Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes (original) (raw)
Abstract
Semaglutide, a glucagon like peptide-1 (GLP-1) receptor agonist, is available as monotherapy in both subcutaneous as well as oral dosage form (first approved oral GLP-1 receptor agonist). It has been approved as a second line treatment option for better glycaemic control in type 2 diabetes and currently under scrutiny for anti-obesity purpose. Semaglutide has been proved to be safe in adults and elderly patients with renal or hepatic disorders demanding no dose modification. Cardiovascular (CV) outcome trials established that it can reduce various CV risk factors in patients with established CV disorders. Semaglutide is well tolerated with no risk of hypoglycaemia in monotherapy but suffers from gastrointestinal adverse effects. A large population affected with COVID-19 infection were diabetic; therefore use of semaglutide in diabetes as well as CV patients would be very much supportive in maintaining health care system during this pandemic situation. Hence, this peptidic drug can be truly considered as a quintessential of GLP-1 agonists for management of type 2 diabetes.
Keywords: GLP-1 agonist, Semaglutide, Type 2 diabetes, SUSTAIN, PIONEER, COVID-19
Introduction
Traditionally, glycaemic control has been the primary objective in managing type 2 diabetes mellitus, but multifactorial approaches like optimizing hyperglycaemia, obesity, hypertension, dyslipidaemia and cardiovascular factors is equally important [1–4]. Insulin deficiency and various pathophysiological changes often leads to other metabolic as well as cardiovascular complications in diabetes patients. Despite of various treatment options, a control on glycaemic level is still very challenging in clinical practice without having side effects like hypoglycaemic episodes [5]. Development of recombinant human proteins and glucagon like peptide-1 (GLP-1) receptor agonists has been a beacon of hope for successful management of diabetes.
GLP-1, a major incretin hormone in humans, acts by numerous mechanisms like augmented insulin secretion (glucose-dependent), inhibition of glucagon release and suppressed hepatic gluconeogenesis [6]. It also causes delayed gastric emptying, reduced appetite and energy intake [7, 8]. Reduction of HbA1c level along with body weight without any risk of hypoglycaemia, provides it a special status for the treatment of obese type 2 diabetic patients. However, Dipeptidyl Peptidase-4 (DPP-4) and Neutral Endopeptidase mediated degradation makes it a candidate with short half-life (1–2 min), which is a major obstacle in its therapeutic utility [8]. Therefore, various GLP-1 receptor agonists like dulaglutide, liraglutide, semaglutide were designed to act in the same way as GLP-1 and are less prone to proteolytic degradation, but they suffer from certain gastrointestinal adverse effects like nausea, vomiting and diarrhoea. Various GLP-1 receptor agonists like exendin based therapies (exenatide, lixisenatide) and GLP-1 analogues (liraglutide, semaglutide and dulaglutide) has been used clinically [3, 9–12]. Recent advancements focus on development of GLP-1 agonists which needs less frequent dosing (once weekly) to improve patient adherence and to reduce the treatment burden [13]. Only three GLP-1 agonists- exenatide extended release, semaglutide and dulaglutide (as albiglutide is withdrawn in 2018) are currently approved for once-weekly dosing [14]. Both the subcutaneous as well as oral dosage form of semaglutide have been shown to achieve significant cardiovascular improvements in clinical studies. Semaglutide, developed by Novo Nordisk, has been launched clinically and marketed as Ozempic ® (subcutaneous injection, weekly-once dosing; available in 0.5, 1.0 mg dose) and Rybelsus ® (oral tablets, once-daily dosing; available in 3, 7, 14 mg dose). Both Ozempic and Rybelsus has been approved by USFDA, Health Canada, European Medicines Agency, Japanese Health ministry and is under scrutiny by several other regulatory authorities [15–22].
Development
At Novo Nordisk, concept of peptide modification and albumin binding for systemic protraction has been utilized in the development of weekly-once GLP-1 agonist- semaglutide.
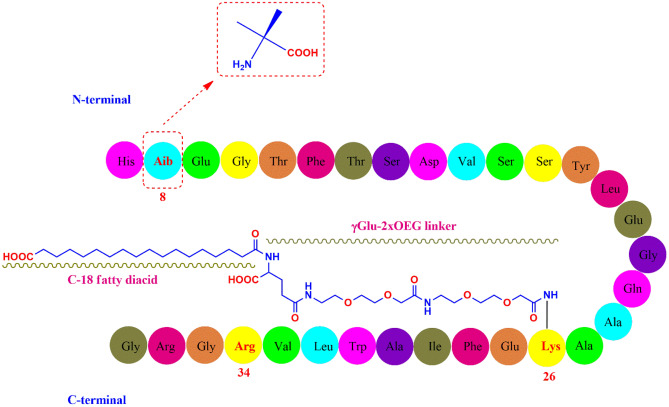
Semaglutide (C187H291N45O59; 4113.58 g/mol) has 31 amino-acid containing peptidic structure (Fig. 1) which is 94% homologous to the native GLP-1 for avoiding immunogenicity [23]. The alanine residue at 8th position is substituted with Aib (2-aminoisobutyric acid) which protects semaglutide from enzymatic degradation by DPP-4 [24]. Similar to liraglutide, the lysine residue at 34th position in native GLP-1 was replaced by arginine in semaglutide too. The arginine substitution at 34th position was helpful in production of GLP-1 analogue through semi-recombinant process [25]. Lysine residue at 26th position was acylated for attachment with a C18 fatty diacid through a hydrophilic linker “γGlu-2xOEG”, which prolonged the systemic half-life through enhanced albumin binding and reduced renal clearance [26].
Fig. 1.
Structure of Semaglutide
Based on the pharmacokinetic studies on Göttingen mini pig model (for moderate insulin deficiency & diabetes) and in vivo efficacy in anti-hyperglycaemic and body weight lowering effect on obese, hyper-insulinemic db/db mice model (for type 2 diabetes), semaglutide was chosen to be a clinical trial candidate [27].
Clinical pharmacology
Mechanism
Semaglutide improves the efficiency of incretin function by activating GLP-1 receptors. It acts by numerous mechanisms like augmented insulin secretion (glucose-dependent), inhibition of glucagon release and suppressed hepatic gluconeogenesis; thereby reducing both fasting as well as postprandial glucose [26]. Semaglutide has shown favorable proinsulin to insulin ratio which suggests improved efficiency of β-cell functioning and augmented production of insulin [28, 29]. It also exhibited improved insulin sensitivity which is likely to be mediated by overall reduction in body weight [30]. Semaglutide also exhibits weight loss which is attributed to reduced energy intake, delay in gastric motility [31].
Mode of administration & posology
Subcutaneous route:
Unlike other weekly-once GLP-1 agonists, dose titration is necessary for weekly-once s.c. semaglutide (Ozempic; 0.5, 1.0 mg). Therapy is initiated with 0.25 mg, then raised to 0.5 mg and 1.0 mg keeping 4 weeks interval. Ozempic pen (red and blue) is available in the strength of 1.34 mg/ml. The red pen delivers 0.25 mg initiation dose and 0.5 mg for maintenance purpose; blue pen is meant for 1.0 mg (maximum maintenance dose) semaglutide [32–34].
Oral route:
In oral dosage form (Rybelsus; 3, 7, 14 mg), semaglutide has been co-formulated with a fatty acid derivative- SNAC (Sodium-N-[8-(2-hydroxybenzoyl)amino]caprylate; Eligen® Technology, Emisphere Technologies), which acts as permeation enhancer. It increases solubility of semaglutide by increasing pH of the local environment and use passive transcellular route to cross cell membranes [29]. It also protects semaglutide against proteolytic degradation at acidic pH of stomach. The tablet has to be swallowed in empty stomach preferably 30 min prior to meal [35, 36].
Transition between Ozempic and Rybelsus: Depending on the patient convenience and requirement, they can be switched from Ozempic to Rybelsus and vice versa [33, 35].
Various pharmacokinetic parameters of both s.c. as well as oral semaglutide have been presented in Table 1.
Table 1.
Pharmacokinetics of semaglutide [33–36]
| Characteristics | Semaglutide (s.c. injection) | Semaglutide (oral) |
|---|---|---|
| Absorption | ||
| Absolute bioavailability | 89% | 0.4–1% |
| Steady state plasma conc | 65 ng/ml (0.5 mg weekly once) | 6.7 nmol/L (7 mg once daily) |
| 123 ng/ml (1 mg weekly once) | 14.6 nmol/L (14 mg once daily) | |
| Time to achieve steady state conc | 4–5 weeks | 4–5 weeks |
| Time to achieve maximum conc | 1–3 days | 01 h |
| Distribution | ||
| Volume of distribution | 12.5 Litres | 8 Litres |
| Protein binding | > 99% | > 99% |
| Metabolic pathway | Proteolytic degradation followed by fatty acid oxidation | Proteolytic degradation followed by fatty acid oxidation |
| Elimination profile | ||
| Elimination t1/2 | 01 week | 01 week |
| Rate of clearance | 0.05 Litres/Hr | 0.04 L/Hr |
Clinical trials for glycaemic control
Phase-I studies
NCT02060266:
Absorption, distribution, metabolism of semaglutide was studied in the blood, plasma, urine, faeces of 7 healthy males (after receiving tritium-labelled (450 μCi/16.7 MBq) 0.5 mg s.c. semaglutide) and compared with that of monkey, rats. Both were found to have similar profile of metabolites and excretory routes [23].
NCT02210871:
A study, conducted on 44 subjects (19 normal, 8 mildly impaired, 10 moderately impaired and 7 with severe hepatic impairment) taking s.c. semaglutide (0.5 mg) proved that hepatic impairment has no apparent effect on its efficacy and needs no modification in dose [37].
NCT02147431:
A placebo-controlled trial (38 type 2 diabetics of either gender received weekly-once s.c. semaglutide) established the safety profile of semaglutide and shown that it has no effect on glucagon action [38].
A placebo controlled, dose escalating trial established various pharmacokinetics and dynamics parameters of oral semaglutide (prepared with SNAC), carried out on 2 groups (6 healthy males in each). Dose dependent increase in plasma concentration of semaglutide was observed along with augmented insulin release. Dose dependent inhibition of ghrelin (hunger hormone) secretion was also observed causing suppression of appetite [39].
NCT01037582:
The first-in-human, single dose, safety study of oral semaglutide was conducted on 135 healthy male persons, who received 2–20 mg of oral semaglutide (2–20 mg) prepared with SNAC (150–600 mg). The optimal dose of SNAC was found to be 300 mg for maximum semaglutide absorption and no safety/tolerability concerns were observed [40].
NCT01686945:
A 10-week long, placebo-controlled trial investigated the safety, tolerability, pharmacokinetics and dynamic effects of oral semaglutide on 107 male subjects (84 healthy and 23 type 2 diabetics). Oral semaglutide was found to be safe and effective for weekly-once dosing with a half-life of 07 days [40].
NCT01572753:
A trial was conducted to assess the outcome of taking food after semaglutide as well as the effect of volume of water taken with oral semaglutide. Participants (158 healthy males) received oral semaglutide with 50-, or 120-ml water and in combination with different after-dose fasting period (15, 30, 60, 120 min). Effective plasma concentration can be achieved by oral intake of semaglutide with 120 ml water in fasting state, followed by fasting for another half an hour [41].
NCT01619345:
A pharmaco-scintigraphic trial was carried out on 24 healthy male subjects to investigate the effect of volume of water taken with oral semaglutide in fasting state on the site of absorption (stomach or proximal small intestine). The site of tablet erosion as well as absorption of semaglutide was found to be stomach. Non-clinical studies carried out on pyloric ligated as well as non-ligated Beagle dogs also supported these results [42].
NCT02172313:
Effect of food on oral semaglutide’s pharmacokinetics was investigated on 78 healthy subjects, who received either once-daily oral semaglutide after a meal, or same dosage in fasting state for 10 days. Results suggested that administration of semaglutide in fasting state is highly essential to achieve therapeutic concentration [43].
NCT02014259:
A multiple-dose trial was conducted on 71 patients (24 healthy, 12 mild, 12 moderate, 12 severe, and 11 with end-stage kidney damage), who received daily-once 5 mg oral semaglutide for 5 days, followed by 10 mg for next 5 days. Dose modification was not necessary as kidney impairment didn’t affect pharmacokinetics of oral semaglutide [44].
NCT02016911:
Another multiple dose trial was conducted on 56 patients (24 normal, 12 mild, 12 moderate, and 8 with severe hepatic damage), who received daily-once 5 mg oral semaglutide for 5 days, followed by 10 mg for next 5 days. The study demonstrated that dose modification is not warranted in case of hepatic impairment [45].
Phase-II studies
NCT02461589:
A 26-week long, dose finding study on 705 subjects compared the efficacy of semaglutide with liraglutide and placebo. Patients were divided into 12 treatment arms (2:2:1) to receive daily-once s.c. semaglutide (0.05, 0.1, 0.2, 0.3 mg), or liraglutide (0.3, 0.6, 1.2, 1.8 mg) or placebo (50, 100, 200, 300 μL). An additional 13th arm explored more flexible semaglutide dosing, where the effects were similar to 0.2 mg semaglutide. Significant reduction in HbA1c and body weight was observed in semaglutide and liraglutide as compared to placebo. Dose–response modelling study revealed that 0.06 mg of semaglutide was equivalent to 1.8 mg of liraglutide [46].
NCT00696657:
A 12-week long trial was carried out on 415 subjects to find the optimal dose of semaglutide. Patients received weekly-once s.c. semaglutide of variable doses ranging from 0.1 to 0.8 mg without dose escalation, or doses of 0.4, 0.8, 1.6 mg with dose escalation, or 1.2 mg and 1.8 mg doses of daily-once liraglutide, or placebo. Significant reduction in HbA1c, fasting plasma glucagon levels and body weight was exhibited by semaglutide, thus 0.5 mg and 1.0 mg dose was selected to be optimal for phase-3 trials [47].
NCT01923181:
A 26-week long, dose finding study was carried out on 632 subjects with five different doses of daily-once 2.5 mg, 5 mg, 10 mg, 20 mg, and 40 mg oral semaglutide. Effect of oral semaglutide (40 mg) was also compared with 1.0 mg s.c. semaglutide and placebo with slow escalation (8-weeks) or fast escalation (2-weeks). A significant fall in HbA1c was exhibited by oral as well as s.c. semaglutide against placebo. Dose-dependent decrease in body weight was reported with oral semaglutide of 10 mg and higher doses. Significant fall in HbA1c, FPG along with weight loss was reported in s.c. as well as 40 mg oral semaglutide. Interestingly, weekly-once s.c. semaglutide (1 mg) was more effective than ≤ 10 mg daily-once oral semaglutide [48].
Phase-III studies
Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) trials were meant for studying weekly-once s.c. semaglutide along with active or placebo comparators (trial design presented in Table 2).
Table 2.
Study design and results of SUSTAIN trials
| SUSTAIN | Clinical trial identification number | Population | Duration(weeks) | Background medication | Semaglutide trial dose & Comparator | % HbA1c reduction | Weight loss (kg) |
|---|---|---|---|---|---|---|---|
| 1 | NCT02054897 | 388 | 30 | None | 0.5 mg | -1.45 | -3.73 |
| 1.0 mg | -1.55 | -4.53 | |||||
| Placebo | -0.02 | -0.98 | |||||
| 2 | NCT01930188 | 1231 | 56 | Metformin and/or Pioglitazone/Rosiglitazone | 0.5 mg | -1.3 | -4.3 |
| 1.0 mg | -1.6 | -6.1 | |||||
| Sitagliptin (100 mg) | -0.5 | -1.9 | |||||
| 3 | NCT01885208 | 813 | 56 | Metformin and/or Thiazolidinediones and/or Sulfonylurea | 1.0 mg | -1.5 | -5.6 |
| Exenatide ER (2.0 mg) | -0.9 | -1.9 | |||||
| 4 | NCT02128932 | 1089 | 30 | Metformin alone or with Sulfonylurea | 0.5 mg | -1.21 | -3.74 |
| 1.0 mg | -1.64 | -5.71 | |||||
| Insulin glargine | -0.83 | +1.15 | |||||
| 5 | NCT02305381 | 397 | 30 | Basal insulin alone or with Metformin | 0.5 mg | -1.4 | -3.7 |
| 1.0 mg | -1.8 | -6.4 | |||||
| Placebo | -0.1 | -1.4 | |||||
| 6 | NCT01720446 | 3297 (83% with CV and/or CKD) | 104 | None | 0.5 mg | -1.1 | -3.6 |
| 1.0 mg | -1.4 | -4.9 | |||||
| Placebo (0.5 mg) | -0.4 | -0.7 | |||||
| Placebo (1.0 mg) | -0.4 | -0.5 | |||||
| 7 | NCT02648204 | 1201 | 40 | Metformin | 0.5 mg | -1.5 | -4.6 |
| 1.0 mg | -1.8 | -6.5 | |||||
| Dulaglutide (0.75 mg) | -1.1 | -2.3 | |||||
| Dulaglutide (1.5 mg) | -1.4 | -3.0 | |||||
| 8 | NCT03136484 | 788 | 52 | Metformin | 1.0 mg | -1.5 | -5.3 |
| Canagliflozin (300 mg) | -1.0 | -4.2 | |||||
| 9 | NCT03086330 | 302 | 30 | SGLT-2 inhibitor alone or with Sulfonylurea/Metformin | 1.0 mg | -1.5 | -4.7 |
| Placebo | -0.1 | -0.9 | |||||
| 10 | NCT03191396 | 577 | 30 | Metformin and Sulfonylurea/SGLT-2 inhibitor | 1.0 mg | -1.7 | -5.8 |
| Liraglutide (1.2 mg) | -1.0 | -1.9 | |||||
| 11 | NCT03689374 | 2275 | 52 | Metformin alone or with Sulfonylurea/Meglitinide/DPP-4 inhibitor/α-glucosidase inhibitor | 1.0 mg | - | - |
| Insulin aspart (4 IU) | - | - | |||||
| Japan-sitagliptin | NCT02254291 | 308 | 30 | None | 0.5 mg | -1.9 | -2.2 |
| 1.0 mg | -2.2 | -3.9 | |||||
| Sitagliptin (100 mg) | -0.7 | 0.0 | |||||
| Japan | NCT02207374 | 601 | 56 | None | 0.5 mg | -1.7 | -1.4 |
| 1.0 mg | -2.0 | -3.2 | |||||
| OAD monotherapy | -0.7 | +0.4 | |||||
| China-MRCT | NCT03061214 | 868 | 30 | Metformin | 0.5 mg | -1.4 | -2.9 |
| 1.0 mg | -1.7 | -4.2 | |||||
| Sitagliptin (100 mg) | -0.9 | -0.4 | |||||
| FORTE | NCT03989232 | 961 | 40 | Metformin alone or with Sulfonylurea | Trial product estimand | Treatment policy estimand | |
| 1.0 mg | -1.9%, 6.0 kg | -1.9%, 5.6 kg | |||||
| 2.0 mg | -2.2%, 6.9 kg | -2.1%, 6.4 kg | |||||
| Placebo | - | - |
SUSTAIN-1:
The study population received either weekly-once s.c. semaglutide of 0.5 mg (128) or 1.0 mg (130), or placebo (129) for 30 weeks through a prefilled PDS290 pen-injector. Both the doses of semaglutide significantly decreased the mean FPG, C-peptide, homeostasis model assessment of β-cell function (HOMA-B) along with HbA1c and body weight against placebo. Apart from gastro-intestinal adverse effects, the safety, efficacy was good [49].
SUSTAIN-2:
A study conducted on 1225 type 2 diabetics (received weekly-once 0.5 mg s.c. semaglutide + daily-once oral placebo, or weekly-once 1.0 mg s.c. semaglutide + daily-once oral placebo, or daily-once 100 mg oral sitagliptin + weekly-once 0.5 mg s.c. placebo, or daily-once 100 mg oral sitagliptin + weekly-once 1.0 mg s.c. placebo for 56 weeks) proved that a significant reduction in HbA1c and body weight was exhibited by semaglutide 0.5 mg and 1.0 mg over sitagliptin [50].
SUSTAIN-3:
A study conducted on 813 subjects, who received 1.0 mg weekly-once s.c. semaglutide or 2.0 mg weekly-once s.c. exenatide ER (extended release) proved the superiority of semaglutide (associated with significant drop in HbA1c and body weight, improved lipid profile, reduced fasting insulin, proinsulin, plasma glucagon and insulin resistance) [28].
SUSTAIN-4:
Effectiveness of semaglutide and insulin glargine was compared on 1089 subjects, who received either weekly-once 0.5 mg s.c. (362), or 1.0 mg s.c. semaglutide (360) and daily-once s.c. insulin glargine, initially 10 IU/day, followed by dose titration (360). A significant reduction of HbA1c and weight loss was achieved with both doses of semaglutide against insulin glargine. Gastrointestinal disorders and skin problems (rashes, pruritus, urticaria) were more frequent with semaglutide and insulin glargine respectively. Four deaths (3 cardiovascular, 1 pancreatic carcinoma) in 0.5 mg semaglutide and two cardiovascular deaths in insulin treated population were reported [51].
SUSTAIN-5:
Another study on 397 type 2 diabetics, who received either weekly-once s.c. semaglutide 0.5 mg (132), or 1.0 mg (131), or placebo (133) proved the superiority of semaglutide in reduction of HbA1c and body weight. Four cases of neoplasm (0.5 mg semaglutide), a malignant neoplasm and a metastatic pancreatic cancer (1.0 mg semaglutide) was also reported [52].
SUSTAIN-6:
An event-driven trial, conducted on 3297 subjects (83% have cardiovascular and/or renal impairment), reported 26% fall in cardiovascular incidents in semaglutide as it checks the progress of atherosclerosis. Participants received either weekly-once 0.5 mg s.c. semaglutide (826), or 1.0 mg (822), or 0.5 mg placebo (824), or 1.0 mg placebo (825) for 104 weeks. Primary outcomes (cardiovascular death, non-fatal stroke/myocardial infarction incidents) were exhibited by 6.6% population of semaglutide. Significant reduction in HbA1c and body weight was observed in semaglutide along with side effects like retinopathy, nephropathy, and acute pancreatitis [53].
SUSTAIN-7:
This study involved 1201 subjects receiving semaglutide 0.5 mg (301), or dulaglutide 0.75 mg (299), or semaglutide 1.0 mg (300), or dulaglutide 1.5 mg (299). Clinically superior reduction in HbA1c and weight was observed in both the semaglutide groups than dulaglutide. Total 11 malignant neoplasms, 9 diabetic retinopathy cases and 6 deaths (one from each dose of semaglutide, and two from each dose of dulaglutide) were reported [54].
SUSTAIN-8:
Effectiveness of semaglutide in reducing HbA1c and body weight over canagliflozin (SGLT-2 inhibitor) was proved by this study on 788 participants receiving weekly-once s.c. semaglutide 1.0 mg, or daily-once oral canagliflozin 300 mg (394 in each group). One death (unrelated to semaglutide treatment) and few cases of retinopathy were reported [55].
A sub-study on 178 patients (88 received weekly-once s.c. 1.0 mg semaglutide and 90 received daily-once oral 300 mg canagliflozin) for 52 weeks involved dual energy X-ray absorptiometry (DXA) scanning. Although improvement in body fat composition was reported by both the groups, but no significantly different changes were observed between semaglutide and canagliflozin [56].
SUSTAIN-9:
This trial compared the efficacy of semaglutide when used along with SGLT-2 inhibitor by taking 302 participants receiving weekly-once 1.0 mg s.c. semaglutide (151) or placebo (151) with a fixed dose escalation period. Semaglutide treatment in conjunction with SGLT-2 inhibitor led to significant reduction in HbA1c and body weight. Mild retinopathy and neoplasms (unrelated to treatment) were reported [57].
SUSTAIN-10:
Effectiveness between semaglutide and liraglutide was studied on 577 participants receiving weekly-once s.c. 1.0 mg semaglutide (290), or daily-once s.c. 1.2 mg liraglutide (287) with dose escalation. Although, retinopathy and neoplasms were reported in both the groups, superiority of semaglutide over liraglutide was established [58].
SUSTAIN-Japan-sitagliptin:
Superior effectiveness (reduction in HbA1c, body weight, FPG, SMBG) of semaglutide over sitagliptin was established in this trial where 308 Japanese subjects received weekly-once s.c. 0.5 mg semaglutide (103) or 1.0 mg (102) or daily-once 100 mg oral sitagliptin (103). One malignant neoplasm and 6 cases of retinopathy reported [59].
SUSTAIN-Japan:
This trial assessed the superior efficacy of semaglutide in reducing HbA1c and body weight over oral antidiabetic drug (OAD) monotherapy, where 601 Japanese subjects received weekly-once s.c. 0.5 mg semaglutide (239), or 1.0 mg semaglutide (241), or OAD (74 DPP-4 inhibitor, 31 biguanide, 7 sulfonyl urea, 4 thiazolidinedione, 3 α-glucosidase inhibitor, 1 glinide). Benign colorectal neoplasms, an event of heart failure, a coronary revascularization, 6 cases of cholelithiasis including 1 death (non-cardiovascular) were reported in semaglutide group [60].
SUSTAIN-China-MRCT:
Superior effectiveness (reduction in HbA1c, body weight, FPG, SMBG, β-cell glucose sensitivity and insulin resistance) of semaglutide over sitagliptin was established in this trial where 868 subjects received 0.5 mg semaglutide + placebo (287), or 1.0 mg semaglutide + placebo (290), or 100 mg oral sitagliptin + placebo (290). Two deaths (unrelated to the treatment) were reported in semaglutide group [61].
SUSTAIN-11:
This on-going trial would explore the effectiveness of semaglutide by comparing various parameters like change in HbA1c, body weight, FPG, SMBG, blood pressure, lipid profile etc. with that of insulin aspart and insulin glargine. Participant would be subjected to 12 weeks of run-in period (receive metformin and daily-once insulin glargine) followed by 52-week treatment period (weekly-once s.c. 1.0 mg semaglutide or 4 units of s.c. insulin aspart by pre-filled pen three times daily before each meal) [62].
SUSTAIN-SWITCH (NCT04287179):
This trial was expected to compare two doses of weekly-once semaglutide and explore the efficacy of a new pen injector, but was cancelled due to the COVID-19 pandemic situation [63].
SUSTAIN-FORTE:
Effectiveness between two doses of s.c. semaglutide (1.0 mg and 2.0 mg) was studied by following 12-week dose escalation period, after which the active comparator group (1.0 mg) received additional placebo s.c. 1.0 mg for masking purpose, whereas 2.0 mg group received weekly two s.c. semaglutide injections of 1.0 mg each [64]. A significant as well as superior reduction in HbA1c and body weight was exhibited by 2.0 mg semaglutide against 1.0 mg group in both trial product and treatment policy estimand [65].
Reduction in HbA1c achieved in the SUSTAIN trials have been depicted in Fig. 2.
Fig. 2.
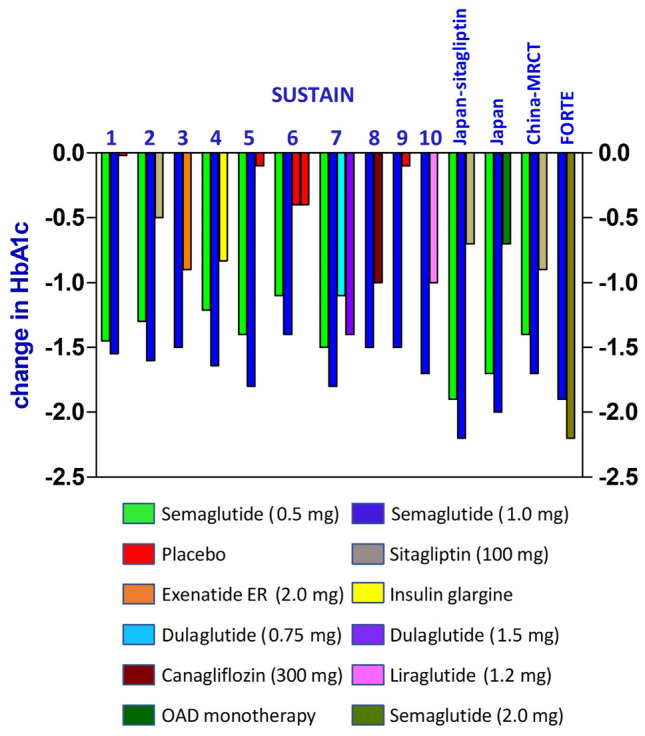
Reduction of HbA1c in SUSTAIN trials
PIONEER (Peptide Innovation for Early Diabetes Treatment) trials for once-daily oral semaglutide
These are phase-3, parallel-group, randomized, active- and/or placebo-controlled trials carried out on type 2 diabetes patients (trial design presented in Table 3).
Table 3.
Study design and results of PIONEER trials
| PIONEER | Clinical trial identification number | Population | Duration(weeks) | Background medication | Semaglutide trial dose & Comparator | Trial product estimand | Treatment policy estimand | ||
|---|---|---|---|---|---|---|---|---|---|
| % HbA1c reduction | Weight loss | % HbA1c reduction | Weight loss | ||||||
| 1 | NCT02906930 | 703 | 26 | None | 3 mg | -0.7 | -0.2 | -0.6 | -0.1 |
| 7 mg | -1.2 | -1.0 | -0.9 | -0.9 | |||||
| 14 mg | -1.4 | -2.6 | -1.1 | -2.3 | |||||
| Placebo | -0.1 | -1.5 | -0.3 | -1.4 | |||||
| 2 | NCT02863328 | 822 | 52 | Metformin | 14 mg | -1.3 | -4.7 | -1.3 | -3.8 |
| Empagliflozin (25 mg) | -0.8 | -3.8 | -0.9 | -3.6 | |||||
| 3 | NCT02607865 | 1864 | 78 | Metformin with/without Sulfonylurea | 3 mg | -0.3 | -1.8 | -0.6 | -1.8 |
| 7 mg | -0.7 | -2.7 | -0.8 | -2.7 | |||||
| 14 mg | -1.1 | -3.5 | -1.1 | -3.2 | |||||
| Sitagliptin (100 mg) | -0.4 | -1.1 | -0.7 | -1.0 | |||||
| 4 | NCT02863419 | 711 | 52 | Metformin with/without SGLT-2 inhibitor | 14 mg Liraglutide | -1.2 | -5.0 | -1.2 | -4.3 |
| (s.c. 1.8 mg) | -0.9 | -3.1 | -0.9 | -3.0 | |||||
| Placebo | +0.2 | -1.2 | -0.2 | -1.0 | |||||
| 5 | NCT02827708 | 324 (with moderate renal impairment) | 26 | Metformin/Sulfonylurea, or both, or Basal insulin with/without Metformin | 14 mg | -1.1 | -3.7 | -1.0 | -3.4 |
| Placebo | -0.1 | -1.1 | -0.2 | -0.9 | |||||
| 6 | NCT02692716 | 3183 (84.7% with CV or CKD) | Event-driven; Median time = 69 weeks | None | 14 mg | -1.0% | 4.2 kg | ||
| Placebo | -0.3% | 0.8 kg | |||||||
| 7 | NCT02849080 | 504 | 52 | One/two amongst Metformin,Sulfonylureas, SGLT-2 inhibitors, or Thiazolidinediones | 14 mg | -1.4 | -2.9 | -1.3 | -2.6 |
| Sitagliptin (100 mg) | -0.7 | -0.8 | -0.8 | -0.7 | |||||
| 8 | NCT03021187 | 731 | 52 | Insulin with/without Metformin | 3 mg | -0.5 | -1.0 | -0.6 | -0.8 |
| 7 mg | -0.8 | -2.9 | -0.8 | -2.0 | |||||
| 14 mg | -1.2 | -4.3 | -1.2 | -3.7 | |||||
| Placebo | 0.0 | +0.6 | -0.2 | +0.5 | |||||
| 9 | NCT03018028 | 243 | 52 | None | 3 mg | -0.9 | 0.0 | -0.9 | -0.3 |
| 7 mg | -1.3 | -0.6 | -1.4 | -0.8 | |||||
| 14 mg | -1.5 | -2.8 | -1.5 | -2.6 | |||||
| Placebo | +0.5 | -1.0 | -0.1 | -0.6 | |||||
| Liraglutide (s.c. 0.9 mg) | -1.1 | +0.4 | -1.2 | 0.0 | |||||
| 10 | NCT03015220 | 458 | 52 | Sulfonylurea/glinide/thia zolidinedione/α-glucosidase inhibitor/SGLT-2 inhibitor monotherapy | 3 mg | -0.7 | +0.1 | -0.9 | 0.0 |
| 7 mg | -1.4 | -1.0 | -1.4 | -0.9 | |||||
| 14 mg | -1.8 | -1.9 | -1.7 | -1.6 | |||||
| Dulaglutide (s.c. 0.75 mg) | -1.3 | +1.1 | -1.4 | +1.0 | |||||
| 11 | NCT04109547 | 664 | 26 | None | 3 mg | - | - | - | - |
| 7 mg | - | - | - | - | |||||
| 14 mg | - | - | - | - | |||||
| Placebo | - | - | - | - | |||||
| 12 | NCT04017832 | 1444 | 26 | Metformin | 3 mg | - | - | - | - |
| 7 mg | - | - | - | - | |||||
| 14 mg | - | - | - | - | |||||
| Placebo | - | - | - | - | |||||
| Sitagliptin (100 mg) | - | - | - | - | |||||
| TEENS | NCT04596631 | 132 (aged 10–17 years) | 52 | Metformin and/or basal insulin | Semaglutide (maximum tolerated dose) Placebo | - | - | - | - |
Pioneer 1:
This trial was conducted on 703 subjects, who received daily-once 3.0 mg oral semaglutide (175), or 7.0 mg (175), or 14.0 mg (175), or placebo (178). All the doses of semaglutide reduced HbA1c and body weight in both trial product estimand and treatment policy estimand, but clinically significant reduction was reported by the 14 mg dose. Apart from gastrointestinal adverse effects, semaglutide exhibited good safety profile [66].
Pioneer 2:
Efficacy of semaglutide and empagliflozin was studied on 703 subjects receiving once-daily oral 14.0 mg semaglutide (412), 25.0 mg empagliflozin (410). A significant decline in HbA1c and body weight was noted for semaglutide against empagliflozin for treatment policy and trial product estimand at 26 weeks. At week 52, semaglutide decreased HbA1c and body weight in trial product estimand than empagliflozin [67].
Pioneer 3:
This trial examined the safety, efficacy of semaglutide and sitagliptin by selecting 1864 subjects who received once-daily oral 3.0 mg semaglutide (466), or 7.0 mg semaglutide (466), or 14.0 mg semaglutide (465), or 100 mg sitagliptin (467) with dose escalation. As compared to sitagliptin, significant decline in HbA1c and body weight was demonstrated by semaglutide 7 and 14 mg but not in 3 mg treatment group. Semaglutide (14 mg) at week 78 was reported to have statistically pronounced fall in HbA1c and body weight compared with sitagliptin [68].
Pioneer 4:
To assess the safety, efficacy of semaglutide and liraglutide, participants received once-daily oral 14.0 mg semaglutide (285), or once-daily s.c. 1.8 mg liraglutide (284) or placebo (142). Mean reduction in HbA1c and body weight was observed by semaglutide in both trial product and treatment policy estimand as compared to liraglutide at week 26 [69].
Pioneer 5:
This trial demonstrated the efficacy of semaglutide on 324 subjects with modest kidney issues, who received either once-daily oral 14.0 mg semaglutide (163), or placebo (161) along with previous antidiabetic therapy. In both trial product and treatment policy estimand, semaglutide demonstrated significant reduction in HbA1c and weight loss against placebo [70].
Pioneer 6:
A cardiovascular safety outcome trial was conducted on 3183 participants (84% above 50 years of age) with cardiovascular disease or moderate renal impairment and received either once-daily oral 14.0 mg semaglutide (1591), or placebo (1592). About 2.3%, 0.8% semaglutide treatment subjects faced nonfatal myocardial infarction and nonfatal stroke respectively, while 0.9% died due to cardiovascular events. With a hazard proportion of 0.79, this cardiovascular effect confirmed noninferiority of oral semaglutide to placebo, along with 80% reduction of cardiovascular risks [71, 72].
Pioneer 7:
This trial compared the efficacy of semaglutide (flexible dosing) and sitagliptin (fixed dose) on 504 subjects with modest kidney dysfunction receiving once-daily oral semaglutide (253) with individualized flexible dosing, or once-daily oral 100 mg sitagliptin (251). In both treatment policy and trial product estimand, flexible dosing of semaglutide achieved reduction in HbA1c and body weight against sitagliptin. Retinopathy and malignant neoplasm cases were reported across all treatment groups [73].
Pioneer 8:
This trial was conducted on 731 participants, who received either once-daily oral 3.0 mg semaglutide (184), or 7.0 mg (182), or 14.0 mg (181), or placebo (184). Dose-dependent decline in HbA1c and body weight was associated with semaglutide than placebo at weeks 26 and 52 for both the treatment policy as well as trial product estimand [74].
Pioneer 9:
Dose–response curve of three different doses of semaglutide, placebo and liraglutide was studied in this trial. Japanese subjects received once-daily oral 3.0 mg semaglutide (49), or 7.0 mg (49), or 14.0 mg (48), or placebo (49), or once-daily s.c. 0.9 mg liraglutide (48). This trial demonstrated dose-dependent superior activity of semaglutide over liraglutide and placebo [75].
Pioneer 10:
This trial evaluated the safety, efficacy of semaglutide with dulaglutide on Japanese subjects who received daily-once oral 3.0 mg semaglutide (131) or 7.0 mg (132) or 14.0 mg (130) or weekly-once s.c. 0.75 mg dulaglutide (65) for 30 weeks. Gastrointestinal adverse effects, infections, retinopathy, malignant neoplasms and 3 cardiovascular adverse events were reported. A significant reduction in HbA1c (with all three doses) and body weight (with 7 mg, 14 mg dose) was noted in semaglutide against dulaglutide [76].
Pioneer 11:
Efficacy of 3 different doses of daily-once oral semaglutide is being examined on 664 subjects receiving oral semaglutide 3 mg, or 7 mg, or 14 mg, or placebo with dose escalation [77].
Pioneer 12:
This on-going trial will compare the safety, efficacy of daily-once semaglutide with sitagliptin. Subjects receive oral semaglutide 3 mg + placebo, or semaglutide 7 mg + placebo, or semaglutide 14 mg + placebo, or oral sitagliptin 100 mg + placebo [78].
Pioneer Teens:
This on-going trial would examine the efficacy, safety of semaglutide in 132 type 2 diabetic children, teenagers (aged 10–17 years) receiving daily-once oral semaglutide (with dose escalation to an individual maximum tolerated dose) or placebo [79].
Reduction in HbA1c achieved in the PIONEER trials have been depicted in Fig. 3.
Fig. 3.
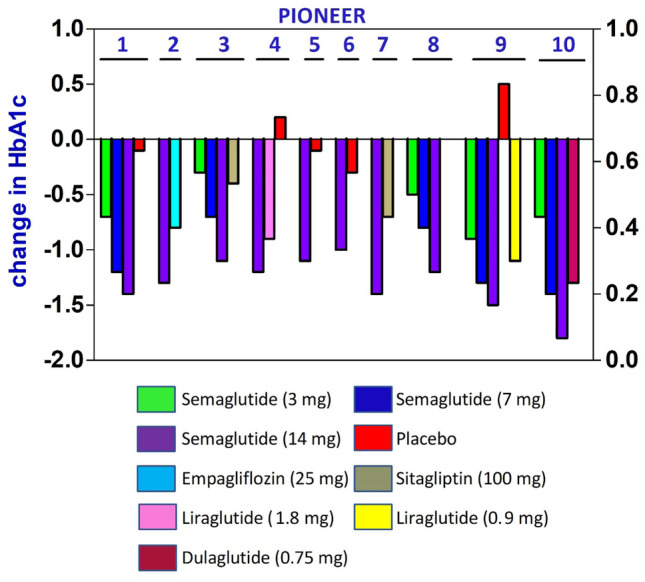
Reduction of HbA1c in PIONEER trials
Indications
Treatment of type 2 diabetes
Individual patient-oriented treatment which needs re-assessment in every 3–6 months, is being advocated by American Diabetes Association 2020 and the ‘Management of Hyperglycaemia in type 2 diabetes” by European Association for the Study of Diabetes consensus report 2019 [80, 81]. It recommends use of semaglutide whenever greater glycaemic control is needed, weight reduction is highly necessary, and also preferred to be used prior to insulin. Semaglutide is highly advisable in cardiovascular diseases (atherosclerotic nature) and in case of intolerance towards SGLT-2 inhibitors. It has also been approved for use in liver and kidney damage, with no requirement of dose adjustment [32, 82].
Semaglutide is an approved second line drug which can be used along with metformin or insulin analogues to achieve treatment intensification. But inadequate glycaemic control or intolerance to metformin may necessitate the use of GLP-1 agonist monotherapy like semaglutide. A reduction in the dose of co-administered insulin or sulfonyl urea is needed when used along with semaglutide to avoid the risk of hypoglycaemia. However, metformin, SGLT-2 inhibitors and thiazolidinedione derivatives doesn’t need any dose adjustment when used along with semaglutide [83, 84]. When semaglutide is used along with DPP-4 inhibitors, it doesn’t provide any synergistic effect and therefore this combination should be avoided [3]. Oral semaglutide presents a better alternative to injectable GLP-1 agonist therapy and increases the patient adherence [85]. The recommended indications for semaglutide have been presented in Table 4 [80, 81, 83, 84].
Table 4.
Indications for Semaglutide (As per American Diabetes Association 2020)
| Semaglutide | ||
|---|---|---|
| Efficacy | High | |
| Cost | High | |
| Oral/Injectable | Both available | |
| Weight loss | Yes | |
| Risk of Hypoglycaemia | No | Semaglutide monotherapy |
| Yes | Combination with insulin or other hypoglycaemic drugs; needs dose reduction | |
| Cardiovascular risk | Reduces cardiovascular risk | |
| Need for dose adjustment in geriatrics, renal and hepatic impairment | Not necessary | |
| Preferred conditions | Requirement of greater glycaemic control Need for injectable therapy to reduce HbA1c Need to switch from injectable to oral therapy If possible, preferred over insulin In cardiovascular diseases of atherosclerotic origin and renal impairment Intolerance to SGLT-2 inhibitors Weight reduction is essential | |
| Precautions | Avoid in- Medullary carcinoma of thyroid, Pancreatitis, Multiple Endocrine Neoplasia Syndrome type 2, Progressive retinopathy, Congestive Heart Failure (As per EMA) |
Based on the encouraging results from SUSTAIN FORTE studies, Novo Nordisk has filed a label expansion request with European Medicines Agency (EMA) on 29th December 2020 to introduce 2.0 mg dose of already approved weekly-once Ozempic® for treatment of hyperglycaemia [86]. A similar submission has been filed with United States Food and Drug Administration (USFDA) on 20th January 2021 for introducing 2.0 mg dose of weekly-once Ozempic [87].
Safety & Tolerability
Adverse effects
Although semaglutide is well tolerated, but it shows dose-dependent, mild to moderately severe gastrointestinal adverse effects (vomiting, nausea, constipation, diarrhoea, and dyspepsia) which usually wears off within 2 weeks of treatment initiation [88]. Other infrequent side effects are nasopharyngitis, headache, infections in urinary tract, upper respiratory tract, and increased pancreatic enzyme (amylase and lipase) levels [49–51, 59]. Occasional cases of acute pancreatitis have been reported which calls for discontinuation of treatment. Cases of hypoglycaemia are very low with semaglutide but concomitant administration of insulin analogues or other hypoglycaemic drugs need dose reduction [83, 84]. Apart from these, very rare cases of transient injection site reactions were also reported [28]. Many common but infrequent adverse effects of GLP-1 agonists like increased pulse rate, heart rate, cholelithiasis, cholecystitis has been observed with semaglutide also [31, 89].
Diabetic retinopathy:
SUSTAIN-6 has reported 76% higher risk for occurrence of event adjudication committee confirmed retinopathy related complications (blindness, vitreous haemorrhage, necessity of photocoagulation, and use of intravitreal agents) in semaglutide treatment group as compared to placebo [53]. Rapid control of hyperglycaemia or decline in HbA1c was responsible for deteriorating retinopathy during initial few weeks of treatment, as proposed by post hoc analysis of SUSTAIN 1–6 and Japanese trials. Moreover, the retinopathy complications were more prevailing in patients receiving insulin therapy but it needs further evidences to confirm [90].
FOCUS (NCT03811561):
A phase-3 trial is being carried out on 1500 subjects to investigate the long-term effects of semaglutide (as an add-on to previous antidiabetic medication) on retinopathy. Participants receive either weekly-once s.c. 1.0 mg semaglutide (with dose escalation) or placebo through a PDS290 pen-injector device [91].
Cardiovascular safety
Inadequate glycaemic control is one of the important factors which boosts the risk of cardiovascular adverse effects in type 2 diabetic subjects. Insulin resistance may alter insulin signalling pathway within myocardial cells to cause heart failure, stroke and myocardial dysfunction [92]. Therefore, cardiovascular risk assessment has been made mandatory by various regulatory authorities for approval of newer antidiabetic agents [93].
A beneficial cardiovascular risk reducing effect of semaglutide has been suggested by both SUSTAIN-6 as well as PIONEER-6 [94]. As compared to placebo, the hazard ratio for cardiovascular adverse effects favoured semaglutide, which clearly indicates cardiovascular benefits (presented in Table 5). A post-hoc analysis has reported about 24% reduction in cardiovascular adverse events was achieved by semaglutide against placebo [94]. Semaglutide has been reported to reduce atherosclerosis by negatively regulating multiple inflammatory pathways in proatherogenic apolipoprotein E-deficient mice (ApoE−/−) and low-density lipoprotein receptor deficient mice (LDLr−/−) model [95]. Moreover, GLP-1 agonists have already been reported to show cardioprotective effect by reducing apoptosis in cardiac cells of rats [96].
Table 5.
Cardiovascular outcomes of semaglutide in SUSTAIN-6 and PIONEER-6 trials
| Cardiovascular Outcomes | Primary outcome | Secondary outcome | Death from any cause, nonfatal MI/Stroke | Death (any cause) | Death (cardiovascular) | Nonfatal MI | Nonfatal stroke | Hospitalization due to unstable angina | Hospitalization due to heart failure | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sustain-6 | Semaglutide (n = 1648) | 6.6 | 12.1 | 7.4 | 3.8 | 2.7 | 2.9 | 1.6 | 1.3 | 3.6 |
| Placebo (n = 1649) | 8.9 | 16.0 | 9.6 | 3.6 | 2.8 | 3.9 | 2.7 | 1.6 | 3.3 | |
| Hazard ratio | 0.74 | 0.74 | 0.77 | 1.05 | 0.98 | 0.74 | 0.61 | 0.82 | 1.11 | |
| Pioneer-6 | Semaglutide (n = 1591) | 3.8 | 5.2 | 4.3 | 1.4 | 0.9 | 2.3 | 0.8 | 0.7 | 1.3 |
| Placebo (n = 1592) | 4.8 | 6.3 | 5.6 | 2.8 | 1.9 | 1.9 | 1.0 | 0.4 | 1.5 | |
| Hazard ratio | 0.79 | 0.82 | 0.77 | 0.51 | 0.49 | 1.18 | 0.74 | 1.56 | 0.86 |
SOUL (NCT03914326):
Long-term cardiovascular outcomes (occurrence of first major cardiovascular event, death, myocardial infarction, non-fatal stroke, coronary revascularization, limb ischemia) are being studied on 9642 subjects with cardiovascular or chronic kidney disease. Participants receive either daily-once semaglutide (dose increased from 3, 7, to 14 mg) or placebo for 3.5 to 5 years [97].
SELECT (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity; NCT03574597):
A 59-month long, phase-3 trial is being conducted on 17,500 obese but healthy subjects with cardiovascular history (age ≥ 45 years), where subjects receive either weekly-once 2.4 mg s.c. semaglutide (starting with 0.24 mg then 0.5, 1.0, 1.7 and finally to 2.4 mg) or placebo. This event-driven trial would assess the efficacy of semaglutide in reducing the risk of cardiovascular problems by examining occurrence of death (cardiovascular reason), non-fatal stroke or myocardial infarction and time taken for cardiovascular death [98, 99].
STRIDE (NCT04560998):
Effect of semaglutide on functional capacity (change in maximum walking distance and pain free walking distance on tread mill, vascular quality) is being examined on 800 participants with peripheral arterial disorder. Participants receive either weekly-once s.c. 1.0 mg semaglutide (dose escalation from 0.25, to 0.5, and then to 1.0 mg) or placebo for 52 weeks [100].
Drug interactions
As semaglutide cause delayed gastric emptying, it can affect the pharmacokinetics of other co-administered drugs. Numerous cross-over trials confirmed non-interference of semaglutide with the bioavailability of oral contraceptives, lisinopril, metformin, warfarin, furosemide, digoxin, atorvastatin, rosuvastatin, and omeprazole [101–105]. Co-administration of levothyroxine with oral semaglutide results in increased exposure of levothyroxine by 33%. Therefore, patients may be advised to take levothyroxine 3 h after the last meal to avoid unnecessary interactions with semaglutide [106].
Antihyperglycemic potential of Semaglutide
Both weekly-once 2.4 mg s.c. semaglutide and daily-once oral semaglutide has been approved by USFDA, Health Canada, European Medicines Agency (EMA), and Japanese Health ministry for use in the management of type 2 diabetes. Semaglutide has also shown weight reduction property in clinical trials, due to which it can provide dual benefit to patients with type 2 diabetes and obesity (diabesity).
Subcutaneous dosage form of semaglutide (semaglutide and inactive ingredients like disodium phosphate dihydrate, propylene glycol, phenol and water for injection) has been studied both as monotherapy and in combination with metformin, metformin + sulfonylureas, metformin and/or thiazolidinedione, and basal insulin in type 2 diabetes patients. In SUSTAIN trials 0.5 and 1.0 mg doses of s.c. semaglutide were studied against placebo, oral DPP-4 inhibitor, other s.c. GLP-1 agonists, insulin, oral SGLT-2 inhibitor. Similarly, the oral dosage form of semaglutide (semaglutide and inactive ingredients like magnesium stearate, microcrystalline cellulose, povidone and salcaprozate sodium) was studied both as monotherapy and in combination with metformin, sulfonylureas, SGLT-2 inhibitor, insulin, and thiazolidinedione derivative. In PIONEER trials 3, 7, and 14 mg doses of oral semaglutide were studied against placebo, oral SGLT-2 inhibitor, oral DPP-4 inhibitor, other s.c. GLP-1 agonists. Efficacy of semaglutide remain unaltered by age, gender, race, ethnicity, body mass index (BMI) and body weight at baseline, diabetes duration and the extent of renal impairment.
Both the dosage forms of semaglutide achieve the steady-state concentration after 4–5 weeks of dose initiation. The s.c. dosage form is taken once in a week, so patients need not take their pills every day. But oral form is more convenient for a significant section of patients and has better patient adherence. Semaglutide appears to be most effective in reducing HbA1c and body weight among the GLP-1 agonists class and also has superior efficacy over other anti-hyperglycemic agents. Semaglutide therapy is initiated with gradual dose escalation to keep the gastro-intestinal adverse effects at bay. It can be safely used in aged persons and in patients with mild to moderate liver or renal impairment. Semaglutide is prescribed as a first line therapy in addition to metformin and/or sulfonyl urea or basal insulin when faster decrease in HbA1c level is required. It is even suggested as monotherapy when metformin can’t be used and also as first line injectable treatment choice over insulins in patient with type 2 diabetes. The benefit of reducing CV risk further makes it a fantastic option for type 2 diabetes patients with established CV disorders.
Future perspectives
High oral doses and different dosing schedule
A phase-1 trial (NCT04524832) is being conducted on 175 healthy subjects to compare and investigate the steady state plasma concentration, Cmax, tmax of high doses of oral semaglutide (25 mg and 50 mg) tablets containing different amounts of SNAC [107].
A phase-1 trial (NCT04513704) is being conducted on 156 healthy subjects to examine various pharmacokinetic parameters (steady state plasma concentration, Cmax, tmax) after daily-once oral semaglutide administration under different dosing schedules. Participants received semaglutide for 10 days (3 mg initially, then 7 mg) under different schedules of A, B, C, D, E; for which the pre-dose and post-dose fasting duration are 2 h 30 min; 4 h, 30 min; 6 h, 30 min; 2 h, overnight fast; overnight fast, 30 min respectively [108].
Reducing myocardial injury in COVID-19 patients
SEMPATICO (NCT04615871): This phase-2 trial aims at reducing the mortality and morbidity of COVID-19 patients. It will assess whether low dose of s.c. semaglutide (0.25 mg on day 0, then 0.5 mg on day 7, 14 and 21) can reduce myocardial injury in COVID-19 patients or those with increased risk (older, obese, hypertensive patients, with established cardiovascular disease, or an elevated troponin level) [109].
Concluding remarks
Semaglutide, a therapeutic peptidic drug, can be truly considered as a quintessential of GLP-1 receptor agonist targeting diabetes. This review has briefly discussed the discovery, development phases, clinical studies, place in pharmacotherapy, practical considerations, recent developments, and efficacy of semaglutide. The anti-hyperglycaemic activity of semaglutide have been firmly established in a series of clinical trials on adults, elderly and obese type 2 diabetic patients with or without renal/hepatic impairment or cardiovascular disorder. Although gastrointestinal side effects are very common with semaglutide, but it’s well tolerated. Semaglutide provides better glycaemic control with low risk of hypoglycaemia in monotherapy and good patient adherence. SUSTAIN-6 and PIONEER-6 studies have assured the cardiovascular safety of semaglutide for long term use in patients having cardiovascular risks. Persons with co-morbid diseases like diabetes have increased susceptibility for COVID-19 infection; so use of semaglutide in diabetic as well as CV patients would be very much supportive in maintaining health care system during this pandemic situation. Hence, semaglutide has been proved to be an indispensable treatment option in the arsenal of physicians for better management of diabetes.
Abbreviations
GLP-1
Glucagon Like Peptide-1
CV
Cardio Vascular
DPP-4
DiPeptidylPeptidase-4
USFDA
United States Food & Drug Administration
Aib
2-Aminoisobutyric acid
SNAC
Sodium-N-[8-(2-hydroxybenzoyl)amino]caprylate
SUSTAIN
Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes
FPG
Fasting Plasma Glucose
HOMA-B
Homeostasis model assessment of β-cell function
ER
Extended release
DXA
Dual energy X-ray Absorptiometry
OAD
Oral Antidiabetic Drugs
SGLT-2
Sodium GLucose co-Transporter-2
SMBG
Self-Monitoring of Blood Glucose
PIONEER
Peptide Innovation for Early Diabetes Treatment
EMA
European Medicines Agency
Authors’ contributions
Conceptualization: Manoj Kumar Mahapatra; Literature search: Manoj Kumar Mahapatra, Muthukumar Karuppasamy; Data analysis: Manoj Kumar Mahapatra, Biswa Mohan Sahoo; Manuscript draft: Manoj Kumar Mahapatra; Critical revision: Muthukumar Karuppasamy, Biswa Mohan Sahoo.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data & material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
Authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 Diabetes Management Algorithm–2017 Executive Summary. Endocr Pract. 2017;23(2):207–238. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RM, Steen O. Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes. Can J Diabetes. 2019;43(2):136–145. doi: 10.1016/j.jcjd.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Astrup A, Finer N. Redefining type 2 diabetes: 'diabesity' or 'obesity dependent diabetes mellitus'? Obes Rev. 2000;1(2):57–59. doi: 10.1046/j.1467-789x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Mauricio D, Meneghini L, Seufert J, Liao L, Wang H, Tong L, Cali A, Stella P, Carita P, Khunti K. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19(8):1155–1164. doi: 10.1111/dom.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasyurek HM, Altunbas HA, Balci MK, Sanlioglu S. Incretins: Their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev. 2014;30:354–371. doi: 10.1002/dmrr.2501. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 8.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 9.Knop FK, Brønden A, Vilsbøll T. Exenatide: pharmacokinetics clinical use and future directions. Expert Opin Pharmacother. 2017;18(6):555–571. doi: 10.1080/14656566.2017.1282463. [DOI] [PubMed] [Google Scholar]
- 10.Leon N, LaCoursiere R, Yarosh D, Patel RS. Lixisenatide (Adlyxin): A Once-Daily Incretin Mimetic Injection for Type-2 Diabetes. Pharm Ther. 2017;42(11):676–682. [PMC free article] [PubMed] [Google Scholar]
- 11.Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised open-label study. Lancet. 2013;381(9861):117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 12.Smith LL, Mosley JF, 2nd, Parke C, Brown J, Barris LS, Phan LD. Dulaglutide (Trulicity): The Third Once-Weekly GLP-1 Agonist. Pharm Ther. 2016;41(6):357–360. [PMC free article] [PubMed] [Google Scholar]
- 13.Billings LK, Handelsman Y, Heile M, Schneider D, Wyne K. Health-Related Quality of Life Assessments with Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes Mellitus. J Manag Care Spec Pharm. 2018;24(9-a Suppl):S30–S41. doi: 10.18553/jmcp.2018.24.9-a.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstock J, Nino A, Soffer J, Erskine L, Acusta A, Dole J, Carr MC, Mallory J, Home P. Impact of a Weekly Glucagon-Like Peptide 1 Receptor Agonist, Albiglutide, on Glycemic Control and on Reducing Prandial Insulin Use in Type 2 Diabetes Inadequately Controlled on Multiple Insulin Therapy: A Randomized Trial. Diabetes Care. 2020;43(10):2509–2518. doi: 10.2337/dc19-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novo Nordisk. Company Announcements: Ozempic® (semaglutide) approved in the US. 2017. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=712. Assessed 2 May 2020.
- 16.Novo Nordisk. Company Announcements: Rybelsus® (semaglutide tablets), the first GLP-1 in a tablet approved in the US. 2019. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=356. Assessed 1 May 2020.
- 17.Novo Nordisk. Press Release: Ozempic® approved in Canada for the treatment of adults with type 2 diabetes. 2018. https://www.novonordisk.ca/content/dam/nncorp/ca/en/press-releases/Ozempic%20press%20release_Eng_01.08.2018_FINAL.pdf. Assessed 1 May 2020.
- 18.Novo Nordisk. Press Release: Health Canada approves RYBELSUS® (semaglutide tablets) the first and only GLP-1 analogue in a pill for the treatment of adults with type 2 diabetes. 2020. https://www.novonordisk.ca/content/dam/Canada/AFFILIATE/www-novonordisk-ca/News/Health-Canada-approves-RYBELSUS-semaglutide-tablets-FINAL-April-9-EN.pdf. Assessed 5 May 2020.
- 19.Novo Nordisk. Company Announcements: Novo Nordisk A/S: Ozempic® (semaglutide) approved in the EU for the treatment of type 2 diabetes. 2018. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=667. Assessed 5 May 2020.
- 20.Novo Nordisk. Company Announcements: Rybelsus® (oral semaglutide) approved for the treatment of adults with type 2 diabetes in the EU. 2020. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=301. Assessed 5 May 2020.
- 21.Novo Nordisk. Company Announcements: Ozempic® approved in Japan for the treatment of type 2 diabetes. 2018. http://hugin.info/2013/R/2178681/841377.pdf. Assessed 5 May 2020.
- 22.Novo Nordisk. Company Announcements: Rybelsus® approved in Japan for the treatment of type 2 diabetes. 2020. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=272. Assessed on 10 May 2020.
- 23.Jensen L, Helleberg H, Roffel A, van Lier JJ, Bjørnsdottir I, Pedersen PJ, Rowe E, Derving Karsbøl J, Pedersen ML. Absorption metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur J Pharm Sci. 2017;104:31–41. doi: 10.1016/j.ejps.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Deacon CF, Knudsen LB, Madsen K, Wiberg FC, Jacobsen O, Holst JJ. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia. 1998;41(3):271–278. doi: 10.1007/s001250050903. [DOI] [PubMed] [Google Scholar]
- 25.Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43(9):1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 26.Knudsen LB, Lau J. The Discovery and Development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne) 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 28.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week Open-Label Randomized Clinical Trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 29.Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10) Pharmaceutics. 2019;11(2):78. doi: 10.3390/pharmaceutics11020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca VA, Capehorn MS, Garg SK, Jódar Gimeno E, Hansen OH, Holst AG, Nayak G, Seufert J. Reductions in insulin resistance are mediated primarily via weight loss in subjects with type 2 diabetes on semaglutide. J Clin Endocrinol Metab. 2019;104(2):4078–4086. doi: 10.1210/jc.2018-02685. [DOI] [PubMed] [Google Scholar]
- 31.Christou GA, Katsiki N, Blundell J, Fruhbeck G, Kiortsis DN. Semaglutide as a promising antiobesity drug. Obes Rev. 2019;20(6):805–815. doi: 10.1111/obr.12839. [DOI] [PubMed] [Google Scholar]
- 32.Romera I, Cebrián-Cuenca A, Álvarez-Guisasola F, Gomez-Peralta F, Reviriego J. A Review of Practical Issues on the Use of Glucagon-Like Peptide-1 Receptor Agonists for the Management of Type 2 Diabetes. Diabetes Ther. 2019;10(1):5–19. doi: 10.1007/s13300-018-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA. Ozempic FDA Prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Assessed 5 May 2020.
- 34.Novo Nordisk. Ozempic Product Monograph. 2018. https://www.novonordisk.ca/content/dam/nncorp/ca/en/products/ozempic-product-monograph.pdf. Assessed 5 May 2020.
- 35.FDA. Rybelsus FDA prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf. Assessed on 5 May 2020.
- 36.Novo Nordisk. Rybelsus Product Monograph. Available at: https://www.novonordisk.ca/content/dam/nncorp/ca/en/products/Rybelsus-PM-EN-monograph.pdf. Assessed 5 May 2020.
- 37.Jensen L, Kupcova V, Arold G, Pettersson J, Hjerpsted JB. Pharmacokinetics and tolerability of semaglutide in people with hepatic impairment. Diabetes Obes Metab. 2018;20(4):998–1005. doi: 10.1111/dom.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korsatko S, Jensen L, Brunner M, Sach-Friedl S, Tarp MD, Holst AG, Heller SR, Pieber TR. Effect of once-weekly semaglutide on the counterregulatory response to hypoglycaemia in people with type 2 diabetes: A randomized placebo-controlled double-blind crossover trial. Diabetes Obes Metab. 2018;20(11):2565–2573. doi: 10.1111/dom.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beglinger C, Poller B, Arbit E, Ganzoni C, Gass S, Gomez-Orellana I, Drewe J. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3-36: a proof-of-concept study in healthy subjects. Clin Pharmacol Ther. 2008;84(4):468–474. doi: 10.1038/clpt.2008.35. [DOI] [PubMed] [Google Scholar]
- 40.Granhall C, Donsmark M, Blicher TM, Golor G, Søndergaard FL, Thomsen M, Bækdal TA. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue Oral Semaglutide in Healthy Subjects and Subjects with Type 2 Diabetes. Clin Pharmacokinet. 2019;58(6):781–791. doi: 10.1007/s40262-018-0728-4. [DOI] [PubMed] [Google Scholar]
- 41.Baekdal TA, Borregaard J, Donsmark M, Breitschaft A, Søndergaard FL. Evaluation of the effects of water volume with dosing and postdose fasting period on pharmacokinetics of oral semaglutide. Diabetes. 2017;66(Suppl 1):A315. [Google Scholar]
- 42.Connor A, Borregaard J, Buckley ST, Donsmark M, Hartoft-Nielsen ML, Maarbjerg SJ, Søndergaard FL, Vegge A, Knudsen LB, Bækdal TA. Site of absorption of an oral formulation of semaglutide. Diabetes. 2017;66(Suppl 1):A315. [Google Scholar]
- 43.ClinicalTrials. Effect of Food on the Pharmacokinetics of Oral Semaglutide in Healthy Subjects. 2014. https://clinicaltrials.gov/ct2/show/NCT02172313. Assessed 15 May 2020.
- 44.Granhall C, Søndergaard FL, Thomsen M, Anderson TW. Pharmacokinetics Safety and Tolerability of Oral Semaglutide in Subjects with Renal Impairment. Clin Pharmacokinet. 2018;57(12):1571–1580. doi: 10.1007/s40262-018-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baekdal TA, Thomsen M, Kupčová V, Hansen CW, Anderson TW. Pharmacokinetics Safety and Tolerability of Oral Semaglutide in Subjects With Hepatic Impairment. J Clin Pharmacol. 2018;58(10):1314–1323. doi: 10.1002/jcph.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lingvay I, Desouza CV, Lalic KS, Rose L, Hansen T, Zacho J, Pieber TR. A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin. Diabetes Care. 2018;41(9):1926–1937. doi: 10.2337/dc17-2381. [DOI] [PubMed] [Google Scholar]
- 47.Nauck MA, Petrie JR, Sesti G, Mannucci E, Courrèges JP, Lindegaard ML, Jensen CB, Atkin SL, Study 1821 Investigators. A Phase 2 Randomized Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog Semaglutide Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes. Diabetes Care. 2016;39(2):231–41. 10.2337/dc15-0165. [DOI] [PubMed]
- 48.Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 2017;318(15):1460–1470. doi: 10.1001/jama.2017.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, Bain SC. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind randomised placebo-controlled parallel-group multinational multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 50.Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin thiazolidinediones or both in patients with type 2 diabetes (SUSTAIN 2): a 56-week double-blind phase 3a randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 51.Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, Rowe E, DeVries JH. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised open-label parallel-group multicentre multinational phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 52.Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, Araki E, Chu PL, Wijayasinghe N, Norwood P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized Controlled Trial. J Clin Endocrinol Metab. 2018;103(6):2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T, SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834–44. 10.1056/NEJMoa1607141. [DOI] [PubMed]
- 54.Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A, SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised open-label phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–86. 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed]
- 55.Lingvay I, Catarig AM, Frias JP, Kumar H, Lausvig NL, le Roux CW, Thielke D, Viljoen A, McCrimmon RJ. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind phase 3b randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834–844. doi: 10.1016/S2213-8587(19)30311-0. [DOI] [PubMed] [Google Scholar]
- 56.McCrimmon RJ, Catarig AM, Frias JP, Lausvig NL, le Roux CW, Thielke D, Lingvay I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63(3):473–485. doi: 10.1007/s00125-019-05065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, Thrasher J, Woo V, Philis-Tsimikas A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 58.Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–9. 10.1016/j.diabet.2019.101117. [DOI] [PubMed]
- 59.Seino Y, Terauchi Y, Osonoi T, Yabe D, Abe N, Nishida T, Zacho J, Kaneko S. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):378–388. doi: 10.1111/dom.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaku K, Yamada Y, Watada H, Abiko A, Nishida T, Zacho J, Kiyosue A. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: A randomized trial. Diabetes Obes Metab. 2018;20(5):1202–1212. doi: 10.1111/dom.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji L, Dong X, Li Y, Li Y, Lim S, Liu M, Ning Z, Rasmussen S, Skjøth TV, Yuan G, Eliaschewitz FG. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week double-blind phase 3a randomized trial. Diabetes Obes Metab. 2021;23(2):404–414. doi: 10.1111/dom.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ClinicalTrials. A Research Study to Compare Semaglutide to Insulin Aspart, When Taken Together With Metformin and Insulin Glargine, in People With Type 2 Diabetes (SUSTAIN 11). 2018. https://clinicaltrials.gov/ct2/show/NCT03689374. Assessed 15 May 2021.
- 63.ClinicalTrials. SUSTAIN SWITCH: A Research Study to Compare Two Dose Schedules of Semaglutide Taken Once Weekly in People With Type 2 Diabetes (SUSTAIN SWITCH). 2020. https://clinicaltrials.gov/ct2/show/NCT04287179. Assessed 10 May 2021.
- 64.ClinicalTrials. A Research Study to Compare Two Doses of Semaglutide Taken Once Weekly in People With Type 2 Diabetes (SUSTAIN FORTE). 2021. https://clinicaltrials.gov/ct2/show/NCT03989232. Assessed 12 May 2021.
- 65.Novo Nordisk. Company Announcement Once-weekly semaglutide 2.0 mg demonstrates superior reduction in HbA1c vs once-weekly semaglutide 1.0 mg in people with type 2 diabetes in the SUSTAIN FORTE trial. 2020. https://ml-eu.globenewswire.com/Resource/Download/2050d050-81c0-4606-98dd-0120429d3587. Assessed 20 May 2021.
- 66.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M, PIONEER 1 Investigators. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019;42(9):1724–32. 10.2337/dc19-0749. [DOI] [PubMed]
- 67.Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, Lingvay I, Søndergaard AL, Treppendahl MB, Montanya E, PIONEER 2 Investigators. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care. 2019;42(12):2272–81. 10.2337/dc19-0883. [DOI] [PubMed]
- 68.Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M, PIONEER 3 Investigators. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults with Type 2 Diabetes Uncontrolled with Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA. 2019;321(15):1466–80. 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed]
- 69.Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ, PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised double-lind, phase 3a trial. Lancet. 2019;394(10192):39–50. 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed]
- 70.Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, Pratley R, Sathyapalan T, Desouza C, PIONEER 5 Investigators. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled randomised phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–27. 10.1016/S2213-8587(19)30192-5. [DOI] [PubMed]
- 71.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC, PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381(9):841–51. 10.1056/NEJMoa1901118. [DOI] [PubMed]
- 72.Bain SC, Mosenzon O, Arechavaleta R, Bogdanski P, Comlekci A, Consoli A, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: Rationale design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21:499–508. doi: 10.1111/dom.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, Wallenstein SOR, Buse JB, PIONEER 7 investigators. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre open-label randomised phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–39. 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed]
- 74.Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, Pedersen KB, Tarp-Johansen MJ, Araki E, PIONEER 8 Investigators. Efficacy Safety and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Trial. Diabetes Care. 2019;42(12):2262–71. 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed]
- 75.Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, Seino Y, PIONEER 9 investigators. Dose-response efficacy and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week phase 2/3a randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8(5):377–91. 10.1016/S2213-8587(20)30075-9. [DOI] [PubMed]
- 76.Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, Inagaki N, PIONEER 10 Investigators. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label randomised active-controlled phase 3a trial. Lancet Diabetes Endocrinol. 2020;8(5):392–406. 10.1016/S2213-8587(20)30074-7. [DOI] [PubMed]
- 77.ClinicalTrials. A Research Study Comparing a New Medicine Oral Semaglutide to Placebo in People With Type 2 Diabetes (PIONEER 11). 2019. https://clinicaltrials.gov/ct2/show/NCT04109547. Assessed 2 Jun 2021.
- 78.ClinicalTrials. A Research Study Comparing a New Medicine Oral Semaglutide to Sitagliptin in People With Type 2 Diabetes (PIONEER 12). 2019. https://clinicaltrials.gov/ct2/show/NCT04017832. Assessed on 16 Jun 2021.
- 79.ClinicalTrials. A Research Study to Compare a New Medicine Oral Semaglutide to a Dummy Medicine in Children and Teenagers With Type 2 Diabetes (PIONEER TEENS). 2020. https://clinicaltrials.gov/ct2/show/NCT04596631. Assessed on 27 Jun 2021.
- 80.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes 2018 A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.American Diabetes Association 9 Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Peralta F, Abreu C. Profile of semaglutide in the management of type 2 diabetes: design development and place in therapy. Drug Des Devel Ther. 2019;13:731–738. doi: 10.2147/DDDT.S165372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.European Medicines Agency. Ozempic; summary of product characteristics. 2020. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004174/WC500244163.pdf. Assessed 18 Jun 2021.
- 84.European Medicines Agency. Rybelsus; summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf. Assessed 18 Jun 2021.
- 85.Brunton SA, Mosenzon O, Wright EE., Jr Integrating oral semaglutide into clinical practice in primary care: for whom when and how? Postgrad Med. 2020;132(sup2):48–60. doi: 10.1080/00325481.2020.1798162. [DOI] [PubMed] [Google Scholar]
- 86.Novo Nordisk. Company announcements: Novo Nordisk files for EU regulatory approval of once-weekly semaglutide 2.0 mg for the treatment of type 2 diabetes. 2020. https://ml-eu.globenewswire.com/Resource/Download/fb1aa331-4c28-4166-967b-fa3034738174. Accessed 28 Jun 2021.
- 87.Novo Nordisk. Company announcements: Novo Nordisk files for regulatory approval in the US of onceweekly semaglutide 2.0 mg for the treatment of type 2 diabetes. 2021. https://ml-eu.globenewswire.com/Resource/Download/9a29821c-a82f-4ef4-b838-3e4b7c794243. Accessed 28 Jun 2021.
- 88.Tan X, Cao X, Zhou M, Zou P, Hu J. Efficacy and safety of once-weekly semaglutide for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2017;26(9):1083–1089. doi: 10.1080/13543784.2017.1360274. [DOI] [PubMed] [Google Scholar]
- 89.O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised double-blind placebo and active controlled dose-ranging phase 2 trial. Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 90.Vilsbøll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simó R, Helmark IC, Wijayasinghe N, Larsen M. Semaglutide reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889–897. doi: 10.1111/dom.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ClinicalTrials. A Research Study to Look at How Semaglutide Compared to Placebo Affects Diabetic Eye Disease in People With Type 2 Diabetes (FOCUS). 2019. https://clinicaltrials.gov/ct2/show/NCT03811561. Assessed 29 Jun 2021.
- 92.Riehle C, Abel ED. Insulin Signaling and Heart Failure. Circ Res. 2016;118(7):1151–1169. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zannad F, Stough WG, Lipicky RJ, Tamargo J, Bakris GL, Borer JS, Alonso García Mde L, Hadjadj S, Koenig W, Kupfer S, McCullough PA, Mosenzon O, Pocock S, Scheen AJ, Sourij H, Van der Schueren B, Stahre C, White WB, Calvo G. Assessment of cardiovascular risk of new drugs for the treatment of diabetes mellitus: risk assessment vs. risk aversion. Eur Heart J Cardiovasc Pharmacother. 2016;2(3):200–5. 10.1093/ehjcvp/pvw007. [DOI] [PMC free article] [PubMed]
- 94.Husain M, Bain SC, Jeppesen OK, Lingvay I, Sørrig R, Treppendahl MB, Vilsbøll T. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22(3):442–451. doi: 10.1111/dom.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, Hecksher-Sørensen J, Ingvorsen C, Polex-Wolf J, Knudsen LB. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE-/- and LDLr-/- Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl Sci. 2018;3(6):844–857. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iorga RA, Bacalbasa N, Carsote M, Bratu OG, Stanescu AMA, Bungau S, Pantis C, Diaconu CC. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (Review) Exp Ther Med. 2020;20(3):2396–2400. doi: 10.3892/etm.2020.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.ClinicalTrials. A Heart Disease Study of Semaglutide in Patients With Type 2 Diabetes (SOUL). 2019. https://clinicaltrials.gov/ct2/show/NCT03914326. Assessed 24 Jun 2021.
- 98.ClinicalTrials. Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT). 2018. https://clinicaltrials.gov/ct2/show/NCT03574597. Assessed 26 Jun 2021.
- 99.Ryan DH, Lingvay I, Colhoun HM, Deanfield J, Emerson SS, Kahn SE, Kushner RF, Marso S, Plutzky J, Brown-Frandsen K, Gronning MOL, Hovingh GK, Holst AG, Ravn H, Lincoff AM. Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 100.ClinicalTrials. A Research Study to Compare a Medicine Called Semaglutide Against Placebo in People With Peripheral Arterial Disease and Type 2 Diabetes (STRIDE). 2020. https://www.clinicaltrials.gov/ct2/show/NCT04560998. Assessed 25 Jun 2021.
- 101.Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide a once-weekly human GLP-1 analog does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497–504. doi: 10.1002/jcph.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hausner H, Derving Karsbøl J, Holst AG, Jacobsen JB, Wagner FD, Golor G, Anderson TW. Effect of Semaglutide on the Pharmacokinetics of Metformin, Warfarin, Atorvastatin and Digoxin in Healthy Subjects. Clin Pharmacokinet. 2017;56(11):1391–1401. doi: 10.1007/s40262-017-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bækdal TA, Borregaard J, Hansen CW, Thomsen M, Anderson TW. Effect of Oral Semaglutide on the Pharmacokinetics of Lisinopril Warfarin Digoxin and Metformin in Healthy Subjects. Clin Pharmacokinet. 2019;58(9):1193–1203. doi: 10.1007/s40262-019-00756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jordy AB, Albayaty M, Breitschaft A, Anderson TW, Christiansen E, Houshmand-Øregaard A, Manigandan E, Bækdal TA. Effect of Oral Semaglutide on the Pharmacokinetics of Levonorgestrel and Ethinylestradiol in Healthy Postmenopausal Women and Furosemide and Rosuvastatin in Healthy Subjects. Clin Pharmacokinet. 2021;60:1171–1185. doi: 10.1007/s40262-020-00976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14(8):869–877. doi: 10.1080/17425255.2018.1488965. [DOI] [PubMed] [Google Scholar]
- 106.Hauge C, Breitschaft A, Hartoft-Nielsen ML, Jensen S, Bækdal T. SAT-140 A Drug-Drug Interaction Trial of Oral Semaglutide with Levothyroxine and Multiple Coadministered Tablets. J Endocr Soc. 2019;3(Suppl 1):SAT-140. 10.1210/js.2019-SAT-140.
- 107.ClinicalTrials. Research Study Comparing New Tablets of Semaglutide in New Doses, in Healthy People. 2020. https://clinicaltrials.gov/ct2/show/NCT04524832. Assessed 27 Jun 2021.
- 108.ClinicalTrials. A Clinical Trial Comparing Semaglutide in Healthy People Who Eat and Take the Medicine at Different Times. 2020. https://clinicaltrials.gov/ct2/show/NCT04513704. Assessed 27 Jun 2021.
- 109.ClinicalTrials. A Clinical Trial Comparing Semaglutide in Healthy People Who Eat and Take the Medicine at Different Times. 2020. https://www.clinicaltrials.gov/ct2/show/NCT04615871. Assessed 27 Jun 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.