How was apical growth regulated in the ancestral land plant? Insights from the development of non-seed plants (original) (raw)
Abstract
Land plant life cycles are separated into distinct haploid gametophyte and diploid sporophyte stages. Indeterminate apical growth evolved independently in bryophyte (moss, liverwort, and hornwort) and fern gametophytes, and tracheophyte (vascular plant) sporophytes. The extent to which apical growth in tracheophytes co-opted conserved gametophytic gene networks, or exploited ancestral sporophytic networks, is a long-standing question in plant evolution. The recent phylogenetic confirmation of bryophytes and tracheophytes as sister groups has led to a reassessment of the nature of the ancestral land plant. Here, we review developmental genetic studies of apical regulators and speculate on their likely evolutionary history.
The combined results of recent developmental genetics and phylogenetics studies suggest that the ancestral sporophyte was more complex than previously thought.
Introduction
The land plants we live among and depend on today look very different to the ancestral green alga that first colonized the land ∼470–515 million years ago (Steemans, 2000; Morris et al., 2018). In response to the new environmental challenges posed by life on land (e.g. desiccation, low water and nutrient availability, high UV radiation, and increased temperature variability), plants evolved a suite of morphological, physiological, and anatomical innovations. The ecological success of these adaptations were such that land plants transformed the planet, resulting in a huge diversity in form as different lineages evolved novel solutions to a changing world.
All land plants share a number of morphological traits—they are multicellular organisms with life cycles that alternate between a haploid gametophyte and a diploid sporophyte stage. Multicellular sporophytes develop within the maternal gametophyte (hence the name embryophytes for land plants) and produce sporopollenin-coated meiospores (Niklas and Kutschera, 2010). Extant members of the non-vascular bryophyte clade (i.e. mosses, liverworts, and hornworts; Figure 1) grow predominantly as haploid gametophytes. Following fertilization, bryophyte sporophytes are dependent on the parent gametophyte for nourishment and persist only briefly before undergoing meiosis and spore production. On the other hand in vascular plants (tracheophytes), the sister lineage to bryophytes consisting of lycophyte, monilophyte, and seed plant clades (Figure 1), the sporophyte stage is dominant and largely independent of the gametophyte (Niklas and Kutschera, 2010).
Figure 1.
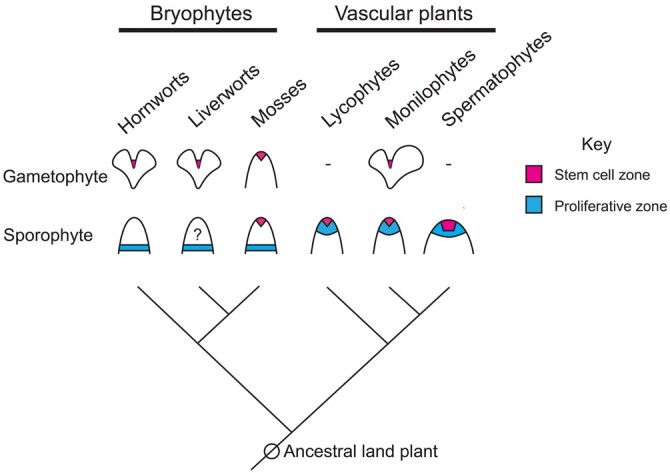
Phylogenetic relationships and representative meristem structure of land plants. Bryophyte gametophytes grow as thalli, leafy shoots or filaments, and meristems comprise a single apical cell. Monilophytes also produce thallus-type gametophytes with a notch meristem (in the case of Ceratopteris hermaphrodite gametophytes). Hornwort sporophytes grow from a persistent basal meristem, moss sporophytes grow initially from an apical initial, and subsequently from an intercalary meristem; the mechanism of proliferative growth in liverwort sporophytes appears to be intercalary meristem-like. Selaginella (lycophyte) and Ceratopteris (monilophyte) apices usually have two and one apical initials, respectively, but other lycophytes and monilophytes have apices with multiple initials. Spermatophyte (seed plant) apices are multicellular and functionally more complex. Figure adapted from Harrison (2017).
The phylogenetic relationship between the bryophytes and the tracheophytes has long been contentious, with almost every bryophyte grouping proposed to be sister lineage to the tracheophytes at some stage (Donoghue et al., 2021). However, wider taxon sampling combined with improved models of sequence evolution now strongly supports bryophytes as monophyletic, with liverworts and mosses forming a clade (the setaphytes) within the bryophytes (Figure 1; Wickett et al., 2014; Puttick et al., 2018; Sousa et al., 2019; Harris et al., 2020; Sousa et al., 2020). Likewise, improved phylogenetic methods have led to a reassessment of which group of the charophyte grade of green algae is the sister lineage to embryophytes. As they exhibit a number of traits characteristic of land plants (e.g. cell walls with plasmodesmata, asymmetric cell division, branching, and zygotic sporopollenin deposition), the Charophyceae and Coleochaetophyceae have historically been considered the likeliest closest embryophyte relatives (Mishler and Churchill, 1984; Mishler and Churchill, 1985). However, phylogenetic analyses of both organelle and nuclear sequences now support the aflagellate and predominantly unicellular Zygnematophyceae as sister lineage to the land plants (Turmel et al., 2006, 2013; Karol et al., 2010; Lemieux et al., 2016; Harris et al., 2020).
A true embryophyte phylogeny is critical to understanding how plants colonized land (Puttick et al., 2018; Donoghue et al., 2021). With the rejection of bryophytes as a paraphyletic grade, it is imperative to reassess previous conclusions about the ancestral state of land plants based on individual bryophyte lineages as sister to tracheophytes. Knowing the phylogenetic relationships between different land plants makes it possible to define which character traits are ancestral, which are derived, and which have been lost in specific lineages. Considering these different evolutionary trajectories, we can now more accurately infer the likely bodyplan, genetic architecture, and growth habit of the ancestral land plant. In this update, we focus on recent results from developmental genetic studies in non-seed plants to make inferences about how shoot apical growth was regulated in the ancestor of embryophytes.
Structure of land plant shoot meristems
Apical growth (i.e. directional proliferative growth facilitated by the self-renewing activity of an undifferentiated meristematic cell or cells) was fundamental to the successful colonization of land as it enabled plants to spread across a solid substrate and, eventually, rise above it. The charophyte sister lineages to the land plants exhibit varied forms of apical growth, including filamentous and thallose habits reminiscent of extant bryophytes. For example, a single apical cell divides transversely in Chara to elongate the main filament, which is further extended by expansion of apical cell derivatives (Pickett-Heaps, 1967), in a mechanism similar to protonemal tip growth in moss (see below). Thus, apical growth evolved prior to the emergence of land plants and aspects of an ancestral body plan were retained following the colonization of land (Delwiche and Cooper, 2015; Harrison, 2017; Moody, 2020).
Mosses
Following spore germination, moss gametophyte growth begins as a 2D filamentous network (the protonema) (Cove, 2005). Initially the protonema consists exclusively of chloroplast-rich chloronemal filaments. Depending on the species and the environment, filaments of longer and narrower caulonemal cells, with fewer chloroplasts, may also emerge. In both cases, filaments extend through tip growth and the uniplanar division of single apical stem cells (Menand et al., 2007). New apical cells initiate from cells subtending the tips of existing filaments (Cove and Knight, 1993; Kofuji and Hasebe, 2014). These side branch initials can either give rise to secondary filaments, therefore elaborating the 2D protonemal network or facilitate a transition to 3D growth through the formation of shoots (gametophores). Despite often initiating on the same parent cell, secondary filaments and gametophores can be distinguished from the orientation of the first cell division and the width of the initial cell (perpendicular/narrow and oblique/wide, respectively) (Harrison et al., 2009; Tang et al., 2020). Gametophore initials undergo three more rounds of cell division to generate a tetrahedral shaped apical cell, which subsequently cleaves spirally to produce a leafy shoot (Harrison et al., 2009). Although protonemal and gametophore apical cells share certain molecular signatures associated with cell proliferation, the multiplanar cell division pattern of gametophore apical cells is reflected in increased transcriptomic complexity (Frank and Scanlon, 2015b).
Moss reproductive organs (female archegonia and male antheridia) derive from single stem cells that initiate at gametophore tips and undergo predictable patterns of cell division, leading to gamete production (Landberg et al., 2013). After fertilization, moss sporophyte growth is characterized by limited rounds of division from an apical stem cell followed by proliferation of a more basal intercalary meristem (Figure 1; Sakakibara et al., 2008; Coudert et al., 2019). The duration and timing of intercalary meristem division is associated with the dramatically different sizes of moss sporophytes observed in different species (French and Paolillo, 1975a, 1975b). A recent transcriptomic study of Physcomitrium patens (sporophyte size ∼1 mm) and Funaria hygrometrica (sporophyte size ∼5 cm) suggests that size differences are largely a consequence of temporal shifts in the expression patterns of conserved genetic networks, rather than species-specific gene gain/loss (Kirbis et al., 2020,).
Liverworts
Relative to mosses, liverworts such as the model system Marchantia polymorpha display only a brief filamentous growth phase following spore germination (Nishihama et al., 2015). Subsequently, liverworts grow in either leafy or thallose forms. In the case of leafy forms, dichotomizing gametophores with ranked leaves (or phyllids) are generated by a single apical cell. In the case of thallose species such as Marchantia, thalli are iterated by the activity of a single wedge-shaped apical cell that resides in an apical notch. Marchantia apical cells divide in four planes, facilitating the formation of multiple cell layers within the thallus and a bifurcating pattern of thallus branching (Shimamura, 2016; Solly et al., 2017). Reproductive organs emerge vertically from the vegetative thallus on separate dioecious plants and, as in mosses, the developing sporophyte is nourished within the archegonia. However, unlike in moss sporophytes, there is no apical stem cell in the Marchantia sporophyte and proliferative growth appears limited to an intercalary meristem-like mechanism (Kienitz-Gerloff, 1874; Dierschke et al., 2021).
Hornworts
Hornworts such as Anthoceros agrestis also grow vegetatively as thalli (Frangedakis et al., 2021). Similar to liverworts, growth is maintained by the activity of individual wedge-shaped apical cells that divide in four planes and are situated in apical notches (Renzaglia et al., 2008). However, apical notches form in greater numbers in Anthoceros and, consequently, thallus growth is irregular. Uniquely within the bryophytes, hornwort reproductive organs are embedded within the thallus. Furthermore, the sporophyte extends from a basal proliferative zone that remains active throughout its life cycle and spore production is continuous (Renzaglia et al., 2008). This is in sharp contrast to setaphytes, in which sporophyte proliferative growth is limited and spore production is terminal.
Tracheophytes
In contrast to bryophytes, vascular plant apical growth occurs almost exclusively in the sporophyte generation, although apical initials have been retained in some fern gametophytes (Banks, 1999). The structure of vascular plant shoot meristems has been reviewed extensively elsewhere (e.g. Gifford and Corson, 1971; Steeves and Sussex, 1989; White and Turner, 1995; Barton, 2010; Spencer et al., 2021). To summarize briefly, in the lycophyte lineage there is considerable variation in shoot meristem organization. For example, in the Selaginellaceae, there are one or two transiently acting apical initials (Harrison et al., 2007), whereas in the Isoëtaceae and Lycopodiaceae, meristems contain multiple apical initials (Philipson, 1990). Monilophytes, including the model systems Ceratopteris, Equisetum, and Nephrolepis (Sanders et al., 2011; Frank and Scanlon, 2015a; Plackett et al., 2015), predominantly exhibit single apical cells; however, ferns with multiple apical cells have also been reported (White and Turner, 1995). Proliferative “core” groups of cells that subtend apical initials and have unique transcriptional signatures have been identified in lycophytes and ferns, suggesting that apical growth in these lineages is coordinated by multiple cell types (Frank et al., 2015; Ambrose and Vasco, 2016).
Shoot apical meristems (SAMs) in seed plants are larger and more complex, consisting of multiple stem cells and distinct functional zones. For example, in flowering plants such as Arabidopsis (Arabidopsis thaliana), meristems consist of three domains: the central (CZ), peripheral (PZ) and rib (RZ) zones. Cells within the CZ self-renew and replenish the surrounding (PZ) and subtending (RZ) zones and are therefore considered the stem cells (functionally equivalent to the apical cells of non-seed plants). Cells within the PZ and RZ proliferate more rapidly than in the CZ and ultimately differentiate to produce lateral organs and ground tissue, respectively (Steeves and Sussex, 1989; Barton, 2010).
It is possible that the ancestral land plant had a multicellular meristem which was subsequently reduced in the bryophyte lineage. However, the similarity of (1) charophyte and bryophyte patterns of growth from single apical initials and (2) extant moss sporophytes and fossils of proto-tracheophytes with apparently simple apical domains (Harrison and Morris, 2017) suggest that meristem complexity is a derived trait in vascular plants. The polyphyletic distribution of multicellular apices within the tracheophytes (i.e. within specific lycophyte and monilophyte lineages), and unique transcriptomic signatures of lycophyte and monilophyte apical cells (Frank et al., 2015), suggest sporophytic meristems may have evolved convergently. How indeterminate vascular plant meristems evolved from an ancestor with a diminutive and determinate sporophyte remains a critical question in the field. The vascular plant SAM may have evolved from persistence and expansion of a transient moss-like sporophyte apical initial (Albert, 1999). Alternatively, a bryophyte-type sporophyte intercalary meristem may have moved apically during evolution and be homologous to SAM proliferative zones (e.g. the core cells of lycophytes and ferns, and PZ/RZ of angiosperms) (Mishler and Churchill, 1984). It is also possible that both these scenarios occurred and the vascular plant SAM evolved from a juxtaposition of the apical and intercalary domains (Harrison, 2017).
It has been proposed that the elaboration of sporophytic apical growth required the large-scale co-option of gametophytic genetic networks (Floyd and Bowman, 2007; Niklas and Kutschera, 2010). Supporting this idea, transcriptomic studies have found homologs of multiple angiosperm SAM regulators to be expressed in Physcomitrium gametophytes (Nishiyama et al., 2003; Frank and Scanlon, 2015a). However, the observation that a number of critical sporophyte stem cell regulators do not function in bryophyte gametophyte apical growth suggests this is not universally true (Sakakibara et al., 2008; Sakakibara et al., 2014; Yip et al., 2016). In the following sections, we review molecular studies into bryophyte development and discuss the likely ancestral roles for regulators of land plant shoot development.
Genetic networks that control apical growth
CLAVATA/WUSCHEL-LIKE HOMEOBOX
Variants of the CLAVATA (CLV)–WUSCHEL (WUS) genetic network are essential regulators of stem cell proliferation in a variety of angiosperm meristematic contexts. In the Arabidopsis SAM (for details of CLV signaling in other developmental contexts, see Fletcher, 2020), the small signaling peptide CLV3 is expressed in the outer cell layers of the CZ (Fletcher et al., 1999). The peptide migrates basally to the center of the SAM where it is bound by its principal receptor—the leucine-rich repeat receptor like kinase (LRR-RLK) CLV1 (Clark et al., 1997; Brand et al., 2000). On the flanks of the SAM, CLV1 functions redundantly with the closely related BARELY ANY MERISTEM (BAM) receptors (Nimchuk et al., 2015). CLV1/BAM receptors, in combination with additional LRR-RLK family members CLV2 (Kayes and Clark, 1998), RECEPTOR-LIKE PROTEIN KINASE 2 (RPK2) (Kinoshita et al., 2010), and CLAVATA3-INSENSITIVE RECEPTOR KINASES (CIKs) (Hu et al., 2018), plus the presumptive pseudokinase CORYNE (CRN) (Müller et al., 2008), function to transcriptionally repress the transcription factor WUS. WUS promotes stem cell proliferation and so, in effect, CLV3 and its downstream signaling components act to limit stem cell number. To maintain stem cell homeostasis, WUS moves apically through plasmodesmata to promote CLV3 expression, thus completing a negative feedback loop (Brand et al., 2000; Schoof et al., 2000). Recently the CLV3-like small peptide CLE40, which is expressed in the PZ and bound by BAM1, has been shown to promote WUS activity (Schlegel et al., 2021). CLV-mediated regulatory networks therefore buffer SAM stem cell number through positive and negative relationships with WUS.
Orthologs of CLV3, CLV1/BAM1, RPK2, and CIK are present in bryophyte genomes, whereas CLV2 and CRN emerged in vascular plants. None of these genes are present in charophyte algae, suggesting that a core CLV signaling network evolved in the ancestor of land plants and was later elaborated during land plant evolution (Whitewoods et al., 2018; Furumizu et al., 2021; Takahashi et al., 2021). In Physcomitrium, loss-of-function Ppclv1a/1b and Pprpk2 mutants produce an over-proliferation of apical cells at the base of gametophores. Moreover, application of synthetic CLV3-like peptides inhibits cell proliferation and causes gametophore dwarfing (Whitewoods et al., 2018). In Marchantia, a CLV3–CLV1–CIK module also regulates gametophytic stem cell proliferation, although in this case positively (Hirakawa et al., 2020; Takahashi et al., 2021). Intriguingly, a CLV3 family member that promotes stem cell proliferation in Arabidopsis has been found to repress apical growth in Marchantia (Hirakawa et al., 2019), suggesting a divergence of signaling mechanisms during evolution. Together, these results support the idea that CLV3 functioned to regulate stem cell proliferation in the common ancestor of land plants. Whether or not CLV signaling was co-opted for sporophytic apical growth in vascular plants, or was already functional in the ancestral sporophyte, will require closer analysis of bryophyte clv mutant sporophytes.
In addition to a role in stem cell proliferation, CLV signaling also appears to regulate stem cell identity in bryophytes. Reduced CLV signaling in Physcomitrium, most notably in Pprpk2 mutants, enhances protonemal spread and the chloronema–caulonemal transition. These observations, and the strong expression of CLV signaling components in protonemal tips, suggest that CLV signaling represses caulonemal apical cell identity (Nemec Venza et al., 2022). A role for the CLV pathway in moss gametophore initiation has also been identified (Whitewoods et al., 2018). Ppclv receptor mutants, and artificial miRNA-targeting of CLV3 homologs, inhibit gametophore formation—in this case not through a defect in stem cell identity specification, but rather through perturbed cell division plane orientation following bud initiation (Whitewoods et al., 2018). CLV signaling also regulates the angle of cell division in Marchantia and Arabidopsis (Whitewoods et al., 2018; Hirakawa et al., 2019), suggesting that this may be a further ancestral function.
With regard to the architecture of the ancestral CLV genetic network, the identity of the downstream targets remains a critical unknown. In angiosperms, CLV signaling is mediated largely by members of the WUS clade of WUS-LIKE HOMEOBOX (WOX) genes. However, the WUS clade of WOX genes emerged after the split between bryophytes and tracheophytes, loss-of-function Ppwox13la/b mutants do not affect Physcomitrium gametophore development, and MpWOX is not required for CLV3 signaling in Marchantia (Nardmann and Werr, 2012; Sakakibara et al., 2014; Hirakawa et al., 2020). These results strongly suggest that the CLV– WOX regulatory interaction is derived. What, then, was the ancestral target(s) of the CLV network? Recent work points to plant hormones as likely candidates. In Physcomitrium, the Pprpk2 and Ppclv1a/1b protonema phenotypes are dependent on auxin transport and it has been proposed that CLV signaling regulates the flow of auxin in protonema tip cells (Nemec Venza et al., 2022). In Arabidopsis, the effects of CLV2/CRN (which are tracheophyte-specific and CLV1-independent) on flower development are auxin-mediated (Jones et al., 2021), suggesting that regulatory interactions between CLV signaling components and auxin have evolved repeatedly in land plants.
Using the observed effects of cytokinin treatments on Ppclv1a/1b and Pprpk2 as inputs for mathematical models, it has also been suggested that PpCLV1A/1B function upstream of a cytokinin signaling pathway (Cammarata et al., 2022). Because WUS is known to mediate both auxin and cytokinin signaling in angiosperms (Leibfried et al., 2005; Ma et al., 2019), these findings support an exciting model in which WUS was co-opted to act as an intermediate between CLV and hormone pathways during vascular plant evolution. As _WUS_-like gene activity appears limited to the vasculature and roots of Selaginella and Ceratopteris, a role for the WUS clade in shoot apical growth appears derived within the vascular plants (Youngstrom et al., 2022).
In bryophytes, following presumed extensive gene loss, members of the WOX family are restricted to the T1 clade that includes homologs of WOX13 (Nardmann and Werr, 2012; Wu et al., 2019). Physcomitrium PpWOX13LA/B are required for embryonic cell division and the de novo formation of stem cells (Sakakibara et al., 2014) and PpWOX13LB is able to rescue callus production defects in the Arabidopsis wus13 mutant (Ikeuchi et al., 2022). Thus, WOX genes appear to function ancestrally both within the diploid generation and in the regulation of stem cell activity. The smaller thalli observed in Marchantia Mpwox mutants (Hirakawa et al., 2020), and effects on both generations of WOX knockdown in a fern (Youngstrom et al., 2019), suggest that WOX genes regulated cell proliferation in both stages of the ancestral land plant life cycle.
HAIRY MERISTEM
The HAIRY MERISTEM (HAM) clade of GRAS family transcription factors are critical regulators of shoot indeterminacy in angiosperms, where they physically and genetically interact with WUS-type WOX proteins to promote stem cell proliferation (Engstrom et al., 2011; Zhou et al., 2015). HAM genes evolved prior to the emergence of land plants (although have since been lost in Marchantia) and have been sufficiently conserved during evolution that moss, lycophyte, and fern HAM orthologs are able to rescue an Arabidopsis ham mutant (Geng et al., 2021). Conservation of function is further supported by a genetic analysis of the Physcomitrium HAM gene PpGRAS12. Loss of PpGRAS12 activity through gene targeting restricts gametophore outgrowth whereas overexpression promotes the formation of ectopic apical cells (Beheshti et al., 2021). This suggests that HAM genes promoted stem cell proliferation in the ancestral land plant and, as with CLV, that their regulatory interaction with WOX genes is derived.
KNOTTED-LIKE HOMEOBOX
Members of the three amino acid loop extension (TALE) superfamily of homeobox transcription factors are essential for apical growth (Hay and Tsiantis, 2010). There are two families of TALE genes in plants: KNOTTED-LIKE HOMEOBOX (KNOX) and BELL-LIKE (BELL). KNOX genes can be subdivided into two classes and members of both classes heterodimerize with BELL partners to regulate development. In angiosperms, Class I and Class II KNOX genes act antagonistically: Class I genes promote meristematic activity and stem cell identity whereas Class II genes promote differentiation (Vollbrecht et al., 1991; Long et al., 1996; Furumizu et al., 2015).
The duplication that generated Class I and Class II KNOX genes occurred in charophyte algae (Frangedakis et al., 2017). In the unicellular chlorophyte Chlamydomonas reinhardtii, a single KNOX and BELL protein are each expressed in distinct gametes, but heterodimerize and activate the zygotic genetic program after gamete fusion (Lee et al., 2008). Thus, the initiation of the diploid genetic program appears to be the ancestral function of KNOX/BELL genes (Bowman et al., 2016). This role has been maintained in bryophytes. Similar to Chlamydomonas, expression of Class I KNOX and BELL genes in gametes is required to establish zygotic development in Marchantia (Dierschke et al., 2021; Hisanaga et al., 2021). Physcomitrium Class II KNOX genes repress gametophytic development during early sporophytic growth (Sakakibara et al., 2013) and PpBELL1 over-expression is able to ectopically induce the diploid program during the gametophytic phase (Horst et al., 2016).
Analyses of moss Class I KNOX genes suggest that their role in sporophyte meristematic growth arose in the ancestral land plant. Loss-of-function Class I KNOX Physcomitrium mutants have no effect on gametophytic growth but sporophyte development is stunted due to reduced proliferation of the intercalary meristem (Singer and Ashton, 2007; Sakakibara et al., 2008; Coudert et al., 2019). In angiosperms, Class I KNOX genes promote stem cell identity via cytokinin signaling (Jasinski et al., 2005; Yanai et al., 2005). This functional interaction also appears to have evolved in the ancestral land plant as Physcomitrium Class I KNOX activity is at least in part dependent on cytokinin (Coudert et al., 2019). The meristematic expression of Class I KNOX genes in diverse land plant lineages (Harrison et al., 2005; Sano et al., 2005; Ambrose and Vasco, 2016; Dierschke et al., 2021), and their broad functional conservation (Frangedakis et al., 2017) suggests that the recruitment of KNOX activity was fundamental to the evolution of indeterminate sporophyte growth.
Class III HD-ZIP
The absence of an obvious gametophytic role for bryophyte Class I KNOX genes—critical regulators of sporophyte meristem development—challenges the hypothesis that indeterminate vascular shoot growth required widescale redeployment of gametophytic apical networks. The view that sporophyte elaboration did not require widescale gametophytic network co-option is supported by a study of Class III HD-ZIP (C3HDZ) genes in Physcomitrium. C3HDZ genes, which arose in the charophytes (Floyd et al., 2006), are expressed in divergent tracheophyte apices (Floyd et al., 2006; Prigge and Clark, 2006) and promote SAM formation in angiosperms (McConnell et al., 2001; Emery et al., 2003). However, loss of C3HDZ function has no effect on apical growth in the gametophore (Yip et al., 2016). Moreover, strong C3HDZ expression in the transient sporophyte apical initial could indicate a pre-existing sporophytic role.
LEAFY
The plant-specific transcription factor LEAFY (LFY) principally regulates meristem identity during reproductive development in angiosperms (Weigel et al., 1992). In Physcomitrium, two LFY genes regulate the first zygotic division and cell divisions at later stages of sporophytic development (Tanahashi et al., 2005). As moss and hornwort LFY proteins bind lineage-specific motifs, whereas liverwort and vascular plant LFY bind a conserved motif, and PpLFY is not able to rescue the Arabidopsis lfy mutant phenotype, it is unclear whether the role of PpLFY is reflective of an ancestral state, or divergent downstream networks (Maizel et al., 2005; Sayou et al., 2014). Gene expression (Rodríguez-Pelayo et al., 2022) and functional (Plackett et al., 2018) analyses in ferns and a lycophyte support an ancestral role for LFY in early embryo development. However, the results of these studies further suggest that LFY was co-opted to regulate cell division in wide-ranging developmental contexts during vascular plant diversification.
TEOSINTE BRANCHED 1
Based on the phylogenetic distribution of branching, it has been proposed that sporophyte branching evolved after the split between the bryophyte and tracheophyte lineages (Harrison, 2017). In angiosperms, shoot branching is regulated by multiple pathways, including by Class II TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR1 (TCP) genes, which repress the outgrowth of axillary meristems (Doebley et al., 1997; Aguilar-Martínez et al., 2007; Koyama et al., 2007). A transcriptomic analysis in moss has revealed that expression of the two Physcomitrium Class II TCP genes (PpTCP5/6) is enriched in the sporophyte and, intriguingly, that loss of PpTCP5 function induces sporophytic branching (Ortiz-Ramírez et al., 2016). Class II TCP genes are thus likely ancestral repressors of sporophyte branching in land plants. Moss sporophyte branching is also observed in plants with perturbed auxin transport (Fujita et al., 2008; Bennett et al., 2014b). In combination, these results suggest that the sporophyte of the ancestral land plant was competent to branch. However, at least in extant lineages, bryophyte branching was either lost, or restricted by conserved developmental networks that were reconfigured during tracheophyte evolution.
AINTEGUMENTA-LIKE
Members of the AINTEGUMENTA (ANT) subfamily of AP2-LIKE transcription factors regulate a variety of processes in angiosperms, including stem cell maintenance, cell proliferation in young organs, and embryogenesis (Elliott et al., 1996; Boutilier et al., 2002; Galinha et al., 2007). A reverse genetic study in Physcomitrium found that four ANT family members (APB1-4) redundantly regulate gametophore development (Aoyama et al., 2012). APB1-4 function downstream of auxin to specify the gametophore branch initial. Due to the expansion and functional diversity of the ANT gene family in angiosperms, it is difficult to surmise ancestral function but, with no report of an Ppapb1-4 sporophyte phenotype (Aoyama et al., 2012), this perhaps represents an example of gametophytic apical network co-option.
DEFECTIVE KERNEL 1
An additional class of genes that regulate gametophore formation is the DEFECTIVE KERNEL 1 (DEK1) family of calpains. Angiosperm dek1 mutants are embryo lethal due to disrupted patterns of cell division (Johnson et al., 2005; Lid et al., 2005). The orientation of cell division appears to be the ancestral role for DEK1 as Physcomitrium Ppdek1 mutants are unable to correctly specify gametophore apical initial position (Perroud et al., 2014). Furthermore, PpDEK1 is able to rescue Arabidopsis dek1 mutant phenotypes (Liang et al., 2013). Based on network analyses of transcriptomic data, it has been inferred that PpDEK1, which is localized to the plasma membrane in recently divided cells (Perroud et al., 2020), functions as a developmental “gatekeeper” of cell fate transitions (Demko et al., 2021). In the case of the transition to 3D growth during gametophyte development, PpDEK1 appears to function partly through repression of APB genes (Demko et al., 2014).
NO GAMETOPHORES
Further regulators of 3D apical growth have been identified by Moody and colleagues using elegant forward genetic screens that use somatic hybridization to facilitate bulk segregant analysis in polyploid Physcomitrium (Moody et al., 2018a). This strategy has revealed NO GAMETOPHORES 1 (NOG1) and NOG2 as critical determinants of gametophore initiation. PpNOG1 encodes an ubiquitin-associated protein that functions upstream of APB genes to promote gametophore bud formation and, subsequently, orient apical cell divisions (Moody et al., 2018b). A lack of functional analyses for NOG1 orthologs in other land plant lineages limits speculation regarding an ancestral function. However, as NOG1 genes are only found in land plants, it has been proposed that NOG1 evolution was fundamental to the establishment of 3D growth (Moody et al., 2018b). Like NOG1, CLV pathway genes are also critical regulators of apical cell division plane orientation that emerged in the ancestor of land plants, and both regulate rotating division planes of apical cells. As rotation of apical cell division plane was a critical land plant innovation that facilitated 3D growth (Zimmerman, 1952), the relative timing, and potential interdependence, of their evolution is a fascinating open question.
PpNOG2, which encodes a shikimate o-hydroxycinnamoyltransferase that functions in the ascorbic acid pathway, restricts gametophore initial cell formation but is required for apical growth of the emerging bud (Moody et al., 2021). Auxin homeostasis and CLV genes are misregulated in Ppnog2, which is proposed to act downstream of NOG1/DEK1/APBs. Inhibition of the Arabidopsis NOG2 ortholog HCT can perturb the ascorbic acid pathway, disrupting flavonoid biosynthesis and consequently limiting auxin transport (Besseau et al., 2007). Thus, the regulation of auxin homeostasis was likely an ancestral function for NOG2.
LATERAL ORGAN SUPRESSOR 1
Demarcation between self-renewing meristematic cells and cells differentiating into lateral organs is critical to apical growth. A number of genes are expressed at the boundary between lateral organs and the SAM in angiosperms, including members of the ALOG family (Hepworth and Pautot, 2015). In addition to regulating lateral organ development cell autonomously, angiosperm ALOG genes also regulate meristem development non-cell autonomously (Takeda et al., 2011; MacAlister et al., 2012; Yoshida et al., 2013). A recent study of the Marchantia ALOG gene LATERAL ORGAN SUPRESSOR 1 (LOS1) suggests both these functions are ancestral in land plants. Expression of MpLOS1 is restricted to lateral organs, however, Mplos1 mutants produce aberrant lateral organs and fail to maintain an apical meristem (Naramoto et al., 2019). Mobile signaling between meristematic and differentiating cells therefore appears to be a fundamental feature of apical growth.
Hormonal coordination of apical growth
Auxin
Many of the genetic modules outlined above are integrated into wider hormonal signaling pathways, not least the auxin network (e.g. Aoyama et al., 2012; Moody et al., 2021; Nemec Venza et al., 2022). Auxin regulates angiosperm development in a myriad of ways. Specifically in regard to shoot apical growth, auxin promotes the differentiation of lateral organs on the periphery of the SAM whereas stem cell activity requires a minimal level of auxin signaling (Ma et al., 2019). Moreover, basipetal transport of auxin from the primary SAM inhibits shoot branching, predominantly through repression of axillary meristem outgrowth (Prusinkiewicz et al., 2009). A role for auxin in meristem maintenance and polar auxin flow from the SAM are conserved across the tracheophytes (Sanders and Langdale, 2013).
Perturbation to auxin transport and signaling has shown that auxin also controls the balance between apical growth and differentiation in bryophytes. Furthermore, auxin signaling is necessary in both gametophytic and sporophytic stages of bryophyte life cycles (Ishizaki et al., 2012; Bennett et al., 2014b; Viaene et al., 2014; Flores-Sandoval et al., 2015; Kato et al., 2015). Charophyte algae have orthologs of many auxin signaling pathway components in their genomes and exhibit a capacity for long distance polar auxin transport (De Smet et al., 2011; Boot et al., 2012). The core molecular components of auxin transport, perception, signaling, and biosynthesis are all found in bryophyte genomes (Paponov et al., 2009; Eklund et al., 2010; Prigge et al., 2010; Bennett et al., 2014a; Flores-Sandoval et al., 2015; Kato et al., 2015; Lavy et al., 2016; Plavskin et al., 2016; Li et al., 2020; Zhang et al., 2020). Together, these results suggest that a basic auxin response module was present in the ancestral land plant and that this module regulated apical growth in the ancestral gametophyte and sporophyte.
Cytokinin
Cytokinin has long been known to promote shoot apical activity cell throughout the land plant lineage (Skoog and Miller, 1957; Ashton et al., 1979; Reski and Abel, 1985). In angiosperms, as referenced above, cytokinin signaling is a critical effector of the WUS and KNOX meristem maintenance pathways. Through conserved genetic networks, cytokinin induces gametophore apical initiation formation in Physcomitrium and regulates multiple aspects of Marchantia thallus development (Flores-Sandoval et al., 2016; von Schwartzenberg et al., 2016; Aki et al., 2019; Hyoung et al., 2020). Importantly, cytokinin also promotes meristematic proliferation in moss sporophytes (French and Paolillo, 1975b; Coudert et al., 2019), suggesting that a role for cytokinin in sporophyte development evolved in the ancestral land plant.
Concluding remarks
The results of the studies summarized above present a complex picture of how apical growth evolved in land plants (Table 1). Together, the findings support models of tracheophyte sporophyte co-option of gametophytic networks (e.g. CLV, HAM, APBs, and ALOG), independent recruitment of ancestral sporophyte networks (e.g. LFY) and the conservation of ancestral sporophyte function (e.g. Class I KNOX, Class II TCP).
Table 1.
Summary of functional studies into apical growth in bryophytes
| Gene family | Function in bryophytes | Function in vascular plant SAMs (sporophytes) | Putative ancestral function |
|---|---|---|---|
| CLV3/CLV1/RPK2 | To regulate cell proliferation, cell identity, and cell division planes in gametophyte (Physcomitrium, Marchantia) | To regulate cell proliferation, cell identity, and cell division planes (angiosperms) | To regulate cell proliferation, cell identity, and cell division planes |
| WOX | To promote cell division in the zygote (Physcomitrium) and gametophyte (Marchantia) | To promote cell proliferation (vascular plants) | To promote cell proliferation |
| HAM | To promote stem cell proliferation in the gametophyte (Physcomitrium) | To promote stem cell proliferation (angiosperms) | To promote stem cell proliferation |
| KNOX | Class I: to promote sporophyte meristematic growth (Physcomitrium) and the transition to the sporophyte stage (Marchantia) Class II: to repress the gametophyte stage (Physcomitrium) | Class I: to promote meristematic growth (vascular plants) Class II: to promote differentiation, in part through antagonizing Class I function (angiosperms) | Pre-duplication: to promote the diploid genetic program and life cycle progression Class I: to promote sporophyte meristematic growth Class II: ? |
| C3HDZ | To promote cell proliferation and regulate tissue patterning in gametophyte leaf development (Physcomitrium) | To promote meristem maintenance and pattern shoots and lateral organs (vascular plants) | Domain specification in lateral organs? |
| LFY | To regulate cell division in the embryo and sporophyte (Physcomitrium) | To promote shoot indeterminacy (Ceratopteris) To promote reproductive development (vascular plants) To maintain indeterminate cell fate in branches (angiosperms) | To regulate sporophytic cell division To promote sporangium formation? |
| Class II TCP | To repress sporophyte branching (Physcomitrium) | To repress sporophyte branching (angiosperms) | To repress sporophyte branching |
| APBs | To specify the gametophore apical initial (Physcomitrium) | To promote stem cell and young tissue proliferation (angiosperms) | To promote stem cell identity |
| DEK1 | To orient gametophore apical cell division (Physcomitrium) | To orient cell division planes during embryogenesis (angiosperms) | To orient cell division planes |
| NOG1 | To promote gametophore initial identity (Physcomitrium) | ? | ? |
| NOG2 | To regulate gametophore formation and auxin homeostasis (Physcomitrium) | To regulate flavonoid biosynthesis (angiosperms) | To regulate auxin homeostasis via flavonoid levels |
| ALOG | Non-cell autonomous regulation of meristem maintenance (Marchantia) | Non-cell autonomous regulation of meristem maintenance (angiosperms) | Non-cell autonomous regulation of meristem maintenance |
With functional genetic data from a highly limited subset of land plant lineages, our assignations of ancestral roles are somewhat speculative and depend on assumptions that may shift with further investigation. In particular, they assume a model of land plant evolution in which similar gene function in bryophytes and tracheophytes reflects a common evolutionary history. It is also possible that a superficially conserved function reflects parallel evolution of a core genetic network. Distinguishing between these evolutionary trajectories is challenging and requires character mapping in additional land plant lineages (see the “Outstanding Questions”). This problem is compounded by high rates of gene loss and morphological simplification within the bryophytes (Harris et al., 2020; Donoghue et al., 2021). The recent strong support for the reclassification of bryophytes as a monophyletic group, however, strengthens the inferences we can now make about ancestral function.
Further characterization of bryophyte development will also improve our understanding of ancestral genetic networks. Specifically, additional functional studies of sporophyte development will enable us to determine whether gene families were functional in the ancestral sporophyte, or have been co-opted from gametophytic apical networks. For example, current data suggest that the CLV and APB networks were co-opted from roles in gametophytic development to regulate apical growth in tracheophyte sporophytes (Aoyama et al., 2012; Whitewoods et al., 2018). More detailed analyses of sporophyte development in Ppclv and Ppapb mutants may reveal ancestral sporophytic roles. The relative intractability of model bryophyte sporophytes makes such experiments difficult. However, recent results demonstrate how important investigating sporophyte development is for elucidating how plants first grew on land. Thanks to a combination of developmental genetic (e.g. Tanahashi et al., 2005; Bennett et al., 2014b; Sakakibara et al., 2014; Ortiz-Ramírez et al., 2016; Coudert et al., 2019) and phylogenetic (e.g. Puttick et al., 2018) studies we now understand the ancestral sporophyte to be far more complex than first envisioned (Figure 2). With a wider diversity of land plant lineages being adopted as model systems (e.g. Plackett et al., 2015; Uddenberg et al., 2015; Conway and Di Stilio, 2020; Di Stilio and Ickert-Bond, 2021; Frangedakis et al., 2021; Spencer et al., 2021), and new fossil discoveries continuing to calibrate morphological trajectories (Edwards et al., 2022a, 2022b), further complexity is likely to be uncovered.
Figure 2.
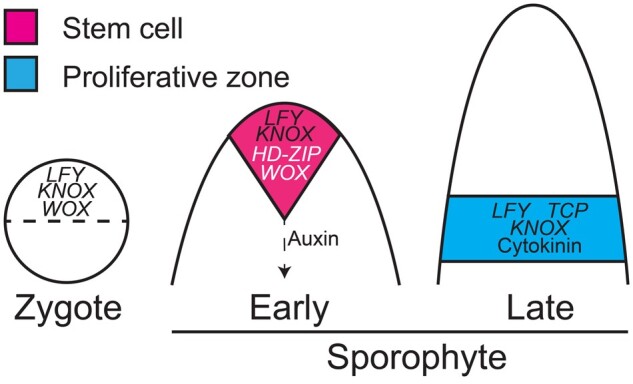
Putative apical regulators in the ancestral sporophyte. Hypothetical ancestral sporophyte based on extant Physcomitrium morphology. Putative roles inferred from bryophyte gene function that are broadly conserved in vascular plants. In zygotes, WOX and LFY genes are required for the first cell division and KNOX genes promote establishment of the sporophyte (Tanahashi et al., 2005; Sakakibara et al., 2013, 2014; Dierschke et al., 2021). In sporophytes, PpLFY genes are expressed throughout development and Pplfy mutants have disrupted cell division planes in apical and basal regions (Tanahashi et al., 2005); Class I KNOX genes regulate cell division in the apical initial and intercalary meristem, their role in the intercalary meristem is dependent on cytokinin signaling (Sakakibara et al., 2008; Coudert et al., 2019); putative roles for HD-ZIP III and WOX genes in apical initial proliferation are based on gene expression patterns rather than functional data (indicated by white lettering) (Sakakibara et al., 2014; Yip et al., 2016); maintenance of an auxin minimum in the apical initial by basipetal auxin transport promotes stem cell identity (Fujita et al., 2008; Bennett et al., 2014b; Thelander et al., 2019; Nemec Venza et al., 2022); Class II TCP genes repress branching in the proliferative zone (Ortiz-Ramírez et al., 2016).
ADVANCES.
- Improved phylogenetic modeling has reclassified the bryophytes and tracheophytes as sister lineages; this updated phylogeny has led to a reassessment of the nature of the ancestral land plant.
- Recent reverse genetic studies into bryophyte models have provided multiple insights into the evolution of core developmental networks in plants.
- The ancestral land plant had a sporophyte that was morphologically far more complex than previously predicted.
- Indeterminate growth in vascular plants was therefore likely more preconditioned by genetic networks in the ancestral sporophyte, and less dependent on gametophytic co-option, than previously thought.
OUTSTANDING QUESTIONS.
- Do genes that regulate cell division in bryophyte zygotes (i.e. WOX, LFY) also regulate later stages of sporophyte development?
- Does the CLV pathway regulate bryophyte sporophyte development?
- How and when were WOX genes co-opted into the CLV signaling network?
- What is the function of Class II KNOX genes in bryophytes?
- How labile is the repression of branching in bryophyte sporophytes?
- How is proliferative growth regulated in the liverwort sporophyte?
- How conserved are core developmental genetic networks in hornworts?
- Are angiosperm SAM stem cells and proliferative zones homologous to the moss sporophyte apical cell and intercalary meristem, respectively?
Acknowledgments
We apologize to authors whose work we were unable to discuss due to space constraints.
Funding
J.P.F. is funded under the European Union Horizon 2020 Programme by a Marie Sklodowska-Curie Individual Fellowship (Grant Agreement 101026955). Work in C.J.H.’s lab is funded by the Leverhulme Trust (RPG-2018-220), Bristol Centre for Agricultural Innovation and the BBSRC.
Conflict of interest statement. None declared.
Contributor Information
Jim P Fouracre, School of Biological Sciences, University of Bristol, Bristol BS8 1TQ, UK.
C Jill Harrison, School of Biological Sciences, University of Bristol, Bristol BS8 1TQ, UK.
J.P.F.: writing—original draft preparation. C.J.H.: writing—reviewing and editing.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Jim Fouracre (jim.fouracre@bristol.ac.uk).
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aki SS, Mikami T, Naramoto S, Nishihama R, Ishizaki K, Kojima M, Takebayashi Y, Sakakibara H, Kyozuka J, Kohchi T, et al. (2019) Cytokinin signaling is essential for organ formation in Marchantia polymorpha. Plant Cell Physiol 60: 1842–1854 [DOI] [PubMed] [Google Scholar]
- Albert VA (1999) Shoot apical meristems and floral patterning: an evolutionary perspective. Trends Plant Sci 4: 84–86 [Google Scholar]
- Ambrose BA, Vasco A (2016) Bringing the multicellular fern meristem into focus. New Phytol 210: 790–793 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Hiwatashi Y, Shigyo M, Kofuji R, Kubo M, Ito M, Hasebe M (2012) AP2-type transcription factors determine stem cell identity in the moss Physcomitrella patens. Development 139: 3120–3129 [DOI] [PubMed] [Google Scholar]
- Ashton NW, Grimsley NH, Cove DJ (1979) Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 144: 427–435 [DOI] [PubMed] [Google Scholar]
- Banks JA (1999) Gametophyte development in ferns. Annu Rev Plant Physiol Plant Mol Biol 50: 163–186 [DOI] [PubMed] [Google Scholar]
- Barton MK (2010) Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Dev Biol 341: 95–113 [DOI] [PubMed] [Google Scholar]
- Beheshti H, Strotbek C, Arif MA, Klingl A, Top O, Frank W (2021) PpGRAS12 acts as a positive regulator of meristem formation in Physcomitrium patens. Plant Mol Biol 107: 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Brockington SF, Rothfels C, Graham SW, Stevenson D, Kutchan T, Rolf M, Thomas P, Wong GK-S, Leyser O, et al. (2014a) Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol Biol Evol 31: 2042–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett TA, Liu MM, Aoyama T, Bierfreund NM, Braun M, Coudert Y, Dennis RJ, O’Connor D, Wang XY, White CD, et al. (2014b) Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr Biol 24: 2776–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot KJM, Libbenga KR, Hille SC, Offringa R, van Duijn B (2012) Polar auxin transport: an early invention. J Exp Bot 63: 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu C-M, van Lammeren AAM, Miki BLA, et al. (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Sakakibara K, Furumizu C, Dierschke T (2016) Evolution in the cycles of life. Annu Rev Genet 50: 133–154 [DOI] [PubMed] [Google Scholar]
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R (2000) Dependence of stem cell fate in Arabidopsis an a feedback loop regulated by CLV3 activity. Science 289: 617–619 [DOI] [PubMed] [Google Scholar]
- Cammarata J, Morales Farfan C, Scanlon MJ, Roeder AHK (2022) Cytokinin–CLAVATA cross-talk is an ancient mechanism regulating shoot meristem homeostasis in land plants. Proc Natl Acad Sci USA 119: e2116860119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575–585 [DOI] [PubMed] [Google Scholar]
- Conway SJ, Di Stilio VS (2020) An ontogenetic framework for functional studies in the model fern Ceratopteris richardii. Dev Biol 457: 20–29 [DOI] [PubMed] [Google Scholar]
- Coudert Y, Novák O, Harrison CJ (2019) A KNOX-cytokinin regulatory module predates the origin of indeterminate vascular plants. Curr Biol 29: 2743–2750.e5 [DOI] [PubMed] [Google Scholar]
- Cove D (2005) The moss Physcomitrella patens. Annu Rev Genet 39: 339–358 [DOI] [PubMed] [Google Scholar]
- Cove DJ, Knight CD (1993) The moss Physcomitrella patens, a model system with potential for the study of plant reproduction. Plant Cell 5: 1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Voß U, Lau S, Wilson M, Shao N, Timme RE, Swarup R, Kerr I, Hodgman C, Bock R, et al. (2011) Unraveling the evolution of auxin signaling. Plant Physiol 155: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche CF, Cooper ED (2015) The evolutionary origin of a terrestrial flora. Curr Biol 25: R899–R910 [DOI] [PubMed] [Google Scholar]
- Demko V, Belova T, Messerer M, Hvidsten TR, Perroud P-F, Ako AE, Johansen W, Mayer KFX, Olsen O-A, Lang D (2021) Calpain DEK1 acts as a developmental switch gatekeeping cell fate transitions. bioRxiv 2021.08.25.457637 [DOI] [PMC free article] [PubMed]
- Demko V, Perroud P-F, Johansen W, Delwiche CF, Cooper ED, Remme P, Ako AE, Kugler KG, Mayer KFX, Quatrano R, et al. (2014) Genetic analysis of DEFECTIVE KERNEL1 loop function in three-dimensional body patterning in Physcomitrella patens. Plant Physiol 166: 903–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stilio VS, Ickert-Bond SM (2021) Ephedra as a gymnosperm evo-devo model lineage. Evol Dev 23: 256–266 [DOI] [PubMed] [Google Scholar]
- Dierschke T, Flores-Sandoval E, Rast-Somssich MI, Althoff F, Zachgo S, Bowman JL (2021) Gamete expression of TALE class HD genes activates the diploid sporophyte program in Marchantia polymorpha. eLife 10: e57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J, Stec A, Hubbard L (1997) The evolution of apical dominance in maize. Nature 386: 485–488 [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Harrison CJ, Paps J, Schneider H (2021) The evolutionary emergence of land plants. Curr Biol 31: R1281–R1298 [DOI] [PubMed] [Google Scholar]
- Edwards D, Morris JL, Axe L, Duckett JG, Pressel S, Kenrick P (2022a) Piecing together the eophytes—a new group of ancient plants containing cryptospores. New Phytol 233: 1440–1455 [DOI] [PubMed] [Google Scholar]
- Edwards D, Morris JL, Axe L, Taylor WA, Duckett JG, Kenrick P, Pressel S (2022b) Earliest record of transfer cells in Lower Devonian plants. New Phytol 233: 1456–1465 [DOI] [PubMed] [Google Scholar]
- Eklund DM, Thelander M, Landberg K, Ståldal V, Nilsson A, Johansson M, Valsecchi I, Pederson ERA, Kowalczyk M, Ljung K, et al. (2010) Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens. Development 137: 1275–1284 [DOI] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an _APETALA2-_like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8: 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL (2003) Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol 13: 1768–1774 [DOI] [PubMed] [Google Scholar]
- Engstrom EM, Andersen CM, Gumulak-Smith J, Hu J, Orlova E, Sozzani R, Bowman JL (2011) Arabidopsis homologs of the petunia HAIRY MERISTEM gene are required for maintenance of shoot and root indeterminacy. Plant Physiol 155: 735–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC (2020) Recent advances in Arabidopsis CLE peptide signaling. Trends Plant Sci 25: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Fletcher LC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Flores-Sandoval E, Dierschke T, Fisher TJ, Bowman JL (2016) Efficient and inducible use of artificial microRNAs in Marchantia polymorpha. Plant Cell Physiol 57: 281–290 [DOI] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Bowman JL (2015) A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genetics 11: e1005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd SK, Bowman JL (2007) The ancestral developmental tool kit of land plants. Int J Plant Sci 168: 1–35 [Google Scholar]
- Floyd SK, Zalewski CS, Bowman JL (2006) Evolution of Class III homeodomain–leucine zipper genes in streptophytes. Genetics 173: 373–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangedakis E, Saint-Marcoux D, Moody LA, Rabbinowitsch E, Langdale JA (2017) Nonreciprocal complementation of KNOX gene function in land plants. New Phytol 216: 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangedakis E, Shimamura M, Villarreal JC, Li F-W, Tomaselli M, Waller M, Sakakibara K, Renzaglia KS, Szövényi P (2021) The hornworts: morphology, evolution and development. New Phytol 229: 735–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MH, Edwards MB, Schultz ER, McKain MR, Fei Z, Sørensen I, Rose JKC, Scanlon MJ (2015) Dissecting the molecular signatures of apical cell-type shoot meristems from two ancient land plant lineages. New Phytol 207: 893–904 [DOI] [PubMed] [Google Scholar]
- Frank MH, Scanlon MJ (2015a) Transcriptomic evidence for the evolution of shoot meristem function in sporophyte-dominant land plants through concerted selection of ancestral gametophytic and sporophytic genetic programs. Mol Biol Evol 32: 355–67 [DOI] [PubMed] [Google Scholar]
- Frank MH, Scanlon MJ (2015b) Cell-specific transcriptomic analyses of three-dimensional shoot development in the moss Physcomitrella patens. Plant J 83: 743–751 [DOI] [PubMed] [Google Scholar]
- French JC, Paolillo DJ (1975a) Intercalary meristematic activity in the sporophyte of Funaria (Musci). Am J Bot 62: 86–96 [DOI] [PubMed] [Google Scholar]
- French JC, Paolillo DJ (1975b) Effect of exogenously supplied growth regulators on intercalary meristematic activity and capsule expansion in Funaria. Bryol 78: 431–437 [Google Scholar]
- Fujita T, Sakaguchi H, Hiwatashi Y, Wagstaff SJ, Ito M, Deguchi H, Sato T, Hasebe M (2008) Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evol Dev 10: 176–186 [DOI] [PubMed] [Google Scholar]
- Furumizu C, Alvarez JP, Sakakibara K, Bowman JL (2015) Antagonistic roles for KNOX1 and KNOX2 genes in patterning the land plant body plan following an ancient gene duplication. PLoS Genet 11: e1004980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumizu C, Krabberød AK, Hammerstad M, Alling RM, Wildhagen M, Sawa S, Aalen RB (2021) The sequenced genomes of nonflowering land plants reveal the innovative evolutionary history of peptide signaling. Plant Cell 33: 2915–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Geng Y, Guo L, Han H, Liu X, Banks JA, Wisecaver JH, Zhou Y (2021) Conservation and diversification of HAIRY MERISTEM gene family in land plants. Plant J 106: 366–378 [DOI] [PubMed] [Google Scholar]
- Gifford EM, Corson GE (1971) The shoot apex in seed plants. Bot Rev 37: 143–229 [Google Scholar]
- Harris BJ, Harrison CJ, Hetherington AM, Williams TA (2020) Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Curr Biol 30: 2001–2012.e2 [DOI] [PubMed] [Google Scholar]
- Harrison CJ (2017) Development and genetics in the evolution of land plant body plans. Phil Trans R Soc B Biol Sci 372: 20150490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Corley SB, Moylan EC, Alexander DL, Scotland RW, Langdale JA (2005) Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature 434: 509–514 [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Morris JL (2017) The origin and early evolution of vascular plant shoots and leaves. Phil Trans R Soc B Biol Sci 373: 20160496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Rezvani M, Langdale JA (2007) Growth from two transient apical initials in the meristem of Selaginella kraussiana. Development 134: 881. [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Roeder AHK, Meyerowitz EM, Langdale JA (2009) Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens. Curr Biol 19: 461–471 [DOI] [PubMed] [Google Scholar]
- Hay A, Tsiantis M (2010) KNOX genes: versatile regulators of plant development and diversity. Development 137: 3153–3165 [DOI] [PubMed] [Google Scholar]
- Hepworth SR, Pautot VA (2015) Beyond the divide: boundaries for patterning and stem cell regulation in plants. Front Plant Sci 6: 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Fujimoto T, Ishida S, Uchida N, Sawa S, Kiyosue T, Ishizaki K, Nishihama R, Kohchi T, Bowman JL (2020) Induction of multichotomous branching by CLAVATA peptide in Marchantia polymorpha. Curr Biol 30: 3833–3840.e4 [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Uchida N, Yamaguchi YL, Tabata R, Ishida S, Ishizaki K, Nishihama R, Kohchi T, Sawa S, Bowman JL (2019) Control of proliferation in the haploid meristem by CLE peptide signaling in Marchantia polymorpha. PLoS Genet 15: e1007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga T, Fujimoto S, Cui Y, Sato K, Sano R, Yamaoka S, Kohchi T, Berger F, Nakajima K (2021) Deep evolutionary origin of gamete-directed zygote activation by KNOX/BELL transcription factors in green plants. eLife 10: e57090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NA, Katz A, Pereman I, Decker EL, Ohad N, Reski R (2016) A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nat Plants 2: 15209. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhu Y, Cui Y, Cheng K, Liang W, Wei Z, Zhu M, Yin H, Zeng L, Xiao Y, et al. (2018) A group of receptor kinases are essential for CLAVATA signalling to maintain stem cell homeostasis. Nat Plants 4: 205–211 [DOI] [PubMed] [Google Scholar]
- Hyoung S, Cho SH, Chung JH, So WM, Cui MH, Shin JS (2020) Cytokinin oxidase PpCKX1 plays regulatory roles in development and enhances dehydration and salt tolerance in Physcomitrella patens. Plant Cell Rep 39: 419–430 [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Ito T, Tanaka H, Favero DS, Kawamura A, Sakamoto S, Wakazaki M, Tameshige T, Fujii H, et al. (2022) Wound-inducible WUSCHEL-RELATED HOMEOBOX 13 is required for callus growth and organ reconnection. Plant Physiol 188: 425–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Nonomura M, Kato H, Yamato KT, Kohchi T (2012) Visualization of auxin-mediated transcriptional activation using a common auxin-responsive reporter system in the liverwort Marchantia polymorpha. J Plant Res 125: 643–651 [DOI] [PubMed] [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15: 1560–5 [DOI] [PubMed] [Google Scholar]
- Johnson KL, Degnan KA, Ross Walker J, Ingram GC (2005) AtDEK1 is essential for specification of embryonic epidermal cell fate. Plant J 44: 114–127 [DOI] [PubMed] [Google Scholar]
- Jones DS, John A, VanDerMolen KR, Nimchuk ZL (2021) CLAVATA signaling ensures reproductive development in plants across thermal environments. Curr Biol 31: 220–227.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karol KG, Arumuganathan K, Boore JL, Duffy AM, Everett KD, Hall JD, Hansen SK, Kuehl JV, Mandoli DF, Mishler BD, et al. (2010) Complete plastome sequences of Equisetum arvense and Isoetes flaccida: implications for phylogeny and plastid genome evolution of early land plant lineages. BMC Evol Biol 10: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ishizaki K, Kouno M, Shirakawa M, Bowman JL, Nishihama R, Kohchi T (2015) Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet 11: e1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes JM, Clark SE (1998) CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125: 3843–3851 [DOI] [PubMed] [Google Scholar]
- Kienitz-Gerloff F (1874) Vergleichende untersuchungen über die entwickelungsgeschichte des lebermoos-sporogoniums. Bot Z 32: 161–172 [Google Scholar]
- Kinoshita A, Betsuyaku S, Osakabe Y, Mizuno S, Nagawa S, Stahl Y, Simon R, Yamaguchi-Shinozaki K, Fukuda H, Sawa S (2010) RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 137: 3911–3920 [DOI] [PubMed] [Google Scholar]
- Kirbis A, Waller M, Ricca M, Bont Z, Neubauer A, Goffinet B, Szövényi P (2020) Transcriptional landscapes of divergent sporophyte development in two mosses, Physcomitrium (Physcomitrella) patens and Funaria hygrometrica. Front Plant Sci 11: 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji R, Hasebe M (2014) Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr Opin Plant Biol 17: 13–21 [DOI] [PubMed] [Google Scholar]
- Koyama T, Furutani M, Tasaka M, Ohme-Takagi M (2007) TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell 19: 473–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landberg K, Pederson ERA, Viaene T, Bozorg B, Friml J, Jönsson H, Thelander M, Sundberg E (2013) The moss Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiol 162: 1406–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M (2016) Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Lin H, Joo S, Goodenough U (2008) Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell 133: 829–840 [DOI] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–5 [DOI] [PubMed] [Google Scholar]
- Lemieux C, Otis C, Turmel M (2016) Comparative chloroplast genome analyses of streptophyte green algae uncover major structural alterations in the Klebsormidiophyceae, Coleochaetophyceae and Zygnematophyceae. Front Plant Sci 7: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-W, Nishiyama T, Waller M, Frangedakis E, Keller J, Li Z, Fernandez-Pozo N, Barker MS, Bennett T, Blázquez MA, et al. (2020) Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat Plants 6: 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Demko V, Wilson RC, Johnson KA, Ahmad R, Perroud P-F, Quatrano R, Zhao S, Shalchian-Tabrizi K, Otegui MS, et al. (2013) The catalytic domain CysPc of the DEK1 calpain is functionally conserved in land plants. Plant J 75: 742–754 [DOI] [PubMed] [Google Scholar]
- Lid SE, Olsen L, Nestestog R, Aukerman M, Brown RC, Lemmon B, Mucha M, Opsahl-Sorteberg H-G, Olsen O-A (2005) Mutation in the Arabidopisis thaliana DEK1 calpain gene perturbs endosperm and embryo development while over-expression affects organ development globally. Planta 221: 339–351 [DOI] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69 [DOI] [PubMed] [Google Scholar]
- Ma Y, Miotk A, Šutiković Z, Ermakova O, Wenzl C, Medzihradszky A, Gaillochet C, Forner J, Utan G, Brackmann K, et al. (2019) WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat Commun 10: 5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister CA, Park SJ, Jiang K, Marcel F, Bendahmane A, Izkovich Y, Eshed Y, Lippman ZB (2012) Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat Genet 44: 1393–1398 [DOI] [PubMed] [Google Scholar]
- Maizel A, Busch MA, Tanahashi T, Perkovic J, Kato M, Hasebe M, Weigel D (2005) The floral regulator LEAFY evolves by substitutions in the DNA binding domain. Science 308: 260–263 [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411: 709–713 [DOI] [PubMed] [Google Scholar]
- Menand B, Calder G, Dolan L (2007) Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J Exp Bot 58: 1843–1849 [DOI] [PubMed] [Google Scholar]
- Mishler BD, Churchill SP (1984) A cladistic approach to the phylogeny of the “Bryophytes.” Brittonia 36: 406–424 [Google Scholar]
- Mishler BD, Churchill SP (1985) Transition to a land flora: phylogenetic relationships of the green algae to bryophytes. Cladistics 1: 305–328 [DOI] [PubMed] [Google Scholar]
- Moody LA (2020) Three-dimensional growth: a developmental innovation that facilitated plant terrestrialization. J Plant Res 133: 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody LA, Kelly S, Clayton R, Weeks Z, Emms DM, Langdale JA (2021) NO GAMETOPHORES 2 is a novel regulator of the 2D to 3D growth transition in the moss Physcomitrella patens. Curr Biol 31: 555–563.e4 [DOI] [PubMed] [Google Scholar]
- Moody LA, Kelly S, Coudert Y, Nimchuk ZL, Harrison CJ, Langdale JA (2018a) Somatic hybridization provides segregating populations for the identification of causative mutations in sterile mutants of the moss Physcomitrella patens. New Phytol 218: 1270–1277 [DOI] [PubMed] [Google Scholar]
- Moody LA, Kelly S, Rabbinowitsch E, Langdale JA (2018b) Genetic regulation of the 2D to 3D growth transition in the moss Physcomitrella patens. Curr Biol 28: 473–478.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ (2018) The timescale of early land plant evolution. Proc Natl Acad Sci USA 115: E2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Bleckmann A, Simon R (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20: 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto S, Jones VAS, Trozzi N, Sato M, Toyooka K, Shimamura M, Ishida S, Nishitani K, Ishizaki K, Nishihama R, et al. (2019) A conserved regulatory mechanism mediates the convergent evolution of plant shoot lateral organs. PLoS Biol 17: e3000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J, Werr W (2012) The invention of _WUS_-like stem cell-promoting functions in plants predates leptosporangiate ferns. Plant Mol Biol 78: 123–134 [DOI] [PubMed] [Google Scholar]
- Nemec Venza Z, Madden C, Stewart A, Liu W, Novák O, Pěnčík A, Cuming AC, Kamisugi Y, Harrison CJ (2022) CLAVATA modulates auxin homeostasis and transport to regulate stem cell identity and plant shape in a moss. New Phytol 234: 149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ, Kutschera U (2010) The evolution of the land plant life cycle. New Phytol 185: 27–41 [DOI] [PubMed] [Google Scholar]
- Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA, Meyerowitz EM (2015) Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 142: 1043–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihama R, Ishizaki K, Hosaka M, Matsuda Y, Kubota A, Kohchi T (2015) Phytochrome-mediated regulation of cell division and growth during regeneration and sporeling development in the liverwort Marchantia polymorpha. J Plant Res 128: 407–421 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K, et al. (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100: 8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ramírez C, Hernandez-Coronado M, Thamm A, Catarino B, Wang M, Dolan L, Feijó JA, Becker JD (2016) A transcriptome atlas of Physcomitrella patens provides insights into the evolution and development of land plants. Mol Plant 9: 205–220 [DOI] [PubMed] [Google Scholar]
- Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, Palme K (2009) The evolution of nuclear auxin signalling. BMC Evol Biol 9: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud P-F, Demko V, Johansen W, Wilson RC, Olsen O-A, Quatrano RS (2014) Defective Kernel 1 (DEK1) is required for three-dimensional growth in Physcomitrella patens. New Phytol 203: 794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud P-F, Meyberg R, Demko V, Quatrano RS, Olsen O-A, Rensing SA (2020) DEK1 displays a strong subcellular polarity during Physcomitrella patens 3D growth. New Phytol 226: 1029–1041 [DOI] [PubMed] [Google Scholar]
- Philipson WR (1990) The significance of apical meristems in the phylogeny of land plants. Plant Syst Evol 173: 17–38 [Google Scholar]
- Pickett-Heaps J (1967) Ultrastructure and differentiation in Chara sp. I. Vegetative cells. Aust J Biol Sci 20: 539–552 [Google Scholar]
- Plackett AR, Conway SJ, Hewett Hazelton KD, Rabbinowitsch EH, Langdale JA, Di Stilio VS (2018) LEAFY maintains apical stem cell activity during shoot development in the fern Ceratopteris richardii. eLife 7: e39625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett ARG, Di Stilio VS, Langdale JA (2015) Ferns: the missing link in shoot evolution and development. Front Plant Sci 6: 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavskin Y, Nagashima A, Perroud P-F, Hasebe M, Quatrano RS, Atwal GS, Timmermans MCP (2016) Ancient trans-acting siRNAs confer robustness and sensitivity onto the auxin response. Dev Cell 36: 276–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Clark SE (2006) Evolution of the class III HD-zip gene family in land plants. Evol Dev 8: 350–361 [DOI] [PubMed] [Google Scholar]
- Prigge MJ, Lavy M, Ashton NW, Estelle M (2010) Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr Biol 20: 1907–1912 [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttick MN, Morris JL, Williams TA, Cox CJ, Edwards D, Kenrick P, Pressel S, Wellman CH, Schneider H, Pisani D, et al. (2018) The interrelationships of land plants and the nature of the ancestral embryophyte. Curr Biol 28: 733–745.e2 [DOI] [PubMed] [Google Scholar]
- Renzaglia KS, Villarreal JC, Duff RJ (2008) New insights into morphology, anatomy, and systematics of hornworts. _In_Shaw AJ, Goffinet B, eds, Bryophyte Biology, Ed 2. Cambridge University Press, Cambridge, pp 139–172 [Google Scholar]
- Reski R, Abel WO (1985) Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165: 354–358 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pelayo C, Ambrose BA, Vasco A, Alzate JF, Pabón-Mora N (2022) Evolution and expression of LEAFY genes in ferns and lycophytes. EvoDevo 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara K, Ando S, Yip HK, Tamada Y, Hiwatashi Y, Murata T, Deguchi H, Hasebe M, Bowman JL (2013) KNOX2 genes regulate the haploid-to-diploid morphological transition in land plants. Science 339: 1067–1070 [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Nishiyama T, Deguchi H, Hasebe M (2008) Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella patens but do function in sporophyte development. Evol Dev 10: 555–566 [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Reisewitz P, Aoyama T, Friedrich T, Ando S, Sato Y, Tamada Y, Nishiyama T, Hiwatashi Y, Kurata T, et al. (2014) _WOX13_-like genes are required for reprogramming of leaf and protoplast cells into stem cells in the moss Physcomitrella patens. Development 141: 1660–1670 [DOI] [PubMed] [Google Scholar]
- Sanders HL, Darrah PR, Langdale JA (2011) Sector analysis and predictive modelling reveal iterative shoot-like development in fern fronds. Development 138: 2925–2934 [DOI] [PubMed] [Google Scholar]
- Sanders HL, Langdale JA (2013) Conserved transport mechanisms but distinct auxin responses govern shoot patterning in Selaginella kraussiana. New Phytol 198: 419–428 [DOI] [PubMed] [Google Scholar]
- Sano R, Juárez CM, Hass B, Sakakibara K, Ito M, Banks JA, Hasebe M (2005) KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evol Dev 7: 69–78 [DOI] [PubMed] [Google Scholar]
- Sayou C, Monniaux M, Nanao MH, Moyroud E, Brockington SF, Thévenon E, Chahtane H, Warthmann N, Melkonian M, Zhang Y, et al. (2014) A promiscuous intermediate underlies the evolution of LEAFY DNA binding specificity. Science 343: 645–648 [DOI] [PubMed] [Google Scholar]
- Schlegel J, Denay G, Wink R, Pinto KG, Stahl Y, Schmid J, Blümke P, Simon RG (2021) Control of Arabidopsis shoot stem cell homeostasis by two antagonistic CLE peptide signalling pathways. eLife 10: e70934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644 [DOI] [PubMed] [Google Scholar]
- von Schwartzenberg K, Lindner A-C, Gruhn N, Šimura J, Novák O, Strnad M, Gonneau M, Nogué F, Heyl A (2016) CHASE domain-containing receptors play an essential role in the cytokinin response of the moss Physcomitrella patens. J Exp Bot 67: 667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M (2016) Marchantia polymorpha: taxonomy, phylogeny and morphology of a model system. Plant Cell Physiol 57: 230–256 [DOI] [PubMed] [Google Scholar]
- Singer SD, Ashton NW (2007) Revelation of ancestral roles of KNOX genes by a functional analysis of Physcomitrella homologues. Plant Cell Rep 26: 2039–2054 [DOI] [PubMed] [Google Scholar]
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11: 118–130 [PubMed] [Google Scholar]
- Solly JE, Cunniffe NJ, Harrison CJ (2017) Regional growth rate differences specified by apical notch activities regulate liverwort thallus shape. Curr Biol 27: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa F, Civáň P, Foster PG, Cox CJ (2020) The chloroplast land plant phylogeny: analyses employing better-fitting tree- and site-heterogeneous composition models. Front Plant Sci 11: 1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa F, Foster PG, Donoghue PCJ, Schneider H, Cox CJ (2019) Nuclear protein phylogenies support the monophyly of the three bryophyte groups (Bryophyta Schimp.). New Phytol 222: 565–575 [DOI] [PubMed] [Google Scholar]
- Spencer V, Nemec Venza Z, Harrison CJ (2021) What can lycophytes teach us about plant evolution and development? Modern perspectives on an ancient lineage. Evol Dev 23: 174–196 [DOI] [PubMed] [Google Scholar]
- Steemans P (2000) Miospore evolution from the Ordovician to the Silurian. Rev Palaeobot Palynol 113: 189–196 [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM (1989) Patterns in Plant Development, Ed 2. Cambridge University Press, Cambridge [Google Scholar]
- Takahashi G, Betsuyaku S, Okuzumi N, Kiyosue T, Hirakawa Y (2021) An evolutionarily conserved coreceptor gene is essential for CLAVATA signaling in Marchantia polymorpha. Front Plant Sci 12: 657548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Hanano K, Kariya A, Shimizu S, Zhao L, Matsui M, Tasaka M, Aida M (2011) CUP-SHAPED COTYLEDON1 transcription factor activates the expression of LSH4 and LSH3, two members of the ALOG gene family, in shoot organ boundary cells. Plant J 66: 1066–1077 [DOI] [PubMed] [Google Scholar]
- Tanahashi T, Sumikawa N, Kato M, Hasebe M (2005) Diversification of gene function: homologs of the floral regulator FLO/LFY control the first zygotic cell division in the moss Physcomitrella patens. Development 132: 1727–1736 [DOI] [PubMed] [Google Scholar]
- Tang H, Duijts K, Bezanilla M, Scheres B, Vermeer JEM, Willemsen V (2020) Geometric cues forecast the switch from two- to three-dimensional growth in Physcomitrella patens. New Phytol 225: 1945–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander M, Landberg K, Sundberg E (2019) Minimal auxin sensing levels in vegetative moss stem cells revealed by a ratiometric reporter. New Phytol 224: 775–788 [DOI] [PubMed] [Google Scholar]
- Turmel M, Otis C, Lemieux C (2006) The chloroplast genome sequence of Chara vulgaris sheds new light into the closest green algal relatives of land plants. Mol Biol Evol 23: 1324–1338 [DOI] [PubMed] [Google Scholar]
- Turmel M, Otis C, Lemieux C (2013) Tracing the evolution of streptophyte algae and their mitochondrial genome. Genome Biol Evol 5: 1817–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddenberg D, Akhter S, Ramachandran P, Sundström JF, Carlsbecker A (2015) Sequenced genomes and rapidly emerging technologies pave the way for conifer evolutionary developmental biology. Front Plant Sci 6: 00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene T, Landberg K, Thelander M, Medvecka E, Pederson E, Feraru E, Cooper ED, Karimi M, Delwiche CF, Ljung K, et al. (2014) Directional auxin transport mechanisms in early diverging land plants. Curr Biol 24: 2786–2791 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350: 241–243 [DOI] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859 [DOI] [PubMed] [Google Scholar]
- White RA, Turner MD (1995) Anatomy and development of the fern sporophyte. Bot Rev 61: 281–305 [Google Scholar]
- Whitewoods CD, Cammarata J, Nemec Venza Z, Sang S, Crook AD, Aoyama T, Wang XY, Waller M, Kamisugi Y, Cuming AC, et al. (2018) CLAVATA was a genetic novelty for the morphological innovation of 3D growth in land plants. Curr Biol 28: 2365–2376.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter E, Matasci N, Ayyampalayam S, Barker MS, Burleigh JG, Gitzendanner MA, et al. (2014) Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc Natl Acad Sci USA 111: E4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-C, Li F-W, Kramer EM (2019) Large-scale phylogenomic analysis suggests three ancient superclades of the WUSCHEL-RELATED HOMEOBOX transcription factor family in plants. PLoS ONE 14: e0223521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15: 1566–1571 [DOI] [PubMed] [Google Scholar]
- Yip HK, Floyd SK, Sakakibara K, Bowman JL (2016) Class III HD-zip activity coordinates leaf development in Physcomitrella patens. Dev Biol 419: 184–197 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Sasao M, Yasuno N, Takagi K, Daimon Y, Chen R, Yamazaki R, Tokunaga H, Kitaguchi Y, Sato Y, et al. (2013) TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc Natl Acad Sci USA 110: 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom CE, Geadelmann LF, Irish EE, Cheng C-L (2019) A fern WUSCHEL-RELATED HOMEOBOX gene functions in both gametophyte and sporophyte generations. BMC Plant Biol 19: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom CE, Withers KA, Irish EE, Cheng C-L (2022) Vascular function of the T3/modern clade WUSCHEL-Related HOMEOBOX transcription factor genes predate apical meristem-maintenance function. BMC Plant Biol 22: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fu X-X, Li R-Q, Zhao X, Liu Y, Li M-H, Zwaenepoel A, Ma H, Goffinet B, Guan Y-L, et al. (2020) The hornwort genome and early land plant evolution. Nat Plants 6: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu X, Engstrom EM, Nimchuk ZL, Pruneda-Paz JL, Tarr PT, Yan A, Kay SA, Meyerowitz EM (2015) Control of plant stem cell function by conserved interacting transcriptional regulators. Nature 517: 377–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman W (1952) Main results of the “Telome Theory.” Paleobotanist 1: 456–470 [Google Scholar]