Combined Risk Assessment of Food-derived Coumarin with in Silico Approaches (original) (raw)
Abstract
Hepatotoxicity associated with food-derived coumarin occurs occasionally in humans. We have, herein, assessed the data of existing clinical and nonclinical studies as well as those of in silico models for humans in order to shed more light on this association. The average intakes of food-derived coumarin are estimated to be 1−3 mg/day, while a ten-times higher level is expected in the worst-case scenarios. These levels are close to or above the tolerable daily intake suggested by a chronic study in dogs. The human internal exposure levels were estimated by a physiologically-based pharmacokinetic model with the use of virtual doses of coumarin in the amounts expected to derive from foods. Our results suggest that: (i) coumarin can be cleared rapidly _via_7-hydroxylation in humans, and (ii) the plasma levels of coumarin and of its metabolite,_o_-hydroxyphenylacetic acid associated with hepatotoxicity, are considerably lower than those yielding hepatotoxicity in rats. Pharmacokinetic data suggest a low or negligible concern regarding a coumarin-induced hepatotoxicity in humans exposed to an average intake from foods. Detoxification of coumarin through the 7-hydroxylation, however, might vary among individuals due to genetic polymorphisms in CYP2A6 enzyme. In addition, the CYP1A2- and CYP2E1-mediated activation of coumarin can fluctuate as a result of induction caused by environmental factors. Furthermore, the daily consumption of food-contained coumarin was implicated in the potential risk of hepatotoxicity by the drug-induced liver injury score model developed by the US Food and Drug Administration. These results support the idea of the existence of human subpopulations that are highly sensitive to coumarin; therefore, a more precise risk assessment is needed. The present study also highlights the usefulness of in silico approaches of pharmacokinetics with the liver injury score model as battery components of a risk assessment.
Key words: coumarin, drug-induced liver injury score model, hepatotoxicity, physiologically based pharmacokinetics, individual susceptibilities
1. Introduction
Coumarin is a naturally occurring organic chemical that is often ingested as part of cinnamon-containing foods. Although the intake of coumarin from foods is generally considered to be safe, coumarin-induced hepatotoxicity has been reported to occasionally occur in humans1,2). Other than through the food intake, a clinical trial of coumarin has been performed on lymphedema patients, but the occurrence of hepatic disorders led to its withdrawal3,4,5). The US Food and Drug Administration (FDA) has banned the use of coumarin as a food additive due to hepatotoxicity concerns.
The occurrence of hepatotoxicity has been observed in experimental animals such as dogs and rats after the administration of coumarin. The coumarin-induced hepatotoxicity is believed to be associated with the metabolism in the body. Coumarin is metabolized to_o_-hydroxyphenylacetic acid (_o_-HPA) through the reactive metabolite coumarin 3,4-epoxide6,7), while the biological 7-hydroxylation is considered to be a process of detoxification. Species differences clearly exist between humans and rats in terms of the coumarin 7-hyroxylation. In fact, the detoxification rates are rapid in humans and slow in rats8,9,10,11). Only CYP2A6 catalyzes coumarin 7-hydroxylation among the major cytochrome P450 enzymes in the human liver. This major detoxification pathway mediated by CYP2A6 is susceptible to genetic polymorphisms, but the influence of such genetic polymorphisms on the hepatotoxicity of coumarin still remains unclarified in vivo in humans12). Moreover, multiple CYP enzymes participate in the 3,4-epoxidation, and the hepatic levels of these enzymes are known to vary among individuals. Therefore, an understanding of inter- and intra-individual differences in terms of their coumarin intake amounts and of their metabolic capacities is necessary in order to evaluate the risk of food-derived coumarin in humans.
In this study, human-relevant data on coumarin were collected, including data regarding its intakes from food, absorption, distribution, metabolism, and excretion (ADME), toxicity, and clinical information in an attempt to refine its risk assessment. Furthermore, two_in silico_ models were introduced in order to provide additional human-relevant information: a physiologically-based pharmacokinetic (PBPK) model11,13), and a drug-induced liver injury (DILI) severity-predicting model developed by the FDA (hereafter referred to as the FDA DILI score model)14).
2. Materials and Methods
2.1 Intake, ADME and Toxicity Data Collection
Data on coumarin (including intake from food, experimental and clinical studies on the ADME and the in vivo and in vitro toxicity of coumarin) were obtained from the relevant regulatory assessment reports1,15,16,17,18,19) and the literature.
2.2 PBPK Modeling
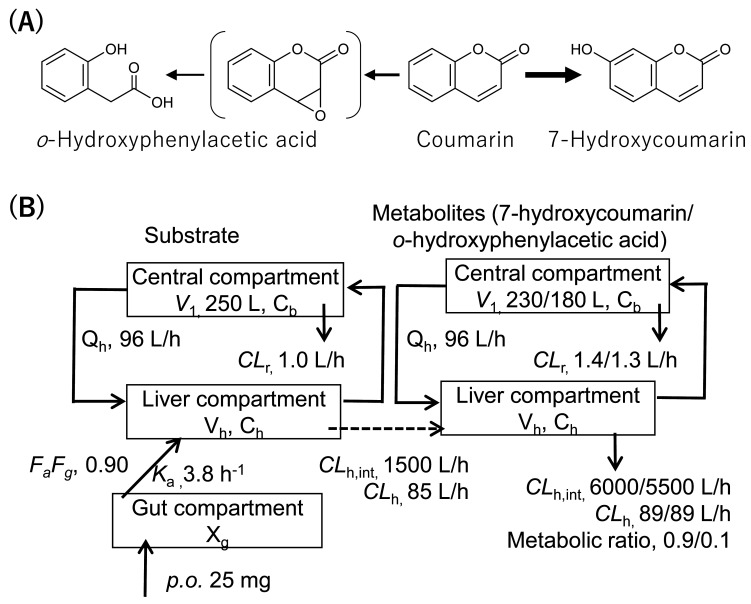
The human PBPK models were constructed based on the rat PBPK model consisting of the gut, the liver, and the central compartments, as described previously11). The blood concentration profiles in rats treated orally with 200 mg/kg of coumarin were reproduced11). A scale-up strategy was applied by using the fixed values of the human body weight (70 kg) and the liver volume (1.5 L). The input parameters for the human PBPK model are presented in detail in Fig. 1. Systems of differential equations were used in order to obtain the concentrations of the substrate coumarin as well as those of the two metabolites (7-hydroxycoumarin and _o_-HPA) in the central compartments, as described previously11).
Fig. 1.
Major metabolic pathways of coumarin (A) and coumarin PBPK model (B). Input parameters for human coumarin PBPK model with two metabolites (7-hydroxycoumarin and_o_-HPA) were calculated previously13). Values for fraction absorbed × intestinal availability (_F_a_F_g), absorption rate constant (_k_a), volume of the systemic circulation (_V_1), hepatic intrinsic clearance (_CL_h,int), hepatic clearance (_CL_h), and renal clearance (_CL_r) values for human PBPK models are shown._X_g represents the amount of compound in the gut compartment, _V_h represents liver volume,_C_h represents hepatic substrate concentration, and_C_b represents blood substrate concentration.
2.3 Application of the FDA DILI Score Model for Coumarin and Related Drugs
Coumarin and related drugs sharing a substructure with coumarin (e.g., warfarin and methoxsalen) were applied to the FDA DILI score model14). The DILI scores were calculated by using the following formula: DILI score = 0.608 × loge (daily dose in mg) + 0.227 × logP + 2.833 (in the case of a present reactive metabolite formation). Prior to the application, the logP (the ratio of the concentration of the unionized compound at equilibrium between organic and aqueous phases) and the molecular weight (MW) were calculated by using the ADMET Predictor version 10.0 (SimulationsPlus, USA). Furthermore, the chemical classes of every substructure of coumarin were compared with those of the set molecules used for the construction of the model (a total of 354 human pharmaceuticals). The chemical classes of the substructures were searched in the public ChemoTyper application (Altamira LLC, USA and Molecular Networks GmbH, Germany). The chemical structures were profiled with the use of the OECD QSAR Toolbox20)(ver. 4.4) for both coumarin and the set molecules.
3. Results
3.1 Collected data of Intake of Coumarin from Foods
In the European Food Safety Authority (EFSA) opinion of 2004, a theoretically-calculated maximum daily intake of coumarin was estimated to be 1.5 mg for an adult with a default body weight of 60 kg (0.025 mg/kg of body weight per day)15). A mean coumarin intake from foods consumed within the Christmas season was estimated to be 5.0 mg per week, and the intakes of the heaviest consumers (six subjects) exceeded 35 mg/week2). In Japan, a consumption of 2.45 g of cinnamon corresponding to the ingestion of 2.73 mg of coumarin from daily foods has been reported21). The total amount of coumarin consumed can reach 5.18 mg/day if daily supplements that contain cinnamon are also consumed21). In the worst-case scenarios, a food-mediated coumarin consumption of around 60 mg/day (based on a body weight of 50 kg) has been reported in Norway18), and an additional exposure of 18 mg/day coumarin was calculated to occur from food supplements in Germany16). These results suggest an average intake of approximately 1−3 mg of coumarin per day, as well as a level equal to ten-times higher the average intake in the worst-case scenario.
3.2 Collected data of Absorption, Distribution, Metabolism, and Excretion of Coumarin
In an in vitro intestinal epithelial cell monolayer system, a fraction-absorbed value of >0.9 was estimated for coumarin based on its apparent permeability. Coumarin is rapidly absorbed after an oral intake of 0.857 mg/kg, but its availability to the systemic circulation is reported to be less than 4%13). The rest of the intake appeared in the form of 7-hydroxycoumarin and the glucuronide in the systemic-circulation, thereby suggesting an extensive first-pass effect. In a study employing an oral administration of 200 mg of coumarin in seven different subjects, 63.4% of the dose was recovered in the 24-h urine in the form of total 7-hydroxycoumarin22). Coumarin can also be absorbed fairly efficiently after a dermal application, and the absorption rates ranged from 54.7% to 66.1% in humans10).
The in vivo metabolic profiles of coumarin are similar among experimental animals and humans. Metabolites deriving from the oxidation of both the phenyl ring (7-hydroxylation) and the lactone ring (3,4-oxidation) can be detected mostly in the urine of mice, rats, dogs10), and human volunteers23); however, certain extents of a fecal excretion have also been observed in rats exposed to high doses10).
7-Hydroxycoumarin and its glucuronic acid conjugate were the major metabolites detected in the urine of most individuals, while the lactone-ring opening metabolite,_o_-HPA, was slightly detected in urine; interestingly, the amount of_o_-HPA was more than that of 7-hydroxycoumarin in the 8-h urine of some individuals after a 2-mg coumarin intake23). o_-Hydroxyphenylacetaldehyde can be formed_in vitro by microsomes from all four human liver samples as the major metabolite of coumarin at a coumarin concentration of 1 mM; however, 7-hydroxycoumarin was the major metabolite detected after an exposure to coumarin concentrations below 50 μM24). These results suggest that both the coumarin concentration and the genetic background can affect coumarin metabolism in humans.
Studies of the toxicity mechanism of coumarin have consistently indicated a role for the reactive intermediate in coumarin-induced hepatotoxicity. The microsomal formation of metabolites bound covalently to hepatic proteins8), the identification of_o_-hydroxyphenylacetaldehyde as a major metabolite of coumarin in the rat hepatic microsomes8), the much lower toxicities of 3- or 4-methylcoumarin and of 3,4-dimethylcoumarin than coumarin25), as well as the reactivity of _o_-hydroxyphenylacetaldehyde26); all support the production of a reactive 3,4-oxide for the facilitation of the coumarin-mediated hepatotoxicity.
In human recombinant CYP systems, CYP1A1, CYP1A2 and CYP2E1 mediate the formation of_o_-hydroxyphenylacetaldehyde, and CYP3A4 may also support this reaction27,28). The 7-hydroxycoumarin formation is supported only by CYP2A6, and no activities are detected with CYP1A1, CYP1A2, CYP2E1, and CYP3A428). CYP2A13 catalyzes both the 3,4-oxide formation and 7-hydroxylation of coumarin29). The low levels of CYP2A13 are expressed selectively in extra-hepatic tissues, while the enzyme’s levels in the liver are negligible30). Population studies suggest that 6% of the UK population is homozygous for the mutant CYP2A6 alleles, whereas the mutant CYP2A6 allele frequency may be as high as 48% in Japanese subjects31).
The production of _o_-HPA was correlated with the CYP1A2 content of human hepatocytes32). The production of o_-HPA in the human liver microsomal system as well as_in vivo, in the humanized-liver mice, was clearly inhibited in the presence of a selective inhibitor of CYP1A2, furafylline33,34). These results suggest, at least partly, the involvement of CYP1A2 in the metabolic activation of coumarin in the human liver. CYP2E1 has also been expected to mediate the 3,4-epoxidation of coumarin in humans27,35,36).
3.3 Collected data of Toxicity of Coumarin
Toxicity data of coumarin have been evaluated and published in the form of review articles2,10,37,38) and risk assessment reports1,15,16,17,18,19). These data consistently indicate the liver as the most sensitive target of coumarin in experimental animals. Hepatotoxicity in rats includes hepatic histopathological lesions along with increased liver enzymes at doses ≥50 mg/kg/day in a 2-year-long carcinogenicity study19). Slight jaundice, marked histopathologic hepatic changes, and distended gall bladder were observed in a 1-year chronic study in dogs39). In baboons, hepatic changes were limited to an increased liver weight and an hepatocyte endoplasmic reticulum hypertrophy observed at the highest coumarin dose (67.5 mg/kg/day) in a 2-year-long chronic toxicity study40). The EFSA determined the tolerable daily intake (TDI) of coumarin to be 0.1 mg/kg/day, based on the no-observed-adverse-effect level (NOAEL) of 10 mg/kg/day found in a chronic toxicity study of coumarin in dogs, with an uncertainty factor of 10015).
Hepatic disorder, characterized as an elevation of the liver enzyme levels, is the most common coumarin-associated adverse finding reported in humans1,2). In one case, a 23-year-old woman was hospitalized with hepatitis after consuming 1–2 g of cinnamon (equivalent to 3.3–6.6 mg of coumarin) daily, for two months2). According to the expert report on the assessment of coumarin in medicinal products1,2), liver damage cannot be ruled out at a daily dose of 25 mg coumarin for a part of the population. In order to extrapolate from this effect level to a human NOAEL, a factor of 5 is considered as justified in the case of a severe effect at the lowest observed adverse effect level. Thus, an exposure to 5 mg of coumarin per day is expected to cause no adverse effects in sensitive subjects. Moreover, a TDI of 0.1 mg/kg body weight was derived1,2,16). This value agreed well with the EFSA value based on animal data15).
The incidence of coumarin-induced hepatotoxicity was estimated to be 0.37% by a clinical trial41). In the National Institutes of Health LiverTox Database, the idiosyncratic, clinically apparent liver injury associated with coumarin was estimated to occur in 2 out of 1,000 patient-years of use42).
3.4 Estimation of the Internal Exposure to Coumarin in Humans Using the PBPK Model
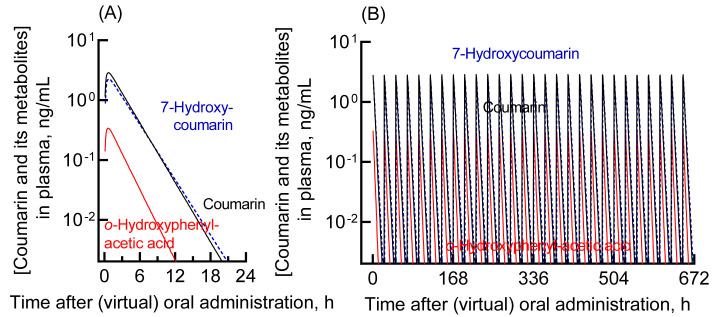
Pharmacokinetic data of coumarin in humans are necessary in order to assess the body exposure in vivo and the subsequent hepatotoxicity. However, the available data that are relevant to the assessment of possible toxic dose levels are limited43). Therefore, the plasma concentrations of coumarin, 7-hydroxycoumarin, and _o_-HPA (generated via a coumarin 3,4-epoxidation) were estimated by using human PBPK models that have been previously developed and validated11). Virtual oral doses of 2.5 mg and 25 mg were applied in the present study. The former dose corresponds approximately to the average intake of food-derived coumarin21), and the latter dose corresponds to an amount close to the maximum estimate of coumarin intake from food or the amount of pharmaceutical administration that cannot exclude hepatotoxicity in a part of the human population2). Plasma concentration curves after a single or a 28-day repeated virtual administration of coumarin in humans were generated by using a human PBPK model (Fig. 2). After a single dosing, the mean and the maximum plasma concentration after an intake of coumarin at a dose of 2.5 mg/day was estimated to be 0.04 and 0.29 ng/mL (0.27 and 2.0 nM), respectively. The results for the 25-mg oral dose are presented in Fig. 2A. The mean and the maximum plasma concentrations of coumarin after a single administration were 0.41 and 2.9 ng/mL (2.8 and 20 nM), respectively. The plasma concentration curves after the repeated virtual administration of coumarin in humans for 28 days were also generated by using the human PBPK model (Fig. 2B). The respective concentrations of coumarin, 7-hydroxycoumarin, and _o_-HPA after a repeated dosing were comparable to those after a single dosing, in consistent with the notion that coumarin is rapidly cleared and does not accumulate in the body.
Fig. 2.
Plasma levels of coumarin and its metabolites in humans estimated using the established human PBPK model. The plasma concentrations of coumarin (black solid line), _o_-HPA (red solid line), and 7-hydroxycoumarin (blue dashed line) after virtual administration of 25 mg of coumarin via the oral route as a single dose (A) or daily doses for 28 days (B) are shown.
3.5 Applicability to FDA DILI Score Model and DILI Scores
The possible hepatotoxicity of coumarin was evaluated by using a FDA DILI score model based on the daily dose of the substance, lipophilicity, and reactive metabolite formation14).
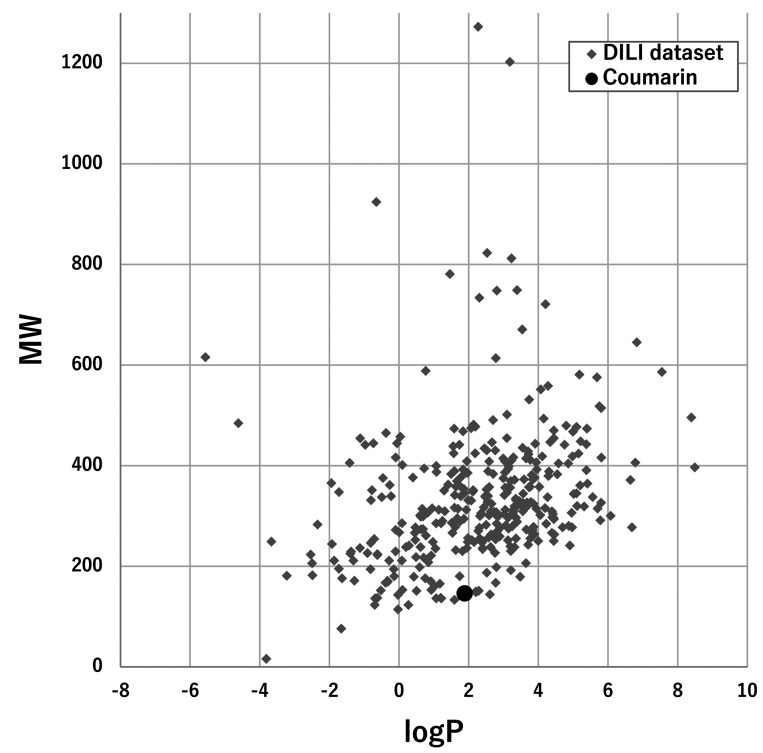
The applicability of coumarin to the FDA DILI score model was assessed by plotting the logP and the MW of coumarin and the set molecules (Fig. 3). The estimated logP value of coumarin (1.39) was in the range of most of the set molecules (from −4 to 8), and the inclusion of the MW of coumarin (146.14) was also confirmed. Coumarin consisted of a total of 13 chemotypes (including benzopyrone), all of which were included in a set of chemotypes of the set molecules (Table 1 and Supplementary Data Table S1). The chemical structure profiling that was undertaken by using the OECD QSAR Toolbox yielded similar results (Supplementary Data Table S2).
Fig. 3.
Application of coumarin to the FDA DILI score model. MW plotted against logP is presented for coumarin (black circle) and the set molecules for constructing the model, with a total of 354 substances (gray diamond).
Table 1. Chemotype of coumarin and duplication in the set of 354 molecules for constructing the FDA DILI score model.
| Chemotype contained in coumarin | No. of duplications in the set molecules |
|---|---|
| bond:C(=O)O_carboxylicEster_alkenyl | 9 |
| bond:C=O_carbonyl_generic | 228 |
| chain:alkeneCyclic_ethene_C_(connect_noZ) | 37 |
| chain:alkeneCyclic_ethene_generic | 67 |
| chain:aromaticAlkane_Ph-C1_cyclic | 83 |
| chain:aromaticAlkene_Ph-C2_cyclic | 11 |
| ring:aromatic_benzene | 264 |
| ring:hetero_[6]_O_pyran_generic | 15 |
| ring:hetero_[6]_Z_1- | 100 |
| ring:hetero_[6]_Z_generic | 152 |
| ring:hetero_[6_6]_O_benzopyran | 3 |
| ring:hetero_[6_6]_O_benzopyrone_(1_2-) | 1 |
| ring:hetero_[6_6]_Z_generic | 52 |
Coumarin and the related drugs sharing a substructure with coumarin were applied to the FDA DILI score model (Table 2). The epoxidation of coumarin and of methoxsalen were assumed to lead to the formation of reactive intermediates15,16). The DILI scores of coumarin were calculated to be 3.71 and 5.11 for the daily consumption levels of 2.5 and 25 mg/day, respectively, and the risk was judged to be moderate (score of 3–6). Warfarin (10 mg/day) was judged to be of low risk based on a DILI score of 1.95, whereas methoxsalen (3 mg/day) was considered to be of medium risk based on a DILI score of 3.92.
Table 2. Application of coumarin and related drugs to the FDA DILI score model.
4. Discussion
In this study, the hepatotoxicity of food-derived coumarin in humans was comprehensively reevaluated by the use of the data of existing clinical and animal studies as well as those of generated by us through in silico approaches for humans.
According to several studies, the average intake of food-derived coumarin is about 1−3 mg/day, and in the worst-case scenarios, the intake is expected to be about ten-times higher than that of the average amount. The levels were found to be almost equal to or above the TDI (0.1 mg/kg/day) that derived from animal studies15).
It has been shown that the majority of coumarin is rapidly converted to 7-hydroxycoumarin, along with low levels of _o_-HPA which is associated with the metabolic activation (Fig. 2A). At a virtual intake of 25 mg of coumarin (that is approximately ten-times more than the average intake), the maximum plasma concentrations of coumarin and _o_-HPA were expected to be 20 nM and 2 nM, respectively, at 0.7 h (Fig. 2A). Based on the plasma-to-liver distribution ratios of coumarin and _o_-HPA of 0.875 and 0.50411), the coumarin and_o_-HPA concentrations in the liver at the same timepoint were estimated to be 17 nM and 1.1 nM, respectively.
The maximum blood concentrations of coumarin and _o_-HPA were estimated to be approximately 200 μM and 80 μM, respectively, at 0.5 h after the administration of a toxic dose of 200 mg/kg44). Therefore, clear differences were observed with regard to both the coumarin and the_o_-HPA levels between the simulated data (at 25 mg/kg) in humans and measured data (at 200 mg/kg) in rats. Based on the pharmacokinetics data, an intake of 25 mg/kg of coumarin in humans may be considered to be of low or subtle concern for the development of hepatotoxicity, as far as the human population maintains average levels of capacity for coumarin metabolism. It should be noted, however, that this PBPK model is based on data deriving from a small number of healthy individuals. The report by Abraham et al. in 20102) claims that hepatotoxicity concerns cannot be ruled out in humans after the ingestion of 25 mg or more of coumarin. These results suggest the existence of a subpopulation that is highly susceptible to coumarin-induced hepatotoxicity. Further investigation of such individual differences in terms of the susceptibility to coumarin is warranted.
The detoxification of coumarin in humans is mainly mediated by CYP2A632,35,45), which may imply variations in the susceptibility to coumarin toxicity as a result of genetic polymorphisms in the metabolizing enzyme46,47,48). The polymorphism of CYP2A6 is rather prevalent in the Japanese population, and the non-wild (poor metabolizer) types were reportedly present in nearly half of the population. In addition, both CYP1A2 and CYP2E1 mediate the production of_o_-hydroxyphenylacetaldehyde, which is probably associated with coumarin toxicity. The hepatic levels of CYP1A2 and CYP2E1 vary under the influence of various environmental factors. Cigarette smoking and alcohol consumption are known to alter the hepatic levels of CYP1A1/2 and CYP2E1 through induction phenomena, respectively. Other dietary components are also known to modulate the activation and the detoxification of coumarin through the processes of inhibition and transport. Therefore, further studies on the impact of individual differences are required in order to refine the evaluation of the coumarin-induced hepatotoxicity in humans.
The FDA continues to compile human hepatotoxicity data of approved or withdrawn drugs, and these data include their daily dose, their lipophilicity, and their reactive metabolite formation. These data are also used for the construction of the QSAR model aiming to predict the severity of clinical liver injury14). Available toxicity data of foods and food ingredients are often not sufficient for the rigid evaluation of their toxicities in humans and, thus, the use of this model is expected to be beneficial. The applicability was at first checked by the comparison of the logP, the MW, and the chemotypes of coumarin with those of a total of 354 molecules that were used in order to construct the model. Coumarin was judged to be applicable, and the daily intakes of coumarin at 2.5 and 25 mg/kg were predicted to be of moderate risk. The structurally related drug, warfarin, was predicted to be of low risk, whereas methoxsalen was predicted to be of moderate risk. The estimated results of the coumarin-related drugs are consistent with hepatotoxicity in humans, thereby offering information on a relative probability for the development of coumarin-induced hepatotoxicity. The DILI score model may also be applicable to food ingredients other than coumarin for the preliminary discrimination or evaluation of potential hepatotoxicity in humans.
In summary, the possibility of developing coumarin-induced hepatotoxicity in humans was, herein, reevaluated through a combined approach that integrated the existing data of clinical and animal studies with data deriving from in silico models. At the current average coumarin intake, the humans can be considered to be safe, at least as far as the coumarin-induced hepatotoxicity is concerned. On the other hand, the existence of a human subpopulation that is highly susceptible to the hepatotoxicity of coumarin is suggested. Further studies are required in order to achieve a more precise risk assessment that would take into account the individual differences in coumarin metabolism as defined by genetic and environmental factors. Moreover, the present study highlights the usefulness of_in silico_ approaches of pharmacokinetics and the liver injury score model as battery components of a risk assessment.
Disclaimer.
This manuscript reflects the views of the authors and does not necessarily reflect those of the National Institute of Health Sciences and the US Food and Drug Administration. Any mention of commercial products is for clarification only and is not intended as approval, endorsement, or recommendation.
Acknowledgments
This study was supported by a grant from the Food Safety Commission, Cabinet Office, Government of Japan (Research program for Risk Assessment Study on Food Safety, No. JPCAFSC20202006).
Footnotes
Conflict of Interest: The authors have no conflict of interest regarding this publication.
References
- 1.European Food Safety Authority. Coumarin in flavourings and other food ingredients with flavouring properties - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2008; 6: 793. Available at https://www.efsa.europa.eu/en/efsajournal/pub/793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham K,Wöhrlin F,Lindtner O,Heinemeyer G,Lampen A. Toxicology and risk assessment of coumarin: Focus on human data. Mol Nutr Food Res. 2010; 54(2): 228–239. , 10.1002/mnfr.200900281 [DOI] [PubMed] [Google Scholar]
- 3.Andréjak M,Gersberg M,Sgro C,et al. French pharmacovigilance survey evaluating the hepatic toxicity of coumarin. Pharmacoepidemiol Drug Saf. 1998; 7(S1, Suppl 1): S45–S50. , [DOI] [PubMed] [Google Scholar]
- 4.Bassett ML andDahlstrom JE. Liver failure while taking coumarin. Med J Aust. 1995; 163(2): 106. , 10.5694/j.1326-5377.1995.tb126130.x [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL,Sloan J,Kugler J. Coumarin-induced hepatotoxicity. J Clin Oncol. 1997; 15(9): 3167–3168. , 10.1200/JCO.1997.15.9.3167 [DOI] [PubMed] [Google Scholar]
- 6.Rietjens IMCM,Punt A,Schilter B,Scholz G,Delatour T,van Bladeren PJ. In silico methods for physiologically based biokinetic models describing bioactivation and detoxification of coumarin and estragole: Implications for risk assessment. Mol Nutr Food Res. 2010; 54(2): 195–207. , 10.1002/mnfr.200900211 [DOI] [PubMed] [Google Scholar]
- 7.Vassallo JD,Morrall SW,Fliter KL,Curry SM,Daston GP,Lehman-McKeeman LD. Liquid chromatographic determination of the glutathione conjugate and ring-opened metabolites formed from coumarin epoxidation. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 794(2): 257–271. , 10.1016/S1570-0232(03)00473-2 [DOI] [PubMed] [Google Scholar]
- 8.Lake BG,Gaudin H,Price RJ,Walters DG. Metabolism of [3-14C]coumarin to polar and covalently bound products by hepatic microsomes from the rat, syrian hamster, gerbil and humans. Food Chem Toxicol. 1992; 30(2): 105–115. , 10.1016/0278-6915(92)90145-B [DOI] [PubMed] [Google Scholar]
- 9.Fentem JH,Fry JR. Species differences in the metabolism and hepatotoxicity of coumarin. Comp Biochem Physiol C Comp Pharmacol. 1993; 104(1): 1–8. , 10.1016/0742-8413(93)90102-Q [DOI] [PubMed] [Google Scholar]
- 10.Lake BG. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 1999; 37(4): 423–453. , 10.1016/S0278-6915(99)00010-1 [DOI] [PubMed] [Google Scholar]
- 11.Miura T,Kamiya Y,Hina S,et al. Metabolic profiles of coumarin in human plasma extrapolated from a rat data set with a simplified physiologically based pharmacokinetic model. J Toxicol Sci. 2020; 45(11): 695–700. , 10.2131/jts.45.695 [DOI] [PubMed] [Google Scholar]
- 12.Farinola N,Piller NB. CYP2A6 polymorphisms: is there a role for pharmacogenomics in preventing coumarin-induced hepatotoxicity in lymphedema patients? Pharmacogenomics. 2007; 8(2): 151–158. , 10.2217/14622416.8.2.151 [DOI] [PubMed] [Google Scholar]
- 13.Ritschel WA,Brady ME,Tan HSI,Hoffmann KA,Yiu IM,Grummich KW. Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Eur J Clin Pharmacol. 1977; 12(6): 457–461. , 10.1007/BF00561066 [DOI] [PubMed] [Google Scholar]
- 14.Chen M,Borlak J,Tong W. A Model to predict severity of drug-induced liver injury in humans. Hepatology. 2016; 64(3): 931–940. , 10.1002/hep.28678 [DOI] [PubMed] [Google Scholar]
- 15.European Food Safety Authority. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 6 (FGE.06): Straight-and branched-chain aliphatic unsatured primary alcohols, aldehydes, carboxylic acids, and esters from chemical groups 1 and 4. EFSA J. 2004; 2(11): 108. . 10.2903/j.efsa.2004.108 [DOI] [Google Scholar]
- 16.Bundesinstitut für Risikobewertung. Consumers, who eat a lot of cinnamon, currently have an overly high exposure to coumarin. In: BfR Health Assessment. ed. Berlin, Germany: Bundesinstitut für Risikobewertung. 2006; No. 043/2006.
- 17.Therapeutic Goods Administration. Safety review: coumarin for use in topical listed medicines. 2019; Version 1.0. Available at: https://www.tga.gov.au/sites/default/files/safety-review-coumarin-listed-medicines.pdf. Accessed on September 4, 2022.
- 18.Fotland TØ,Paulsen JE,Sanner T,Alexander J,Husøy T. Risk assessment of coumarin using the bench mark dose (BMD) approach: Children in Norway which regularly eat oatmeal porridge with cinnamon may exceed the TDI for coumarin with several folds. Food Chem Toxicol. 2012; 50(3-4): 903–912. , 10.1016/j.fct.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 19.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Coumarin (CAS No. 91-64-5) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser. 1993; 422: 1–340. [PubMed] [Google Scholar]
- 20.Dimitrov SD,Diderich R,Sobanski T,et al. QSAR Toolbox – workflow and major functionalities. SAR QSAR Environ Res. 2016; 27(3): 203–219. , 10.1080/1062936X.2015.1136680 [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki Y,Tabata S,Iida K,et al. Determination of coumarin in cinnamon foods. Ann Rep Tokyo Metr Inst Pub Health. 2008; 59: 143–148. [Google Scholar]
- 22.Moran E,O’Kennedy R,Thornes RD. Analysis of coumarin and its urinary metabolites by high-performance liquid chromatography. J Chromatogr, Biomed Appl. 1987; 416(1): 165–169. , 10.1016/0378-4347(87)80499-1 [DOI] [PubMed] [Google Scholar]
- 23.Hadidi H,Irshaid Y,Broberg Vågbø C,et al. Variability of coumarin 7- and 3-hydroxylation in a Jordanian population is suggestive of a functional polymorphism in cytochrome P450 CYP2A6. Eur J Clin Pharmacol. 1998; 54(5): 437–441. , 10.1007/s002280050489 [DOI] [PubMed] [Google Scholar]
- 24.Fentem JH,Fry JR. Metabolism of coumarin by rat, gerbil and human liver microsomes. Xenobiotica. 1992; 22(3): 357–367. , 10.3109/00498259209046647 [DOI] [PubMed] [Google Scholar]
- 25.Fernyhough L,Kell SW,Hammond AH,Thomas NW,Fry JR. Comparison of in vivo and_in vitro_ rat hepatic toxicity of coumarin and methyl analogues, and application of quantitative morphometry to toxicity in vivo. Toxicology. 1994; 88(1-3): 113–125. , 10.1016/0300-483X(94)90114-7 [DOI] [PubMed] [Google Scholar]
- 26.Born SL,Hu JK,Lehman-McKeeman LD. o-hydroxyphenylacetaldehyde is a hepatotoxic metabolite of coumarin. Drug Metab Dispos. 2000; 28(2): 218–223. [PubMed] [Google Scholar]
- 27.Born SL,Caudill D,Fliter KL,Purdon MP. Identification of the cytochromes P450 that catalyze coumarin 3,4-epoxidation and 3-hydroxylation. Drug Metab Dispos. 2002; 30(5): 483–487. , 10.1124/dmd.30.5.483 [DOI] [PubMed] [Google Scholar]
- 28.Zhuo X,Gu J,Zhang QY,Spink DC,Kaminsky LS,Ding X. Biotransformation of coumarin by rodent and human cytochromes P-450: metabolic basis of tissue-selective toxicity in olfactory mucosa of rats and mice. J Pharmacol Exp Ther. 1999; 288(2): 463–471. [PubMed] [Google Scholar]
- 29.Von Weymarn LB,Murphy SE. CYP2A13-catalysed coumarin metabolism: comparison with CYP2A5 and CYP2A6. Xenobiotica. 2003; 33(1): 73–81. , 10.1080/0049825021000022302 [DOI] [PubMed] [Google Scholar]
- 30.Su T,Bao Z,Zhang QY,Smith TJ,Hong JY,Ding X. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000; 60(18): 5074–5079. [PubMed] [Google Scholar]
- 31.Fernandez-Salguero P,Hoffman SM,Cholerton S,et al. A genetic polymorphism in coumarin 7-hydroxylation: sequence of the human CYP2A genes and identification of variant CYP2A6 alleles. Am J Hum Genet. 1995; 57(3): 651–660. [PMC free article] [PubMed] [Google Scholar]
- 32.Murayama N,Yamazaki H. Metabolic activation and deactivation of dietary-derived coumarin mediated by cytochrome P450 enzymes in rat and human liver preparations. J Toxicol Sci. 2021; 46(8): 371–378. , 10.2131/jts.46.371 [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki H,Horiuchi K,Takano R,et al. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int J Environ Res Public Health. 2010; 7(9): 3406–3421. , 10.3390/ijerph7093406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura T,Uehara S,Shimizu M,Murayama N,Suemizu H,Yamazaki H. Roles of human cytochrome P450 1A2 in coumarin 3,4-epoxidation mediated by untreated hepatocytes and by those metabolically inactivated with furafylline in previously transplanted chimeric mice. J Toxicol Sci. 2021; 46(11): 525–530. , 10.2131/jts.46.525 [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki H,Mimura M,Sugahara C,Shimada T. Catalytic roles of rat and human cytochrome P450 2A enzymes in testosterone 7α- and coumarin 7-hydroxylations. Biochem Pharmacol. 1994; 48(7): 1524–1527. , 10.1016/0006-2952(94)90579-7 [DOI] [PubMed] [Google Scholar]
- 36.Yamazoe Y,Murayama N,Yoshinari K. Refined CYP2E1* Template**system to decipher the ligand-interactions. Drug Metab Pharmacokinet. 2021; 41: 100413. , 10.1016/j.dmpk.2021.100413 [DOI] [PubMed] [Google Scholar]
- 37.Felter SP,Vassallo JD,Carlton BD,Daston GP. A safety assessment of coumarin taking into account species-specificity of toxicokinetics. Food Chem Toxicol. 2006; 44(4): 462–475. , 10.1016/j.fct.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 38.Api AM,Belmonte F,Belsito D,et al. RIFM fragrance ingredient safety assessment, coumarin, CAS Registry Number 91-64-5. Food Chem Toxicol. 2019; 130 (Suppl 1): 110522. , 10.1016/j.fct.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 39.Hagan EC,Hansen WH,Fitzhugh OG,et al. Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet Toxicol. 1967; 5(2): 141–157. , 10.1016/S0015-6264(67)82961-4 [DOI] [PubMed] [Google Scholar]
- 40.Evans JG,Gaunt IF,Lake BG. Two-year toxicity study on coumarin in the baboon. Food Cosmet Toxicol. 1979; 17(3): 187–193. , 10.1016/0015-6264(79)90280-3 [DOI] [PubMed] [Google Scholar]
- 41.Cox D,O’Kennedy R,Thornes RD. The rarity of liver toxicity in patients treated with coumarin (1,2-benzopyrone). Hum Toxicol. 1989; 8(6): 501–506. , 10.1177/096032718900800612 [DOI] [PubMed] [Google Scholar]
- 42.Warfarin. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK548837/. Accessed on June 15, 2022. [PubMed]
- 43.Mielke H,Abraham K,Götz M,et al. Physiologically based toxicokinetic modelling as a tool to assess target organ toxicity in route-to-route extrapolation—The case of coumarin. Toxicol Lett. 2011; 202(2): 100–110. , 10.1016/j.toxlet.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 44.Tanaka Y,Fujii W,Hori H,Kitagawa Y,Ozaki K. Changes in coumarin kinetics and subcellular localization of CYP2E1 contribute to bile duct damage and reduce hepatocellular damage after repeated administration of coumarin in rats. Toxicol Lett. 2017; 280: 99–105. , 10.1016/j.toxlet.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 45.Pelkonen O,Rautio A,Raunio H,Pasanen M. CYP2A6: a human coumarin 7-hydroxylase. Toxicology. 2000; 144(1-3): 139–147. , 10.1016/S0300-483X(99)00200-0 [DOI] [PubMed] [Google Scholar]
- 46.Ujjin P,Satarug S,Vanavanitkun Y,et al. Variation in coumarin 7-hydroxylase activity associated with genetic polymorphism of cytochrome P450 2A6 and the body status of iron stores in adult Thai males and females. Pharmacogenetics. 2002; 12(3): 241–249. , 10.1097/00008571-200204000-00009 [DOI] [PubMed] [Google Scholar]
- 47.Peamkrasatam S,Sriwatanakul K,Kiyotani K,et al. In vivo evaluation of coumarin and nicotine as probe drugs to predict the metabolic capacity of CYP2A6 due to genetic polymorphism in Thais. Drug Metab Pharmacokinet. 2006; 21(6): 475–484. 10.2133/dmpk.21.475 [DOI] [PubMed] [Google Scholar]
- 48.Kiyotani K,Yamazaki H,Fujieda M,et al. Decreased coumarin 7-hydroxylase activities and CYP2A6 expression levels in humans caused by genetic polymorphism in CYP2A6 promoter region (CYP2A6*9). Pharmacogenetics. 2003; 13(11): 689–695. 10.1097/00008571-200311000-00005 [DOI] [PubMed] [Google Scholar]