Muscle Biopsy-proven Drug-induced Microscopic Polyangiitis in a Patient with Tuberculosis (original) (raw)
Abstract
We herein report a case of muscle biopsy-proven microscopic polyangiitis (MPA) in a patient with tuberculosis. The patient had developed a persistent fever after the initiation of treatment for tuberculosis and was positive for myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA). However, because conventional symptoms were lacking, determination of the biopsy site was difficult. Based on the findings of a biopsy of the biceps femoris, which confirmed small vessel vasculitis, the patient was diagnosed with MPA. The fever was alleviated by glucocorticoids. Tuberculosis and antituberculosis drugs can cause ANCA-associated vasculitis (AAV). A muscle biopsy is useful for the diagnosis of AAV.
Keywords: antineutrophil cytoplasmic antibody, antineutrophil cytoplasmic antibody-associated vasculitis, microscopic polyangiitis, muscle biopsy, tuberculosis
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is an autoimmune disease characterized by systemic small-vessel vasculitis. Two types of ANCA, myeloperoxidase (MPO)-ANCA and proteinase 3-ANCA, are mainly associated with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA). AAV typically involves the kidneys and lungs and causes renal failure, interstitial pneumonia, and diffuse alveolar hemorrhaging (DAH).
However, while AAV is a life-threatening disease (1,2), its diagnosis is often difficult, especially when typical symptoms are lacking. A kidney biopsy is performed when renal involvement is suggested. Alternatively, a lung biopsy, although less common, can also be performed. However, it is difficult to perform a biopsy when there are no classical symptoms indicative of the site. Furthermore, infectious diseases and drugs can cause AAV, which makes the diagnosis even more complicated.
We herein report a case of MPA in a patient with tuberculosis, in which a muscle biopsy was useful for the diagnosis.
Case Report
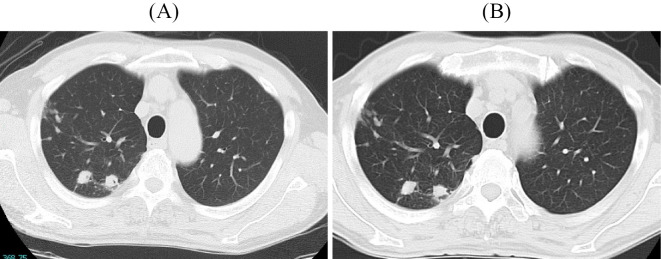
The patient was a 70-year-old man with no notable medical history. Chest abnormal shadows were detected by chest X-ray during a health examination. Chest computed tomography (CT) performed at another hospital for a close examination detected nodular and granular shadows suggestive of lung tuberculosis in the right upper lobe (Fig. 1A). The patient was asymptomatic. Sputum culture was positive for Mycobacterium tuberculosis, and the diagnosis of lung tuberculosis was made.
Figure 1.
Chest computed tomography showing nodular and granular shadows in the right upper lobe. (A) At the time of the diagnosis. (B) At the time of the first examination in our hospital.
Antituberculosis treatment was initiated with isoniazid, rifampicin, ethambutol, and pyrazinamide in June 2021. He developed a persistent fever three months after the initiation of treatment, and the drugs were discontinued, as drug fever was suspected. However, the fever persisted, and the cause remained unknown. Therefore, he was referred to our hospital in November 2021 for a further examination.
During the first examination, he was febrile and exhausted due to the persistent fever. A laboratory examination showed an elevated MPO-ANCA titer of 36.1 IU/mL and a normal level of creatinine. The drug-induced lymphocyte stimulation (DLST) test was positive for isoniazid. No abnormal findings were detected on a urinalysis (Table). Purpura, arthralgia, neuropathy, and fecal occult blood were not confirmed. Chest CT showed nodular and granular shadows in the right upper lobe, along with no findings suggestive of interstitial pneumonia (Fig. 1B). Chest shadows slightly improved compared with those confirmed at the diagnosis. Enhanced abdominal CT and magnetic resonance imaging (MRI) of the spine showed no findings that could cause a fever, such as intra-abdominal abscess or tuberculous spondylitis. Transthoracic echocardiography revealed no vegetations, and no significant microorganisms were detected in blood, sputum, or urine cultures, including M. tuberculosis.
Table.
Laboratory Findings during the First Examination in Our Hospital.
| Blood cell count | Blood chemistry | ||
|---|---|---|---|
| WBC (/μL) | 9,400 | TP (g/dL) | 7.2 |
| Neutrophils (%) | 84.5 | T-Bil (mg/dL) | 0.4 |
| Eosinophils (%) | 1.8 | AST (U/L) | 55 |
| Basophils (%) | 0.5 | ALT (U/L) | 84 |
| Lymphocytes (%) | 7.9 | LDH (U/L) | 140 |
| Monocytes (%) | 5.3 | γ-GTP (U/L) | 118 |
| RBC (104/μL) | 376 | BUN (mg/dL) | 20 |
| Hemoglobin (g/dL) | 10.1 | Cre (mg/dL) | 0.71 |
| Hematocrit (%) | 31.4 | CK (U/L) | 21 |
| Plt (/μL) | 37.3 | Na (mEq/L) | 132 |
| K (mEq/L) | 4.2 | ||
| Cl (mEq/L) | 95 | ||
| CRP (mg/dL) | 14.43 | ||
| HbA1c (%) | 6.0 | ||
| F-T4 (ng/dL) | 1.11 | ||
| TSH (μIU/mL) | 7.41 | ||
| IgG (mg/dL) | 2,009 | ||
| IgA (mg/dL) | 294 | ||
| IgM (mg/dL) | 41 | ||
| IgE (IU/mL) | 151 | ||
| IgG4 (mg/dL) | 18 | ||
| C3 (mg/dL) | 120 | ||
| C4 (mg/dL) | 189 | ||
| CH50 (U/mL) | 47.7 | ||
| RF (IU/mL) | 17 | ||
| AAN | 160 (homogenous) | ||
| MPO-ANCA (U/mL) | 36.1 | ||
| PR3-ANCA (U/mL) | <1.0 | ||
| sIL2R (U/mL) | 1,792 | ||
| Infection | |||
| β-D-Glucan (pg/mL) | 9.3 | ||
| Aspergillus antigen | Negative | ||
| Cryptococcus antigen | Negative | ||
| HIV antibody | Negative | ||
| Urinalysis | |||
| RBC (/HPF) | 1-4 | ||
| WBC (/HPF) | 1-4 | ||
| Protein | Negative | ||
| DLST | |||
| Isoniazid | Negative | ||
| Rifampicin | Negative | ||
| Ethambutol | Negative |
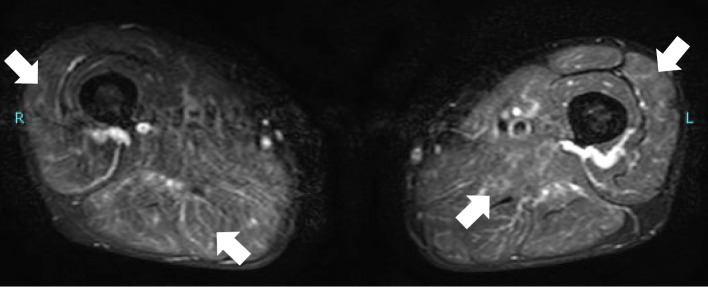
Since the patient complained of mild grasp pain in his thighs, MRI of the lower extremities was performed, and fat-suppression T2 imaging showed a linear and reticular high signal, suggestive of vasculitis in the thigh muscles of both sides (Fig. 2). Based on these findings and an elevated MPO-ANCA titer, MPA was suspected. Therefore, a muscle biopsy was performed on the right biceps femoris. A histopathological examination revealed monocyte infiltration around the small vessels and fibrinoid necrosis of the vessel walls (Fig. 3). According to the classification criteria for MPA proposed by the American College of Rheumatology/European Alliance of Associations for Rheumatology (3), the present case had a classification score of six and was thus classified as having MPA.
Figure 2.
Fat-suppression T2 images of magnetic resonance imaging showing a linear and reticular high signal in the thigh muscles (arrows).
Figure 3.
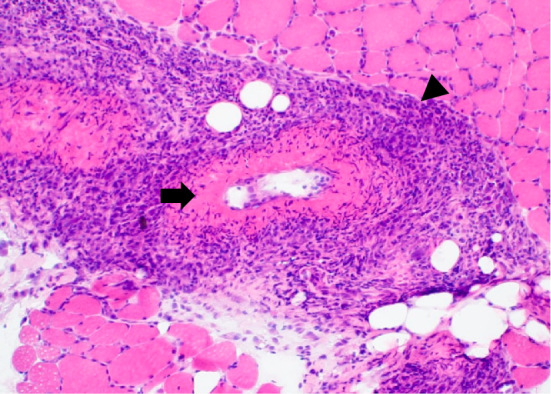
A histopathological examination of the right biceps femoris showing monocyte infiltration around the small vessels (arrowhead) and fibrinoid necrosis of the vessel walls (arrow) (Hematoxylin and Eosin staining, ×10).
Radiological worsening of lung tuberculosis lesions and signs of extrapulmonary tuberculosis were not detected, and sputum culture was negative for M. tuberculosis. The patient had used drugs that could induce MPA. Therefore, we excluded active tuberculosis mimicking MPA and made a diagnosis of drug-induced MPA.
The fever and grasp pain were alleviated immediately after the administration of prednisolone 30 mg/day, accompanied by a decrease in the MPO-ANCA titer. Antituberculosis drugs rifabutin, levofloxacin, and ethionamide were administered. Tapering of prednisolone and treatment for tuberculosis will continue accordingly.
Discussion
We herein report a case of MPA diagnosed by a muscle biopsy in a patient with lung tuberculosis. AAV, which includes MPA, is a systemic autoimmune vasculitis that affects small vessels and most often involves the kidneys and lungs. Glomerulonephritis develops in approximately 77% of patients (4) and typically presents as rapidly progressive glomerulonephritis. Interstitial pneumonia and DAH occur in 15% and 12% of patients with MPA, respectively (5,6). DAH causes acute respiratory failure, and mechanical ventilation is required in 47% of cases (1).
As the lack of typical symptoms at disease presentation often leads to difficulties in making a diagnosis, considering the increasing prevalence of AAV (5), an appropriate diagnostic approach is warranted. In other studies, approximately 74.5% of patients with MPA and 92% with GPA were ANCA-positive (7,8). Certain drugs, including propylthiouracil and hydralazine, can cause AAV (9). Rifampicin and ethambutol have also been reported to cause AAV (10). In our case, the fever persisted, along with the increased ANCA titer, even after discontinuation of antituberculosis drugs. Although drug-induced AAV usually resolves and ANCA level falls after discontinuation of the causative drugs (9), in some cases, vasculitis can persist even after discontinuation of drugs and can progress to end-stage renal failure (10). Therefore, drug-induced MPA was suspected, and we changed the antituberculosis drugs.
In addition to drugs, ANCA can be detected in patients with infectious diseases, such as infective endocarditis (11). ANCA, especially MPO-ANCA rather than PR3-ANCA, has been associated with tuberculosis (12), similar to the present case. Furthermore, Toyoshima et al. reported a case of GPA with positive ANCA that reacted with antituberculosis drugs (13). Why ANCA levels are elevated in patients with tuberculosis is unclear. However, this phenomenon strongly suggests that acid-fast bacilli are associated with the onset of AAV. In the present case, not only antituberculosis drugs but also M. tuberculosis infection might have been associated with the development of MPA. The significance of the positive DLST findings for isoniazid is unclear, as the diagnostic value of a DLST for drug-induced AAV has not been studied.
Muscle is a well-vascularized tissue that can be easily accessed, and the usefulness of a muscle biopsy for vasculitis has been established (14). In a study that examined the utility of a muscle biopsy for confirming vasculitis, the sensitivity and specificity were 67.7% and >99%, respectively, although this study was not restricted to AAV (15). More recently, Lacou et al. (16) reported that predictive factors for positivity of a muscle biopsy in AAV included MPO-ANCA, female sex, and an elevated neutrophil count; conversely, the CK level, biopsy site, and presence of myalgia were not associated. In our case, the patient had positive MPO-ANCA findings, and the CK levels were not elevated.
This study highlights the utility of a muscle biopsy for successfully diagnosing a case of MPA. Despite its inability to predict the renal prognosis, relapse, and death (16), a muscle biopsy remains a useful technique for diagnosing AAV, specifically in cases with a strong suspicion of AAV but where it is difficult to determine the biopsy site. Furthermore, physicians should be aware that AAV may develop in patients with tuberculosis.
Written informed consent for the publication of this case report was obtained from the patient. A copy of the written consent form is available for review by the Editor-in-Chief of the journal on request.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Cartin-Ceba R, Diaz-Caballero L, Al-Qadi MO, et al. Diffuse alveolar hemorrhage secondary to antineutrophil cytoplasmic antibody-associated vasculitis: predictors of respiratory failure and clinical outcomes. Arthritis Rheumatol 68: 1467-1476, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 12: 1680-1691, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suppiah R, Robson JC, Grayson PC, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 116: 488-498, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Watts RA, Mahr A, Mohammad AJ, et al. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transplant 30: i14-i22, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Schirmer JH, Wright MN, Vonthein R, et al. Clinical presentation and long-term outcome of 144 patients with microscopic polyangiitis in a monocentric German cohort. Rheumatology (Oxford) 55: 71-79, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Guillevin L, Durand-Gasselin B, Cevallos R, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum 42: 421-430, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Finkielman JD, Lee AS, Hummel AM, et al. ANCA are detectable in nearly all patients with active severe Wegener's granulomatosis. Am J Med 120: 643.e9-643.e14, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Savige J, Davies D, Falk RJ, et al. Antineutrophil cytoplasmic antibodies and associated diseases: a review of the clinical and laboratory features. Kidney Int 57: 846-862, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Oshiro Y, Kawanaka N, et al. A case of MPO-ANCA-related nephritis caused by an anti-tuberculosis drug. Nihon Jinzo Gakkai Shi 55: 172-176, 2013(in Japanese). [PubMed] [Google Scholar]
- 11.Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol 66: 1672-1677, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Sherkat R, Mostafavizadeh K, Zeydabadi L, et al. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol 8: 52-57, 2011. [PubMed] [Google Scholar]
- 13.Toyoshima M, Chida K, Suda T, et al. Wegener's granulomatosis responding to antituberculous drugs. Chest 119: 643-645, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Said G, Lacroix-Ciaudo C, Fujimura H, et al. The peripheral neuropathy of necrotizing arteritis: a clinicopathological study. Ann Neurol 23: 461-465, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Hervier B, Durant C, Masseau A, et al. Use of muscle biopsies for diagnosis of systemic vasculitides. J Rheumatol 38: 470-474, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Lacou M, Leroy M, Le Lan N, et al. Muscle biopsy in anti-neutrophil cytoplasmic antibody-associated vasculitis: diagnostic yield depends on anti-neutrophil cytoplasmic antibody type, sex, and neutrophil count. Rheumatology (Oxford) 60: 699-707, 2021. [DOI] [PubMed] [Google Scholar]