Glycoalkaloids of Plants in the Family Solanaceae (Nightshade) as Potential Drugs (original) (raw)
Abstract
Worldwide interest in medicinal plants and related drugs is growing because of the increased spectrum of new synthetic drugs. In this context, secondary plant metabolites are most significant. This review analyzes data on the structures and biosyntheses of metabolites such as glycoalkaloids; methods for their extraction from plants of the family Solanaceae, particularly potato S. tuberosum; their qualitative and quantitative analysis; biological activity; and toxicity. This information could be useful in the selection of methods for sample preparation and extraction of glycoalkaloids during the search for new plant sources with prospects of creating effective and safe pharmacological agents.
Keywords: glycoalkaloids, α-solanine, α-chaconine, potato, pharmacological activity
The demand for medicinal plants as such and products based on them is currently increasing in many countries [1, 2]. In Russia, the interest in medicinal plants as sources of raw material for drug manufacturing aligns with global trends, i.e., the number of manufacturers producing drugs of plant origin and the number of consumers using phytotherapy as a milder and complex treatment method are increasing [3].
Plants contain carbohydrates, amino acids, nucleotides, fatty acids, and chlorophylls, which are called primary metabolites, and broad spectra of compounds not involved in growth, development, and reproduction, which are usually called secondary metabolites or compounds of secondary origin. Secondary metabolites can be specific for one or several plant species, in contrast to primary metabolites, which are present in all plant cells [4]. These compounds most often fulfill ecological functions, i.e., protect the plant from various pests and pathogens, impart color and fragrance to flowers and fruit, and facilitate interaction of plants among themselves and with other organisms in the ecosystem. Several secondary metabolites absorb ultraviolet radiation, thereby having an antioxidant effect, in addition to protecting plants from bacteria, fungi, and viruses [5]. Several secondary metabolites are extremely toxic.
Plants produce large quantities of secondary metabolites that are divided into three main groups depending on their biosynthetic pathway. These are terpenoids, phenolic compounds, and alkaloids [6]. Alkaloids are especially interesting.
Plants frequently contain alkaloids in a glycoside form as glycoalkaloids (GAs). Representatives of the family Solanaceae contain significant amounts of them and include many agricultural crops that are useful for man, e.g., tobacco (Nicotiana spp.); bell pepper (Capsicum annuum); eggplant (Solanum melongena); tomato (S. lycopersicum); and the most significant plant of all, potato (S. tuberosum) [7]. They all are used primarily as sources of carbohydrates in animal husbandry and the food industry. They are promising for use as raw materials for GA production. It is noteworthy that none of the above plants of the family are official, their phytochemical analyses are not given, and detailed descriptions of extraction methods for target compounds and their physicochemical analyses are lacking.
A literature search showed that many scientific articles published before 2000 report the physicochemical properties of GAs, their extraction methods from plant raw material, and their pharmacological effects. The research results given in them (or like them) are often not cited in more contemporary sources. An attempt was made in the literature review to systematize data on GAs from plants of the family Solanaceae considering both earlier articles and contemporary information obtained using highly sensitive methods and technologies.
Structures and biosyntheses of GAs
GAs consist of two structural components, i.e., the aglycon structure based on the C27 cholestane skeleton with additional N-containing rings that provide basicity and an oligosaccharide fragment. The aglycons are divided into five categories depending on their structure, i.e., solanidanes, spirosolanes [8], epiminochloestanes, α-epiminocyclohemiketals, and 3-aminospirostanes [9].
At least 90 structurally unique steroidal alkaloids were identified in >350 species in the genus Solanum.
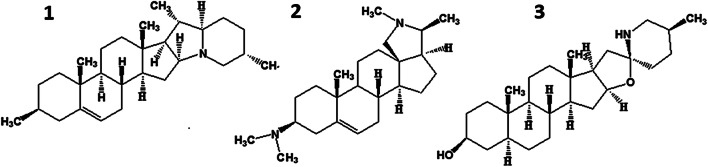
Nitrogen can be added as a primary NH2 (free or methylated) to form simple steroidal bases (e.g., conessine), with a closed ring incorporating a skeletal or side-chain C atom as a secondary NH (e.g., tomatidine), or annelated in two rings as a tertiary N (e.g., solanidine) (Fig. 1) [10].
Fig. 1.
Structural formulas of the aglycons solanidine (1), conessine (2), and tomatidine (3).
The structures of the oligosaccharide moieties are responsible for much of the biological activity of GAs. The carbohydrate part of steroidal alkaloids can include _D_-glucose, _D_-galactose, _L_-rhamnose, L_-arabinose, D_-xylose, and _L_-fructose. The biosynthesis of GAs involves a mechanistic pathway that consists of three steps [11]. Primary metabolites are synthesized in the first two steps and form cycloartanol and cholesterol, respectively. GAs are formed from the common precursor cholesterol in the third step. Cholesterol is transformed into the aglycon solanidine, which is then transformed into solanine and chaconine via formation of glycoside bonds to the sugars [12].
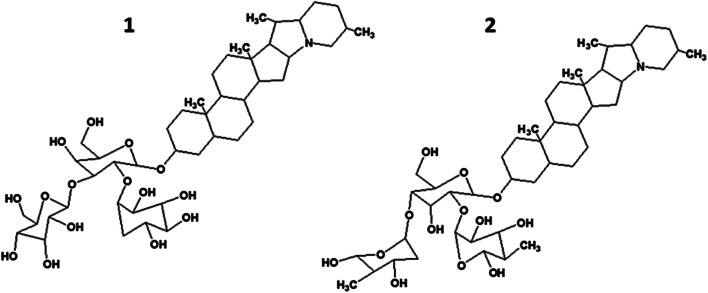
The first GA isolated from potato (S. tuberosum) was solanine. It was subsequently proven that solanine was a mixture of two components, i.e., α-solanine and α-chaconine (Fig. 2) [13]. Both GAs function as natural pesticides, protecting the plant from fungi, herbivorous animals, and insects. Sunlight, mechanical damage, aging, and other stress factors increase their synthesis [14]. They have the same aglycon, solanidine, but different oligosaccharide fragments. α-Chaconine has a branched carbohydrate side chain, α-chacotriose (bis-α-_L_-rhamnopyranosyl-β-_D_-glucopyranose), bonded to the aglycon 3-OH group. α-Solanine has a side chain of α-solatriose (α-_L_-rhamnosyl-β-_D_-glucopyranosyl-β-galactopyranose) also bonded to the same aglycon 3-OH group.
Fig. 2.
Structural formulas of potato (Solanum tuberosum) GAs α-solanine (1) and α-chaconine (2).
The letters α, β, and γ in named GAs denote an inactive trisaccharide, disaccharide, or monosaccharide, respectively, that is formed upon hydrolysis of the glycoside bonds in their molecules [15].
The GA yields in various studies are difficult to compare because the contents of target compounds in potato are highly variable due to various habitats and subsequent storage conditions and several extraction methods [16]. However, researchers agree that the highest levels of GAs occur in flowers (according to various data from 3000 mg/kg for fresh mass to 30,000 mg/kg upon drying), sprouts (from 2000 mg/kg for fresh mass to 10,000 mg/kg upon drying), bitter tuber skin (from 1500 to 2200 mg/kg of fresh mass), and potatoes [17].
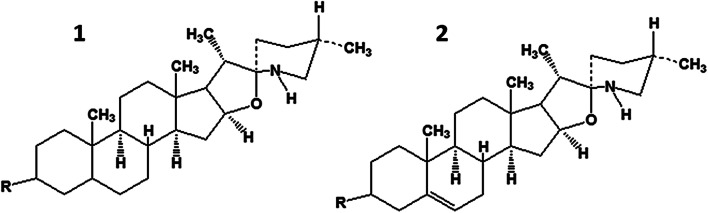
About 100 steroidal alkaloids were detected in various tissues in early development stages of tomato plants (S. lycopersicum) [18, 19]. Representatives of this family contained GAs of the spirosolane (α-tomatine) and dehydrotomatine types (differs by a double bond in steroid ring B) (Fig. 3). Both GAs had the same tetrasaccharide side chain (lycotetraose) but structurally different aglycons. α-Tomatine had the aglycon tomatidine; dehydrotomatine, tomatidenol [20].
Fig. 3.
Structural formulas of tomato (Solanum lycopersicum) alkaloids α-tomatine (1) and dehydrotomatine (2).
All parts of the tomato plant, including leaves, stems, and fruit, contain tomatine and dehydrotomatine. Unripe green tomatoes contain up to 500 mg of α-tomatine per kg of fruit. However, tomatine decomposes significantly as the fruit ripens to 5 mg/kg of mass of fresh red tomatoes [21]. The contents of dehydrotomatine/α-tomatine in various parts of tomato (mg/kg of fresh mass) were 14/144 in large unripe green fruit; 54/465, small unripe green fruit; 33/118, roots, 33/118; 71/975, leaves; and 190/1100, flowers [7].
Isolation of the spirosolane GA esculeoside from ripe cherry tomatoes was also reported [22].
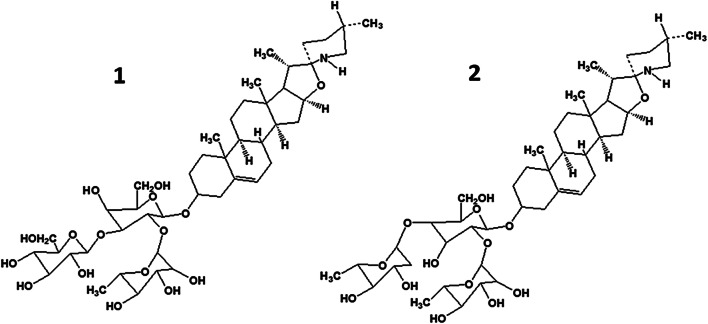
Eggplant (S. melongena) contained the two steroidal GAs solasonine and solamargine (Fig. 4). They were constructed from the same aglycon (solasonine) but had different trisaccharide side chains. Solasonine included solatriose (α-_L_-rhamnopyranosyl-β-_D_-glucopyranosyl-β-galactopyranose); solamargine, chacotriose [23].
Fig. 4.
Structural formulas of eggplant (Solanum melongena) glycoalkaloids solasonine (1) and solamargine (2).
Thus, eggplant GAs differed from those observed in potato only by the structure of the steroid part. They had identical carbohydrate side chains. A study of several eggplant varieties showed that the GA contents usually increased during development and ripening of the fruit [24]. A calorimetric study of 21 S. melongena varieties showed that the GA contents varied from 6.25 to 20.5 mg/100 g of fresh mass (average 11.3 mg/100 g) [25].
Plants of the genus Petunia, which also belong to the Solanaceae family, may be of interest as raw material for GA production. Cultivated varieties of petunia have broad areal distributions. It is not a pharmacopoeial plant and is not used in medicine. The phytochemical composition and pharmacological properties are practically unstudied. However, alkaloids in seeds from fruit of this plant have been reported in the scientific literature.
Thus, GAs are widely distributed in plants of the family Solanaceae and accumulate in various amounts in all their parts. They have different chemical structures that are responsible for the broad spectrum of possible pharmacological effects.
Further discussion will focus on potato GAs as potential pharmacological compounds because of the significant amount of waste from food manufacturing (70 – 140 tons of potato peels yearly around the world) (besides the broad spectrum of identified biological effects) that is discarded in dumps and leads to ecological problems or is used as low-quality feed for animals [26].
Methods for extraction of potato GAs
GAs are polar poorly stable compounds that are slightly soluble in H2O at pH ≥ 7 while the aglycon solanidine is nonpolar [27]. Several researchers ascribed its greater absorption from the gastrointestinal tract (GIT) to its nonpolar nature [28]. A typical method for extraction of GAs is liquid extraction at atmospheric pressure [29]. Methods for extraction of solanine from plant raw material using acidified aqueous solutions or organic solvents are based on the chemical properties of GAs. Simultaneous treatment with acids and heating is rarely used in order to avoid hydrolysis of the glycoside bonds. The solvents recommended by researchers are HOAc (2%, for dried tubers), MeOH–CHCl3 (2:1, for fresh tubers), or THF–H2O–MeCN–HOAc (glacial) (500:300:200:10 v/v, for lyophilized plants) [13].
Liquid extraction methods under pressure using low-boiling solvents or their mixtures at elevated temperatures (up to 200°C) and pressures (up to 214 atm) are also known. This increases the solubility of the target compound, diffusion rate of the solvent, and mass transfer and decreases the surface tension and extractant viscosity [16, 30].
MeOH, EtOH, and HOAc (5%) showed the greatest percent extraction of GAs from potato peels by liquid extraction under pressure; MeCN and CHCl3, the least. An increased temperature and increased MeOH concentration increased the yield of target compounds [16]. Heating increased the solubility of the compounds and destroyed the binding of the compounds to matrix components via van-der-Waals bonds, H-bonds, and dipole interactions.
A method for liquid extraction from lyophilized potato peel by MeOH under pressure was reported [16]. The extracts were an MeOH–CHCl3 mixture with added aqueous Na2SO4 to facilitate phase separation. The procedure used subsequent removal of the CHCl3 layer, distillation of the MeOH, dissolution of the dry residue in anhydrous MeOH, filtration to remove insoluble Na2SO4, distillation of the MeOH, and hydrolysis by H2SO4 (2 N) on a water bath. The solution was neutralized and extracted with C6H6. The C6H6 was vacuum distilled. The dry residue was dissolved in MeOH [31].
The degree of GA extraction was shown to increase if sonication was used [32].
Several methods are primarily used to purify GAs. These use precipitation by ammonium hydroxide (unsuitable for α-chaconine because of low solubility, 1 g per 100 – 1000 mL of solvent, in aqueous solutions at pH 10), separation by aqueous Na2SO4 or water-saturated _n_-BuOH (unsuitable for extracts by anhydrous solvents), and ion-pair chromatography or several solid-phase extraction methods [13].
Potato parts such as runners, tubers, and peels are quickly spoiled sources of GAs because of high water contents and significant carbohydrate contents that promote microorganism growth. The biologically active compounds in this raw material are also susceptible to microbial and enzymatic degradation and destruction by external factors during storage. These drawbacks are eliminated by drying, which also helps to reduce the particle sizes and to accumulate the target compounds [17].
Drying potato runners at 14°C in the dark at 10% humidity led to a statistically significant increase in the content of steroidal alkaloids by the seventh day of >70% as compared to fresh plants [17]. This may have resulted from the involvement of the phytohormone ethylene as a response to stress [33] because the long and mild drying process preserved the viability of the plant cells. Other drying methods (16-hour drying in a vacuum oven at 70°C and 0.6 atm, 72-hour drying from the frozen state at – 54°C and 6 × 10–5 atm) also increased the yield of target compounds; however, not as significantly as drying in air [17], possibly because of degradation of the target compound because of the temperature and pressure (α-chaconine is more resistant to these effects).
A procedure for obtaining solanine in high yield from callus culture medium of potato that was produced using growth medium with added amino acid tryptophan, which helped to increase solanine synthesis [34], was demonstrated.
Factors affecting the GA content in the plant raw material and; correspondingly, the yield of target compounds could include the plant variety (species), growth conditions, degree of ripeness, mechanical damage, storage conditions (lighting, temperature), etc. [14].
Data for the effect of temperature on GA accumulation are contradictory. In one study, a doubling of the GA level in potato tubers after storage for six weeks at 4 – 6°C as compared to tubers that were stored at 12 – 15°C was reported [35]. It was also reported that the GA content increased at 10°C and changed only insignificantly if the temperature was reduced further to 4.4°C [36].
The GA content could vary as the result of the action of various light sources (daylight, UV) [37].
Potato tubers subjected to UV irradiation had the highest GA level [35]. Sunlight or artificial light could increase GA synthesis in potatoes by 3 – 4 times as compared to those stored in the dark [38].
Other researchers reported that the blue part of the spectrum (with wavelength <500 nm, especially UV light with λ < 300 nm) and IR light ( λ > 1300 nm) were activators of GA synthesis [39].
Heat treatment (boiling, frying, baking) did not lower the amount of GAs in potatoes because they are very thermally stable [40]. However, some data indicate their content was reduced by 15% after cooking in a microwave oven [41].
Thus, various methods for extraction of GAs from plant raw material are known although they have several drawbacks such as toxic solvents, long processing times, etc. The most significant factor influencing the GA content is the action of various light sources that can be used to increase the yield of the target compounds.
Qualitative and quantitative analysis of potato GAs
Physicochemical analytical methods are primarily used for qualitative and quantitative analysis of GAs because they have important advantages, e.g., high sensitivity, rapidity, universality, economy, and the possibility for automation. On the other hand, several traditional color reactions can be used to identify solanine. These are the reactions with Wagner’s reagent, Dragendorff’s reagent, Sonnenschein’s reagent, and Mayer’s reagent.
An IR spectroscopic method was proposed for qualitative analysis of solanine, chaconine, and solanidine [42].
Colorimetric analytical methods rely on the reaction of solanine with several reagents because the molecule lacks significant chromophores. They include Mayer’s reagent, a mixture of H3PO4 (85%) and paraformaldehyde (1%), a mixture of antimony(III) chloride and conc. HCl [43] (nonspecific methods suitable for all steroids with a double bond on C-5), bromothymol blue (after hydrolysis), and methyl orange (after hydrolysis) [13].
A titrimetric method after hydrolysis was also proposed for quantitative determination of potato GAs. The method consisted of titration of solanine by phenol with an indicator of bromophenol blue. The color changed when all solanine formed a complex with phenol [44].
TLC was used as a quick and simple screening method for analyzing many samples. Several systems were often proposed as the mobile phase, e.g., CHCl3–EtOH–NH4 OH [13, 27], _n_-BuOH–HOAc–H2O (2:1:1), CH2Cl2–MeOH– H2O–conc. NH4 OH (70:30:4:0.4), etc. Detection was made by H2SO4 (50%) [45], I2 vapor (nonspecific reversible detector), anisaldehyde, Sb(III) chloride, Dragendorff’s reagent, Clarke’s reagent (nonspecific but gives different colors with different GAs) [13]. Quantitative determination at 507 nm could be performed by scanning the reflectance [46].
Gas chromatography was also used for analysis of aglycons and GAs after the appropriate derivatization (e.g., permethylation) [47, 48].
Amethod for quantitative determination of the two major potato GAs, α-solanine and α-chaconine, and their aglycon form solanidine was developed using liquid chromatography with mass spectrometry and a single quadrupole sensor in single-ion monitoring mode [49].
High-performance liquid chromatography (HPLC) with UV spectrophotometric detection was the most popular method for quantitative analysis of GAs. However, the target compounds absorbed UV light in the range 200 – 215 nm, which reduced the selectivity of the analysis [50, 51].
Several authors used HPLC in combination with tandem mass spectrometry. This significantly increased the sensitivity of the analysis [49, 52].
Methods for radioimmune [53] and immunoenzyme analysis [54, 55] were also described.
Thus, methods for qualitative and quantitative analysis of GAs isolated from plant raw material have been described and can provide a basis for developing new determination methods.
Pharmacological activity of potato GAs
Potato GAs are used in several pharmacotherapeutic areas. For example, solanidine is a precursor for the synthesis of hormonal compounds and other pharmacologically active compounds [45].
The antitumor potential of potato GAs has been demonstrated in many investigations (primarily in vitro) using activation of mitochondrial pathways of apoptosis or autophagy, delay of the cell cycle, inhibition of angiogenesis and metastasis (by reducing expression of genes coding metalloproteinases, E-cadgerin, etc.), and induction of lipid peroxidation [26].
For example, the antitumor activity of solanine was demonstrated in vitro against hepatocarcinoma (HepG2, most sensitive culture), stomach carcinoma (SGC-7901), and colon cell culture (LS174) via stimulation of apoptosis in a dose-dependent manner [56]. Solanidine derivatives could stop the G0/G1 and G2/M phases of the cell cycle [1]. Inhibition of MMP-2 and MMP-9 by solanine was associated with suppression of migration and invasion of A2058 human melanoma cell line and enhanced its antimetastatic potential [57]. Synthetic analogs of solanidine prepared from pregnenolone acetate exhibited antiproliferative activity against HL-60 human leukemia cell culture [58].
The viability of the following cancer cell lines was reduced by GAs: HeLa cervical, HepG2 liver, U937 lymphoma, and AGS and KATO III stomach. This effect was concentration dependent (0.1 – 10 μg/mL) with α-chaconine exhibiting greater efficacy than α-solanine [14, 59].
Solanine at 3.6 and 9 μg/mL suppressed metastasis in pancreatic cancer cells [60]. It eliminated multi-drug resistance by reducing expression of transporter MRP1 in K56/ADM human myelogenous leukemia cell culture [61]. Several biologically active compounds of plant origin (e.g., a chemically modified polysaccharide complex) demonstrated the ability to inhibit in vivo protein-transporter glycoprotein- P [62]. GAs may display analogous activity, thereby manifesting their antitumor effect.
The mechanism of chaconine antitumor activity against HT-29 colon cancer cells involves initiation of apoptosis by stimulating caspase-3 via reduced phosphorylation of extracellular signal-related kinase ERK1/2 [63].
It is noteworthy that α-solanine appeared safe for normal cells (fibroblasts and keratinocytes) up to a concentration of 18.4 μM [57].
Several researchers demonstrated the potential of using α-solanine as adjuvant therapy for potentiating the in vitro sensitivity of tumor cells to radiotherapy [26].
The in vivo antitumor activity of GAs has been little studied. For example, intraperitoneal (i.p.) administration of α-solanine at a dose of 5 mg/kg to mice inhibited the development of transplanted breast and prostate tumors. The toxicity of the compound at this dose was not apparent because the LD50 was 40 mg/kg [64]. Pancreatic tumors transplanted to mice were suppressed by a two-week course of α-solanine at a dose of 2 μg/g once per day because of reduced expression of vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA) [65]. Analyses of the antitumor activity of α-solanine upon peroral administration were not found in the scientific literature.
The therapeutic potential of potato GAs is not limited to antitumor activity.
Anti-inflammatory activity was found in vitro for α-chaconine and solanidine via inhibiting the production of interleukins 2 and 8. α-Solanine, solanidine, and potato peel extract also reduced NO production [66]. The anti-inflammatory activity was more pronounced for the aglycon solanidine [29]. The glycosides had antitumor potential [67].
Several studies showed antimicrobial, insecticidal, and fungicidal properties of potato Gas.
Research proved that α-chaconine and α-solanine were highly active against three pathogenic strains of trichomonads. α-Solanine was several times more active than α-chaconine [68]. Several other researchers disagreed. In any case, synergism was found between the compounds [69]. Computer modeling demonstrated the inhibitory activity of solanidine against the key trypanosome metabolic enzyme trypanothione reductase [70].
α-Chaconine and α-solanine were experimentally demonstrated not to have a substantial direct inhibitory effect on the growth of the potato blight Phytophthora infestans while non-glycosylated solanidine exhibited potent inhibitory activity [71]. The influence of GAs on fungi depends on not only the compound structure but also the pest species (particularly the sterol composition in their membranes), cultivation conditions, and plant growth stage [69, 72].
The antimalarial activity of α-chaconine was studied. It showed dose-dependent suppression of malaria infection [73].
Antibacterial activity against several Gram-positive bacteria, especially Staphylococcus aureus and Escherichia coli was found for the extract of S. tuberosum peels. However, it was active against only one Gram-negative bacterium, namely Pseudomonas aeruginosa [74, 75]. The mechanism of action of the alkaloids could be explained by disruption of H-bonds in DNA molecules of the bacteria [76] and damage to the cell wall [77].
α-Solanine exhibited inhibitory activity against several isoforms of liver microsome enzymes, cholinesterase, and several other enzymes after i.p. administration at a dose of 20 mg/kg of rat mass [78]. Inhibition of acetylcholinesterase was also found for human and bovine erythrocytes when used at a concentration of 100 μM [79].
The pharmacokinetic properties of potato GAs have been analyzed primarily in animals and were found to be highly species-specific.
Most animal studies were consistent with low bioavailability of GAs upon peroral administration [80].
The maximum plasma concentration of α-chaconine after peroral administration to female mice at a dose of 10 mg/kg of mass was 0.82 μg/mL after 14 h. The concentration dropped slowly and was 0.31 μg/mL after 120 h. The peak concentration in liver was observed already after 6 h (2.97 μg/g) with a repeat peak after 120 h that was indicative of enterohepatic circulation [81]. The peak concentrations of α-chaconine in hamsters (10 mg/kg internally) in most organs and blood plasma were observed after 12 h; in heart and kidneys, after 24 h. The maximum content of α-chaconine after administration to male rats at a dose of 5 mg/kg was observed in liver (1.3% of the radioactivity) and plasma (0.2% of the radioactivity) after 6 – 12 h. The decrease of radioactivity in feces 24 and 48 h after administration was 60 and 80%, respectively. Ten percent of the radioactive compound was excreted with urine after 1 d. The liven content after 24 h was only 1.29% of the injected dose; plasma, 0.17% [82].
Peroral administration to rats of radioactively labeled α-solanine (5 mg/kg) led to 84% of the injected dose being eliminated mainly with feces (65% as solanidine) and urine (6% as the aglycon) over 4 d. The greatest content of the compound (~1.5% of the injected dose) was observed after 24 h in liver and then in blood, kidneys, and lungs. α-Solanine was excreted (15 – 20% of the injected dose) with urine and feces 24 h after intraperitoneal administration (5 – 15 mg/kg) [83].
The bioavailability of labeled α-solanine upon peroral administration to rats and hamsters at a dose of 170 μg/kg was 1.6 and 3.2%, respectively (for the unlabeled compound). However, the level of total radioactivity was 29 and 57%, respectively. The elimination half-life of unlabeled α-solanine was faster in rats (7.79 ± 0.83 h) than in hamsters (19.7 ± 4.2 h). The elimination half-lives calculated from the total radioactivity in the animals were similar (82.2 ± 6.1 h for rats and 94 ± 33 h for hamsters). The total excretion from rats 7 d after peroral administration of the compound was 89% (3% urine and 86% feces); from hamsters, 39% (10% urine and 29% feces) [84].
The peak plasma concentrations of α-solanine and α-chaconine after peroral administration of potato peels (in an amount providing ~1 mg/kg of mass of GAs) to seven male volunteers were reached after 5.1 and 6.0 h, respectively. The maximum content of solanidine was reached after 8 h. The elimination half-lives were 11 and 19 h, respectively [50]. The times to reach the maximum concentration of α-solanine and α-chaconine were 4 and 8 h, respectively, regardless of the peroral doses of GA in solutions of various concentrations or ground potato peels of various masses (with about equal GA contents) administered to six males and eight females. The elimination half-lives of the compounds had distinct person-to-person variations and were 27 – 84 h for α-chaconine and 5 – 42 h for α-solanine [85].
Solanidine demonstrated a significant elimination half-life in people. It was detected in blood plasma after 2 – 3 weeks on a potato-free diet. Binding in plasma to steroidal compounds was proposed [53]. Solanidine was shown to accumulate in human liver [13].
Characteristics of drugs containing biologically active potato ( S. tuberosum ) compounds
Several drugs based on biologically active potato compounds are currently used in the clinic. However, they all are a mixture of compounds extracted from the plant without an indication of the actual pharmacological compound. Therefore, the pharmacokinetics of the drugs have not been studied and standardization of them is challenging.
For example, the antiviral and immunomodulating drug Panavir® (OOO National Research Company, Russia) (solution for intravenous injection, rectal suppositories, vaginal suppositories, gel for local and external use) contains a complex of S. tuberosum runner polysaccharides as the active ingredient and is used in complex therapy for herpes, cytomegalovirus, and papillomavirus infections; tick-borne encephalitis; chronic bacterial prostatitis; corona virus infections in cats; and other infections [86]. The mechanisms of the pharmacological activity of the drug have been described only in research of domestic researchers. It was found to induce interferon synthesis by α- and γ-leukocytes of peripheral blood [87] and to reduce production of interleukin-2, -4, -5, and -10 and tumor necrosis factor alpha in patients with atopic dermatitis. However, these effects often appeared only in vivo [88]. The use of Panavir is even more critical because specific drugs against papillomavirus infection are lacking. The basic strategy of its therapy is aimed at the use of nonspecific antiviral drugs with immunomodulating effects [89].
Immunomax™ (lyophilizate for preparation of solution for intravenous injection, Avexima JSC, Russia) is a preparation based on an acidic peptidoglycan from potato sprouts. It possesses antiviral and immunomodulating activity in vivo and is used for pharmacotherapy of infections caused by human papillomavirus, mycoplasma, chlamydia, ureaplasma, and several other vectors [90]. GAs have not been implicated in Panavir and Immunomax compositions.
Gamma-plant (Vector SRC of Virology and Biotechnology, Russia) is an anti-inflammatory agent with immunomodulating and antiviral activity. Its active ingredient is also an aqueous extract of fresh potato sprouts containing a glycoprotein fraction. The dosage form is a solution for subcutaneous injection. It is used for rheumatoid arthritis resistant to nonsteroidal anti-inflammatory drugs or with contraindications to them [91].
The antiulcer agent Immeran (SPC Gemma-B JSC, Russia) (solution for intravenous injection) is another drug based on potato polysaccharide complex containing small amounts of protein (aqueous extract). Its mechanism of action includes modulation of the levels of pro- and anti-inflammatory cytokines of stomach mucous and the duodenum [92]. The composition, dosage form, and instructions for use of Ultsep preparation (SOLAFARM OOO, Russia) are analogous to those of Immeran [93].
The manufacturing cycle of drugs based on biologically active potato compounds is an internal document of the manufacturer as a part of the industrial (technical) regulation [94] and is not available for review.
Quality control of these drugs complies with the requirements for their dosage forms. Also, the qualitative and quantitative characteristics of the total biologically active ingredients (e.g., polysaccharides for Panavir) are analyzed.
Compounds extracted from potatoes contain several biologically active additives (Panavir inlight, Inderma) and cosmetic agents (face masks, hand cream, several others) besides the drugs. Indications that they contain GAs were not found.
Toxicity of potato GAs
GAs are not toxic in vivo if plants of the family Solanaceae grown under standard conditions and usually containing ≤100 mg/kg of GAs are used in food. However, some factors (growth conditions, harvesting methods, processing methods) can cause the GA content to increase to toxic levels [11]. Poisoning by GAs (particularly solanine) is frequently encountered if plants of the family Solanaceae are added to children’s food, which is also explained by the bitter taste of plant parts with high contents of them. Nevertheless, several supervisory organizations in various countries regulate the GA content in potatoes [80].
The GA concentration increased significantly (up to 50%) in potatoes grown under drought stress conditions [95]. Cold and moist [96] or hot and dry conditions during growth of the plants also caused the GA concentration to increase [97]. It is noteworthy that GA production continued during storage of harvested plant parts. Also, the compounds were not destroyed during culinary operations [98].
The leading cause of GA toxicity is the ability to bind cholesterol in biological membranes [15], to destroy the cell membrane potential, and to inhibit acetylcholinesterase [99]. However, their influence on the human and animal body is not limited to these effects.
The approximate lethal dose of solanine for humans is 2 – 5 mg/kg [15, 80]. Lower concentrations of solanine are toxic for humans. The compound (but not solanidine) inhibits acetylcholinesterase, destroys the integrity of cell membranes, and lowers the membrane potential and transport of Ca ions [80]. Intravenous injection of it causes hemolysis [100]. The symptoms of poisoning include headache, dizziness, stomachache, difficulty breathing, nausea, vomiting, diarrhea, and several specific signs such as itching in the neck, hyperesthesia, and shortness of breath.
GA toxicity also manifests in animals. However, it is less pronounced (it is like that in people only for hamsters [101]). For example, the LD50 for mice upon peroral administration of α-solanine was >1000 mg/kg of mass. However, it was only 27 mg/kg (30 for α-chaconine and 34 mg/kg for tomatine) upon i.p. injection. This parameter for rabbits upon i.p. injection was twice the value [102]. GAs caused a teratogenic effect in rats [103] and monkeys [104]. However, several researchers refuted this by using various animals [13, 105, 106]. Also, deaths of offspring increased if pregnant rats were fed feed with increased contents of solanine [107]. In another study with rats, a diet with a moderate GA content did not show a harmful effect. They retarded the fetal and postnatal growth rates although they did not cause malformations in the offspring [108].
Solanidine, solasodine, and tomatidine (with a double bond between C5 and C6 in the B ring) caused hepatomegaly upon addition to feed for nonpregnant and pregnant mice for two weeks. Also, weight gain of the mother and offspring decreased in pregnant animals. Miscarriages were also observed after administration of solanidine [109]. Formation of cranium defects and estrogenic activity were demonstrated in hamsters under the influence of solanidine [110] although an analogous effect was not found for the glycosides [111]. Peroral administration of the glycosides could also be associated with symptoms of solanidine poisoning, probably because of hydrolysis by stomach acid and glycosidases of the intestinal bacterial microflora [80]. Use in food of sprouted potato tubers in the period before conception increased the risk of developing nerve defects and orofacial creases in fetuses [111]. Research on the genotoxicity of potato GAs was not found. Also, analyses of chronic toxicity and genotoxicity were not reported [80].
The ability of potato GAs to cause itching of scars was demonstrated in a small blind prospective clinical trial [112]. Cardiotoxicity of solanidine-like GAs from Veratrum taliense related to blockage of cardiac sodium channels was shown in mice [31, 100].
α-Chaconine is considered more toxic than α-solanine. The combination of these GAs can cause a synergistic toxic effect. The elimination half-life of α-chaconine in mice was ~44 h, which is longer than that of α-solanine [85].
Thus, potato GAs are promising pharmacological substances. However, their significant toxicity requires detailed pharmacokinetic and pharmacodynamic studies. The medicinal raw material requires careful standardization.
Footnotes
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 7, pp. 25 – 34, July, 2022.
References
- 1.Dey P, Kundu A, Chakraborty HJ, et al. Int. J. Cancer. 2019;145(7):1731–1744. doi: 10.1002/ijc.31965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.I. V. Chernykh, E. E. Kirichenko, A. V. Shchul’kin, et al., Ross. Med.-Biol. Vestn. im. Akad. I. P. Pavlova, 26(2), 305 – 316 (2018).
- 3.Boiko NN, Bondarev AV, Zhilyakova ET, et al. Nauchn. Resul’tat Med. Farm. 2017;3(4):30–38. [Google Scholar]
- 4.Harborne JB. Introduction to Ecological Biochemistry. London: Academic Press; 1993. [Google Scholar]
- 5.Kennedy DO, Wightman EL. Adv. Nutr. 2011;2(1):32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazaki K. Curr. Opin. Plant Biol. 2005;8(3):301–307. doi: 10.1016/j.pbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Milner SE, Brunton NP, Jones PW, et al. J. Agric. Food Chem. 2011;59(8):3454–3484. doi: 10.1021/jf200439q. [DOI] [PubMed] [Google Scholar]
- 8.Mazid M, Khan TA, Mohammad F. Biol. Med. 2011;3(2):232–249. [Google Scholar]
- 9.T. Vaananen, Academic Dissertation, Helsinki (2007).
- 10.Dinan L, Harmatha J, Lafont R. J. Chromatogr. A. 2001;935(1–2):105–123. doi: 10.1016/S0021-9673(01)00992-X. [DOI] [PubMed] [Google Scholar]
- 11.Ginzberg I, Tokuhisa JG, Veilleux RE. Potato Res. 2009;52(1):1–15. doi: 10.1007/s11540-008-9103-4. [DOI] [Google Scholar]
- 12.Ivanova KA, Gerasimova SV, Khlestkina EK. Vavilov. Zh. Genet. Sel. 2018;22(1):25–34. [Google Scholar]
- 13.Friedman M, McDonald GM, Filadelfi-Keszi M. Crit. Rev. Plant Sci. 1997;16(1):55–132. doi: 10.1080/07352689709701946. [DOI] [Google Scholar]
- 14.M. A. B. Siddique and N. Brunton, in: Alkaloids - Their Importance in Nature and Human Life, J. Kurek (ed.), IntechOpen, London (2019), p. 47.
- 15.Nepal B, Stine KJ. Processes. 2019;7(8):513. doi: 10.3390/pr7080513. [DOI] [Google Scholar]
- 16.Hossain MB, Rawson A, Aguilo-Aguayo I, et al. Molecules. 2015;20(5):8560–8573. doi: 10.3390/molecules20058560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossain MB, Brunton NP, Rai DK. Molecules. 2016;21(4):403. doi: 10.3390/molecules21040403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mintz-Oron S, Mandel T, Rogachev I, et al. Plant Physiol. 2008;147(2):823–851. doi: 10.1104/pp.108.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwahn K, de Souza LP, Fernie AR, Tohge T. J. Integr. Plant Biol. 2014;56(9):864–875. doi: 10.1111/jipb.12274. [DOI] [PubMed] [Google Scholar]
- 20.Friedman M. J. Agric. Food Chem. 2013;61(40):9534–9550. doi: 10.1021/jf402654e. [DOI] [PubMed] [Google Scholar]
- 21.Friedman M. J. Chromatogr. A. 2004;1054(1–2):143–155. doi: 10.1016/j.chroma.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara Y, Takaki A, Uehara Y, et al. Tetrahedron. 2004;60(22):4915–4920. doi: 10.1016/j.tet.2004.03.088. [DOI] [Google Scholar]
- 23.Blankemeyer JT, McWilliams ML, Rayburn JR, et al. Food Chem. Toxicol. 1998;36(5):383–389. doi: 10.1016/S0278-6915(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 24.Mennella G, Lo Scalzo R, Fibiani M, et al. J. Agric. Food Chem. 2012;60(47):11821–11831. doi: 10.1021/jf3037424. [DOI] [PubMed] [Google Scholar]
- 25.Bajaj KL, Kaur G, Chadha ML. J. Plant Foods. 1979;3(3):163–168. doi: 10.1080/0142968X.1979.11904224. [DOI] [Google Scholar]
- 26.Hassan SH, Gul S, Zahra HS, et al. Nutr. Cancer. 2021;73(9):1541–1552. doi: 10.1080/01635581.2020.1803932. [DOI] [PubMed] [Google Scholar]
- 27.Nikolic NC, Stankovic MZ. J. Agric. Food Chem. 2003;51(7):1845–1849. doi: 10.1021/jf020426s. [DOI] [PubMed] [Google Scholar]
- 28.Harvey MH, Mcmillan M, Morgan MRA, Chan HWS. Hum. Toxicol. 1985;4(2):187–194. doi: 10.1177/096032718500400209. [DOI] [PubMed] [Google Scholar]
- 29.Suhaj M. J. Food Compos. Anal. 2006;19(6–7):531–537. doi: 10.1016/j.jfca.2004.11.005. [DOI] [Google Scholar]
- 30.Denery JR, Dragull K, Tang C, Li QX. Anal Chim Acta. 2004;501(2):175–181. doi: 10.1016/j.aca.2003.09.026. [DOI] [Google Scholar]
- 31.Wang G, Rong MQ, Li Q, et al. Toxins. 2016;8(1):12. doi: 10.3390/toxins8010012. [DOI] [Google Scholar]
- 32.Tata A, Perez CJ, Hamid TS, et al. J. Am. Soc. Mass Spectrom. 2014;26(4):641–648. doi: 10.1007/s13361-014-1039-0. [DOI] [PubMed] [Google Scholar]
- 33.Zemlyanskaya EV, Omel’yanchuk NA, Ermakov AA, Mironova VV. Vavilov. Zh. Genet. Sel. 2016;20(3):386–395. [Google Scholar]
- 34.A. S. Rylkova, in: Proceedings of a Scientific Conference with International Participation “Higher School of Biotechnology and Food Technologies” [in Russian], St. Petersburg (2016), pp. 34 – 36.
- 35.Machado RMD, Toledo MCF, Garcia LC. Food Control. 2007;18(5):503–508. doi: 10.1016/j.foodcont.2005.12.008. [DOI] [Google Scholar]
- 36.Love SL, Herrman TJ, Thompsonjohns A, Baker TP. Potato Res. 1994;37(1):77–85. doi: 10.1007/BF02360434. [DOI] [Google Scholar]
- 37.Percival G, Dixon G, Sword A. J. Sci. Food Agric. 1994;66(2):139–144. doi: 10.1002/jsfa.2740660206. [DOI] [Google Scholar]
- 38.Salunkhe DK, Wu MT, Jadhav SJ. J. Food Sci. 1972;37(6):969–970. doi: 10.1111/j.1365-2621.1972.tb03718.x. [DOI] [Google Scholar]
- 39.Jadhav SJ, Sharma RP, Salunkhe DK, Crit CRC. Rev. Toxicol. 1981;9(1):21–104. doi: 10.3109/10408448109059562. [DOI] [PubMed] [Google Scholar]
- 40.Porter WL. Am. Potato J. 1972;49(10):403–406. doi: 10.1007/BF02864839. [DOI] [Google Scholar]
- 41.Takagi K, Toyoda M, Fujiyama Y, Saito Y. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1990;31(1):67–73. doi: 10.3358/shokueishi.31.67. [DOI] [Google Scholar]
- 42.Glossman-Mitnik D. Spectrochim. Acta, Part A. 2007;66(1):208–211. doi: 10.1016/j.saa.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Wang SL, Bedford CL, Thompson NR. Am. Potato J. 1972;49(8):302–308. doi: 10.1007/BF02861668. [DOI] [Google Scholar]
- 44.Fitzpatrick TJ, Osman SF. Am. Potato J. 1974;51(10):318–323. doi: 10.1007/BF02851505. [DOI] [Google Scholar]
- 45.Hennessy RC, Jorgensen NO, Scavenius C, et al. Front. Microbiol. 2018;9:2648. doi: 10.3389/fmicb.2018.02648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodart P, Kabengera C, Noirfalise A, et al. J. AOAC Int. 2000;83(6):1468–1473. doi: 10.1093/jaoac/83.6.1468. [DOI] [PubMed] [Google Scholar]
- 47.Laurila J, Laakso I, Vaananen T, et al. J. Agric. Food Chem. 1999;47(7):2738–2742. doi: 10.1021/jf981009b. [DOI] [PubMed] [Google Scholar]
- 48.Moreau RA, Nystrom L, Whitaker BD, et al. Prog. Lipid Res. 2018;70:35–61. doi: 10.1016/j.plipres.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen SD, Schmidt JM, Kristiansen GH, et al. Foods. 2020;9(4):416. doi: 10.3390/foods9040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellenas KE, Nyman A, Slanina P, et al. J. Chromatogr. B: Biomed. Sci. Appl. 1992;573(1):69–78. doi: 10.1016/0378-4347(92)80476-7. [DOI] [PubMed] [Google Scholar]
- 51.Yuan B, Byrnes DR, Dinssa FF, et al. J. Food Sci. 2019;84(2):235–243. doi: 10.1111/1750-3841.14424. [DOI] [PubMed] [Google Scholar]
- 52.Baur S, Frank O, Hausladen H, et al. Food Chem. 2021;365:130461. doi: 10.1016/j.foodchem.2021.130461. [DOI] [PubMed] [Google Scholar]
- 53.Zhou X, Gao Q, Pratico G, et al. Genes Nutr. 2019;14(1):1–15. doi: 10.1186/s12263-019-0631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driedger DR, LeBlanc RJ, LeBlanc EL, Sporns P. J. Agric. Food Chem. 2000;48(4):1135–1139. doi: 10.1021/jf990680t. [DOI] [PubMed] [Google Scholar]
- 55.Badowski P, Urbanek-Karlowska B. Rocz. Panstw. Zakl. Hig. 1999;50(1):69–75. [PubMed] [Google Scholar]
- 56.JI YB, Gao SY. Chin. Herb. Med. 2012;4(2):126–135. [Google Scholar]
- 57.Lu M-K, Shih Y-W, Chien T-TC, et al. Biol. Pharm. Bull. 2010;33(10):1685–1691. doi: 10.1248/bpb.33.1685. [DOI] [PubMed] [Google Scholar]
- 58.Zupko I, Molnar J, Rethy B, et al. Molecules. 2014;19(2):2061–2076. doi: 10.3390/molecules19022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman M, Lee KR, Kim HJ, et al. J. Agric. Food Chem. 2005;53(15):6162–6169. doi: 10.1021/jf050620p. [DOI] [PubMed] [Google Scholar]
- 60.Ji YB, Gao SY, Ji CF, Zou X. J. Ethnopharmacol. 2008;115(2):194–202. doi: 10.1016/j.jep.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 61.Yi YJ, Jia XH, Zhu C, et al. Oncology Lett. 2018;15(6):10070–10076. doi: 10.3892/ol.2018.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chernykh IV, Shchul’kin AV, Yakusheva EN, et al. Nauka Molod. (Eruditio Juvenium) 2019;7(3):349–357. doi: 10.23888/HMJ201973349-357. [DOI] [Google Scholar]
- 63.Zuber T, Holm D, Byrne P, et al. Food Funct. 2015;6(1):72–82. doi: 10.1039/C4FO00649F. [DOI] [PubMed] [Google Scholar]
- 64.Pan B, Zhong W, Deng Z, et al. Cancer Med. 2016;5(11):3214–3222. doi: 10.1002/cam4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lv C, Kong H, Dong G, et al. PLoS One. 2014;9(2):e87868. doi: 10.1371/journal.pone.0087868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenny OM, McCarthy CM, Brunton NP, et al. Life Sci. 2013;92(13):775–782. doi: 10.1016/j.lfs.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Friedman M. J. Agric. Food Chem. 2006;54(23):8655–8681. doi: 10.1021/jf061471t. [DOI] [PubMed] [Google Scholar]
- 68.Friedman M, Huang V, Quiambao Q, et al. J. Agric. Food Chem. 2018;66(30):7942–7947. doi: 10.1021/acs.jafc.8b01726. [DOI] [PubMed] [Google Scholar]
- 69.Fewell AM, Roddick JG. Mycol. Res. 1997;101(5):597–603. doi: 10.1017/S0953756296002973. [DOI] [Google Scholar]
- 70.Arguelles AJ, Cordell GA, Maruenda H. Nat. Prod. Commun. 2016;11(1):57–62. [PubMed] [Google Scholar]
- 71.Dahlin P, Muller MC, Ekengren S, et al. Mol. Plant Microbe Interact. 2017;30(7):531–542. doi: 10.1094/MPMI-09-16-0186-R. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Maldonado AF, Schieber A, Ganzle MG. J. Appl. Microbiol. 2016;120(4):955–965. doi: 10.1111/jam.13056. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Li S, Sun F, et al. Pharm. Biol. 2010;48(9):1018–1024. doi: 10.3109/13880200903440211. [DOI] [PubMed] [Google Scholar]
- 74.Amanpour R, Abbasi-Maleki S, Neyriz-Naghadehi M, Asadi-Samani M. J. HerbMed Pharmacol. 2015;4(2):45–48. [Google Scholar]
- 75.Ismail SA, Abdullah VS, Kamel FH. Plant Arch. 2019;19(2):4009–4014. [Google Scholar]
- 76.Mabhiza D, Chitemerere T, Mukanganyama S. Int. J. Med. Chem. 2016;2016:1–7. doi: 10.1155/2016/6304163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burdiek EM. Econ. Bot. 1971;25(4):363–365. doi: 10.1007/BF02985202. [DOI] [Google Scholar]
- 78.Dalvi RR. Jpn J. Vet. Sci. 1985;47(4):657–659. doi: 10.1292/jvms1939.47.657. [DOI] [PubMed] [Google Scholar]
- 79.Roddick JG. Phytochemistry. 1989;28(10):2631–2634. doi: 10.1016/S0031-9422(00)98055-5. [DOI] [Google Scholar]
- 80.EFSA Panel on Contaminants in the Food Chain (CONTAM), D. Schrenk and M. Bignami, et al., EFSA J., 18(8), e06222 (2020). [DOI] [PMC free article] [PubMed]
- 81.Sharma RP, Taylor MJ, Bourcier DR. Drug Chem. Toxicol. 1983;6(2):219–234. doi: 10.3109/01480548309016026. [DOI] [PubMed] [Google Scholar]
- 82.Norred WP, Nishie K, Osman SF. Res. Commun. Chem. Pathol. Pharmacol. 1976;13(2):161–171. [PubMed] [Google Scholar]
- 83.Nishie K, Gumbmann MR, Keyl A. Toxicol. Appl. Pharmacol. 1971;19(1):81–92. doi: 10.1016/0041-008X(71)90192-X. [DOI] [PubMed] [Google Scholar]
- 84.Groen K, Pereboom-de Fauw DPKH, Besamusca P, et al. Xenobiotica. 1993;23(9):995–1005. doi: 10.3109/00498259309057038. [DOI] [PubMed] [Google Scholar]
- 85.Mensinga TT, Sips AJ, Rompelberg CJ, et al. Regul. Toxicol. Pharmacol. 2005;41(1):66–72. doi: 10.1016/j.yrtph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Kalinina TS, Zlenko DV, Kiselev AV, et al. Int. J. Biol. Macromol. 2020;161:936–938. doi: 10.1016/j.ijbiomac.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nosik NN, Kolobukhina LV, Merkulova LN, et al. Tsitokiny Vospalenie. 2009;8(2):49–52. [Google Scholar]
- 88.S. V. Stovbun and L. V. Yakovenko, Vestn. Mosk. Univ., Ser. 3: Fiz., Astron., No. 6, 101 – 106 (2014).
- 89.Dovletkanova ER, Prilepskaya VN, Letunovskaya AB. Eff. Farmakoter. Endokrinol. 2018;3(26):20–23. [Google Scholar]
- 90.Pichugin AV, Bagaev AV, Chulkina MM, et al. Immunologiya. 2015;36(4):200–205. [Google Scholar]
- 91.Chekanovskaya LA, Generalov AV. Pharm. Chem. J. 2000;34(3):155–160. doi: 10.1007/BF02524589. [DOI] [Google Scholar]
- 92.Generalov EA. Aktual. Vopr. Biol. Fiz Khim. 2019;4(1):85–89. [Google Scholar]
- 93.Khomyakova TI, Zolotova NA, Tsyganova SO, et al. Eksp. Klin. Gastroenterol. 2015;117(5):118. [Google Scholar]
- 94.OST 64-02-003-2002 Products of the Medical Industry. Technological Regulations of Manufacturing. Contents, Order of Development, Agreement, and Approval; //https://base.garant.ru/.
- 95.Bejarano L, Mignolet E, Devaux A, et al. J. Sci. Food Agric. 2000;80(14):2096–2100. doi: 10.1002/1097-0010(200011)80:14<2096::AID-JSFA757>3.0.CO;2-6. [DOI] [Google Scholar]
- 96.Sinden SL, Webb RE. Am. Potato J. 1972;49(9):334–338. doi: 10.1007/BF02861777. [DOI] [Google Scholar]
- 97.Morris SC, Petermann JB. Food Chem. 1985;18(4):271–282. doi: 10.1016/0308-8146(85)90108-6. [DOI] [Google Scholar]
- 98.Milner SE, Brunton NP, Jones PW, et al. J. Agric. Food Chem. 2011;59(8):3454–3484. doi: 10.1021/jf200439q. [DOI] [PubMed] [Google Scholar]
- 99.Patel K, Patel DK. J. Coastal Life Med. 2017;5(3):134–140. doi: 10.12980/jclm.5.2017J6-256. [DOI] [Google Scholar]
- 100.Azim A, Shaikh HA, Ahmad R. J. Pharm. (Univ. Karachi) 1984;3:43–49. [Google Scholar]
- 101.Van Gelder WMJ, Tuinistra LGMTH, Van der Greef J, Scheffer JJC. J. Chromatogr. A. 1989;482(1):13–22. doi: 10.1016/S0021-9673(01)93203-0. [DOI] [Google Scholar]
- 102.Nishie K, Gumbmann MR, Keyl AC. Toxicol. Appl. Pharmacol. 1971;19(1):81–92. doi: 10.1016/0041-008X(71)90192-X. [DOI] [PubMed] [Google Scholar]
- 103.Renwick JH. Br. J. Prev. Soc. Med. 1972;26(2):67–88. doi: 10.1136/jech.26.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poswillo DE, Sopher D, Mitchell SJ, et al. Teratology. 1973;8(3):339–347. doi: 10.1002/tera.1420080317. [DOI] [PubMed] [Google Scholar]
- 105.Ruddick JA, Harwig J, Scott PM. Teratology. 1974;9(2):165–168. doi: 10.1002/tera.1420090207. [DOI] [PubMed] [Google Scholar]
- 106.Sharma RP, Willhite CC, Wu MT, Salunkhe DK. Teratology. 1978;18(1):55–61. doi: 10.1002/tera.1420180109. [DOI] [PubMed] [Google Scholar]
- 107.Kline BE, Von Elbe H, Dahle NA, Kupchan SM. Proc. Soc. Exp. Biol. Med. 1961;107(4):807–809. doi: 10.3181/00379727-107-26762. [DOI] [PubMed] [Google Scholar]
- 108.Taciak M, Tusnio A, Pastuszewska B. J. Anim. Physiol. Anim. Nutr. 2011;95(5):556–563. doi: 10.1111/j.1439-0396.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- 109.Friedman M, Henika PR, Mackey BE. Food Chem. Toxicol. 2003;41(1):61–71. doi: 10.1016/S0278-6915(02)00205-3. [DOI] [PubMed] [Google Scholar]
- 110.Gaffield W, Keeler RF. Chem. Res. Toxicol. 1996;9(2):426–433. doi: 10.1021/tx950091r. [DOI] [PubMed] [Google Scholar]
- 111.Ni W, Tian T, Zhang L, et al. Nutr. J. 2018;17(112):1–8. doi: 10.1186/s12937-018-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alonso PE, Rioja LF. Burns. 2016;42(3):535–540. doi: 10.1016/j.burns.2015.09.019. [DOI] [PubMed] [Google Scholar]