ACG Clinical Guideline: Guidelines for the Diagnosis and Management of Gastroesophageal Reflux Disease (original) (raw)
. Author manuscript; available in PMC: 2023 Jan 1.
Abstract
Gastroesophageal reflux disease (GERD) continues to be among the most common diseases seen by gastroenterologists, surgeons, and primary care physicians. Our understanding of the varied presentations of GERD, enhancements in diagnostic testing, and approach to patient management have evolved. During this time, scrutiny of proton pump inhibitors (PPI) has increased considerably. While PPIs remain the medical treatment of choice for GERD, multiple publications have raised questions about adverse events, raising doubts about the safety of long-term use and increasing concern about over-prescribing of PPIs. New data regarding the potential for surgical and endoscopic interventions have emerged. In this new document, we provide updated, evidence-based recommendations and practical guidance for the evaluation and management of GERD, including pharmacologic, lifestyle, surgical, and endoscopic management. The GRADE system was used to evaluate the evidence and the strength of recommendations. Key concepts and suggestions that as of this writing do not have sufficient evidence to grade are also provided.
Introduction
A lot has changed, much remains the same. Gastroesophageal reflux disease (GERD) continues to be among the most common diseases seen by gastroenterologists, surgeons, and primary care physicians. Since publication of the last American College of Gastroenterology guideline on reflux management [1], clinically important advances in surgical and endoscopic therapy of GERD have emerged. Our understanding of the varied presentations of GERD, enhancements in diagnostic testing, and approach to patient management have evolved. During this time, scrutiny of proton pump inhibitors (PPI) has increased considerably. While PPIs remain the medical treatment of choice for GERD, multiple publications have raised questions about adverse events, raising doubts about the safety of long-term use and increasing concern about over-prescribing of PPIs. In this new document, we provide updated, evidence-based recommendations and practical guidance for the evaluation and management of GERD, including pharmacologic, lifestyle, surgical, and endoscopic management. The management of functional heartburn and other functional upper GI symptoms is beyond the scope of this guideline. Additional detail regarding esophageal physiologic testing is covered in other guidelines.
Summary and strength of the recommendations can be found in Table 1 with Key Concepts summarized in Table 2.
Table 1.
Summary and strength of recommendations
| GRADE qualityof evidence | GRADE strength ofrecommendation | |
|---|---|---|
| Diagnosis of GERD | ||
| For patients with classic GERD symptoms of heartburn and regurgitation who have no alarm symptoms, we recommend an 8-wk trial of empiric PPIs once daily before a meal. | Moderate | Strong |
| We recommend attempting to discontinue the PPIs in patients whose classic GERD symptoms respond to an 8-wk empiric trial of PPIs. | Low | Conditional |
| In patients with chest pain who have had adequate evaluation to exclude heart disease, objective testing for GERD (endoscopy and/or reflux monitoring) is recommended. | Low | Conditional |
| We do not recommend the use of a barium swallow solely as a diagnostic test for GERD. | Low | Conditional |
| We recommend endoscopy as the first test for evaluation of patients presenting with dysphagia or other alarm symptoms (weight loss and GI bleeding) and for patients with multiple risk factors for Barrett’s esophagus. | Low | Strong |
| In patients for whom the diagnosis of GERD is suspected but not clear, and endoscopy shows no objective evidence of GERD, we recommend reflux monitoring be performed off therapy to establish the diagnosis. | Low | Strong |
| We suggest against performing reflux monitoring off therapy solely as a diagnostic test for GERD in patients known to have endoscopic evidence of LA grade C or D reflux esophagitis or in patients known to have long-segment Barrett’s esophagus. | Low | Strong |
| GERD management | ||
| We recommend weight loss in overweight and obese patients for improvement of GERD symptoms. | Moderate | Strong |
| We suggest avoiding meals within 2–3 hr of bedtime. | Low | Conditional |
| We suggest avoidance of tobacco products/smoking in patients with GERD symptoms. | Low | Conditional |
| We suggest avoidance of “trigger foods” for GERD symptom control. | Low | Conditional |
| We suggest elevating head of bed for nighttime GERD symptoms. | Low | Conditional |
| We recommend treatment with PPIs over treatment with H2RA for healing EE. | High | Strong |
| We recommend treatment with PPIs over H2RA for maintenance of healing for EE. | Moderate | Strong |
| We recommend PPI administration 30–60 min before a meal rather than at bedtime for GERD symptom control. | Moderate | Strong |
| For patients with GERD who do not have EE or Barrett’s esophagus, and whose symptoms have resolved with PPI therapy, an attempt should be made to discontinue PPIs | Low | Conditional |
| For patients with GERD who require maintenance therapy with PPIs, the PPIs should be administered in the lowest dose that effectively controls GERD symptoms and maintains healing of reflux esophagitis. | Low | Conditional |
| We recommend against routine addition of medical therapies in PPI nonresponders. | Moderate | Conditional |
| We recommend maintenance PPI therapy indefinitely or antireflux surgery for patients with LA grade C or D esophagitis. | Moderate | Strong |
| We do not recommend baclofen in the absence of objective evidence of GERD. | Moderate | Strong |
| We recommend against treatment with a prokinetic agent of any kind for GERD therapy unless there is objective evidence of gastroparesis. | Low | Strong |
| We do not recommend sucralfate for GERD therapy except during pregnancy. | Low | Strong |
| We suggest on-demand/or intermittent PPI therapy for heartburn symptom control in patients with NERD. | Low | Conditional |
| Extraesophageal GERD symptoms | ||
| We recommend evaluation for non-GERD causes in patients with possible extraesophageal manifestations before ascribing symptoms to GERD. | Moderate | Strong |
| We recommend that patients who have extraesophageal manifestations of GERD without typical GERD symptoms (e.g., heartburn and regurgitation) undergo reflux testing for evaluation before PPI therapy. | Moderate | Strong |
| For patients who have both extraesophageal and typical GERD symptoms, we suggest considering a trial of twice-daily PPI therapy for 8–12 wk before additional testing. | Low | Conditional |
| We suggest that upper endoscopy should not be used as the method to establish a diagnosis of GERD-related asthma, chronic cough, or LPR. | Low | Conditional |
| We suggest against a diagnosis of LPR based on laryngoscopy findings alone and recommend additional testing should be considered. | Low | Conditional |
| In patients treated for extraesophageal reflux disease, surgical or endoscopic antireflux procedures are only recommended in patients with objective evidence of reflux. | Low | Conditional |
| Refractory GERD | ||
| We recommend optimization of PPI therapy as the first step in management of refractory GERD. | Moderate | Strong |
| We recommend esophageal pH monitoring (Bravo, catheter-based, or combined impedance-pH monitoring) performed OFF PPIs if the diagnosis of GERD has not been established by a previous pH monitoring study or an endoscopy showing long-segment Barrett’s esophagus or severe reflux esophagitis (LA grade C or D). | Low | Conditional |
| We recommend esophageal impedance-pH monitoring performed ON PPIs for patients with an established diagnosis of GERD whose symptoms have not responded adequately to twice-daily PPI therapy. | Low | Conditional |
| For patients who have regurgitation as their primary PPI-refractory symptom and who have had abnormal gastroesophageal reflux documented by objective testing, we recommend consideration of antireflux surgery or TIF. | Low | Conditional |
| Surgical and endoscopic options for GERD | ||
| We recommend antireflux surgery performed by an experienced surgeon as an option for long-term treatment of patients with objective evidence of GERD. Those who have severe reflux esophagitis (LA grade C or D), large hiatal hernias, and/or persistent, troublesome GERD symptoms who are likely to benefit most from surgery. | Moderate | Strong |
| We recommend consideration of MSA as an alternative to laparoscopic fundoplication for patients with regurgitation who fail medical management. | Moderate | Strong |
| We recommend consideration of RYGB as an option to treat GERD in obese patients who are candidates for this procedure and who are willing to accept its risks and requirements for lifestyle alterations. | Low | Conditional |
| Because data on the efficacy of radiofrequency energy (Stretta) as an antireflux procedure is inconsistent and highly variable, we cannot recommend its use as an alternative to medical or surgical antireflux therapies. | Low | Conditional |
| We suggest consideration of TIF for patients with troublesome regurgitation or heartburn who do not wish to undergo antireflux surgery and who do not have severe reflux esophagitis (LA grade C or D) or hiatal hernias >2 cm. | Low | Conditional |
Table 2.
Key concept statements
| Diagnosis of GERD |
|---|
| We do not recommend HRM solely as a diagnostic test for GERD. |
| GERD management |
| There is conceptual rationale for a trial of switching PPIs for patients who have not responded to one PPI. For patients who have not responded to one PPI, more than one switch to another PPI cannot be supported. |
| Use of the lowest effective dose is recommended and logical but must be individualized. One area of controversy relates to abrupt PPI discontinuation and potential rebound acid hypersecretion, resulting in increased reflux symptoms. Although this has been demonstrated to occur in healthy controls, strong evidence for an increase in symptoms after abrupt PPI withdrawal is lacking. |
| Extraesophageal GERD |
| Although GERD may be a contributor to extraesophageal symptoms in some patients, careful evaluation for other causes should be considered for patients with laryngeal symptoms, chronic cough, and asthma. |
| Diagnosis, evaluation, and management of potential extraesophageal symptoms of GERD is limited by lack of a gold-standard test, variable symptoms, and other disorders which may cause similar symptoms |
| Endoscopy is not sufficient to confirm or refute the presence of extraesophageal GERD. |
| Because of difficulty in distinguishing between patient with laryngeal symptoms and normal controls, salivary pepsin testing is not recommended for evaluation of patients with extraesophageal reflux symptoms |
| For patients whose extraesophageal symptoms have not responded to a trial of twice-daily PPIs, we recommend upper endoscopy, ideally off PPIs for 2–4 wk. If endoscopy is normal, consider reflux monitoring. If EGD shows EE, that does not confirm that the extraesophageal symptoms are from GERD. Patients still may need pH-impedance testing |
| For patients with extraesophageal symptoms, we do not routinely recommend oropharyngeal or pharyngeal pH monitoring. |
| Refractory GERD |
| It is important to stop PPI therapy in patients whose off-therapy reflux testing is negative, unless another indication for continuing PPIs is present. In 1 study, 42% of patients reported continuing PPI treatment after a negative evaluation for refractory GERD, which included negative endoscopy and pH-impedance monitoring [2]. |
| Esophageal manometry should be considered as part of the evaluation for patients with refractory GERD in patients with a normal endoscopy and pH monitoring study and for patients being considered for surgical or endoscopic treatment. |
| If not already performed off PPIs, we recommend diagnostic upper endoscopy with esophageal biopsies after discontinuing PPI therapy, ideally for 2 to 4 wk |
| For patients with PPI-refractory symptoms who have a normal pH monitoring test OFF PPIs or a normal impedance-pH monitoring test ON PPIs (including a negative SI and SAP), we recommend discontinuation of PPIs unless there is an indication for PPI therapy other than the refractory symptoms. |
| Surgical and endoscopic therapy |
| We recommend HRM before antireflux surgery or endoscopic therapy to rule out achalasia and absent contractility. For patients with ineffective esophageal motility, HRM should include provocative testing to identify contractile reserve (e.g., multiple rapid swallows). |
| We recommend a careful evaluation and caution before proceeding with invasive therapy for patients with PPI-refractory GERD symptoms other than regurgitation. |
| Before performing invasive therapy for GERD, a careful evaluation is required to ensure that GERD is present and as best as possible determine is the cause of the symptoms to be addressed by the therapy, to exclude achalasia (which can be associated with symptoms such as heartburn and regurgitation that can be confused with GERD), and to exclude conditions that might be contraindications to invasive treatment such as absent contractility. |
| Long-term PPI issues |
| Regarding the safety of long-term PPI usage for GERD, we suggest that patients should be advised as follows: “PPIs are the most effective medical treatment for GERD. Some medical studies have identified an association between the long-term use of PPIs and the development of numerous adverse conditions including intestinal infections, pneumonia, stomach cancer, osteoporosis-related bone fractures, chronic kidney disease, deficiencies of certain vitamins and minerals, heart attacks, strokes, dementia, and early death. Those studies have flaws, are not considered definitive, and do not establish a cause-and-effect relationship between PPIs and the adverse conditions. High-quality studies have found that PPIs do not significantly increase the risk of any of these conditions except intestinal infections. Nevertheless, we cannot exclude the possibility that PPIs might confer a small increase in the risk of developing these adverse conditions. For the treatment of GERD, gastroenterologists generally agree that the well-established benefits of PPIs far outweigh their theoretical risks.” |
| Switching PPIs can be considered for patients who experience minor PPI side effects including headache, abdominal pain, nausea, vomiting, diarrhea, constipation, and flatulence. |
| For patients with GERD on PPIs who have no other risk factors for bone disease, we do not recommend that they raise their intake of calcium or vitamin D or that they have routine monitoring of bone mineral density. |
| For patients with GERD on PPIs who have no other risk factors for vitamin B12 deficiency, we do not recommend that they raise their intake of vitamin B12 or that they have routine monitoring of serum B12 levels. |
| For patients with GERD on PPIs who have no other risk factors for kidney disease, we do not recommend that they have routine monitoring of serum creatinine levels. |
| For patients with GERD on clopidogrel who have LA grade C or D esophagitis or whose GERD symptoms are not adequately controlled with alternative medical therapies, the highest quality data available suggest that the established benefits of PPI treatment outweigh their proposed but highly questionable cardiovascular risks. |
| PPIs can be used to treat GERD in patients with renal insufficiency with close monitoring of renal function or consultation with a nephrologist. |
Methods
The guideline is structured in the format of statements that are considered to be clinically important by the content authors for evaluation and treatment of GERD. The authors developed PICO questions and performed a literature search for each question with assistance from a research librarian. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) process was used to assess the quality of evidence for each statement [2]. The quality of evidence is expressed as high (we are confident in the effect estimate to support a particular recommendation), moderate, low, or very low (we have very little confidence in the effect estimate to support a particular recommendation) based on the risk of bias of the studies, evidence of publication bias, heterogeneity among studies, directness of the evidence and precision of the estimate of effect [3]. A strength of recommendation is given as either strong (recommendations) or conditional (suggestions) based on the quality of evidence, risks versus benefits, feasibility, and costs taking into account perceived patient and population-based factors [4]. Furthermore, a narrative evidence summary for each section provides important details for the data supporting the statements.
Our goal is to showcase a document that offers best practice recommendations for clinicians caring for patients with GERD.
Diagnosis of GERD
Recommendations
- For patients with classic GERD symptoms of heartburn and regurgitation who have no alarm symptoms, we recommend an 8-week trial of empiric proton pump inhibitor (PPI) once daily before a meal. (Strong recommendation, moderate level of evidence)
- We recommend attempting to discontinue the PPIs in patients whose classic GERD symptoms respond to an 8-week empiric trial of PPIs. (conditional recommendation, low level of evidence)
- We recommend diagnostic endoscopy, ideally after PPIs are stopped for 2 to 4 weeks, in patients whose classic GERD symptoms do not respond adequately to an 8-week empiric trial of PPIs, or whose symptoms return when PPIs are discontinued. (Strong recommendation, low level of evidence)
- In patients who have chest pain without heartburn and who have had adequate evaluation to exclude heart disease, objective testing for GERD (endoscopy and/or reflux monitoring) is recommended. (Conditional recommendation, low level of evidence)
- We do not recommend the use of a barium swallow solely as a diagnostic test for GERD. (Conditional recommendation, low level of evidence)
- We recommend endoscopy as the first test for evaluation of patients presenting with dysphagia or other alarm symptoms (weight loss, GI bleeding), and for patients with multiple risk factors for Barrett’s esophagus. (Strong recommendation, low level of evidence)
- In patients for whom the diagnosis of GERD is suspected but not clear, and endoscopy shows no objective evidence of GERD, we recommend reflux monitoring be performed off therapy to establish the diagnosis. (Strong recommendation, low level of evidence)
- We recommend against performing reflux monitoring off therapy solely as a diagnostic test for GERD in patients known to have endoscopic evidence of Los Angeles grade C or D reflux esophagitis, or in patients with long-segment Barrett’s esophagus. (Strong recommendation, low level of evidence)
Key Concept
- We do not recommend high resolution manometry (HRM) solely as a diagnostic test for GERD.
Defining GERD
A single unifying definition of GERD is difficult. In preparing this guideline, we have blended the multiple definitions in the literature to create the following: GERD is the condition in which the reflux of gastric contents into the esophagus results in symptoms and/or complications. GERD is objectively defined by the presence of characteristic mucosal injury seen at endoscopy and/or abnormal esophageal acid exposure demonstrated on a reflux monitoring study.
Pathophysiology of GERD
The pathophysiology of GERD includes a poorly functioning esophagogastric junction; the antireflux barrier composed of the lower esophageal sphincter and crural diaphragm, coupled with impaired esophageal clearance and alterations in esophageal mucosal integrity. Reflux esophagitis develops when refluxed gastric juice triggers the release of cytokines and chemokines that attract inflammatory cells, and that also might contribute to symptoms. Other contributors to GERD symptoms may include decreased salivary production, delayed gastric emptying, and esophageal hypersensitivity. As such, GERD can no longer be approached as a single disease, but one with multiple phenotypic presentations and different diagnostic considerations.
Symptoms
Typical symptoms of GERD include heartburn and regurgitation. Heartburn is the most common GERD symptom, and is described as substernal burning sensation rising from the epigastrium up toward the neck. Regurgitation is the effortless return of gastric contents upward toward the mouth, often accompanied by an acid or bitter taste. While both heartburn and regurgitation are major symptoms of GERD, the genesis of these symptoms are not the same, and the diagnostic and management approaches vary depending on which symptom predominates. Chest pain, indistinguishable from cardiac pain, may present in conjunction with heartburn and regurgitation, or as the only GERD symptom. The symptoms of GERD are nonspecific and may overlap or be confused with those of other disorders such as rumination, achalasia, eosinophilic esophagitis, reflux hypersensitivity, functional disease, cardiac or pulmonary disease, and paraesophageal hernia.
Extraesophageal manifestations of GERD can include laryngeal and pulmonary symptoms such as hoarseness, throat clearing, and chronic cough, and conditions such as laryngitis, pharyngitis, and pulmonary fibrosis. It also has been proposed that GERD might exacerbate asthma. These extraesophageal manifestations are challenging for patients and physicians because, while they may result from GERD, they may also be due to a host of other causes. Even in patients with established GERD, it can be difficult to establish that GERD is the cause of these extraesophageal problems.
There is no gold standard for the diagnosis of GERD. Thus, the diagnosis is based on a combination of symptom presentation, endoscopic evaluation of esophageal mucosa, reflux monitoring, and response to therapeutic intervention. Heartburn and regurgitation remain the most sensitive and specific symptoms for GERD, although not as reliable as one might believe. A well-done but older systematic review found a variable sensitivity of heartburn and regurgitation for erosive esophagitis (30-76%), with the specificity ranging from 62-96% [5]. Most consensus statements and guidelines advocate a trial of therapy with a proton pump inhibitor (PPI) as a diagnostic “test” in patients with the typical symptoms of heartburn and regurgitation, with the underlying assumption that a PPI response establishes the diagnosis of GERD. While this a practical and efficient approach it is limited by a pooled sensitivity of 78% and specificity of only 54% (using endoscopy and pH monitoring as the reference standard) based on a meta-analysis and prospective study [6, 7].
Chest pain is commonly listed as a symptom of GERD. Similar to heartburn, a PPI trial has often been used for diagnosis of suspected GERD-related chest pain [8]. However, a systematic review of PPI treatment of non-cardiac chest pain found that symptom improvement with a PPI trial was effective only in patients with erosive esophagitis or abnormal pH monitoring [9]. There was no significant response to PPIs compared with placebo when endoscopy and pH monitoring were normal, and the symptoms of chest pain and heartburn did not reliably predict a PPI response [10].
Atypical and extraesophageal symptoms and conditions such as chronic cough, dysphonia, asthma, sinusitis, laryngitis, and dental erosions have been associated with GERD. However, these symptoms and conditions have poor sensitivity and specificity for the diagnosis of GERD. Diagnoses of GERD by extraesophageal symptoms alone or by their response to PPIs are unreliable due to poor sensitivity and specificity for GERD and not recommended (see additional discussion in the Extraesophageal GERD section below).
Barium radiography
Barium radiographs should not be used solely as a diagnostic test for GERD. The presence of reflux on a barium esophagram or upper GI series has poor sensitivity and specificity for GERD when compared to pH testing. In a recent prospective study, only about one-half of patients with abnormal reflux on a barium study were found to have abnormal pH monitoring [11, 12]. The finding of barium reflux above the thoracic inlet with or without provocative maneuvers (including the water siphon test) somewhat increases the sensitivity for reflux, but not sufficiently for barium esophagram to be recommended as a diagnostic test for GERD [13].
Endoscopy
Upper endoscopy is the most widely used objective test for evaluating the esophageal mucosa. For patients with GERD symptoms who also have alarm symptoms such as dysphagia, weight loss, bleeding, vomiting, and/or anemia, endoscopy should be performed as soon as feasible. The endoscopic findings of erosive esophagitis (EE) and Barrett’s esophagus are specific for the diagnosis of GERD. The Los Angeles (LA) classification of EE is the most widely used and validated scoring system [14]. Recent expert consensus statements concluded that LA grade A EE is not sufficient for a definitive diagnosis of GERD, as it is not reliably differentiated from normal [15, 16]. LA B EE can be diagnostic of GERD in the presence of typical GERD symptoms and PPI response, while LA grade C is virtually always diagnostic of GERD. In outpatients, LA grade D EE is a manifestation of severe GERD, but LA grade D EE might not be a reliable index of GERD severity in hospitalized patients. The finding of any Barrett’s esophagus segment >3 cm with intestinal metaplasia on biopsy is diagnostic of GERD and obviates the need for pH testing merely to confirm that diagnosis. In patients with LA grade C and D EE, endoscopy is recommended after PPI treatment to ensure healing and to evaluate for Barrett’s esophagus, which can be difficult to detect when severe EE is present.
For patients having endoscopy for typical GERD symptoms, normal mucosa is the most common finding. There are limited data on the frequency of finding EE in patients undergoing endoscopy while taking PPIs but, since PPIs are highly effective for healing EE, underlying EE clearly can be missed in this setting. Consequently, a diagnosis of non-erosive reflux disease (NERD) should only be made if endoscopy is performed off PPIs. In order to maximize the yield of GERD diagnosis and assess for EE, diagnostic endoscopy should ideally be performed after PPIs have been stopped for 2 weeks, and perhaps as long as 4 weeks if possible. In a small prospective study assessing relapse of EE in patients with LA C EE that was healed with PPIs, discontinuation of PPI therapy led to return of EE in as little as one week [17]. Stopping PPIs for 2 to 4 weeks also will facilitate a diagnosis of eosinophilic esophagitis (EoE), which is a diagnostic consideration when endoscopy is performed for patients with symptoms that are thought to be due to GERD but are not eliminated by PPIs [18]. While esophageal biopsies have little value as a diagnostic test for GERD, they are required to establish a diagnosis of EoE. Since PPIs can eliminate the endoscopic and histologic features of EoE, the diagnosis of EoE cannot be excluded if endoscopy is performed while the patient is taking PPIs [18]. Patients should be advised that they can take antacids for symptom relief during this period of 2 to 4 weeks off PPIs. Some patients will not be able to tolerate discontinuing their PPI therapy, but the diagnostic advantages discussed above warrant an attempt at stopping PPIs before performing diagnostic endoscopy for GERD.
Esophageal Manometry
High resolution manometry (HRM) can be used to assess motility abnormalities associated with GERD, but HRM is not alone a diagnostic test for GERD. Weak LES pressure and ineffective esophageal motility often accompany severe GERD, but no manometric abnormality is specific for GERD. For esophageal impedance-pH monitoring, HRM is used to locate the LES for positioning of transnasal pH-impedance catheters. HRM also has a role in the evaluation of patients considering surgical or endoscopic antireflux procedures, primarily to evaluate for achalasia. Patients with achalasia can have heartburn and regurgitation that are mistaken for GERD symptoms, and antireflux procedures performed for such a mistaken diagnosis of GERD can result in devastating dysphagia. Thus, HRM should ideally be performed in all patients prior to any antireflux procedure. Although esophageal manometry has been proposed as a means to “tailor” antireflux operations, with Nissen (complete) fundoplication reserved for patients with normal peristalsis and partial fundoplication used for those with ineffective esophageal motility, studies on this issue have not supported the efficacy of this approach. Nevertheless, absent contractility is for most a contraindication to fundoplication. Newer developments in HRM include physiologic assessment of esophagogastric junction morphology and provocative testing with multiple rapid swallows or the rapid drink challenge. In patients undergoing surgical treatment of GERD, reduced contractile reserve documented by multiple rapid swallows on HRM is associated with post-operative dysphagia [19]. More data are needed to clarify the role of altered motility on outcomes after magnetic sphincter augmentation and transoral incisionless fundoplication. Until those are forthcoming, a preoperative HRM is recommended. HRM is part of the diagnostic work up for patients unresponsive to PPIs when an etiology for symptoms cannot be demonstrated by impedance pH monitoring and in patients with non-cardiac chest pain especially those not responsive to a PPI trial to assess for motility abnormalities
Reflux monitoring
Ambulatory reflux monitoring (pH or impedance-pH) allows for assessment of esophageal acid exposure to establish or refute a diagnosis of GERD, and for correlating symptoms with reflux episodes using the symptom index or symptom association probability. The main methods of reflux testing include a wireless telemetry capsule (Bravo Reflux Capsule, Medtronic, Minneapolis, MN) attached to the esophageal mucosa during endoscopy and transnasal catheter-based testing, and there are strengths and weaknesses to each approach. With transnasally-positioned pH and pH/impedance catheters, the monitoring period generally is limited to 24 hours, while wireless pH telemetry capsule monitoring can last from 48 to 96 hours. In addition, the capsule avoids the physical discomfort and embarrassment of a transnasal catheter, and so patients are more likely to carry on normal daily activities during capsule pH monitoring [20, 21]. There is no capsule system available for impedance monitoring, which requires a transnasal catheter. Dual-pH sensor transnasal catheters and a hypopharyngeal pH probe are also available to document acid reflux into the proximal esophagus and oropharynx, but the utility of these techniques is highly questionable with studies reporting widely disparate results (see extraesophageal section). Several factors are assessed during reflux testing, including acid exposure time, number of reflux events, and symptom correlation. Impedance pH testing also allows for measurement of weakly acidic and nonacid reflux, assessment of bolus clearance, and extent of proximal reflux. Reflux symptom association on impedance pH testing may help predict symptom response to therapy and may help in diagnosing reflux hypersensitivity [22]. With both wireless capsule and catheter-based reflux tests, the most consistently reliable variables include the total acid exposure time and the composite DeMeester score.
The relationship between symptoms and reflux events can be assessed using the symptom index (SI) or symptom association probability (SAP). To calculate SI, the total number of reflux episodes associated with symptom episodes is divided by the total number of symptom episodes during the entire monitoring period; an SI ≥50% is considered positive. To determine the SAP, the 24-hour monitoring period is divided into 720 two-minute increments, and each increment is evaluated for the occurrence of reflux and symptom episodes. A Fisher’s exact test is performed to determine a p-value for the probability that reflux and symptom events are randomly distributed, and the SAP is determined by subtracting the calculated p value from 1, and multiplying the remainder by 100%; an SAP >95% is considered positive. The validity of both of these indices has been questioned, and neither has been demonstrated superior to the other for clinical purposes. The sensitivity and specificity of reflux monitoring is high in GERD patients with erosive esophagitis, though perhaps not as accurate in those with a normal endoscopy. Impedance monitoring that enables detection of weakly acidic and non-acidic reflux has been shown to be useful in identifying patients with reflux hypersensitivity who might respond to antireflux surgery [23].
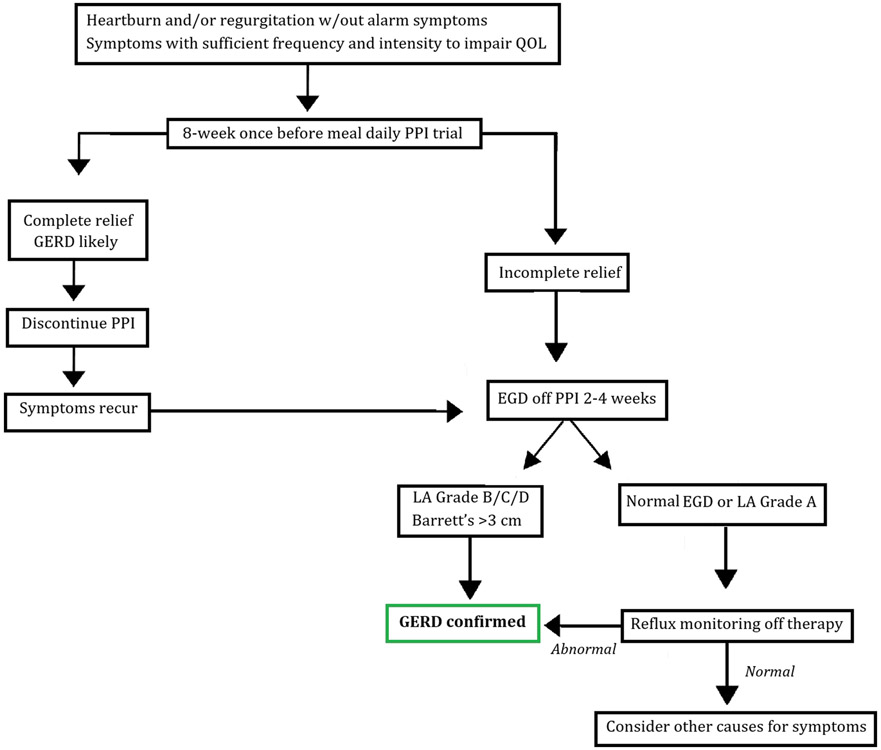
An issue that frequently arises is whether esophageal pH monitoring should be performed on or off PPI therapy. It is generally recommended to monitor after PPIs are stopped for 7 days if the diagnosis of GERD is not clear, and prior to antireflux surgery or endoscopic therapy for GERD to document abnormal acid reflux [16]. This recommendation includes testing with either the telemetry capsule (48-96 hours) or impedance-pH catheter. Reflux monitoring while on PPI therapy is suggested in patients who have had the diagnosis of GERD established by previous objective evidence (i.e. erosive esophagitis, Barrett’s esophagus, prior pH testing off PPI) but who have symptoms potentially reflux-related that have not responded to PPIs. In these patients, impedance/pH testing is recommended to document reflux hypersensitivity for weakly acidic or non-acidic reflux as well as for acid reflux. Figure 1 outlines an overall approach to the diagnosis of GERD.
Figure 1:
Diagnosis of Gastroesophageal Reflux Disease
Diagnosis of GERD in Pregnancy
Approximately two-thirds of pregnant women experience heartburn, which can begin in any trimester [24]. Most patients do not have a previous diagnosis of GERD [25], though a history of GERD may increase the likelihood of GERD occurring during pregnancy. Despite its frequent occurrence during pregnancy, heartburn usually resolves after delivery [26]. Pregnancy and the amount of weight gain during pregnancy are risk factors for frequent GERD symptoms one-year post delivery [26]. Heartburn is the only GERD symptom that has been studied in pregnancy, and the diagnosis of GERD is almost always symptom based. Endoscopy and pH monitoring are rarely needed.
New developments
A recently approved device for evaluation of GERD uses a catheter-based balloon lined by sensors that measure mucosal impedance during endoscopy. This technique has shown promise for differentiating GERD from EoE and may develop to be a useful adjunct to endoscopy in the diagnosis of GERD [27].
GERD Medical Management
Recommendations
- We recommend weight loss in overweight and obese patients for improvement of GERD symptoms. (Strong recommendation, moderate level of evidence)
- We suggest avoiding meals within 2-3 hours of bedtime. (Conditional recommendation, low level of evidence)
- We suggest avoidance of tobacco products/smoking in patients with GERD symptoms. (Conditional recommendation, low level of evidence)
- We suggest avoidance of "trigger foods" for GERD symptom control. Conditional recommendation, low level of evidence)
- We suggest elevating head of bed for nighttime GERD symptoms. (Conditional recommendation, low level of evidence)
- We recommend treatment with PPI over treatment with H2RA for healing erosive esophagitis. (Strong recommendation, high level of evidence)
- We recommend treatment with PPI over H2RA for maintenance of healing from erosive esophagitis. (Strong recommendation, moderate level of evidence)
- We recommend PPI administration 30 to 60 minutes prior to a meal rather than at bedtime for GERD symptom control. (Strong recommendation, moderate level of evidence)
- For GERD patients who do not have erosive esophagitis or Barrett’s esophagus, and whose symptoms have resolved with PPI therapy, an attempt should be made to discontinue PPIs or to switch to on-demand therapy in which PPIs are taken only when symptoms occur and discontinued when they are relieved. (Conditional recommendation, low level of evidence)
- For GERD patients who require maintenance therapy with PPIs, the PPIs should be administered in the lowest dose that effectively controls GERD symptoms and maintains healing of reflux esophagitis. (Conditional recommendation, low level of evidence)
- We recommend against routine addition of medical therapies in PPI non-responders. (Conditional recommendation, moderate level of evidence)
- We recommend maintenance PPI therapy indefinitely or antireflux surgery for patients with Los Angeles grade C or D esophagitis. (Strong recommendation, moderate level of evidence)
- We do not recommend Baclofen in the absence of objective evidence of GERD. (Strong recommendation, moderate level of evidence)
- We recommend against treatment with a prokinetic agent of any kind for GERD therapy unless there is objective evidence of gastroparesis. (Strong recommendation, low level of evidence)
- We do not recommend sucralfate for GERD therapy except during pregnancy. (Strong recommendation, low level of evidence)
- We suggest on-demand or intermittent PPI therapy for heartburn symptom control in patients with non-erosive reflux disease. (Conditional recommendation, low level of evidence)
Key Concepts
- There is conceptual rationale for a trial of switching PPIs for patients who have not responded to one PPI. For patients who have not responded to one PPI, more than one switch to another PPI cannot be supported.
- Use of the lowest effective PPI dose is recommended and logical but must be individualized. One area of controversy relates to abrupt PPI discontinuation and potential rebound acid hypersecretion resulting in increased reflux symptoms. Although this has been demonstrated to occur in healthy controls, strong evidence for an increase in symptoms after abrupt PPI withdrawal is lacking.
Management of GERD requires a multifaceted approach, taking into account the symptom presentation, endoscopic findings, and likely physiological abnormalities. Management decisions may differ depending on hiatal hernia type and size, on the presence of erosive esophagitis and/or Barrett’s esophagus, body mass index and on accompanying physiologic abnormalities such as gastroparesis or ineffective motility with absence of contractile reserve. Medical management includes lifestyle modifications as well as pharmacologic therapy, principally with medications that reduce gastric acid secretion. Surgical and endoscopic options are discussed in other sections.
Non-pharmacologic lifestyle modifications include recommendations for diet modification (content and timing), body positioning with meals and while sleeping, and weight management (Table 3).
Table 3.
Recommendations based on results of a review of studies involving lifestyle modifications
| Lifestyle modification | Strength of scientific evidence | Pathophysiologically conclusive? | Recommendable? |
|---|---|---|---|
| Avoid fatty meals | Equivocal | Equivocal | Yes |
| Avoid carbonated beverages | Moderate | Yes | Yes |
| Select decaffeinated beverages | Equivocal | Equivocal | Not generally |
| Avoid citrus | Weak | Yes | Yes, if citrus triggers symptoms |
| Eat smaller meals | Weak | Yes | Yes |
| Lose weight | Equivocal | Equivocal | Yesa |
| Avoid alcoholic beverages | Weak | Mechanisms not understood; different alcoholic beverages have different effects | Not generally |
| Stop smoking | Weak | Yes | Yesa |
| Avoid excessive exercise | Weak | Yes | Yes |
| Sleep with head elevated | Equivocal | Equivocal | Yes |
| Sleep on the left side | Unequivocal | Yes | Yes |
Diet and Lifestyle Changes
Common recommendations include weight loss for overweight patients, elevating the head of the bed, tobacco and alcohol cessation, avoidance of late night meals and bedtime snacks, staying upright during and after meals, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods such as citrus and tomatoes, and foods with high fat content [28]. Supporting data for these recommendations are limited and variable, often involving only small and uncontrolled studies, and rarely as the only intervention, making interpretation and definitive recommendations difficult. However, multiple studies, including several randomized controlled trials, have demonstrated improvement in nocturnal GERD symptoms and nocturnal esophageal acid exposure with head of bed elevation or sleeping on a wedge. Also, compared to lying left-side down, lying right-side down increases nocturnal reflux and reflux after meals, presumably because right-sided recumbency places the EGJ in a dependent position relative to the pool of gastric contents that favors reflux [29, 30].Thus, patients might be advised to avoid sleeping right side down [31-34].
Several studies have evaluated the effects of various foods on LES pressure to try to determine which items might lead to GERD. In laboratory studies, coffee, caffeine, citrus, and spicy food had little to no effect on LES pressure [35, 36]. However, some of these items might have irritant effects that could evoke GERD symptoms without influencing reflux. Alcohol consumption, tobacco smoking, chocolate, peppermint, and high-fat foods do reduce LES pressure in the laboratory, but few studies document the benefits of avoiding these foods and practices. Smoking cessation was shown to improve GERD symptoms in a large cohort study [37]. Patients in a smoking cessation study had GERD symptoms measured by validated questionnaire, and those who successfully quit smoking for a year had 44% improvement in GERD symptoms, compared to 18% in those who continued to smoke [38].
A recent paper, using data collected from the prospective Nurses Health Study, evaluated women without a known history of GERD for the impact of coffee, tea, soda, milk, water and juice on reflux symptoms. Six servings of coffee, tea, and soda were associated with increased reflux symptoms compared to zero servings per day. In contrast, milk and juice were not associated with increased reflux symptoms, despite the acidic nature of some of these beverages [39]. Substituting water for two servings of coffee, tea and soda was associated with a decrease in GERD symptoms, suggesting that substitution of water for these beverages might be helpful in the management of GERD.
The timing of food intake can also affect GERD symptoms. A short interval (<3 hours) between eating and bedtime or lying supine is associated with increased GERD symptoms and need for medication [40]. Weight gain has been associated with new onset of GERD symptoms [41], even in those with a normal BMI at baseline. Obesity increases the risk of GERD, possibly due to a combination of eating a diet high in fat and other foods that promote reflux, increased intra-abdominal pressure that promotes reflux due to increased intra-abdominal fat, and physiologic changes induced by products of visceral fat [42]. Several studies have examined the role of weight and weight loss on GERD. A population-based study in Norway assessed weight and GERD symptoms at baseline and 10 years later, and identified a dose-dependent improvement in GERD symptoms with weight loss [43]. Prospective and cohort studies also have shown improvement in GERD with weight loss. One study documented a 40% reduction in frequent GERD symptoms in women who reduced their BMI by 3.5 or more compared with controls [44]. A meta-analysis suggests that weight loss in overweight patients, avoidance of eating prior to going to sleep, and smoking cessation are effective in relief of GERD symptoms [45].
Medications
The backbone of pharmacologic therapy for GERD are medications that are directed at neutralization or reduction of gastric acid. Agents in this class include antacids, histamine H2-receptor antagonists (H2RA), and proton pump inhibitors. Antacids are used exclusively for on-demand symptom relief with little evidence to favor one type over another. Studies with an alginic acid preparation manufactured in the United Kingdom suggest potential efficacy in symptom relief compared to other products, but alginate content of preparations sold in other countries is variable [46].
Proton Pump Inhibitors
PPIs are the most commonly prescribed medication based on ample data demonstrating consistently superior heartburn and regurgitation relief, as well as improved healing compared to H2RAs. A meta-analysis (published when only two PPIs were available) provides important insight into PPI efficacy. PPIs showed a significantly faster healing rate (12%/week) vs. H2RAs (6%/week), and faster, more complete heartburn relief (11.5%/week) vs. H2RAs (6.4%/week) [47, 48].
Studies on GERD treatment typically last only 8-12 weeks, in part because symptom relief and healing appear to peak in that time frame. The healing rates of erosive esophagitis are not linear; thus, clinicians and patients need to understand that symptom relief and healing may not be rapid. PPIs are associated with a greater rate of “complete” symptom relief (usually assessed at 4 weeks) in patients with erosive esophagitis (~70-80%) compared to patients with so called non-erosive reflux disease (NERD) in which symptom relief approximates 50-60% [49]. Trials in NERD patients are based on symptoms of frequent heartburn and the absence of erosions on an index endoscopy without objective documentation of GERD by reflux monitoring. There are likely many patients included in NERD who have functional heartburn and thus unlikely to respond to PPI.
Meta-analyses suggest that overall GERD symptom relief and healing rates differ little among the seven available PPIs, despite studies demonstrating differences in pH control. A meta-analysis examining efficacy of different PPIs for healing of erosive esophagitis included 10 studies (15,316 patients) [50]. At 8 weeks, there was a 5% (RR, 1.05; 95% CI, 1.02-1.08) relative increase in the probability of healing of erosive esophagitis with esomeprazole, yielding an absolute risk reduction of 4% and number needed to treat (NNT) of 25, a number unlikely to be clinically meaningful. Although all the PPIs are effective for healing reflux esophagitis when given in their standard dosages, there are wide variations in the acid-suppression potency of the different PPI preparations. If relative acid-suppression potencies of individual PPIs (based on their effects on mean 24-hour intragastric pH) are standardized to omeprazole to yield ‘omeprazole equivalents’ (OEs, with omeprazole having an OE of 1.00), the relative potencies of standard-dose pantoprazole, lansoprazole, omeprazole, esomeprazole and rabeprazole have been estimated at 0.23, 0.90, 1.00, 1.60 and 1.82 OEs, respectively [51] [52].
PPIs can bind only to proton pumps that are actively secreting acid. Since meals stimulate proton pump activity, enteric-coated PPIs control intra-gastric pH best when given before a meal (30-60 minutes before breakfast for once-daily dosing, 30-60 minutes before breakfast and dinner for twice-daily dosing [53, 54]. Bedtime dosing is discouraged as this is less effective than a pre-dinner dose in acid control [55]. Dexlansoprazole , a dual delayed release PPI, in which first absorption is in the duodenum, then partially further down the small bowel appears to have similar efficacy in pH control regardless of meal timing. An omeprazole-sodium bicarbonate combination that is not enteric coated provides good control of intragastric pH in the first four hours of sleep when dosed at bedtime [56]. There appears to be a wide variation in individual intragastric pH control between PPIs, a rationale for considering switching PPIs in patients with incomplete response [57]. In a study of 282 patients with persistent heartburn on lansoprazole 30 mg once daily who were randomized either to double the dose of lansoprazole or to switch to esomeprazole 40 mg once daily, the two strategies were equally effective, with approximately 55% of patients in both groups experiencing a decrease in the percentage of heartburn-free days [58]. Studies suggest that genetic differences in CYP2C19 metabolism affect PPI response, however genetic testing in this regard has no established role in practice. If one is considering a PPI switch, changing to a PPI that does not rely on CYP2C19 for primary metabolism (rabeprazole) might be considered.
Maintenance PPI therapy should be administered for patients with GERD complications including severe erosive esophagitis (LA C or D) and Barrett’s esophagus[59]. For patients without erosive esophagitis or Barrett’s esophagus who continue to have symptoms when PPI therapy is discontinued, consideration can be given to on-demand therapy in which PPIs are taken only when symptoms occur and discontinued when they are relieved [60, 61]. Two-thirds of patients with nonerosive disease responsive to PPIs will demonstrate symptomatic relapse when PPIs are stopped. With LA grade C esophagitis, nearly 100% will relapse within 6 months [62]. Recurrence of erosive esophagitis after discontinuation can occur in as little as 1-2 weeks, particularly in patients with prior LA C erosive esophagitis [17]. Patients with LA grade C or D erosive esophagitis should remain on long-term PPI therapy to maintain healing.
In some cases, patients with NERD and otherwise non-complicated GERD can be managed successfully with on-demand or intermittent PPI therapy. In one randomized controlled trial, 83% of NERD patients randomized to 20 mg of omeprazole on demand were in remission at 6 months compared with 56% of patient on placebo [63]. In a systematic review of randomized controlled trials comparing on-demand PPI vs placebo, symptom-free days for NERD patients in the on-demand arm were equivalent to rates for patients on continuous PPI therapy, and both on-demand and continuous PPI were superior to placebo. On-demand PPI therapy was not better than continuous PPI therapy for patients with erosive esophagitis. Step-down therapy to H2RAs is another acceptable option for management, particularly in NERD patients [64, 65].
Use of the lowest effective dose is recommended and logical but must be individualized. One area of controversy relates to abrupt PPI discontinuation and potential rebound acid hypersecretion resulting in increased reflux symptoms. Although rebound acid hypersecretion has been demonstrated to occur in healthy controls, strong evidence for an increase in symptoms after abrupt PPI withdrawal is lacking [66-68].
Histamine-2-Receptor Antagonists Taken at Bedtime
Medical options for GERD patients with incomplete symptom response on PPI therapy are limited. The addition of bedtime H2RA has been suggested for patients on PPI with persistent nocturnal symptoms. This approach gained popularity after several studies demonstrated improved overnight intragastric pH control with the addition of an H2RA [69], although a well-done study demonstrated loss of pH control (tachyphylaxis) after a month of bedtime H2RA therapy [70]. Based on these data, use of a bedtime H2RA may be beneficial if dosed on an as-needed basis for patients with nocturnal symptoms and for patients with objective evidence of nocturnal acid reflux on pH monitoring despite PPI treatment.
Prokinetics
There are limited data on the use of prokinetic agents for patients with GERD. Metoclopramide has been shown to increase lower esophageal sphincter pressure, enhance esophageal peristalsis, and augment gastric emptying. However, data on its efficacy in GERD are scant, and significant adverse events have been reported with long-term and high-dose metoclopramide use, including central nervous system side effects such as drowsiness, agitation, irritability, depression, dystonic reactions, and tardive dyskinesia [71, 72]. Thus, we do not recommend using metoclopramide solely for the treatment of GERD. Prucalopride, a 5 HT agonist FDA-approved for treatment of constipation, was shown in one off-label use study to improve gastric emptying and reduce esophageal acid exposure in patients with GERD. In the future, this may be a potential add-on therapy for patients with GERD on PPIs found to have delayed gastric emptying [73].
Baclofen
Baclofen, a GABAB agonist, reduces the transient LES relaxations that enable reflux episodes. Baclofen decreases the number of post-prandial acid and non-acid reflux events, nocturnal reflux activity, and belching episodes [74-76]. A trial of baclofen at a dosage of 5-20 mg three times a day can be considered in patients with objective documentation of continued symptomatic reflux despite optimal PPI therapy. Short-term randomized controlled trials have demonstrated symptomatic improvement with baclofen [74-76]. A randomized, placebo-controlled trial of medical therapy (including baclofen) vs antireflux surgery for PPI-refractory heartburn found no significant benefit for baclofen compared to placebo at one year, but the study was not sufficiently powered to detect a small but potentially important effect for baclofen [23]. Usage is limited by side effects of dizziness, somnolence, and constipation.
Sucralfate
Sucralfate is a mucosal protective agent, but few data document its efficacy in GERD. Limited studies have suggested similar efficacy to H2RAs, but there are no comparative data to PPIs, nor any combination studies with these agents. Sucralfate is largely unabsorbed and has no systemic toxicity. There is little to recommend for this agent in GERD outside of pregnancy.
Treatment of GERD during Pregnancy
A small randomized controlled trial found that sucralfate was superior to dietary and lifestyle modifications for relieving heartburn and regurgitation in pregnant women [77]. Approximately two-thirds of pregnant women experience heartburn. It has been recommended that treatment of GERD during pregnancy should start with lifestyle modifications. When lifestyle modifications fail, antacids (aluminum-, calcium-, or magnesium-containing), alginates, and sucralfate are the first-line therapeutic agents. All histamine H2-blockers are FDA Category B, and all PPIs are FDA Category B except omeprazole, which is FDA Category C.
Extraesophageal GERD Symptoms
Recommendations
- We recommend evaluation for non-GERD causes in patients with possible extra-esophageal manifestations before ascribing symptoms to GERD. (Strong recommendation, moderate level of evidence)
- We recommend that patients who have extra-esophageal manifestations of GERD without typical GERD symptoms (e.g. heartburn, regurgitation) undergo reflux testing for evaluation prior to PPI therapy. (Strong recommendation, moderate level of evidence)
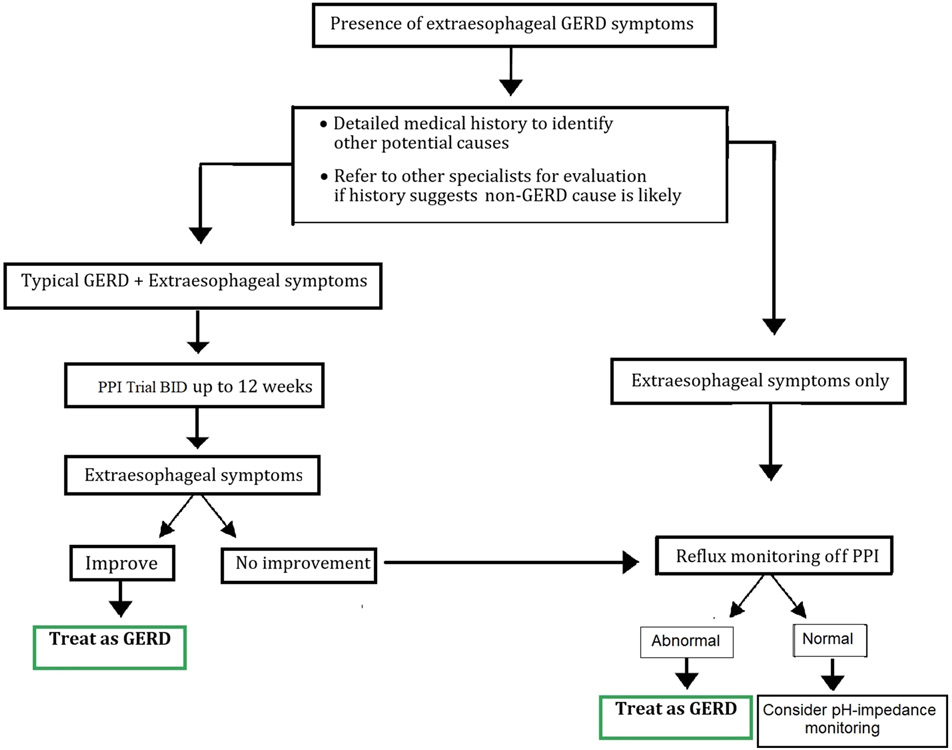
- For patients who have both extraesophageal and typical GERD symptoms we suggest considering a trial of twice-daily PPI therapy for 8 to 12 weeks prior to additional testing. (Conditional recommendation, low level of evidence)
- We suggest that upper endoscopy should not be used as the method to establish a diagnosis of GERD-related asthma, chronic cough, or laryngopharyngeal reflux. (Conditional recommendation, low level of evidence)
- We suggest against a diagnosis of laryngopharyngeal reflux based on laryngoscopy findings alone and recommend additional testing should be considered. (Conditional recommendation, low level of evidence)
- In patients treated for extraesophageal reflux disease, surgical or endoscopic anti-reflux procedures are only recommended in patients with objective evidence of reflux. (Conditional recommendation, low level of evidence)
Key Concepts
- While GERD may be a contributor to extraesophageal symptoms in some patients, careful evaluation for other causes should be considered for patients with laryngeal symptoms, chronic cough, and asthma.
- Diagnosis, evaluation, and management of potential extraesophageal symptoms of GERD is limited by lack of a gold standard test, variable symptoms, and other disorders which may cause similar symptoms
- Due to difficulty distinguishing between patient with laryngeal symptoms and normal controls, salivary pepsin testing is not recommended for evaluation of patients with extraesophageal reflux symptoms
- For patients whose extraesophageal symptoms have not responded to a trial of twice-daily PPIs, we recommend upper endoscopy, ideally off PPIs for 2 to 4 weeks. If endoscopy is normal, consider reflux monitoring. Demonstration of erosive esophagitis by endoscopy establishes a diagnosis of GERD, but does not confirm that GERD is the cause of the extraesophageal symptoms. Confirmation may require pH/impedance testing.
- For patients with extraesophageal symptoms, we do not routinely recommend oropharyngeal or pharyngeal pH monitoring.
Numerous extraesophageal symptoms and conditions have been attributed to GERD, including chronic cough, throat-clearing, hoarseness, globus, asthma, and laryngitis. These are vexing for patients as well as physicians, as the symptoms ascribed to extra-esophageal GERD are often non-specific and overlap with other disorders. Evaluation by otorhinolaryngology, allergy, and pulmonary specialists should be considered in these patients, depending on the constellation of symptoms. Currently available diagnostic tools to establish GERD as the cause of extraesophageal symptoms have substantial limitations. PPI treatment is relied upon as both a diagnostic tool and treatment for extraesophageal GERD symptoms, but is often ineffective and prolonged treatment trials with PPI may delay diagnosis and care for patients with non-reflux laryngeal and pulmonary disorders.
Symptoms
The association between GERD and extraesophageal symptoms has been examined in multiple studies. In a case-control study of veterans, patients with esophagitis or esophageal strictures were more likely to have a diagnosis of laryngitis (OR 2.01), aphonia (OR 1.81), asthma (OR 1.51), and pharyngitis (OR 1.48) compared to control patients [78]. In a U.S. survey study, 26% of patients reported both GERD and laryngeal symptoms [79]. Of this group with both GERD and laryngeal complaints, 38% reported voice disorders and 44% had occasional breathing difficulties. Some studies have suggested that chronic cough may be due to GERD in 21-41% of cases [80]. However, due to the wide variety of causes of chronic cough, the American College of Chest Physicians guideline for evaluation of chronic cough suggests looking for other sources before attributing chronic cough to GERD [81].
GERD may also have a role in asthma, with one systematic review of 28 studies identifying GERD symptoms in 59% of asthma patients and abnormal pH testing in 51% [82]. However, data from several randomized controlled trials suggest that PPI treatment is ineffective for many patients with asthma, which brings in to question the role of acid reflux in asthma symptoms [83, 84].
Endoscopy
Endoscopy is frequently used for assessing classic symptoms of GERD, such as heartburn and regurgitation, but its role in assessment of extraesophageal GERD symptoms is less clear. In patients with extraesophageal GERD symptoms, the reported frequency of erosive esophagitis ranges from 18% to 52% [85, 86]. However, the presence of erosive esophagitis does not confirm GERD as a cause of extraesophageal symptoms, as erosive esophagitis has been found in 16% of patients with no typical or extraesophageal GERD symptoms in a general population who were undergoing periodic health checkup [87]. Nevertheless, if LA grade C or D erosive esophagitis is present, this establishes a diagnosis of severe GERD and justifies a trial of PPI therapy.
Laryngoscopy
Laryngoscopy performed by an otorhinolaryngologist (ENT) is commonly used to assess for signs of extraesophageal GERD, in particular, laryngopharyngeal reflux (LPR). Findings on laryngoscopy that are associated with reflux include posterior commissure hypertrophy, laryngeal and arytenoid inflammation, vocal cord edema, and endolaryngeal mucus. Several scoring systems have been developed for grading the laryngoscopic findings, the most common of which is the reflux finding score (RFS) [88]. However, correlation between symptoms, laryngoscopic findings, and other objective testing such as pH and pH-impedance monitoring, is low. In a systematic review evaluating different reported signs of LPR and relevant clinical outcomes, 29 different LPR signs and multiple scoring systems were evaluated. LPR signs on laryngoscopy were found to have low specificity, with validation hampered by the lack of a gold standard for diagnosis [89]. Inter-rater reliability for laryngeal findings was also found to be low for multiple laryngoscopic features attributed to LPR [90]. In one study of patients originally thought to have LPR, a careful review of laryngoscopic findings by study investigators identified other causes of the laryngeal complaints including cancer, muscle tension dysphonia, vocal cord paresis, and benign mucosal lesions [91]. In one recent pediatric study, the laryngoscopic RFS did not correlate with pH-impedance findings, the presence of erosive esophagitis, or quality of life [92]. This lack of correlation between laryngoscopic findings and symptoms also been documented in adults. In one study of 105 normal, asymptomatic volunteers, 86% had findings associated with reflux on laryngoscopy, with some signs of LPR seen in 70% of participants [93]. A second study of normal, asymptomatic volunteers found at least one sign of inflammation in 93% of participants who underwent flexible laryngoscopy [94]. The use of laryngoscopy for diagnosis of LPR has substantial limitations, with inflammation seen in asymptomatic volunteers, low reproducibility, and lack of correlation between laryngoscopic findings and symptoms. While ENT physicians often treat LPR based on laryngoscopy findings, a poor response to medical therapy should not be surprising.
Reflux testing
Multichannel pH-impedance testing, traditional catheter-based pH testing, and wireless pH testing have been used to evaluate patients with extraesophageal GERD symptoms. Reflux testing using pH-impedance can detect acidic (pH <4), weakly acidic (pH 4-7), and nonacidic reflux (pH >7), and determine the extent of proximal reflux, which may be important in the evaluation of extra-esophageal GERD symptoms. pH-impedance testing in patients with LPR symptoms is abnormal in 40% of cases [95]. pH-Impedance monitoring has been used in several studies of patients with LPR symptoms, and those with abnormal pH impedance results were found to be more likely to respond to PPI treatment than patients with normal testing [96, 97]. Studies in which pH-impedance monitoring was used to identify the relationship between reflux events and cough episodes have shown that chronic cough can be associated with weakly acidic and nonacidic reflux events [98, 99]. In a study of 21 patients with globus and 12 with heartburn alone who were evaluated by pH-impedance testing performed on PPI therapy, proximal reflux was noted to be more common in the globus patients [100]. Use of pH-impedance in this study increased the yield of standard pH testing by 28%, and identified proximal esophageal reflux as a significant predictor of globus.
Presently, the clinical significance of proximal reflux is unclear, and studies have varied in their criteria for defining this entity [101]. One study found that extraesophageal symptoms were not more frequently associated with proximal esophageal reflux than typical GERD symptoms and that, irrespective of symptoms, half of all reflux events extended to the proximal esophagus [102]. In a study of 237 patients with extraesophageal symptoms refractory to medical therapy, traditional reflux parameters were better predictors of fundoplication outcome than impedance testing, with the presence of heartburn and acid exposure times >12% increasing the probability of surgical success [103]. In a retrospective study of 33 patients with refractory reflux symptoms (typical and atypical) evaluated by pH-impedance monitoring on PPIs, only a positive SAP for heartburn or regurgitation was associated with improvement after surgery [104]. In the absence of a clear definition of ‘normal’ proximal esophageal reflux, interpretation of impedance results for extraesophageal GERD is problematic, and surgical outcomes appear to be predicted better by traditional reflux parameters.
The choice to test on or off PPI in patients with extraesophageal symptoms has no clear answer. Testing off PPI can be used to determine whether pathologic esophageal acid exposure is present and should be considered when the pre-test probability for GERD is low. Testing on PPI can be considered in patients already known to have pathologic acid exposure, such as those with Barrett’s esophagus or with LA C or D erosive esophagitis [105]. One proposed model for determining which patients should undergo pH testing on or off a PPI was developed using a population of 471 patients with refractory heartburn or extraesophageal GERD [106]. Risk factors for abnormal esophageal acid exposure in patients with suspected extraesophageal reflux included BMI >25, hiatal hernia, and presence of heartburn. In patients with extraesophageal symptoms persistent after 2 months of BID PPI, the investigators suggest calculation of the Heartburn, Asthma, and BMI Extraesophageal Reflux (HAs-BEER) score – 1 point each for BMI >25, asthma, and heartburn, but no points for cough or hoarseness. pH impedance testing on PPI was recommended for patients with a HAs-BEER score of 3, whereas testing off PPI was recommended for those with scores ≤2. Other studies attempting to address the question of testing on or off PPI have found that the total number of reflux episodes detected by impedance is similar between testing on and off PPI [107, 108], while one study found that patients were more likely to have a positive SAP off PPI [107].
Wireless pH testing also has been used for evaluation of patients with extraesophageal symptoms. In one series of patients with extraesophageal GERD who had wireless pH testing, 81% had abnormal acid exposure, typically mild to moderate reflux, and more often in the upright position [109]. However, as wireless pH testing focuses on distal acid reflux only, it is not a reliable index for laryngeal acid exposure. However, if normal over 96 hours of testing, it provides evidence against acid reflux as a cause of symptoms.
Pharyngeal and Oropharyngeal Reflux Monitoring
Catheter-based pharyngeal pH monitoring with dual sensor probes, and oropharyngeal pH monitoring have been proposed as methods to better detect LPR compared to traditional pH monitoring and pH-impedance. However, the reliability of pharyngeal pH measurement has been questioned, and proximal sensor data may be unreliable due to placement issues [110-113]. Similar to pH-impedance testing, the amount of proximal reflux considered abnormal varies by study [114-117].A systematic review found no significant differences in dual-channel pH testing results between normal controls and patients with laryngeal symptoms [118].
Early studies of oropharyngeal pH testing were promising, and appeared to predict success of antireflux surgery [119, 120]. However, subsequent studies have failed to identify a significant correlation between oropharyngeal reflux events and pH-impedance reflux events, suggesting that decreases in oropharyngeal pH may be due to factors other than gastroesophageal reflux [121-125]. One study of adults with laryngeal symptoms evaluated patients using the reflux symptom index, video laryngoscopy, and oropharyngeal pH monitoring, followed by a PPI trial [126]. There were no significant differences in oropharyngeal acid exposure between PPI responders, partial responders, and nonresponders. Lack of correlation between oropharyngeal pH events and pH impedance events was seen in another study of adults with suspected LPR - oropharyngeal pH test results were unable in distinguishing asymptomatic volunteers from patients with laryngeal irritation [127].
Salivary pepsin testing
Salivary pepsin testing has been proposed as a non-invasive method of detecting LPR. A recent meta-analysis of 11 observational studies examined the role of salivary pepsin testing in diagnosing LPR [128]. Significant heterogeneity was found, with varying reference standards for LPR diagnosis (pH monitoring, symptoms, laryngoscopic signs), different pepsin assays, variable definitions of abnormal tests, and number of pepsin tests performed. Another study found pooled sensitivity of pepsin testing for LPR was 64% and specificity was 68%, with an AUC of 0.71 [129]. Another meta-analysis of pepsin as a marker of LPR reached similar conclusions, and noted that control patients often had elevated salivary pepsin levels [130]. Salivary pepsin levels also may vary by time of day, with higher levels in the morning, which limits interpretation [131]. A study of children with GERD found no correlation between multichannel pH-impedance and salivary pepsin testing results [129]. In a study of adults with laryngeal complaints, pepsin was found in the saliva of 78% of those with laryngoscopic signs of laryngeal inflammation, but in 47% of patients with normal laryngoscopy [130]. In another study, pepsin testing was unable to distinguish between healthy adult volunteers and patients with extra-esophageal reflux symptoms [132].
PPIs and Extraesophageal Symptoms
A clinical response to PPI therapy has been proposed as a method to both diagnose and treat extra-esophageal GERD [133-135], and has been evaluated in numerous observational studies and randomized controlled trials, with 4 meta-analyses and 1 systematic review compiling the results. The efficacy of PPIs in LPR remains unclear, as two meta-analyses found no significant benefit of PPIs [136, 137], while two found some benefit [138, 139]. In one recent meta-analysis of 10 RCTs of PPI treatment for LPR, the pooled relative risk of improvement with any PPI treatment was 1.31, with a stronger PPI effect seen in studies that excluded dietary management of LPR (RR 1.42) [138]. Another meta-analysis found improved symptoms in LPR patients treated with PPI compared to placebo, with improvements in symptom index, but not in the laryngoscopy reflux finding score [139]. These analyses showed that the diagnostic criteria for LPR varied substantially between studies, as did clinical outcomes, treatment regimens, and treatment duration, making recommendations for use of PPI in LPR challenging [138, 140].
While PPI treatment is often the first step in the management of LPR, this approach may need to be reconsidered. One study comparing up-front reflux testing for LPR patients rather than starting them on empiric PPI therapy found that overall evaluation and treatment costs were lower with initial pH-impedance and esophageal manometry testing [141]. Also, a comparison of several algorithms for managing LPR revealed that total costs of therapy were lower in LPR patients treated with initial twice-daily PPI dosing rather than once-daily PPI [141].
Recent studies have questioned the role of PPI therapy for patients with asthma. Two randomized controlled trials, one in adults and one in children, showed no benefit in controlling asthma symptoms in patients on twice-daily PPIs [83, 84]. One systematic review on the role of PPIs in asthma found a small improvement in morning peak expiratory flow that was unlikely to be clinically meaningful [142]. One RCT did show improved asthma symptoms in patients on twice-daily PPIs, but only in GERD patients with nocturnal respiratory symptoms [143]. Chronic cough has also been attributed to GERD, but recent studies and systematic reviews suggest that PPIs are not effective in treating chronic cough in the majority of patients [144] [145-147].
Surgery
Antireflux surgery has been used to treat patients with extraesophageal GERD symptoms, but outcomes are inferior to those of antireflux surgery for patients with traditional GERD symptoms. Two systematic reviews (involving primarily studies that were small, retrospective, and uncontrolled) have examined the relationship among extraesophageal GERD symptoms, esophageal acid exposure, and surgical outcomes, [148, 149]. The range of reported improvement in extraesophageal symptoms was wide, ranging from 15% to 95%, with extraesophageal symptoms having poorer response to surgical treatment than typical GERD symptoms.
In one study, patients for whom PPIs provided only incomplete relief of laryngeal symptoms despite normalizing esophageal acid exposure were offered antireflux surgery. At one year, only 10% of patients who underwent surgery and 7% of patients who continued medical therapy for GERD had improvement in laryngeal symptoms. However, two-thirds of patients who pursued non-surgical, non-GERD treatments for laryngeal symptoms had improved symptoms at 1 year [150]. This study illustrates the importance of pursuing non-GERD treatments for unexplained laryngeal symptoms.
Several observational studies and one randomized controlled trial have suggested that antireflux surgery can improve asthma symptoms. In the one RCT, 74% of surgically-treated patients (n=16) had improvement in asthma symptoms compared to 9% on H2RAs and 4.2% in the control group [151]. Observational studies of antireflux surgery for asthma patients suggest that asthma symptoms can improve, but improvement in pulmonary function tests and objective parameters is inconsistent [151-153]. Furthermore, heterogeneity in inclusion criteria and surgical techniques among studies make it difficult to draw meaningful conclusions about the efficacy of antireflux surgery for treating asthma.
Predicting which patients with extraesophageal symptoms will improve with antireflux surgery is challenging. In one study of patients with extraesophageal symptoms, predictors of symptomatic improvement after surgery included the presence of heartburn with or without regurgitation, and abnormal acid exposure time on pH testing [103]. Recurrence of extraesophageal symptoms after surgical therapy is also a concern. One retrospective cohort study compared adults with extraesophageal GERD (n=36) and typical reflux symptoms (n=79), all of whom had abnormal distal esophageal acid exposure. Recurrence of symptoms after surgery was more likely in patients with extraesophageal symptoms and in those who had a poor response to preoperative PPI therapy [154]. Patients with extraesophageal symptoms that do not respond to PPI and patients without objective evidence of reflux should avoid surgical or endoscopic treatment of GERD.
Refractory GERD
Recommendations
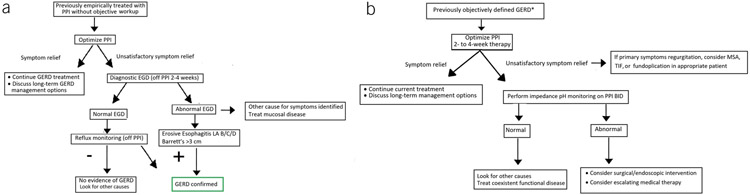
- We recommend optimization of PPI therapy as the first step in management of refractory GERD. (Strong recommendation, moderate level of evidence)
- We suggest esophageal pH monitoring (Bravo, catheter-based, or combined impedance-pH monitoring) performed OFF PPIs if the diagnosis of GERD has not been established by a prior pH monitoring study or an endoscopy showing long-segment Barrett’s esophagus or severe reflux esophagitis (Los Angeles grade C or D). (Conditional recommendation, low level of evidence)
- We suggest esophageal impedance pH monitoring performed ON PPIs for patients with an established diagnosis of GERD whose symptoms have not responded adequately to twice-daily PPI therapy. (Conditional recommendation, low level of evidence)
- For patients who have regurgitation as their primary PPI-refractory symptom and who have had abnormal gastroesophageal reflux documented by objective testing, we suggest consideration of anti-reflux surgery or TIF. (Conditional recommendation, low level of evidence)
Key Concepts
- It is important to stop PPI therapy in patients whose off-therapy reflux testing is negative, unless another indication for continuing PPI is present.
- Esophageal manometry should be considered as part of the evaluation for refractory GERD in patients with a normal endoscopy and pH monitoring study, and for patients being considered for surgical or endoscopic treatment.
- If not already performed off PPIs, we recommend diagnostic upper endoscopy after discontinuing PPI therapy, ideally for two to four weeks. Esophageal biopsies should be performed even if endoscopy reveals normal mucosa.
- We recommend performing high-resolution esophageal manometry in patients with refractory GERD if reflux monitoring and endoscopy are unrevealing.
It has been suggested that up to 40% of patients treated with PPI will report persistent symptoms of heartburn and regurgitation, with negative effects on quality of life [155-157]. One systematic review of GERD studies found that persistent GERD symptoms were present in 32% of patients participating in primary care-based randomized trials of GERD therapy, with 45% of patients in observational studies having persistent symptoms [156]. Although there is limited data evaluating the benefit of twice-daily PPIs for patients with GERD symptoms refractory to once-daily PPIs [158], GERD generally has not been considered “PPI-refractory” unless the patient has been on PPIs BID. The most commonly accepted definition of refractory GERD is persistent heartburn and/or regurgitation despite 8 weeks of double-dose PPI therapy [159]. Other authorities consider persistent symptoms after 12 weeks on double-dose PPI to be refractory GERD [160]. These patient-driven definitions, while pragmatic, are broad. Similarly, the terms “complete relief/response”, “partial relief/response”, and “no response” have been arbitrarily and poorly defined, and duration of symptoms and PPI dosing vary across studies [156, 161]. GERD is a disease with multiple symptom presentations that respond variably to PPIs. Heartburn is more likely to respond to PPIs than regurgitation or extraesophageal symptoms. As such, it is clinically useful to separate refractory heartburn, regurgitation, and extraesophageal symptoms when thinking about these patients. Table 4 lists four potential mechanisms of refractory GERD.
Table 4.
Potential mechanisms underlying symptoms suspected due to GERD but refractory to PPI therapy
| Despite PPI therapy abnormal acid reflux persists and is causing symptoms |
|---|
| There is reflux hypersensitivity, a condition in which PPIs have normalized esophageal acid exposure, but “physiologic” reflux episodes (acidic or nonacidic) nevertheless are strongly associated with and evoke symptoms |
| The symptoms are not due to GERD, but are caused by esophageal disorders other than GERD (e.g., EoE and achalasia) |
| The symptoms are not due to GERD, but are caused by nonesophageal disorders (e.g., gastroparesis, rumination, and heart disease) |
| The symptoms are functional (i.e., not because of GERD or any other identifiable histopathologic, motility, or structural abnormality). |
There are two broad groups of patients with symptoms despite PPI therapy. One group is patients with symptoms suspected to be GERD-related who have been empirically treated with a PPI (typically once- then increased to twice-daily) yet remain symptomatic. The second group of patients have objective evidence of GERD, with endoscopic findings of erosive esophagitis or Barrett’s esophagus and/or reflux testing showing abnormal esophageal acid exposure, who have incomplete or no response to PPI. When discussing the overall approach to patients with GERD symptoms not relieved by PPIs, it is prudent to discuss management of these two groups separately.
History and Physical Exam
The evaluation of refractory GERD should begin with a careful history and physical examination. This will enable the clinician to make a meaningful assessment of the likelihood that GERD is causing the bothersome symptoms and may provide clues to the presence of non-esophageal disorders. If no obvious non-esophageal disorders are present, then optimization of PPI therapy is recommended. This is critical for managing patients with persistent GERD symptoms, regardless of whether the patient has been empirically treated or carries an objective diagnosis of GERD.
Optimization of PPI Therapy
Optimization of PPI therapy includes verifying compliance, confirming that the PPI is taken 30 to 60 minutes before the first meal of the day for daily dosing and before the first and dinner meal for twice daily dosing [162]. A study analyzing data from randomized trials in which gastric pH monitoring was performed in patients receiving various PPI formulations concluded that twice-daily PPI therapy is superior to once-daily double-dose PPI therapy in maintaining gastric pH above 4 during a 24-hour monitoring period [52]. We analyzed data from randomized clinical trials that performed pH testing in patients receiving solid-dose PPI formulations. In one study, patients with good symptom control took daily PPI on 84% of days, compared to patients with poor symptom control, who took PPI on only 55% of days [163], with similar findings seen in other studies [80]. In a recent randomized, multicenter trial of Veterans with heartburn refractory to PPI treatment, 42 of 366 (11.4%) participants had ≥50% improvement in GERD symptoms when omeprazole use was optimized, with dosing 30 minutes before breakfast and dinner[23]. Another study of nonerosive reflux disease patients with typical GERD symptoms despite PPI use found that 35% responded to daily esomeprazole when dosed correctly [164]. A smaller trial examined the effects of optimizing daily omeprazole compared to ad lib dosing, and found improvement in symptoms and GERD quality of life scores in those receiving education on proper dosing of daily PPI [165]. Some studies have found that doubling the PPI dose or dosing twice daily can help with persistent typical symptoms of GERD, as can switching to a different PPI [58, 166]. Regardless of dose, a small, but clinically significant number of patients will have symptom improvement with the simple, low-cost intervention of optimizing PPI therapy.
Endoscopy
Endoscopy is the next step to investigate persistent GERD symptoms and evaluate alternative diagnoses. Performing endoscopy after a PPI holiday might increase the yield for identifying erosive esophagitis or determine if an alternative diagnosis, such as eosinophilic esophagitis, is responsible for symptoms. A recent study found that erosive esophagitis can relapse within two weeks after stopping PPI, with some patients even developing Los Angeles grade C erosive esophagitis [62]. Eosinophilic esophagitis has been seen in 1-8% of patients with refractory GERD [23, 167-170]. Temporarily discontinuing PPIs prior to endoscopy in these patients may unmask the eosinophilic esophagitis histology, which could be obscured if endoscopy is performed on PPI, missing an opportunity to identify the cause of ongoing GERD symptoms. In patients with persistent GERD symptoms on PPIs, there is a low likelihood of finding reflux esophagitis if PPIs are not stopped prior to endoscopy [16, 171].
Reflux monitoring
Regardless of symptom presentation, it is imperative to document the presence of abnormal or ongoing reflux in order to plan treatment options for patients with persistent GERD symptoms. Reflux monitoring can identify patients with ongoing acid reflux, weakly acidic, non-acidic reflux, adequate acid control but ongoing symptoms, and normal reflux parameters. Depending on the clinical situation, performing monitoring off PPI for seven days or testing for acid, weakly acidic, and nonacid reflux while on PPI, can be considered.
The choice of test and whether to test on or off PPI is dependent on the question being asked. If the patient has been empirically treated (never had an objective diagnosis of GERD), or the clinician believes the likelihood that reflux is the cause of symptoms is low, or for patients considering surgery, an off-therapy study should be considered [16, 159]. A recent study investigated the utility of 96 hour capsule based pH monitoring off PPI therapy in patients with persistent typical symptoms despite PPI treatment to determine if PPIs could be stopped. Patients with two or more days with esophageal acid exposure time >4 percent were unlikely to be able to stop PPIs. Those with a normal study on all four days were the group with the highest likelihood of being able to discontinue PPIs [172].
On-therapy monitoring is suggested prior to surgery or endoscopic intervention in patients with previous objective findings of GERD (such as Barrett’s esophagus or Los Angeles grade C/D erosive esophagitis) who have continued symptoms despite PPI treatment [16]. Although retrospective studies suggest that patients with GERD symptoms unresponsive to PPIs who are proven to have GERD by off-therapy pH monitoring can respond to surgery [107], and some interventionalists endorse antireflux procedures based on off-therapy pH monitoring for such patients [173], the documentation of persistent abnormal acid reflux on PPIs or of a positive association between symptoms and reflux episodes offers “reassurance” that surgery or endoscopic therapy can be successful in the PPI-refractory patient.
Several studies have attempted to address the question of testing on or off PPI in patients with persistent GERD. The total number of reflux episodes detected by impedance is similar between testing on and off PPI [107, 174, 175]. Other studies have used reflux testing to guide therapy for patients with refractory GERD symptoms. Reflux testing combined with other testing, such as esophageal manometry, gastric emptying studies, and endoscopy, identified a diagnosis of GERD in only 34.5% of cases in one study [176]. In a multicenter study, only 21% of patients with persistent heartburn on PPIs were found to have truly refractory GERD [23]. Overall, the balance of data suggests that few patients with refractory GERD symptoms on PPIs have continued reflux as the cause for symptoms, suggesting value for a tailored approach using impedance-pH monitoring prior to intervention [23]. For on-therapy reflux monitoring, we recommend that PPIs be taken twice-daily, the approach used in the randomized trial of medical vs surgical therapy for PPI-refractory reflux disease [23]. Furthermore, impedance-pH monitoring rather than pH monitoring alone is recommended for on-therapy reflux monitoring, both because the yield of pH monitoring in this setting is so low (fewer than 10% of patients on twice-daily PPIs have persistently abnormal acid reflux [160]) and because impedance monitoring enables correlation between symptoms and non-acid reflux episodes. It is important to stop PPI therapy in patients whose off-therapy reflux testing is negative, unless a previous diagnosis of GERD had been made or another indication for continuing PPI is present. In one study, 42% of patients reported continuing PPI treatment after a negative evaluation for refractory GERD, which included negative endoscopy and pH impedance monitoring [177].
Esophageal Manometry
Some patients with motility disorders such as achalasia or esophageal spasm will report heartburn symptoms. In studies of patients with refractory GERD, 1-3% of patients are found to have achalasia when manometry is performed [23, 178]. Patients with esophageal aperistalsis are identified in roughly 3% of manometry tests performed for evaluation of GERD [178]. These patients often report heartburn symptoms and have a poor response to antireflux surgery. Other disorders, such as rumination and supragastric belching, may also detected by esophageal manometry.
Surgery
Recent publications have changed the thought process on surgical intervention for some patients with refractory GERD. Randomized trials compared magnetic sphincter augmentation to continued medical therapy in patients with regurgitation refractory to PPIs. MSA improved symptoms more than continued medical therapy in patients with objective documentation of abnormal reflux [179, 180]. Two randomized trials with transoral incisionless fundoplication (TIF) also demonstrate better improvement in regurgitation with TIF compared to high dose PPI, though the magnitude of improvement was not as great as with MSA [181]. A recent study illustrates the challenge of managing refractory GERD. In this study of medical versus surgical treatment for 366 patients with heartburn that failed to respond to PPIs, extensive evaluation revealed that heartburn symptoms were truly PPI-refractory and reflux-related in only 78 patients (21%) [23].
Identifying patients with true refractory GERD is crucial as surgery (or endoscopic treatment) may truly be best in this group. For patients with regurgitation refractory to PPI therapy, care should be taken to distinguish regurgitation from rumination, a functional disorder characterized by effortless food regurgitation during or soon after eating, typically with rechewing, reswallowing, or spitting out of the regurgitated material. Surgical treatment is not recommended for patients with rumination [182]. A detailed discussion of the management of functional heartburn and other functional upper gastrointestinal symptoms exceeds the scope of this guideline.
Surgical and Endoscopic Options for GERD
Recommendations
- We recommend antireflux surgery performed by an experienced surgeon as an option for long-term treatment of patients with objective evidence of GERD, especially those who have severe reflux esophagitis (LA grades C or D), large hiatal hernias, and/or persistent, troublesome GERD symptoms. (Strong recommendation, moderate level of evidence)
- We recommend consideration of magnetic sphincter augmentation (MSA) as an alternative to laparoscopic fundoplication for patients with regurgitation who fail medical management. (Strong recommendation, moderate level of evidence)
- We suggest consideration of Roux-en-Y gastric bypass (RYGB) as an option to treat GERD in obese patients who are candidates for this procedure and who are willing to accept its risks and requirements for lifestyle alterations. (Conditional recommendation, low level of evidence)
- Since data on the efficacy of radiofrequency energy (Stretta) as an antireflux procedure is inconsistent and highly variable, we cannot recommend its use as an alternative to medical or surgical antireflux therapies. (Conditional recommendation, low level of evidence)
- We suggest consideration of transoral incisionless fundoplication (TIF) for patients with troublesome regurgitation or heartburn who do not wish to undergo antireflux surgery and who do not have severe reflux esophagitis (Los Angeles grades C or D) or hiatal hernias >2 cm. (Conditional recommendation, low level of evidence)
Key Concepts
- We recommend high resolution manometry prior to antireflux surgery or endoscopic therapy to rule out achalasia and absent contractility. For patients with ineffective esophageal motility, high resolution manometry should include provocative testing to identify contractile reserve (e.g. multiple rapid swallows).
- Prior to performing invasive therapy for GERD, a careful evaluation is required to ensure that GERD is present and as best as possible determine is the cause of the symptoms to be addressed by the therapy, to exclude achalasia (which can be associated with symptoms such as heartburn and regurgitation that can be confused with GERD), and to exclude conditions that might be contraindications to invasive treatment such as absent contractility.
In the majority of patients, the symptoms and endoscopic signs of GERD resolve readily with medical treatment, and invasive antireflux therapies are neither required nor desired by patients. However, GERD is a chronic disease, and patients often require protracted medical treatment, which is inconvenient and carries some risk. Severe reflux esophagitis (LA grades C and D) does not heal reliably with any medical therapy other than PPIs, and studies have demonstrated that severe erosive esophagitis returns quickly in the large majority of patients when PPIs are stopped [17, 183, 184]. It might be possible to reduce or even eliminate medical therapy for patients with mild forms of GERD (e.g. no reflux esophagitis worse than Los Angeles grade B), but patients with severe reflux esophagitis (Los Angeles grades C or D) will require PPI therapy indefinitely to maintain healing. In light of recent concerns regarding the safety of long-term PPI usage, many patients are uncomfortable with the prospect of lifelong PPI treatment. Although antireflux procedures have their own well-established risks, some of which are serious, there are a number of patients who prefer to opt for those over the putative risks and inconvenience of lifelong PPI therapy.
GERD that fails to respond to medical therapy is another valid indication for antireflux procedures, but one that requires meticulous pre-procedure evaluation to achieve good surgical outcomes. Prior to the advent of PPIs, failure to respond to medical therapy was the major indication for antireflux surgery. Today, however, PPI therapy is so effective for treating typical GERD symptoms like heartburn and regurgitation that failure to respond to PPIs should be regarded as a red flag that GERD may not be the underlying cause. Indeed, patients who have the best response to antireflux surgery are those with typical GERD symptoms who respond well to PPIs [185, 186], presumably because these patients clearly have abnormal gastroesophageal reflux, and antireflux surgery is highly effective at controlling that problem. Although it is claimed that 30% to 40% of patients treated with PPIs for GERD have persistent “GERD symptoms” [187, 188], in many cases those PPI-resistant symptoms are mistakenly assumed to be caused by reflux. Symptoms that are not reflux-related will not respond to antireflux procedures, yet these procedures often have been used (and failed) in patients who had little or no objective evidence of underlying GERD. It is critical to establish that “refractory GERD symptoms” are indeed reflux-related before recommending invasive antireflux treatment.
In a recent study of medical versus surgical treatment for PPI-refractory heartburn that included 366 patients referred to GI clinics because of heartburn that failed to respond to PPIs, extensive work-up revealed that the heartburn was truly PPI-refractory and reflux-related in only 78 patients (21%) [23]. Among the other 288 patients, heartburn was relieved in 42 (12%) when they were given a trial of twice-daily omeprazole with explicit instructions on how to take the medication properly, 70 (19%) were unwilling or unable to complete the rigorous preoperative work-up required for trial entry, 54 (15%) were excluded for miscellaneous reasons, 23 (6%) had non-GERD esophageal disorders such as eosinophilic esophagitis and achalasia, and 99 (27%) had functional heartburn. For the 78 patients in whom rigorous work-up established that the PPI-refractory heartburn was indeed reflux-related, treatment success (≥50% improvement in GERD Health-Related Quality of Life symptom scores at one year) for laparoscopic Nissen fundoplication (18/27, 66.7%) was significantly superior to active medical (7/25, 28.0%, p=.007) and placebo medical (3/26, 11.5%, p<0.001) treatments.
Even though heartburn is the cardinal symptom of GERD, the aforementioned study shows that PPI-refractory heartburn is uncommonly due to GERD. As discussed above establishing a clear causal relationship with GERD can be even more difficult for so-called “extraesophageal GERD symptoms” such as throat clearing, hoarseness, and chronic cough. Surgical treatment of extraesophageal GERD is reviewed in detail in the ‘extraesophageal GERD section’. Few high-quality data have established the benefit of invasive treatments for patients with these extraesophageal GERD symptoms, and physicians should be extra cautious in recommending such treatments for patients with laryngopharyngeal reflux and other “extraesophageal GERD symptoms. Only persistent abnormal acid reflux and reflux hypersensitivity are likely to benefit from antireflux procedures.
Fundoplication
Fundoplication, especially Nissen fundoplication, is widely regarded as the “gold-standard” among the antireflux procedures for its efficacy in improving the physiologic parameters of GERD such as LES pressure and esophageal acid exposure time [189]. Fundoplication creates a barrier to the reflux of all gastric material (acidic and non-acidic), and therefore should be an effective treatment for any GERD symptom that is reflux related.
Interest in surgical antireflux therapy intensified in the 1980s when observational studies described >90% efficacy for fundoplication in controlling GERD symptoms over a 10-year period [190]. Interest in fundoplication was further fueled by a randomized trial conducted by the VA in the late 1980s (when antireflux surgery was performed as an open procedure and before PPIs were available) which found that open Nissen fundoplication was significantly more effective than ranitidine-based medical therapy in healing the symptoms and endoscopic signs of complicated GERD for the two-year duration of the study [191]. However, a long-term follow-up investigation published in 2001 showed that after 10 to 13 years, 23 (62%) of 37 surgical patients for whom follow-up was available reported that they were once again taking antireflux medications on a regular basis to treat their GERD symptoms, and surgically-treated patients had decreased long-term survival due largely to excess deaths from heart disease [192]. This report and other developments resulted in a long decline in the use of operative treatment for GERD.
Laparoscopic antireflux surgery (LARS) was introduced in 1991, and this has since become the standard operative approach to fundoplication, essentially replacing open antireflux surgery. Studies focusing on the durability of modern surgical technique have found a wide range of GERD recurrence rates. Cohort studies often found high rates of postoperative antireflux medication usage (up to 43%) [193-197], while several randomized trials of LARS vs. medical therapy conducted at specialized centers described lower GERD recurrence rates (10%-27% during follow-up periods of 3 to 5 years) [198-200].
A recent systematic review and meta-analysis focusing on patient-relevant outcomes of fundoplication versus PPI-based medical management of GERD found that heartburn and regurgitation were less frequent with surgical than with medical therapy and, although a considerable proportion of patients still needed antireflux medications after fundoplication, surgical patients were significantly more satisfied with their treatment in the short and medium term [201]. However, a more recent Cochrane review concluded that there is considerable uncertainty in the balance of benefits versus harms of laparoscopic fundoplication compared to long term PPI therapy, and called for further randomized, controlled trials [202]. Compared to Nissen (complete) fundoplication, partial fundoplications (e.g. Toupet, Dor) appear to have similar efficacy in relieving GERD symptoms, but result in less postoperative dysphagia, gas-bloat, and inability to belch and vomit [203-206]. However, partial fundoplication also might have a higher rate of recurrent GERD [206].
A recent report of a retrospective, population-based cohort study has shed considerable light on the outcome of LARS performed in a “real-world” setting [207]. The study involved 2,655 patients identified in the Swedish Patient Registry as having had primary LARS performed between 2005 and 2014. During a mean follow-up period of 5.1 years, 470 patients (17.7%) had a reflux recurrence (i.e., 393 used PPIs/H2RAs for >6 months, 77 had repeat antireflux surgery). Within 30 days of surgery, 109 patients (4.1%) had complications such as infection, bleeding, and esophageal perforation, and there were only 2 deaths (0.1%), neither of which was directly related to the operation. Postoperative dysphagia was documented in 21 patients (0.8%), including 14 (0.5%) who required endoscopic dilatation. This report suggests that LARS can be performed with a relatively low rate of morbidity, and with a very low mortality rate, considerably lower than that of the old open antireflux surgery. The study did not assess patient reported outcomes or the use of over-the-counter medications, and it is well known that LARS occasionally can have catastrophic short- and long-term complications. Nevertheless, it appears to be well tolerated in the large majority of cases, and the observation that >80% of patients did not resume the use of antireflux medications suggests that the operation provides long-lasting relief of GERD symptoms for most patients. How patients and physicians view the 17.7% recurrence rate is a matter of personal perspective.
In summary, modern medical antireflux therapy and laparoscopic fundoplication appear to have similar efficacy in healing the symptoms and endoscopic signs of GERD. Recent concerns about the safety of long-term PPI therapy and refinements in surgical technique that have substantially decreased its morbidity and mortality have rekindled interest in fundoplication. Clearly, antireflux surgery is not a permanent cure for GERD in all patients as it was once touted to be, and the operation occasionally can have severe adverse effects. Nevertheless, most patients obtain long-term benefit from fundoplication, and patient satisfaction with successful surgery appears to be greater than that for chronic medical therapy. The major question for patients considering antireflux surgery is this: Does the >80% possibility of long-term freedom from PPIs and their attendant risks warrant the 4% risk of acute complications of fundoplication and its 17.7% risk of GERD recurrence? [208]
Magnetic sphincter augmentation
Magnetic sphincter augmentation (MSA) with the LINX® Reflux Management System, a necklace of titanium beads with magnetic cores that encircles the distal esophagus to bolster the LES and prevent reflux, was developed as a less invasive and more readily reversible GERD treatment than fundoplication. The initial target population for MSA was GERD patients with abnormal acid reflux documented by esophageal pH monitoring (off PPIs) who experienced only partial relief with PPIs, and who did not have large hiatal hernias or severe reflux esophagitis [209]. For 100 such patients in an early pilot study with no control group, 92% achieved ≥50% improvement in quality-of-life scores, 93% reduced their PPI usage by ≥50%, and 64% had ≥50% reduction in esophageal acid exposure at one year [209]. Dysphagia was the most frequent adverse event, experienced by 68% of patients in the postoperative period, by 11% at one year, and by 4% at three years. Six patients had serious adverse events, and six eventually had the device removed. In a 5-year follow-up of patients in this study, there were no device erosions or migrations, 85% of patients had discontinued their use of PPIs, and all patients reported the ability to belch and vomit [210].
Although large hiatal hernias and severe reflux esophagitis were contraindications to MSA in early studies of the technique, subsequent studies have found that the short-term clinical outcomes of MSA for patients with those conditions are similar to those described for patients with less severe forms of GERD [211-213]. Unlike the minimal surgical dissection required for implantation of the device in patients with small hiatal hernias, however, patients with large hiatal hernias require a more extensive dissection and repair of the crural diaphragm.
One problem with implantation of the metallic MSA device is that patients cannot have MRI with scanning systems >1.5 Tesla. An early concern regarding MSA was that the device would erode into the esophagus. A recent study of data provided by the manufacturer (Torax Medical, Inc.) and the MAUDE database on 9,453 devices placed between 2007 and 2017 found that the risk of erosion was 0.3% at 4 years [214]. The median time to erosion was 26 months, and most occurred between 1 to 4 years after device implantation. Most of the eroded devices were removed by a combination of endoscopy and laparoscopy, and there were no serious complications of device removal. Thus, device erosion appears to be infrequent and safely managed.
To date, there has been no publication of a randomized trial directly comparing MSA with the gold-standard surgical treatment of laparoscopic fundoplication. However, observational cohort studies have compared the techniques, and systematic reviews and meta-analyses of those reports have arrived at generally similar conclusions [215-218]. Compared to fundoplication, MSA has shorter operative times and shorter durations of hospital stays. There appear to be no significant differences between MSA and fundoplication in rates of GERD symptom control, postoperative PPI usage, major complications including dysphagia, and rates of reoperation. Most, but not all reports suggest that MSA results in less gas-bloat and greater ability to belch and vomit than fundoplication.
A recent randomized trial has established the unequivocal superiority of MSA over twice-daily PPIs for the control of regurgitation [180]. In this study, 152 patients with moderate to severe regurgitation despite once-daily PPI therapy were randomly assigned to receive twice-daily PPIs (n = 102) or MSA (n = 50), and MSA was offered to patients in the twice-daily PPI group who had persistent regurgitation after 6 months of treatment. At one year, control of regurgitation was achieved in 72 of 75 patients (96%) in the MSA group, but in only 8 of 43 patients treated with PPIs (19%). MSA was not associated with any peri-operative events, device explants, erosions, or migrations.
MSA also appears to have a role in the treatment of GERD that worsens or develops after bariatric operations such as sleeve gastrectomy and Roux-en-Y gastric bypass [219]. These operations alter gastric anatomy in a way that can preclude performance of a standard fundoplication. Limited data suggest that MSA is safe and effective for treating GERD in this setting.
In summary, MSA appears to be a safe and effective alternative to laparoscopic fundoplication. Clinical outcomes of the two procedures are similar, and both have unique advantages and disadvantages. The minimal surgical dissection required for MSA results in greater technical ease, shorter operative times, and shorter durations of hospital stays than for fundoplication. MSA is also easier to reverse, and MSA may result in less gas-bloat and greater ability to belch and vomit than fundoplication. The MRI restriction after MSA is a disadvantage and, compared to fundoplication, there is a paucity of long-term data on MSA outcomes. With no randomized trials comparing the two procedures, it is difficult to recommend one over the other at this time.
Roux-en-Y gastric bypass
GERD is strongly associated with obesity. Compared to individuals with a normal body mass index (BMI), the prevalence of GERD in those whose BMI exceeds 35 is increased up to six-fold [220]. Obesity poses technical challenges to the performance of fundoplication surgery. In addition, the elevated intra-abdominal pressure associated with obesity might put strain on the diaphragmatic hiatus, resulting in fundoplication disruption and herniation, increased surgical complications, and poor outcomes. Roux-en-Y gastric bypass (RYGB) can control GERD in obese patients, presumably because the small gastric pouch fashioned during RYGB produces far less acid than an intact stomach, and because the accompanying long alimentary loop prevents the reflux of bile. Due to the widespread perception among surgeons that fundoplication has poor outcomes in obese patients, and the fact that RYGB has been shown both to control reflux and induce weight loss, RYGB has come to be considered the antireflux surgery of choice for obese patients, in whom it is used both as a primary antireflux procedure and as a means for correction of a failed fundoplication [221, 222]. However, there is now considerable controversy regarding the role of RYGB as an antireflux procedure.
One reason for the controversy is the substantial variability in results of studies on outcomes and rates of complications for fundoplication in obese patients. Some studies have documented poorer results of fundoplication in the obese [223], while others have found no differences in complications and outcomes between obese and non-obese patients [224]. A recent systematic review and meta-analysis on this issue found no significant differences between obese and non-obese patients in the rates of perioperative complications, redo surgery, and conversion from laparoscopic to open surgery, but the recurrence of reflux after fundoplication was significantly lower in the non-obese patients (OR 0.28, 95% CI 0.13-0.61, p=0.001) [225]. Other reasons for controversy on the role of RYGB include the lack of randomized trials comparing it directly with fundoplication, and the fact that, although RYGB can have numerous beneficial effects, it is a technically difficult operation that produces major alterations in anatomy, which can result in serious early and late complications [226]. In addition, a recent, nationwide cohort study of all adults with preoperative reflux who underwent gastric bypass in Sweden between 2006 and 2015 found that, in 2,454 participants followed for median 4.6 years, reflux recurred in 48.8% (95% CI 46.8-51.0) within 2 years of the operation [227]. The authors concluded that the efficacy of gastric bypass for GERD symptoms might have been overestimated. Finally, reports have documented the occasional new development of GERD after RYGB [219].
With all the above-noted uncertainty, an argument can be made to regard RYGB primarily as a highly effective weight loss operation that has the added potential benefit of controlling acid reflux, rather than as an antireflux operation primarily. Obese patients with GERD should be adequately counseled and willing to accept the risks and lifestyle demands of bariatric surgery before undergoing RYGB for control of GERD.
Endoscopic antireflux therapies
A number of endoscopic devices for treating GERD have been introduced over the past two decades, and most have been withdrawn from the marketplace because of concerns regarding safety and efficacy. Presently, the only endoscopic GERD treatments still widely available are radiofrequency antireflux treatment (Stretta, Restech corporation, Houston Texas) and transoral incisionless fundoplication (TIF, endogastric solutions). Studies of the endoscopic procedures generally have excluded patients with hiatal hernias >2 cm, grade C and D erosive esophagitis, esophageal strictures, and long-segment Barrett’s esophagus. Consequently, if these devices are to be used at all, based on data their use should be limited to patients with milder forms of GERD.
The Stretta procedure is difficult to evaluate, in part because it is not totally clear how it functions as an antireflux therapy. Initially, it was thought to control reflux by inducing swelling and mechanical alteration at the esophagogastric junction. However, an early, sham-controlled trial found that, 6 months after treatment, Stretta had significantly improved gastroesophageal reflux disease symptoms and quality of life, but it did not decrease esophageal acid exposure [228]. This raised the possibility that the procedure might alleviate GERD symptoms by altering sensation in the distal esophagus. Systematic reviews and meta-analyses have arrived at contradictory conclusions regarding Stretta’s efficacy. One meta-analysis that evaluated only randomized controlled trials found that Stretta did not produce significant changes in esophageal acid exposure, quality of life, or the ability to stop PPIs [229], while another meta-analysis that included both controlled and cohort studies concluded that Stretta significantly reduced esophageal acid exposure, improved quality of life, and decreased PPI usage [230]. Nevertheless, in 2013, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) gave Stretta a strong recommendation for use in patients who refuse laparoscopic Nissen fundoplication [231].
TIF attempts to create a flap valve involving 180° to 270° of the circumference of the esophagogastric junction by plicating a portion of the proximal stomach using a series of T-fasteners. Randomized trials have shown that TIF is effective for treating troublesome regurgitation [181, 232], but the long-term benefit of TIF is not established and questionable [218]. One recent systematic review and meta-analysis on the use of TIF for refractory GERD found that TIF resulted in significant improvements in GERD health-related quality of life and DeMeester scores, enabling 89% of patients to discontinue PPIs [233]. However, another systematic review and meta-analysis on the use of TIF for the treatment of GERD found that although symptoms responded to TIF significantly more often that to PPIs/sham, TIF did not result in significant improvement in esophageal acid exposure and most patients resumed PPIs at reduced dosages during long-term follow-up. The incidence of serious adverse events (perforation and bleeding) was 2.4%, and the rate of total satisfaction with TIF was 69% by 6 months [234].
Long-Term PPI Issues
- Regarding the safety of long-term PPI usage for GERD, we suggest that patients should be advised as follows: “Proton pump inhibitors (PPIs) are the most effective medical treatment for gastroesophageal reflux disease (GERD). Some medical studies have identified an association between the long-term use of PPIs and the development of numerous adverse conditions including intestinal infections, pneumonia, stomach cancer, osteoporosis related bone fractures, chronic kidney disease, deficiencies of certain vitamins and minerals, heart attacks, strokes, dementia and early death. Those studies have flaws, are not considered definitive, and do not establish a cause-and-effect relationship between PPIs and the adverse conditions. High-quality studies have found that PPIs do not significantly increase the risk for any of these conditions except intestinal infections. Nevertheless, we cannot exclude the possibility that PPIs might confer a small increase in the risk of developing these adverse conditions. For the treatment of GERD, gastroenterologists generally agree that the well-established benefits of PPIs far outweigh their theoretical risks.”
- Switching PPIs can be considered for patients who experience minor side PPI effects including headache, abdominal pain, nausea, vomiting, diarrhea, constipation and flatulence.
- For GERD patients on PPIs who have no other risk factors for bone disease, we do not recommend that they raise their intake of calcium or vitamin D, or that they have routine monitoring of bone mineral density.
- For GERD patients on PPIs who have no other risk factors for vitamin B12 deficiency, we do not recommend that they raise their intake of vitamin B12, or that they have routine monitoring of serum B12 levels.
- For GERD patients on PPIs who have no other risk factors for kidney disease, we do not recommend that they have routine monitoring of serum creatinine levels.
- For GERD patients on clopidogrel who have Los Angeles grade C or D esophagitis or whose GERD symptoms are not adequately controlled with alternative medical therapies, the highest quality data available suggest that the established benefits of PPI treatment outweigh their proposed but highly questionable cardiovascular risks.
- PPIs can be used to treat GERD in patients with renal insufficiency with close monitoring of renal function or consultation with a nephrologist.
PPIs are widely considered the mainstay of medical treatment for GERD. Side effects of PPIs that have been identified in clinical trials and listed on FDA labels as the “most common adverse reactions” include headache, abdominal pain, nausea, vomiting, diarrhea, constipation, and flatulence. These relatively minor side effects occur infrequently and abate when the medications are stopped. Limited data also suggest that these side effects sometimes can be PPI preparation-specific and, for patients who experience them, a trial of switching from one PPI to another is a reasonable management strategy [235]. Of far more concern to patients and physicians alike are the growing number of serious putative adverse effects of chronic PPI therapy that have been identified predominantly through weak associations found in observational studies [236, 237].
Table 5 lists the major putative adverse effects of chronic PPI therapy and the proposed underlying mechanisms. Some of these effects are assumed to be a consequence of PPI-induced suppression of gastric acid secretion. For example, gastric acid suppression can enable ingested pathogens that ordinarily would have been destroyed by gastric acid to survive and cause enteric infections, or to be aspirated and cause pneumonia [237]. Reduced gastric acidity can impair the uptake of certain vitamins (e.g. B12) and minerals (e.g. calcium) and can elevate serum levels of gastrin, a growth factor with pro-proliferative effects that might predispose to carcinogenesis [237]. Mechanisms other than gastric acid inhibition have been proposed to underlie a number of other adverse effects that have been associated with PPI usage such as kidney disease and cardiovascular events (Table 5).
Table 5.
Major putative adverse effects of chronic PPI therapy
| Putative adverse effect | Meta-analysisreference numbers | HRa or ORb (95% CI)found in recent RCT (94) | Major proposed mechanisms |
|---|---|---|---|
| Cardiovascular events | (237-240) | 1.04a (0.93–-1.15) | PPIs block metabolism of ADMA, which accumulates and inhibits NO synthase, thus blocking endothelial production of NO needed for vascular homeostasis |
| (All) MI | 0.94a (0.79–1.12) | ||
| Stroke | 1.16a (0.94–1.44) | ||
| Cardiovascular death | 1.03a (0.89–1.20) | ||
| Cardiovascular events in patients on clopidogrelc | (241-260) | NA | PPIs are metabolized by the same enzyme needed to activate clopidogrel (CYP2C19), so concomitant use of these drugs might decrease the antiplatelet effect of clopidogrel |
| Kidney disease | (261-265) | NA | AIN develops as an idiosyncratic drug reaction and progresses to chronic kidney disease |
| (All) AIN | NA | ||
| Chronic kidney disease | 1.17b (0.94–1.45) | ||
| Enteric infection (other than Clostridium. difficile) | (266,267) | 1.33b (1.01–1.75) | Reduced gastric acid enables ingested enteric pathogens to survive passage through the stomach |
| C. difficile | (268-276) | 2.26b (0.70–7.34) | Reduced gastric acid enables survival of ingested C. difficile vegetative forms and prevents conversion of salivary nitrite to ROS that suppress C. difficile spores; PPIs may enhance C. difficile toxin expression and cause microbiome alterations that promote C. difficile colitis |
| SIBO | (277,278) | NA | Reduced gastric acid enables increased bacterial colonization of the UGI tract |
| Spontaneous bacterial peritonitis in patients with cirrhosis | (279-283) | NA | Increased bacterial colonization of the UGI tract and PPI-induced increases in UGI tract permeability predispose to bacterial translocation; PPIs also might interfere with inflammatory cell functions that ordinarily would prevent infection |
| Pneumonia | (284-289) | 1.02b (0.87–1.19) | Reduced gastric acid enables UGI tract colonization with pulmonary pathogens that can be aspirated; PPIs also might interfere with inflammatory cell functions that ordinarily would prevent infection |
| Dementia | (290-293) | 1.20b (0.81–1.78) | PPIs block vacuolar H+-ATPase needed to acidify microglial lysosomes, thereby preventing their degradation of cerebral amyloid-β peptide; PPI-induced B12 deficiency also might contribute to dementia |
| Bone fracture | (294-302) | 0.96b (0.79–1.17) | Reduced gastric acid causes calcium malabsorption leading to decreased bone mineral density; PPIs might reduce bone resorption by blocking vacuolar H+-ATPase in osteoclasts; PPIs cause hypergastrinemia that might cause parathyroid hyperplasia |
| Gastric atrophy | (303-305) | 0.73b (0.40–1.32) | PPIs promote corpus-predominant H. pylori gastritis that results in gastric atrophy with loss of parietal cells |
| Gastric cancer | (306-308) | NA | PPIs promote gastric atrophy and inflammation in _H. pylori_–infected patients, resulting in intestinal metaplasia predisposed to malignancy; reduced gastric acid enables overgrowth of bacteria that convert dietary nitrates to potentially carcinogenic N-nitroso compounds; PPI-induced hypergastrinemia causes gastric epithelial cell proliferation that promotes carcinogenesis |
| Vitamin B12 deficiency | (309) | NA | Reduced gastric acid results in malabsorption of protein-bound cobalamin; gastric atrophy results in decreased intrinsic factor production |
| Hypomagnesemia | (310-312) | NA | PPI effects in elevating intestinal pH may interfere with magnesium absorption, perhaps because the affinity of the enterocyte magnesium transporter TRPM6/7 for magnesium decreases in a higher pH environment |
| All-cause mortality | (313) | 1.03a (0.92–1.15) | Potentially all of above |
One area of considerable persistent controversy relates to the association between chronic PPI use and hypomagnesemia. Two meta-analyses on this issue concluded that long-term PPI use is significantly associated with hypomagnesemia [238, 239], while another two concluded that the risk of PPI-induced hypomagnesemia was unclear because of significant heterogeneity among studies [240, 241]. A recent AGA Best Practice Recommendation concluded that long-term PPI users should not routinely screen or monitor serum magnesium levels [242], whereas the FDA suggests that health care providers should consider monitoring magnesium levels prior to initiation of PPI treatment and then periodically (http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm). We feel that presently there is insufficient data to make a meaningful recommendation regarding the need for monitoring of magnesium levels in patients on chronic PPI therapy.
It is important to appreciate that the mere identification of an association between PPIs and adverse conditions in observational studies cannot establish a cause-and-effect relationship, and that such studies are highly susceptible to biases that can prejudice results. Observational studies on potential PPI side effects are especially susceptible to the biases of confounding by indication (in which the medical indication for a PPI, not the PPI itself, is responsible for the adverse effect) and protopathic bias (in which the PPI does not cause an adverse condition, but is prescribed to treat symptoms of that already-present yet unrecognized condition) [243, 244].
The epidemiologist/statistician Sir Austin Bradford Hill, in his Presidential Address to the Section of Occupational Medicine of the Royal Society of Medicine in 1965, proposed 9 criteria that can strengthen the case for a cause-and-effect relationship in associations between exposures and diseases identified through observational studies [245]. These so-called Bradford-Hill criteria include 1) Strength of the association, 2) Consistency of the observation, 3) Specificity of the exposure for the disease, 4) Temporality (i.e. exposure preceded disease), 5) Biological gradient (dose response), 6) Plausibility of the proposed mechanism for how the exposure might cause disease, 7) Coherence among epidemiologic and other types of data, 8) Experimental data support a cause-and-effect relationship, and 9) Analogy with the effects of similar types of exposures. In 2017, Vaezi and colleagues reported that no proposed PPI adverse effect fulfilled all 9 of the Bradford-Hill criteria, and most fulfilled fewer than 4 [246].
It has been noted that most reported associations in observational clinical research are spurious, and the minority that are real are often exaggerated [247]. Experts caution that weak associations found in such studies are more likely to result from bias than from cause-and-effect relationships and, unless relative risks (RRs) in cohort studies exceed 2-3 or odds ratios (ORs) in case-control studies exceed 3-4, the findings generally should not be considered credible [247]. Reports of observational studies that have identified potential PPI side effects typically have described weak associations with RRs or ORs <2 [248]. Furthermore, even strong associations in such studies do not establish cause-and-effect relationships. For example, some observational studies have found a strong association (ORs >4) between PPI usage and esophageal adenocarcinoma, an association that is likely due to confounding by indication (i.e., PPIs were prescribed to treat GERD, which was the real risk factor for the cancer that subsequently developed) [249]. Observational studies also have found a strong association between PPI usage and development of community-acquired pneumonia, an association that may well have been the result of protopathic bias (i.e., PPIs were prescribed for symptoms of cough and chest discomfort that were mistakenly attributed to GERD but in fact were caused by an unrecognized, early pneumonia) [250].
A recent, large, placebo-controlled randomized trial reported by Moayyedi et al. has shed considerable light on the issue of PPI safety [251]. In this exceptionally high-quality study, 17,598 patients aged 65 years or older with stable cardiovascular or peripheral artery disease treated with rivaroxaban and/or aspirin were randomly assigned to receive the PPI pantoprazole (40 mg daily, n-8,791) or placebo (n=8,807). Following randomization, data were collected at 6-month intervals over a period of 3 years specifically with the intent of identifying potential PPI side effects including pneumonia, C. difficile infection, other enteric infections, fractures, gastric atrophy, chronic kidney disease, dementia, cardiovascular disease, cancer, and all-cause mortality. The investigators found no significant differences between the PPI and placebo groups in rates of occurrence for any of those potential side effects except for enteric infections (1.4% vs 1.0% in the PPI and placebo groups, respectively; OR 1.33; 95% confidence interval 1.01-1.75). Table 5 lists the hazard ratios (HRs) and ORs for all the putative adverse events evaluated in this study. The authors concluded that the use of pantoprazole for 3 years was not associated with any adverse event other than a modestly increased risk of developing enteric infections.
Moayyedi’s report provides high-quality evidence to suggest that most of the associations between PPI usage and adverse events that have been identified in observational studies were the result of residual confounding and other biases, and unlikely to represent cause-and-effect relationships. Reassuring as this study is, it is important to consider several caveats. First, the trial had a maximum follow-up of five years, which might not be sufficient time for some adverse events to develop (e.g., gastric cancer) [252]. Next, despite the large size of the study, some adverse events (e.g. gastric atrophy, _C. difficile_-associated diarrhea) occurred so infrequently that conclusions regarding possible PPI involvement are limited. Finally, and perhaps most important, the 95% confidence intervals around some of the HRs and ORs observed in this prospective trial, large as it is, still are relatively wide. It is reassuring that the HRs and ORs for some events (pneumonia, fracture, cardiovascular disease, dementia, and all-cause mortality) are even lower than the lower limits of the 95% confidence intervals reported in earlier observational studies. Nevertheless, this study cannot exclude the possibility that PPIs confer a modest risk for any of these adverse events (i.e., the upper limit of the 95% confidence intervals all are >1), and even a modest risk for such serious events is cause for concern. As the authors themselves acknowledge, the possibility that PPIs confer a modest risk for these putative adverse events can never be excluded no matter how large the study sample size [251].
Summary
We have made every effort to review and grade all available evidence to develop this guideline. Much is new and different compared to the 2013 guideline, particularly as it relates to approaching extraesophageal symptoms, refractory GERD, and surgical and endoscopic therapies. Each section provides a separate review of the evidence supporting our recommendations, therefore some repetition was necessary to do this effectively. Our algorithms offer an overall approach to diagnosis and management of the major presentations of the disease, and reflect our discussion in the body of the manuscript. We have attempted to address all the key issues in PPI management and adverse events so clinicians will have a comprehensive, go-to source in the guideline. . We have done our best to present a thorough review of the evidence for our recommendations and key concepts, and to provide an evidence-based approach to GERD that can be used effectively in everyday practice.
We expect that new diagnostic tools and treatments will be developed and those that we have will be further refined. Mucosal integrity testing, for example, is available commercially but is not developed sufficiently to warrant discussion in this guideline. Esophageal function testing is addressed in detail in another guideline, while other extensive reviews focus on valuable additions to our clinical armamentarium such as magnetic sphincter augmentation and TIF. Potassium-competitive acid blockers (PCABs) are exciting potential new agents for pharmacologic treatment of GERD. One, currently available in Japan, presently is undergoing Phase 3 trials in the US as we complete this document, and may well be approved for clinical use soon after this review is published! Future research with advanced endoscopic techniques, data on long-term efficacy of surgical intervention, and advances in artificial intelligence and basic science will almost certainly change the way we manage GERD going forward.
Figure 2:
Diagnostic Algorithm for Extraesophageal Gastroesophageal Reflux Disease symptoms
Figure 3:
Management Algorithm of Symptoms Suspected Due to GERD Incompletely Responsive to Proton Pump Inhibitors
Footnotes
Potential competing Interests:
Katz PO: Has served as Consultant for Phathom Pharma and Medtronic
Research support: Diversatek
Royalties: Up to Date
Dunbar K: None currently. Previous research funding from Ironwood.
Schnoll-Sussman FH: Consultant for Ethicon, Interpace Biosciences and Medtronic
Greer KB: None
Yadlapati, R: Consultant thru institutional agreement: Medtronic, Ironwood, Diversatek. Research support: Ironwood. Advisory Board: Phathom Pharma: Stock Options: RJS Mediagnostics
Spechler SJ: Has served as a consultant for Interpace Diagnostics, Cernostics, Phathom Pharmaceuticals, Takeda and Ironwood Pharmaceuticals.
Royalties: Up To Date.
Contributor Information
Philip O. Katz, Weill Cornell Medical Center, Jay Monahan Center for Gastrointestinal Health, New York, NY.
Kerry Dunbar, University of Texas Southwestern Medical Center, Gastroenterology and Hepatology, Dallas VA Medical Center, Dallas, TX.
Felice H. Schnoll-Sussman, Jay Monahan Center for Gastrointestinal Health, Weill Cornell Medical Center, New York, NY.
Katarina B. Greer, Case Western Reserve University School of Medicine, Cleveland Louis Stokes VA Medical Center, Cleveland, OH.
Rena Yadlapati, Division of Gastroenterology, University of California San Diego, La Jolla, CA.
Stuart Jon Spechler, Division of Gastroenterology, Center for Esophageal Diseases, Baylor University Medical Center at Dallas, Dallas, TX.
References:
- 1.Katz PO, Gerson LB, and Vela MF, Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol, 2013. 108(3): p. 308–28; quiz 329. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt G, et al. , GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol, 2011. 64(4): p. 383–94. [DOI] [PubMed] [Google Scholar]
- 3.Balshem H, et al. , GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol, 2011. 64(4): p. 401–6. [DOI] [PubMed] [Google Scholar]
- 4.Andrews JC, et al. , GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol, 2013. 66(7): p. 726–35. [DOI] [PubMed] [Google Scholar]
- 5.Numans ME, et al. , Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med, 2004. 140(7): p. 518–27. [DOI] [PubMed] [Google Scholar]
- 6.Cremonini F, et al. , Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a metaanalysis. Am J Gastroenterol, 2005. 100(6): p. 1226–32. [DOI] [PubMed] [Google Scholar]
- 7.Kahrilas PJ, Hughes N, and Howden CW, Response of unexplained chest pain to proton pump inhibitor treatment in patients with and without objective evidence of gastro-oesophageal reflux disease. Gut, 2011. 60(11): p. 1473–8. [DOI] [PubMed] [Google Scholar]
- 8.Moayyedi P, et al. , Can the clinical history distinguish between organic and functional dyspepsia? Jama, 2006. 295(13): p. 1566–76. [DOI] [PubMed] [Google Scholar]
- 9.Hirano I and Richter JE, ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol, 2007. 102(3): p. 668–85. [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, et al. , American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology, 2008. 135(4): p. 1383–1391, 1391.e1-5. [DOI] [PubMed] [Google Scholar]
- 11.Dent J, et al. , Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut, 2005. 54(5): p. 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston BT, et al. , Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease. Am J Gastroenterol, 1996. 91(6): p. 1181–5. [PubMed] [Google Scholar]
- 13.Richter JE and Castell DO, Gastroesophageal reflux. Pathogenesis, diagnosis, and therapy. Ann Intern Med, 1982. 97(1): p. 93–103. [DOI] [PubMed] [Google Scholar]
- 14.Lundell LR, et al. , Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut, 1999. 45(2): p. 172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyawali CP, et al. , Modern diagnosis of GERD: the Lyon Consensus. Gut, 2018. 67(7): p. 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyawali CP and Fass R, Management of Gastroesophageal Reflux Disease. Gastroenterology, 2018. 154(2): p. 302–318. [DOI] [PubMed] [Google Scholar]
- 17.Dunbar KB, et al. , Association of Acute Gastroesophageal Reflux Disease With Esophageal Histologic Changes. Jama, 2016. 315(19): p. 2104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odiase E, et al. , New Eosinophilic Esophagitis Concepts Call for Change in Proton Pump Inhibitor Management Before Diagnostic Endoscopy. Gastroenterology, 2018. 154(5): p. 1217–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoikes N, et al. , The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg Endosc, 2012. 26(12): p. 3401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iluyomade A, et al. , Interference with daily activities and major adverse events during esophageal pH monitoring with bravo wireless capsule versus conventional intranasal catheter: a systematic review of randomized controlled trials. Dis Esophagus, 2017. 30(3): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 21.Kessels SJM, et al. , Safety and Efficacy of Wireless pH Monitoring in Patients Suspected of Gastroesophageal Reflux Disease: A Systematic Review. J Clin Gastroenterol, 2017. 51(9): p. 777–788. [DOI] [PubMed] [Google Scholar]
- 22.Gyawali CP, et al. , ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol, 2020. 115(9): p. 1412–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spechler SJ, et al. , Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N Engl J Med, 2019. 381(16): p. 1513–1523. [DOI] [PubMed] [Google Scholar]
- 24.Marrero JM, et al. , Determinants of pregnancy heartburn. Br J Obstet Gynaecol, 1992. 99(9): p. 731–4. [DOI] [PubMed] [Google Scholar]
- 25.Richter JE, Review article: the management of heartburn in pregnancy. Aliment Pharmacol Ther, 2005. 22(9): p. 749–57. [DOI] [PubMed] [Google Scholar]
- 26.Rey E, et al. , Gastroesophageal reflux symptoms during and after pregnancy: a longitudinal study. Am J Gastroenterol, 2007. 102(11): p. 2395–400. [DOI] [PubMed] [Google Scholar]
- 27.Patel DA, et al. , Development and Validation of a Mucosal Impedance Contour Analysis System to Distinguish Esophageal Disorders. Gastroenterology, 2019. 156(6): p. 1617–1626.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaltenbach T, Crockett S, and Gerson LB, Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med, 2006. 166(9): p. 965–71. [DOI] [PubMed] [Google Scholar]
- 29.Katz LC, Just R, and Castell DO, Body position affects recumbent postprandial reflux. J Clin Gastroenterol, 1994. 18(4): p. 280–3. [DOI] [PubMed] [Google Scholar]
- 30.Khoury RM, et al. , Influence of spontaneous sleep positions on nighttime recumbent reflux in patients with gastroesophageal reflux disease. Am J Gastroenterol, 1999. 94(8): p. 2069–73. [DOI] [PubMed] [Google Scholar]
- 31.Allampati S, et al. , Use of a positional therapy device significantly improves nocturnal gastroesophageal reflux symptoms. Dis Esophagus, 2017. 30(3): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Person E, et al. , A Novel Sleep Positioning Device Reduces Gastroesophageal Reflux: A Randomized Controlled Trial. J Clin Gastroenterol, 2015. 49(8): p. 655–9. [DOI] [PubMed] [Google Scholar]
- 33.Khan BA, et al. , Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol, 2012. 27(6): p. 1078–82. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton JW, et al. , Sleeping on a wedge diminishes exposure of the esophagus to refluxed acid. Dig Dis Sci, 1988. 33(5): p. 518–22. [DOI] [PubMed] [Google Scholar]
- 35.El-Serag HB, Satia JA, and Rabeneck L, Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut, 2005. 54(1): p. 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newberry C and Lynch K, The role of diet in the development and management of gastroesophageal reflux disease: why we feel the burn. J Thorac Dis, 2019. 11(Suppl 12): p. S1594–s1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ness-Jensen E, et al. , Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol, 2014. 109(2): p. 171–7. [DOI] [PubMed] [Google Scholar]
- 38.Kohata Y, et al. , Long-Term Benefits of Smoking Cessation on Gastroesophageal Reflux Disease and Health-Related Quality of Life. PLoS One, 2016. 11(2): p. e0147860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta RS, et al. , Association Between Beverage Intake and Incidence of Gastroesophageal Reflux Symptoms. Clin Gastroenterol Hepatol, 2020. 18(10): p. 2226–2233.e4. [DOI] [PubMed] [Google Scholar]
- 40.Ness-Jensen E, et al. , Lifestyle Intervention in Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol, 2016. 14(2): p. 175–82.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobson BC, et al. , Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med, 2006. 354(22): p. 2340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraser-Moodie CA, et al. , Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol, 1999. 34(4): p. 337–40. [DOI] [PubMed] [Google Scholar]
- 43.Mathus-Vliegen LM and Tytgat GN, Twenty-four-hour pH measurements in morbid obesity: effects of massive overweight, weight loss and gastric distension. Eur J Gastroenterol Hepatol, 1996. 8(7): p. 635–40. [PubMed] [Google Scholar]
- 44.Ness-Jensen E, et al. , Weight loss and reduction in gastroesophageal reflux. A prospective population-based cohort study: the HUNT study. Am J Gastroenterol, 2013. 108(3): p. 376–82. [DOI] [PubMed] [Google Scholar]
- 45.Hampel H, Abraham NS, and El-Serag HB, Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med, 2005. 143(3): p. 199–211. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson J, et al. , Randomized clinical trial: a double-blind, placebo-controlled study to assess the clinical efficacy and safety of alginate-antacid (Gaviscon Double Action) chewable tablets in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol, 2019. 31(1): p. 86–93. [DOI] [PubMed] [Google Scholar]
- 47.Wang WH, et al. , Head-to-head comparison of H2-receptor antagonists and proton pump inhibitors in the treatment of erosive esophagitis: a meta-analysis. World J Gastroenterol, 2005. 11(26): p. 4067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan M, et al. , Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev, 2007(2): p. Cd003244. [DOI] [PubMed] [Google Scholar]
- 49.Robinson M, et al. , A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Multicentre Investigational Group. Aliment Pharmacol Ther, 1995. 9(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 50.Gralnek IM, et al. , Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol, 2006. 4(12): p. 1452–8. [DOI] [PubMed] [Google Scholar]
- 51.Kirchheiner J, et al. , Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol, 2009. 65(1): p. 19–31. [DOI] [PubMed] [Google Scholar]
- 52.Graham DY and Tansel A, Interchangeable Use of Proton Pump Inhibitors Based on Relative Potency. Clin Gastroenterol Hepatol, 2018. 16(6): p. 800–808.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatlebakk JG and Berstad A, Pharmacokinetic optimisation in the treatment of gastro-oesophageal reflux disease. Clin Pharmacokinet, 1996. 31(5): p. 386–406. [DOI] [PubMed] [Google Scholar]
- 54.Lee RD, et al. , The effect of time-of-day dosing on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR: evidence for dosing flexibility with a Dual Delayed Release proton pump inhibitor. Aliment Pharmacol Ther, 2010. 31(9): p. 1001–11. [DOI] [PubMed] [Google Scholar]
- 55.Gunaratnam NT, et al. , Sub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophageal reflux disease. Aliment Pharmacol Ther, 2006. 23(10): p. 1473–7. [DOI] [PubMed] [Google Scholar]
- 56.Katz PO, et al. , Comparison of the effects of immediate-release omeprazole oral suspension, delayed-release lansoprazole capsules and delayed-release esomeprazole capsules on nocturnal gastric acidity after bedtime dosing in patients with night-time GERD symptoms. Aliment Pharmacol Ther, 2007. 25(2): p. 197–205. [DOI] [PubMed] [Google Scholar]
- 57.Hatlebakk JG, et al. , Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. Aliment Pharmacol Ther, 2000. 14(10): p. 1267–72. [DOI] [PubMed] [Google Scholar]
- 58.Fass R, et al. , Treatment of patients with persistent heartburn symptoms: a double-blind, randomized trial. Clin Gastroenterol Hepatol, 2006. 4(1): p. 50–6. [DOI] [PubMed] [Google Scholar]
- 59.Savarino V, et al. , Pathophysiology, diagnosis, and pharmacological treatment of gastro-esophageal reflux disease. Expert Rev Clin Pharmacol, 2020. 13(4): p. 437–449. [DOI] [PubMed] [Google Scholar]
- 60.Boghossian TA, et al. , Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev, 2017. 3(3): p. Cd011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talley NJ, et al. , Esomeprazole 20 mg maintains symptom control in endoscopy-negative gastro-oesophageal reflux disease: a controlled trial of 'on-demand' therapy for 6 months. Aliment Pharmacol Ther, 2001. 15(3): p. 347–54. [DOI] [PubMed] [Google Scholar]
- 62.Schindlbeck NE, et al. , Three year follow up of patients with gastrooesophageal reflux disease. Gut, 1992. 33(8): p. 1016–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lind T, et al. , On demand therapy with omeprazole for the long-term management of patients with heartburn without oesophagitis--a placebo-controlled randomized trial. Aliment Pharmacol Ther, 1999. 13(7): p. 907–14. [DOI] [PubMed] [Google Scholar]
- 64.Pace F, et al. , Systematic review: maintenance treatment of gastro-oesophageal reflux disease with proton pump inhibitors taken 'on-demand'. Aliment Pharmacol Ther, 2007. 26(2): p. 195–204. [DOI] [PubMed] [Google Scholar]
- 65.Inadomi JM, et al. , Step-down management of gastroesophageal reflux disease. Gastroenterology, 2001. 121(5): p. 1095–100. [DOI] [PubMed] [Google Scholar]
- 66.Juul-Hansen P and Rydning A, Clinical and pathophysiological consequences of on-demand treatment with PPI in endoscopy-negative reflux disease. Is rebound hypersecretion of acid a problem? Scand J Gastroenterol, 2011. 46(4): p. 398–405. [DOI] [PubMed] [Google Scholar]
- 67.Metz DC, et al. , Withdrawing PPI therapy after healing esophagitis does not worsen symptoms or cause persistent hypergastrinemia: analysis of dexlansoprazole MR clinical trial data. Am J Gastroenterol, 2011. 106(11): p. 1953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reimer C, et al. , Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology, 2009. 137(1): p. 80–7, 87.e1. [DOI] [PubMed] [Google Scholar]
- 69.Peghini PL, et al. , Nocturnal recovery of gastric acid secretion with twice-daily dosing of proton pump inhibitors. Am J Gastroenterol, 1998. 93(5): p. 763–7. [DOI] [PubMed] [Google Scholar]
- 70.Fackler WK, et al. , Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology, 2002. 122(3): p. 625–32. [DOI] [PubMed] [Google Scholar]
- 71.Rao AS and Camilleri M, Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther, 2010. 31(1): p. 11–9. [DOI] [PubMed] [Google Scholar]
- 72.Ren LH, et al. , Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol, 2014. 20(9): p. 2412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kessing BF, et al. , Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil, 2014. 26(8): p. 1079–86. [DOI] [PubMed] [Google Scholar]
- 74.Grossi L, et al. , Effect of baclofen on oesophageal motility and transient lower oesophageal sphincter relaxations in GORD patients: a 48-h manometric study. Neurogastroenterol Motil, 2008. 20(7): p. 760–6. [DOI] [PubMed] [Google Scholar]
- 75.Koek GH, et al. , Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut, 2003. 52(10): p. 1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vela MF, et al. , Baclofen decreases acid and non-acid post-prandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment Pharmacol Ther, 2003. 17(2): p. 243–51. [DOI] [PubMed] [Google Scholar]
- 77.Ranchet G, Gangemi O, and Petrone M, Sucralfate in the treatment of gravid pyrosis. G Ital Obstet Ginecol, 1990. 12: p. 1–16. [Google Scholar]
- 78.el-Serag HB and Sonnenberg A, Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology, 1997. 113(3): p. 755–60. [DOI] [PubMed] [Google Scholar]
- 79.Connor NP, et al. , Symptoms of extraesophageal reflux in a community-dwelling sample. J Voice, 2007. 21(2): p. 189–202. [DOI] [PubMed] [Google Scholar]
- 80.Irwin RS, Curley FJ, and French CL, Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis, 1990. 141(3): p. 640–7. [DOI] [PubMed] [Google Scholar]
- 81.Kahrilas PJ, et al. , Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. Chest, 2016. 150(6): p. 1341–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Havemann BD, Henderson CA, and El-Serag HB, The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut, 2007. 56(12): p. 1654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mastronarde JG, et al. , Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med, 2009. 360(15): p. 1487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holbrook JT, et al. , Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. Jama, 2012. 307(4): p. 373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poelmans J, et al. , The yield of upper gastrointestinal endoscopy in patients with suspected reflux-related chronic ear, nose, and throat symptoms. Am J Gastroenterol, 2004. 99(8): p. 1419–26. [DOI] [PubMed] [Google Scholar]
- 86.Ronkainen J, et al. , High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol, 2005. 40(3): p. 275–85. [DOI] [PubMed] [Google Scholar]
- 87.Lei WY, et al. , Predictive factors of silent reflux in subjects with erosive esophagitis. Dig Liver Dis, 2015. 47(1): p. 24–9. [DOI] [PubMed] [Google Scholar]
- 88.Belafsky PC, Postma GN, and Koufman JA, The validity and reliability of the reflux finding score (RFS). Laryngoscope, 2001. 111(8): p. 1313–7. [DOI] [PubMed] [Google Scholar]
- 89.Lechien JR, et al. , Instruments evaluating the clinical findings of laryngopharyngeal reflux: A systematic review. Laryngoscope, 2019. 129(3): p. 720–736. [DOI] [PubMed] [Google Scholar]
- 90.Branski RC, Bhattacharyya N, and Shapiro J, The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope, 2002. 112(6): p. 1019–24. [DOI] [PubMed] [Google Scholar]
- 91.Rafii B, et al. , Incidence of underlying laryngeal pathology in patients initially diagnosed with laryngopharyngeal reflux. Laryngoscope, 2014. 124(6): p. 1420–4. [DOI] [PubMed] [Google Scholar]
- 92.Rosen R, et al. , The Edematous and Erythematous Airway Does Not Denote Pathologic Gastroesophageal Reflux. J Pediatr, 2017. 183: p. 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hicks DM, et al. , The prevalence of hypopharynx findings associated with gastroesophageal reflux in normal volunteers. J Voice, 2002. 16(4): p. 564–79. [DOI] [PubMed] [Google Scholar]
- 94.Milstein CF, et al. , Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs. flexible laryngoscope). Laryngoscope, 2005. 115(12): p. 2256–61. [DOI] [PubMed] [Google Scholar]
- 95.de Bortoli N, et al. , How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related? World J Gastroenterol, 2012. 18(32): p. 4363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nennstiel S, et al. , pH/multichannel impedance monitoring in patients with laryngopharyngeal reflux symptoms - Prediction of therapy response in long-term follow-up. Arab J Gastroenterol, 2016. 17(3): p. 113–116. [DOI] [PubMed] [Google Scholar]
- 97.Wang AJ, et al. , Comparison of patients of chronic laryngitis with and without troublesome reflux symptoms. J Gastroenterol Hepatol, 2012. 27(3): p. 579–85. [DOI] [PubMed] [Google Scholar]
- 98.Blondeau K, et al. , Improved diagnosis of gastro-oesophageal reflux in patients with unexplained chronic cough. Aliment Pharmacol Ther, 2007. 25(6): p. 723–32. [DOI] [PubMed] [Google Scholar]
- 99.Blondeau K, et al. , The relationship between gastroesophageal reflux and cough in children with chronic unexplained cough using combined impedance-pH-manometry recordings. Pediatr Pulmonol, 2011. 46(3): p. 286–94. [DOI] [PubMed] [Google Scholar]
- 100.Anandasabapathy S and Jaffin BW, Multichannel intraluminal impedance in the evaluation of patients with persistent globus on proton pump inhibitor therapy. Ann Otol Rhinol Laryngol, 2006. 115(8): p. 563–70. [DOI] [PubMed] [Google Scholar]
- 101.Lechien JR, et al. , Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol Head Neck Surg, 2019. 160(5): p. 762–782. [DOI] [PubMed] [Google Scholar]
- 102.Roberts JR, et al. , Extraesophageal gastroesophageal reflux disease (GERD) symptoms are not more frequently associated with proximal esophageal reflux than typical GERD symptoms. Dis Esophagus, 2012. 25(8): p. 678–81. [DOI] [PubMed] [Google Scholar]
- 103.Francis DO, et al. , Traditional reflux parameters and not impedance monitoring predict outcome after fundoplication in extraesophageal reflux. Laryngoscope, 2011. 121(9): p. 1902–9. [DOI] [PubMed] [Google Scholar]
- 104.Desjardin M, et al. , 24-hour pH-impedance monitoring on therapy to select patients with refractory reflux symptoms for antireflux surgery. A single center retrospective study. Neurogastroenterol Motil, 2016. 28(1): p. 146–52. [DOI] [PubMed] [Google Scholar]
- 105.Roman S, et al. , Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil, 2017. 29(10): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 106.Patel DA, et al. , Model to Select On-Therapy vs Off-Therapy Tests for Patients With Refractory Esophageal or Extraesophageal Symptoms. Gastroenterology, 2018. 155(6): p. 1729–1740.e1. [DOI] [PubMed] [Google Scholar]
- 107.Hemmink GJ, et al. , Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: 'on' or 'off' proton pump inhibitor? Am J Gastroenterol, 2008. 103(10): p. 2446–53. [DOI] [PubMed] [Google Scholar]
- 108.Blonski W, Vela MF, and Castell DO, Comparison of reflux frequency during prolonged multichannel intraluminal impedance and pH monitoring on and off acid suppression therapy. J Clin Gastroenterol, 2009. 43(9): p. 816–20. [DOI] [PubMed] [Google Scholar]
- 109.Fletcher KC, et al. , Significance and degree of reflux in patients with primary extraesophageal symptoms. Laryngoscope, 2011. 121(12): p. 2561–5. [DOI] [PubMed] [Google Scholar]
- 110.Desjardin M, et al. , Pharyngeal pH alone is not reliable for the detection of pharyngeal reflux events: A study with oesophageal and pharyngeal pH-impedance monitoring. United European Gastroenterol J, 2013. 1(6): p. 438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noordzij JP, et al. , Correlation of pH probe-measured laryngopharyngeal reflux with symptoms and signs of reflux laryngitis. Laryngoscope, 2002. 112(12): p. 2192–5. [DOI] [PubMed] [Google Scholar]
- 112.Vaezi MF, Schroeder PL, and Richter JE, Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol, 1997. 92(5): p. 825–9. [PubMed] [Google Scholar]
- 113.McCollough M, et al. , Proximal sensor data from routine dual-sensor esophageal pH monitoring is often inaccurate. Dig Dis Sci, 2004. 49(10): p. 1607–11. [DOI] [PubMed] [Google Scholar]
- 114.Golub JS, et al. , Comparison of an oropharyngeal pH probe and a standard dual pH probe for diagnosis of laryngopharyngeal reflux. Ann Otol Rhinol Laryngol, 2009. 118(1): p. 1–5. [DOI] [PubMed] [Google Scholar]
- 115.Ayazi S, et al. , Proximal esophageal pH monitoring: improved definition of normal values and determination of a composite pH score. J Am Coll Surg, 2010. 210(3): p. 345–50. [DOI] [PubMed] [Google Scholar]
- 116.Hoppo T, et al. , How much pharyngeal exposure is "normal"? Normative data for laryngopharyngeal reflux events using hypopharyngeal multichannel intraluminal impedance (HMII). J Gastrointest Surg, 2012. 16(1): p. 16–24; discussion 24-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oelschlager BK, et al. , Gastroesophageal and pharyngeal reflux detection using impedance and 24-hour pH monitoring in asymptomatic subjects: defining the normal environment. J Gastrointest Surg, 2006. 10(1): p. 54–62. [DOI] [PubMed] [Google Scholar]
- 118.Joniau S, et al. , Reflux and laryngitis: a systematic review. Otolaryngol Head Neck Surg, 2007. 136(5): p. 686–92. [DOI] [PubMed] [Google Scholar]
- 119.Worrell SG, et al. , Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc, 2013. 27(11): p. 4113–8. [DOI] [PubMed] [Google Scholar]
- 120.Wiener GJ, et al. , Oropharyngeal pH monitoring for the detection of liquid and aerosolized supraesophageal gastric reflux. J Voice, 2009. 23(4): p. 498–504. [DOI] [PubMed] [Google Scholar]
- 121.Hayat JO, et al. , Objective detection of esophagopharyngeal reflux in patients with hoarseness and endoscopic signs of laryngeal inflammation. J Clin Gastroenterol, 2014. 48(4): p. 318–27. [DOI] [PubMed] [Google Scholar]
- 122.Ummarino D, et al. , Gastroesophageal reflux evaluation in patients affected by chronic cough: Restech versus multichannel intraluminal impedance/pH metry. Laryngoscope, 2013. 123(4): p. 980–4. [DOI] [PubMed] [Google Scholar]
- 123.Mazzoleni G, et al. , Correlation between oropharyngeal pH-monitoring and esophageal pH-impedance monitoring in patients with suspected GERD-related extra-esophageal symptoms. Neurogastroenterol Motil, 2014. 26(11): p. 1557–64. [DOI] [PubMed] [Google Scholar]
- 124.Chiou E, et al. , Diagnosis of supra-esophageal gastric reflux: correlation of oropharyngeal pH with esophageal impedance monitoring for gastro-esophageal reflux. Neurogastroenterol Motil, 2011. 23(8): p. 717–e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Plocek A, et al. , Esophageal Impedance-pH Monitoring and Pharyngeal pH Monitoring in the Diagnosis of Extraesophageal Reflux in Children. Gastroenterol Res Pract, 2019. 2019: p. 6271910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yadlapati R, et al. , Oropharyngeal pH Testing Does Not Predict Response to Proton Pump Inhibitor Therapy in Patients with Laryngeal Symptoms. Am J Gastroenterol, 2016. 111(11): p. 1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yadlapati R, et al. , Abilities of Oropharyngeal pH Tests and Salivary Pepsin Analysis to Discriminate Between Asymptomatic Volunteers and Subjects With Symptoms of Laryngeal Irritation. Clin Gastroenterol Hepatol, 2016. 14(4): p. 535–542.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, et al. , Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: a meta-analysis. Eur Arch Otorhinolaryngol, 2018. 275(3): p. 671–678. [DOI] [PubMed] [Google Scholar]
- 129.Spyridoulias A, et al. , Detecting laryngopharyngeal reflux in patients with upper airways symptoms: Symptoms, signs or salivary pepsin? Respir Med, 2015. 109(8): p. 963–9. [DOI] [PubMed] [Google Scholar]
- 130.Calvo-Henríquez C, et al. , Is Pepsin a Reliable Marker of Laryngopharyngeal Reflux? A Systematic Review. Otolaryngol Head Neck Surg, 2017. 157(3): p. 385–391. [DOI] [PubMed] [Google Scholar]
- 131.Na SY, et al. , Optimal timing of saliva collection to detect pepsin in patients with laryngopharyngeal reflux. Laryngoscope, 2016. 126(12): p. 2770–2773. [DOI] [PubMed] [Google Scholar]
- 132.Dy F, et al. , Salivary Pepsin Lacks Sensitivity as a Diagnostic Tool to Evaluate Extraesophageal Reflux Disease. J Pediatr, 2016. 177: p. 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ford CN, Evaluation and management of laryngopharyngeal reflux. Jama, 2005. 294(12): p. 1534–40. [DOI] [PubMed] [Google Scholar]
- 134.Gupta N, Green RW, and Megwalu UC, Evaluation of a laryngopharyngeal reflux management protocol. Am J Otolaryngol, 2016. 37(3): p. 245–50. [DOI] [PubMed] [Google Scholar]
- 135.Koufman JA, et al. , Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg, 2002. 127(1): p. 32–5. [DOI] [PubMed] [Google Scholar]
- 136.Megwalu UC, A systematic review of proton-pump inhibitor therapy for laryngopharyngeal reflux. Ear Nose Throat J, 2013. 92(8): p. 364–71. [DOI] [PubMed] [Google Scholar]
- 137.Qadeer MA, et al. , Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol, 2006. 101(11): p. 2646–54. [DOI] [PubMed] [Google Scholar]
- 138.Lechien JR, et al. , Clinical outcomes of laryngopharyngeal reflux treatment: A systematic review and meta-analysis. Laryngoscope, 2019. 129(5): p. 1174–1187. [DOI] [PubMed] [Google Scholar]
- 139.Guo H, Ma H, and Wang J, Proton Pump Inhibitor Therapy for the Treatment of Laryngopharyngeal Reflux: A Meta-Analysis of Randomized Controlled Trials. J Clin Gastroenterol, 2016. 50(4): p. 295–300. [DOI] [PubMed] [Google Scholar]
- 140.Karkos PD and Wilson JA, Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope, 2006. 116(1): p. 144–8. [DOI] [PubMed] [Google Scholar]
- 141.Carroll TL, et al. , Rethinking the laryngopharyngeal reflux treatment algorithm: Evaluating an alternate empiric dosing regimen and considering up-front, pH-impedance, and manometry testing to minimize cost in treating suspect laryngopharyngeal reflux disease. Laryngoscope, 2017. 127 Suppl 6: p. S1–s13. [DOI] [PubMed] [Google Scholar]
- 142.Chan WW, et al. , The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med, 2011. 171(7): p. 620–9. [DOI] [PubMed] [Google Scholar]
- 143.Kiljander TO, et al. , Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med, 2006. 173(10): p. 1091–7. [DOI] [PubMed] [Google Scholar]
- 144.Kahrilas PJ, et al. , Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. Chest, 2016. 150(6): p. 1341–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang AB, et al. , Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev, 2011. 2011(1): p. Cd004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kahrilas PJ, et al. , Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest, 2013. 143(3): p. 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaheen NJ, et al. , Randomised clinical trial: high-dose acid suppression for chronic cough - a double-blind, placebo-controlled study. Aliment Pharmacol Ther, 2011. 33(2): p. 225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iqbal M, et al. , Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A, 2008. 18(6): p. 789–96. [DOI] [PubMed] [Google Scholar]
- 149.Sidwa F, et al. , Surgical Treatment of Extraesophageal Manifestations of Gastroesophageal Reflux Disease. World J Surg, 2017. 41(10): p. 2566–2571. [DOI] [PubMed] [Google Scholar]
- 150.Swoger J, et al. , Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol, 2006. 4(4): p. 433–41. [DOI] [PubMed] [Google Scholar]
- 151.Sontag SJ, et al. , Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol, 2003. 98(5): p. 987–99. [DOI] [PubMed] [Google Scholar]
- 152.Kiljander T, et al. , Comparison of the effects of esomeprazole and fundoplication on airway responsiveness in patients with gastro-oesophageal reflux disease. Clin Respir J, 2013. 7(3): p. 281–7. [DOI] [PubMed] [Google Scholar]
- 153.Komatsu Y, Hoppo T, and Jobe BA, Proximal reflux as a cause of adult-onset asthma: the case for hypopharyngeal impedance testing to improve the sensitivity of diagnosis. JAMA Surg, 2013. 148(1): p. 50–8. [DOI] [PubMed] [Google Scholar]
- 154.Krill JT, et al. , Association Between Response to Acid-Suppression Therapy and Efficacy of Antireflux Surgery in Patients With Extraesophageal Reflux. Clin Gastroenterol Hepatol, 2017. 15(5): p. 675–681. [DOI] [PubMed] [Google Scholar]
- 155.Kahrilas PJ, Boeckxstaens G, and Smout AJ, Management of the patient with incomplete response to PPI therapy. Best Pract Res Clin Gastroenterol, 2013. 27(3): p. 401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.El-Serag H, Becher A, and Jones R, Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther, 2010. 32(6): p. 720–37. [DOI] [PubMed] [Google Scholar]
- 157.Becher A and El-Serag H, Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther, 2011. 34(6): p. 618–27. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H, et al. , A Meta-Analysis and Systematic Review of the Efficacy of Twice Daily PPIs versus Once Daily for Treatment of Gastroesophageal Reflux Disease. Gastroenterol Res Pract, 2017. 2017: p. 9865963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yadlapati R, et al. , Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol, 2018. 113(7): p. 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sifrim D and Zerbib F, Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut, 2012. 61(9): p. 1340–54. [DOI] [PubMed] [Google Scholar]
- 161.Charbel S, Khandwala F, and Vaezi MF, The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol, 2005. 100(2): p. 283–9. [DOI] [PubMed] [Google Scholar]
- 162.Hatlebakk JG, et al. , Nocturnal gastric acidity and acid breakthrough on different regimens of omeprazole 40 mg daily. Aliment Pharmacol Ther, 1998. 12(12): p. 1235–40. [DOI] [PubMed] [Google Scholar]
- 163.Dickman R, et al. , Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil, 2011. 17(4): p. 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ribolsi M, et al. , Prevalence and clinical characteristics of refractoriness to optimal proton pump inhibitor therapy in non-erosive reflux disease. Aliment Pharmacol Ther, 2018. 48(10): p. 1074–1081. [DOI] [PubMed] [Google Scholar]
- 165.Waghray A, et al. , Optimal Omeprazole Dosing and Symptom Control: A Randomized Controlled Trial (OSCAR Trial). Dig Dis Sci, 2019. 64(1): p. 158–166. [DOI] [PubMed] [Google Scholar]
- 166.Fass R, et al. , Omeprazole 40 mg once a day is equally effective as lansoprazole 30 mg twice a day in symptom control of patients with gastro-oesophageal reflux disease (GERD) who are resistant to conventional-dose lansoprazole therapy-a prospective, randomized, multi-centre study. Aliment Pharmacol Ther, 2000. 14(12): p. 1595–603. [DOI] [PubMed] [Google Scholar]
- 167.Anis K, et al. , Retrospective Analysis of Eosinophilic Esophagitis in Patients with Refractory Gastroesophageal Reflux Disease. Cureus, 2019. 11(7): p. e5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.García-Compeán D, et al. , Prevalence of eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease symptoms: A prospective study. Dig Liver Dis, 2011. 43(3): p. 204–8. [DOI] [PubMed] [Google Scholar]
- 169.Poh CH, et al. , Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc, 2010. 71(1): p. 28–34. [DOI] [PubMed] [Google Scholar]
- 170.Veerappan GR, et al. , Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol, 2009. 7(4): p. 420–6, 426.e1-2. [DOI] [PubMed] [Google Scholar]
- 171.Gaddam S, et al. , The impact of pre-endoscopy proton pump inhibitor use on the classification of non-erosive reflux disease and erosive oesophagitis. Aliment Pharmacol Ther, 2010. 32(10): p. 1266–74. [DOI] [PubMed] [Google Scholar]
- 172.Yadlapati R, et al. , Ambulatory Reflux Monitoring Guides Proton Pump Inhibitor Discontinuation in Patients With Gastroesophageal Reflux Symptoms: A Clinical Trial. Gastroenterology, 2021. 160(1): p. 174–182 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hamdy E, et al. , Outcome of laparoscopic Nissen fundoplication for gastroesophageal reflux disease in non-responders to proton pump inhibitors. J Gastrointest Surg, 2014. 18(9): p. 1557–62. [DOI] [PubMed] [Google Scholar]
- 174.Shi Y, et al. , Predictors of proton pump inhibitor failure in non-erosive reflux disease: A study with impedance-pH monitoring and high-resolution manometry. Neurogastroenterol Motil, 2016. 28(5): p. 674–9. [DOI] [PubMed] [Google Scholar]
- 175.Xiao Y, et al. , Tailored therapy for the refractory GERD patients by combined multichannel intraluminal impedance-pH monitoring. J Gastroenterol Hepatol, 2016. 31(2): p. 350–4. [DOI] [PubMed] [Google Scholar]
- 176.Galindo G, et al. , Multimodality evaluation of patients with gastroesophageal reflux disease symptoms who have failed empiric proton pump inhibitor therapy. Dis Esophagus, 2013. 26(5): p. 443–50. [DOI] [PubMed] [Google Scholar]
- 177.Gawron AJ, et al. , Many patients continue using proton pump inhibitors after negative results from tests for reflux disease. Clin Gastroenterol Hepatol, 2012. 10(6): p. 620–5; quiz e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chan WW, Haroian LR, and Gyawali CP, Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc, 2011. 25(9): p. 2943–9. [DOI] [PubMed] [Google Scholar]
- 179.Bell R, et al. , Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc, 2019. 89(1): p. 14–22.e1. [DOI] [PubMed] [Google Scholar]
- 180.Bell R, et al. , Magnetic Sphincter Augmentation Superior to Proton Pump Inhibitors for Regurgitation in a 1-Year Randomized Trial. Clin Gastroenterol Hepatol, 2020. 18(8): p. 1736–1743.e2. [DOI] [PubMed] [Google Scholar]
- 181.Hunter JG, et al. , Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology, 2015. 148(2): p. 324–333.e5. [DOI] [PubMed] [Google Scholar]
- 182.Murray HB, et al. , Diagnosis and Treatment of Rumination Syndrome: A Critical Review. Am J Gastroenterol, 2019. 114(4): p. 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hetzel DJ, et al. , Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology, 1988. 95(4): p. 903–12. [DOI] [PubMed] [Google Scholar]
- 184.Klinkenberg-Knol EC, et al. , Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology, 2000. 118(4): p. 661–9. [DOI] [PubMed] [Google Scholar]
- 185.Oelschlager BK, et al. , Long-term outcomes after laparoscopic antireflux surgery. Am J Gastroenterol, 2008. 103(2): p. 280–7; quiz 288. [DOI] [PubMed] [Google Scholar]
- 186.Power C, Maguire D, and McAnena O, Factors contributing to failure of laparoscopic Nissen fundoplication and the predictive value of preoperative assessment. Am J Surg, 2004. 187(4): p. 457–63. [DOI] [PubMed] [Google Scholar]
- 187.Fass R and Sifrim D, Management of heartburn not responding to proton pump inhibitors. Gut, 2009. 58(2): p. 295–309. [DOI] [PubMed] [Google Scholar]
- 188.Scarpellini E, et al. , Management of refractory typical GERD symptoms. Nat Rev Gastroenterol Hepatol, 2016. 13(5): p. 281–94. [DOI] [PubMed] [Google Scholar]
- 189.Spechler SJ, Surgery for gastroesophageal reflux disease: esophageal impedance to progress? Clin Gastroenterol Hepatol, 2009. 7(12): p. 1264–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.DeMeester TR, Bonavina L, and Albertucci M, Nissen fundoplication for gastroesophageal reflux disease. Evaluation of primary repair in 100 consecutive patients. Ann Surg, 1986. 204(1): p. 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Spechler SJ, Comparison of medical and surgical therapy for complicated gastroesophageal reflux disease in veterans. The Department of Veterans Affairs Gastroesophageal Reflux Disease Study Group. N Engl J Med, 1992. 326(12): p. 786–92. [DOI] [PubMed] [Google Scholar]
- 192.Spechler SJ, et al. , Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. Jama, 2001. 285(18): p. 2331–8. [DOI] [PubMed] [Google Scholar]
- 193.Bonatti H, et al. , Use of acid suppressive medications after laparoscopic antireflux surgery: prevalence and clinical indications. Dig Dis Sci, 2007. 52(1): p. 267–72. [DOI] [PubMed] [Google Scholar]
- 194.Ciovica R, et al. , The use of medication after laparoscopic antireflux surgery. Surg Endosc, 2009. 23(9): p. 1938–46. [DOI] [PubMed] [Google Scholar]
- 195.Gee DW, Andreoli MT, and Rattner DW, Measuring the effectiveness of laparoscopic antireflux surgery: long-term results. Arch Surg, 2008. 143(5): p. 482–7. [DOI] [PubMed] [Google Scholar]
- 196.Papasavas PK, et al. , Effectiveness of laparoscopic fundoplication in relieving the symptoms of gastroesophageal reflux disease (GERD) and eliminating antireflux medical therapy. Surg Endosc, 2003. 17(8): p. 1200–5. [DOI] [PubMed] [Google Scholar]
- 197.Wijnhoven BP, et al. , Use of antireflux medication after antireflux surgery. J Gastrointest Surg, 2008. 12(3): p. 510–7. [DOI] [PubMed] [Google Scholar]
- 198.Anvari M, et al. , A randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for the treatment of patients with chronic gastroesophageal reflux disease (GERD): 3-year outcomes. Surg Endosc, 2011. 25(8): p. 2547–54. [DOI] [PubMed] [Google Scholar]
- 199.Grant AM, et al. , Minimal access surgery compared with medical management for gastro-oesophageal reflux disease: five year follow-up of a randomised controlled trial (REFLUX). Bmj, 2013. 346: p. f1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lundell L, et al. , Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut, 2008. 57(9): p. 1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rickenbacher N, et al. , Fundoplication versus medical management of gastroesophageal reflux disease: systematic review and meta-analysis. Surg Endosc, 2014. 28(1): p. 143–55. [DOI] [PubMed] [Google Scholar]
- 202.Garg SK and Gurusamy KS, Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev, 2015(11): p. Cd003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tian ZC, et al. , A Meta-Analysis of Randomized Controlled Trials to Compare Long-Term Outcomes of Nissen and Toupet Fundoplication for Gastroesophageal Reflux Disease. PLoS One, 2015. 10(6): p. e0127627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Du X, et al. , A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270°) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol, 2016. 16(1): p. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Memon MA, et al. , Laparoscopic anterior versus posterior fundoplication for gastro-esophageal reflux disease: a meta-analysis and systematic review. World J Surg, 2015. 39(4): p. 981–96. [DOI] [PubMed] [Google Scholar]
- 206.Du X, et al. , Laparoscopic Nissen (total) versus anterior 180° fundoplication for gastro-esophageal reflux disease: A meta-analysis and systematic review. Medicine (Baltimore), 2017. 96(37): p. e8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Maret-Ouda J, et al. , Association Between Laparoscopic Antireflux Surgery and Recurrence of Gastroesophageal Reflux. Jama, 2017. 318(10): p. 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Spechler SJ, The Durability of Antireflux Surgery. Jama, 2017. 318(10): p. 913–915. [DOI] [PubMed] [Google Scholar]
- 209.Ganz RA, et al. , Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med, 2013. 368(8): p. 719–27. [DOI] [PubMed] [Google Scholar]
- 210.Ganz RA, et al. , Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol, 2016. 14(5): p. 671–7. [DOI] [PubMed] [Google Scholar]
- 211.Rona KA, et al. , Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc, 2017. 31(5): p. 2096–2102. [DOI] [PubMed] [Google Scholar]
- 212.Ayazi S, et al. , Magnetic sphincter augmentation (MSA) in patients with hiatal hernia: clinical outcome and patterns of recurrence. Surg Endosc, 2020. 34(4): p. 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Buckley FP 3rd, et al. , Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc, 2018. 32(4): p. 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Alicuben ET, et al. , Worldwide Experience with Erosion of the Magnetic Sphincter Augmentation Device. J Gastrointest Surg, 2018. 22(8): p. 1442–1447. [DOI] [PubMed] [Google Scholar]
- 215.Aiolfi A, et al. , Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis. Int J Surg, 2018. 52: p. 82–88. [DOI] [PubMed] [Google Scholar]
- 216.Chen MY, et al. , Efficacy of Magnetic Sphincter Augmentation versus Nissen Fundoplication for Gastroesophageal Reflux Disease in Short Term: A Meta-Analysis. Can J Gastroenterol Hepatol, 2017. 2017: p. 9596342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Guidozzi N, et al. , Laparoscopic magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and pooled analysis. Dis Esophagus, 2019. 32(9). [DOI] [PubMed] [Google Scholar]
- 218.Skubleny D, et al. , LINX(®) magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc, 2017. 31(8): p. 3078–3084. [DOI] [PubMed] [Google Scholar]
- 219.Riva CG, et al. , Magnetic Sphincter Augmentation After Gastric Surgery. Jsls, 2019. 23(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nilsson M, et al. , Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. Jama, 2003. 290(1): p. 66–72. [DOI] [PubMed] [Google Scholar]
- 221.Kim M, et al. , Minimally invasive Roux-en-Y gastric bypass for fundoplication failure offers excellent gastroesophageal reflux control. Am Surg, 2014. 80(7): p. 696–703. [PubMed] [Google Scholar]
- 222.Patterson EJ, et al. , Comparison of objective outcomes following laparoscopic Nissen fundoplication versus laparoscopic gastric bypass in the morbidly obese with heartburn. Surg Endosc, 2003. 17(10): p. 1561–5. [DOI] [PubMed] [Google Scholar]
- 223.Perez AR, Moncure AC, and Rattner DW, Obesity adversely affects the outcome of antireflux operations. Surg Endosc, 2001. 15(9): p. 986–9. [DOI] [PubMed] [Google Scholar]
- 224.Ng VV, et al. , Laparoscopic anti-reflux surgery is effective in obese patients with gastro-oesophageal reflux disease. Ann R Coll Surg Engl, 2007. 89(7): p. 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abdelrahman T, et al. , Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg, 2018. 51: p. 76–82. [DOI] [PubMed] [Google Scholar]
- 226.Lim R, et al. , Early and late complications of bariatric operation. Trauma Surg Acute Care Open, 2018. 3(1): p. e000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Holmberg D, et al. , Gastric bypass surgery in the treatment of gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther, 2019. 50(2): p. 159–166. [DOI] [PubMed] [Google Scholar]
- 228.Corley DA, et al. , Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology, 2003. 125(3): p. 668–76. [DOI] [PubMed] [Google Scholar]
- 229.Lipka S, Kumar A, and Richter JE, No evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol, 2015. 13(6): p. 1058–67.e1. [DOI] [PubMed] [Google Scholar]
- 230.Fass R, et al. , Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc, 2017. 31(12): p. 4865–4882. [DOI] [PubMed] [Google Scholar]
- 231.Auyang ED, et al. , SAGES clinical spotlight review: endoluminal treatments for gastroesophageal reflux disease (GERD). Surg Endosc, 2013. 27(8): p. 2658–72. [DOI] [PubMed] [Google Scholar]
- 232.Trad KS, et al. , Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO Randomized Clinical Trial. Surg Innov, 2015. 22(1): p. 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McCarty TR, et al. , Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy, 2018. 50(7): p. 708–725. [DOI] [PubMed] [Google Scholar]
- 234.Testoni S, et al. , Long-term outcomes of transoral incisionless fundoplication for gastro-esophageal reflux disease: systematic-review and meta-analysis. Endosc Int Open, 2021. 9(2): p. E239–e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kahrilas PJ, Shaheen NJ, and Vaezi MF, American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology, 2008. 135(4): p. 1392–1413, 1413.e1-5. [DOI] [PubMed] [Google Scholar]
- 236.Islam MM, et al. , Adverse outcomes of long-term use of proton pump inhibitors: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol, 2018. 30(12): p. 1395–1405. [DOI] [PubMed] [Google Scholar]
- 237.Laine L, et al. , Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther, 2000. 14(6): p. 651–68. [DOI] [PubMed] [Google Scholar]
- 238.Srinutta T, et al. , Proton pump inhibitors and hypomagnesemia: A meta-analysis of observational studies. Medicine (Baltimore), 2019. 98(44): p. e17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cheungpasitporn W, et al. , Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail, 2015. 37(7): p. 1237–41. [DOI] [PubMed] [Google Scholar]
- 240.Liao S, Gan L, and Mei Z, Does the use of proton pump inhibitors increase the risk of hypomagnesemia: An updated systematic review and meta-analysis. Medicine (Baltimore), 2019. 98(13): p. e15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Park CH, et al. , The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One, 2014. 9(11): p. e112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Freedberg DE, Kim LS, and Yang YX, The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology, 2017. 152(4): p. 706–715. [DOI] [PubMed] [Google Scholar]
- 243.Bosco JL, et al. , A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol, 2010. 63(1): p. 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Horwitz RI and Feinstein AR, The problem of "protopathic bias" in case-control studies. Am J Med, 1980. 68(2): p. 255–8. [DOI] [PubMed] [Google Scholar]
- 245.Hill AB, THE ENVIRONMENT AND DISEASE: ASSOCIATION OR CAUSATION? Proc R Soc Med, 1965. 58(5): p. 295–300. [PMC free article] [PubMed] [Google Scholar]
- 246.Vaezi MF, Yang YX, and Howden CW, Complications of Proton Pump Inhibitor Therapy. Gastroenterology, 2017. 153(1): p. 35–48. [DOI] [PubMed] [Google Scholar]
- 247.Grimes DA and Schulz KF, False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol, 2012. 120(4): p. 920–7. [DOI] [PubMed] [Google Scholar]
- 248.Wijarnpreecha K, et al. , Associations of Proton-Pump Inhibitors and H2 Receptor Antagonists with Chronic Kidney Disease: A Meta-Analysis. Dig Dis Sci, 2017. 62(10): p. 2821–2827. [DOI] [PubMed] [Google Scholar]
- 249.García Rodríguez LA, Lagergren J, and Lindblad M, Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut, 2006. 55(11): p. 1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sarkar M, Hennessy S, and Yang YX, Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med, 2008. 149(6): p. 391–8. [DOI] [PubMed] [Google Scholar]
- 251.Moayyedi P, et al. , Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology, 2019. 157(3): p. 682–691.e2. [DOI] [PubMed] [Google Scholar]
- 252.Targownik LE, et al. , Use of proton pump inhibitors and risk of osteoporosis-related fractures. Cmaj, 2008. 179(4): p. 319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Batchelor R, et al. , Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther, 2018. 48(8): p. 780–796. [DOI] [PubMed] [Google Scholar]
- 254.Dahal K, et al. , Efficacy and Safety of Proton Pump Inhibitors in the Long-Term Aspirin Users: A Meta-Analysis of Randomized Controlled Trials. Am J Ther, 2017. 24(5): p. e559–e569. [DOI] [PubMed] [Google Scholar]
- 255.Li S, et al. , Real-World Relationship Between Proton Pump Inhibitors and Cerebro-Cardiovascular Outcomes Independent of Clopidogrel. Int Heart J, 2019. 60(4): p. 910–918. [DOI] [PubMed] [Google Scholar]
- 256.Sun S, et al. , Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil, 2017. 29(2). [DOI] [PubMed] [Google Scholar]
- 257.Bundhun PK, et al. , Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012 - 2016). BMC Cardiovasc Disord, 2017. 17(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cardoso RN, et al. , Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart, 2015. 2(1): p. e000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen J, et al. , Pharmacodynamic impacts of proton pump inhibitors on the efficacy of clopidogrel in vivo--a systematic review. Clin Cardiol, 2013. 36(4): p. 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Demcsák A, et al. , PPIs Are Not Responsible for Elevating Cardiovascular Risk in Patients on Clopidogrel-A Systematic Review and Meta-Analysis. Front Physiol, 2018. 9: p. 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Farhat N, et al. , Systematic review and meta-analysis of adverse cardiovascular events associated with proton pump inhibitors used alone or in combination with antiplatelet agents. Crit Rev Toxicol, 2019. 49(3): p. 215–261. [DOI] [PubMed] [Google Scholar]
- 262.Gerson LB, et al. , Lack of significant interactions between clopidogrel and proton pump inhibitor therapy: meta-analysis of existing literature. Dig Dis Sci, 2012. 57(5): p. 1304–13. [DOI] [PubMed] [Google Scholar]
- 263.Hu W, et al. , Influence of proton pump inhibitors on clinical outcomes in coronary heart disease patients receiving aspirin and clopidogrel: A meta-analysis. Medicine (Baltimore), 2018. 97(3): p. e9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Huang B, et al. , Adverse cardiovascular effects of concomitant use of proton pump inhibitors and clopidogrel in patients with coronary artery disease: a systematic review and meta-analysis. Arch Med Res, 2012. 43(3): p. 212–24. [DOI] [PubMed] [Google Scholar]
- 265.Hulot JS, et al. , Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol, 2010. 56(2): p. 134–43. [DOI] [PubMed] [Google Scholar]
- 266.Khan MY, et al. , Reduction in postpercutaneous coronary intervention angina in addition to gastrointestinal events in patients on combined proton pump inhibitors and dual antiplatelet therapy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol, 2018. 30(8): p. 847–853. [DOI] [PubMed] [Google Scholar]
- 267.Khan SU, et al. , Meta-Analysis of Efficacy and Safety of Proton Pump Inhibitors with Dual Antiplatelet Therapy for Coronary Artery Disease. Cardiovasc Revasc Med, 2019. 20(12): p. 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kwok CS, et al. , No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: meta-analysis. Int J Cardiol, 2013. 167(3): p. 965–74. [DOI] [PubMed] [Google Scholar]
- 269.Kwok CS and Loke YK, Meta-analysis: the effects of proton pump inhibitors on cardiovascular events and mortality in patients receiving clopidogrel. Aliment Pharmacol Ther, 2010. 31(8): p. 810–23. [DOI] [PubMed] [Google Scholar]
- 270.Malhotra K, et al. , Cerebrovascular Outcomes With Proton Pump Inhibitors and Thienopyridines: A Systematic Review and Meta-Analysis. Stroke, 2018. 49(2): p. 312–318. [DOI] [PubMed] [Google Scholar]
- 271.Melloni C, et al. , Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes, 2015. 8(1): p. 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Niu Q, et al. , Combination Use of Clopidogrel and Proton Pump Inhibitors Increases Major Adverse Cardiovascular Events in Patients With Coronary Artery Disease: A Meta-Analysis. J Cardiovasc Pharmacol Ther, 2017. 22(2): p. 142–152. [DOI] [PubMed] [Google Scholar]
- 273.Pang J, et al. , Efficacy and safety of clopidogrel only vs. clopidogrel added proton pump inhibitors in the treatment of patients with coronary heart disease after percutaneous coronary intervention: A systematic review and meta-analysis. Int J Cardiol Heart Vasc, 2019. 23: p. 100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Serbin MA, Guzauskas GF, and Veenstra DL, Clopidogrel-Proton Pump Inhibitor Drug-Drug Interaction and Risk of Adverse Clinical Outcomes Among PCI-Treated ACS Patients: A Meta-analysis. J Manag Care Spec Pharm, 2016. 22(8): p. 939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sherwood MW, et al. , Individual Proton Pump Inhibitors and Outcomes in Patients With Coronary Artery Disease on Dual Antiplatelet Therapy: A Systematic Review. J Am Heart Assoc, 2015. 4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Siller-Matula JM, et al. , Effect of proton pump inhibitors on clinical outcome in patients treated with clopidogrel: a systematic review and meta-analysis. J Thromb Haemost, 2010. 8(12): p. 2624–41. [DOI] [PubMed] [Google Scholar]
- 277.Nochaiwong S, et al. , The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant, 2018. 33(2): p. 331–342. [DOI] [PubMed] [Google Scholar]
- 278.Qiu T, Zhou J, and Zhang C, Acid-suppressive drugs and risk of kidney disease: A systematic review and meta-analysis. J Gastroenterol Hepatol, 2018. [DOI] [PubMed] [Google Scholar]
- 279.Sun J, et al. , The use of anti-ulcer agents and the risk of chronic kidney disease: a meta-analysis. Int Urol Nephrol, 2018. 50(10): p. 1835–1843. [DOI] [PubMed] [Google Scholar]
- 280.Yang Y, et al. , Proton-pump inhibitors use, and risk of acute kidney injury: a meta-analysis of observational studies. Drug Des Devel Ther, 2017. 11: p. 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bavishi C and Dupont HL, Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther, 2011. 34(11-12): p. 1269–81. [DOI] [PubMed] [Google Scholar]
- 282.Hafiz RA, et al. , The Risk of Community-Acquired Enteric Infection in Proton Pump Inhibitor Therapy: Systematic Review and Meta-analysis. Ann Pharmacother, 2018. 52(7): p. 613–622. [DOI] [PubMed] [Google Scholar]
- 283.Arriola V, et al. , Assessing the Risk of Hospital-Acquired Clostridium Difficile Infection With Proton Pump Inhibitor Use: A Meta-Analysis. Infect Control Hosp Epidemiol, 2016. 37(12): p. 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cao F, et al. , Updated meta-analysis of controlled observational studies: proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect, 2018. 98(1): p. 4–13. [DOI] [PubMed] [Google Scholar]
- 285.Deshpande A, et al. , Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol, 2012. 10(3): p. 225–33. [DOI] [PubMed] [Google Scholar]
- 286.Janarthanan S, et al. , Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol, 2012. 107(7): p. 1001–10. [DOI] [PubMed] [Google Scholar]
- 287.Kwok CS, et al. , Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol, 2012. 107(7): p. 1011–9. [DOI] [PubMed] [Google Scholar]
- 288.Oshima T, et al. , Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol, 2018. 53(1): p. 84–94. [DOI] [PubMed] [Google Scholar]
- 289.Tariq R, et al. , Association of Gastric Acid Suppression With Recurrent Clostridium difficile Infection: A Systematic Review and Meta-analysis. JAMA Intern Med, 2017. 177(6): p. 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tleyjeh IM, et al. , Association between proton pump inhibitor therapy and clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS One, 2012. 7(12): p. e50836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Trifan A, et al. , Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol, 2017. 23(35): p. 6500–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lo WK and Chan WW, Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol, 2013. 11(5): p. 483–90. [DOI] [PubMed] [Google Scholar]
- 293.Su T, et al. , Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol, 2018. 53(1): p. 27–36. [DOI] [PubMed] [Google Scholar]
- 294.Deshpande A, et al. , Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol, 2013. 28(2): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 295.Khan MA, et al. , Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol, 2015. 27(11): p. 1327–36. [DOI] [PubMed] [Google Scholar]
- 296.Trikudanathan G, et al. , Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract, 2011. 65(6): p. 674–8. [DOI] [PubMed] [Google Scholar]
- 297.Xu HB, et al. , Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res, 2015. 14(3): p. 7490–501. [DOI] [PubMed] [Google Scholar]
- 298.Yu T, et al. , Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: A meta-analysis. Dig Liver Dis, 2016. 48(4): p. 353–9. [DOI] [PubMed] [Google Scholar]
- 299.Eom CS, et al. , Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. Cmaj, 2011. 183(3): p. 310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Filion KB, et al. , Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut, 2014. 63(4): p. 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Giuliano C, Wilhelm SM, and Kale-Pradhan PB, Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol, 2012. 5(3): p. 337–44. [DOI] [PubMed] [Google Scholar]
- 302.Johnstone J, Nerenberg K, and Loeb M, Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther, 2010. 31(11): p. 1165–77. [DOI] [PubMed] [Google Scholar]
- 303.Lambert AA, et al. , Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One, 2015. 10(6): p. e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wang CH, et al. , Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf, 2019. 18(3): p. 163–172. [DOI] [PubMed] [Google Scholar]
- 305.Batchelor R, et al. , Dementia, cognitive impairment and proton pump inhibitor therapy: A systematic review. J Gastroenterol Hepatol, 2017. 32(8): p. 1426–1435. [DOI] [PubMed] [Google Scholar]
- 306.Hussain S, et al. , No association between proton pump inhibitor use and risk of dementia: Evidence from a meta-analysis. J Gastroenterol Hepatol, 2020. 35(1): p. 19–28. [DOI] [PubMed] [Google Scholar]
- 307.Li M, et al. , Proton pump inhibitor use and risk of dementia: Systematic review and meta-analysis. Medicine (Baltimore), 2019. 98(7): p. e14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Song YQ, et al. , Proton pump inhibitor use does not increase dementia and Alzheimer's disease risk: An updated meta-analysis of published studies involving 642305 patients. PLoS One, 2019. 14(7): p. e0219213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Eom CS, et al. , Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med, 2011. 9(3): p. 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hussain S, et al. , Proton pump inhibitors' use and risk of hip fracture: a systematic review and meta-analysis. Rheumatol Int, 2018. 38(11): p. 1999–2014. [DOI] [PubMed] [Google Scholar]
- 311.Kwok CS, Yeong JK, and Loke YK, Meta-analysis: risk of fractures with acid-suppressing medication. Bone, 2011. 48(4): p. 768–76. [DOI] [PubMed] [Google Scholar]
- 312.Liu J, et al. , Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. Life Sci, 2019. 218: p. 213–223. [DOI] [PubMed] [Google Scholar]
- 313.Ngamruengphong S, et al. , Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol, 2011. 106(7): p. 1209–18; quiz 1219. [DOI] [PubMed] [Google Scholar]
- 314.Poly TN, et al. , Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int, 2019. 30(1): p. 103–114. [DOI] [PubMed] [Google Scholar]
- 315.Yu EW, et al. , Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med, 2011. 124(6): p. 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhou B, et al. , Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int, 2016. 27(1): p. 339–47. [DOI] [PubMed] [Google Scholar]
- 317.Ye X, et al. , Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol, 2011. 23(9): p. 794–800. [DOI] [PubMed] [Google Scholar]
- 318.Eslami L and Nasseri-Moghaddam S, Meta-analyses: does long-term PPI use increase the risk of gastric premalignant lesions? Arch Iran Med, 2013. 16(8): p. 449–58. [PubMed] [Google Scholar]
- 319.Li Z, et al. , Effect of long-term proton pump inhibitor administration on gastric mucosal atrophy: A meta-analysis. Saudi J Gastroenterol, 2017. 23(4): p. 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lundell L, et al. , Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther, 2015. 42(6): p. 649–63. [DOI] [PubMed] [Google Scholar]
- 321.Ahn JS, et al. , Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol, 2013. 19(16): p. 2560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tran-Duy A, et al. , Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol, 2016. 14(12): p. 1706–1719.e5. [DOI] [PubMed] [Google Scholar]
- 323.Wan QY, et al. , Long-term proton pump inhibitors use and risk of gastric cancer: a meta-analysis of 926 386 participants. Gut, 2019. 68(4): p. 762–764. [DOI] [PubMed] [Google Scholar]
- 324.Jung SB, et al. , Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis. Intern Med J, 2015. 45(4): p. 409–16. [DOI] [PubMed] [Google Scholar]
- 325.Baik SH, Fung KW, and McDonald CJ, The Mortality Risk of Proton Pump Inhibitors in 1.9 Million US Seniors: An Extended Cox Survival Analysis. Clin Gastroenterol Hepatol, 2021. [DOI] [PubMed] [Google Scholar]