rubidge BS. 2019. A new tusked cistecephalid dicynodont (Therapsida, Anomodontia) from the upper Permian upper Madumabisa Mudstone Formation, Luangwa Basin, Zambia. Pap. Palaeontol. 7, 1-42. [Google Scholar]
- 47.Angielczyk KD, Kammerer CF. 2017. The cranial morphology, phylogenetic position and biogeography of the upper Permian dicynodont Compsodon helmoedi van Hoepen (Therapsida, Anomodontia). Pap. Palaeontol. 3, 513-545. ( 10.1002/spp2.1087) [DOI] [Google Scholar]
- 48.Kammerer C. 2019. A new dicynodont (Anomodontia: Emydopoidea) from the terminal Permian of KwaZulu-Natal, South Africa. Palaeontol. Afr. 53, 179-191. [Google Scholar]
- 49.Boonstra LD. 1962. The dentition of the titanosuchian dinocephalians. Ann. South Afr. Mus. 46, 57-112. [Google Scholar]
- 50.Whitney MR, Sidor CA. 2019. Histological and developmental insights into the herbivorous dentition of tapinocephalid therapsids. PLoS ONE 14, 1-21. ( 10.1371/journal.pone.0223860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angielczyk KD, Sullivan C. 2008. Diictodon feliceps (Owen, 1876), a dicynodont (Therapsida, Anomodontia) species with a Pangaean distribution. J. Vertebr. Paleontol. 28, 788-802. ( 10.1671/0272-4634(2008)28[788:DFOADT]2.0.CO;2) [DOI] [Google Scholar]
- 52.Keyser AW. 1993. A re-evaluation of the smaller endothiodontidae. Geol. Surv. South Afr. Mem. 82, 1-53. [Google Scholar]
- 53.King GM, Rubidge B. 1993. A taxonomic revision of small dicynodonts with post-canine teeth. Zool. J. Linn. Soc. 107, 131-154. ( 10.1111/j.1096-3642.1993.tb00218.x) [DOI] [Google Scholar]
- 54.Smith RMH, Rubidge BS, Van der Walt MVM. 2012. Early evidence of molariform hypsodonty in a Triassic stem-mammal therapsid biodiversity patterns and palaeoenvirnoments of the Karoo Basin, South Africa. In Forerunners of mammals (eds Chinsamy-Turan A), pp. 31-64. Bloomington, IN: Indiana University Press. [Google Scholar]
- 55.Jinnah ZA, Rubidge B. 2007. A double-tusked dicynodont and its biostratigraphic significance. S. Afr. J. Sci. 103, 51-53. [Google Scholar]
- 56.Carrington R. 1958. Elephants: a short account of their natural history. In Evolution and influence on mankind. London, UK: Chatto and Windus. [Google Scholar]
- 57.Cluver MA. 1970. THe palate and mandible in some specimens of Dicynodon testudirostris Broom & Hotton (Reptilia, Therapsida). Ann. South Afr. Mus. 56, 133-153. [Google Scholar]
- 58.Smith RMH, Angielczyk KD, Benoit J, Fernandez V. 2021. Neonate aggregation in the Permian dicynodont Diictodon (Therapsida, Anomodontia): evidence for a reproductive function for burrows? Palaeogeogr. Palaeoclimatol. Palaeoecol. 569, 110311. ( 10.1016/j.palaeo.2021.110311) [DOI] [Google Scholar]
- 59.Hopson JA. 1963. Tooth replacement in cynodont, dicynodont and therocephalian reptiles. Proc. Zool. Soc. 142, 625-654. ( 10.1111/j.1469-7998.1964.tb04632.x) [DOI] [Google Scholar]
- 60.Kermack KA. 1956. Tooth replacement in mammal-like reptiles of the suborders gorgonopsia and therocephalia. Phil. Trans. R. Soc. Lond. B 240, 95-136. ( 10.1098/rstb.1956.0013) [DOI] [Google Scholar]
- 61.Olroyd SL, LeBlanc ARH, Araújo R, Angielczyk KD, Duhamel A, Benoit J, Amaral M. 2021. Histology and μCT reveal the unique evolution and development of multiple tooth rows in the synapsid Endothiodon. Sci. Rep. 11, 16875. ( 10.1038/s41598-021-95993-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans AR, Wilson GP, Fortelius M, Jernvall J. 2007. High-level similarity of dentitions in carnivorans and rodents. Nature 445, 78-81. ( 10.1038/nature05433) [DOI] [PubMed] [Google Scholar]
- 63.Kangas AT, Evans AR, Thesleff I, Jernvall J. 2004. Nonindependence of mammalian dental characters. Nature 432, 211-214. ( 10.1038/nature02927) [DOI] [PubMed] [Google Scholar]
- 64.Melstrom KM, Irmis RB. 2019. Repeated evolution of herbivorous crocodyliforms during the Age of Dinosaurs. Curr. Biol. 29, 2389-2395.e3. ( 10.1016/j.cub.2019.05.076) [DOI] [PubMed] [Google Scholar]
- 65.Bertin TJC, Thivichon-Prince B, LeBlanc ARH, Caldwell MW, Viriot L. 2018. Current perspectives on tooth implantation, attachment, and replacement in amniota. Front. Physiol. 9, 1630. ( 10.3389/fphys.2018.01630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Nievelt AFH, Smith KK. 2005. To replace or not to replace: the significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 31, 324-346. ( 10.1666/0094-8373(2005)031[0324:TRONTR]2.0.CO;2) [DOI] [Google Scholar]
- 67.Olroyd SL, Sidor CA, Angielczyk KD. 2017. New materials of the enigmatic dicynodont Abajudon kaayai (Therapsida, Anomodontia) from the lower Madumabisa Mudstone Formation, middle Permian of Zambia. J. Vertebr. Paleontol. 37, e1403442. ( 10.1080/02724634.2017.1403442) [DOI] [Google Scholar]
- 68.Olivier C, Houssaye A, Jalil NE, Cubo J. 2017. First palaeohistological inference of resting metabolic rate in an extinct synapsid, Moghreberia nmachouensis (Therapsida: Anomodontia). Biol. J. Linn. Soc. 121, 409-419. ( 10.1093/biolinnean/blw044) [DOI] [Google Scholar]
- 69.Kammerer CF, Viglietti PA, Hancox PJ, Butler RJ, Choiniere JN. 2019. A new kannemeyeriiform dicynodont (Ufudocyclops mukanelai, gen. et sp. nov.) from Subzone C of the Cynognathus Assemblage Zone, Triassic of South Africa, with implications for biostratigraphic correlation with other African Triassic faunas. J. Vertebr. Paleontol. 39, 1596921. ( 10.1080/02724634.2019.1596921) [DOI] [Google Scholar]
- 70.Angielczyk KD, Liu J, Yang W. 2021. A redescription of Kunpania scopulusa, a bidentalian dicynodont (Therapsida, Anomodontia) from the ?Guadalupian of northwestern China. J. Vertebr. Paleontol. 41, e1922428. ( 10.1080/02724634.2021.1922428) [DOI] [Google Scholar]
- 71.Janis CM, Fortelius M. 1988. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol. Rev. 63, 197-230. ( 10.1111/j.1469-185X.1988.tb00630.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are histological images and are figured. Per standard practice, individual, high-resolution images are deposited on MorphoBank (project no. P4097) and are publicly available at http://morphobank.org/permalink/?P4097.