Evidence of prehistoric human activity in the Falkland Islands (original) (raw)
Interdisciplinary evidence indicates human activity in the Falkland Islands before European exploration of the South Atlantic.
Abstract
When Darwin visited the Falkland Islands in 1833, he noted the puzzling occurrence of the islands’ sole terrestrial mammal, Dusicyon australis (or “warrah”). The warrah’s origins have been debated, and prehistoric human transport was previously rejected because of a lack of evidence of pre-European human activity in the Falkland Islands. We report several lines of evidence indicating that humans were present in the Falkland Islands centuries before Europeans, including (i) an abrupt increase in fire activity, (ii) deposits of mixed marine vertebrates that predate European exploration by centuries, and (iii) a surface-find projectile point made of local quartzite. Dietary evidence from D. australis remains further supports a potential mutualism with humans. The findings from our study are consistent with the culture of the Yaghan (Yámana) people from Tierra del Fuego. If people reached the Falkland Islands centuries before European colonization, this reopens the possibility of human introduction of the warrah.
INTRODUCTION
When Charles Darwin visited the Falkland Islands in 1833, he noted the unusual occurrence of a single terrestrial mammal species: Dusicyon australis, a fox-like canid known as the Falkland Islands wolf or “warrah” (1). In his journals, Darwin observed the warrah’s lack of fear and its inquisitive nature, hypothesizing that “within a very few years … this fox will be classed with the dodo, as an animal which has perished from the face of the earth” (2). By 1876, the warrah became the first canid to go extinct in the global historic period, as a result of overhunting (3). While the warrah’s extinction is well documented, the nature and timing of its initial dispersal to the Falkland Islands has been the subject of speculation for centuries. Genetic evidence indicates that D. australis diverged from Dusicyon avus, a South American mainland species of fox, between 31 and 8 thousand years (ka) BP (before present; 4). When the first Europeans arrived in 1690, they found no evidence of people nor have any archaeological sites been discovered that support a pre-European human presence in the islands to date. It has thus been hypothesized that D. australis evolved from populations of D. avus that crossed the South Atlantic during the Last Glacial Maximum, when sea levels were ~125 m lower and the exposed continental shelf reduced the distance of a transoceanic crossing (4). However, this hypothesis is undermined by a paucity of evidence for a sea-ice bridge, as well as the lack of any other native terrestrial mammals in the Falkland Islands (4). Alternately, if D. australis diverged from D. avus in mainland South America, perhaps via domestication, then it could have arrived in the Falkland Islands during the Holocene via transport by Yaghan (Yámana) or other Indigenous peoples before European exploration of the South Atlantic (1).
The position of the Falkland Islands relative to the nutrient-rich Falkland Current (Fig. 1) could have facilitated the islands’ accessibility to capable seafaring peoples, who may have taken advantage of the current’s rich biodiversity as a food resource (5). By 6400 BP, most of the Indigenous communities of the Beagle Channel and the Strait of Magellan subsisted primarily on marine resources (6). Both vertebrate remains preserved in shell middens and historic ethnographic accounts indicate that Indigenous Fuegians had developed the capacity to hunt pinnipeds in open water from canoes (6). Several lines of evidence indicate the possibility of a pre-European human presence in the Falkland Islands, including (i) anecdotal evidence of canoes during Darwin’s visit (2), (ii) a previously reported increase in fossil charcoal centered at 4800 BP near the present-day town of Stanley (7), (iii) reports of lithics and a bone harpoon (8, 9), and (iv) the presence of the warrah, the Falkland Islands’ lone terrestrial mammal. In 1979, a local landowner found a stone projectile point on the north side of New Island (Fig. 2). This artifact is made of locally sourced quartzite abundant throughout the Falkland Islands (10) and is consistent with lithic technology used in Tierra del Fuego over the past 1000 years (Fig. 2) (8). However, in the absence of archaeological data, the origin of the stone point (and the canoes observed by Darwin in 1833) remained an enigma.
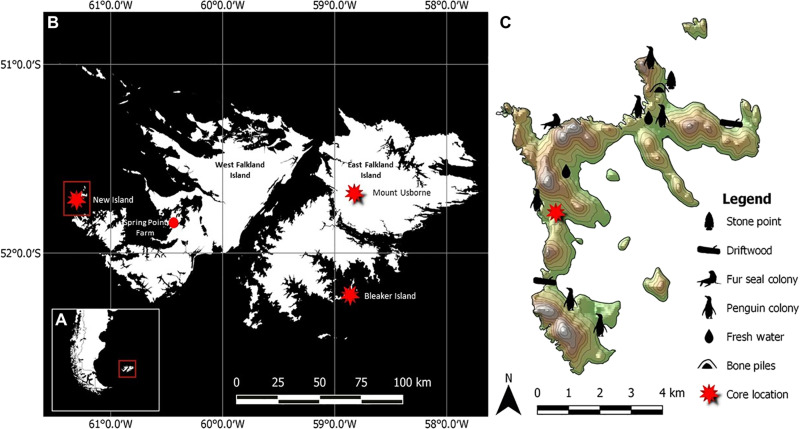
Fig. 1. Map of the study areas in the Falkland Islands and important resource locations on New Island.
(A) Position of the Falkland Islands in relation to southern South America and the Falklands Current (inset). (B) Map of the Falkland Islands with peat core locations indicated by red stars, including New Island, Mount Usborne, and Bleaker Island. (C) Map of New Island with locations of key resources relative to the location of the New Island core.
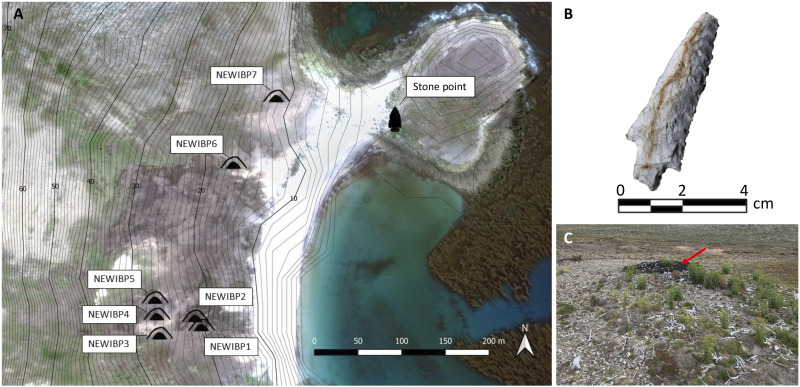
Fig. 2. New Island site map and images.
(A) New Island site map depicting bone pile and stone point locations; NEWIBP6 and NEWIBP7 were fully excavated in 2018 and analyzed for this project. (B) A three-dimensional model of the New Island lithic point found in 1976. (C) Image of NEWIBP6 before excavation. The bone pile was primarily preserved under a dark layer of peat (red arrow). The area has undergone considerable surface erosion resulting in downslope scatter and exposure of bone elements. Photo credit: Kit Hamley, University of Maine. Map data: 2015 Google.
Establishing the timing of human arrival on islands can be challenging, as small or transient populations may not leave behind cultural material (11). However, the paleoecological record can provide evidence of anthropogenic impacts on sensitive island ecosystems, including increases in fire activity, species introductions, or extinctions (11–13). Of these, fire has proven to be a particularly sensitive indicator of initial human activity in island ecosystems (11). Sedimentary charcoal accumulation rates (CHAR) have been found to increase by orders of magnitude immediately following human arrival, ultimately declining to an elevated baseline relative to the preanthropogenic background (11, 14, 15). By providing indirect proxy evidence of human impacts integrated across whole landscapes, the paleoecological record may be more sensitive than the archaeological record at detecting initial or ephemeral human activities on island ecosystems. To leverage this potential, we combined methods from paleoecology and archaeology to investigate a potential prehistoric human presence in the Falkland Islands. Our interdisciplinary investigation re-opens the question of a potential human prehistory of the Falkland Islands, which are an important biodiversity hotspot and the site of the first canid extinction in the global historic record.
RESULTS
In 2018, we conducted a ground surface survey on New Island in the area where a stone point made of locally sourced quartzite had been found on the surface by a local landowner in 1979. During the survey, we identified seven distinct deposits of disarticulated seabird and marine mammal bones just upslope of the location where the stone point was found (Fig. 2). We excavated two of these deposits, which were primarily composed of Otaria flavescens [South American sea lion; New Island bone pile 6 (NEWIBP6): minimum number of individuals (MNI) = 61; NEWIBP7: MNI = 73; table S3] and Eudyptes chrysocome (southern rockhopper penguin; NEWIBP6: MNI = 9; NEWIBP7: MNI = 77; table S3). We dated 10 unique elements from these piles, representing 10 O. flavescens individuals (table S1). The New Island bone piles yielded dates between 675 and 530 BP (1275 to 1420 CE) [all dates reported as calibrated calendar years before present (1950), except when indicated as CE or otherwise noted].
We analyzed charcoal records from three sites for evidence of prehistoric anthropogenic burning: a peat column from New Island, a peat core from a bog on Mount Usborne (705 m; East Falkland), and a coastal peat column from Bleaker Island (Fig. 1). Mount Usborne is the highest location in the Falkland Islands, located in the interior of East Falkland; its isolation, exposure, and distance to coastal resources make it an unlikely candidate for past human land use, and the site is not visited frequently today. We thus consider the Mount Usborne charcoal record to be a control sample representing the natural, climate-driven fire regime for the Falkland Islands. In contrast, Bleaker Island is a small (20.7 km2) outlying island to the south of East Falkland. We included this location because Darwin noted several dugout canoes on a beach at Bleaker in 1833. He speculated that the Falklands Current was the most likely explanation for the presence of the canoes but did not rule out Indigenous visitors (2).
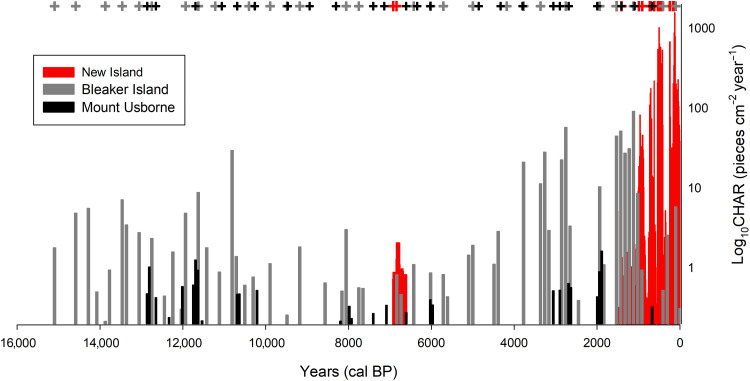
The Mount Usborne fire history was relatively uniform (Fig. 3), with low CHAR (<1 piece cm−2 year−1; Fig. 3) throughout the 13,000-year record. The 15,000-year record at Bleaker Island is similar, with only a slight increase in CHAR and fire frequency at ~7000 BP. In contrast, the New Island charcoal record shows a marked increase in CHAR at ~1800 BP that is not seen at the other two sites (Fig. 3). From 550 to 400 BP, CHAR abruptly increased by two orders of magnitude, contemporaneous with the radiocarbon distributions of the dated O. flavescens bones (Fig. 4). A similar abrupt increases in CHAR occurred from 180 to 120 BP during the initial arrival of Europeans in New Island, after which CHAR values were reduced but fire activity remained constant until the present day (Fig. 4). The increases in CHAR at 550 and 180 BP contain the highest values of any of the records that we analyzed throughout the Falkland Islands and were several orders of magnitude greater than the maximum CHAR values from Mount Usborne or Bleaker Island (Fig. 3), as well as from a previously published charcoal record from Surf Bay on East Falkland (16).
Fig. 3. CHAR for New Island (red), Bleaker Island (gray), and Mount Usborne (black), with significant fire events indicated (+).
CHAR data are plotted on a log axis due to a three orders of magnitude difference in CHAR values among locations. Years are reported in calibrated years before present (cal BP).
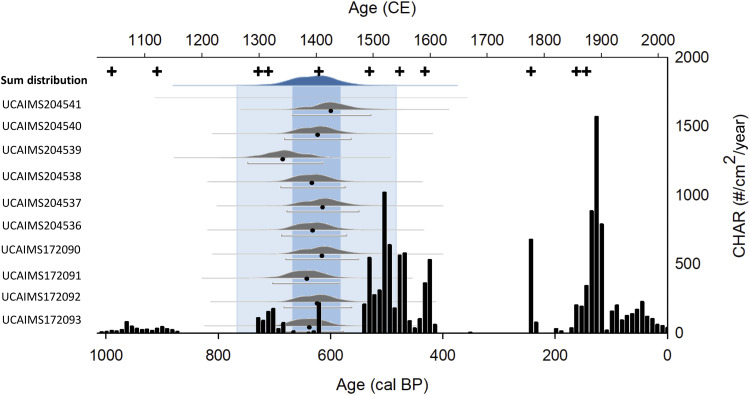
Fig. 4. New Island CHAR (black bars) and significant fire events (+), showing an increase in fire activity beginning in 1000 BP, with clusters of high-magnitude peaks occurring at 550 to 400 BP and 180 BP to 2016 CE present.
Probability distribution curves for calibrated radiocarbon dated O. flavescens samples from NEWIBP6 and NEWIBP7 (gray curves), 95% confidence ranges (gray brackets), and median values of the resulting calibrated likelihoods (black circles) for each sample. Sum distribution (top blue curve) of calibrated radiocarbon likelihood distributions for all O. flavescens bone pile samples (n = 10). Colored bars mark the highest probability range (dark blue; 95% probability range) of bone pile deposition, likelihood age range (light blue; 62% probability range) of human habitation as determined by bone pile deposition and fire events, and the period of European arrival and colonization of New Island specifically (gray). Sample IDs are noted at the left edge of the figure and correspond with the adjacent probability distribution curves.
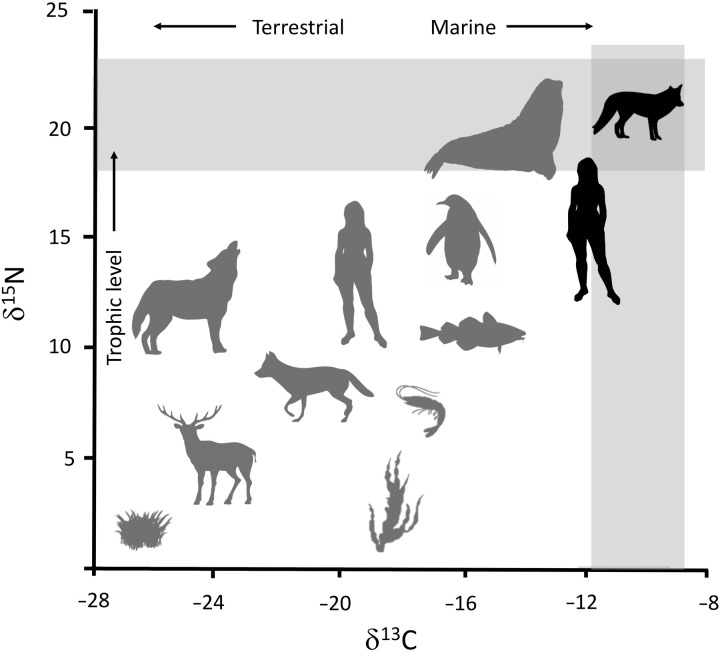
To assess the possibility of a human introduction of the warrah, we analyzed six D. australis elements from a shallow, seasonal pond at Spring Point Farm, West Falkland. Spring Point is the only location where fossil D. australis bones have been found in the Falkland Islands despite large populations of the canids reported in historic accounts. The Falkland Islands are largely composed of acidic peat, which is poor for bone preservation and may account for the apparent scarcity of warrah fossils. Of the six samples sent for accelerator mass spectrometry (AMS) dating, only three yielded sufficient collagen for dating (table S1). Of these, a lower M1 tooth yielded the oldest known age for D. australis, 3396–3752 BP (3790 ± 25 14C BP), which is currently the minimum arrival date of the warrah in the Falkland Islands. The other two D. australis dates were 236–500 BP (895 ± 15 14C BP) and 264–511 BP (925 ± 15 14C BP) (table S1). Carbon and nitrogen isotopes suggest that the three D. australis individuals that we analyzed had a marine-based diet consisting primarily of apex marine predators; δ13C values were −9.6, −12.3, and −12.2 per mil (‰) (Fig. 5 and table S1), and δ15N values were 22, 17.3, and17.7 ‰, respectively. These δ15N values place the warrah on par with sea lions, fur seals, and cetaceans, which range from 11 to 22‰ (17–19); typically, a 3 to 5‰ enrichment in δ15N is observed with each increase in trophic level (20). While these results may be consistent with selective coastal scavenging, they could also indicate a commensal relationship with humans, subsisting on littoral resources such as O. flavescens and E. chrysocome (21).
Fig. 5. Stable carbon and nitrogen isotope ratios for reference and studied terrestrial and marine taxa.
D. australis (black canid silhouette) exhibits δ15N and δ13C values (gray bars; table S1) consistent with marine-based diets from high trophic levels. Pre- and postcontact hunter-gatherer remains from Tierra del Fuego (black human silhouette) had a similar trophic signature (38). Mid-Holocene hunter-gatherer bone samples from the Pampas region in northern Argentina (gray human silhouette) suggest a more mixed terrestrial diet (40).
DISCUSSION
Multiple independent lines of evidence from New Island indicate that people were in the Falkland Islands centuries before European colonization: an orders-of-magnitude increase in charcoal that overlaps in age with multiple mixed vertebrate bone deposits and a locally sourced stone point consistent with technology used by Indigenous Fuegian cultures. The earliest possible European sightings of the Falkland Islands date to the 16th century (22), and the first confirmed landing was in 1690 CE (Supplementary Text) (23). The Falkland Islands were not permanently settled until 1764 CE (7); there are no recorded landings at New Island until 1774 CE (24). Critically, while the earliest dates for European sighting of the Falkland Islands are debated, the first landing was not made until more than 250 years after the charcoal peaks and bone piles reported in this study (1275 to 1420 CE).
The fire history at New Island is consistent with that seen in other island systems following human arrival (11), indicated by an orders-of-magnitude increase in charcoal (Fig. 3). While fire can have natural or human origins, we interpret the increase in fire activity ~550 BP at New Island to be anthropogenic for several reasons. First, there are only two potential ignition sources in the Falkland Islands: lightning strikes and people. Fires caused by lightning strikes throughout the Falkland Islands are rare today (7). As in Tierra del Fuego, sustained Westerlies counteract the strong convective currents that produce thunderstorms (although the strength and position of the Westerlies have shifted through time) (25). An average of four thunderstorms per year (7), coupled with low average temperatures (29° to −9°C) and low precipitation (642 mm/year) (26), creates conditions in which lightning-caused fires are rare. Lightning is thus unlikely to produce the prolonged periods of high-frequency fire events seen in the New Island charcoal record, and it is unlikely that lightning strikes would be more common on New Island than on Mount Usborne or Bleaker Island. Furthermore, the observed increase in fire at New Island in the last millennium cannot be explained by changes in grassland productivity, temperature (27), or precipitation (28), none of which increased over the last millennium (fig. S1), so we rule these out as drivers.
The precontact increase in CHAR at 550 BP is similar in magnitude to the later increase at 1790 CE, when European and American sailors regularly used fire to improve visibility on New Island, as well as to flush seals and penguins for hunting and to render whale, seal, and penguin oil (24, 29). This historic increase in CHAR demonstrates the sensitivity of our peat record to anthropogenic burning and provides a useful basis of comparison for the pre-European increase in CHAR (Fig. 3). The gap in fire events between 550 and 180 BP is also consistent with the documented absence of human habitants in the Falkland Islands at the time of early European exploration of the region. While the earliest fire peaks at 1000 BP appear small compared to subsequent intervals, they represent an order-of-magnitude increase in CHAR over earlier values in the New Island record, when fires were rare and small by comparison, and may represent an earlier prehistoric human presence (Figs. 3 and 4).
We also interpret the mixed New Island bone assemblages as anthropogenic for several reasons: First, E. chrysocome is a prey species for O. flavescens, and it is unlikely that these two species would be found together in abundance in natural death assemblages (table S3). Second, the conspicuous absence of O. flavescens stomach contents, including fish bones or otoliths, cephalopod beaks, and bone fragments in the surrounding matrix, suggests that these bone piles were not the original death sites (30). Furthermore, the piles are located on a 9° slope undergoing active erosion, with no depressions that would promote accumulation over time (Fig. 2C). These features appear more consistent with a secondary processing or midden site rather than natural death assemblages. No cut marks or other evidence of butchering were found during our excavations. However, the absence of cut marks does not necessarily preclude processing by people (30, 31). The presence of processing marks depend on several factors, including the skill of the butchers, carcass size, tool materials, the elements in an assemblage, and the taphonomy of the site (31, 32). Preferential use of bone and shell tools by several Indigenous Fuegian cultures (33) may explain the lack of butchery marks, as bone and mussel shell tools likely would have been too soft to leave easily observable cut marks on bone. It may also explain the absence of lithic debris across the site.
Of the several groups inhabiting Tierra del Fuego during the Holocene, the evidence at New Island is most consistent with culture of the Yaghan (Yámana) people, highly capable seafarers subsisting on a marine diet composed largely of seals, penguins, and shellfish (33). They hunted sea lions and seals with bone harpoons hafted to wooden handles, and meat and skins were processed using mussel shells hafted to wooden handles (33). While stone points were used by the Yaghan, they were used infrequently and typically only for arrows, which they sometimes used to hunt penguins (33). Before European colonization, the Yaghan were highly mobile people without permanent settlements, and families (including canids) traveled together by dugout canoe as far as the Diego Ramírez Islands, located 105 km southwest of Cape Horn in the Drake Passage (33). Fire was an integral part of Yaghan culture, whose fires may even have given Tierra del Fuego (“Land of Fire”) its name (33). According to contemporary ethnographic accounts, the Yaghan wore little clothing despite the harsh climate and kept constant fires centrally located for warmth and cooking (33). They brought fire with them when they traveled by sea, where a small hearth was stoked by children who crouched in the middle of their canoes (33). The Yaghan also used signal fires to alert neighboring bands of whale carcasses or other important resources or when someone had died (33). While there are no ethnographic accounts of the Yaghan using fire to flush game, as later Europeans did in the Falkland Islands (dense tussac grass is less common in Tierra del Fuego), it is possible that Indigenous visitors used fire as a hunting tool in the Falkland Islands. It is also possible that accidental burns resulted from smaller, intentional fires, as dry tussac grass and peat are highly flammable.
Based on ethnographic accounts of Yaghan people, it follows that evidence of their presence in the Falkland Islands would include (i) increased fire activity, (ii) utilization of coastal resources such as sea lions and penguins, and (iii) the presence of canids. The findings reported here are consistent with these expectations, although we are unable to determine whether these were intentional visits. We think it unlikely that prehistoric peoples had a sustained presence in the Falkland Islands. Rather, the evidence supports one or more temporary visitations or accidental landings that left behind little cultural material but resulted in a discernable anthropogenic fingerprint in the fossil record. While the evidence from New Island suggests a local arrival of people between 1000 and 550 BP, we cannot rule out earlier visits elsewhere across the Falkland Islands. Both the presence of the warrah on West Falkland by at least 3400 BP and an increase in charcoal abundance recorded in a previous peat record near Stanley from 5400 BP (7) to ~3500 BP (16) could indicate an earlier arrival.
The possibility of a prehistoric human presence in the Falkland Islands reopens the debate about the origin of the warrah and could explain the long-debated anomaly of a sole terrestrial mammal on an isolated archipelago. Southern South America has a long history of close human-canid interactions (34–36), including the extinct Fuegian dog [a domesticated form of the culpeo fox, _Lycalopex culpaeus_ (36)], and foxes played an important role in Yaghan culture. According to ethnographic accounts, each family kept several canids, who routinely traveled in canoes and were never intentionally left behind when traveling (33). The Fuegian dog bore a close morphological similarity to the culpeo and played an active role in the hunt for otters, birds, and foxes, as well as a protective role when unfamiliar humans were present. Even in times of resource scarcity, these canids were never eaten and their skins were never used, as their role in the family was more akin to hunting partner or companion (33). Ethnographic accounts and recent genetic studies both suggest that L. culpaeus underwent self-domestication through the construction of a new ecological niche shared with humans (33, 36). While D. australis was not closely related to L. culpaeus, its closest relative, D. avus, is the most commonly identified canid in Fuego-Patagonian archaeological sites until its extinction by ~400 BP (3, 4, 34). At the Loma de los Muertos mortuary site, a D. avus individual (2792 ± 50 14C BP) was found to be the primary subject of an interment, which suggests an intimate and mutualistic interspecies relationship (35). It has been suggested that the warrah may have been a semi-domesticated form of D. avus (1), which could explain the observed docile and curious nature of the warrah described by Darwin and others, as well as some of the warrah’s physical traits (2, 37).
When combined with the long history of human marine adaptation, semi-domestication of the warrah in mainland South America could help explain its presence in the Falkland Islands. The δ15N values reported here reflect a marine diet at higher trophic levels, including seals, which the small canid was unlikely to have hunted. While the warrah may have been a selective coastal scavenger, their enriched δ15N values could also have resulted from a mutualism with humans who were primarily subsisting on sea lions, penguins, and other marine predators (21). Both pre- and post-contact hunter-gatherers from Tierra del Fuego had isotopic values (δ13C = −12.8, δ15N = 17.2; Fig. 5), reflecting their specialization on marine resources from high tropic levels (38, 39). In contrast, hunter-gatherers in the more interior Pampas region of northern Argentina exhibited much lower values (δ13C = −17.4, δ15N = 11.9; Fig. 5) associated with more terrestrial diets (40). While the dietary isotopic signatures of the warrah samples do not unequivocally prove human introduction, neither do they rule out a mutualism with people. Future studies of warrah remains collected during the European period, when abundant terrestrial food sources (e.g., sheep, cattle, horses, rabbits, pigs, rats, and mice) had been introduced to the islands, may provide further insight into the warrah’s dietary preferences and potential interaction with humans.
Ecological changes follow closely on the heels of human arrival, particularly in sensitive island ecosystems (although it is important to note that these impacts are not necessarily negative) (41). Although our study did not directly quantify human impacts on native flora and fauna, our findings raise questions about the influence of fire, hunting, or the arrival of the warrah on the Falkland Islands’ flora and fauna. Oceanic islands are often hotspots of biodiversity, and many suffered extensive extinctions throughout the Holocene, which began during initial human settlement and accelerated during European colonization (42). Ground-nesting birds were hit especially hard by Holocene extinctions, particularly because of the introduction of predators (42, 43), so resolving the timing and origin of the warrah is especially relevant. Today, the Falkland Islands are globally important breeding grounds for many species of ground-nesting seabirds that are threatened by multiple global changes, including sea level rise, habitat loss, and climate change (44). Further analysis of vertebrate fossil deposits, sedimentary archives (including ancient DNA), and historic museum specimens will be critical for establishing historic biodiversity baselines and assessing the long-term resilience of Falkland Islands biodiversity to global changes (16).
A pre-European human presence in the Falkland Islands broadens our understanding of the human history of not only the Falkland Islands but also the South Atlantic more generally and highlights the skill and adaptability of southern South America’s adept seafaring peoples. New Island has many features that would have been attractive to seafarers (a sheltered sandy cove, fresh water, seabird, and pinniped colonies; Fig. 1); these are shared with many other islands in the Falkland Islands and elsewhere. The highly mobile lifestyles and material cultures of people such as the Yaghan may mask their presence in the archaeological record, limiting our ability to fully recognize the complex histories of human migration. Our work supports interdisciplinary investigations where conspicuous evidence of human activity remains absent and highlights the utility of paleoecological proxies in identifying the indirect signatures of initial, ephemeral, or nomadic peopling. Future research may yield new potential sites in the Falkland Islands and beyond. Ideally, this work should be done in collaboration with regional Indigenous communities, whose oral histories and traditional ecological knowledge represent millennia of expertise in the prehistory of Tierra del Fuego and the South Atlantic.
MATERIALS AND METHODS
Site description
The Falkland Islands are located in the South Atlantic Ocean (51°42′S, 57°51′W), north of the Antarctic Convergence (Fig. 1). The archipelago is made up of two large islands (East and West Falkland) and 776 smaller outlying islands. The Falkland Islands lie between the temperate zone that characterizes the southernmost part of South America and the Antarctic polar environment that characterizes many sub-Antarctic islands. The islands have lacked native tree species since at least the Pliocene. The inland vegetation is dominated by Cortaderia pilosa (whitegrass) and Empetrum rubrum (diddle-dee). Poa flabellata (tussac) grasslands fringe the coasts of larger islands and completely cover many smaller islands (45). P. flabellata grows up to 3.5 m in height on pedestals and forms abundant peat (45). Tussac grasslands provide critical habitat and shelter for many ground-nesting seabirds and South American sea lions (45). Falkland Islands’ tussac peat provides a high-resolution record of past environments (46).
New Island is the westernmost island in the Falkland Islands archipelago. Because of its position in the nutrient-rich Falkland Current, the island was one of the first to be heavily used by European and American sealers arriving as early as 1770 (47). Elevation on New Island ranges from 0 to 244 m. Sheer cliffs dominate the western margins of the island, whereas the eastern shores are characterized by lower elevations with deep bays that shelter sandy beaches from the persistent Westerly winds (47). The island supports the largest global population of South American fur seals (Arctocephalus australis), South American sea lions (O. flavescens), black-browed albatross (Thalassarche melanophris), gentoo penguins (Pygoscelis papua), rockhopper penguins (E. chrysocome), and rock shags (Phalacrocorax magellanicus), as well as many other smaller seabird populations, natural fresh water sources, and driftwood-collecting beaches that provide the natural source of firewood on the islands (Fig. 1). New Island bedrock is made of the Port Stephens Formation, which is primarily characterized by quartzite (10). Similar to many islands here, New Island was subject to historic sheep grazing, and many parts of the island have undergone subsequent erosion. There are several wetter areas on the island that have retained a >2-m layer of peat. Bleaker Island is a low-lying island (peak elevation, 27 m) to the south of East Falkland (Fig. 1), characterized by low cliffs on its eastern shores interspersed with sand and pebble beaches, as well as low-lying western shores and pebble beaches. The island has several natural freshwater sources, breeding populations of rockhopper, gentoo and Magellanic penguins, king and rock cormorants, and sea lions. Sheep have grazed the island for over a century, resulting in the loss of much of the native tussac grass. Several low areas have maintained deep peat deposits that are several meters deep. Mount Usborne is the highest point in the Falkland Islands at 705 m above mean sea level (AMSL). It is located in the western interior of East Falkland and shows evidence of cirque glaciation (48). The ground is primarily mixed boggy terrain with intermittent large stone runs (10). This heavily exposed, high-elevation, interior location has no history of human habitation and is unlikely to have supported prehistoric human populations, as food resources are concentrated on the coasts. We thus interpret this record as the climatic null model for the natural fire regime of the Falkland Islands.
Core acquisition, description, and sampling
Sediment columns were collected from New Island (223 cm; 51°43′09.5″S, 61°18′20.2″W; 12 m AMSL) and Bleaker Island (208 cm; 52°13′02.6″S, 58°51′35.0″W; 21 m AMSL) during January and February 2016. Column sections were extracted as 10-cm square blocks, sampled continuously from the surface of hand-dug exposures using trowels and shovels. Each column ended in light gray clay at the bottom. Column sections were measured and described in the field, wrapped in plastic wrap and aluminum foil, and taken to the National Lacustrine Core Facility at the University of Minnesota, where they were imaged with high-resolution photography. Column blocks were brought to the University of Maine for analysis, sliced at 1-cm intervals, and stored at 4°C in gallon Ziploc bags for later subsampling. In March 2016, a peat core was collected from Mount Usborne (280 cm; 51°42′14.0″S, 58°48′50.6″W) with a square-rod piston corer. The core was measured and described in the field, wrapped in plastic wrap and encased in polyvinyl chloride tubing, and stored at 4°C at the University of Maine.
Core chronology
We selected three terrestrial plant macrofossils from the Bleaker Island peat column, six terrestrial plant macrofossils from the New Island peat column, and five terrestrial plant macrofossils from the Mount Usborne peat core for AMS radiocarbon dating. All but two terrestrial plant samples were processed at the University of California Irvine Keck Laboratory (table S2). Two samples from the Mount Usborne core were processed at the National Ocean Sciences Accelerator Mass Spectrometry facility (table S2, OS). Calibrated age ranges were identified using Calib 8.2 and the SHCal20 calibration curve (49, 50). Column chronologies were produced using the R package “clam” for classical age depth modeling (figs. S2 to S4) (51). Models were produced using linear interpolation between dated levels.
Charcoal
Subsamples (1 cm3) were taken at contiguous 1-cm intervals from the New Island, Bleaker Island, and Mount Usborne cores and processed for charcoal using a modification by Whitlock and Larsen (52). Samples were first treated with 7% H2O2 at 50°C for 24 hours to disaggregate sediments and bleach organic matter. Samples were then screened through 125-μm sieves, and the collected material was rinsed into plastic petri dishes with a few drops of 7% H2O2 and placed in the drying oven at 50°C until all liquid was evaporated (minimum, 24 hours). Processed petri dishes were then placed on gridded platforms, and individual charcoal fragments were counted under a stereomicroscope (52).
Raw charcoal concentrations (particles/cm3) were converted to CHAR (particles/cm2/year) by multiplying concentrations by the sedimentation rate (years cm−3) established from the age-depth models. Charcoal data were separated into background and peak components using CharAnalysis version 1.1 for MATLAB (53). CHAR values were interpolated to 20-year time steps to account for uneven sampling intervals caused by variations in sedimentation rates or core gaps. The background influx of charcoal was estimated using a robust 500-year Lowess smoothing window. CharAnalysis identified peaks using a locally fitted Gaussian mixture model and then determined whether those peaks differed significantly from the smallest non-significant peak sample in the preceding five samples by using a two-sample Poisson test based on the presmoothed charcoal concentrations (particles/cm3) (53).
Loss on ignition
Subsamples (1 cm3) were taken for loss-on-ignition analysis at contiguous 1-cm intervals from the New Island, Bleaker Island, and Mount Usborne cores and processed following the work of Heiri et al. (54). Samples were weighed in ceramic crucibles (wet weight) and heated in a muffle furnace at 100°C for 24 hours, 550°C for 4 hours, and 925°C for 2 hours. Each sample was weighed after each burn to calculate the percent weight lost. Loss on ignition is interpreted as representing the relative fractions of organic carbon (550°C), carbonates (925°C), and minerals (remaining fraction).
Radiocarbon dating and isotopic analysis of bone
Seventeen bone and tooth samples were processed for AMS radiocarbon dating at the University of California Irvine Keck Laboratory using ultrafiltration methods outlined by Beaumont et al. (55) on collagen from bone and tooth samples (tables S1 and S2). D. australis bones were calibrated using the Marine20 calibration curve (56) and SHCal20 curve (49) in Calib 8.2 (50) (table S1). To establish a marine reservoir correction for O. flavescens bone dates, we also submitted four samples from Denver Museum of Nature and Science specimens collected in 1926 from the Santa Cruz Provence in Argentina. The resulting Δ_R_ value of −10 ± 33 was determined by using the Marine Reservoir Correction tool provided in Calib 8.2 (50). This correction age and the Marine20 calibration curve (56) were applied to all O. flavescens and D. australis samples (table S1). Probability distribution curves and a sum probability distribution for O. flavescens samples were determined using OxCal (Fig. 4) (57). All dates reported in the text represent calibrated calendar years before present (1950).
Archaeological survey, excavation, and determination of MNI
We conducted a two-person pedestrian survey of New Island. Although not comprehensive because of the extent of the island and our limited personnel numbers, we identified resource locations including fresh water, seabird and pinniped colonies, driftwood accumulation sites, bone piles, and artifact locations (Fig. 1). We identified seven bone piles on the north end of New Island and excavated two of the piles in February 2018 (NEWIBP6 and NEWIBP7). The disarticulated bones were in a mixed sandy peaty matrix overlaying a dense clay. Bones were examined for surface modifications and breakage features, and care was taken to avoid any marking of bone surfaces during excavation. All sediment was screened through ¼-inch wire mesh to isolate small bone fragments and lithic material. O. flavescens skulls, scapulae, and mandibles were classified by size to assess age and sex in the determination of MNI for each pile (table S3). E. chrysocome crania, sterna, and caudal vertebrae were used to determine the MNI for each pile. Because NEWIBP6 lacked stratigraphic differentiation, elements were lumped for MNI calculation while the NEWIBP7 contained three visible strata from which MNI were derived. Each bone pile was characterized by limited fragmentation. None of the bird specimens showed evidence of tooth marks or digestion that would indicate that they were present in the pile as a result of having been ingested by the individual sea lions present in the piles.
Acknowledgments
We are grateful to the Falkland Islanders who lent expertise and graciously allowed access to field sites and samples: the Evans, Strange, Rendell, Smith, and Rowland families and S. Massam. We thank the Denver Museum of Nature and Science for providing historic O. flavescens samples. The South Atlantic Environmental Research Institute, New Island Conservation Trust, Falkland Islands Museum and National Trust, and Tony Smith of Discovery Falklands for providing invaluable logistical support. Columns were scanned at LacCore (National Lacustrine Core Facility), Department of Earth Sciences, University of Minnesota-Twin Cities. A. Cassels created the three-dimensional model of the stone point. We are grateful to three anonymous reviewers whose comments greatly improved this manuscript.
Funding: This material is based on work supported by the NSF Graduate Research Fellowship under grant no.1840992 to K.M.H., an Explorer’s Club Student Research Grant, a Rolex/Explorer’s Club grant, the Dan and Betty Churchill Exploration Fund, and >200 crowdfunders on experiment.com. J.L.G. was supported by NSF CAREER grant EAR-1753186.
Author contributions: K.M.H., J.L.G., and D.H.S. conceptualized this study. K.M.H., J.L.G., D.V.G., K.E.K., J.R.S., T.V.L., and B.L.H. conducted field and laboratory analyses. K.M.H., J.L.G., D.V.G., K.E.K., B.L.H., D.H.S., J.R.S., P.B., and T.V.L. contributed to the interpretation and wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper (LOI, charcoal, radiocarbon dates, and δ15N and δ13C isotopes) are present in the paper, the Supplementary Materials, and the Neotoma Paleoecology Database (www.neotomadb.org/).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S4
Tables S1 to S3
References
REFERENCES AND NOTES
- 1.Clutton-Brock J., Man-made dogs. Science 197, 1340–1342 (1977). [DOI] [PubMed] [Google Scholar]
- 2.C. Darwin, Journal of Researches During the Voyage of HMS "Beagle" (Collins, 1860).
- 3.Prevosti F. J., Ramírez M. A., Schiaffini M., Martin F., Sauthier D. E. U., Carrera M., Sillero-Zubiri C., Pardinas U. F. J., Extinctions in near time: New radiocarbon dates point to a very recent disappearance of the South American fox Dusicyon avus (Carnivora: Canidae ). Biol. J. Linn. Soc. 116, 704–720 (2015). [Google Scholar]
- 4.Austin J. J., Soubrier J., Prevosti F. J., Prates L., Trejo V., Mena F., Cooper A., The origins of the enigmatic Falkland Islands wolf. Nat. Commun. 4, 1552 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Chiessi C. M., Ulrich S., Mulitza S., Pätzold J., Wefer G., Signature of the Brazil-Malvinas Confluence (Argentine Basin) in the isotopic composition of planktonic foraminifera from surface sediments. Mar. Micropaleontol. 64, 52–66 (2007). [Google Scholar]
- 6.Orquera L. A., Piana E. L., Sea nomads of the beagle channel in southernmost South America: Over six thousand years of coastal adaptation and stability. J. Isl. Coast. Archaeol. 4, 61–81 (2009). [Google Scholar]
- 7.Buckland P. C., Edwards K. J., Palaeoecological evidence for possible pre-European settlement in the Falkland Islands. J. Archaeol. Sci. 25, 599–602 (1998). [Google Scholar]
- 8.Philpott R. A., Eshelman R. E., A Fuegian projectile point found on Steeple Jason, Falkland Islands. Falkl. Islands J. 8, 46–58 (2004). [Google Scholar]
- 9.Hattersley-Smith G., Fuegian Indians in the Falkland Islands. Polar Rec. (Gr. Brit). 21, 605–606 (1983). [Google Scholar]
- 10.D. T. Aldiss, E. J. Edwards, The Geology of the Falkland Islands (British Geological Survey, 1999).
- 11.Burney D. A., Burney L. P., MacPhee R. D. E., Holocene charcoal stratigraphy from Laguna Tortuguero, Puerto Rico, and the timing of human arrival on the island. J. Archaeol. Sci. 21, 273–281 (1994). [Google Scholar]
- 12.Grayson D., The archaeological record of human impacts on animal populations. J. World Prehistory. 15, 1–68 (2001). [Google Scholar]
- 13.Gosling W. D., Sear D. A., Hassall J. D., Langdon P. G., Bönnen M. N. T., Driessen T. D., Kemenade Z. R., Noort K., Leng M. J., Croudace I. W., Bourne A. J., McMichael C. N. H., Human occupation and ecosystem change on Upolu (Samoa) during the Holocene. J. Biogeogr. 47, 600–614 (2020). [Google Scholar]
- 14.Mcwethy D. B., Whitlock C., Wilmshurst J. M., McGlone M. S., Fromont M., Li X., Dieffenbacher-Krall A., Hobbs W. O., Fritz S. C., Cook E. R., Rapid landscape transformation in South Island, New Zealand, following initial Polynesian settlement. Proc. Natl. Acad. Sci. U.S.A. 107, 21343–21348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirch P. V., Ellison J., Palaeoenvironmental evidence for human colonization of remote Oceanic islands. Antiquity. 68, 310–321 (1994). [Google Scholar]
- 16.Groff D. V., Hamley K. M., Lessard T. J. R., Greenawalt K. E., Yasuhara M., Brickle P., Gill J. L., Seabird establishment during regional cooling drove a terrestrial ecosystem shift 5000 years ago. Sci. Adv. 6, eabb2788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drago M., Cresp E. A., Aguilar A., Cardona L., Garcia N. A., Dans S. L., Goodall R. N. P., Historic diet change of the South American sea lion in Patagonia as revealed by isotopic analysis. Mar. Ecol. Prog. Ser. 384, 273–286 (2009). [Google Scholar]
- 18.Baylis A. M. M., Orben R. A., Costa D. P., Tierney M., Brickle P., Staniland I. J., Habitat use and spatial fidelity of male South American sea lions during the nonbreeding period. Ecol. Evol. 7, 3992–4002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riccialdelli L., Newsome S. D., Fogel M. L., Goodall R. N. P., Isotopic assessment of prey and habitat preferences of a cetacean community in the southwestern South Atlantic Ocean. Mar. Ecol. Prog. Ser. 418, 235–248 (2010). [Google Scholar]
- 20.Vander Zanden M. J., Rasmussen J. B., Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 46, 2061–2066 (2001). [Google Scholar]
- 21.Guiry E. J., Dogs as analogs in stable isotope-based human paleodietary reconstructions: A review and considerations for future use. J. Archaeol. Method Theory 19, 351–376 (2012). [Google Scholar]
- 22.Gallagher R. E., Byron’s journal of his circumnavigation 1764-1766. Hakluyt Soc. 64, 742–744 (1964). [Google Scholar]
- 23.Shackleton L., The Falkland Islands and their history. J. Geogr. 149, 1–4 (1983). [Google Scholar]
- 24.I. J. Strange, New Island, Falkland Islands: A South Atlantic Wildlife Sanctuary for Conservation Management (Design in Nature, 2007).
- 25.Lamy F., Kilian R., Arz H. W., Francois J. P., Kaiser J., Prange M., Steinke T., Holocene changes in the position and intensity of the southern westerly wind belt. Nat. Geosci. 3, 695–699 (2010). [Google Scholar]
- 26.J. Turner, S. Pendlebury, The International Antarctic Weather Forecasting Handbook (British Antarctic Survey, 2004).
- 27.WAIS Divide Project Members , Precise interpolar phasing of abrupt climate change during the last ice age. Nature 520, 661–665 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Bjorck S., Rundgren M., Ljung K., Unkel I., Wallin A., Multi-proxy analyses of a peat bog on Isla de los Estados, easternmost Tierra del Fuego: A unique record of the variable Southern Hemisphere Westerlies since the last deglaciation. Quat. Sci. Rev. 42, 1–14 (2012). [Google Scholar]
- 29.Falklands Conservation, New Island Trust (2020).
- 30.Lyman R. L., Prehistoric seal and sea-lion butchering on the southern Northwest Coast. Soc. Am. Archaeol. 57, 246–261 (1992). [Google Scholar]
- 31.Haynes G., Elephant-butchering at modern mass-kill sites in Africa. Curr. Res. Pleistocene. 4, 75–77 (1987). [Google Scholar]
- 32.Domínguez-Rodrigo M., Yravedra J., Why are cut mark frequencies in archaeofaunal assemblages so variable? A multivariate analysis. J. Archaeol. Sci. 36, 884–894 (2009). [Google Scholar]
- 33.M. Gusinde, F. Schütze, in Die Feuerland-Indianer (The Fuegian Indians) (1937), pp. 365–1185; https://ehrafworldcultures.yale.edu.
- 34.Stahl P. W., Interactions between humans and endemic canids in Holocene South America. Source J. Ethnobiol. 32, 108–127 (2012). [Google Scholar]
- 35.Prates L., Crossing the boundary between humans and animals: The extinct fox Dusicyon avus from a hunter-gatherer mortuary context in Patagonia (Argentina). Antiquity 88, 1201–1212 (2014). [Google Scholar]
- 36.Petrigh R. S., Fugassa M. H., Molecular identification of a Fuegian dog belonging to the Fagnano Regional Museum ethnographic collection, Tierra del Fuego. Quat. Int. 317, 14–18 (2013). [Google Scholar]
- 37.R. E. Gallagher, Byron’s Journal of His Circumnavigation, 1764–1766 (Hakluyt Society, 2017).
- 38.Tafuri M. A., Zangrando A. F. J., Tessone A., Kochi S., Cecchi J. M., Vincenzo F. D., Profico A., Manzi G., Dietary resilience among hunter-gatherers of Tierra del Fuego: Isotopic evidence in a diachronic perspective. PLOS ONE 12, e0175594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yesner D. R., Figuerero Torres M. J., Guichon R. A., Borrero L. A., Stable isotope analysis of human bone and ethnohistoric subsistence patterns in Tierra del Fuego. J. Anthropol. Archaeol. 22, 279–291 (2003). [Google Scholar]
- 40.Politis G. G., Scabuzzo C., Tykot R. H., An approach to pre-hispanic diets in the Pampas during the early/middle Holocene. Int. J. Osteoarchaeol. 19, 266–280 (2009). [Google Scholar]
- 41.Ellis E. C., Gauthier N., Goldewijk K. K., Bird R. B., Boivin N., Díaz S., Fuller D. Q., Gill J. L., Kaplan J. O., Kingston N., Locke H., McMichael C. N. H., Ranco D., Rick T. C., Rebecca Shaw M., Stephens L., Svenning J.-C., Watson J. E. M., People have shaped most of terrestrial nature for at least 12,000 years. Proc. Natl. Acad. Sci. U.S.A. 118, e2023483118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood J. R., Alcover J. A., Blackburn T. M., Bover P., Duncan R. P., Hume J. P., Louys J., Meijer H. J. M., Rando J. C., Wilmshurst J. M., Island extinctions: Processes, patterns, and potential for ecosystem restoration. Environ. Conserv. 44, 348–358 (2017). [Google Scholar]
- 43.Blackburn T. M., Cassey P., Duncan R. P., Evans K. L., Gaston K. J., Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958 (2004). [DOI] [PubMed] [Google Scholar]
- 44.McDowall R. M., Falkland Islands biogeography: Converging trajectories in the South Atlantic Ocean. J. Biogeogr. 32, 49–62 (2005). [Google Scholar]
- 45.Armstrong P. H., Human impact on the Falkland Islands environment. Environmentalist 14, 215–231 (1994). [Google Scholar]
- 46.Payne R. J., Ring-Hrubesh F., Rush G., Sloan T. J., Evans C. D., Mauquoy D., Peatland initiation and carbon accumulation in the Falkland Islands. Quat. Sci. Rev. 212, 213–218 (2019). [Google Scholar]
- 47.I. J. Strange, New Island: Falkland Islands (Design In Nature, 2007).
- 48.Clapperton C. M., Evidence of cirque glaciation in the Falkland Islands. J. Glaciol. 10, 121–125 (1971). [Google Scholar]
- 49.Hogg A. G., Heaton T. J., Hua Q., Palmer J. G., Turney C. S. M., Southon J., Bayliss A., Blackwell P. G., Boswijk G., Bronk Ramsey C., Pearson C., Petchey F., Reimer P., Reimer R., Wacker L., SHCal20 Southern Hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 62, 759–778 (2020). [Google Scholar]
- 50.Stuiver M., Reimer P. J., Extended 14C data base and revised calib 3.0 14C age calibration program. Radiocarbon 35, 215–230 (1993). [Google Scholar]
- 51.Blaauw M., Methods and code for “classical” age-modelling of radiocarbon sequences. Quat. Geochronol. 5, 512–518 (2010). [Google Scholar]
- 52.C. Whitlock, C. P. S. Larsen, in Tracking Environmental Change Using Lake Sediments Vol 3: Terrestrial, Algal and Siliceous Indicators, J. P. Smol, H. J. B. Birks, W. M. Last, Eds. (Kluwer Academic Publishers, 2001), pp. 75–97. [Google Scholar]
- 53.Higuera P. E., Gavin D. G., Bartlein P. J., Hallett D. J., Peak detection in sediment-charcoal records: Impacts of alternative data analysis methods on fire-history interpretations. Int. J. Wildl. Fire. 19, 996–1014 (2010). [Google Scholar]
- 54.Heiri O., Lotter A. F., Lemcke G., Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 25, 101–110 (2001). [Google Scholar]
- 55.Beaumont W., Beverly R., Southon J., Taylor R. E., Bone preparation at the KCCAMS laboratory. Nucl. Instrum. Methods Phys. Res. B 268, 906–909 (2010). [Google Scholar]
- 56.Heaton T. J., Köhler P., Butzin M., Bard E., Reimer R. W., Austin W. E. N., Bronk Ramsey C., Grootes P. M., Hughen K. A., Kromer B., Reimer P. J., Adkins J., Burke A., Cook M. S., Olsen J., Skinner L. C., Marine20—The marine radiocarbon age calibration curve (0–55,000 cal BP). Radiocarbon 62, 779–820 (2020). [Google Scholar]
- 57.Ramsey C. B., Methods for summarizing radiocarbon datasets. Radiocarbon 59, 1809–1833 (2017). [Google Scholar]
- 58.Turney C. S. M., Jones R. T., Fogwill C., Hatton J., Williams A. N., Hogg A., Thomas Z. A., Palmer J., Mooney S., Reimer R. W., A 250-year periodicity in Southern Hemisphere westerly winds over the last 2600 years. Clim. Past. 12, 189–200 (2016). [Google Scholar]
- 59.Henniker-Heaton H., Did Sir Richard Hawkins visit the Falkland Islands? R. Geogr. Soc. 67, 52–57 (1926). [Google Scholar]
- 60.Reimer P. J., Bard E., Bayliss A., Beck J. W., Blackwell P. G., Ramsey C. B., Buck C. E., Cheng H., Edwards R. L., Friedrich M., Grootes P. M., Guilderson T. P., Haflidason H., Hajdas I., Hatté C., Heaton T. J., Hoffmann D. L., Hogg A. G., Hughen K. A., Kaiser K. F., Kromer B., Manning S. W., Niu M., Reimer R. W., Richards D. A., Scott E. M., Southon J. R., Staff R. A., Turney C. S. M., van der Plicht J., IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 55, 1869–1887 (2013). [Google Scholar]
- 61.Hua Q., Barbetti M., Rakowski A. Z., Atmospheric radiocarbon for the period 1950–2010. Radiocarbon 55, 2059–2072 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S4
Tables S1 to S3
References