Effectiveness of Sodium Bicarbonate Infusion on Mortality in Critically Ill Children With Metabolic Acidosis (original) (raw)
Abstract
Objective: Metabolic acidosis often occurs in the paediatric intensive care unit (PICU). Although sodium bicarbonate (SB) has been widely used in paediatrics, data on the effect of SB on children with metabolic acidosis in the PICU are scarce.
Methods: Patients with metabolic acidosis who were treated with SB within 48 h of PICU admission were screened. Multivariate logistic regression, subgroup analysis, and propensity score matching (PSM) were used to investigate the relationships between SB infusion and clinical outcomes.
Results: A total of 1,595 patients with metabolic acidosis were enrolled in this study. In the multivariate logistic regression model, SB infusion was not correlated with in-hospital mortality (odds ratio (OR) 0.87, 95% confidence interval (CI) 0.47–1.63, p = 0.668), but was significantly correlated with hypernatraemia (OR 1.98, 95% CI 1.14–3.46, p = 0.016), hypokalaemia (OR 2.01, 95% CI 1.36–2.96, p < 0.001), and hypocalcaemia (OR 4.29, 95% CI 2.92–6.31, p < 0.001). In the pH value, lactate level, acute kidney injury level, age grouping, and anion gap level subgroups, the ORs for SB and in-hospital mortality were not statistically significant. After PSM, the results remained unchanged.
Conclusion: SB infusion does not reduce the in-hospital mortality of severely ill children with metabolic acidosis and increases the risk of hypernatraemia, hypokalaemia, and hypocalcaemia. More effort should be focused on eliminating the causes of metabolic acidosis rather than SB infusion.
Keywords: sodium bicarbonate, pediatric intensive care unit, metabolic acidosis, prognostic value, cohort study
Introduction
Metabolic acidosis is a disorder of the body’s acid-base balance characterized by a primary decrease in plasma bicarbonate concentration (BC) caused by an increase in hydrogen ions or a loss of bicarbonate in the extracellular fluid (Fujii et al., 2019). Metabolic acidosis can be divided into lactic acidosis, ketoacidosis, hyperchloremic acidosis and so on and has a high incidence in the paediatric intensive care unit (PICU) (Kraut, 2018). Diseases such as sepsis, severe hypoxemia, and cardiogenic shock are the main causes of metabolic acidosis (Velissaris et al., 2015; Haas et al., 2016). Metabolic acidosis can cause haemodynamic instability, decrease myocardial contractility and arterial vasodilation, decrease the cellular oxygen supply and mitochondrial oxygen consumption, impair responsiveness to catecholamines, and induce insulin resistance, leading to increased mortality (Schotola et al., 2012; Kraut and Madias, 2014).
At present, the most basic treatment for metabolic acidosis is to eliminate the underlying causes, such as treating infections and increasing the oxygen supply. However, some institutions have used sodium bicarbonate (SB) or other alkaline drugs after ineffective treatments. Theoretically, SB infusion can neutralize excess acid, thereby restoring the blood pH (Loomba et al., 2020). However, several studies have shown that SB infusions in critically ill patients with metabolic acidosis do not reduce mortality and increases the risk of adverse reactions. It is highly controversial whether SB should be infused in critically ill patients with metabolic acidosis. Existing studies mostly focus on adults, and there have been few reports on severely ill children; therefore, PICU physicians have no clear guidance regarding the use of SB. As such, the purpose of this study was to evaluate whether SB infusion could improve the prognosis of critically ill children with metabolic acidosis.
Materials and Methods
Database Introduction
The Paediatric Intensive Care (PIC) database is a completely open, single-centre, bilingual Chinese-English database developed by the Children’s Hospital of Zhejiang University School of Medicine (Zeng et al., 2020). It is a database specifically for PICU patients and includes diagnostic, testing, and monitoring data for non-adult patients aged 0–18 years from multiple intensive care units (ICUs). This database contains information regarding 13,499 hospital stays for 12,881 paediatric patients. The PIC database is based on the widely used Medical Information Mart for Intensive Care (MIMIC) database and can be downloaded and used after registration, application, and certification. The data used in this study were collected in adherence to Health Insurance Portability and Accountability Act (HIPAA) standards. This project was approved by the Institutional Review Committee of the Children’s Hospital of Zhejiang University School of Medicine and exempt from obtaining patient informed consent.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) patients between 1 month and 18 years old; 2) if a patient was hospitalized multiple times, only information from the first hospitalization was selected; and 3) patients with metabolic acidosis in the first 48 h of ICU admission (pH < 7.35, BC < 22 mmol/L).
The exclusion criteria were as follows: 1) patients in the neonatal intensive care unit; 2) lack of chart event data in the database; 3) patients undergoing cardiac surgery; and 4) patients with combined respiratory acidosis (partial pressure of carbon dioxide (PaCO2) > 50 mmHg).
If pH, BC, and PaCO2 were measured multiple times within the first 48 h of ICU entry, the minimum pH and BC values and the maximum PaCO2 value during this period were used.
Data Extraction and Definition of the Primary and Secondary Outcomes
The extracted demographic information included age, sex, and type of ICU admission; experimental variables included white blood cell (WBC) count, platelet (PLT) count, activated partial thromboplastin time (APTT), anion gap, and lactic acid concentration, and the initial values at ICU admission were used for the above variables. Comorbidities included anaemia, hypertension, acute kidney injury (AKI), liver dysfunction, and ketoacidosis. All comorbidities were diagnosed within 48 h of ICU admission. We used the pROCK criterion, which defines AKI as an increase in creatinine levels of ≥20 μmol/L and ≥30% within 7 days (Xu et al., 2018). The pROCK classifies AKI stages 2 and 3 as creatinine increases of ≥40 μmol/L and ≥60% and ≥80 μmol/L and ≥120%, respectively. The diagnostic criteria of other comorbidities are provided in Supplementary Table S1. The percentage of missing values of each variable was less than 5%; therefore, the mean or median was used to replace the missing value.
The primary outcome measure was in-hospital mortality, defined as death during hospitalization. The secondary outcome measures included hypernatraemia, hypokalaemia, hypocalcaemia, hospitalization length of stay (LOS) and 30-days mortality. Only hypernatraemia (serum sodium >150 mmol/L), hypokalaemia (serum potassium <3.5 mmol/L), and hypocalcaemia (free calcium <1.2 mmol/L) that occurred after the use of SB were counted. The maximum sodium value and minimum potassium and calcium values were selected.
Statistical Analysis
Continuous variables with a normal distribution are presented as the mean ± standard deviation, and continuous variables with a nonnormal distribution are presented as the median (quartile). Student’s t test, the Wilcoxon rank sum test, or the chi-squared test was used to compare differences between two groups. A backward stepwise method was used to screen the covariates for multivariate logistic regression. Considering that pH, lactate, and AKI are important factors that affect the use of SB in clinical practice and that different physiopathological situations may arise at different ages and anion gaps, the above factors were used for subgroup analysis. To verify the interaction between SB and these variables, multiplicative interaction terms were incorporated in the regression model.
Propensity score matching (PSM) was used to minimize the influence of confounding factors that may cause bias in the results. PSM scores were assigned based on the probability of patients receiving SB treatment and were estimated using a multivariate logistic regression model. The 1:1 nearest neighbour matching algorithm was used for matching, and the caliper value was set to 0.05. Based on Table 1, the following variables were selected to generate the PSM score: age, ICU type, APTT, anion gap, anaemia, hypertension, and AKI.
TABLE 1.
Comparisons of the baseline characteristics between patients with or without sodium bicarbonate use.
| Variable | All patients | Non-SB group | SB group | P |
|---|---|---|---|---|
| (n = 1,595) | (n = 1,045) | (n = 550) | ||
| Age, months | 17 (5–50) | 17 (5–52) | 16 (5–43) | 0.593 |
| Age, n (%) | — | — | — | 0.077 |
| <12 months | 692 (43.4) | 452 (43.3) | 240 (43.6) | — |
| ≥12 months and <60 months | 598 (35.6) | 365 (34.9) | 203 (36.9) | — |
| ≥60 months and <120 months | 207 (13.0) | 131 (12.5) | 76 (13.8) | — |
| ≥120 months | 128 (8.0) | 97 (9.3) | 31 (5.6) | — |
| Male, n (%) | 904 (56.7) | 598 (57.2) | 306 (55.6) | 0.543 |
| ICU type, n (%) | — | — | — | 0.033 |
| PICU | 417 (26.1) | 291 (27.8) | 126 (22.9) | — |
| SICU | 1,178 (73.9) | 754 (72.2) | 424 (77.1) | — |
| Laboratory data | — | — | — | — |
| WBC (<4 or >12, 109/L) | 679 (42.6) | 435 (41.6) | 244 (44.4) | 0.293 |
| Platelet (<100, 109/L) | 100 (6.3) | 66 (6.3) | 34 (6.2) | 0.916 |
| Lactate (≥2.0, mmol/L) | 625 (39.2) | 412 (39.4) | 213 (38.7) | 0.786 |
| APTT (>45, s) | 246 (15.4) | 186 (17.8) | 60 (10.9) | <0.001 |
| PH (<7.2) | 81 (5.1) | 56 (5.4) | 25 (4.5) | 0.482 |
| BC (<14, mmol/L) | 173 (10.8) | 113 (10.8) | 60 (10.9) | 0.953 |
| Anion gap (8–16, mmol/L) | 510 (32.0) | 404 (38.7) | 106 (19.3) | <0.001 |
| Comorbidities, n (%) | ||||
| Anemia | 1,093 (68.5) | 693 (66.3) | 400 (72.7) | 0.009 |
| Hypertension | 349 (21.9) | 183 (17.5) | 166 (30.2) | <0.001 |
| AKI | 181 (11.3) | 133 (12.7) | 48 (8.7) | 0.017 |
| AKI stage ≥2 | 48 (3.0) | 16 (1.5) | 32 (5.8) | 0.865 |
| Liver dysfunction | 309 (19.4) | 198 (18.9) | 111 (20.2) | 0.553 |
| Diabetic ketoacidosis | 117 (7.3) | 76 (7.3) | 41 (7.5) | 0.895 |
| Clinical outcome | ||||
| Hypernatremia | 59 (3.7) | 31 (3.0) | 28 (5.1) | 0.033 |
| Hypokalaemia | 121 (7.6) | 64 (6.1) | 57 (10.4) | 0.002 |
| Hypocalcemia | 135 (8.5) | 51 (4.9) | 84 (15.3) | <0.001 |
| Hospital LOS (day) | 11 (7–18) | 11 (7–18) | 12 (6–19) | 0.667 |
| 30 days mortality, n (%) | 58 (3.6) | 40 (3.8) | 18 (3.3) | 0.574 |
| Hospital mortality, n (%) | 61 (3.8) | 44 (4.2) | 17 (3.1) | 0.268 |
A p value <0.05 was considered statistically significant. Statistical analysis was performed using STATA (V.16), SPSS (V.24), and R (V.3.6.3).
Results
Baseline Characteristics
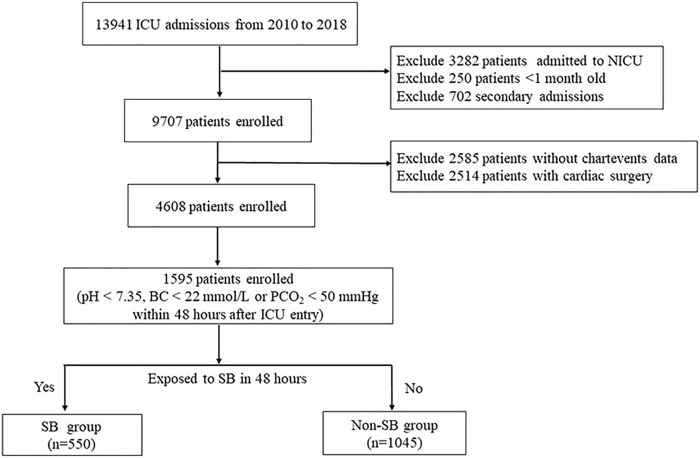
A total of 1,595 patients were enrolled; the study flowchart is shown in Figure 1. Table 1 provides the baseline characteristics data. The overall median age was 17 months. A total of 56.7% of patients were male, and the in-hospital mortality was 3.8%. Comparisons between the SB and non-SB groups indicated that there were significant differences in ICU type, APTT, anion gap, anaemia, hypertension, and AKI. The occurrence of hypernatraemia (5.1 vs. 3.0%, p = 0.033), hypokalaemia (10.4 vs. 6.1%, p = 0.002), and hypocalcaemia (15.3 vs. 4.9%, p < 0.001) in the SB group was higher than that in the non-SB group. However, there were no significant differences between the two groups in LOS, 30-days mortality, or in-hospital mortality.
FIGURE 1.
Flow diagram of patient recruitment. NICU, neonatal intensive care unit; SB, sodium bicarbonate.
Primary Outcome
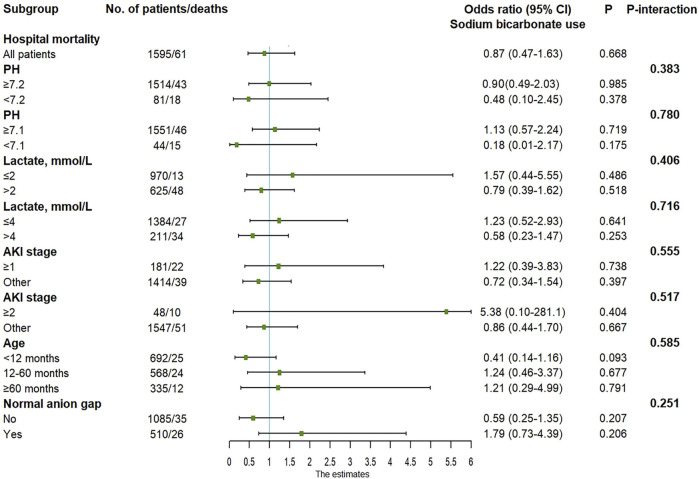
The results of univariate and multivariate logistic regressions with in-hospital mortality as the outcome variable are shown in Table 2. Regardless of univariate analysis or multivariate analysis, there was no significant correlation between SB use and in-hospital mortality. In the multivariate analysis, ICU type, lactate level, APTT, and AKI were independent risk factors for in-hospital death. Subgroup analysis was performed based on pH, lactate level, AKI, age and anion gap (Figure 2). When constructing a logistic regression model for the subgroup with age ≥120 months, the small number of deaths in this subgroup precluded statistical analysis. Therefore, we set age ≥60 months as a subgroup. The odds ratios (ORs) for SB and in-hospital death in the different subgroups were not statistically significant. In addition, the p-interaction between SB and pH, lactate, AKI, age, or anion gap was greater than 0.05.
TABLE 2.
Logistic regressions of sodium bicarbonate use for in-hospital mortality.
| Crude OR | 95% CI | P | Adjusted OR | 95% CI | P | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <12 months | Ref.- | Ref | ||||
| ≥12 months and <60 months | 1.18 | 0.67–2.08 | 0.576 | 1.26 | 0.67–2.37 | 0.472 |
| ≥60 months and <120 months | 1.07 | 0.48–2.42 | 0.866 | 1.12 | 0.46–2.69 | 0.805 |
| ≥120 months | 0.86 | 0.29–2.52 | 0.784 | 0.58 | 0.18–1.83 | 0.351 |
| Gender (female) | 0.84 | 0.50–1.42 | 0.523 | 0.67 | 0.39–1.17 | 0.160 |
| ICU type (PICU) | 6.31 | 3.65–10.91 | <0.001 | 3.54 | 1.96–6.42 | <0.001 |
| WBC (<4 or >12, 109/L) | 2.00 | 1.19–3.36 | 0.009 | 1.54 | 0.88–2.67 | 0.127 |
| Platelet (<100, 109/L) | 3.57 | 1.80–7.10 | <0.001 | 1.45 | 0.66–3.18 | 0.358 |
| Lactate (≥2.0, mmol/L) | 6.12 | 3.29–11.40 | <0.001 | 4.39 | 2.32–8.31 | <0.001 |
| APTT (>45, s) | 3.83 | 2.25–6.53 | <0.001 | 2.30 | 1.30–4.06 | 0.004 |
| Anion gap (8–16, mmol/L) | 1.61 | 0.96–2.71 | 0.071 | 1.04 | 0.58–1.84 | 0.903 |
| Anemia | 0.94 | 0.54–1.62 | 0.822 | 0.87 | 0.48–1.57 | 0.635 |
| Hypertension | 0.45 | 0.20–1.00 | 0.051 | 0.54 | 0.23–1.23 | 0.142 |
| Acute kidney injury | 4.88 | 2.82–8.44 | <0.001 | 2.26 | 1.24–4.11 | 0.007 |
| Liver dysfunction | 3.53 | 2.09–5.94 | <0.001 | 1.50 | 0.83–2.70 | 0.180 |
| Diabetic ketoacidosis | 2.29 | 1.10–4.76 | 0.027 | 1.19 | 0.53–2.66 | 0.673 |
| Sodium bicarbonate use | 0.73 | 0.41–1.28 | 0.270 | 0.87 | 0.47–1.63 | 0.668 |
FIGURE 2.
Adjusted odds ratio in the subgroup analysis. AKI, acute kidney injury; CI, confidence interval.
Secondary Outcomes
Multivariate logistic regression was performed using hypernatraemia, hypokalaemia, hypocalcaemia, LOS, and 30-day mortality as the outcome variables, as shown in Table 3. LOS was converted to a dichotomous variable based on the median value (11.7 days). The use of SB was associated with an increased risk for hypernatraemia (OR 1.98, 95% CI 1.14–3.46, p = 0.016), hypokalaemia (OR 2.01, 95% CI 1.36–2.96, p < 0.001) and hypocalcaemia (OR 4.29, 95% CI 2.92–6.31, p < 0.001). The OR values for other covariates are provided in Supplementary Table S2.
TABLE 3.
Multivariate logistic regression of sodium bicarbonate infusion for secondary outcome.
| Secondary outcome | OR | 95% CI | P |
|---|---|---|---|
| Hypernatremia | 1.98 | 1.14–3.46 | 0.016 |
| Hypokalaemia | 2.01 | 1.36–2.96 | <0.001 |
| Hypocalcemia | 4.29 | 2.92–6.31 | <0.001 |
| Longer hospital LOS (>11.3 days) | 0.96 | 0.77–1.20 | 0.726 |
| 30 days mortality | 1.03 | 0.55–1.90 | 0.937 |
PSM Results
Utilizing the 1:1 matching algorithm, there were 518 matched pairs (Table 4). The overall quality of the matched samples was assessed by examining the propensity scores between groups (Supplementary Figure S1). All covariates were balanced between the two groups. There were no significant differences in in-hospital mortality, 30-days mortality, or LOS between the two groups; however, in the SB group, there were increased incidences of hypernatraemia (5.4 vs. 2.7%, p = 0.027), hypokalaemia (10.6 vs. 6.0%, p = 0.007), and hypocalcaemia (15.8 vs. 6.2%, p < 0.001).
TABLE 4.
Comparisons of the covariates after propensity score matching.
| Variable | All patients | Non-SB group | SB group | P |
|---|---|---|---|---|
| (n = 1,036) | (n = 518) | (n = 518) | ||
| Age, months | 17 (5–44) | 17 (5–43) | 17 (5–47) | 0.525 |
| ICU type, n (%) | — | — | — | 1.000 |
| PICU | 240 (23.2) | 120 (23.2) | 120 (23.2) | — |
| SICU | 796 (76.8) | 398 (76.8) | 398 (76.8) | — |
| APTT (> 45, s) | 115 (11.1) | 55 (10.6) | 60 (11.6) | 0.621 |
| Anion gap (8–16, mmol/L) | 207 (20.0) | 101 (19.5) | 106 (20.5) | 0.698 |
| Comorbidities, n (%) | ||||
| Anemia | 735 (71.0) | 367 (70.9) | 368 (71.0) | 0.945 |
| Hypertension | 261 (25.2) | 127 (24.5) | 134 (25.9) | 0.616 |
| Acute kidney injury | 92 (8.9) | 44 (8.5) | 48 (9.3) | 0.662 |
| Clinical outcome | ||||
| Hypernatremia | 42 (4.1) | 14 (2.7) | 28 (5.4) | 0.027 |
| Hypokalaemia | 86 (8.3) | 31 (6.0) | 55 (10.6) | 0.007 |
| Hypocalcemia | 114 (11.0) | 32 (6.2) | 82 (15.8) | <0.001 |
| Hospital LOS (day) | 12 (7–19) | 12 (7–18) | 12 (6–19) | 0.767 |
| 30 days mortality, n (%) | 39 (3.8) | 22 (4.3) | 17 (3.3) | 0.414 |
| Hospital mortality, n (%) | 40 (3.9) | 24 (4.6) | 16 (3.1) | 0.197 |
Sensitivity Analysis
To test the reliability of the results after PSM, different covariates were included for the sensitivity analysis. As seen in Table 2, the relationships between the use of SB and clinical outcomes were confounded to some extent by some experimental variables, such as WBC count, PLT count, and lactate level. Therefore, these three variables were added for PSM; there were no significant differences in the covariates after PSM (Supplementary Table S3, Supplementary Figure S2), and the results remained stable.
Discussion
The main purpose of this study was to evaluate the effect of SB infusion on the prognosis of PICU patients with metabolic acidosis. To the best of our knowledge, this is the largest study on this subject ever conducted in a PICU. The results indicated that SB infusion was not correlated with changes in mortality in critically ill children with metabolic acidosis, even in subgroups with pH < 7.1, AKI and hyperlactacidaemia. Additionally, SB infusion was associated with an increased risk of hypernatraemia, hypokalaemia, and hypocalcaemia.
Currently, whether SB infusion improves survival in patients with metabolic acidosis is debated. Several studies have shown that SB treatment does not improve patient prognosis but increases the incidence of adverse reactions and even mortality. Loomb et al. conducted a systematic review and meta-analysis, which included 341 children from six studies, to determine whether SB treatment can improve haemodynamics, gas exchange, and blood oxygen saturation in infants with metabolic acidosis (Loomba et al., 2020). The results suggested that the use of SB did not improve oxygen saturation as measured by pulse oximetry, heart rate, blood pressure (BP), pH, or oxygen partial pressure. Kim et al. retrospectively analysed 103 patients with lactic acidosis and found that the use rate of SB in the deceased group was higher than that in the survival group (p = 0.006); multivariate logistic regression suggested that the administration of SB was an independent risk factor for in-hospital death (OR 6.27, 95% CI 1.1–35.78) (Kim et al., 2013). In terms of basic research, there are two experiments with dogs with lactic acidosis. Compared with those in dogs in the control group, the blood pH and mortality did not improve in dogs that received SB treatment; however, BP and cardiac output decreased (Arieff et al., 1982; Graf et al., 1985).
It seems intuitive to add an alkaline agent to acidic blood to increase the pH; however, the actual treatment approach is much more complicated (Quade et al., 2021). The inability of SB to improve the prognosis of children with metabolic acidosis can be explained as follows. First, although SB infusion can increase blood pH in a short period of time (Mintzer et al., 2015), it can also increase CO2 production and aggravate intracellular acidosis in children with severe circulatory failure (Kim et al., 2013; Collins and Sahni, 2017). Second, SB treatment can lead to fluid overload and electrolyte disorders, including hypocalcaemia, which in turn affects the function of the heart and vascular smooth muscle (Kimmoun et al., 2015; Drumheller and Sabolick, 2021), resulting in cerebral and cardiovascular haemodynamic fluctuations and functional abnormalities (Aschner and Poland, 2008; Berg et al., 2010; Katheria et al., 2017). Studies have reported that SB infusion can increase the risk of cerebral oedema in paediatric patients with diabetic ketoacidosis (Glaser et al., 2001). Third, SB infusion may change the oxyhaemoglobin saturation relationship and increase the production of lactic acid in anaerobic glycolysis (Forsythe and Schmidt, 2000). The results of this study are consistent with the above view that SB cannot reduce the mortality of children with metabolic acidosis and that SB treatment causes hypernatraemia, hypokalaemia, and hypocalcaemia.
As described above, many studies have shown that patients with metabolic acidosis do not benefit from SB infusion. However, some patients with specific diseases or severe conditions may benefit from SB infusion. In a multicentre, phase III randomized controlled trial (RCT) that included 389 patients with severe metabolic acidaemia (pH ≤ 7.20), there was no significant difference in the survival rate at day 28 between the control group and the SB group. However, for patients with an AKI network score of two to three points, the survival rate in the SB group was higher than that in the control group on day 28 (63 vs. 46%, p = 0.028) (Jaber et al., 2018). Zhang et al. retrospectively analysed 1718 sepsis patients with metabolic acidosis in the MIMIC database (500 patients in the SB group and 1,218 patients in the non-SB group) (Zhang et al., 2018). The results indicated that there was no significant difference in mortality between the two groups overall (hazard ratio (HR) 1.04, 95% CI 0.86–1.26, p = 0.670) but that SB use benefitted the population with grade 2 or three AKI and pH < 7.2 (HR 0.74, 95% CI 0.51–0.86, p = 0.021). However, in this study, for PICU patients, SB infusion did not benefit patients in the severe acidosis (pH < 7.2 or pH < 7.1) and AKI subgroups. Notably, the causes of metabolic acidosis or AKI in children are different from those in adults, and different aetiologies may cause different patient responses to SB treatment (Joannes-Boyau and Forni, 2018).
Our research presents some advantages. Thus far, this is the largest study on the efficacy of SB in the PICU; therefore, enough confounding variables were included, and subgroup analysis was performed. However, this study also has limitations. First, this is a retrospective study. Although PSM and sensitivity scores were used to balance the important confounding factors, there may still be some unknown confounding factors that affected the results. Second, multiple subgroup analyses were conducted, but the small number of cases in the pH < 7.1 subgroup may lead to false negative results. Third, due to limitations with respect to the database, data for some treatments were not available, such as balanced salt solution and haemodialysis, which would affect the acid-base balance of patients. Fourth, because of database limitations, we could not precisely classify the type of metabolic acidosis in each patient. For example, organic acidemias are mostly diagnosed by some sophisticated laboratory tests, such as tandem mass spectrometry and gas chromatography–mass spectrometry, which are not available in databases. Therefore, we could not clarify whether the patient had organic acidemia.
Conclusion
Acidaemia in critically ill children is a common concern of paediatricians. Based on the conclusions of this study, SB infusion may not be beneficial. More effort should be focused on eliminating the causes of metabolic acidosis rather than SB infusion. Notably, RCTs targeting specific disease populations and different types of acidosis are needed.
Acknowledgments
We are grateful to the PIC database for providing clinical data. We also thank Li Chikong for the assistance and suggestion of this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Committee of the Children’s Hospital of Zhejiang University School of Medicine. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
LZ and CC conceptualized and designed the study and reviewed and revised the manuscript. HW coordinated and supervised data collection, drafted the initial manuscript, and reviewed and revised the manuscript. RL carried out the analyses, interpreted the results and drafted the initial manuscript. TL, SC, and YZ contributed to data collection and reviewed and revised the manuscript. All authors contributed to manuscript revision and read and approved the submitted version. HW and RL contributed equally to this work.
Funding
This research was supported by the Sanming Project of Medicine in Shenzhen (SZSM202011004), the Natural Science Foundation of Guangdong Province (2020A1515010151), and the Clinical Funding of the Seventh Affiliated Hospital, Sun Yat-sen University (ZSQYLCKYJJ202024).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.759247/full#supplementary-material.
References
- Arieff A. I., Leach W., Park R., Lazarowitz V. C. (1982). Systemic Effects of NaHCO3 in Experimental Lactic Acidosis in Dogs. Am. J. Physiol. 242 (6), F586–F591. 10.1152/ajprenal.1982.242.6.F586 [DOI] [PubMed] [Google Scholar]
- Aschner J. L., Poland R. L. (2008). Sodium Bicarbonate: Basically Useless Therapy. Pediatrics 122 (4), 831–835. 10.1542/peds.2007-2400 [DOI] [PubMed] [Google Scholar]
- Berg C. S., Barnette A. R., Myers B. J., Shimony M. K., Barton A. W., Inder T. E. (2010). Sodium Bicarbonate Administration and Outcome in Preterm Infants. J. Pediatr. 157 (4), 684–687. 10.1016/j.jpeds.2010.05.019 [DOI] [PubMed] [Google Scholar]
- Collins A., Sahni R. (2017). Uses and Misuses of Sodium Bicarbonate in the Neonatal Intensive Care Unit. Semin. Fetal Neonatal. Med. 22 (5), 336–341. 10.1016/j.siny.2017.07.010 [DOI] [PubMed] [Google Scholar]
- Drumheller B. C., Sabolick E. E. (2021). Hemodynamic Instability and Abnormal Vasopressor Responsiveness in the Setting of Severe Metabolic Acidosis Treated with Adapted Alkalinization and Continuous Renal Replacement Therapy in the Emergency Department. J. Emerg. Med. 60 (1), 67–72. 10.1016/j.jemermed.2020.09.022 [DOI] [PubMed] [Google Scholar]
- Forsythe S. M., Schmidt G. A. (2000). Sodium Bicarbonate for the Treatment of Lactic Acidosis. Chest 117 (1), 260–267. 10.1378/chest.117.1.260 [DOI] [PubMed] [Google Scholar]
- Fujii T., Udy A., Licari E., Romero L., Bellomo R. (2019). Sodium Bicarbonate Therapy for Critically Ill Patients with Metabolic Acidosis: A Scoping and a Systematic Review. J. Crit. Care 51, 184–191. 10.1016/j.jcrc.2019.02.027 [DOI] [PubMed] [Google Scholar]
- Glaser N., Barnett P., McCaslin I., Nelson D., Trainor J., Louie J., et al. (2001). Risk Factors for Cerebral Edema in Children with Diabetic Ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N. Engl. J. Med. 344 (4), 264–269. 10.1056/NEJM200101253440404 [DOI] [PubMed] [Google Scholar]
- Graf H., Leach W., Arieff A. I. (1985). Evidence for a Detrimental Effect of Bicarbonate Therapy in Hypoxic Lactic Acidosis. Science 227 (4688), 754–756. 10.1126/science.3969564 [DOI] [PubMed] [Google Scholar]
- Haas S. A., Lange T., Saugel B., Petzoldt M., Fuhrmann V., Metschke M., et al. (2016). Severe Hyperlactatemia, Lactate Clearance and Mortality in Unselected Critically Ill Patients. Intensive Care Med. 42 (2), 202–210. 10.1007/s00134-015-4127-0 [DOI] [PubMed] [Google Scholar]
- Jaber S., Paugam C., Futier E., Lefrant J. Y., Lasocki S., Lescot T., et al. (2018). Sodium Bicarbonate Therapy for Patients with Severe Metabolic Acidaemia in the Intensive Care Unit (BICAR-ICU): a Multicentre, Open-Label, Randomised Controlled, Phase 3 Trial. Lancet 392 (10141), 31–40. 10.1016/S0140-6736(18)31080-8 [DOI] [PubMed] [Google Scholar]
- Joannes-Boyau O., Forni L. G. (2018). Time to Treat Metabolic Acidosis in the ICU with Sodium Bicarbonate? Anaesth. Crit. Care Pain Med. 37 (6), 493–494. 10.1016/j.accpm.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Katheria A. C., Brown M. K., Hassan K., Poeltler D. M., Patel D. A., Brown V. K., et al. (2017). Hemodynamic Effects of Sodium Bicarbonate Administration. J. Perinatol 37 (5), 518–520. 10.1038/jp.2016.258 [DOI] [PubMed] [Google Scholar]
- Kim H. J., Son Y. K., An W. S. (2013). Effect of Sodium Bicarbonate Administration on Mortality in Patients with Lactic Acidosis: a Retrospective Analysis. PLoS One 8 (6), e65283. 10.1371/journal.pone.0065283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmoun A., Novy E., Auchet T., Ducrocq N., Levy B. (2015). Hemodynamic Consequences of Severe Lactic Acidosis in Shock States: from Bench to Bedside. Crit. Care 19, 175. 10.1186/s13054-015-0896-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut J. A., Madias N. E. (2014). Lactic Acidosis. N. Engl. J. Med. 371 (24), 2309–2319. 10.1056/NEJMra1309483 [DOI] [PubMed] [Google Scholar]
- Kraut J. A. (2018). Treatment of Acute Acidaemia in the Seriously Ill Patient: Should Base Be Given? Anaesth. Crit. Care Pain Med. 37 (6), 495–497. 10.1016/j.accpm.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Loomba R. S., Abdulkarim M., Bronicki R. A., Villarreal E. G., Flores S. (2020). Impact of Sodium Bicarbonate Therapy on Hemodynamic Parameters in Infants: a Meta-Analysis. J. Matern. Fetal Neonatal. Med., 1–7. 10.1080/14767058.2020.1786051 [DOI] [PubMed] [Google Scholar]
- Mintzer J. P., Parvez B., Alpan G., LaGamma E. F. (2015). Effects of Sodium Bicarbonate Correction of Metabolic Acidosis on Regional Tissue Oxygenation in Very Low Birth Weight Neonates. J. Perinatol 35 (8), 601–606. 10.1038/jp.2015.37 [DOI] [PubMed] [Google Scholar]
- Quade B. N., Parker M. D., Occhipinti R. (2021). The Therapeutic Importance of Acid-Base Balance. Biochem. Pharmacol. 183, 114278. 10.1016/j.bcp.2020.114278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotola H., Toischer K., Popov A. F., Renner A., Schmitto J. D., Gummert J., et al. (2012). Mild Metabolic Acidosis Impairs the β-adrenergic Response in Isolated Human Failing Myocardium. Crit. Care 16 (4), R153. 10.1186/cc11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velissaris D., Karamouzos V., Ktenopoulos N., Pierrakos C., Karanikolas M. (2015). The Use of Sodium Bicarbonate in the Treatment of Acidosis in Sepsis: A Literature Update on a Long Term Debate. Crit. Care Res. Pract. 2015, 605830. 10.1155/2015/605830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Nie S., Zhang A., Jianhua M., Liu H. P., Xia H., et al. (2018). A New Criterion for Pediatric AKI Based on the Reference Change Value of Serum Creatinine. J. Am. Soc. Nephrol. 29 (9), 2432–2442. 10.1681/ASN.2018010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Yu G., Lu Y., Tan L., Wu X., Shi S., et al. (2020). PIC, a Paediatric-specific Intensive Care Database. Sci. Data 7 (1), 14. 10.1038/s41597-020-0355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhu C., Mo L., Hong Y. (2018). Effectiveness of Sodium Bicarbonate Infusion on Mortality in Septic Patients with Metabolic Acidosis. Intensive Care Med. 44 (11), 1888–1895. 10.1007/s00134-018-5379-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.