The HOX Homeodomain Proteins Block CBP Histone Acetyltransferase Activity (original) (raw)
Abstract
Despite the identification of PBC proteins as cofactors that provide DNA affinity and binding specificity for the HOX homeodomain proteins, HOX proteins do not demonstrate robust activity in transient-transcription assays and few authentic downstream targets have been identified for these putative transcription factors. During a search for additional cofactors, we established that each of the 14 HOX proteins tested, from 11 separate paralog groups, binds to CBP or p300. All six isolated homeodomain fragments tested bind to CBP, suggesting that the homeodomain is a common site of interaction. Surprisingly, CBP-p300 does not form DNA binding complexes with the HOX proteins but instead prevents their binding to DNA. The HOX proteins are not substrates for CBP histone acetyltransferase (HAT) but instead inhibit the activity of CBP in both in vitro and in vivo systems. These mutually inhibitory interactions are reflected by the inability of CBP to potentiate the low levels of gene activation induced by HOX proteins in a range of reporter assays. We propose two models for HOX protein function: (i) HOX proteins may function without CBP HAT to regulate transcription as cooperative DNA binding molecules with PBX, MEIS, or other cofactors, and (ii) the HOX proteins may inhibit CBP HAT activity and thus function as repressors of gene transcription.
The HOX homeodomain (HD) proteins have long been recognized as master developmental regulators. However, despite intensive efforts, their mechanism of action remains obscure. Soon after HOX genes were first described, the isolated 60-amino-acid HD was shown to bind DNA (27), and a paradigm that the HOX proteins function as transcription factors was quickly established (17). However, many full-length HOX proteins bind DNA very poorly in in vitro assays and/or exhibit little binding specificity (44). An apparent answer to both of these problems was the demonstration that HOX proteins form cooperative DNA binding complexes with the PBC HD proteins, including PBX/EXD and MEIS/PREP/HTX (reviewed in reference 23). These PBC-HOX interactions increased the DNA binding affinity of HOX proteins and defined an apparent specificity code for DNA binding across the 13 paralog groups into which HOX proteins can be assigned on the basis of amino acid homology (6, 47). The most compelling evidence for a model in which HOX proteins function as DNA binding factors is presented in a series of papers showing that modification of putative PBX-HOX recognition sites in the upstream regulatory regions of several HOXB genes causes changes in lacZ reporter gene expression in transgenic mice (22, 33, 50). Persuasive data for HOX proteins acting as DNA binding proteins has also been obtained in Drosophila, which carries reporter genes in various mutant backgrounds (40). New data also suggest that HOX proteins function with a PBX-like protein to regulate zebra fish morphogenesis (35). However, other recent studies have questioned the concept that HOX-PBC binding sites confer target gene regulatory specificity (19).
Despite the intensive efforts of many laboratories, few authentic downstream targets for HOX proteins functioning as transcription activators or repressors have been described, and the mechanism of action of HOX proteins remains unclarified. However, there have been a few recent papers which describe apparent HOX protein targets, albeit not in the context of cooperative DNA binding with the PBC proteins (37, 38). Among the most intriguing possible cofactors that might enhance HOX protein transcriptional activity are CBP and p300. These proteins have been the subject of intense study and have been shown to potentiate the activity of a number of transcription factors (9, 24, 26, 28, 36, 54). Indeed, CBP was reported to interact specifically with and enhance HOXB7 protein activity (8), as well as to enhance the transcriptional activity of HOXD4 (41). Among their several functions, CBP and p300 are thought to increase general transcription through the activity of a histone acetyltransferase (HAT) domain (24, 28). One current model for the role of CBP and p300 in transcription is that they function by mediating the acetylation of histones within the nucleosome core, thus facilitating and/or stabilizing steric changes which permit increased access of the general transcriptional machinery to target genes (14). An alternative model is that CBP and p300 directly acetylate transcription factors, thereby altering their DNA binding capacities (1, 11, 53).
In an attempt to explain our inability to observe substantial transcriptional activity for HOX proteins, we began to explore whether CBP/p300 could potentiate HOX protein activity in a range of reporter-cell systems. We have demonstrated that HOX proteins from each of the 11 paralog groups tested can interact with CBP and/or p300. However, the anticipated formation of CBP-HOX complexes on consensus HOX DNA binding sites were not observed. On the contrary, addition of CBP to preformed HOX-DNA complexes results in the disappearance of electrophoretic mobility shift assay (EMSA) bands. Consistent with this observation, CBP did not potentiate HOX or PBX-HOX activity with a range of reporter genes in transient-transfection assays. Although HOX proteins are not substrates for CBP, they can inhibit CBP HAT activity in vitro and in vivo. These observations have led us to consider a change in the paradigm that HOX proteins always function as DNA binding transcription factors, and we now propose that an additional mechanism of action may be to modulate the HAT activity of CBP and p300.
MATERIALS AND METHODS
In vitro coprecipitation assays.
For immunoprecipitation, bacterially expressed glutathione _S_-transferase (GST)-CBP (13) (amino acids 1098 to 1877 [see Fig. 4D for structures]) or control GST protein was affinity purified by adsorption to glutathione-Sepharose 4B (Pharmacia, Piscataway, N.J.). Flag-p300 was produced from a baculovirus vector (4) in SF9 cells and affinity purified on M2 monoclonal anti-Flag beads (Sigma, St. Louis, Mo.). 35S-labeled HOX, MEIS1, and PBX1a proteins were synthesized as previously described (44) using the TNT system (Promega, Madison, Wis.). The labeled proteins were incubated with immobilized GST-CBP or control GST beads, or Flag-p300 bound to M2 beads or M2 control beads, at 4°C for 4 to 16 h in binding buffer consisting of 10 mM Tris-HCl (pH 7.5), 75 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol (DTT), 6% glycerol, 1% bovine serum albumin (BSA), and 1% NP-40 with 1× proteinase inhibitor cocktail (Roche, Indianapolis, Ind.). Following extensive washing in a solution of 15 mM Tris-HCl 75 (pH 7.5), 75 mM NaCl, 1% BSA, and 0.15% Triton X-100 with 1× proteinase inhibitor cocktail, the beads were boiled with 2× Laemmli buffer, and the precipitated proteins were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels and subjected to autoradiography. For coprecipitation experiments (see Fig. 4), 35S-labeled CBP CH3 subfragment or HOXD4-HD, prepared by in vitro transcription-translation, was coprecipitated using antisera to the T7 tag or Flag epitope fused to the HOX proteins or to the Flag epitope fused to CBP HAT (Sigma).
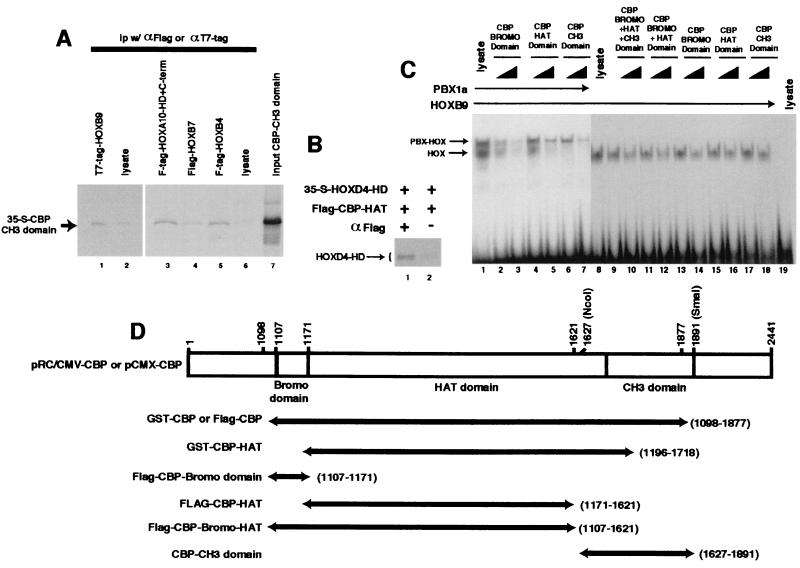
FIG. 4.
Different CBP domains interact with HOX proteins. (A) The CBP CH3 domain interacts with HOX proteins. Coimmunoprecipitation was performed using 35S-labeled CBP CH3 domain together with immobilized T7 or Flag epitope-tagged HOX proteins (lanes 1, 3, 4, and 5) or control beads (lanes 2 and 6). The CBP CH3 polypeptide was specifically precipitated by full-length HOXB9 (compare lanes 1 and 2), as well as HOXB4 and HOXB7 (compare lane 4 or 5 with 6) or the HOXA10 HD plus a short C-terminal (C-term) region (compare lanes 3 and 6). (B) The HOXD4 homeodomain binds to the CBP HAT domain. 35S-labeled T7 epitope-tagged HOXD4-HD and unlabeled Flag-CBP HAT domain protein fragments, synthesized by in vitro translation, were preincubated and subjected to coprecipitation in the presence (+; lane 1) or absence (−; lane 2) of antiserum to the Flag epitope. (C) HOXB9 interacts with the CBP HAT, bromo, and CH3 domains. CBP domains (shown schematically in panel D) were expressed as Flag epitope-tagged proteins by using an in vitro transcription-translation system and used in competitive EMSA assays at approximately one to threefold molar ratio to the HOXB9 protein, as described in Materials and Methods. Each domain was capable of specifically blocking HOXB9-DNA interactions, as reflected by diminution of the EMSA band intensities. (D) CBP expression clones.
DNA precipitation assays were performed using a 32P-labeled oligonucleotide containing an Abd-B HOX protein consensus binding site (TTACGAC) (47) with T7 epitope-tagged HOXB9 or HOXB13 and Flag epitope-tagged CBP in the same binding buffer and wash solutions. HOX proteins were preincubated with the target DNA for 30 min at 4°C followed by incubation with Flag-CBP for an additional 30 min and then immunoprecipitated with anti-Flag antibody (Sigma) and protein G beads.
EMSA interference by CBP.
PBX1a and the HOX or MEIS1 proteins, synthesized using the TNT system, were coincubated with a 32P-labeled probe at 4°C for 30 min in binding buffer prior to addition of increasing amounts (approximate molar ratio, 0.5 to 2) of Flag-CBP or Flag-CBP fragments, prepared by in vitro transcription-translation. Following an additional 30-min incubation, the reaction mixtures were subjected to EMSA analysis. In some experiments, the HOX proteins were incubated with increasing amounts of Flag-CBP prior to addition of the DNA, with identical results. The target oligonucleotides contained a PBX site (ATGAT) and either a consensus site that can be bound by proteins from HOX paralog groups 1 through 8 (TAAT), a binding site for the higher HOX paralog proteins (TTAC) (47), or a MEIS1 binding site (TGACAG) (46).
Acetylation assay.
Histone H3 (2 μg; Roche) was incubated with immobilized GST-CBP HAT (amino acids 1196–1718) in the presence or absence of approximately equal amounts of graded concentrations of His-tagged full-length HOXB1 or HOXD4 protein, or the HOXA9 or HOXA10 HD motif, in 50 mM Tris-HCl (pH 8.0), 1 mM DTT, 10 mM Na butyrate, 10% glycerol, and 14C-labeled acetyl-coenzyme A (28). These proteins were prepared as pET (Novagen, Madison, Wis.) derivatives in bacteria and affinity purified on Ni-nitrilotriacetic acid-His beads (Novagen). Affinity-purified HOXB6-maltose binding protein was prepared as a bacterial fusion product, and purified maltose binding protein was used as a control for nonspecific inhibition of HAT activity. Affinity-purified GST protein was also used as a control for nonspecific inhibition of HAT activity. Based on Coomassie blue staining, the HOX proteins were used at approximate ratios relative to histone H3 of 0.03 to 0.1 for HOXB1 and HOXA10-HD, 0.03 to 0.3 for HOXD4 (in separate experiments), and 0.1 for HOXB6 and HOXA9-HD.
HOX protein-mediated transcription assays.
The reporter constructs included (i) pTCBS, which contains an 8-mer of an oligonucleotide containing a consensus TAAT HOX binding site in the pT109luc plasmid, which has been shown to confer activation by HOXD9 (52) and by HOXB7 (8); (ii) pTHCR, which contains a 90-bp cross-regulatory HOX response element sequence from the upstream region of the HOXD9 gene cloned into the pT81 luciferase vector (51); (iii) pSX and pNB, 6.1-kb and 217-bp fragments from the upstream autoregulatory region of the HOXD4 gene cloned into the pXP2 luciferase reporter (34); and (iv) pPBX-HOX9, which contains a 3-mer of an oligonucleotide containing a consensus ATGAT-TTACGAC recognition sequence for PBX and the AbdB HOX proteins inserted into the pGL3 luciferase reporter vector (47). pCMX-CBP-HA expresses a full-length CBP protein with a C-terminal hemagglutinin epitope tag fusion (4), while pRC/CMV-CBP expresses a full-length CBP. For transient assays, all plasmids, including the reporter DNA (total, less than 3 μg/60-mm-diameter dish) were transfected into cells with Lipofectamine (Life Technologies, Gaithersburg, Md.), and after 36 h, the cell lysates were assayed for luciferase or chloramphenicol acetyltransferase (CAT) reporter gene activity, which was normalized to β-galactosidase (β-Gal) activity resulting from a pRSV–β-Gal transfection control plasmid. In a few transient and stable assays, the histone deacetylase inhibitor Trichostatin A (Wako Biochemicals, Richmond, Va.) was included in the cell culture media at concentrations of 20 to 1,000 nM. Although the addition of Trichostatin A did not alter reporter gene activity at any level assayed, concentrations above 300 nM were found to be toxic to cells. Most of the assays for HOXB7 activity used a cytomegalovirus expression plasmid used in our previous studies (6). For several assays with the pTCBS plasmid, a second HOXB7 clone in the pcDNA3 expression plasmid (8) (from V. Bours) was used. For stable assays, the pNB or pTCBS reporter genes were subcloned into the pTK-Hygro vector (Clontech, Palo Alto, Calif.). To make pNB-hygro and pTCBS-hygro, the hygro region from pTK-Hygro was excised with _Ava_I/blunt and _Hin_dIII and cloned into pNB and pTCBS cut with _Bam_HI/blunt plus _Hin_dIII. Following establishment of stable lines, the HOX-CBP expression plasmids and the RSV–β-Gal transfection control plasmid were transiently transfected into cells, and reporter gene activity was measured after 24 h. Total DNA was held constant by the addition of parental vectors for each experiment. Data are reported relative to the activity measured for the reporter gene in the absence of exogenous transcription factors. All assays were performed in duplicate.
In vivo GAL4 DBD-CBP HAT-mediated transcription assays.
Assays using the pCDNA-GAL4-DNA binding domain (DBD)-CBP-HAT expression plasmid and the GAL4-pML-CAT reporter construct in 293T cells (Fig. 5A) were performed as previously described (24), with a constant total DNA concentration maintained by the addition of the appropriate parental plasmids, using a pRSV-β-Gal expression vector to control for transfection efficiency. Data are reported as CAT activity relative to the levels detected for the pML-CAT vector in the absence of GAL4 DBD-CBP HAT, which was arbitrarily assigned a value of 1.0. Means and standard deviations are reported (see below). Statistical significance was calculated using the Student t test. Western blot analysis was used to confirm expression of HOX protein fragments and that the expression of HOX proteins did not alter GAL4 DBD-CBP HAT protein levels. Parallel transfections of 293T cells were harvested in 10 mM NaPO4 (pH 7.2)–1% β-mercaptoethanol–1% SDS–6 M urea. Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), followed by immunoblotting with anti-Flag serum to detect HOXB7, anti-GAL4 (Santa Cruz Biotechnology, Santa Cruz, Calif.) to detect GAL4 DBD-CBP HAT, or a previously described affinity-purified serum used to detect HOXB6 (16), using an ECL kit (Amersham, Piscataway, N.J.). To test for possible HOX protein binding to the GAL4 DBD as a source of transcriptional repression, in vitro coimmunoprecipitation experiments were performed as described above, using 35S-labeled GAL4 DBD or GAL4 DBD-CBP HAT proteins together with Flag-tagged HOXB7. Antiserum to the Flag epitope fused to HOXB7 specifically precipitated GAL4 DBD-CBP HAT but was unable to precipitate GAL4 DBD alone, indicating that HOXB7 interacts with CBP HAT but not with the GAL4 DBD (not shown). As an additional control for possible HOX protein interference in the binding of the GAL4 DBD to a DNA target, EMSA was performed with a consensus GAL4 binding site (43) together with bacterially expressed GST-GAL4 DBD (Santa Cruz Biotechnology) in the absence or presence of one-, two-, and threefold molar excess of bacterially expressed HOXD4 or HOXB1. The HOX proteins did not compete GAL4 DBD interactions, as reflected by a constant intensity of EMSA bands (not shown).
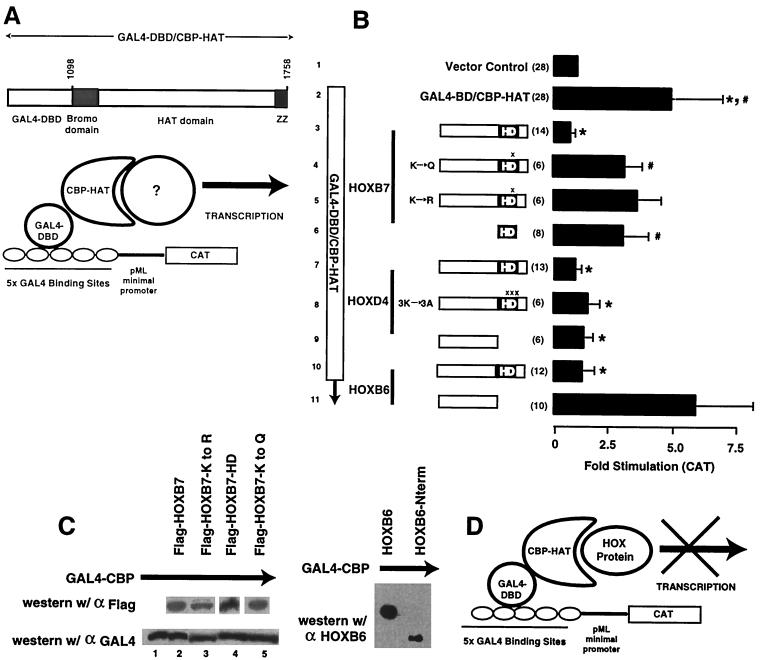
FIG. 5.
HOX proteins block in vivo CBP HAT activity. (A) Schematic representation of the assay system used to demonstrate the in vivo importance of CBP HAT activity for reporter gene transcription. A previously described system for demonstrating the importance of HAT activity for the transcriptional effects of CBP (24) was studied in the presence and absence of HOX proteins using transient-transfection assays in 293T cells. (B) HOX proteins block in vivo CBP HAT-mediated gene transcription. In this transient-transfection system, expression of a GAL4-CBP HAT fusion protein stimulates reporter gene expression from a plasmid containing multiple GAL4 binding sites (compare bars 1 and 2). Cotransfection of full-length Flag-HOXB7 (bar 3), HOXD4 (bar 7), or Flag-HOXB6 (bar 10) resulted in complete inhibition of the GAL4-CBP-induced activity (∗, P < 0.001). Flag-HOXB7 proteins containing mutations at Lys-55 within helix 3 of the HD (bars 4 and 5) showed partial inhibition of CBP HAT (#, P < 0.03 for K→Q HOXB7; P = 0.06 for K→R HOXB7), suggesting the importance of this amino acid for CBP interactions. In contrast, a HOXD4 protein containing Lys-Ala substitutions at positions 55, 57, and 58 of the HD (bar 8) still inhibited CBP HAT. Consistent with this finding, the observation that the N-terminal region of HOXD4 lacking the HD (bar 9) also blocks in vivo CBP HAT suggests that this protein does not require the HD for interaction with CBP. However, the N-terminal region of HOXB6 does not block CBP HAT activity (bar 11), suggesting that the HD is required for this HOX-CBP interaction. The isolated Flag-HOXB7 HD fragment (bar 6) exhibited reduced effectiveness in blocking CBP HAT-mediated activation in a statistically significant manner (#, P < 0.03), suggesting that the HOXB7 N-terminal flanking region is required for full in vivo functional interaction with CBP. Statistical differences were calculated compared to the vector control (bar 2). Means and standard deviations are reported. The approximate locations of point mutations in HOXB7 and HOXD4 are denoted by X above the HD. (C) Western blot analysis demonstrating the expression of approximately equal amounts of the various HOX proteins and equal expression of the GAL4-CBP protein in transient-transfection assays. Parallel transfection dishes from the experiments shown in panel B were subjected to Western blotting with antiserum to either the epitope Tag (HOXB7) or the GAL4 fusion moiety (GAL4-CBP HAT) or with antiserum against the N-terminal region of the HOXB6 protein (HOXB6-Nterm). w/, with. (D) Model for HOX protein inhibition of CBP HAT-mediated transcriptional activation. CBP HAT potentiates gene transcription by an unknown mechanism previously shown to depend on CBP HAT activity (panel A). In the model, HOX proteins are proposed to bind to and inhibit the CBP HAT domain activity.
In vivo coprecipitation assays.
293T cells were transiently transfected with either a Flag-HOXB7, a Flag-HOXB6, or a HOXD4 expression plasmid alone or together with the pCDNA-GAL4-DBD-CBP/HAT expression plasmid. After 48 h of culture, the cells were lysed by sonication in 2× binding buffer (150 mM NaCl, 2 mM EDTA, 2 mM DTT, 20 mM Tris-HCl [pH 7.5], 12% glycerol, 2% BSA, and 2% NP-40). The lysates were diluted 1:1 with distilled water and incubated for 4 to 16 h at 4°C with antisera to GAL4 or control sera or antisera to Flag. Following adsorption of antibodies to protein G beads and washing (75 mM NaCl, 0.15 mM Tris.HCl [pH 7.5], 1% BSA, 0.15% Triton X-100), the precipitated proteins were subjected to SDS-PAGE and Western blotting with anti-Flag or HOXB6 or anti-CBP and visualized by electrochemiluminescence. Antisera to HOXD4 were prepared in rabbits.
Plasmids and vectors.
Flag-CBP, Flag-CBP bromo domain, Flag-CBP HAT, and CBP CH3 were all produced by standard PCR amplification and cloned with _Bam_HI and _Hin_dIII linkers into either the parental pSP65 vector or a derivative into which a Flag epitope had been inserted. pCMV-HOXB6 (45) and pCMV-HOXB7 (49) express the respective full-length proteins. p4.2 and p4.2FS encode a full-length HOXD4 protein and a partial protein containing a stop codon within helix 3 of the HD, respectively (39). pSG5-HOXD9 and pSG5-HOXD10 (51) and pRC/CMV-HOXA9 and pRC/CMV-HOXB9 (48) express full-length proteins. pCMV-HOXB7-HD (amino acids 147 to 207), pCMV-HOXB7-Nterm (amino acids 1 to 146), pCMV-HOXB6-Nterm (amino acids 1 to 135), p4.2K-HOXD4-Nterm (amino acids 1 to 152), and pCMV-HOXB6-HD (amino acids 145 to 206) were prepared by standard PCR cloning. Point mutations in the full-length HOXB7 and the HOXB7 proteins (K-207 to R-207 or K-207 to Q-207) and the triple HOXD4 mutation (K-208, K-210, and K-211 to A-208, A-210, and A-211) were made using the Excite PCR kit and standard methods.
A full-length cDNA encoding the human HOXD10 protein (51) was cloned into pET28a. pET-HOXB3-HD consists of amino acids 176 to 281; pCMV-HOXB6-HD consists of the HD plus the preceding 8 N-terminal and the last 18 C-terminal amino acids; pET-Flag-HOXA9 consists of the HD plus 7 C-terminal amino acids; pET-HOXA10-HD+C-term represents the protein product encoded by an alternatively spliced cDNA (20) and contains the HD plus 24 N-terminal and 15 C-terminal amino acids. These partial proteins were expressed as Flag or T7 epitope-tagged fusion proteins from pET vectors, from the T3 promoter within the pCMV vector, or from the SP6 promoter within the pSP65 vector. Δ-TALE-PBX1a and R to K were prepared by PCR mutagenesis and cloned in the pSG5 (Promega) vector. All of the other HOX, PBX, and MEIS clones were cloned into either pET, Bluescript (Stratagene), or pSG5 for use in in vitro transcription as described previously (44, 46, 47).
RESULTS
HOX proteins interact with CBP and/or p300.
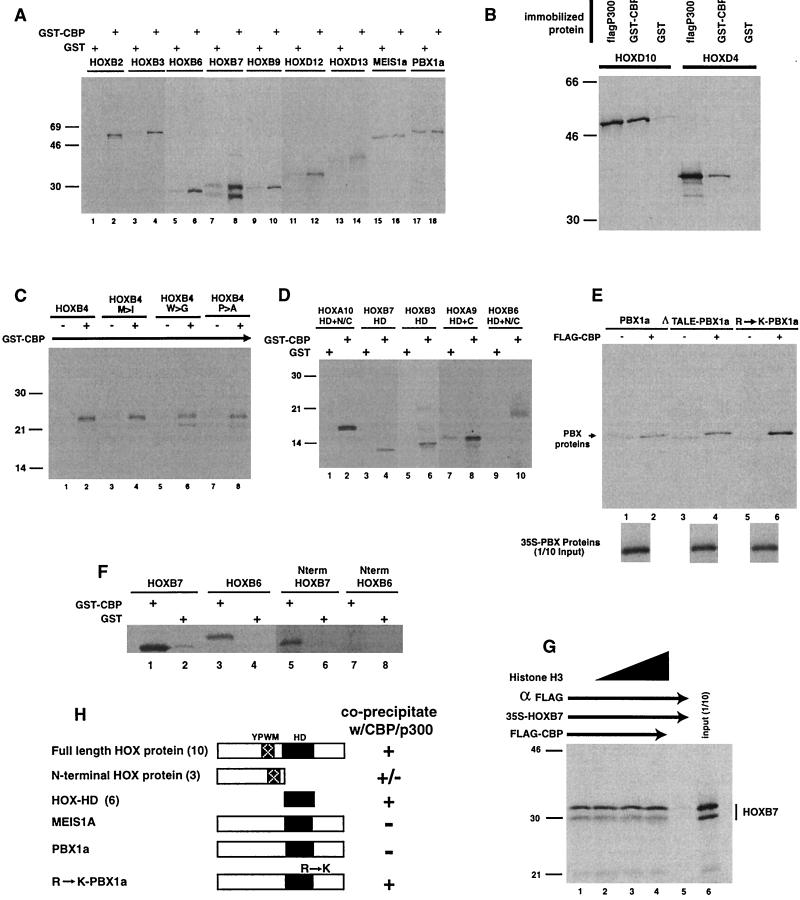
In previous studies noted above, other transcription factors have been shown to interact with CBP and/or p300. In order to test whether the HOX homeodomain proteins interacted with CBP and p300, we first studied the coprecipitation of HOXB2 and HOXB3 with a partial GST-CBP protein containing the bromo, HAT, and CH3 domains (Fig. 1). Both HOX proteins bound to immobilized GST-CBP but not to control GST beads (Fig. 1A). To determine how universal the interactions with HOX proteins were, we then examined a series of full-length HOX proteins from various paralog groups. While the 39 HOX proteins are classified into 13 paralog groups on the basis of sequence conservation within the HD, within each paralog, they exhibit moderate to substantial sequence homology outside of the HD (3). Representative HOX proteins from each paralog group tested, including HOX6, HOX7, HOX9, HOX12, and HOX13, were coprecipitated with immobilized GST-CBP when compared with the background precipitation with control GST protein beads (Fig. 1A). A full-length p300 protein, as well as GST-CBP, was also shown to bind to members of two additional paralog groups (HOX4 and HOX10) in coprecipitation assays (Fig. 1B). In contrast, two non-HOX HD proteins, MEIS and PBX, appeared to react to a much lesser extent in coprecipitation assays. In most experiments there was no difference in the binding of the PBX and MEIS proteins to GST-CBP versus control GST beads (Fig. 1A). However, in a few experiments there appeared to be weak binding of these proteins to GST-CBP (Fig. 1E).
FIG. 1.
HOX proteins coprecipitate with CBP and p300 and interact through the HD. Coprecipitation experiments were performed by incubating 35S-labeled HOX or TALE HD proteins, prepared by in vitro transcription-translation, with immobilized GST-CBP (bromo, HAT, and CH3 domains) (see Fig. 4D for structures), Flag-p300, or GST or with M2 control beads. Following extensive washing, the pellets were solubilized and run on SDS-PAGE gels followed by autoradiography. (A) Representative members of HOX paralog groups bind to CBP. In contrast, two TALE HD proteins, PBX and MEIS, exhibit reduced binding compared to background (lanes 15 to 18; also panel E, lanes 1 and 2). (B) HOX proteins also bind to the full-length p300 protein. (C) The highly conserved YPWM motif within the HOX proteins is not required for CBP binding. Changing the M, W, or P does not prevent HOXB4 interactions with CBP. (D) The HOX HD is sufficient for CBP binding. The constructs are described in Materials and Methods, but all contain the HD alone or with short N-terminal or C-terminal flanking regions. (E) The TALE motif does not prevent PBX binding to CBP, but changing an arginine to lysine within HD helix 3 increases CBP binding to PBX. Δ-TALE PBX represents a mutant PBX protein in which the three-amino-acid loop within the HD has been deleted. R→K-PBX1a represents a mutant PBX protein in which arginine-55 (underlined) within an RYKK motif (numbering according to the Antennapedia HD system) has been changed to lysine-55 to match the KXKK motif found in all of the HOX proteins. (F) HOXB7 N-terminal protein (Nterm HOXB7), but not the HOXB6 N-terminal region (Nterm HOXB6), coprecipitates with CBP. 35S-labeled HOXB7 N-terminal protein (lane 5 versus 6) coprecipitates with GST-CBP. In contrast, HOXB6 N-terminal protein does not bind GST-CBP (lane 7 versus 8). Full-length HOXB6 and HOXB7 proteins were included as positive controls for coprecipitation with GST-CBP (lane 1 to 4). (G) Histone H3 does not effectively compete with HOXB7 for binding to CBP. Approximately 10- to 100-fold excess of purified histone H3 was added to the coprecipitation mixture of Flag-CBP and 35S-labeled HOXB7. The order of addition did not affect the data shown. (H) Summary of the interactions of Hox proteins with CBP/p300. The number of different HOX proteins tested is shown in parentheses. w/, with; +, precipitation; −, no precipitation.
HOX-CBP interaction blocks HOX-DNA binding.
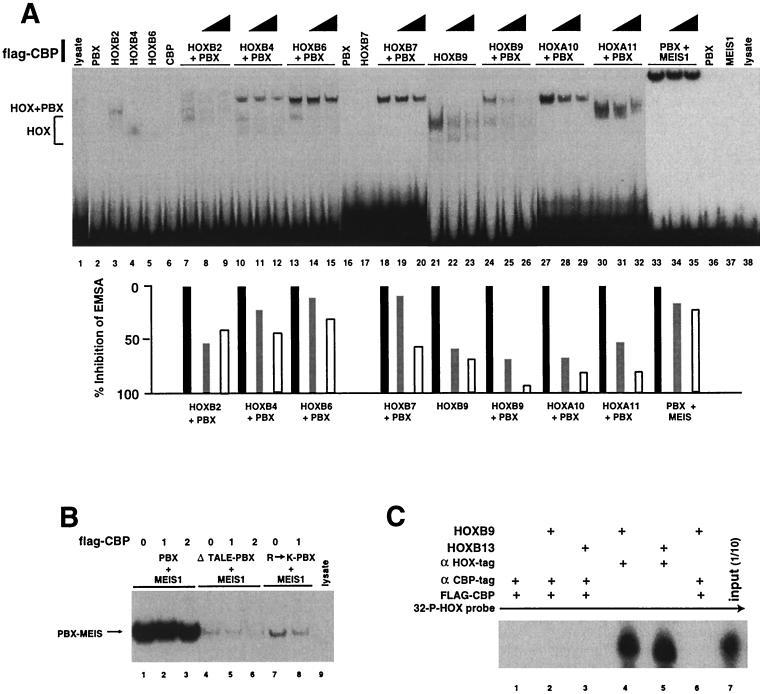
Based on previous studies showing that interactions of transcription factors with CBP could enhance reporter gene transcription (reviewed in reference 10), we anticipated that CBP would form DNA binding complexes with the HOX proteins. We therefore utilized EMSA assays in an attempt to visualize these proteins complexed to DNA targets. Previous studies had shown that HOX proteins from paralog groups 9 to 13 bind to DNA in the absence of cofactors (47), while HOX proteins from paralog groups 1 to 8 require PBX to form stable gel shift complexes (44). Since there is no evidence for direct CBP binding to a DNA consensus sequence, we used a series of oligonucleotide targets containing HOX-PBX consensus binding sites in an attempt to detect supershifted EMSA bands, representing CBP-HOX–PBX-DNA complexes. We first performed EMSA with an oligonucleotide containing a consensus PBX-HOXB9 site to visualize the possible binding of CBP to HOXB9-DNA and HOXB9-PBX-DNA complexes (Fig. 2A). However, contrary to our expectations, addition of Flag-CBP reduced the DNA binding of either HOXB9 protein alone or HOXB9-PBX complexes in a dose-dependent manner without a concomitant appearance of new EMSA bands, representing formation of triple complexes in which CBP was tethered to the DNA through the HOX proteins. The loss of HOX binding did not appear to be due to direct competitive DNA binding by CBP, which did not produce a gel shift when tested alone (Fig. 2A, lane 6). While these results did not confirm our working model in which DNA-bound HOX proteins would recruit CBP to a transcription site, they did provide indirect confirmation for the observation that the HOX proteins can interact with CBP. Because these results were at variance with our working hypothesis of how CBP would potentiate HOX transcription factor activity, we performed EMSA assays with other HOX paralog proteins. HOXB2, HOXB4, HOXB6, HOXB7, HOXA10, and HOXA11 also exhibited variably reduced DNA binding in the presence of CBP (Fig. 2A). The higher-paralog AbdB proteins tested (groups 9 through 11) seemed to show somewhat greater sensitivity to CBP blocking of DNA binding than the lower-paralog proteins from groups 1 to 8. This difference may reflect the capacity of the AbdB-like HOX proteins to bind DNA without cofactors, while HOX proteins from paralogs 1 to 8 require PBX and/or PREP1 or other cofactors for strong DNA interactions. CBP may be relatively ineffective in competing with PBX for HOX proteins. Consistent with the coimmunoprecipitation data, CBP did not show a substantial inhibitory effect on a PBX-MEIS-DNA gel shift complex (Fig. 2A and B), confirming the weak interaction of CBP with these proteins.
FIG. 2.
CBP prevents DNA binding by HOX proteins. (A and B) EMSA analysis was performed with 32P-labeled oligonucleotides containing cooperative binding sites for PBX and the respective HOX or MEIS proteins. (A) CBP blocks HOX protein DNA binding in EMSA. HOX, PBX, and MEIS proteins were preincubated with 1 or 4 μl of a Flag epitope-tagged CBP protein containing the bromo, HAT, and CH3 domains, synthesized by in vitro transcription-translation, or equivalent amounts of control lysate. The lysate controls did not shift any of the oligonucleotide probes (lanes 1 and 38 and data not shown). Some of the HOX proteins shift DNA alone (lanes 3, 4, and 21), while other HOX proteins do not (lanes 5 and 17). All of the HOX proteins form moderate or strong EMSA bands with PBX (lanes 7, 10, 13, 18, 24, 27, and 30). In each case, addition of approximately 0.5- to 2-fold molar ratio of Flag-CBP resulted in a dose-dependent decrease in the intensities of the EMSA bands, ascribed to binding by either HOX alone or the HOX-PBX complexes. However, Flag-CBP did not compete with DNA-bound PBX-MEIS1a complexes (lanes 33 to 35). The probes used contained the following consensus binding sites: TGATTGAT for lysate (lane 1), PBX1a (lane 2), CBP (lane 6), and HOXB2 through HOXB6 (lanes 3 to 5 and 7 to 15); TGATTTAC for HOXB7 through HOXA11 (lanes 16 to 32); and TGATTGACAG for PBX with MEIS (lanes 33 to 37) and lysate (lane 38). Densitometry scans of EMSA bands are shown below the lanes. Solid bars, no Flag-CBP; shaded and open bars, 1 and 4 μl of Flag-CBP, respectively. (B) The TALE motif does not alter CBP-PBX interactions, but changing R-55 to K-55 increases PBX-CBP binding. PBX and MEIS proteins form a heterodimeric EMSA complex on a consensus DNA target (compare lanes 1 and 9). Removing the three-amino-acid loop (TALE) from PBX1a weakened protein-DNA binding, as measured by EMSA (compare lanes 1 to 3 with 4 to 6), but did not enhance interactions with CBP, which would be demonstrated by reduced gel shift with increasing Flag-CBP (compare lanes 5 and 6 with 4). In contrast, changing arginine-55 to lysine-55 (underlined) to produce a KYKK motif, as described in the legend to Fig. 1, results in a PBX protein that shows reduced gel shift band intensity in the presence of Flag-CBP (compare lanes 7 and 8). This result indicates interaction of these proteins, as reflected by competitive inhibition of R→K PBX molecule binding to DNA by CBP. (C) Immunoprecipitation of CBP-HOX complexes does not coprecipitate DNA prebound to the HOX protein. 32P-labeled DNA in preformed HOXB9-DNA or HOXB13-DNA complexes could be precipitated with antiserum to the HOX epitope tag (lanes 4 and 5). Following addition of Flag-CBP, precipitation with antiserum to the Flag epitope did not yield DNA (lanes 2 and 3), confirming that CBP does not bind to HOX proteins in preformed HOX-DNA binding complexes in vitro. In lane 6, the proteins were preincubated prior to the addition of the DNA, demonstrating that the order of addition did not influence complex formation. Prolonged exposure of the autoradiograph yielded extremely faint bands in lanes 2 and 3, which represented less than 1% of the DNA bound by the HOX proteins. Lane 1, immunoprecipitation control; lane 7, 1/10 input oligonucleotide. +, present.
We hypothesized that perhaps putative CBP-HOX-DNA complexes were unstable under the experimental conditions of the EMSA analysis. We therefore tested whether it was possible to detect the presence of a CBP-HOX-DNA complex by coprecipitation of 32P-labeled DNA from preformed HOX-DNA complexes, using antisera to a Flag epitope fused to CBP. As a control, we used specific antisera to the T7 epitope tag present on the HOXB9 and HOXB13 proteins to demonstrate that they were bound to the radiolabeled DNA target (Fig. 2C). When Flag-CBP was added to the preformed HOX-DNA complex, antisera to the Flag epitope did not coprecipitate HOX-bound DNA (Fig. 2C). Incubation of the Flag-CBP protein with HOXB9 prior to addition of the DNA target did not result in coprecipitation of DNA (Fig. 2C, lane 6). This experiment confirms that the HOX and CBP proteins do not form DNA binding complexes.
The HOX homeodomain motif interacts with CBP.
Having detected interaction of 14 different HOX proteins with CBP, we made a working assumption that one or more conserved motifs might mediate HOX protein interactions with CBP and p300. To define such a region(s) of the HOX proteins, we first focused on a conserved YPWM sequence previously identified as a PBX interaction domain (7, 21, 30, 44). Since the PBX and MEIS proteins do not contain this motif, we first examined the possible importance of the YPWM motif for CBP binding by using a series of mutant HOXB4 proteins. HOXB4 derivatives containing changes in the W, P, or M, which had previously been shown to disrupt PBX binding (44), still coprecipitated with CBP, indicating that this region was not required for interactions with the CBP protein (Fig. 1C). A HOXB7 mutant protein containing changes in the YPWM motif was also used in conjunction with the type of EMSA assay shown in Fig. 2 to confirm that CBP-HOX interactions are not disrupted by alterations within this domain (data not shown).
In the previously described assays and others presented below, we show that representative members of 11 of the 13 HOX paralog groups (HOX5 and HOX8 were not tested) interact with CBP and/or p300. Sequence comparisons of the HOX proteins from the 11 paralog groups that exhibited interactions with CBP did not reveal any areas of substantial conservation outside the HD. We therefore tested whether the HD itself was sufficient for interaction with CBP in coprecipitation assays. A series of five partial HOX proteins containing only the HD with very short or no flanking regions were tested for interaction with GST-CBP (Fig. 1D). Constructs containing only the HD (HOXB7-HD, and HOXB3-HD), the HD with a short C-terminal flanking region (HOXA9-HD+C), or the HD with short N- and C-terminal arms (HOXB6-HD+N/C and HOXA10-HD+N/C) are all coprecipitated with GST-CBP but not with GST control beads. Additional experiments demonstrating that the HD interacts with CBP are presented below. Taken together, these data establish that the HD can interact directly with CBP. However, additional experiments, presented below, reveal that for at least some HOX proteins, additional flanking regions also bind to CBP.
To further explore the mechanism of HOX-CBP binding, we next focused on the observation that the non-HOX PBX and MEIS proteins exhibit much weaker interaction with CBP. One of the major differences between the HOX homeodomains and those of PBX or MEIS is the presence of a three-amino-acid loop (the TALE domain) in the last two proteins, which is the docking site for the YPWM motif with in the HOX proteins (29, 32). We therefore tested the possibility that the TALE motif was inhibiting interactions between the MEIS or PBX proteins and CBP. Removal of the TALE sequence did not enhance the weak interaction of PBX1a with CBP in the coprecipitation assay (Fig. 1E). The removal of the TALE loop greatly decreased the apparent DNA binding avidity of the PBX-MEIS protein complex (Fig. 2B). However, the resulting weak gel shift band was not altered by exogenous CBP, confirming that the presence of the TALE motif did not result in the observed weak PBX-MEIS interactions with CBP.
Although the HD motif is defined on the basis of relative sequence homology, even the relatively conserved HOX HDs vary considerably. Only helix 3 shows an extended conservation across the paralog groups shown to interact with CBP. Examination of helix 3 reveals that all the HOX proteins contain a highly conserved KXKK sequence. Lysine 55 (underlined), according to the Drosophila Antennapedia HD numbering scheme, is absolutely conserved within the HOX proteins but is altered in the helix 3 sequences of both PBX (RYKK) and MEIS (RIVQ) proteins. Furthermore, a KXKK motif was recently identified as a consensus acetylation recognition site within the GATA1 protein for the CBP HAT domain (1, 13). We therefore tested the binding of Flag-CBP to a mutant PBX protein in which the arginine corresponding to position 55 was changed to lysine (PBX-R to K). The PBX-R to K protein exhibited an increased coprecipitation with CBP (Fig. 1E) as well as increased ability to interact with CBP, as reflected by CBP-mediated reduction in the intensity of a PBX-MEIS EMSA gel shift complex (Fig. 2B). These data suggest that a lysine at position 55 of the homeodomain may be part of the binding surface of the HD that interacts with CBP. However, experiments presented below reveal that some HOX proteins also utilize additional interaction domains to bind with CBP and p300.
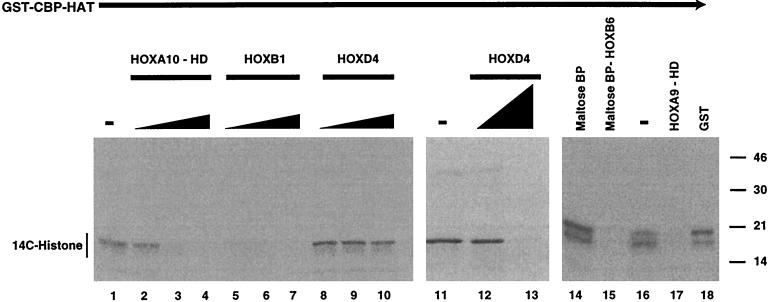
HOX proteins are not acetylated by CBP HAT.
Since the HOX proteins exhibit binding to CBP and p300, one possible mechanism by which CBP might prevent HOX protein DNA binding might be by direct acetylation of the HD. We noted that the KXKK sequence in helix 3, the conserved HD motif that functions as part of the DNA recognition surface, matches a known acetylation site within the GATA1 protein for CBP HAT (1, 13). We thus examined whether the HOX proteins were substrates for the acetylation activity associated with CBP and p300. In repeated experiments in the presence or absence of a histone H3 substrate, neither CBP HAT (Fig. 3) nor full-length Flag-p300 (not shown) was capable of acetylating bacterially expressed, affinity-purified full-length HOXB1, HOXD4, or HOXB6 protein or the HOXA9 or HOXA10 HD fragments. Thus, none of the HOX protein constructs with their respective fusion tags, which migrate between approximately 15 and 55 kDa, are labeled by CBP HAT under conditions in which histone H3 incorporates 14C-labeled acetyl groups (Fig. 3). Parallel experiments in which the histone H3 protein was omitted to ensure that it was not acting as a competitive inhibitor also showed no acetylation of the various HOX proteins (data not shown). Previous studies have shown that HOX proteins undergo conformational changes upon interactions with PBX and/or DNA (5, 42). We therefore repeated the acetylation assays of HOXB1 and HOXD4 in the presence of either a specific oligonucleotide containing a HOX binding site (TAAT) or cofactor PBX protein. However, acetylation of the HOX proteins was not detected under any of these conditions (data not shown).
FIG. 3.
The HOX proteins competitively inhibit CBP HAT activity through the homeodomain but are not substrates. Purified preparations of immobilized GST-CBP HAT domain were incubated with histone H3 substrate in the presence of 14C-labeled acetyl coenzyme A. Assays were performed in the absence (−) of HOX proteins (lanes 1, 11, and 16); with purified bacterially expressed preparations of the full-length, T7-tagged HOXB1 (0.1- to 0.3-fold protein ratio to H3) (lanes 5 to 7); or, in two separate experiments, with HOXD4 protein (0.1- to 1.0-fold protein ratio to H3) (lanes 8 to 10, 12, and 13) or a maltose-binding-HOXB6 fusion protein (0.2-fold ratio to H3) (lane 15). The band of labeled histone, representing CBP HAT activity, was diminished in a dose-dependent manner by the addition of the full-length HOXB1, HOXD4, or HOXB6 protein. Note that new bands representing acetylation of the HOX proteins, which would migrate in the region from approximately 30 to 55 kDa, were not detected, indicating that these proteins are not substrates for CBP HAT. Similar experiments were performed with GST-CBP HAT or Flag-p300 and the HOX proteins in the absence of histone to confirm that HOX proteins were not acetylated (not shown). Affinity-purified bacterially expressed HOXA10 and HOXA9 HD fragments (0.1- to 0.3-fold or 0.2-fold protein ratio to H3) also acted as competitive inhibitors of CBP HAT activity (lanes 2 to 4 and 17, respectively). Again, no bands for 14C-labeled HD fragments, which would migrate at approximately 8 to 10 kDa, were observed. Control affinity-purified maltose binding protein (BP) or GST did not inhibit CBP HAT activity at concentrations similar to those used for the HOX fusion proteins (lanes 14 and 18).
The HOX homeodomain inhibits CBP HAT activity.
Although none of the HOX proteins were substrates for CBP HAT, all three of the full-length HOX proteins, as well as both of the isolated HDs tested, were capable of inhibiting the acetyltransferase activity of the CBP HAT domain towards histone H3 substrate in a dose-dependent manner (Fig. 3). Two control proteins, GST and maltose binding protein, did not affect HAT activity at similar concentrations. Since the HOX proteins inhibited CBP histone acetylation, we asked whether a putative in vivo target, such as histone H3, was capable of acting as a competitive inhibitor to block HOX-CBP interactions. However, increasing amounts of histone H3 were not effective in preventing HOXB7-CBP binding, as measured in a coprecipitation assay (Fig. 1G). These data suggest that HOX-CBP interactions are strong compared to CBP-histone binding.
Multiple regions of the CBP protein interact with the HOX proteins.
Previous studies have demonstrated that CBP and p300 interact with numerous proteins through at least eight separate sites (reviewed in reference 10). To identify the region(s) within the CBP molecule that binds to HOX proteins, we first utilized a series of truncated CBP proteins in coprecipitation assays. The CBP CH3 domain was specifically coprecipitated by a series of Flag-HOX proteins, immobilized using antisera against the Flag tag (Fig. 4A). Similar experiments using either the Flag-CBP bromo domain or the Flag-CBP HAT domain (Fig. 4D shows structures) with a full-length T7 tag-HOXB7 protein did not yield clear evidence of specific precipitation compared to control beads (not shown). However, the Flag-CBP HAT domain exhibited specific binding to an untagged HOXD4-HD fragment in a coprecipitation assay (Fig. 4B), providing a sixth example of direct interaction of a HOX HD motif with a CBP protein containing the HAT region. The CBP CH3, bromo, and HAT domains were each capable of specific, but relatively weak, interaction with the full-length HOXB9 protein, as reflected by their capacity to interfere in an EMSA assay, with or without PBX (Fig. 4C). Taken together, these data demonstrate relatively weak interactions between isolated CBP domains and the HOX proteins, with stronger interactions being observed for the combined bromo-HAT-CH3 motifs present in the GST- and Flag-CBP proteins used in the experiments shown in Fig. 1 and 2. However, the capacity of HOX proteins, including isolated HDs, to inhibit the acetyltransferase activity of the GST-CBP HAT protein, which contains the HAT and partial bromo and CH3 domains (Fig. 3), together with the specific coprecipitation of the HOXD4-HD with the Flag-CBP HAT domain, suggest that HOX proteins bind to the HAT motif through the HD.
CBP does not potentiate HOX transcriptional activity on consensus DNA targets in transient or stable assays.
We have been unsuccessful in attempts to detect robust HOX protein transcriptional activity using numerous transient-transfection systems (Table 1). Although our current data indicated that HOX proteins do not form stable complexes with CBP while bound to DNA, we hypothesized that increased local concentrations of HOX proteins following dissociation from a consensus DNA binding site might increase the regional concentration of CBP, leading to increased gene transcription. We therefore tested the influence of exogenous full-length CBP on a series of reporters containing synthetic PBX-HOX sites as well as four biological HOX targets which had been previously reported to exhibit transcriptional activity in transient assays in response to HOX proteins (34, 51). While some of these reporters showed moderate (up to fourfold) response to HOX proteins alone, addition of CBP did not yield statistically significant changes in reporter gene activity using plasmid-based reporter genes in transient assays (Table 1). HOXB9 and HOXA9 behaved as weak repressors on PBX-HOX9 sites. HOXB9 with PBX1a yielded the largest effect (4.9-fold repression), and addition of exogenous CBP yielded mild potentiation (2-fold) of this repression rather than producing gene activation, as would be anticipated from a HOX “tether” model. Addition of the histone deacetylase inhibitor Trichostatin A did not significantly alter the transcription activity either with or without exogenous CBP protein.
TABLE 1.
CBP does not potentiate HOX protein activity in transient- or stable-transfection assays
| HOX gene | Cell line | No. of expta | Reporter | Fold changeb | |
|---|---|---|---|---|---|
| −CBPc | +CBP | ||||
| B9 | F9 | 2 | p(B9+PBX)6 | −3.2 | −2.9 |
| B9+PBX | F9 | 2 | p(B9+PBX)6 | −4.9 | −9.6 |
| A9+PBX | F9 | 1 | p(B9+PBX)6 | −1.7 | −1.5 |
| B6 | MDA-MB231 | 1 | pTCBS | 1.4 | 2.1 |
| B7 | MDA-MB231 | 5 | pTCBS | 2.9 | 3.4 |
| D4 | MDA-MB231 | 4 | pTCBS | 4.5 | 4.5 |
| D4 | MDA-MB231 | 1 | pTHCR | 2.6 | 2.0 |
| D10 | MDA-MB231 | 1 | pTHCR | 1.0 | 0.8 |
| B7 | P19 | 2 | pTCBS | 1.6c | 1.8 |
| B7 | P19-stable | 4 | pTCBS | 1.8c | 2.6 |
| D4 | P19 | 4 | pNB | 4.0c | 4.1 |
| D4 | P19-stable | 4 | pNB | 2.4c | 2.8 |
| D4 | MDA-MB231 | 1 | pSX | 1.0 | 0.33 |
| D4 | P19 | 2 | pSX | 2.8 | 2.2 |
We hypothesized that if the CBP protein modulates transcription by acetylation of histones within a highly condensed chromatin structure, it might be necessary for the target reporter sequence to be integrated into the host chromosome. We therefore made stable P19 cell lines in which the pTCBS reporter plasmid (51) or the pNB reporter plasmid (34) was integrated, respectively. These plasmids contain putative HOX binding sites and have been used previously to demonstrate modest reporter gene activity in transient assays. However, transient transfection of HOXB7 into cells containing the integrated pTCBS reporter yielded only a modest 1.8-fold activation compared to the parental plasmid, and addition of CBP together with HOXB7 produced a statistically similar 2.6-fold stimulation (Table 1). In a similar fashion, HOXD4 activated the integrated pNB reporter approximately 2.4-fold without CBP and 2.8-fold with CBP. These data suggest that CBP does not directly stimulate the transcriptional activity of HOX proteins bound to their cognate DNA recognition sites within chromatin.
HOX proteins inhibit CBP HAT activity in vivo.
In order to determine whether HOX proteins are capable of blocking CBP acetyltransferase activity in vivo, we utilized a previously described system in which a partial CBP protein consisting of the HAT, bromo, and ZZ domains was fused to the GAL4 DBD (Fig. 5A) (24). In the original studies, this fusion protein activated a CAT reporter gene containing five upstream GAL4 binding sites and a minimal pML promoter (Fig. 5A). In these studies, a small deletion within the HAT domain abrogated transcriptional activation, demonstrating a dependence on acetyltransferase activity for in vivo CBP modulation of gene transcription (24). Consistent with the previous report, we observed that the GAL4 DBD-CBP HAT fusion protein yielded a fivefold activation of reporter gene activity when cotransfected into 293T cells compared to the activity of the reporter alone (Fig. 5B). Cotransfection of constructs encoding full-length HOXB7, HOXD4, or HOXB6 protein completely abrogated the effect of GAL4 DBD-CBP HAT (Fig. 5B), suggesting that the HOX proteins block in vivo CBP HAT activity (P < 0.001). A number of controls were employed to support this conclusion. Western blot analysis was performed to demonstrate that addition of HOXB7 did not alter the GAL4 DBD-CBP HAT protein concentration (Fig. 5C). Similar data were obtained for the full-length HOXB6 and HOXD4 proteins (data not shown). To demonstrate that HOX proteins do not bind to the GAL4 DBD moiety, HOXB7 was coprecipitated with GAL4 DBD-CBP HAT in vitro but was not coprecipitated with GAL4 DBD alone (not shown). In addition, we showed that bacterially expressed HOXD4 and HOXB1 were not capable of blocking GAL4 DBD binding to DNA in an EMSA assay (not shown).
The homeodomain is required for some but not all in vivo HOX-CBP HAT interactions.
To test the role of the HD in in vivo interactions with CBP HAT, we first studied truncated N-terminal HOXB7 and HOXB6 proteins lacking the HD. The N-terminal HOXB7 protein was not stable in vivo (data not shown), so its ability to bind to CBP could not be assessed in this assay. In contrast, Western blotting demonstrated that the N-terminal HOXB6 protein was stable in the transfection system (Fig. 5C). The N-terminal HOXB6 protein could not block CBP HAT-mediated gene transcription under conditions in which the full-length protein was active (Fig. 5B). In agreement with this result, an in vitro-synthesized HOXB6 N-terminal fragment was not coprecipitated with GST-CBP under conditions in which the full-length HOXB6 protein was bound to GST-CBP (Fig. 1F). These data demonstrate that the HOXB6 HD is required for interaction with CBP HAT. In contrast, the N-terminal region of HOXD4, lacking the HD, can efficiently block in vivo CBP HAT activity (Fig. 5B). This result was confirmed using a HOXD4-FS mutant containing a stop codon at the start of helix 3 of the HD that was also capable of inhibiting in vivo CBP HAT-mediated CAT activity (not shown). These data are supported by the independent observation that the HOXD4 N-terminal region binds to CBP in a coprecipitation assay (41). To further explore HD interactions with CBP HAT, we attempted to test the HOXB7 and HOXB6 HD fragments directly in the in vivo assay system. The HOXB6-HD protein fragment, while stable when synthesized in a reticulocyte lysate system, was unstable in vivo and could not be detected in 293T cell lysates. The HOXB7-HD fragment, which was stable (Fig. 5C), exhibited reduced but detectable interaction with CBP compared to the full-length HOXB7 protein in blocking in vivo CBP HAT activity (Fig. 5B). We hypothesize that the isolated HD adopts a shape in vivo that is not sufficient for maximal interaction with CBP HAT. In this regard, previous studies have shown that the N-terminal region influences the conformation of the HOX HD (5).
We hypothesized that changing individual amino acids within the HD might allow expression of stable proteins, providing an alternative approach to testing the importance of the HD in blocking CBP HAT. Building on the apparent importance of Lys-55 of the HD for interaction with CBP, as reflected by the R-to-K PBX protein, this amino acid was changed in the full-length HOXB7 protein to either Gln (K to Q) or Arg (K to R), and the resulting proteins were tested in the in vivo reporter gene system. Western blotting showed that the mutant HOXB7 proteins were produced in concentrations equal to that of the wild-type HOXB7 protein (Fig. 5C). Both mutant proteins were substantially less effective in blocking CBP HAT-mediated reporter activation (Fig. 5B), with the K-to-Q mutant showing a significant difference (P < 0.03), while the K-to-R mutant protein just missed statistical significance (P = 0.06). These data suggest that for the HOXB7 protein, Lys-55 of the HD is required for efficient in vivo binding to CBP HAT. However, HOXB7 proteins containing either mutation at Lys-55 of the HD, produced in vitro, are still capable of coprecipitation with GST-CBP (data not shown), suggesting that other regions of the protein may also bind CBP. To confirm this possibility, the N-terminal HOXB7 protein lacking the HD was shown to coprecipitate with GST-CBP (Fig. 1F). Mutation of Lys-55, along with the two additional lysines of the KXKK HAT recognition motif in HOXD4, to alanine (3K to 3A) did not block the capacity of HOXD4 to bind to CBP HAT, as reflected by inhibition of CAT activity (Fig. 5). This result is consistent with the data presented above showing that the N-terminal region of HOXD4, lacking the HD, is sufficient for in vivo interaction with CBP HAT. In summary, the HOXB6 protein appears to be dependent on the HD for interaction with CBP, while the HOXD4 and HOXB7 HDs can bind to CBP but are not required for interaction with CBP, which can also be mediated by their respective N-terminal regions.
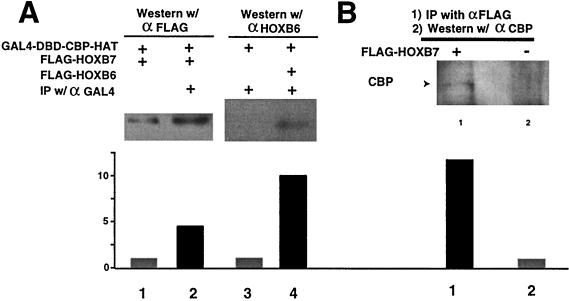
HOX proteins interact with CBP in vivo
Coimmunoprecipitation experiments were used to demonstrate in vivo interactions of transfected HOXB7 and HOXB6 proteins with the GAL4 DBD-CBP HAT protein (Fig. 6A). Specific antisera to the GAL4 fusion moiety were used to precipitate GAL4-CBP HAT protein from transfected 293T cells. Subsequent Western blotting with antisera to the Flag epitope on HOXB7 revealed a band that was greatly diminished when the anti-GAL4 serum was omitted (Fig. 6A). In a similar fashion, precipitation of the GAL4 DBD-CBP HAT protein brought down a protein, detected by the anti-Flag sera, that migrated in the position of Flag-HOXB6, while lysates in which Flag-HOXB6 were not expressed did not reveal this band (Fig. 6A). In order to detect interaction of exogenous HOX proteins with endogenous CBP, 293T cells transfected with Flag-HOXB7 were subjected to coimmunoprecipitation with anti-Flag sera. Western blotting with antisera to CBP revealed a band that was absent in cell lysates from which the Flag-HOXB7 vector was omitted (Fig. 6B). These data indicate that HOXB7 is capable of interacting with native CBP under in vivo conditions.
FIG. 6.
HOX proteins bind CBP in vivo. (A) Coimmunoprecipitation of exogenous CBP with exogenous Flag-tagged HOXB7 and HOXB6. Transiently transfected GAL4 DBD-CBP HAT was precipitated from whole 293T cell lysates using antiserum to the GAL4 fusion partner. Following resolution of the precipitated proteins by SDS-PAGE, exogenous Flag-HOXB7 or HOXB6, brought down by coprecipitation, was visualized using specific antiserum to the Flag epitope fused to HOXB7 (lane 2) or antiserum to HOXB6 (lane 4). Minimal or no protein bands were visualized in control lysates lacking the antiserum for GAL4 (lane 1) or HOXB6 (lane 3) protein. (B) Coimmunoprecipitation of transiently transfected HOXB7 and endogenous CBP. Following precipitation of Flag-HOXB7 protein from whole 293T cell lysates with antiserum to the Flag epitope, endogenous CBP protein was detected by Western blotting of precipitated proteins with CBP-specific antiserum (lane 1), while cell lysates containing a control expression vector showed no band for CBP protein (lane 2). Densitometry scans of electrochemiluminescence band intensities are shown below the lanes. +, present; −, absent. y axis shows relative intensity.
DISCUSSION
There is now a sizeable literature demonstrating a role for HOX proteins in the regulation of many fundamental developmental and differentiation pathways. Nevertheless, despite extensive efforts, a clear mechanism of action for HOX proteins remains elusive. In our own laboratory, we have been unable to demonstrate robust activation or repression of transcription by numerous full-length HOX proteins on a broad range of targets in standard transient-transfection assays in an array of cell lines (summarized in Table 1). These targets include natural biological promoter-enhancers containing putative HOX binding sites, as well as synthetic PBX-HOX or MEIS-HOX consensus binding sites. Other investigators have made similar observations (C. Hauser, personal communication) or confirmed that the types of regulatory events documented for HOX proteins in transgenic mice have not been duplicated in transient assays using reporter gene systems (R. Krumlauf, personal communication). These largely negative studies suggest that either (i) HOX proteins do not function as conventional transcriptional activators or repressors, (ii) reporter constructs or cellular milieu have not been optimal for detection of biologically relevant HOX protein transcriptional activity, or (iii) other cofactors are required to enable the regulatory function of HOX proteins in transcription.
Our present studies began with the anticipation that addition of CBP-p300, which had been shown to act as a cofactor for other transcription systems (2), would potentiate HOX protein transcriptional activity. Indeed, we find that all 14 HOX proteins studied, representing 11 of the 13 paralog groups, interact with CBP or p300. Since paralog members exhibit relatively conserved amino acid sequences, these data make it likely that all 39 HOX proteins bind CBP. However, our data demonstrate that while CBP does recognize HOX proteins in preformed DNA complexes, their interaction paradoxically disrupts HOX protein-DNA binding. This observation was confirmed by our demonstration that addition of CBP to a range of HOX protein and target reporter gene systems did not potentiate transcriptional activity. While this work was in progress, Chariot et al. reported that CBP dramatically enhanced the transcriptional activity of HOXB7 towards the pTCBS reporter gene in transient assays in MDA-MB231 cells (8). We therefore added this system to the others that we report in Table 1. However, we were unable to detect any effect of CBP with this reporter using the same reagents in MDA-MB231 or P19 cells. We currently have no explanation for these differences. If the low levels of reporter activation described in Table 1 are indeed due to direct DNA binding by the HOX proteins, our EMSA data (Fig. 2) would suggest that addition of CBP to these systems would be inhibitory. The fact that no such inhibitory effects were observed may be due to the well-documented overall positive influence of CBP on gene transcription by mechanisms that remain opaque. It seems possible that additional positive actions of CBP might mask repressive effects of CBP blocking the low levels of HOX-mediated gene activation observed in these complex systems.
Chariot et al. also reported that interaction of the HOXB7 protein with CBP was localized to the N-terminal region of the HOXB7 protein, and they were not able to detect interaction between CBP and the HOXB7 C-terminal region that contains the HD (8). In addition, Saleh et al. recently demonstrated CBP binding to the HOXD4-N-terminal region (41). We present data confirming that the N-terminal regions of HOXB7 and HOXD4 do interact with CBP. Thus, it appears that for HOXD4 and HOXB7, portions of the protein other than the HD are sufficient for interactions with CBP. However, we also show that both the HOXB7 and the HOXD4 HDs bind to CBP in two different assays. Taken as a whole, our data suggest that many, if not all, HOX proteins interact with CBP through the homeodomain. These data include (i) coprecipitation of each of the six isolated HD polypeptides tested with CBP HAT protein fragments (summarized in Fig. 1H), (ii) the inhibition of in vitro CBP HAT activity toward a histone substrate by the isolated HOXA9 and HOXA10 HDs, (iii) partial loss of in vivo inhibitory function of HOXB7 toward CBP HAT activity by specific point mutations within the HD, (iv) loss of in vivo inhibitory activity of HOXB6 when the HD is deleted, and (v) inhibition of in vivo CBP HAT activity by the HOXB7 HD.
A number of transcription factors have been reported to exhibit enhanced DNA binding following acetylation by CBP HAT (1, 11, 13, 53). Following our observation that the binding of HOX proteins to DNA was blocked by CBP, we asked whether this inhibition was mediated by acetylation of the HOX proteins by CBP HAT activity. However, we have been unable to demonstrate acetylation of five bacterially expressed full-length or partial HD-containing HOX proteins under conditions in which histone substrates are readily modified by GST-CBP HAT. Interestingly, helix 3 of the HOX homeodomain contains a conserved KXKK motif that has been identified as an acetylation site within the GATA1 zinc finger (13). HOX proteins are thought to undergo conformational changes upon interacting with PBX and/or binding to DNA (5, 42). We considered the possibility that the lack of observable acetylation of HOX proteins by CBP might be due to an inappropriate conformation when studied alone. However, prebinding of the HOX proteins to the PBX cofactor protein and/or an oligonucleotide containing a consensus binding site did not facilitate acetylation by CBP HAT. The recent reports that Trichostatin A, a deacetylase inhibitor, stimulates HOXB7- and HOXD4-mediated reporter gene transcription (8, 41) suggest that acetylation events, including modification of the HOX proteins, may play a role in their regulation and activity. We have no explanation for the fact that we were unable to observe Trichostatin A effects on reporter gene activity other than to note that relatively low levels of this compound (greater than 300 nM) were severely toxic to a range of cell types. Taken together with the finding that HOX proteins inhibit CBP HAT activity towards histone substrates, these data suggest that the HOX homeodomain is bound by the CBP HAT domain but the HOX proteins are not biological substrates for the CBP and p300 acetylases.
Most studies have suggested that CBP interactions with transcription factors lead to enhanced gene transcription. The mechanism(s) of this potentiation effect remains unknown but is thought to involve acetylation of histones and/or other components of the transcriptional machinery. There are several previous examples of proteins that function by blocking CBP and p300 HAT activity (4, 12). These include the bHLH transcription factor, Twist, which binds to and inhibits the HAT domain of p300 (12). In addition, the E1A oncoprotein has been reported to inhibit the acetyltransferase activity of p300 and CBP (4). Furthermore, while this work was in review, the MSX3 HD protein was reported to specifically inhibit the HAT activity of CBP and p300 (25). This study did not define the region of MSX3 that binds to CBP and p300, but we note that the MSX3 protein contains a Lys-55 within the context of a KXKK motif within the HD.
A review of the voluminous HOX literature suggests that HOX proteins function as both transcriptional activators and repressors. Pinsonneault et al. suggested that EXD and PBX function to switch the HOX proteins from repressors to activators (31). This model would be consistent with our hypothesis that HOX proteins function by at least two alternative mechanisms. In the widely accepted model, the PBX and EXD proteins function to facilitate DNA binding by the HOX proteins, which might then participate in activation (and/or repression) of gene targets. We now propose an alternative model in which HOX proteins function to repress gene transcription by blocking the activity of CBP and p300 (Fig. 5D). One model would be that HOX proteins block CBP HAT activity in a non-DNA-dependent mechanism. Alternatively, local HOX protein concentrations within chromatin regions might be increased by equilibrium HOX-DNA interactions. HOX proteins released from DNA complexes might subsequently inhibit local concentrations of CBP and p300, resulting in repression of local gene transcription. We note that there are several other examples of HOX proteins apparently functioning in a non-DNA-dependent fashion to repress gene transcription by binding to TF-IID (18) and the Maf oncoprotein (15).
ACKNOWLEDGMENTS
This work was supported by the Medical Research Service of the Veterans Administration and by NIH grants 1R01CA80029-1(C.L.) and NIH 1R01GM55814001A2 (C.L.). Antisera to HOXD4 were prepared in rabbits under a collaborative NIH-Small Business Award to BAbCo, Richmond, Calif. (NIH N44-DK-4-2219).
We thank T. Kouzarides, D. Chakravarti, R. M. Evans, Y. Nakatani, and G. A. Blobel for CBP derivatives, V. Bours for a pcDNA3-HOXB7 expression plasmid, M. Featherstone for the HOXD4 and HOXD4-FS expression plasmids and the pNB and pSX reporter plasmids, and V. Zappavigna for the pTCBS and pTHCR reporter plasmids. We thank S. Dorsam for reading the manuscript.
REFERENCES
- 1.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 2.Brownell J E, Allis C D. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184. doi: 10.1016/s0959-437x(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 3.Burglin T. A comprehensive classification of homeobox genes. In: Duboule D, editor. Guidebook to the homeobox genes. Oxford, England: Oxford University Press; 1994. pp. 25–71. [Google Scholar]
- 4.Chakravarti D, Ogryzko V, Kao H Y, Nash A, Chen H, Nakatani Y, Evans R M. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell. 1999;96:393–403. doi: 10.1016/s0092-8674(00)80552-8. [DOI] [PubMed] [Google Scholar]
- 5.Chan S K, Popperl H, Krumlauf R, Mann R S. An extradenticle-induced conformational change in a HOX protein overcomes an inhibitory function of the conserved hexapeptide motif. EMBO J. 1996;15:2476–2487. [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C-P, Brocchieri L, Shen W-F, Largman C, Cleary M L. Pbx modulation of Hox homeodomain N-terminal arms establishes a gradient of DNA-binding specificities across the Hox locus. Mol Cell Biol. 1996;16:1734–1745. doi: 10.1128/mcb.16.4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C-P, Shen W-F, Rozenfeld S, Lawrence H J, Largman C, Cleary M L. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 8.Chariot A, van Lint C, Chapelier M, Gielen J, Merville M P, Bours V. CBP and histone deacetylase inhibition enhance the transactivation potential of the HOXB7 homeodomain-containing protein. Oncogene. 1999;18:4007–4014. doi: 10.1038/sj.onc.1202776. [DOI] [PubMed] [Google Scholar]
- 9.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 10.Goodman R H, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 11.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 12.Hamamori Y, Sartorelli V, Ogryzko V, Puri P L, Wu H Y, Wang J Y, Nakatani Y, Kedes L. Regulation of histone acetyltransferases p300 and PCAF by the bHLH protein twist and adenoviral oncoprotein E1A. Cell. 1999;96:405–413. doi: 10.1016/s0092-8674(00)80553-x. [DOI] [PubMed] [Google Scholar]
- 13.Hung H L, Lau J, Kim A Y, Weiss M J, Blobel G A. CREB-binding protein acetylates hematopoietic transcription factor GATA-1 at functionally important sites. Mol Cell Biol. 1999;19:3496–3505. doi: 10.1128/mcb.19.5.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Ikehara T, Nakagawa T, Kraus W L, Muramatsu M. p300-mediated acetylation facilitates the transfer of histone H2A-H2B dimers from nucleosomes to a histone chaperone. Genes Dev. 2000;14:1899–1907. [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka K, Yoshitomo-Nakagawa K, Shioda S, Nishizawa M. A set of Hox proteins interact with the Maf oncoprotein to inhibit its DNA binding, transactivation, and transforming activities. J Biol Chem. 2001;276:819–826. doi: 10.1074/jbc.M007643200. [DOI] [PubMed] [Google Scholar]
- 16.Kömüves L G, Shen W-F, Kwong A, Stelnicki E, Rozenfeld S, Oda Y, Blink A, Krishnan K, Lau B, Mauro T, Largman C. Changes in HOXB6 homeodomain protein structure and localization during human epidermal development and differentiation. Dev Dynamics. 2001;218:636–647. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1014>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Levine M, Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988;55:537–540. doi: 10.1016/0092-8674(88)90209-7. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Manley J L. Even-skipped represses transcription by binding TATA binding protein and blocking the TFIID-TATA box interaction. Mol Cell Biol. 1998;18:3771–3781. doi: 10.1128/mcb.18.7.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Veraksa A, McGinnis W. A sequence motif distinct from Hox binding sites controls the specificity of a Hox response element. Development. 1999;126:5581–5589. doi: 10.1242/dev.126.24.5581. [DOI] [PubMed] [Google Scholar]
- 20.Lowney P, Corral J, Detmer K, LeBeau M M, Deaven L, Lawrence H J, Largman C. A human Hox 1 homeobox gene exhibits myeloid-specific expression of alternative transcripts in human hematopoietic cells. Nucleic Acids Res. 1991;19:3443–3449. doi: 10.1093/nar/19.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Q, Kamps M P. Structural determinants within Pbx1 that mediate cooperative DNA binding with pentapeptide-containing Hox proteins: proposal for a model of a Pbx1-Hox-DNA complex. Mol Cell Biol. 1996;16:1632–1640. doi: 10.1128/mcb.16.4.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maconochie M K, Nonchev S, Studer M, Chan S K, Popperl H, Sham M H, Mann R S, Krumlauf R. Cross-regulation in the mouse HoxB complex: the expression of Hoxb2 in rhombomere 4 is regulated by Hoxb1. Genes Dev. 1997;11:1885–1895. doi: 10.1101/gad.11.14.1885. [DOI] [PubMed] [Google Scholar]
- 23.Mann R S, Chan S K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Balbas M A, Bannister A J, Martin K, Haus-Seuffert P, Meisterernst M, Kouzarides T. The acetyltransferase activity of CBP stimulates transcription. EMBO J. 1998;17:2886–2893. doi: 10.1093/emboj/17.10.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra-Chaudhary R, Matsui H, Raghow R. Msx3 protein recruits histone deacetylase to down-regulate the Msx1 promoter. Biochem J. 2001;353:13–22. [PMC free article] [PubMed] [Google Scholar]
- 26.Merika M, Williams A J, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 27.Muller M, Affolter M, Leupin W, Otting G, Wuthrich K, Gehring W J. Isolation and sequence-specific DNA binding of the Antennapedia homeodomain. EMBO J. 1988;7:4299–4304. doi: 10.1002/j.1460-2075.1988.tb03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 29.Passner J M, Ryoo H D, Shen L, Mann R S, Aggarwal A K. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 30.Phelan M L, Rambaldi I, Featherstone M S. Cooperative interactions between HOX and PBX proteins mediated by a conserved peptide motif. Mol Cell Biol. 1995;15:3989–3997. doi: 10.1128/mcb.15.8.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinsonneault J, Florence B, Vaessin H, McGinnis W. A model for extradenticle function as a switch that changes HOX proteins from repressors to activators. EMBO J. 1997;16:2032–2042. doi: 10.1093/emboj/16.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper D E, Batchelor A H, Chang C-P, Cleary M L, Wolberger C. Structure of a HOXB1-PBX1 heterodimer bound to DNA: role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell. 1999;96:587–597. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 33.Popperl H, Bienz M, Studer M, Chan S-K, Aparicio S, Brenner S, Mann R S, Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- 34.Popperl H, Featherstone M S. An autoregulatory element of the murine HOX-4.2 gene. EMBO J. 1992;11:3673–3680. doi: 10.1002/j.1460-2075.1992.tb05452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popperl H, Rikhof H, Chang H, Haffter P, Kimmel C B, Moens C B. Iazarus is a novel pbx gene that globally mediates hox gene function in zebrafish. Mol Cell. 2000;6:255–267. doi: 10.1016/s1097-2765(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 36.Puri P L, Avantaggiati M L, Balsano C, Sang N, Graessmann A, Giordano A, Levrero M. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 1997;16:369–383. doi: 10.1093/emboj/16.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raman V, Martensen S A, Reisman D, Evron E, Odenwald W F, Jaffee E, Marks J, Sukumar S. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405:974–978. doi: 10.1038/35016125. [DOI] [PubMed] [Google Scholar]
- 38.Raman V, Tamori A, Vali M, Zeller K, Korz D, Sukumar S. HOXA5 regulates expression of the progesterone receptor. J Biol Chem. 2000;275:26551–26555. doi: 10.1074/jbc.C000324200. [DOI] [PubMed] [Google Scholar]
- 39.Rambaldi I, Kovacs E N, Featherstone M S. A proline-rich transcriptional activation domain in murine HOXD-4 (HOX-4.2) Nucleic Acids Res. 1994;22:376–382. doi: 10.1093/nar/22.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryoo H D, Mann R S. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleh M, Rambaldi I, Yang X J, Featherstone M S. Cell signaling switches HOX-PBX complexes from repressors to activators of transcription mediated by histone deacetylases and histone acetyltransferases. Mol Cell Biol. 2000;20:8623–8633. doi: 10.1128/mcb.20.22.8623-8633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez M, Jennings P A, Murre C. Conformational changes induced in Hoxb-8/Pbx-1 heterodimers in solution and upon interaction with specific DNA. Mol Cell Biol. 1997;17:5369–5376. doi: 10.1128/mcb.17.9.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz M L, Baeuerle P A. A vector, pHisGal, allowing bacterial production of proteins fused to a hexahistidine-tagged Gal4 DNA-binding domain. BioTechniques. 1994;17:714–716. , 718. [PubMed] [Google Scholar]
- 44.Shen W-F, Chang C-P, Rozenfeld S, Sauvageau G, Humphries R K, Lu M, Lawrence H J, Cleary M L, Largman C. HOX homeodomain proteins exhibit selective complex stabilities with Pbx and DNA. Nucleic Acids Res. 1996;24:898–906. doi: 10.1093/nar/24.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen W-F, Largman C, Lowney P, Corral J, Detmer K, Hauser C A, Simonitch T A, Hack F M, Lawrence H J. Lineage-restricted expression of homeobox-containing genes in human hematopoietic cell lines. Proc Natl Acad Sci USA. 1989;86:8536–8540. doi: 10.1073/pnas.86.21.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen W-F, Montgomery J C, Rozenfeld S, Lawrence H J, Buchberg A, Largman C. The Abd-B-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol. 1997;17:6448–6558. doi: 10.1128/mcb.17.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen W-F, Rozenfeld S, Lawrence H J, Largman C. The Abd-B-like Hox homeodomain proteins can be subdivided by the ability to form complexes with Pbx1a on a novel DNA target. J Biol Chem. 1997;272:8198–8206. doi: 10.1074/jbc.272.13.8198. [DOI] [PubMed] [Google Scholar]
- 48.Shen W F, Rozenfeld S, Kwong A, Komuves L, Lawrence H J, Largman C. HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol Cell Biol. 1999;19:3051–3061. doi: 10.1128/mcb.19.4.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simeone A, Mavilio F, Acampora D, Giampaolo A, Faiella A, Zappavigna V, D'Esposito M, Pannese M, Russo G, Boncinelli E, Peschle C. Two human homeobox genes, C1 and C8: structure analysis and expression in embryonic development. Proc Natl Acad Sci USA. 1987;84:4914–4918. doi: 10.1073/pnas.84.14.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiting J, Marshall H, Cook M, Krumlauf R, Rigby P W J, Stott D, Alleman R K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox 2.6 gene expression. Genes Dev. 1991;5:2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- 51.Zappavigna V, Renucci A, Izpisua-Belmonte J C, Urier G, Peschle C, Duboule D. HOX4 genes encode transcription factors with potential auto- and cross-regulatory capacities. EMBO J. 1991;10:4177–4187. doi: 10.1002/j.1460-2075.1991.tb04996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zappavigna V, Sartori D, Mavilio F. Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev. 1994;8:732–744. doi: 10.1101/gad.8.6.732. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Bieker J J. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc Natl Acad Sci USA. 1998;95:9855–9860. doi: 10.1073/pnas.95.17.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong H, Voll R E, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]