In vitro gastrointestinal digestion studies on total phenols, flavonoids, anti-oxidant activity and vitamin C in freeze-dried vegetable powders (original) (raw)
Abstract
In the present research study, the impact of digestion process on the levels of total phenols, flavonoids, vitamin C as well as anti-oxidant activity in freeze dried powders of mustard greens (MG) and roselle leaves (RL) was investigated. In addition, physicochemical and functional properties of MG and RL samples also evaluated. The digestion of freeze-dried vegetable powders was achieved through in vitro digestive procedure using various enzymes. From the study, it was observed that the digestion process increased the availability of phenols in both powders, where the digested vegetable powders possessed higher levels of total phenols and flavonoids. In contrast, the levels of vitamin C and anti-oxidant activity of vegetable powders (MG & RL) was found to be decreased minimally. Our research study suggests that in vitro digestion could enhance the TPC and TFC in mustard greens and roselle leaves. Therefore, MG and RL can be considered as a functional ingredient in the development of new products with better nutritional and functional characteristics. Further, the data on the physicochemical, functional and bioactive compounds in MG and RL may be used as reference for the enhancement of quality of products developed from MG and RL.
Keywords: In vitro digestion, Vegetable powders, Roselle leaves, Mustard greens, Vitamin C
Introduction
Non communicable diseases (NCDs) such as cardiovascular disease (CVD), diabetes, and cancer are one of the major causes of premature death. According to World Health Organization (WHO), the health risks associated with these diseases can be reduced by consuming fruits, vegetables, and fiber compounds. Fruits and vegetables are also high in vitamins, minerals, and phytochemicals, all of which can benefit the consumer's health. Aside from that, polyphenols which are commonly present in fruits and vegetables has gained popularity owing to their proven ability to neutralize free radicals and provide protection and prevention against cardiovascular disease, diabetes, and cancer (Arfaoui, 2021). However, the beneficial activities of phenolic compounds are highly relied on their bioavailability. Therefore, it is vital to understand the bioavailability of these polyphenols in order to evaluate their activity in various food matrixes (Manach et al., 2004).
Among the different classes of vegetables, leafy green vegetables are a great source of certain polyphenols and dietary fibers. Mustard greens (Brasicca juncea) are commonly known as Chinese mustard, nutritious leafy vegetable that has abundant amount of bioactive compounds like glucosinolates, flavonoids, anthocyanin as well as large amount of dietary fibers, ascorbic acid, chlorophyll, minerals and β-carotene (Tian & Deng, 2020). Apart from its antioxidant capacity, it also exhibits anti-cancer, anti-obesity (Lee et al., 2018), anti-bacterial, anti-hyperglycemia, anti-depressant as well as therapeutic activities making it a very good food as well and folk medicine.
Roselle leaves (Hibiscus sabdariffa), commonly known as red sorrel are found in warm countries like India, Saudi Arabia, Malaysia, Egypt, Mexico, etc. (Khan, 2017). The young leaves and stems of roselle can be consumed as raw and the seeds are used as a protein source in human meal. They have numerous medical applications against various diseases including high blood pressure, pyrexia, liver diseases and leukaemia attributable to content of protocatechuic acid in leaves (Mohd-Esa et al., 2010). Research reports also indicated its positive effect on prevention of cancer and improving digestibility in human beings. Anti-oxidant activity against low density lipoprotein (LDL) also has been found from the in vivo studies (Hirunpanich et al., 2006).
The bioavailability of the phenolic compounds is one of the greater interests in recent years. Various research works have been conducted to study the bioavailability of phenolic compounds and fibers. The phenolics in fruits and vegetable are bound to the dietary fibers and needs to be liberated for their health beneficial action (Gouw et al., 2017). This process is normally achieved in the gastrointestinal tract (GIT).
Discharge of phenolic substances from food products has been occurring in GIT through direct solubilization in GIT secretions and by enzymatic activity. During this process, a portion of phenolic components (low molecular weight) are absorbed in smaller intestine area and a section of high molecular weight phenolic substances are fermented by the microbes in GIT and a portion of phenolic components are absorbed by gut epithelial cells that act as pro-oxidants (Jung et al., 2015).
In vitro digestibility studies are useful in determining the efficacy of the release of phenolics in GIT. As compared to in vivo experiments, in vitro studies are economical and produces results quickly. In vivo experiments are suitable substitutes to animal and human models for prompt selection of nutritive components. A suitable in vivo digestibility method would offer precise results in a shorter time period and it will be useful in the examination of different food products for their bioavailability as well as digestibility studies (He et al., 2017).
Being a rising field in research and experiments many research studies have been conducted to study the in vitro digestibility of fruits and vegetables in recent times. The influence of in vitro digestibility on various bioactive components comprising antioxidants have been examined in many fruits and vegetables (Gouw et al., 2017; Nayak et al., 2020a).
The primary objective of this study was to observe the changes in bio active compounds including phenolic, flavonoid and vitamin C before and after in vitro digestion of green leafy vegetable powders produced from mustard greens and roselle leaves. The research study also involved in the analysis of physiochemical and functional properties of two green leafy vegetable (mustard greens and roselle leaves) powders.
Materials and methods
Materials
Fresh green leafy vegetables (mustard greens (MG), roselle leaves (RL)) were procured from a local farm situated near to CIT, Kokrajhar, Assam, India. Plant materials were cleaned in running tap water and unwanted portions were removed. Samples (MG & RL) were subjected to blanching treatment (100 °C for 2 min) followed by air drying in blotting paper. Then the samples were shifted to lyophilizer which was maintained at −75 °C.
After lyophilization, samples were crushed in a domestic mixer grinder and collected in separate zip-lock bags and stored in desiccator for further experiments. Chemicals, reagents and enzymes used in the analysis of MG & RL samples were procured from Sigma Aldrich, Bengaluru, Inida, Himedia and Merck, Mumbai, India.
Physiochemical analysis of wet and dry MG, RL
Moisture content and water activity
Moisture content of samples (MG & RL) was estimated according to the method of AOAC, 1995. 10 g of MG & RL samples were measured and placed in a hot air oven which was maintained at 105 °C. After 3 h of drying, samples were weighed and every 30 min the weight of the samples were estimated till no change in the samples weight of observed. The final moisture content of the samples was calculated on a dry basis.
Water activity of MG & RL samples was computed with the help of a water activity meter.
pH, titratable acidity (TA) and total soluble solid (TSS)
Wet and dry samples of MG and RL were tested for pH, TA, and TSS using the method followed by Cavender et al., 2014. Wet and dried samples (5 g) of MG and RL were weighed and mixed thoroughly with 45 ml of distilled water. It was then thoroughly mixed before being filtered through Whatman # 1 filter paper. The filtrate was collected for examining physico-chemical properties of MG and RL samples. A pH metre was used to measure the pH of the sample by taking 20–30 ml of resultant filtrate. Titratable acidity TA) of MG & RL was analysed by titrating the filtrate against 0.1 M NaOH. A handheld refractometer (MASTER-500, Atago, Japan) was used to calculate the TSS of MG & RL samples and the results of the experiments were given in terms of brix.
Color measurement
Color values of MG & RL samples was examined by using Hunter colorimeter as described by Nayak et al., (2018). The results of color analysis were given in terms of hue angle and Chroma.
Functional properties of dried vegetable powders (VP)
Oil adsorption Capacity (OAC)
The OAC of dried samples (MG & RL) was estimated by following the procedure of Nayak et al., (2020a). In a centrifuge tube, 1 g of MG & RL sample was taken along with olive oil (10 ml) and placed in the laboratory till next day morning. After overnight incubation, the mixtures were centrifuged for 5 min at 1500 rpm. The surplus oil was drained. The dried oil-adsorbed sample was reweighed. The OAC was measured in g oil/g dry weight (DW).
Water- holding capacity (WHC)
The computation of WHC of dried MG & RL samples was performed using the method suggested by Nayak et al., (2020a). 1 g of MG or RL sample was taken along with 30 ml of distilled water and mixed well. The mixture was subjected to centrifugation at 5000 rpm for 30 min. The supernatant was discarded and the dried sample was reweighed after it had absorbed water. The WHC was calculated as g water/g DW.
Swelling ability (SA)
The SA of MG & RL samples was analyzed using the method recommended by Nayak et al., (2020a). 1 g of MG & RL sample was taken and mixed well with 20 ml of distilled water and kept in the room temperature overnight to permit the fibers to swell. The excess water was drained, and the remaining residues were weighed again. The SA was calculated as mL/g DW.
Extraction of phenolic compounds from dried samples
Extraction of phenols from dried samples was carried out by using methanol and glacial acetic acid. During the extraction process, 3 g of MG & RL sample was incorporated into 60% ethanol and 1% glacial acetic acid (30 ml). The resultant mixture was kept in an ultrasonictor bath for 20 min. After ultrasonication, the mixture was filtered and the filtrate was placed in rotary vacuum evaporator to perform the evaporation at 50 °C for 10 min. The remaining sample was mixed with 25 ml of distilled water for analysis (Gouw et al., 2017).
Total phenolic content (TPC)
The estimation of total phenols has been carried out by using the method followed by.
Cavender et al., (2014). The results of the experiments were expressed in terms of mg of gallic acid equivalents (GAE)/g DW.
Total flavonoids content (TFC)
Colorimetric method was applied for the assessment of TFC in the MG & RL extracts (Krishnan et al., 2020). TFC values were shown as mg of quercetin equivalents (QE)/g DW.
Radical scavenging activity (RSA)
RSA was assessed using a colorimetric assay (DPPH method) with a slight modification (Babuskin et al., 2015). The absorbance obtained was expressed as a percentage of absorbance.
Vitamin C Analysis
Vitamin C of the dried samples were calculated by 2, 6-dichloroindophenol titrimetric method (Nayak et al., 2020b).
In vitro gastrointestinal study of MG and RL powders
In vitro studies of vegetable powders (MG & RL) were performed according to the method followed by Nayak et al., 2020a. The whole digestion process was split into 3 stages with respect to the areas at which it happens in our body namely, mouth, stomach and intestines, respectively.
Digestion in mouth has been initiated by adding 1 g of vegetable powder (MG & RL) with 8.5 ml of phosphate buffer (pH-7, 0.05 M), 0.5 ml of -amylase (20 FAU/g) and 150 µl of 50 mM CaCl2. The mixture was vortexed well and incubated in an incubator-shaker water bath (LabTech, Daihan Labtech, India) at a temperature of 37 °C with 50 rpm for 2 min.
In order to proceed to stomach digestion, the sample was removed after 2 min and 5 ml of distilled water, 1 ml of 0.2% porcine pepsin solution and 30 µl of 50 mM CaCl2 were incorporated into the reaction mixture. 1 M HCl was added to the mixture to achieve the pH of 3. Then, the volume of the mixture was moved up to 20 ml by including distilled water into the mixture and placed in a shaking water bath which was maintained at 37 °C with 200 rpm for 2 h.
After 2 h, the sample was removed from the water bath to undergo intestinal digestion with simulated intestinal fluid (SIF). The sample from the gastric compartment was mixed with SIF containing 10 ml of phosphate buffer (pH-7, 0.05 M), 3 ml of intestinal juice (12.5 g of bile and 2 g of porcine pancreatin in 0.1 M NaHCO3) and 50 mM of CaCl2 (240 µl). 1 M of NaOH was incorporated into the mixture to achieve the pH of 7. The mixture was placed in a shaking water bath (37 °C) for 2 h at 200 rpm.
After the incubation period, the samples were instantly centrifuged in order to avoid further enzymatic activity. The supernatant was separated after centrifuging at 6000 RPM at 4 °C for 20 min in a cooling centrifuge. Supernatant portion was stored at 4 °C for further evaluation of vegetable powder samples.
Statistical analysis
The experiments and analysis in our work were performed in triplicates. The results from the research work are expressed in mean value ± SD. The experimental data was analyzed using ANOVA (SPSS Statistics 17.0) at p < 0.05.
Result and discussion
Physiochemical analysis
The physicochemical properties of dried samples of MG and RL were represented in Table 1. The moisture content (MC) of the wet samples was decreased from 95.26 ± 3.97 and 96.13 ± 2.76% to 12.36 ± 0.54 and 10.25 ± 0. 38% for MG and RL samples respectively. The aw values of the samples were also observed to be lowered from 0.98 ± 0.02 and 0.99 ± 0.03 to 0.21 ± 0.02 and 0.20 ± 0.02 for wet and dry MG & RL samples.
Table 1.
Physicochemical properties of wet & dried vegetable samples
| SAMPLE/PARAMETRES | Mustard greens (MG) | Roselle leaves (RL) | ||
|---|---|---|---|---|
| WMG | DMG | WRL | DRL | |
| Moisture content (%) | 95.26 ± 3.97 | 12.36 ± 0.54 | 96.13 ± 2.76 | 10.25 ± 0. 38 |
| Water Activity(aw) | 0.98 ± 0.01 | 0.21 ± 0.02 | 0.99 ± 0.01 | 0.20 ± 0.02 |
| Total soluble solids (°brix) | 3.00 ± 0.00 | 2.00 ± 0.10 | 5.00 ± 0.10 | 2.00 ± 0.10 |
| pH | 4.52 ± 0.11 | 4.78 ± 0.08 | 4.01 ± 0.09 | 4.98 ± 0.10 |
| Titratable acidity (TA, %) | 0.34 ± 0.02 | 0.26 ± 0.01 | 0.63 ± 0.02 | 0.42 ± 0.01 |
| Color values | ||||
| Chroma | 15.26 ± 0.42 | 13.88 ± 0.36 | 14.52 ± 0.52 | 13.65 ± 0.39 |
| Hue | 54.36 ± 1.21 | 59.33 ± 1.31 | 65.82 ± 1.39 | 69.46 ± 1.24 |
The MC and aw values of MG & RL samples significantly influence the stability of the powders, storage characteristics and other technical characteristics (Wang et al., 2020). After freeze drying process, the aw values of MG & RL powders were decreased. The aw value of the food products designate the free water presents in foods, which also has the detrimental impact on the keeping quality. From the aw values of freeze-dried MG & RL powders, it can be understood that the aw levels were within the projected range for the food powders as well as the aw values of MG & RL powders within the suggested range (< 0.60) to assure the microbial safety (Wang et al., 2020).
Research works indicated that lowering both moisture content and water can be helpful in increasing the shelf life of vegetable powder by inhibiting microbial infestation. Further, the combined effect of aw and moisture content was reported to restrict the growth of Salmonella in wheat flour. Similarly leafy vegetables which has high moisture content can be preserved only by lowering their moisture content and aw using different drying or dehydration techniques (Garces-Vega et al., 2019).
The TSS content of the wet and dry samples of MG was recorded as 3.00 ± 0.00 and 2.00 ± 0.10°brix, whereas RL samples (wet and dry) showed the TSS level of 5.00 ± 0.10 and 2.00 ± 0.10, before and after drying, respectively. From the data, it can be understood that the total solid content of the leafy vegetables was found to be very low. Fasoyiro et al., (2005) reported an approximate TSS content of Roselle extract as 3.20 ± 0.633°brix, which is consistent with our findings.
The pH of the samples was slightly increased after freezing dehydration, but the difference was not statistically significant for either. Vegetables and its powders with a neutral pH range (4–6) can help to extend the shelf life by impeding microbial growth and escalation (Shin et al., 2015).The titratable acidity of the MG and RL samples, on the other hand, was found to be reduced from 0.34 and 0.63 percent to 0.26 and 0.42 percent. RL was found to have higher titratable acidity than RL, but studies have found an average of 2.400.38% titratable acidity in Roselle calyxes (Fasoyiro et al., 2005). In this study, only Roselle leaves were used to make RL powder, which could be the titratable acidity is much lower in our case.
Prior to drying, the chroma values of MG and RL samples were obtained as 15.26 ± 0.42 and 14.52 ± 0.52, respectively and the values were found to be decreased to 13.88 ± 0.36 and 13.65 ± 0.39, respectively after drying for MG and RL samples (Table 1). An increment in hue values was observed in both samples and it can be attributed to the method of drying (freeze drying) used in the study. While conventional drying methods (like hot air drying) can affect the color pigments like chlorophyll present in leaf and degrade at higher temperatures, but freeze drying does not directly affect the color pigments which may be resulted in a low increment of hue values. The low temperature drying may helped in the retention of color pigments resulting in minor difference between chroma values of wet and dried sample (Shin et al., 2015).
Overall, the physicochemical properties of wet MG and RL samples are affected by the variety and species of fruit, as well as the source of origin. These properties can be preserved with the right treatments. As a result of the various treatments, dried samples will have slightly different properties than wet samples. The difference in dried sample is frequently attributed to the effect of processing parameters and factors, as well as the method at which the samples have been processed (Raghavendra et al., 2006).
Functional properties
The functional properties of both the MG and RL samples has been displayed in Table 2. The knowledge of functional properties is frequently required for product development (Soria-Hernández et al., 2015). During product development, hydration properties such as water holding capacity (WHC) and swelling ability (SA) can be used to determine processing parameters such as syneresis and viscosity. While oil absorption capacity (OAC) is significant due to the fact that fats present in foods frequently act as flavor retainers and contribute to oxygen mediated spoilage reactions (Requena et al., 2016).
Table 2.
Functional properties of dried vegetable powders
| Sample/Properties | Roselle leaves (RL) | Mustard greens (MG) |
|---|---|---|
| OAC (g oil/g dry weight (DW)) | 1.85 ± 0.02 | 2.01 ± 0.03 |
| WHC (g water/g DW) | 2.57 ± 0.02 | 3.30 ± 0.02 |
| SA (ml/g DW) | 13.89 ± 0.31 | 12.52 ± 0.27 |
The oil absorption capacity (OAC) of MG and RL powders was found to be 1.85 ± 0.02 and 2.01 ± 0.03 g oil/g DW, respectively. OAC can be defined as the capacity of the materials to conserve the oil in foods and it has been highly related to the particle size of food materials. Dietary fibres which are bind to fats, oils, and water in foods are the main components which can influence OAC of the vegetable powder. The differences in OAC of MG and RL powders can be attributed to the presence of dietary fibres, which can entrap both protein and non-protein components (Nayak et al., 2020a).
The water holding capacity (WHC) of MG and RL were found to be 2.57 ± 0.02 and 3.30 ± 0.02 water/g DW, respectively. According to Alfredo et al., (2009), WHC can be defined as the ability of the material (vegetable powder) to retain water when subjected to an external centrifugal gravity force or compression. Swelling ability (SA) of MG and RL powders was obtained as 13.89 ± 0.31 and 12.52 ± 0.27 ml/g DW, respectively (Table 2). WHC and SA of vegetable powders has been linked with the amount of dietary fibre present in it. Further, SA of vegetable powders has been depend on the characteristics of individual components as well as the physical structure of the matrix, such as porosity and crystallinity (Raghavendra et al., 2006).
The WHC and SA of the MG and RL powders has been found to be low as compared to the data reported by the researchers for various vegetables. It may be due to the fact that the WHC and SA are directly proportional to the amount of dietary fibre present in the vegetables, the levels of WHC and SA may be higher in fibrous vegetables than leafy plants like MG and RL. It has to be mentioned that the higher WHC values corelated to higher hydration in foods, especially it is important in food products such as soups and supplements (Soria-Hernández et al., 2015).
Effect of in-vitro digestion (IVD) on TPC, TFC, RSA and vitamin C levels
The data obtained for TPC, TFC, RSA and vitamin C levels in MG & RL powders (before & after in-vitro digestion) was presented in Fig. 1(a), (d).
Fig. 1.
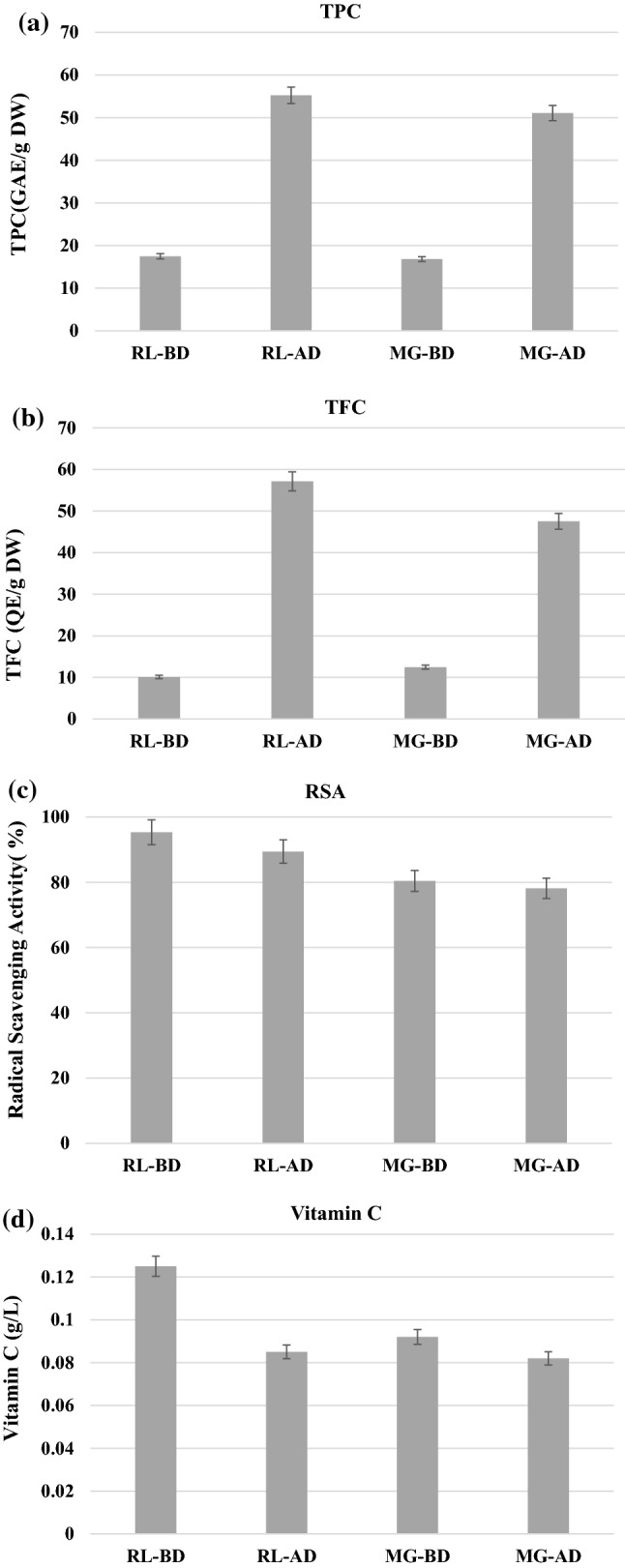
a TPC of vegetable powders before and after in-vitro digestion, b TFC of vegetable powders before and after in-vitro digestion, c RSA of vegetable powders before and after in-vitro digestion, d Vitamin C contents of vegetable powders before and after in-vitro digestion. RL-BD – roselle leaves (before digestion); RL- AD – roselle leaves (after digestion); MG-BD – mustard greens (before digestion); MG-AD – mustard greens (after digestion)
Before digestion, the TPC of MG and RL was found to be 16.83 ± 0.72 and 17.50 ± 0.69 mg GAE/g DW, respectively (Fig. 1a). The yield of phenolic compounds was influenced by the solvent type, mode of extraction, and the content of phenolic compounds in the different species of fruits and vegetables (Wang et al., 2016). In addition, the loss of phenolic compounds may also occur during the extraction process also. Mustard greens (Brassica juncea var. gemmifera), which has many edible parts including buds and leaves, had the highest TPC of 17.66 mg GAE/g DW in the lateral leaf buds (Sun et al., 2018). Other studies have reported a TPC of 12.8–16.10 ± 0.344 mg GAE/g DW in mustard green sprouts (Sun et al., 2018). The findings of our study are consistent with previously published reports. The species and variants of mustard greens can also have an effect on the TPC levels as confirmed by the results obtained in various studies. Puangkam et al., (2017) discovered high TPC level (197.80 ± 01 mg GAE/g DW) in mustard green leaves which were grown in Thailand. It can be concluded that, in addition to cultivars, the source and treatment of raw vegetables can have an impact on the TPC of samples.
TPC of MG & RL powders has been increased to 51.08 ± 2.05 and 55.22 ± 2.12 mg GAE/g DW, respectively (Fig. 1a), after in vitro digestion, which was approximately by three-fold than the TPC levels before digestion. A study published by Puangkam et al., (2017) showed a minimal change in the TPC of mustard greens after in vitro digestion from 14.12 to 13.33 g GAE/100 g DW. In the study of Puangkam et al., (2017), it was was stated that the change in TPC level may be due to the fact that the dietary supplements were sensitive to alkaline solutions and were thus affected by the intestinal pH of the simulation process. As our research work has been carried out in the pH level of 7 in the intestinal phase, the decrease in TPC level was not observed after digestion process. In fruits, TPC was found to be increased after in vitro digestion, indicating that TPC of fruit is unaffected by such facts (Nayak et al 2020a).
The TFC of the MG and RL followed a similar trend as TPC where it was found to be increased from 10.11 ± 0.44 and 12.48 ± 0.52 mg of QE/g DW in dry sample to 57.15 ± 2.31 and 47.54 ± 2.05 mg of QE/g DW after digestion process in MG and RL (Fig. 1b). The increase in TFC levels in MG and RL after digestion process can be attributed to the fact that the dietary fibers in leafy vegetables were untangled during the digestion process freeing the phenols and flavonoids and resulting in an sharp increase in their amounts (Gouw et al., 2017). Further, the increase in TFC levels in MG & RL may also be ascribed to modifications in phenolic compounds in each stage of in vitro digestion process (Nayak et al 2020a). Flavonoids are well known for their antioxidant and anti-inflammatory health benefits. The high quantity of flavonoids in fruits or vegetables can also aid against lung and oral cavity cancer (Pant et al., 2020). Although an increase in TPC and TFC was obtained in our study, some researchers have found a decrease in polyphenols and flavonoids, as well as antioxidant capacity, in some fruits and vegetables, citing phenomena such as polymerization, oxidation, and interaction between different dietary compounds as the cause (Sun et al., 2018).
The radical scavenging activity (RSA) of the samples was determined by measuring the powders' ability to reduce free DPPH radicals, and the results were shown in Fig. 1c. From the figure, it can be detected that RSA values of MG & RL samples were found to be higher prior to drying and the RSA values were recorded as 95.30 ± 3.91 and 80.40 ± 3.12%. However, after the in vitro digestion process, the RSA values were reduced to om 89.42 ± 3.38 and 78.13 ± 2.73%. The decrease in the DPPH activity after in vitro digestion was also reported in other literatures also. The decrease in RSA values may be ascribed to the sensitivity of antioxidant compounds to pH as well as the degradation antioxidant compounds due to the presence of enzymes. The pH of a substance can also affects the racemization of molecules, resulting in a change in configuration and rendering the molecule to inactive state in the digestion process. This can also be the result of presence of various antiradical compounds in the matrix of the vegetable powder enabling their mechanism during digestion process (Bermúdez-Soto et al., 2007). Puangkam et al., (2017) also observed a decrease from 8.18 ± 0.10 to 4.93 ± 0.04 mM TE/100 g after digestion process in MG powder samples. They discovered a significant difference in the change in RSA when compared to TPC and concluded that phenolic compounds are not the only ones that provide antioxidant activity; rather, those responsible for antioxidant activity reduction are also susceptible to digestion condition. Our previous work in the in vitro digestion of fruit pomaces also produced a similar trend in RSA values by DPPH assay (Nayak et al., 2020b).
The vitamin C content of the samples before and after in vitro digestion was presented in Fig. 1d. From the figure, it can be understood that an insignificant variation in vitamin C level of MG and RL samples can be seen before and after digestion process. Prior to digestion, the vitamin C level of MG and RL sample was registered as 0.125 ± 0.005 and 0.092 ± 0.004 and it was seemed to be decreased to 0.085 ± 0.002 and 0.082 ± 0.002, respectively for MG and RL samples. The decrease in vitamin C values was found to follow the same trend as the RSA values of MG and RL samples, after in vitro digestion. The reduction in vitamin C levels after digestion may be due to that the acidic environment in gastric digestion may influence the decrease in vitamin C. Further, the oxidation of vitamin C owing to its pro-oxidant nature also resulted in its decrease, after digestion process. It may also be connected with the production of metal oxygen ascorbate complexes which leads to the decrease in vitamin C levels in fruits and vegetables (Rodríguez-Roque et al., 2013).
Conclusions
The present study focused on the physicochemical properties, functional properties, and in vitro digestion of a freeze-dried samples of mustard greens and roselle leaves. The results from the physicochemical and functional analysis may be useful in the functional food product development processes. The analysis of phenolic compounds and antioxidants before and after in vitro digestion provided an overall view about the various factors which causes its changes before and after the digestion process. The phenolic contents of the MG and RL powders is based on the extraction efficiency and is also related to the morphology and fibre content of the vegetables. Phenolic compounds which are mainly trapped inside the dietary fibres of the vegetables has been be released through stimulated gastrointestinal digestion by the action of pH and enzymes. The results also showed that in vitro digestion increased total phenol and flavonoid content. Further research into the bioavailability of vegetable powders can aid in the development of new functional food products, as they are reported to be important natural antioxidants for preventing diseases caused by oxidative stress, such as cardiovascular disease and diabetes mellitus.
Acknowledgements
Authors sincerely expresses the greatest gratitude to Central Institute of Technology Kokrakhar, Deemed to be University under MoE, Govt. of India for carrying out this research work.
Abbreviations
MG
Mustard greens
RL
Roselle leaves
NCD
Non communicable diseases
CVD
Cardio vascular diseases
WHO
World Health Organization
LDL
Low density lipoprotein
GIT
Gastrointestinal tract
TA
Titratable acidity
TSS
Total soluble solids
VP
Vegetable powders
OAC
Oil absorption capacity
DW
Dry weight
WHC
Water holding capacity
SA
Swelling ability
TPC
Total phenolic content
GAE
Gallic acid equivalents
TFC
Total flavonoid content
QE
Quercetin equivalents
RSA
Radical scavenging activity
DPPH
2, 2-Diphenyl-1-picrylhydrazyl
SIF
Simulated intestinal fluid
ANOVA
Analysis of variance
MC
Moisture content
aw
Water activity
WMG
Wet Mustard greens
DMG
Dry Mustard greens
WRL
Wet Roselle leaves
DRL
Dry Roselle leaves
RL-BD
Roselle Leaves (before digestion);
RL- AD
Roselle Leaves (after digestion);
MG-BD
Mustard Greens (before digestion)
MG-AD
Mustard Greens (after digestion)
Author’s Contributions
PKN: Formal analysis; investigation; methodology; supervision; visualization; writing-review & editing. AS: Data curation; investigation; methodology; validation; visualization; writing original draft. RKK: Conceptualization; data curation; investigation; methodology; supervision; visualization; writing-original draft; writing review & editing.
Funding
None.
Data availability
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research. Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Code availability not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
All authors gave their consent to participate in the publication, as well as the responsible authorities at the institute where the work has been carried out. All authors gave their consent for publication of the work.
Ethical approval
Ethics approval was not required for this research. This study does not involve any human or animal testing.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . Official methods of analysis. Washington DC: Association of official analytical chemists; 1995. [Google Scholar]
- Alfredo VO, Gabriel RR, Luis CG, David BA. Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.) LWT-Food Sci Technol. 2009;42(1):168–173. doi: 10.1016/j.lwt.2008.05.012. [DOI] [Google Scholar]
- Arfaoui L. Dietary plant polyphenols: effects of food processing on their content and bioavailability. Molecules. 2021;26(10):2959. doi: 10.3390/molecules26102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babuskin S, Radhakrishnan K, Babu PAS, Sukumar M, Fayidh MA, Sabina K, Archana G, Sivarajan M. Effects of rosemary extracts on oxidative stability of chikkis fortified with microalgae biomass. J Food Sci Technol. 2015;52(6):3784–3793. doi: 10.1007/s13197-014-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102(3):865–874. doi: 10.1016/j.foodchem.2006.06.025. [DOI] [Google Scholar]
- Cavender G, Liu M, Hobbs D, Frei B, Strik B, Zhao Y. Effects of different organic weed management strategies on the physicochemical, sensory, and antioxidant properties of machine-harvested blackberry fruits. J Food Sci. 2014;79(10):S2107–S2116. doi: 10.1111/1750-3841.12639. [DOI] [PubMed] [Google Scholar]
- Fasoyiro SBB, Babalola SOO, Owosibo T. Chemical Composition and Sensory Quality of Fruit-Flavoured Roselle (Hibiscus sabdariffa) Drinks. World J Agric Sci. 2005;1(2):161–164. [Google Scholar]
- Garces-Vega FJ, Ryser ET, Marks BP. Relationships of water activity and moisture content to the thermal inactivation kinetics of salmonella in low-moisture foods. J Food Prot. 2019;82(6):963–970. doi: 10.4315/0362-028X.JFP-18-549. [DOI] [PubMed] [Google Scholar]
- Gouw VP, Jung J, Zhao Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT-Food Sci Technol. 2017;80:136–144. doi: 10.1016/j.lwt.2017.02.015. [DOI] [Google Scholar]
- He M, Zeng J, Zhai L, Liu Y, Wu H, Zhang R, Li Z, Xia E. Effect of in vitro simulated gastrointestinal digestion on polyphenol and polysaccharide content and their biological activities among 22 fruit juices. Food Res Inter. 2017;102:156–162. doi: 10.1016/j.foodres.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, Suthisisang C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol. 2006;103(2):252–260. doi: 10.1016/j.jep.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Jung J, Cavender G, Zhao Y. Impingement drying for preparing dried apple pomace flour and its fortification in bakery and meat products. J Food Sci Technol. 2015;52(9):5568–5578. doi: 10.1007/s13197-014-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. Nutritional and health importance of hibiscus sabdariffa: a review and indication for research needs. J Nutri Health Food Eng. 2017 doi: 10.15406/jnhfe.2017.06.00212. [DOI] [Google Scholar]
- Krishnan KR, Rayaguru K, Nayak PK. Ultra-sonicated vacuum drying's effect on antioxidant activity, TPC, TFC and color of elephant apple slices. Food Biosci. 2020;36:100629. doi: 10.1016/j.fbio.2020.100629. [DOI] [Google Scholar]
- Lee JJ, Kim HA, Lee J. The effects of Brassica juncea L Leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet. Nutr Res Pract. 2018;12(4):298–306. doi: 10.4162/nrp.2018.12.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Mohd-Esa N, Hern FS, Ismail A, Yee CL. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010;122(4):1055–1060. doi: 10.1016/j.foodchem.2010.03.074. [DOI] [Google Scholar]
- Nayak PK, Chandrasekar CM, Sundarsingh A, Kesavan RK. Effect of in-vitro digestion on the bio active compounds and biological activities of fruit pomaces. J Food Sci Technol. 2020;57(12):4707–4715. doi: 10.1007/s13197-020-04507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak PK, Mohan C, Radha Krishnan K. Effect of microwave pretreatment on the color degradation kinetics in mustard greens (Brassica juncea) Chem Eng Commun. 2018;205:1261–1273. doi: 10.1080/00986445.2018.1446003. [DOI] [Google Scholar]
- Nayak PK, Basumatary B, Chandrasekar CM, Seth D, Kesavan RK. Impact of thermosonication and pasteurization on total phenolic contents, total flavonoid contents, antioxidant activity, and vitamin C levels of elephant apple (Dillenia indica) juice. J Food Process Eng. 2020;43(8):e13447. doi: 10.1111/jfpe.13447. [DOI] [Google Scholar]
- Pant U, Bhajan R, Singh A, Kulshesthra K, Singh AK, Punetha H. Green leafy mustard: a healthy alternative. Electron J Plant Breed. 2020;11(1):267–270. doi: 10.37992/2020.1101.045. [DOI] [Google Scholar]
- Puangkam K, Muanghorm W, Konsue N. Stability of bioactive compounds and antioxidant activity of thai cruciferous vegetables during In vitro digestion. Curr Res Nutr Food Sci. 2017;5(2):100–108. doi: 10.12944/CRNFSJ.5.2.06. [DOI] [Google Scholar]
- Raghavendra SN, Ramachandra Swamy SR, Rastogi NK, Raghavarao KSMS, Kumar S, Tharanathan RN. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J Food Eng. 2006;72(3):281–286. doi: 10.1016/j.jfoodeng.2004.12.008. [DOI] [Google Scholar]
- Requena MC, González CNA, Barragán LAP, Correia T, Esquivel JCC, Herrera RR. Functional and physico-chemical properties of six desert-sources of dietary fiber. Food Biosci. 2016;16:26–31. doi: 10.1016/j.fbio.2016.08.001. [DOI] [Google Scholar]
- Rodríguez-Roque MJ, Rojas-Graü MA, Elez-Martínez P, Martín-Belloso O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice. J Agric Food Chem. 2013;61(8):1859–1867. doi: 10.1021/jf3044204. [DOI] [PubMed] [Google Scholar]
- Shin LER, Zzaman W, Kuang YT, Bhat R. Influence of dehydration techniques on physicochemical, antioxidant and microbial qualities of ipomoea aquaticaforsk: an underutilized green leafy vegetable. J Food Process Preserv. 2015;39(6):1118–1124. doi: 10.1111/jfpp.12326. [DOI] [Google Scholar]
- Soria-Hernández C, Serna-Saldívar S, Chuck-Hernández C. Physicochemical and functional properties of vegetable and cereal proteins as potential sources of novel food ingredients. Food Technol Biotechnol. 2015;53(3):269–277. doi: 10.17113/ftb.53.03.15.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Tian YX, Jiang M, Yuan Q, Chen Q, Zhang Y, Luo Y, Zhang F, Tang HR. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var gemmifera) RSC Adv. 2018;8(59):33845–33854. doi: 10.1039/C8RA05504A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Deng F. Phytochemistry and biological activity of mustard (Brassica juncea): a review. CYTA J Food. 2020;18(1):704–718. doi: 10.1080/19476337.2020.1833988. [DOI] [Google Scholar]
- Wang W, Jung J, Tomasino E, Zhao Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT-Food Sci Technol. 2016;72:229–238. doi: 10.1016/j.lwt.2016.04.041. [DOI] [Google Scholar]
- Wang H, Tong X, Yuan Y, Peng X, Zhang Q, Zhang S, Xie C, Zhang X, Yan S, Xu J, Jiang L. Effect of spray-drying and freeze-drying on the properties of soybean hydrolysates. J Chem. 2020 doi: 10.1155/2020/9201457. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research. Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Code availability not applicable.