Neural mobilization in low back and radicular pain: a systematic review (original) (raw)
ABSTRACT
Background
Low back pain can present with radicular pain caused by lumbosacral nerve root pathology. Neural mobilization (NM) is a treatment technique used to treat low back and radicular pain (LBRP).
Purpose
To evaluate the effectiveness of NM interventions in improving pain, disability, and function in adults with LBRP.
Data Sources
CINAHL Plus, MEDLINE (Ovid), Physiotherapy Evidence Database, and Cochrane databases were searched.
Study Selection
Randomized controlled trials assessing the effect of NM on pain, disability, and/or function in adults with LBRP.
Data Extraction
Authors reviewed studies and used the PEDro scale and the revised Cochrane risk-of-bias tool to assess methodological quality and risk of bias.
Data Synthesis
Eight studies were included. Six of the eight studies found the addition of NM to conservative treatment improved all measured outcomes. One study found improvements in some but not all functional measures, and delayed improvements in pain. One study found improvements in measures of neural sensitivity, but not overall pain and disability.
Conclusions
NM may be an effective tool for short-term improvements in pain, function, and disability associated with LBRP. Additional high quality research is needed.
Study registration
: This systematic review protocol was registered with PROSPERO (registration number: CRD42020192338).
KEYWORDS: Neural mobilization, nerve root, low back pain, sciatica, radicular
Introduction
Low Back and Radicular Pain (LBRP), defined as pain due to compression, irritation, or other pathology of one or more lumbosacral nerve roots, is one of the most common forms of low back pain [1–3]. Annual prevalence has been reported as high as 25% and lifetime prevalence as high as 43% [1,4]. Not only is LBRP common it is significantly debilitating. Compared to other forms of low back pain, LBRP is associated with a variety of poor outcomes. These outcomes include more severe and persistent pain, increased cost of care, and longer periods of disability and absence from work [1,5–7]. Therefore there is a need to treat this promptly and effectively.
In terms of isolating the cause of pain, lumbar disc herniation is widely considered the most common cause of LBRP [8–10]. However, LBRP may also be caused by osteophytes, spondylolisthesis, and other local factors. Symptom presentation of LBRP usually involves pain characterized as sharp, burning, dull, aching or lancinating that radiates from the lower back below the gluteal fold and follows a dermatomal distribution based on the level of spinal root pathology[11]. In addition to pain, other symptoms may also exist and include paresthesia, weakness, and diminished ankle and/or knee reflexes[9]. No single assessment serves as the gold standard for diagnosis of LBRP, so a medical diagnosis of LBRP is therefore only reached after a combination of imaging studies, a detailed review of the patient’s symptoms, and the performance of a physical examination[9]. Given the variety of causative factors involved, range of symptom presentation, and difficulty in reaching an accurate diagnosis, LBRP is a challenging condition to diagnose and treat.
Treatment options for LBRP include conservative care, pharmaceutical interventions and surgery. Conservative care options are varied and may include a variety of exercise protocols, electrical modalities such as transcutaneous electrical nerve stimulation, and techniques aimed at mobilizing the affected tissue such as spinal mobilization, and neural mobilization (NM)[12].
Neural mobilization refers to the therapeutic practice of applying mechanical forces to nerves in the body, with the goal of restoring healthy movement. Nerves must be able to move within the nerve bed (i.e. surrounding tissue) for normal movement to occur, and tolerate strain, compression, and transverse movement along the nerve bed[13]. Some NM techniques directly mobilize the neural tissue (e.g. nerve glide, nerve flossing) via either active (e.g. exercise) or passive (e.g. manual therapy) techniques[14]. Others indirectly mobilize the nerves via movement of the surrounding tissues (e.g. spinal mobilization)[15].
NM may be helpful for decreasing pain and restoring nerve function in a variety of musculoskeletal conditions, but its effects on LBRP are not well-studied[16–21]. A 2014 critical review by Efstathiou et al [22] concluded that the existing literature was too sparse and low-quality in determining the effectiveness of treating lumbar radiculopathy with NM. More recently, a 2016 systematic review by Su and Lim [23] found that NM interventions provided pain relief and reduction in disability for people with nerve-related chronic musculoskeletal disorders, but found no significant differences between NM and other conservative treatments. Finally, a 2017 systematic review by Basson et al [14] on the efficacy of treating various musculoskeletal conditions with NM found that a variety of NM techniques can improve pain and disability in these participants.
Given the lack of consensus on the functional effects of NM on LBRP, this field of research would benefit from a systematic review focused solely on a population with lumbar radicular pain. The objective of this systematic review was to evaluate the effectiveness of NM interventions in improving pain, disability, and function in adults with LBRP.
Methods
Study registration
Study registration: This systematic review protocol was registered with PROSPERO (registration number: CRD42020192338), and was performed in line with the PRISMA declaration guidelines.
Search strategy
The databases searched in this systematic review were CINAHL Plus, MEDLINE (Ovid), Physiotherapy Evidence Database, and Cochrane Central Register of Controlled Trials. All searches were performed in May 2020. Our search strategy targeted clinical trials with two primary variables of interest, a specific participant population (people with LBRP) and our specific intervention (neural mobilizations). Our search strategy included terms related to LBRP, including sciatica, neurogenic, and disc herniation, as well as related terms for neural mobilization interventions, including nerve modality, nerve tension, and nerve flossing. For CINAHL Plus, we also limited our searches to randomized controlled trials (RCTs) and excluded MEDLINE records. No filters related to publication date were used for any of our searches, and no limitations on outcome measurement timeline were applied prospectively. See Appendix 1 for an example search strategy.
Eligibility criteria
RCTs, available in English, assessing the effect of NM on pain, function, or disability in adults with LBRP were eligible for inclusion.
Included studies met the following criteria: (1) Participant populations presented with symptoms, radiological findings, or other clinical findings of LBRP. These included radiological findings of lumbar disc herniation or degeneration, back pain radiating down into at least one lower extremity, reproduction of symptoms with passive straight leg raise (PSLR) or slump test, myotomal weakness, dermatomal change in sensation, or hyporeflexia in a lumbosacral innervation pattern. (2) NM intervention was provided using methods that directly mobilize the neural tissue (e.g. nerve glide, nerve flossing) via either active (e.g. exercise) or passive (e.g. manual therapy) techniques. Eligible comparator/control conditions included no treatment, sham treatment, or conservative treatment not involving neural mobilization. (3) Outcomes assessed at least one of our primary outcomes of interest (pain, disability, and function), via scales such as the Numeric Pain Rating Scale (NPRS), Visual Analog Scale (VAS), Oswestry Disability Index (ODI), Roland-Morris Disability Questionnaire (RMDQ), or the Short Form Health Survey (SF-36 or SF-12).
Animal studies, case reports, cohort studies, and studies on healthy participants were excluded. Studies with patient populations including evidence or indication of specific spinal pathology (including but not limited to vertebral fracture, neoplasm, cauda equina syndrome, and spinal infection), systemic neurological disorders or lesions, or low back pain without radicular qualities were also excluded. Studies comparing two separate interventions with no control condition were excluded, as were studies assessing NM only performed by indirect mobilization of the nerves via movement of the surrounding tissues (e.g. spinal mobilization).
Two reviewers (SD and MP) independently screened titles and abstracts of records identified via the described search strategy using these inclusion and exclusion criteria. Full-text of appropriate studies were screened independently for inclusion by both reviewers. Discrepancies were resolved by consensus meeting. In the case of continued disagreement, a third reviewer would have been used to determine eligibility for inclusion.
Data extraction and quality assessment
Data were systematically extracted from the abstract and full-text of each study. The following items were included: author name and year of publication, participant demographics, experimental and control treatment protocols, all outcome parameters including assessment timing, and main results.
Articles that met the inclusion criteria were assessed using the PEDro scale and the revised Cochrane risk-of-bias tool (RoB2). The PEDro scale is a valid and reliable tool for measuring the methodological quality of clinical trials[24]. The scale provides a rating for each study between zero and ten based on a series of “yes or ‘no’ questions, with a score of >8 considered excellent, a score of 6 to 8 considered good, a score of 4 to 5 considered fair, and a score of <4 considered poor[25]. The RoB2 is a tool for assessing the risk of bias in randomized trials. Five separate domains of potential bias are assessed and scored as ‘low risk’, ‘some concerns’, or ‘high risk’, after which the separate domains are combined to give an overall assessment of each study’s risk of bias as ‘low risk’, ‘some concerns’, or ‘high risk’[26].
Data synthesis and analysis
To assess the appropriateness of performing a meta-analysis, we compared the timeframe and method of outcome measurement in the eight included studies. No more than two studies used the same combination of intervention, outcome measure, and assessment timeframe, and we therefore concluded that meta-analysis was not appropriate.
Study selection
The database searches resulted in 704 records. After removing duplicates and screening at title/abstract and full-text levels, eight studies remained. Figure 1 shows a summary of the study selection process.
Figure 1.
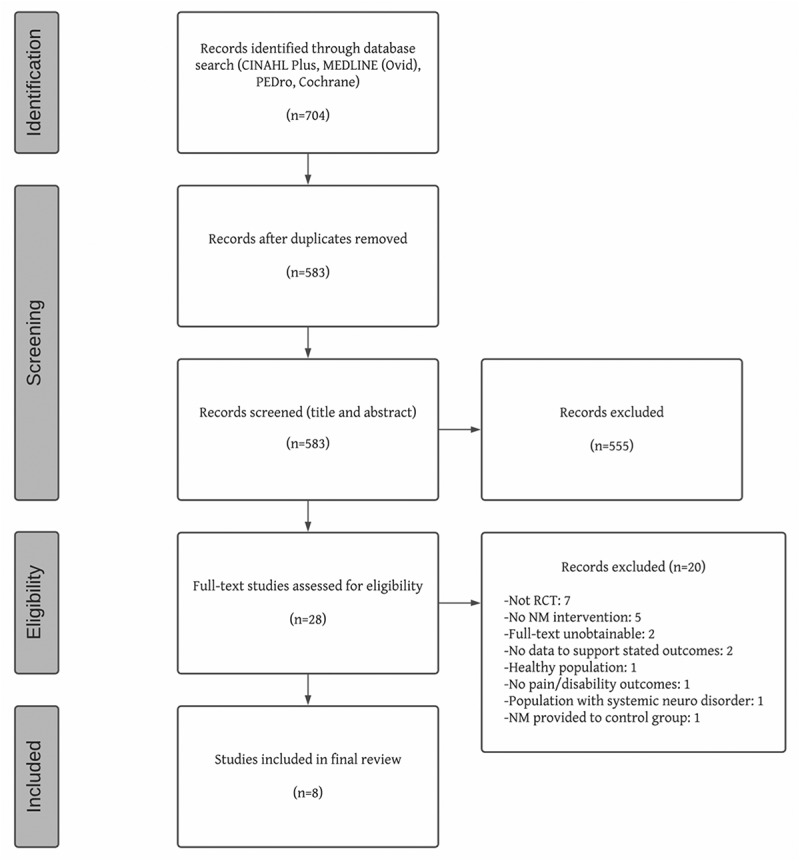
PRISMA flowchart.
Study characteristics
Table 1 reports sample size, gender distribution, age, and symptom duration for each study’s intervention and control group. The studies included in this review involved a total of 322 participants. The six studies that reported participant gender involved a total of 137 female participants and 105 male participants. Table 2 reports intervention details, outcome measures, frequency of intervention and assessment, and results of each of the eight included studies.
Table 1.
Demographics of Included Studies.
| | | Intervention Group | Control Group | | | | | | | ------------------------------- | -------------------------- | ------------- | ------------------ | -------------------------- | ----------- | ------------------ | -------------------------- | | Study | total sample size | sample size | age mean (SD) | symptom duration mean (SD) | sample size | age mean (SD) | symptom duration mean (SD) | | Ahmed et al. 2013 | n = 30: 14 female, 16 male | n = 15 | 53.0 (1.9) years | 4.87 (1.50) weeks | n = 15 | 52.6 (1.6) years | 5.26 (1.75) weeks | | Cleland et al. 2006 | n = 30: 9 female, 21 male | n = 16 | 40.0 (12.2) years | 14.5 (8.0) weeks | n = 14 | 39.4 (11.3) years | 18.5 (12.5) weeks | | Ferreira et al. 2016 | n = 60: 45 female, 15 male | n = 30 | 43.9 (14.5) years | 302.4 weeks | n = 30 | 40.3 (12.9) years | 104.3 weeks | | Jeong et al. 2016 | n = 30: 14 female, 16 male | n = 15 | 35.1 (6.4) years | – | n = 15 | 41.6 (11.1) years | – | | Nagrale et al. 2013 | n = 60: 39 female, 21 male | n = 30 | 38.2 (3.47) years | 66.31 (11.16) weeks | n = 30 | 37.76 (4.70) years | 64.14 (7.78) weeks | | Pallipamula & Singaravelan 2012 | n = 42 | n = 21 | 42.53 (6.99) years | 9.09 (1.89) weeks | n = 21 | 40.2 (7.55) years | 8.91 (1.80) weeks | | Plaza-Manzano et al. 2020 | n = 32: 16 female, 16 male | n = 16 | 45.4 (6.0) years | 75.2 (6.1) weeks | n = 16 | 47.0 (8.0) years | 74.7 (6.5) weeks | | Satishkumar et al. 2017 | n = 38 | n = 19 | 34.11 (8.36) years | 32.02 (12.38) weeks | n = 19 | 35.47 (8.40) years | 31.55 (11.12) weeks |
Table 2.
Intervention Protocols, Outcome Measures, and Results of Included Studies.
| | Protocol | | | Outcome Measures | | Results | | | | ------------------------------- | ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | -------------------------------------------------------------------------------------------------------------------------------------------------------------- | ------------------------------------------------------------------------- | -------------------------------------------------------------------------------- | ------------------------------------------------------------------ | ---------------------------------------------------------------------------------------------------------------------------------- | ---------------------------------------------------------------------------------------------------------------------------------------------------------- | | Study | Nerve Mobilization Treatment | Control Group Treatment | Frequency, Duration, Sessions | OMs used (* denotes translated version) | OM timing (* denotes translated version) | Significant findings | Non-significant findings | | Ahmed et al. 2013 | passive neurodynamic slider in supine PSLR position with bias to peroneal or tibial nerves; HEP with nerve flossing technique | flexion or extension exercises, 30 min per day, 10 reps, 2–3 sets of 10 reps per exercise; TENS – along sciatic nerve tract, 100 Hz for 30 minutes per session | 3x/wk; 2 wks; 6 in-person treatments | NPRS; SF-12 | at baseline after wk 3* | IG treatment favored for both outcomes | | | Cleland et al. 2006 | static slump stretch with overpressure in long sitting; Daily HEP with same technique | 5-min exercise bike; grade III–IV lumbar spine mobilizations; standardized exercise program targeting low back pain; HEP of standardized exercise program | 2x/wk; 3 wks; 6 in-person treatments 18 HEP sessions | ODI; NPRS; Location of symptoms (body chart) | at baseline after wk 3* | IG treatment favored for all outcomes | | | Ferreira et al. 2016 | grade III lumbar foramen opening mobilizations; passive neurodynamic slider in sidelying; progressed to active neurodynamic slider in slump sitting; Daily HEP with sliding and tensioning techniques | advice to remain active | 2x/wk; 2 wks; 4 in-person treatments 14 HEP sessions | NPRS (leg and low back); ODI 2.0*; PSFS; Location of symptoms (body chart); GPE | at baseline after wk 2* after wk 4 | IG treatment favored for PSFS and GPE after 2 weeks; IG treatment favored for leg NPRS, low back NPRS, PSFS, and GPE after 4 weeks | No significant BG differences for ODI 2.0 and location of symptoms at any timepoints; No significant BG differences for leg or low back NPRS after 2 weeks | | Jeong et al. 2016 | active neurodynamic tensioner in seated position | lumbar segmental stabilization exercise program targeting transversus abdominis and multifidus | 3x/wk; 6 wks; 18 treatments | SF-36 PF and GH subscores | at baseline after wk 6* | IG treatment favored for both outcomes | | | Nagrale et al. 2013 | static slump stretch with overpressure in long sitting; Daily HEP with same technique | 5-min exercise bike; grade III–IV lumbar spine mobilizations; standardized exercise program targeting low back pain | 2x/wk; 3 wks; 6 in-person treatments 18 HEP sessions | NPRS; ODI; FABQ | at baseline after wk 1 after wk 2 after wk 3* after wk 6 | IG treatment favored for NPRS and FABQ after weeks 1 and 2; IG treatment favored for all outcomes after weeks 3 and 6 | No significant BG difference for ODI after weeks 1 and 2 | | Pallipamula & Singaravelan 2012 | active neurodynamic slider in seated slump position | TENS along the area of symptoms; mechanical lumbar traction | 1x daily; 6 days; 6 sessions of NM and TENS 3 sessions of lumbar traction | VAS; Sciatica Bothersomeness Scale; PSLR; Active Lumbar Flexion; Modified ODI | at baseline after 6 days* | IG treatment favored for all outcomes | | | Plaza-Manzano et al. 2020 | passive neurodynamic slider in supine targeting sciatic nerve | motor control exercise program targeting transversus abdominis and multifidus; HEP with same exercises | 2x/wk; 4 wks; 8 treatments | NPRS; S-LANSS; RMDQ; PSLR; PPT | at baseline after 2 wks after 4 wks* 2 months after final session | IG treatment favored for S-LANSS and SLR | No significant group*time interactions for NPRS, RMDQ, and PPT | | Satishkumar et al. 2017 | active neurodynamic slider in seated slump position | lumbar stabilization exercises, progressed weekly | 5x/wk; 4 wks; 20 treatments | NPRS; RMDQ*; PSLR; FABQ* | at baseline after wk 1 after wk 2 after wk 4* | IG treatment favored for all outcomes after weeks 1, 2 and 4 | |
Five of the eight studies [27–31] performed neurodynamic slider techniques, two [32,33] performed a passive nerve stretch, and one [34] performed a neurodynamic tensioner. A slider is a technique in which multiple joints are moved to alternate tension and slack on opposing ends of a nerve tract, resulting in a ‘flossing’ effect that maximizes neural excursion while keeping tension relatively constant. A tensioner is a technique in which multiple joints are moved to pull on a nerve tract from both ends, moving dynamically between a position of low tension and a position of high tension. A static nerve stretch places a nerve tract in a position of tension and holds there for a set duration with no movement. One study [28] further attempted to reduce pressure on the affected nerve roots by performing a foramen opening technique in addition to the neurodynamic slider.Control interventions varied widely. Five of eight studies [30–34] provided an exercise program intended to improve lumbar stability and motor control, alone or along with lumbar spinal mobilization. Other control interventions included TENS, traction, and advice to remain active. All study participants in NM intervention groups received the control interventions in addition to the NM interventions.
The frequency of intervention and assessment was highly variable between studies. The duration of the intervention ranged from six straight days of intervention to six weeks of intervention three times per week. Four studies [28,32–34] included a home exercise program in addition to in-person treatment sessions. Four studies [27,29,32,34] assessed outcomes only at baseline and at the final treatment session. Others included assessments at intermediate time points[31], at a follow-up after the end of treatment[28], or both [30,33].
The populations captured by the inclusion and exclusion criteria of the eight studies were also significantly heterogeneous. Three studies [29–31] required MRI confirmation of lumbosacral disc herniation for inclusion, while others used symptom-based criteria including radiating pain and symptom provocation with nerve tension tests. There was a notable lack of consensus regarding the level of neural sensitivity considered to be appropriate for inclusion and NM intervention. Four papers had inclusion criteria of reproduction of radiating symptoms with PSLR of 30–70 degrees [31,34], 40–70 degrees[30], or >35 degrees[27], while two papers [32,33] excluded participants with a positive PSLR of <45 degrees. Duration of symptoms prior to the start of intervention ranged from an average of 4.87 weeks in one study to 5.8 years in another.
Methodological quality
Of the eight included trials, four were good quality via the PEDro quality assessment [28,30,32,33], three were fair [27,29,31], and one was poor[34]. The RoB2 risk-of-bias assessments corresponded well with the PEDro quality assessments, with the fair and poor quality papers judged to be high risk, and the four good quality studies judged to have some concerns. Quality assessment scores for all studies are available in Table 3.
Table 3.
PEDro and Cochrane Quality Assessment.
| | PEDro | | Cochrane | | | | | | | | | | | | | | | ------------------------------- | - | -------- | - | - | - | - | - | - | -- | -- | ------------- | ---- | --------------------- | --------------------------------------------------------------------------- | ------------- | | | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | total score* | | Subscores Domains 1–5 | Overall Risk | | | Ahmed et al. 2013 | y | y | y | y | n | n | n | n | n | y | y | 5/10 | | 1: low risk 2: high risk 3: some concerns 4: high risk 5: some concerns | high risk | | Cleland et al. 2006 | y | y | y | y | n | n | y | y | y | y | y | 8/10 | | 1: low risk 2: low risk 3: low risk 4: low risk 5: some concerns | some concerns | | Ferreira et al. 2016 | y | y | y | y | n | n | y | y | y | y | y | 8/10 | | 1: low risk 2: low risk 3: some concerns 4: low risk 5: low risk | some concerns | | Jeong et al. 2016 | y | y | n | n | n | n | n | n | n | y | y | 3/10 | | 1: some concerns 2: high risk 3: some risk 4: low risk 5: high risk | high risk | | Nagrale et al. 2013 | y | y | y | y | n | n | y | y | y | y | y | 8/10 | | 1: low risk 2: low risk 3: low risk 4: low risk 5: some concerns | some concerns | | Pallipamula & Singaravelan 2012 | y | y | n | y | n | n | n | y | n | y | y | 5/10 | | 1: some concerns 2: low risk 3: some concerns 4: high risk 5: some concerns | high risk | | Plaza-Manzano et al. 2020 | y | y | y | y | n | n | y | y | y | y | y | 8/10 | | 1: low risk 2: low risk 3: low risk 4: low risk 5: some concerns | some concerns | | Satishkumar et al. 2017 | y | y | y | y | n | n | n | n | n | y | y | 5/10 | | 1: low risk 2: high risk 3: some risk 4: high risk 5: some concerns | high risk |
No trials were able to blind the therapist to the intervention they were delivering. This reflects an ongoing challenge in the field rather than a failing of these studies in particular. Several studies that performed physical assessments as outcome measures did not specify whether the assessor was blinded to the intervention group. Only one paper [28] pre-published their study design in order to allow assessment of result reporting bias.
Results
Table 2 describes the results of each individual study included in this systematic review. All eight included studies measured disability or function as a primary outcome. Four [28,29,32,33] used the standard, modified, or revised version of the ODI, two [30,31] used the RMDQ, and one study each used the Patient Specific Functional Scale (PSFS)[28], SF-12[27], and select subscales of the SF-36[34]. Seven of the studies included pain as a primary outcome measure, with six [27,28,30–33] using the 11-point NPRS. Only three studies [28,30,33] re-assessed outcomes at a follow-up later than the final treatment session, and only one [30] assessed any outcomes later than six weeks following initial treatment.
Disability/Function
Five of the eight included studies found significant between-group differences favoring NM treatment for all outcomes related to function or disability at all post-baseline measurements [27,29,31,32,34]. Of the remaining three studies, Nagrale et al [33] found significant between-group differences favoring NM treatment for the ODI after weeks 3 and 6. Ferreira et al [28] had mixed results, with the PSFS showing significant between-group differences favoring NM treatment after week 2 and week 4, but no significant differences for the ODI. Plaza-Manzano et al [30] found no significant group*time interaction for the RMDQ.
Pain
Of the seven studies that included at least one measure of pain, five found significant between-group differences favoring NM treatment for all outcomes related to function or disability at all post-baseline measurements [27,29,31–33]. Of the remaining two studies, Ferreira et al [28] found significant between-group differences favoring NM treatment after week 4 in leg and low back NPRS scores. Plaza-Manzano et al [30] found significant group*time interactions for the PSLR and the Leeds Assessment of Neuropathic Symptoms and Signs (a measure of neuropathic pain), but not for the NPRS or pressure pain threshold.
Discussion
The goal of this systematic review was to determine the effectiveness of NM in treating adults with LBRP. This subset of low back pain patients experiences worse outcomes on average[1], but has been inconsistently defined pathologically in prior research, limiting the ability to draw conclusions about effective treatments. NM was chosen as the intervention of interest because it has been shown to be effective in treating nerve-related pain in other body regions[14], but evidence related to lumbosacral pain is limited.
Six of the eight RCTs included in this review found that adding an NM intervention to a conservative treatment plan improved all outcomes. The results of the remaining two papers favored NM treatment for some outcomes; Plaza-Manzano et al [30] found improvements in measures of neural sensitivity, but not overall pain and disability, while Ferreira et al [28] found improvements in some but not all functional measures, and delayed improvements in pain. Furthermore, no studies found any evidence of NM treatment having a detrimental effect on any outcomes. Overall, the collective results of these eight studies indicate that NM may be recommended for treatment of LBRP, although data are still too limited to determine the extent to which NM contributes to the effectiveness of a multi-modal treatment plan.
The low quality and inconsistent rigor of the included studies is a limitation of this review. Only four of the eight included studies achieved a quality rating of good on the PEDro scale and a RoB2 risk assessment lower than ‘high.’ Several studies failed to include critical information such as the proportion of randomized participants who completed the entire study, and the details of the concealed allocation process used for randomization. Ahmed et al [27] stated that participants were given a home exercise program but gave no information about the frequency with which they performed it. Jeong et al [34] described the steps of the NM technique performed, but gave no indication of whether the technique was performed for multiple repetitions within a treatment session, and provided no rationale for their choice of the general health and physical functioning subscales of the SF-36 as sole outcome measures.
The substantial variability in intervention design, frequency, and duration, as well as symptom intensity and duration in participant populations, limits our ability to draw conclusions about the efficacy of the use of any specific NM protocol. Our conclusions regarding long-term effects are also limited because only three studies re-assessed outcomes after the final treatment session. The breadth of positive findings when NM is added to a wide variety of control interventions does confirm that NM can be an appropriate part of a multi-modal treatment plan for people with LBRP.
The lack of consensus regarding language to describe LBRP limits the ability of researchers and clinicians to understand and discuss this unique population. The term ‘sciatica,’ while easily recognized, is not anatomically descriptive. Its continued use to describe both nerve root pathology and peripheral nerve involvement conflates two very different clinical presentations. The use of the term ‘radicular’ is also inconsistent across studies. The word’s literal meaning is ‘pertaining to the nerve root,’ but it has been conflated with ‘radiculopathy’ and therefore assumed to only apply to severe nerve root compromise. For example, Cleland et al [32] and Nagrale et al [33] go so far as to claim that their study populations have ‘non-radicular low back pain’ by nature of excluding participants who have diminished reflexes, sensation, or strength, despite all participants exhibiting low back pain that radiates below the gluteal fold and is reproduced with slump testing. This level of inconsistency in the use of the word radicular has the potential to actively create confusion.
Additional high quality RCTs on this subject are necessary to further explore the treatment of LBRP with NM. It is especially important that future RCTs on this topic use precise and consistent criteria to define the participant population. We recommend future studies use a predetermined duration of symptoms, a standardized method of identifying radicular symptoms, and longer follow-up periods. Standardized intervention protocols would also significantly reduce the overall study heterogeneity and improve the extent to which these findings can be generalized.
In conclusion, our findings suggest that NM may be an effective tool for short-term improvements in pain, function, and disability associated with LBRP. Robust claims regarding the utility of particular intervention protocols will require publication of additional high-quality RCTs with detailed, homogenous study protocols.
Supplementary Material
Supplemental Material
Notes on Contributors
Mica Peacock earned her Bachelor of Arts in Biology from Reed College, and received her Doctor of Physical Therapy degree at Samuel Merritt University.
Samuel Douglas received his Bachelor of Arts in Neuroscience and Behavior from Wesleyan University, and received his Doctor of Physical Therapy degree at Samuel Merritt University.
Preeti Nair earned her Bachelor of Physiotherapy at Pune University in India. She then earned a PhD in Rehabilitation Sciences at the University of Florida, with a research focus on Biomechanics and Neurophysiology of walking in individuals with neurological impairment. She is currently an Associate Professor in the Department of Physical Therapy at Samuel Merritt University.
Supplementary material
Supplemental data for this article can be accessed here.
Disclosure statement
Authors report no conflict of interest.
References
- [1].Konstantinou K, Dunn KM.. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33(22):2464–2472. [DOI] [PubMed] [Google Scholar]
- [2].Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332(7555):1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ropper AH, Zafonte RD. Sciatica. N Engl J Med. 2015;372(13):1240–1248. [DOI] [PubMed] [Google Scholar]
- [4].Van Boxem K, Cheng J, Patijn J, et al. Lumbosacral Radicular Pain. Pain Pract. 2010;10(4):339–358. [DOI] [PubMed] [Google Scholar]
- [5].Hill JC, Konstantinou K, Egbewale BE, et al. Clinical outcomes among low back pain consulters with referred leg pain in primary care. Spine. 2011;36(25):2168–2175. [DOI] [PubMed] [Google Scholar]
- [6].Tubach F, Beauté J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol. 2004;57(2):174–179. [DOI] [PubMed] [Google Scholar]
- [7].Grotle M, Brox JI, Glomsrød B, et al. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain. 2007;11(3):290–298. [DOI] [PubMed] [Google Scholar]
- [8].Deyo RA, Mirza SK. Herniated lumbar intervertebral disk. N Engl J Med. 2016;374(18):1763–1772. [DOI] [PubMed] [Google Scholar]
- [9].Bardin LD, King P, Maher CG. Diagnostic triage for low back pain: a practical approach for primary care. Med J Aust. 2017;206(6):268–273. [DOI] [PubMed] [Google Scholar]
- [10].Rogerson A, Aidlen J, Jenis LG. Persistent radiculopathy after surgical treatment for lumbar disc herniation: causes and treatment options. Int Orthop. 2019;43(4):969–973. [DOI] [PubMed] [Google Scholar]
- [11].Hancock MJ, Koes B, Ostelo R, et al. Diagnostic accuracy of the clinical examination in identifying the level of herniation in patients with sciatica. Spine. 2011;36(11):E712–E719. [DOI] [PubMed] [Google Scholar]
- [12].Lewis RA, Williams NH, Sutton AJ, et al. Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses. Spine J. 2015;15(6):1461–1477. [DOI] [PubMed] [Google Scholar]
- [13].Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86(1):92–109. [DOI] [PubMed] [Google Scholar]
- [14].Basson A, Olivier B, Ellis R, et al. The effectiveness of neural mobilization for neuromusculoskeletal conditions: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(9):593–615. [DOI] [PubMed] [Google Scholar]
- [15].Leininger B, Bronfort G, Evans R, et al. Spinal manipulation or mobilization for radiculopathy: a systematic review. Phys Med Rehabil Clin N Am. 2011;22(1):105–125. [DOI] [PubMed] [Google Scholar]
- [16].Kavlak Y, Uygur F. Effects of nerve mobilization exercise as an adjunct to the conservative treatment for patients with tarsal tunnel syndrome. J Manipulative Physiol Ther. 2011;34(7):441–448. [DOI] [PubMed] [Google Scholar]
- [17].Dabholkar AS, Kalbande VM, Yardi S. Neural tissue mobilisation using ultt2b and radial head mobilisation v/s exercise programme in lateral epicondylitis. Indian J Physiother Occup Ther. 2013;7(4):247. [Google Scholar]
- [18].Bialosky JE, Bishop MD, Price DD, et al. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J Orthop Sports Phys Ther. 2009;39(10):709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nee RJ, Vicenzino B, Jull GA, et al. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: a randomised trial. J of Physiotherapy. 2012;58(1):23–31. doi: 10.1016/s1836-9553(12)70069-3 [DOI] [PubMed] [Google Scholar]
- [20].Nar NH. Effect of neural tissue mobilization on pain in cervical radiculopathy patients. Indian Journal of Physiotherapy & Occupational Therapy-An International Journal. 2014;8(1):144–148 [Google Scholar]
- [21].Gupta R, Sharma S. Effectiveness of median nerve slider’s neurodynamics for managing pain and disability in cervicobrachial pain syndrome. Indian J of Physiother & Occup Ther. 2012;6(1). [Google Scholar]
- [22].Efstathiou MA, Stefanakis M, Savva C, et al. Effectiveness of neural mobilization in patients with spinal radiculopathy: a critical review. J Bodyw Mov Ther. 2015;19(2):205–212. [DOI] [PubMed] [Google Scholar]
- [23].Su Y, Lim ECW. Does evidence support the use of neural tissue management to reduce pain and disability in nerve-related chronic musculoskeletal pain? A systematic review with meta-analysis. Clin J Pain. 2016;32(11):991–1004. [DOI] [PubMed] [Google Scholar]
- [24].de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. [DOI] [PubMed] [Google Scholar]
- [25].Foley NC, Teasell RW, Bhogal SK, et al. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10(1):1–7. [PubMed] [Google Scholar]
- [26].Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [27].Ahmed N, Tufel S, Khan MH, et al. Effectiveness of neural mobilization in the management of sciatica. J Musculoskelet Res. 2013;16(3):1350012 [Google Scholar]
- [28].Ferreira G, Stieven F, Araujo F, et al. Neurodynamic treatment did not improve pain and disability at two weeks in patients with chronic nerve-related leg pain: a randomised trial. J Physiother. 2016;62(4):197–202. [DOI] [PubMed] [Google Scholar]
- [29].Pallipamula K, Singaravelan RM. Efficacy of nerve flossing technique on improving sciatic nerve function in patients with sciatica – a randomized controlled trial. Rom J Phys Ther. 2012;18(30):13–22. [Google Scholar]
- [30].Plaza-Manzano G, Cancela-Cilleruelo I, Fernández-de-las-peñas C, et al. Effects of adding a neurodynamic mobilization to motor control training in patients with lumbar radiculopathy due to disc herniation: a randomized clinical trial. Am J Phys Med Rehabil. Published online 2019 Sept 5. doi: 10.1097/PHM.0000000000001295 [DOI] [PubMed] [Google Scholar]
- [31].Satishkumar BS, Dibyendunarayan BD, Ramalingam TA. Effectiveness of nerve flossing technique in chronic lumbar radiculopathy. Indian J Physiother Occup Ther. 2017;11(1):44–49 [Google Scholar]
- [32].Cleland JA, Childs JD, Palmer JA, et al. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006;11(4):279–286. [DOI] [PubMed] [Google Scholar]
- [33].Nagrale AV, Patil SP, Gandhi RA, et al. Effect of slump stretching versus lumbar mobilization with exercise in subjects with non-radicular low back pain: a randomized clinical trial. J Man Manip Ther. 2012;20(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jeong UC, Kim CY, Park YH, et al. The effects of self-mobilization techniques for the sciatic nerves on physical functions and health of low back pain patients with lower limb radiating pain. J Phys Therapy Sci. 2016;28(1):46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material