Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post‐hoc analysis of a randomised controlled trial (original) (raw)
Abstract
Magnesium status and vitamin B6 intake have been linked to mental health and/or quality of life (QoL). In an 8‐week Phase IV randomised controlled study in individuals with low magnesemia and severe/extremely severe stress but who were otherwise healthy, greater stress reduction was achieved with magnesium combined with vitamin B6 than with magnesium alone. We present a previously unreported secondary analysis of the effect of magnesium, with and without vitamin B6, on depression, anxiety, and QoL. Adults with Depression Anxiety Stress Scales (DASS‐42) stress subscale score >18 were randomised 1:1 to magnesium + vitamin B6 combination (Magne B6®; daily dose 300 and 30 mg, respectively) or magnesium alone (Magnespasmyl®; daily dose 300 mg). Outcomes included changes from baseline in DASS‐42 depression and anxiety scores, and QoL (Short Form‐36 Health Survey). DASS‐42 anxiety and depression scores significantly improved from baseline to week 8 with both treatments, particularly during the first 4 weeks. Improvement in QoL continued over 8 weeks. Participants' perceived capacity for physical activity in daily life showed greater improvement with magnesium + vitamin B6 than magnesium alone (Week 4). In conclusion, magnesium supplementation, with or without vitamin B6, could provide a meaningful clinical benefit in daily life for individuals with stress and low magnesemia.
Keywords: anxiety, depression, magnesium supplementation, quality of life, stress, vitamin B6 supplementation
1. INTRODUCTION
Advances in understanding the neurobiology of stress have demonstrated an interplay between disturbances in biochemical processes and physical and mental symptoms (McEwen, 1998). The protective biological responses that occur in response to stressors (allostasis) usually involve activation of neural, neuroendocrine and neuroendocrine‐immune mechanisms (McEwen, 2005). However, over long periods of time, allostatic overload can occur, resulting in mood disorders, chronic illness and reduced quality of life (QoL; Juster et al., 2010; McEwen, 2005; McEwen & Wingfield, 2003). Furthermore, components of the allostatic system have been associated with depression‐ and anxiety‐like behaviours (McEwen, 2015). Since the cumulative effect of daily stress has been linked to symptoms of anxiety and depression up to 10 years later (Charles et al., 2013), it follows that chronic stress, anxiety and depression could be viewed as a continuum of the same condition.
Magnesium status has been shown to be linked to anxiety, depression and mood changes (Boyle et al., 2017; Derom et al., 2013; Forsyth et al., 2011; Jung et al., 2010). During periods of stress, catecholamines and corticosteroids are released; prolonged release of these stress‐associated hormones then cause a progressive loss of magnesium from body stores (Galland, 1991‐1992). Since low magnesium status results in further release of catecholamines and corticosteroids a positive feedback loop that exacerbates magnesium depletion is created (Cuciureanu & Vink, 2011).
As magnesium is an enzymatic cofactor in over 600 biochemical reactions (de Baaij et al., 2015), magnesium deficiency could affect allostatic regulation in multiple ways. Magnesium influences activity of the hypothalamic‐pituitary‐adrenal axis, which instigates various responses to cope with stress demands (Murck, 2002). Magnesium also reduces central adrenocorticotrophic hormone (Murck, 2002) and peripheral (cortisol) endocrine responses (Held et al., 2002), thereby decreasing anxiety. Additionally, magnesium may help reduce presynaptic glutamate release (Papadopol & Nechifor, 2011) and glutamatergic activity that has been implicated in fear, anxiety and panic responses (Boyle et al., 2017; Clerc et al., 2013).
Magnesium levels have also been linked with general health and QoL; a retrospective study (n = 81) showed that the higher magnesium levels were associated with improved QoL scores in all 10 categories of the Short Form‐36 Health Survey (SF‐36) measuring QoL and health (Viebahn et al., 2016). Magnesium supplementation is therefore of interest not only as a potential aid to coping with stress, but also as a treatment for anxiety and depression (Botturi et al., 2020; Boyle et al., 2017; Kirkland et al., 2018). However, to date, no evidence has shown that magnesium supplementation can result in improved QoL.
Pyridoxine (vitamin B6) plays an important role in numerous physiological processes. It acts as a cofactor in over 100 enzymatic reactions, including in the synthesis of neurotransmitters such as gamma‐aminobutyric acid, serotonin and dopamine (Sato, 2018). In addition to modulating neurobiological mechanisms associated with mood disorders such as depression and anxiety (McCarty, 2000), vitamin B6 may have other stress‐reducing properties, including hypotensive effects and may reduce the physiological consequences of corticosteroid release (McCarty, 2000).
Inadequate intake of vitamin B6 has recently been linked to an increased risk of anxiety and depression in a cross‐sectional study of over 3,000 individuals (Kafeshani et al., 2019). Furthermore, vitamin B6 supplementation has demonstrated beneficial effects on emotional symptoms, such as reducing irritability, depression and tiredness (Doll et al., 1989). Vitamin B6 may also modulate magnesium levels, with some evidence showing increased circulating and tissue magnesium concentrations following high‐dose vitamin B6 supplementation (Abraham et al., 1981; Iezhitsa et al., 2011; Majumdar & Boylan, 1989). As both vitamin B6 and magnesium modulate neurobiological mechanisms, it has been hypothesized that they may have a synergistic effect (De Souza et al., 2000; Iezhitsa et al., 2011; Pouteau et al., 2018).
In the primary analysis of a Phase IV randomised controlled study (Pouteau et al., 2018), vitamin B6 augmented the beneficial effect of magnesium supplementation on stress relief, in healthy adults with low magnesemia and severe or extremely severe subjective stress at baseline. Over the 8‐weeks study period, a marked reduction in stress levels from baseline was observed with magnesium supplementation. In addition, magnesium supplementation combined with vitamin B6 resulted in greater improvements than magnesium alone. Based on these findings and other evidence from the literature, we conducted a secondary, post‐hoc analysis of the study by Pouteau and colleagues (2018) to explore whether magnesium supplementation improves anxiety and depression (Depression Anxiety Stress Scales [DASS‐42]) and QoL (SF‐36) in this cohort of stressed but otherwise healthy subjects. Furthermore, we explored whether addition of vitamin B6 to magnesium supplementation enhances any observed effects on mental health and QoL.
2. METHODS
2.1. Trial design
This 8‐weeks Phase IV, randomised, investigator‐blinded, parallel‐group trial (EudraCT Number: 2015‐003749‐24) compared the combination of magnesium and vitamin B6 with magnesium alone. Participants were recruited at four clinical trial centres in France. Eligible participants were adults aged 18–50 years with moderate to extremely severe stress at screening, defined as having a DASS‐42 stress subscale score of >18 and with suboptimal serum magnesium levels (range 0.66–0.84 mmol/L; Pouteau et al., 2018). Details of participant demographics and characteristics at baseline have previously been reported (Pouteau et al., 2018; Noah et al., 2020). Briefly, mean (standard deviation [SD]) age was 31.6 (8.5) years, 74% were female and mean (SD) DASS‐42 stress score was 27.7 (7.1) (severe stress: 26–33). Mean (SD) serum level of magnesium and vitamin B6 was 0.80 (0.04) mmol/L and 48.56 (52.27) nmol/L, respectively (levels after 4 and 8 weeks are described elsewhere [Noah et al., 2020]). Demographic and clinical characteristics were similar between treatment groups.
Participants were randomised 1:1 to treatment with either the magnesium + vitamin B6 combination (Magne B6®; 300 mg as magnesium lactate dihydrate and 30 mg vitamin B6 daily) or magnesium alone (Magnespasmyl®; 300 mg daily as magnesium lactate dihydrate). Participants could follow their regular diet during the 8‐weeks study period, and were asked to maintain monotherapy (magnesium + vitamin B6 or magnesium alone) for the study duration and not to take medications known to affect magnesium status (e.g., magnesium‐containing salts, levodopa or tetracyclines, phosphate or calcium salts, nonsteroidal anti‐inflammatory drugs) or to consume vitamin/mineral supplements or magnesium‐rich foods (e.g., dark chocolate, <50 g per day) or drinks (≤2 glasses per day; Noah et al., 2020). Investigators remained blinded with regard to the assigned study treatment until the database lock (Pouteau et al., 2018). The primary endpoint (subjective stress rating) was reported in Pouteau et al. (2018).
This article presents key secondary endpoints focused on anxiety and depression, based on a post‐hoc analysis. For full details of inclusion and exclusion criteria, treatments and primary trial endpoints, see Pouteau et al. (2018). Ethical approval for the trial and all analyses planned within the protocol was obtained from the Ethics Committee of Clermont‐Ferrand University Hospital, France (Comité de Protection des Personnes Sud Est 6; reference number: AU 1239, date of approval 01 March 2016), and all patients provided written informed consent.
2.2. Assessments and endpoints
The objective of these secondary and post‐hoc analyses was to explore the impact of magnesium (and vitamin B6) supplementation on mental and physical health in the participants by analysing DASS‐42 scores and SF‐36 scores at baseline, Week 4 and Week 8.
The self‐reported DASS‐42 is a 42‐item questionnaire that includes three subscales designed to measure the negative emotional states of depression, anxiety and stress and has been validated for clinical conditions (Brown et al., 1997; Crawford & Henry, 2003). In this study, DASS‐42 scores for anxiety and depression, as well as a total score were assessed at baseline, Week 4 and Week 8, and the change from baseline to Week 4 or Week 8 was calculated for each of the endpoints.
The SF‐36, validated for measuring QoL in a general practice population, examined the inter‐relationship between mental and physical health (Brazier et al., 1992). The 36‐item questionnaire comprises eight domains: physical role functioning (a measure of perceived capacity to participate in ordinary activities), bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well‐being, social functioning, energy/fatigue, and general health perceptions. Scores for each domain were derived following the guidance of Ware and Sherbourne (1992). Scores range from 0 to 100, where higher scores indicate better QoL, and scores <50 indicate poor QoL. The following endpoints were assessed at baseline, Week 4 and Week 8: separate SF‐36 scores for all eight domains, an overall SF‐36 physical score summary, and a SF‐36 mental score summary. The change from baseline to Week 4 or Week 8 was calculated for each of the endpoints. Reference data from a historical cross‐sectional survey of the French metropolitan population (Leplège et al., 2001) were retrieved to provide context for the current study population.
2.3. Statistical analyses
Analyses were performed on the intent‐to‐treat (ITT) population (all randomised participants with at least one consumption of study product), comprising 264 patients (132 patients in each treatment group; Pouteau et al., 2018).
Data collected from the DASS‐42 and SF‐36 assessments were analysed using the same statistical approach. For the two treatment groups separately and after pooling (Overall group), descriptive statistics including the mean and SD were used to initially summarize scores at each visit (baseline, Week 4, and Week 8) and the change in score from baseline to Week 4 and Week 8. Subsequently, a Model Mixed for Repeated Measures (MMRM) including sex and visit as categorical fixed effects, DASS‐42 or SF‐36 at baseline as a continuous fixed effect, and subject as a random effect, was used to estimate differences over time within each treatment group and the overall group. Adjusted means, standard errors (SE), 95% confidence intervals (CI) and _p_‐values were calculated. Differences between the two treatment groups in terms of change from baseline to Week 4 and Week 8 for DASS‐42 and SF‐36 were also assessed using the above MMRM model but modified to include treatment group as a further categorical fixed effect and two interaction terms: DASS‐42 or SF‐36 at baseline x treatment group, and visit x treatment group. Data are reported as adjusted means, SE, 95% CI and _p_‐values. An alpha level of 5% with a two‐sided test was used for all comparisons. As these post‐hoc analyses were intended to identify trends and be hypothesis generating, correction for multiple testing was not performed (to minimize type II errors). Correcting for multiple testing is a very conservative approach that reduces the likelihood of making a type I error (false positive); however, it is of no less importance that doing so simultaneously increases the likelihood of type II errors (false negatives), which is especially relevant for exploratory, post‐hoc analyses such as these. Analyses were performed using SAS version 9.4 software.
3. RESULTS
3.1. Anxiety and depression
At the baseline time point, DASS‐42 anxiety and depression scores were similar for the two treatment groups, magnesium + vitamin B6 and magnesium alone (Table 1). Adjusted mean DASS‐42 anxiety and depression subscale scores at each visit are in Figure 1.
TABLE 1.
DASS‐42 anxiety and depression scores at baseline time point in two magnesium supplemented groups and in the overall ITT population
| Parameter | Magnesium + vitamin B6 (N = 132) | Magnesium (N = 132) | Total (N = 264) |
|---|---|---|---|
| DASS‐42 anxiety | n (%) | n (%) | n (%) |
| Normal (scale 0–7) | 25 (18.9) | 26 (19.7) | 51 (19.3) |
| Mild (8–9) | 7 (5.3) | 10 (7.6) | 17 (6.4) |
| Moderate (10–14) | 31 (23.5) | 27 (20.5) | 58 (22.0) |
| Severe (15–19) | 25 (18.9) | 24 (18.2) | 49 (18.6) |
| Extremely severe (20–42) | 44 (33.3) | 45 (34.1) | 89 (33.7) |
| DASS‐42 depression | n (%) | n (%) | n (%) |
| Normal (scale 0–9) | 47 (35.6) | 43 (32.6) | 90 (34.1) |
| Mild (10–13) | 18 (13.6) | 20 (15.2) | 38 (14.4) |
| Moderate (14–20) | 33 (25.0) | 33 (25.0) | 66 (25.0) |
| Severe (21–27) | 16 (12.1) | 23 (17.4) | 39 (14.8) |
| Extremely severe (28–42) | 18 (13.6) | 13 (9.8) | 31 (11.7) |
FIGURE 1.
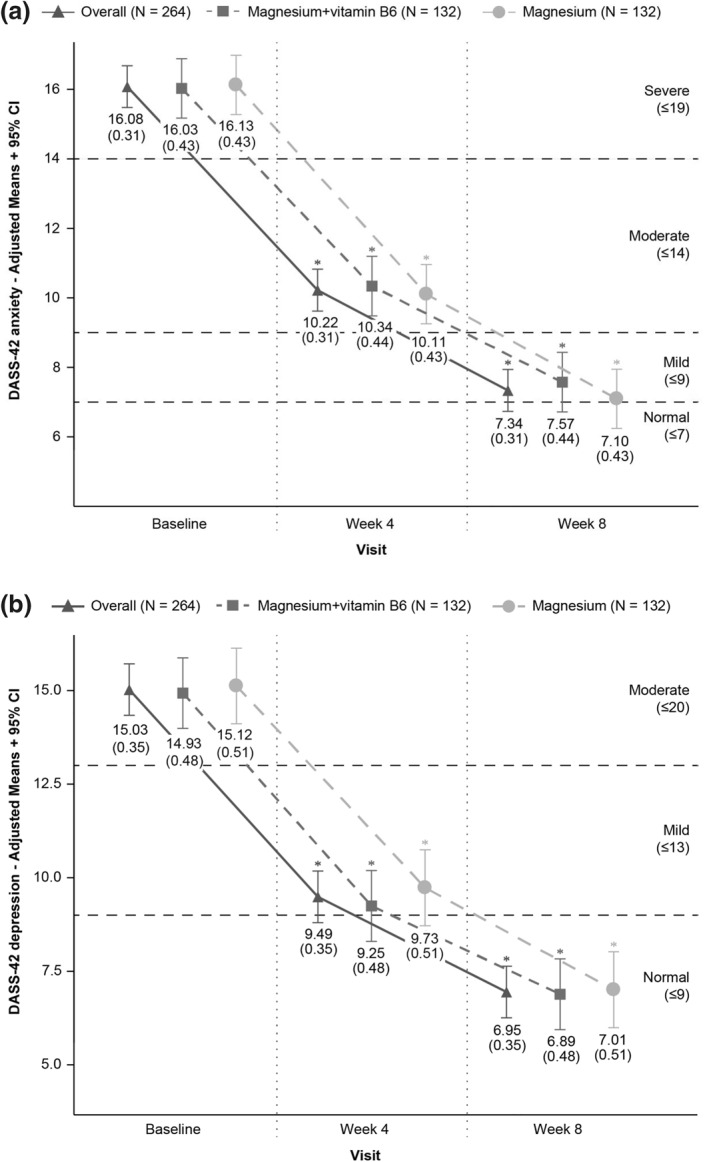
DASS‐42 scores (adjusted means) for (a) anxiety and (b) depression by treatment and visit. *Statistically significant (p < 0.05) differences between baseline and a given post‐baseline visit using MMRM. Horizontal dashed lines represent the upper limit of each DASS‐42 category. Abbreviations: CI, confidence interval; DASS‐42, Depression Anxiety Stress Scales; MMRM, Model Mixed for Repeated Measures
Both treatment groups showed improved anxiety scores over the course of the 8‐weeks study, with mean anxiety scores reducing from a severe level to a near normal level (Figure 1). Greatest improvement in mean anxiety score occurred in the first 4 weeks of treatment; the overall group mean score decreased from baseline by −5.86 (95% CI −6.67; −5.04). However, no significant difference was observed between the magnesium + vitamin B6 and magnesium alone groups at either Week 4 or 8 (Table 2).
TABLE 2.
DASS‐42 anxiety and depression scores (adjusted means) by visit – change from baseline and treatment difference for two magnesium supplemented groups and overall ITT population
| Parameter | Statistics | Magnesium + vitamin B6 (N = 132) | Magnesium (N = 132) | Overall group a (N = 264) | Treatment difference |
|---|---|---|---|---|---|
| DASS‐42 anxiety | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | −5.69 (0.59) b | −6.02 (0.58) b | −5.86 (0.41) b | 0.26 (0.66) |
| Change from baseline to Week 8 | Adjusted mean (SE) | −8.45 (0.59) b | −9.03 (0.59) b | −8.74 (0.41) b | 0.54 (0.66) |
| DASS‐42 depression | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | −5.69 (0.65) b | −5.39 (0.69) b | −5.54 (0.47) b | −0.41 (0.75) |
| Change from baseline to Week 8 | Adjusted mean (SE) | −8.04 (0.65) b | −8.12 (0.69) b | −8.08 (0.47) b | −0.05 (0.75) |
Across the overall population, participants in both treatment groups improved DASS‐42 depression scores over the course of the 8‐weeks study (Figure 1b), such that, depression scores reduced from moderate to normal (≤9). In both groups, most of the improvement in the depression score occurred in the first 4 weeks of treatment (mean decrease from baseline −5.54 [95% CI −6.47; −4.61 overall]). No significant difference was observed between magnesium + vitamin B6 and magnesium alone groups at either Week 4 or 8 (Table 2).
3.2. Quality of life
QoL changes from baseline, by visit and by treatment group, assessed using the SF‐36 questionnaire, are shown in Table 3. Table S1 presents the SF‐36 scores by visit and by treatment group. The adjusted mean physical QoL summary score at baseline was similar for both treatment groups (52.6 for the magnesium + vitamin B6 group; 53.1 for magnesium alone) (Table 3). The adjusted mean mental summary score also did not differ between the treatment groups at the initial visit (30.4 for the magnesium + vitamin B6 group; 29.6 for magnesium alone). These baseline mental summary scores were low compared to the initial physical summary scores, which is reflective of the stressed status of this otherwise healthy study population.
TABLE 3.
SF‐36 change from baseline (adjusted mean differences) by visit with comparison between both magnesium supplemented groups – ITT population
| Parameter | Statistics | Magnesium + vitamin B6 (N = 132) | Magnesium (N = 132) | Overall group a (N = 264) | Treatment difference |
|---|---|---|---|---|---|
| Physical domains | |||||
| Physical functioning | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 1.8 (0.9) b | 1.7 (1.0) | 1.7 (0.7) b | −0.3 (1.2) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 4.2 (0.9) b | 5.0 (1.0) b | 4.6 (0.7) b | −1.2 (1.2) |
| Physical role functioning | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 14.6 (2.5) b | 4.6 (2.7) | 9.6 (1.9) b | 7.8 (3.4) c |
| Change from baseline to Week 8 | Adjusted mean (SE) | 22.4 (2.5) b | 13.7 (2.7) b | 18.1 (1.9) b | 6.5 (3.5) |
| Bodily pain | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 6.2 (1.5) b | 7.0 (1.4) b | 6.6 (1.0) b | 0.1 (1.9) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 7.4 (1.5) b | 10.3 (1.4) b | 8.8 (1.0) b | −1.9 (1.9) |
| General health | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 4.6 (1.0) b | 8.1 (1.1) b | 6.3 (0.7) b | −2.7 (1.4) c |
| Change from baseline to Week 8 | Adjusted mean (SE) | 7.7 (1.0) b | 9.3 (1.1) b | 8.5 (0.7) b | −0.8 (1.4) |
| Physical summary score | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 0.1 (0.5) | 0.2 (0.5) | 0.2 (0.3) | 0.0 (0.6) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 0.8 (0.5) | 1.0 (0.5) b | 0.9 (0.3) b | −0.2 (0.6) |
| Mental domains | |||||
| Vitality | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 13.6 (1.4) b | 11.4 (1.3) b | 12.5 (1.0) b | 0.9 (1.8) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 18.4 (1.4) b | 17.1 (1.3) b | 17.7 (0.9) b | 0.0 (1.8) |
| Social functioning | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 12.8 (1.6) b | 14.2 (1.8) b | 13.5 (1.2) b | −0.4 (2.3) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 21.5 (1.6) b | 21.1 (1.8) b | 21.3 (1.2) b | 1.5 (2.3) |
| Emotional role functioning | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 27.4 (3.0) b | 22.3 (3.3) b | 24.9 (2.2) b | 4.4 (4.2) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 35.3 (3.0) b | 33.7 (3.3) b | 34.5 (2.2) b | 1.0 (4.2) |
| Emotional well being | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 14.0 (1.3) b | 11.4 (1.3) b | 12.7 (0.9) b | 1.7 (1.7) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 19.8 (1.3) b | 17.3 (1.3) b | 18.6 (0.9) b | 1.5 (1.7) |
| Mental summary score | |||||
| Change from baseline to Week 4 | Adjusted mean (SE) | 9.4 (0.8) b | 8.3 (0.9) b | 8.9 (0.6) b | 0.7 (1.1) |
| Change from baseline to Week 8 | Adjusted mean (SE) | 13.0 (0.8) b | 12.0 (0.9) b | 12.5 (0.7) b | 0.6 (1.1) |
3.3. SF‐36 domains
Figure 2 represents the SF‐36 data as a radar plot. All mental domain scores (role limitations due to personal or emotional problems, emotional well‐being, social functioning, energy/fatigue) were very low at baseline with regard to the reference population (historical cross‐sectional population from the French metropolitan area [Leplège et al., 2001]). Across the 8 weeks of magnesium supplementation, ongoing improvements were observed in all SF‐36 domains, reaching levels close to those of the reference population. Improvements were most pronounced in the mental domain, but were also observed in the physical domain. For example, a relevant improvement was observed for the emotional role functioning domain where participants had a baseline score of 34.3 (95% CI 30.6; 38.0) and ended up with a score of 69.9 (95% CI 66.2; 73.6) after the 8‐weeks study period (Table 3).
FIGURE 2.
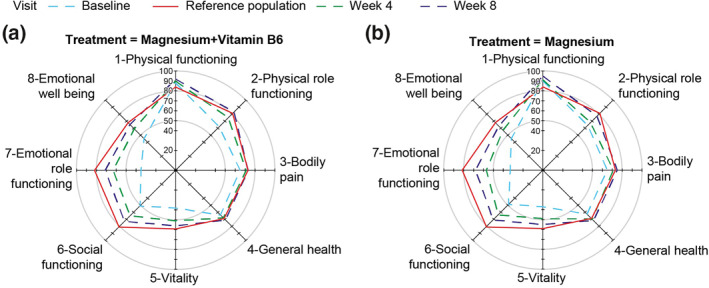
SF‐36 scores for all domains at each visit (3 time points) among (a) patients treated with magnesium + vitamin B6; (b) patients treated with magnesium alone. Note: Means of SF‐36 scores are adjusted by sex and SF‐36 at baseline. Data calculated using MMRM including sex and visit as categorical fixed effects and SF‐36 score at baseline as continuous fixed effect and subject as random effect. For context, the red line shows reference data from a survey of a cross‐section of the French metropolitan population (Leplège et al., 2001; SF‐36 validation population in manual of SF‐36 in France) (see discussion). Abbreviations: MMRM, Model Mixed for Repeated Measures; SF‐36, Short Form‐36 Health Survey
Although improvements from baseline were observed in the SF‐36 physical role functioning domain in both treatment groups, a significantly greater improvement at the 4‐week time point occurred in participants who received magnesium + vitamin B6 compared with those who received magnesium alone (change from baseline to Week 4: 14.6 ± 2.5 vs. 4.6 ± 2.7, respectively; p = 0.025; Table 3). There was continued improvement with both treatments at Week 8, with the mean change from baseline increasing by 7.8 points from Week 4 to Week 8 for the magnesium + vitamin B6 group and by 9.1 points for the magnesium alone group. The difference between treatments in change from baseline to Week 8 was not statistically significant (p = 0.059; Table 3). The general health domain score improved from baseline in both treatment groups, with participants receiving magnesium alone showing a significantly greater improvement at Week 4 than those receiving magnesium + vitamin B6 (change from baseline to Week 4: 8.1 ± 1.1 vs. 4.6 ± 1.0, respectively; p = 0.046; Table 3).
4. DISCUSSION
This secondary analysis shows that magnesium (with or without vitamin B6) improved mood and anxiety, and improved associated QoL, in stressed but otherwise healthy adults. Magnesium (with or without vitamin B6) significantly improved baseline depression and anxiety scores of the DASS‐42 scale to normal or near normal levels by Week 8, with greatest change observed during the first 4 weeks. Improvements in SF‐36 QoL after 4 to 8 weeks were observed with both treatments, for all mental domains and most physical domains. Of note, participants' perception of being physically limited in their daily activities (physical role functioning) improved significantly more at Week 4 (similar trend at Week 8) with magnesium supplementation when combined with vitamin B6 than without. These clinical data support magnesium as a treatment for improving stress‐related mental health in individuals with suboptimal magnesemia and the further evaluation of a potential additional benefit with vitamin B6.
4.1. Effect of magnesium on mental health and QoL
Our findings support earlier findings, which demonstrate the potential of magnesium in the treatment of anxiety and depression (Botturi et al., 2020; Derom et al., 2013; Jung et al., 2010) as well as stress (Pouteau et al., 2018). They are also consistent with the findings of Forsyth et al. (2011) who reported that worse self‐reported depression was associated with lower magnesium intake (as a percentage of estimated average requirements) in a cohort of adults prior to treatment for depression and anxiety. Furthermore, our findings are consistent with previous reports of beneficial effects of magnesium supplementation on depression (Eby & Eby, 2006; Rajizadeh et al., 2017; Ryszewska‐Pokraśniewicz et al., 2018; Tarleton et al., 2017), anxiety (Boyle et al., 2017) and mood (Derom et al., 2013; Szewczyk et al., 2008). We saw marked improvement in both mental and physical aspects of QoL. Despite the importance of magnesium in human physiology, data on the effect of magnesium on QoL are scarce. To our knowledge, there has been only one previous study showing that magnesium prophylaxis for migraine improved QoL in a paediatric population (Kovacevic et al., 2017). In our study, physical domain SF‐36 scores at baseline were shown to be higher than reference data from a historical survey of a cross‐section of the French metropolitan population (Leplège et al., 2001; Figure 2). This may be due to the relatively young age of our cohort (mean 31.6 ± 8.5 years) or perhaps due to a slight improvement of the physical QoL of the French population in recent years. Nevertheless, our observations appear consistent with data published more recently by Briançon et al. (2011) from the SU.VI.MAX cohort.
Study participants with moderate to extremely severe stress at baseline were selected for inclusion. Many of these participants also presented with severe or extremely severe anxiety (52.3%), and moderate to extremely severe depression (51.5%) both of which frequently occur together. Study participants had reduced QoL at baseline (primarily impaired mental subdomain scores, but also impaired physical domain scores). Additionally, these participants presented with suboptimal serum magnesium levels (range 0.66–0.84 mmol/L). These baseline observations likely reflect known associations between chronic stress and dysregulation of the allostatic system resulting in worse mood and anxiety and are consistent with allostatic dysregulation in magnesium deficient subjects (McEwen, 2005, 2015). Indeed, the correlation between stress and physical symptoms, such as fatigue, is well documented (Doerr et al., 2015; Kocalevent et al., 2011), and thus the presence of stress in combination with suboptimal magnesium levels may help explain these baseline observations.
4.2. Effect of vitamin B6 in addition to magnesium on mental health and QoL
Consistent trends in favour of the magnesium and vitamin B6 combination observed for the SF‐36 physical role functioning domain, which were significant at Week 4, suggest that vitamin B6 augments participants' perception of improvement in physical capacity to perform activities, over and above the effect of magnesium alone. This represents an interesting clinical insight, and further exploration of this finding, which may help to elucidate the underlying mechanism of action of the combination, is warranted. We observed greater improvement in the SF‐36 general health domain with magnesium alone relative to magnesium + vitamin B6 at Week 4. However, the difference between groups was small and unlikely to be clinically meaningful.
A beneficial additive effect of magnesium and vitamin B6 treatment has been previously demonstrated on anxiety (for a review see Boyle et al., 2017), stress (Pouteau et al., 2018) and premenstrual syndrome (Fathizadeh et al., 2010; De Souza et al., 2000). However, we did not observe an additive effect; improvement in anxiety and depression scores with magnesium + vitamin B6 was equivalent to that seen with magnesium alone. One possible reason for the discordance could be the variation in anxiety and depression reported by our study participants at baseline. We have previously observed a synergistic effect for magnesium and vitamin B6 in stress reduction in a severely/extremely severely stressed population (Pouteau et al., 2018). Secondly, the lack of observed differential effect of addition of vitamin B6 in our study may be influenced by magnesium status at baseline. Vitamin B6 has been postulated to enhance magnesium absorption or cell penetration, but in the present study, very few participants (6/264; 2.3%) had frank hypomagnesaemia (serum magnesium levels <0.7 mmol/L, by definition) despite all having suboptimal magnesemia (<0.85 mmol/L). Further comparison with data from the literature is difficult since magnesium levels were not consistently reported in previous studies (Boyle et al., 2017; De Souza et al., 2000; Fathizadeh et al., 2010).
4.3. Limitations
Our analysis has some limitations. It is a post‐hoc analysis of a study not being primarily designed for the analyses of subjective anxiety, depression and QoL. The selected nature of our study sample, and that data collected using both the SF‐36 and DASS‐42 questionnaires are self‐reported, requires that our findings be interpreted with a degree of caution. Furthermore, the study did not include a placebo group and it was not possible to collect the ethnicity of the participants due to French ethical legislation. While we did request that participants abstained from taking medications known to affect magnesium levels and from consuming magnesium‐rich foods and water, dietary intake remains a potential limitation. With these limitations in mind, our findings should be considered exploratory and require confirmation in an appropriately designed follow‐up study.
5. CONCLUSION
The analyses presented suggest that magnesium treatment has potential benefit for the symptomatic treatment of stress‐associated mood and anxiety, and may improve self‐reported QoL. There now exists a growing body of evidence supporting the use of magnesium to improve stress‐related mental health, and further investigation is warranted to explore whether the addition of vitamin B6 may also offer additional benefits in such populations.
CONFLICT OF INTEREST
Lionel Noah, Béatrice Bois De Fer, and Etienne Pouteau are employees of Sanofi‐Aventis. Gisèle Pickering reports consultancy fees from Sanofi, unrelated to this publication. André Mazur reports consultancy fees from Sanofi, unrelated to this publication. Louise Dye has received research funding from Sanofi and consultancy payments unrelated to this publication.
AUTHORS CONTRIBUTION
Lionel Noah: Conceptualisation, Investigation, Methodology, Project administration, Writing. Etienne Pouteau: Conceptualisation, Investigation, Methodology, Project administration, Writing. Béatrice Bois De Fer: Formal analysis, Validation, Writing. André Mazur: Investigation, Writing. Louise Dye: Investigation, Writing. Gisèle Pickering.: Investigation, Writing.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Marmar Kabir‐Ahmadi for the statistical analysis and validation of the primary analysis, Professor Claude Dubray for his contribution as principal investigator of the superiority clinical trial (Pouteau et al., 2018) and Dr Lamia Achour for her contribution in the development of this manuscript. We acknowledge Ranjana Whitlock (Ashfield Healthcare Communications, Macclesfield, UK) for assistance in draughting the manuscript and coordinating and collating author contributions, which was funded by Sanofi‐Aventis Groupe, Gentilly, France, in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The study was funded by Sanofi‐Aventis Group, Gentilly, France, the manufacturer of MagneB6. The Sanofi‐Aventis Group took an active a role in all aspects of this study, including the design, data collection and analysis, decision to publish, and the preparation of the manuscript.
Noah, L. , Dye, L. , Bois De Fer, B. , Mazur, A. , Pickering, G. , & Pouteau, E. (2021). Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post‐hoc analysis of a randomised controlled trial. Stress and Health, 37(5), 1000–1009. 10.1002/smi.3051
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial subjects. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.
REFERENCES
- Abraham, G. E. , Schwartz, U. D. , & Lubran, M. M. (1981). Effect of vitamin B‐6 on plasma and red blood cell magnesium levels in premenopausal women. Annals of Clinical and Laboratory Science, 11(4), 333–336. http://www.annclinlabsci.org/cgi/content/abstract/11/4/333 [PubMed] [Google Scholar]
- Botturi, A. , Ciappolino, V. , Delvecchio, G. , Boscutti, A. , Viscardi, B. , & Brambilla, P. (2020). The role and the effect of magnesium in mental disorders: A systematic review. Nutrients, 12(6), 1661. 10.3390/nu12061661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, T. , Lawton, C. , & Dye, L. (2017). Part 2.2–overview. Nutrients, 9(5), 429. 10.3390/nu9050429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier, J. E. , Harper, R. , Jones, N. M. , O'Cathain, A. , Thomas, K. J. , Usherwood, T. , & Westlake, L. (1992). Validating the SF‐36 health survey questionnaire: New outcome measure for primary care. BMJ, 305(6846), 160–164. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briançon, S. , Boini, S. , Bertrais, S. , Guillemin, F. , Galan, P. , & Hercberg, S. (2011). Long‐term antioxidant supplementation has no effect on health‐related quality of life: The randomized, double‐blind, placebo‐controlled, primary prevention SU.VI.MAX trial. International Journal of Epidemiology, 40(6), 1605–1616. 10.1093/ije/dyr161 [DOI] [PubMed] [Google Scholar]
- Brown, T. A. , Chorpita, B. F. , Korotitsch, W. , & Barlow, D. H. (1997). Psychometric properties of the depression anxiety stress scales (DASS) in clinical samples. Behaviour Research and Therapy, 35(1), 79–89. 10.1016/S0005-7967(96)00068-X [DOI] [PubMed] [Google Scholar]
- Charles, S. T. , Piazza, J. R. , Mogle, J. , Sliwinski, M. J. , & Almeida, D. M. (2013). The wear and tear of daily stressors on mental health. Psychological Science, 24(5), 733–741. 10.1177/0956797612462222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc, P. , Young, C. A. , Bordt, E. A. , Grigore, A. M. , Fiskum, G. , & Polster, B. M. (2013). Magnesium sulfate protects against the bioenergetic consequences of chronic glutamate receptor stimulation. PLoS One, 8(11), e79982. 10.1371/journal.pone.0079982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, J. R. , & Henry, J. D. (2003). The depression anxiety stress scales (DASS): Normative data and latent structure in a large non‐clinical sample. British Journal of Clinical Psychology, 42(2), 111–131. 10.1348/014466503321903544 [DOI] [PubMed] [Google Scholar]
- Cuciureanu, M. D. , & Vink, R. (2011). Magnesium and stress. In: Vink R. & Nechifor M. (Eds.), Magnesium in the Central Nervous System [Internet]. Adelaide, Australia: University of Adelaide Press. https://www.ncbi.nlm.nih.gov/books/NBK507250/ [Google Scholar]
- de Baaij, J. H. F. , Hoenderop, J. G. J. , & Bindels, R. J. M. (2015). Magnesium in man: Implications for health and disease. Physiological Reviews, 95(1), 1–46. 10.1152/physrev.00012.2014 [DOI] [PubMed] [Google Scholar]
- De Souza, M. C. , Walker, A. F. , Robinson, P. A. , & Bolland, K. (2000). A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety‐related premenstrual symptoms: A randomized, double‐blind, crossover study. Journal of Women's Health & Gender‐Based Medicine, 9(2), 131–139. 10.1089/152460900318623 [DOI] [PubMed] [Google Scholar]
- Derom, M.‐L. , Sayón‐Orea, C. , Martínez‐Ortega, J. M. , & Martínez‐González, M. A. (2013). Magnesium and depression: A systematic review. Nutritional Neuroscience, 16(5), 191–206. 10.1179/1476830512Y.0000000044 [DOI] [PubMed] [Google Scholar]
- Doerr, J. M. , Ditzen, B. , Strahler, J. , Linnemann, A. , Ziemek, J. , Skoluda, N. , Hoppmann, C. A. , & Nater, U. M. (2015). Reciprocal relationship between acute stress and acute fatigue in everyday life in a sample of university students. Biological Psychology, 110, 42–49. 10.1016/j.biopsycho.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Doll, H. , Brown, S. , Thurston, A. , & Vessey, M. (1989). Pyridoxine (vitamin B6) and the premenstrual syndrome: A randomized crossover trial. The Journal of the Royal College of General Practitioners, 39(326), 364–368. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1711872/ [PMC free article] [PubMed] [Google Scholar]
- Eby, G. A. , & Eby, K. L. (2006). Rapid recovery from major depression using magnesium treatment. Medical Hypotheses, 67(2), 362–370. 10.1016/j.mehy.2006.01.047 [DOI] [PubMed] [Google Scholar]
- Fathizadeh, N. , Ebrahimi, E. , Valiani, M. , Tavakoli, N. , & Yar, M. H. (2010). Evaluating the effect of magnesium and magnesium plus vitamin B6 supplement on the severity of premenstrual syndrome. Iranian Journal of Nursing and Midwifery Research, 15(Suppl. 1), 401–405. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3208934/ [PMC free article] [PubMed] [Google Scholar]
- Forsyth, A. K. , Williams, P. G. , & Deane, F. P. (2011). Nutrition status of primary care patients with depression and anxiety. Australian Journal of Primary Health, 18(2), 172–176. 10.1071/PY11023 [DOI] [PubMed] [Google Scholar]
- Galland, L. (1991‐1992). Magnesium, stress and neuropsychiatric disorders. Magnesium and Trace Elements, 10(2–4), 287–301. https://pubmed.ncbi.nlm.nih.gov/1844561/ [PubMed] [Google Scholar]
- Held, K. , Antonijevic, I. A. , Künzel, H. , Uhr, M. , Wetter, T. C. , Golly, I. C. , Steiger, A. , & Murck, H. (2002). Oral Mg2+ supplementation reverses age‐related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry, 35(4), 135–143. 10.1055/s-2002-33195 [DOI] [PubMed] [Google Scholar]
- Iezhitsa, I. N. , Spasov, A. A. , Kharitonova, M. V. , & Kravchenko, M. S. (2011). Effect of magnesium chloride on psychomotor activity, emotional status, and acute behavioural responses to clonidine, D‐amphetamine, arecoline, nicotine, apomorphine, and L‐5‐hydroxytryptophan. Nutritional Neuroscience, 14(1), 10–24. 10.1179/174313211X12966635733277 [DOI] [PubMed] [Google Scholar]
- Jung, K. I. , Ock, S. M. , Chung, J. H. , & Song, C. H. (2010). Associations of serum Ca and Mg levels with mental health in adult women without psychiatric disorders. Biological Trace Element Research, 133(2), 153–161. 10.1007/s12011-009-8421-y [DOI] [PubMed] [Google Scholar]
- Juster, R.‐P. , McEwen, B. S. , & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kafeshani, M. , Feizi, A. , Esmaillzadeh, A. , Keshteli, A. H. , Afshar, H. , Roohafza, H. , & Adibi, P. (2019). Higher vitamin B6 intake is associated with lower depression and anxiety risk in women but not in men: A large cross‐sectional study. International Journal for Vitamin and Nutrition Research, 90(5–6), 484–492. 10.1024/0300-9831/a000589 [DOI] [PubMed] [Google Scholar]
- Kirkland, A. , Sarlo, G. , & Holton, K. (2018). The role of magnesium in neurological disorders. Nutrients, 10(6), 730. 10.3390/nu10060730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocalevent, R. D. , Hinz, A. , Brähler, E. , & Klapp, B. F. (2011). Determinants of fatigue and stress. BMC Research Notes, 4, 238. 10.1186/1756-0500-4-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic, G. , Stevanovic, D. , Bogicevic, D. , Nikolic, D. , Ostojic, S. , Tadic, B. V. , Nikolic, B. , Bosiocic, I. , Ivancevic, N. , Jovanovic, K. , Samardzic, J. , & Jancic, J. (2017). A 6‐month follow‐up of disability, quality of life, and depressive and anxiety symptoms in pediatric migraine with magnesium prophylaxis. Magnesium Research, 30(4), 133–141. 10.1684/mrh.2018.0431 [DOI] [PubMed] [Google Scholar]
- Leplège, A. , Ecosse, E. , Pouchot, J. , Coste, J. , & Perneger, T. (2001). Le questionnaire MOS SF‐36 : manuel de l’utilisateur et guide d’interprétation des scores. Paris: ESTEM; 2001. [Google Scholar]
- Majumdar, P. , & Boylan, L. M. (1989). Alteration of tissue magnesium levels in rats by dietary vitamin B6 supplementation. International Journal for Vitamin and Nutrition Research, 59(3), 300–303. https://pubmed.ncbi.nlm.nih.gov/2599796/ [PubMed] [Google Scholar]
- McCarty, M. F. (2000). High‐dose pyridoxine as an 'anti‐stress' strategy. Medical Hypotheses, 54(5), 803–807. 10.1054/mehy.1999.0955 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338(3), 171–179. 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (2005). Stressed or stressed out: What is the difference? Journal of Psychiatry & Neuroscience, 30(5), 315–318. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1197275/ [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. , Bowles, N. P. , Gray, J. D. , Hill, M. N. , Hunter, R. G. , Karatsoreos, I. N. , & Nasca, C. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. , & Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. 10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- Murck, H. (2002). Magnesium and affective disorders. Nutritional Neuroscience, 5(6), 375–389. 10.1080/1028415021000039194 [DOI] [PubMed] [Google Scholar]
- Noah, L. , Pickering, G. , Mazur, A. , Dubray, C. , Hitier, S. , Dualé, C. , & Pouteau, E. (2020). Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: Secondary data from a randomized controlled trial. Magnesium Research, 33(3), 45–57. 10.1684/mrh.2020.0468 [DOI] [PubMed] [Google Scholar]
- Papadopol, V. , & Nechifor, M. (2011). Magnesium in neuroses and neuroticism. In: Vink R. & Nechifor M. (Eds.), Magnesium in the Central Nervous System [Internet], Adelaide, Australia: University of Adelaide Press. https://www.ncbi.nlm.nih.gov/books/NBK507254/ [PubMed] [Google Scholar]
- Pouteau, E. , Kabir‐Ahmadi, M. , Noah, L. , Mazur, A. , Dye, L. , Hellhammer, J. , Pickering, G. , & Dubray, C. (2018). Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single‐blind clinical trial. PLoS One, 13(12), e0208454. 10.1371/journal.pone.0208454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajizadeh, A. , Mozaffari‐Khosravi, H. , Yassini‐Ardakani, M. , & Dehghani, A. (2017). Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double‐blind, placebo‐controlled trial. Nutrition, 35, 56–60. 10.1016/j.nut.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Ryszewska‐Pokraśniewicz, B. , Mach, A. , Skalski, M. , Januszko, P. , Wawrzyniak, Z. M. , Poleszak, E. , Nowak, G. , Pilc, A. , & Radziwoń‐Zaleska, M. (2018). Effects of magnesium supplementation on unipolar depression: A placebo‐controlled study and review of the importance of dosing and magnesium status in the therapeutic response. Nutrients, 10(8), 1014. 10.3390/nu10081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K. (2018). Why is vitamin B6 effective in alleviating the symptoms of autism? Medical Hypotheses, 115, 103–106. 10.1016/j.mehy.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Szewczyk, B. , Poleszak, E. , Sowa‐Kućma, M. , Siwek, M. , Dudek, D. , Ryszewska‐Pokraśniewicz, B. , Radziwoń‐Zaleska, M. , Opoka, W. , Czekaj, J. , Pilc, A. , & Nowak, G. (2008). Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacological Reports, 60(5), 588–599. https://pubmed.ncbi.nlm.nih.gov/19066406/ [PubMed] [Google Scholar]
- Tarleton, E. K. , Littenberg, B. , MacLean, C. D. , Kennedy, A. G. , & Daley, C. (2017). Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS One, 12, e0180067. 10.1371/journal.pone.0180067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebahn, I. , Porta, S. , & Kisters, K. (2016). Magnesium status correlates with health and quality of life. Trace Elements and Electrolytes, 33, 70–73. 10.5414/TEX01420 [DOI] [Google Scholar]
- Ware, J. E., Jr. , & Sherbourne, C. D. (1992). The MOS 36‐ltem short‐form health survey (SF‐36). Medical Care, 30(6), 473–483. https://pubmed.ncbi.nlm.nih.gov/1593914/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial subjects. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.