Perforin-independent β-cell destruction by diabetogenic CD8+ T lymphocytes in transgenic nonobese diabetic mice (original) (raw)
Abstract
Autoimmune diabetes in nonobese diabetic (NOD) mice results from destruction of pancreatic β cells by T lymphocytes. It is believed that CD8+ cytotoxic T lymphocytes (CTLs) effect the initial β-cell insult in diabetes, but the mechanisms remain unclear. Studies of NOD.lpr mice have suggested that disease initiation is a Fas-dependent process, yet perforin-deficient NOD mice rarely develop diabetes despite expressing Fas. Here, we have investigated the role of perforin and Fas in the ability of β cell–reactive CD8+ T cells bearing a T-cell receptor (8.3-TCR) that is representative of TCRs used by CD8+ CTLs propagated from the earliest insulitic lesions of NOD mice, and that targets an immunodominant peptide/H-2Kd complex on β cells, to effect β-cell damage in vitro and in vivo. In vitro, 8.3-CTLs killed antigenic peptide–pulsed non–β-cell targets via both perforin and Fas, but they killed NOD β cells via Fas exclusively. Perforin-deficient 8.3-TCR–transgenic NOD mice expressing an oligoclonal or monoclonal T-cell repertoire developed diabetes even more frequently than their perforin-competent littermates. These results demonstrate that diabetogenic CD8+ CTLs representative of CTLs putatively involved in the initiation of autoimmune diabetes kill β cells in a Fas-dependent and perforin-independent manner.
Introduction
Development of spontaneous autoimmune diabetes in nonobese diabetic (NOD) mice is the result of a complex CD4+ and CD8+ T cell–dependent process directed against pancreatic β cells (1, 2). Although the role of T cells as effectors of β-cell destruction in autoimmune diabetes is well established, the mechanisms that initiate diabetogenesis in susceptible mice remain poorly understood.
Adoptive T-cell transfer studies using spleen cells from prediabetic NOD mice have demonstrated that transfer of diabetes into immunodeficient NOD mice requires both CD4+ and CD8+ T cells (3–6). Because splenic CD4+ T cells from diabetic NOD mice and preactivated β cell–specific CD4+ T-cell clones can home into pancreatic islets and kill β cells in the absence of CD8+ T cells (6–13), it has been proposed that naive autoreactive CD4+ T cells may differentiate into effector cells by engaging autoantigens shed from the β cells by a CD8+ T cell–mediated insult, in the context of MHC class II molecules on local antigen-presenting cells (APCs) (14, 15). This view is supported by several lines of evidence. First, β2 microglobulin (MHC class I)–deficient and anti-CD8 mAb–treated NOD mice, which do not bear mature CD8+ T cells, develop neither diabetes nor islet inflammation (16–19). Second, restoring expression of MHC class I molecules on the β cells of β2 microglobulin–deficient NOD mice restores insulitis susceptibility (20). Finally, splenocytes from prediabetic NOD mice cannot efficiently transfer insulitis into β2 microglobulin–deficient NOD.scid mice (21).
The mechanism through which diabetogenic CD8+ T cells effect this initial β-cell insult in autoimmune diabetes, however, is unknown. CD8+ CTLs usually kill target cells through one of two alternative lytic pathways (22–27). In the perforin pathway, cell death is caused by direct effects of perforin and granzymes on the target cell. In the Fas pathway, a T-cell membrane ligand (FasL), which is upregulated when the T-cell receptor (TCR) is engaged, binds a target cell surface receptor (Fas) that induces apoptosis when ligated (28). Previous studies in a virus-induced model of autoimmune diabetes indicated that CD8+ T cells specific for a transgenic viral neoantigen required perforin to effect β-cell damage and to initiate diabetogenesis in transgenic C57BL/6 mice (29). On the other hand, adoptive T-cell transfer studies of a highly diabetogenic, perforin-positive CD8+ T-cell clone into NOD.lpr/lpr mice suggested that CD8+ T cell–induced destruction of β cells in NOD mice was mediated via Fas exclusively (30). Studies of perforin- or Fas-deficient NOD mice also yielded apparently contradictory results. Perforin-deficient NOD mice developed severe insulitis but rarely became diabetic (31), and NOD.lpr/lpr mice developed neither diabetes nor insulitis despite expressing perforin (32). It is conceivable that the paradoxical outcomes of these studies were attributable to differences in the genetic backgrounds of the mice under study (NOD vs. C57BL/6), to genetic heterogeneity with respect to diabetes-susceptibility loci (i.e., NOD mice congenic for the perforin or lpr mutations might have inherited diabetes-resistance genes from the mice donating the mutations), to differences in the nature of the target autoantigens (transgenic vs. endogenous), and/or to pleiotropic effects of the perforin and Fas deficiencies on CD4+ and/or CD8+ T-cell development and function. These findings could be reconciled, however, if the CD8+ clonotypes that purportedly effect the initial β-cell insult that initiates diabetogenesis in NOD mice require Fas do so and if those clonotypes that are recruited to the pancreas later on in the disease process require perforin to effect clinically significant β-cell destruction.
Here, we have tested this hypothesis by investigating the role of perforin and Fas in the ability of CD8+ T cells bearing a representative highly diabetogenic, β cell–reactive TCR (8.3-TCR) (33) to effect β-cell damage in vitro and in vivo. This TCR specificity uses a TCRα rearrangement that is frequently used by CD8+ T cells propagated from the earliest insulitic lesions of young NOD mice (Vα17 and Jα42 elements, often joined by the N-region sequence M-R-D/E; ref. 34), and targets an antigenic peptide/H-2Kd complex that is also recognized by ∼50% of the CD8+ clonotypes that can be isolated from the islets of acutely diabetic NOD mice (Anderson, B., et al., manuscript submitted for publication). To determine whether CD8+ T cells bearing this TCR require perforin to effect β-cell damage, we compared the functional activity and diabetogenic potential of 8.3-CD8+ T cells from perforin-deficient (8.3-NOD.PO–/–) and perforin-competent (8.3-NOD.PO+/–) 8.3-TCR–transgenic NOD mice. We found that (a) in vitro, 8.3-CTLs killed antigenic peptide–pulsed non–β-cell targets via both perforin and Fas but killed NOD β cells via Fas exclusively; (b) unlike NOD.PO–/– mice, which were diabetes resistant, 8.3-NOD.PO–/– mice and recombination activating gene-2–deficient (RAG-2–/–) 8.3-NOD.PO–/– mice developed diabetes even more frequently than 8.3-NOD.PO+/– or RAG-2–/– 8.3-NOD.PO+/+ mice, respectively; and (c) 8.3-CTLs from 8.3-NOD.PO–/– mice were as diabetogenic as 8.3-CTLs from 8.3-NOD.PO+/+ mice when adoptively transferred into immunodeficient NOD mice. These results therefore demonstrate that diabetogenic, TCR-transgenic CD8+ CTLs representative of CTLs involved in the initiation of autoimmune diabetes in NOD mice kill β cells in a Fas-dependent and perforin-independent manner. On the basis of these results, we propose that initiation of autoimmune diabetes by CD8+ CTLs is a Fas-dependent and perforin-independent process.
Methods
Mice.
8.3-NOD mice and RAG-2–/– 8.3-NOD mice, expressing the TCRαβ rearrangements of the H-2Kd–restricted, β cell–reactive CD8+ T-cell clone NY8.3, have been described (33). Perforin knockout (PO–/–) C57BL/6 (B6) mice (27) were provided by W. Clark (University of California–Los Angeles, Los Angeles, California, USA). Rat insulin promoter B7.1-transgenic NOD.scid mice (RIP-B7.1-NOD.scid) were provided by D. Serreze (The Jackson Laboratory, Bar Harbor, Maine, USA), with permission from R. Flavell (Yale University, New Haven, Connecticut, USA) (35). NOD/Lt, B6, and B6.MRL-Faslpr mice were produced from stocks purchased from The Jackson Laboratory. The perforin mutation of B6.PO–/– mice was backcrossed onto the NOD background for 10 generations. NOD.PO+/– mice of the 10th backcross were intercrossed to generate NOD.PO–/– mice. NOD.PO–/– mice were outcrossed with 8.3-NOD mice and RAG-2–/– 8.3-NOD mice to generate 8.3-NOD.PO+/– and RAG-2+/– 8.3-NOD.PO+/– mice. 8.3-NOD.PO+/– and RAG-2+/– 8.3-NOD.PO+/– mice were then backcrossed with NOD.PO–/– mice, or were intercrossed, to generate 8.3-NOD.PO–/– and RAG-2–/– 8.3-NOD.PO–/– mice, respectively. NOD.lpr/lpr mice were generated by backcrossing the Faslpr gene of B6.MRL-Faslpr mice onto the NOD background for eight generations and by intercrossing NOD.lpr+/– mice. Mice were screened for inheritance of wild-type and mutated perforin and Fas genes by PCR. Mice were housed under specific pathogen–free conditions.
Diabetes.
Diabetes was monitored by measuring urine glucose levels with Diastix (Miles Canada Inc., Ontario, Canada) twice a week. Animals were considered diabetic after two consecutive readings ≥3+. Blood glucose measurements in ≥3+ glycosuric mice are consistently >15 mmol/l. Absence of diabetes in healthy mice sacrificed at the end of the study was confirmed by measuring blood glucose levels using Glucostix (Miles Canada Inc.) and a glucometer (<10 mmol/l).
Cell lines, antibodies, and flow cytometry.
L2120-Fas+ and L2120-Fas– cells were provided by P. Goldstein (Centre National de la Recherche Scientifique, Marseille, France). H-2Kd–transfected RMA-S cells (RMA-SKd) were provided by B. Wipke and M. Bevan (University of Washington, Seattle, Washington, USA). The hybridoma secreting the GK1.5 mAb (anti-CD4) was obtained from the American Type Culture Collection (Rockville, Maryland, USA). Anti–Lyt-2 (CD8α)/phycoerythrin (PE) (53-6.7), anti-L3T4/FITC (IM7), anti-L3T4/biotin (CD4) (H129.19), anti-CD2/biotin (RM2-5), anti-CD5 (53-7.3)/biotin, anti-CD11a/biotin (M17/4), anti-CD24/biotin (M1/69), anti-CD28/biotin (37.51), anti-CD44/FITC (IM7), anti-CD45RB/FITC (23G2), anti–L-selectin/biotin (CD62L) (MEL-14), anti-CD69/biotin (H1.2F3), anti-Vβ8.1/8.2-FITC (MR5-2), and anti-H-2Kd/FITC (SF1-1.1) were all from PharMingen (San Diego, California, USA). Anti–IL-2R/FITC (CD25) (AMT13) was purchased from Cedarlane Labs Ltd. (Hornby, Ontario, Canada). Mouse IgG–absorbed FITC- or biotin-conjugated goat anti-rat IgG and FITC-conjugated goat anti-mouse IgG were obtained from Caltag Laboratories Inc. (San Francisco, California, USA) and Becton Dickinson Immunocytometry Systems (San Jose, California, USA), respectively. Streptavidin-PerCP was obtained from Becton Dickinson. Thymi, spleens, and islet-derived T-cell lines were analyzed by three-color flow cytometry using a FACScan (Becton Dickinson) (33).
Peptides.
The synthetic peptides NRP (NOD-relevant peptide; Anderson, B., et al., manuscript submitted for publication) and the tumor-derived, negative-control peptide tum (36) were prepared using 9-fluorenyl methyloxy carbonyl (FMOC) chemistry and purified through reverse-phase HPLC to >90% purity. NRP, a peptide of unknown antigen source that was defined using combinatorial peptide libraries, is a powerful agonist of the 8.3-TCR and is also recognized by ∼50% of the CD8+ CTLs that can be propagated from the pancreatic islets of acutely diabetic nontransgenic NOD mice. Peptides were resuspended at 1 mg/ml in 0.1% acetic acid (Fisher Scientific Co., Fair Lawn, New Jersey, USA). Additional dilutions were done with RPMI-1640 containing 0.25% BSA.
Generation of spleen- and islet-derived CD8+ T-cell lines and clones.
Spleen cells from 8.3-NOD.PO+/– or 8.3-NOD.PO–/– mice were depleted of CD4+ T cells using GK1.5 mAb–coated magnetic beads (37), adjusted to 2 × 104 CD8+ T cells per 100 μl of complete medium (RPMI-1640 containing 10% heat-inactivated FBS [GIBCO BRL, Grand Island, New York, USA], 50 U/ml penicillin, 50 μg/ml streptomycin [Flow Laboratories, McLean, Virginia, USA], and 50 μM 2-ME [Sigma Chemical Co., St. Louis, Missouri, USA]), stimulated with NRP-pulsed irradiated NOD splenocytes (1 μg/ml, 105 cells per well) for three to four days, and expanded in complete medium containing 0.5 U/ml of recombinant IL-2 (rIL-2; Takeda Co., Osaka, Japan) for seven to 14 days. The growing cells (8.3-CD8+ CTLs >95% CD8+ pure) were used as effectors in cytotoxicity assays. Islet-derived CD8+ T-cell lines were generated from diabetic mice within one day of diabetes onset, as described previously (37). Briefly, purified islets were cultured in media containing rIL-2. Cells migrating out of islets within the first six days of culture were depleted of CD4+ T cells and cloned by limiting dilution. Growing clones were assayed for serine esterase content, and serine esterase+ clones were expanded by stimulation with irradiated NOD islets and rIL-2.
51Cr release assays.
RMA-SKd cells (preincubated at 26°C overnight) and L1210-Fas+ and L1210-Fas– cells were labeled with [51Cr]sodium chromate (Du Pont NEN Research Products, Boston, Massachusetts, USA) for two hours (at 26°C for RMA-SKd cells or at 37°C for other cells), washed twice with RPMI-1640, and resuspended at 105 cells/ml in RPMI-1640 containing 0.25% BSA. Pancreatic islet cells were prepared from five- to eight-week-old NOD/Lt, B6, and NOD.lpr /lpr mice (37); labeled with [51Cr]sodium chromate for two hours at 37°C; and seeded at 104 cells per well. RMA-SKd, L1210-Fas+, and L1210-Fas– cells labeled with 51Cr were seeded at 104 cells/100 μl/well, pulsed with NRP or tum (1 μg/ml) for one hour at 37°C, and used as target cells in 51Cr release assays. Effector cells (peptide-activated splenic CD8+ T cells or islet-derived CD8+ T-cell clones; 100 μl) were added to each well in duplicate at different target/effector ratios (1:10 and 1:3, respectively). Cultures of RMA-SKd cells with peptides but no T cells were used as controls to confirm that the peptides were not cytotoxic. Plain medium or 1% Triton X-100 was added to sets of target cells for examination of spontaneous and total cell lysis, respectively. The plates were incubated at 37°C for eight hours, and the supernatants were collected for determination of specific 51Cr release (38).
Proliferation assays.
Naive splenic CD8+ T cells from 8.3-NOD.PO+/– or 8.3-NOD_.PO–/–_ mice (2 × 104) were incubated, in triplicate, with γ-irradiated (3,000 rad) islet cells (105 per well) for three days at 37°C in 5% CO2. Cultures were pulsed with 1 μCi of [3H]thymidine during the last 18 h of culture and harvested.
Cytokine secretion.
Naive splenic CD8+ T cells from 8.3-NOD.PO+/– or 8.3-NOD.PO–/– mice (2 × 104 per well) were incubated with γ-irradiated NOD islet cells (105 per well) and splenocytes (105 per well) in 96-well plates for 48 h at 37°C. The supernatants (100 μl/well) were assayed for IL-2, IL-4, and IFN-γ content by ELISA, using commercially available kits (Genzyme Diagnostics, Cambridge, Massachusetts, USA).
Adoptive T-cell transfer.
Naive 8.3-CD8+ T cells were first activated with NRP in vitro, as already described here, and then injected into the tail veins of five- to seven-week-old RIP-B7-NOD.scid mice (4–5 × 106 cells per mouse).
Statistical analyses.
Statistical analyses were performed using the Mann-Whitney U and χ2 tests.
Results
Spontaneous autoimmune diabetes in NOD.PO–/– mice.
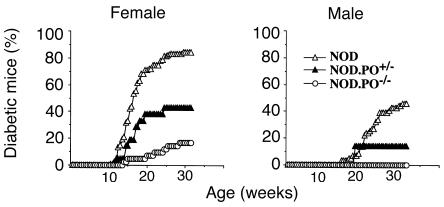
The first set of experiments investigated whether our NOD.PO–/– mice (produced by intercrossing N10 heterozygotes) were as resistant to development of diabetes as mice from a previously described NOD.PO–/– stock (produced by intercrossing N7 heterozygotes; ref. 31). As shown in Figure 1, the incidence of diabetes in NOD.PO+/– mice and NOD.PO–/– mice was significantly lower than in NOD/Lt mice (females: 43% vs. 17% vs. 84%, P < 0.0001, respectively; males: 14% vs. 0% vs. 46%, respectively, _P_ < 0.0001). The few female NOD._PO–/–_ mice that developed diabetes did so slightly later than the female NOD._PO+/–_ and NOD/Lt mice that became diabetic, but these differences were not statistically significant (155 ± 38 vs. 124 ± 24 vs. 119 ± 6 days, respectively). Most of the islets in pancreata from old, nondiabetic NOD._PO–/–_ mice (>30 weeks) displayed severe insulitis, but a large fraction of the insulitis lesions of these mice were nondestructive and contained significant amounts of endocrine tissue (data not shown). Thus, like the NOD.PO–/– mice of Kagi et al. (31), our NOD.PO–/– mice are resistant to development of massive β-cell loss and diabetes but remain susceptible to insulitis. As in this previous study, we also found a dosage effect of the perforin mutation on diabetes incidence.
Figure 1.
Incidence of autoimmune diabetes in NOD/Lt, NOD.PO+/–, and NOD.PO–/– mice. Data corresponds to 41 NOD.PO–/– females, 31 NOD.PO–/– males, 21 NOD.PO+/– females, seven NOD.PO+/– males, 114 NOD/Lt females, and 59 NOD/Lt males. See the text for statistics.
Phenotypic properties of 8.3-CD8+ T cells from 8.3-NOD.PO–/– mice.
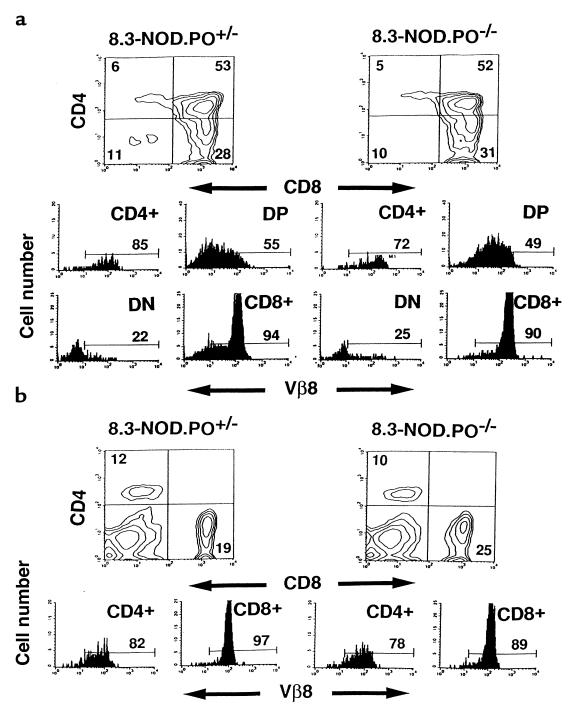
We next backcrossed the 8.3-TCR from 8.3-NOD mice into NOD.PO–/– mice and observed the fate of CD8+ T cells bearing the 8.3-TCR in the absence of perforin. Three-color cytofluorometric studies of thymocytes and splenic cells from 8.3-NOD.PO–/– and 8.3-NOD.PO+/– mice indicated that 8.3-CD8+ T cells maturing in a perforin-deficient NOD background were phenotypically normal. The percentages of CD4+CD8+ and CD4–CD8+ thymocytes expressing high levels of the transgenic TCRβ chain were similar in both types of mice (Figure 2a). Thymocyte development was skewed toward the CD8+ T-cell subset in both 8.3-NOD.PO–/– and 8.3-NOD.PO+/– mice (Figure 2a). As a result, the spleens (Figure 2b) and lymph nodes (data not shown) of 8.3-NOD.PO–/– mice had similar percentages of CD8+ T cells, Vβ8.1+CD8+ T cells, and CD4+ T cells as the spleens of 8.3-NOD.PO+/– mice. Normal positive selection of 8.3-thymocytes in 8.3-NOD.PO–/– mice was confirmed by comparing the thymocyte profiles of RAG-2–/– 8.3-NOD.PO–/– and RAG-2–/– 8.3-NOD.PO+/– mice, which cannot rearrange endogenous TCR genes. RAG-2–/– 8.3-NOD.PO–/– mice selected as many Vβ8.1+CD8+ T cells as RAG-2–/– 8.3-NOD.PO+/– mice (data not shown). Additional cytofluorometric studies of thymocytes from 8.3-NOD.PO–/– and 8.3-NOD.PO+/– mice using mAb’s against several differentiation markers, including the transgenic TCRβ chain, MHC class I (H-2Kd), CD5, CD24, CD44, and CD69, revealed that the CD4+CD8+ and CD4–CD8+ thymocytes of both types of mice were phenotypically similar (data not shown). Likewise, no phenotypic differences were noted between the peripheral CD8+ T cells of 8.3-NOD.PO–/– mice and those of 8.3-NOD.PO+/– mice with respect to several cell surface markers, including Vβ8.1/8.2, CD2, CD11a, CD28, CD44, CD45RB, CD62L, and CD69 (data not shown). Taken together, these data suggested that 8.3-CD8+ T cells mature normally in a perforin-deficient background.
Figure 2.
Flow cytometric profiles of thymocytes and splenocytes from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice. CD4, CD8, and Vβ8.1/8.2 profiles of thymocytes (a) and splenic cells (b) from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice (n = 4–6 mice per group, 8–12 weeks old). Upper panels show CD4 vs. CD8 contour plots of cells stained with anti-CD8/PE, anti–Vβ8.1/8.2-FITC, and anti-CD4/biotin plus Streptavidin-PerCP. The lower panels show the Vβ8.1/8.2 fluorescence histograms of each T-cell subset after electronic gating. Numbers indicate the average percentage of cells (upper panels) or number of Vβ8.1/8.2+ cells (lower panels) in each subset. DN, double-negative cells. DP, double-positive cells.
Functional responsiveness of peripheral CD8+ T cells from 8.3-NOD.PO–/– mice.
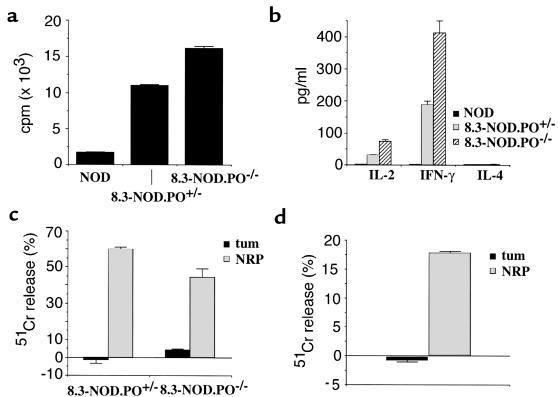
To investigate whether the peripheral 8.3-CD8+ T cells of 8.3-NOD.PO–/– mice were responsive to stimulation with β-cell autoantigen, we compared the proliferative and cytokine secretion activities of naive splenic CD8+ T cells from 8.3-NOD.PO–/– and 8.3-NOD.PO+/– mice in response to stimulation with irradiated NOD islet cells. As shown in Figure 3, a and b, the CD8+ T cells from 8.3-NOD.PO–/– mice proliferated better and secreted greater amounts of IFN-γ and IL-2 than the CD8+ T cells from 8.3-NOD.PO+/– mice. The 8.3-CD8+ T cells from these mice did not secrete IL-4, confirming their Tc1 phenotype. The NOD islet reactivity of 8.3-CD8+ T cells is H-2Kd–restricted and β cell–specific, as it did not occur when using B6 islet cells (H-2Kb) or H-2Kd–expressing non–β-cell targets as a source of antigen (ref. 33 and data not shown). Furthermore, these responses are driven by the 8.3-TCR, as they did not occur when using splenic CD8+ T cells from nontransgenic NOD mice as responders (Figure 3). The lack of nontransgenic NOD T-cell responses in these short-term in vitro assays does not imply that NRP-reactive CD8+ T cells do not exist in NOD mice; in short-term assays, nontransgenic NOD CD8+ splenocytes also fail to proliferate or secrete cytokines above background levels in response to syngeneic (NOD) or allogeneic (B6) islet cells (Figure 3a and data not shown). In any case, the results obtained with 8.3-CD8+ T cells indicate that 8.3-NOD.PO–/– mice export functional β cell–reactive CD8+ T cells to the periphery and that these CD8+ T cells are slightly hyperreactive to antigenic stimulation in vitro.
Figure 3.
Functional activity of 8.3-CD8+ T cells from 8.3-NOD.PO–/– mice. (a) Proliferation of CD8+ T cells from NOD/Lt, 8.3-NOD.PO+/–, and 8.3-NOD.PO–/– mice in response to NOD islet cells. (b) Cytokine secretion by splenic CD8+ T cells from NOD/Lt, 8.3-NOD.PO+/–, and 8.3-NOD.PO–/– mice in response to stimulation with NOD islet cells. (c) Differentiation of 8.3-CTLp from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice into CTL. Splenic CD8+ T cells were incubated with NRP-pulsed NOD splenocytes for seven days and then used as effectors in 51Cr release assays employing NRP- or tum-pulsed RMA-SKd cells as targets (at a 1:10 target/effector ratio). Bars show the SEMs. (d) Cytotoxic activity of islet-derived CD8+ T-cell clones from 8.3-NOD.PO–/– mice at a 1:3 target/effector ratio.
To investigate whether the 8.3-CD8+ T cells of 8.3-NOD.PO–/– mice could differentiate into cytotoxic T cells upon antigenic challenge in vitro, we stimulated the splenic CD8+ T cells from 8.3-NOD.PO–/– and 8.3-NOD.PO+/– mice with the peptide ligand of the 8.3-TCR (NRP; Anderson, B., et al., manuscript submitted for publication) in the presence of irradiated NOD splenocytes as APCs. We then tested the ability of the responding cells to lyse Fas+, H-2Kd cDNA–transfected RMA-S cells (RMA-SKd) in the presence of either NRP or an irrelevant H-2Kd–binding peptide (tum; ref. 36). As shown in Figure 3c, NOD islet–stimulated 8.3-CD8+ T-cell lines from 8.3-NOD.PO–/– mice killed NRP-pulsed, but not tum-pulsed, RMA-SKd targets almost as efficiently as 8.3-CD8+ T-cell lines from 8.3-NOD.PO+/– mice. These responses were 8.3-TCR–specific and activation-dependent, as they were not observed when using islet-stimulated CD8+ T cells from nontransgenic NOD mice or when using naive CD8+ T cells from 8.3-NOD.PO+/+ mice as effector cells (data not shown). To confirm that the cytotoxic response observed in these assays was representative of the cytotoxic activity of in vivo–activated 8.3-CD8+ T cells, we generated 8.3-CD8+ T-cell clones from the pancreatic islets of 8.3-NOD.PO–/– mice and tested their ability to kill NRP- or tum-pulsed RMA-SKd cells in vitro. As shown in Figure 3d, these clones efficiently killed NRP-pulsed, but not tum-pulsed, RMA-SKd cells. It thus appears that the 8.3-CD8+ T cells of 8.3-NOD.PO–/– mice can differentiate into CTLs both in vitro and in vivo.
8.3-CD8+ CTLs can kill non–β-cell targets via both perforin and Fas.
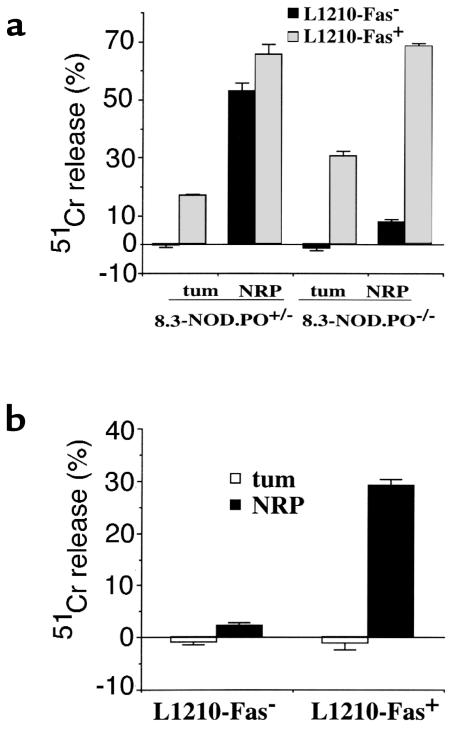
We next asked whether 8.3-CD8+ CTLs could lyse non–β-cell targets via perforin, Fas, or both. This was done by investigating whether NRP-differentiated 8.3-CD8+ CTLs from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice could kill peptide-pulsed L1210-Fas+ and/or peptide-pulsed L1210-Fas– cells in vitro (L1210 cells are H-2Kd+). As shown in Figure 4a (left), NRP-activated 8.3-CD8+ CTLs from 8.3-NOD.PO+/– mice efficiently killed NRP-pulsed L1210-Fas+ and NRP-pulsed L1210-Fas– targets. These CTLs were slightly cytotoxic to tum-pulsed L1210-Fas+ cells (bystander lysis) but could not kill tum-pulsed L1210-Fas– cells. This indicated that the cytotoxicity of 8.3-CD8+ CTLs on L1210 cells was, for the most part, antigen-specific. Experiments using 8.3-CD8+ CTLs from 8.3-NOD.PO–/– mice revealed that these CTLs were able to kill NRP-pulsed L1210-Fas+ targets (and, to a much lesser degree, tum-pulsed L1210-Fas+ targets), but they could not kill NRP- or tum-pulsed L1210-Fas– targets (Figure 4a, right). To confirm that these responses were not an artifact of in vitro stimulation with NRP, we investigated whether 8.3-CD8+ CTLs clones derived from pancreatic islets of 8.3-NOD.PO–/– mice exhibited the same pattern of cytotoxicity on these target cells. As shown in Figure 4b, these clones killed NRP-pulsed L1210-Fas+ cells but could not kill tum-pulsed L1210-Fas+ cells, tum-pulsed L1210-Fas– cells, or NRP-pulsed L1210-Fas– cells. Taken together, these data demonstrated that 8.3-CTLs can kill antigen-pulsed L1210 targets via both perforin and Fas and confirmed that the 8.3-CD8+ CTLs from 8.3-NOD.PO–/– mice cannot kill Fas-deficient targets.
Figure 4.
Cytotoxic activity of 8.3-CTLs from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice against peptide-pulsed L1210-Fas+ and L1210-Fas– cells. 8.3-CTL lines (a) and clones (b) were generated as in Figure 3 and tested at a 1:10 (for lines) or 1:3 (for clones) target/effector ratio . Bars show the SEMs.
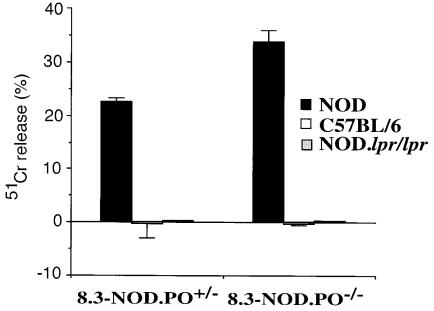
Perforin-deficient 8.3-CD8+ CTLs can kill NOD, but not NOD.lpr, β cells.
We next asked whether NRP-differentiated 8.3-CD8+ CTLs from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice could kill NOD (H-2Kd, Db), C57BL/6 (H-2Kb, Db), and NOD.lpr/lpr (H-2Kd, Db) islet cells in vitro. As shown in Figure 5, 8.3-CD8+ CTLs from both 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice killed NOD, but not B6, islet cells. Surprisingly, CTLs from both types of mice were unable to kill NOD.lpr/lpr islet cells. Although these results cannot exclude the possibility that 8.3-CD8+ CTLs may also be able to kill β cells via non-perforin/non-Fas mechanisms, they strongly suggest that 8.3-CD8+ CTLs can only lyse β cells that express Fas.
Figure 5.
β-cell cytotoxicity of 8.3-CTLs from 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice. NRP-differentiated 8.3-CTLs were tested in cytotoxicity assays against islet cells from NOD/Lt, C57BL/6, and NOD.lpr/lpr mice (at a 1:10 target/effector ratio).
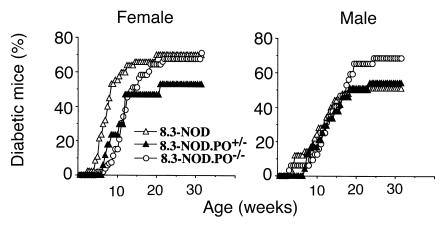
Spontaneous autoimmune diabetes in 8.3-NOD.PO–/– and 8.3-NOD.PO+/– mice.
The results of the in vitro experiments predicted that diabetogenesis in 8.3-NOD mice would be independent of perforin expression. To investigate this, we observed 8.3-NOD.PO+/+, 8.3-NOD.PO+/–, and 8.3-NOD.PO–/– mice for development of diabetes. As shown in Figure 6, female 8.3-NOD.PO–/– mice developed diabetes more frequently than 8.3-NOD.PO+/– mice (71% vs. 53%; P < 0.0001) and as frequently as 8.3-NOD.PO+/+ mice (71% vs. 72%, respectively). On average, female 8.3-NOD.PO–/– mice developed diabetes later than 8.3-NOD.PO+/+ mice (97 ± 37 vs. 61 ± 24 days; P < 0.0001) but only slightly later than female 8.3-NOD.PO+/– mice (97 ± 37 vs. 80 ± 31 days; nonsignificant differences). Male 8.3-NOD.PO–/– mice also developed diabetes more frequently than 8.3-NOD.PO+/– mice and 8.3-NOD.PO+/+ mice (69% vs. 54% vs. 52%, respectively; P < 0.0001). No significant differences were noted in the average age at onset of the disease among male mice from these three strains (100 ± 36 vs. 94 ± 35 vs. 83 ± 35 days, respectively). Taken together, these results demonstrated that 8.3-CD8+ CTL–induced diabetogenesis is, for the most part, independent of perforin expression and that absence of perforin actually increases the penetrance of the disease in 8.3-NOD mice.
Figure 6.
Spontaneous diabetes in 8.3-NOD.PO+/– and 8.3-NOD.PO–/– mice. Data corresponds to 17 8.3-NOD.PO+/– females, 24 8.3-NOD.PO+/– males, 44 8.3-NOD.PO–/– females, and 27 8.3-NOD.PO–/– males. See the text for statistics.
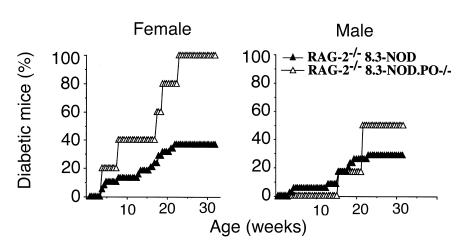
Spontaneous diabetes in monoclonal 8.3-NOD.PO–/– mice.
It could be argued, however, that β-cell destruction in 8.3-NOD.PO–/– mice was effected by CD8+ or CD4+ T cells bearing endogenous TCRs, rather than by 8.3-CD8+ CTLs. To confirm that 8.3-CD8+ T cells from 8.3-NOD.PO–/– mice could destroy β cells in vivo independently of other T-cell specificities, we compared the natural history of diabetes in RAG-2–/– 8.3-NOD.PO–/– and RAG-2–/– 8.3-NOD.PO+/+ mice, which cannot rearrange endogenous TCRs and thus bear a monoclonal TCR repertoire. As shown in Figure 7, RAG-2–/– 8.3-NOD.PO–/– mice developed diabetes even more frequently than RAG-2–/– 8.3-NOD.PO+/+ mice, both in females (100% vs. 37%; P < 0.001) and in males (50% vs. 29%; P < 0.05). The average age at onset of diabetes in these two types of mice was comparable (females: 85 ± 51 vs. 92 ± 47 days; males: 141 ± 25 vs. 107 ± 45 days).
Figure 7.
Spontaneous diabetes in RAG-2–/– 8.3-NOD.PO+/+ vs. RAG-2–/– 8.3-NOD.PO–/– mice. Data corresponds to five RAG-2–/– 8.3-NOD.PO–/– females, six RAG-2–/– 8.3-NOD.PO–/– males, 38 RAG-2–/– 8.3-NOD.PO+/+ females, and 34 RAG-2–/– 8.3-NOD.PO+/+ males. See text for statistics.
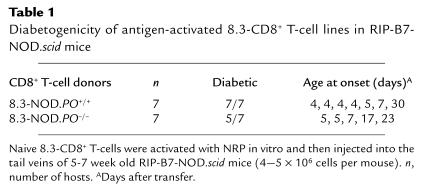
Diabetogenic activity of perforin-deficient 8.3-CTLs in immunodeficient NOD mice.
Finally, to confirm that perforin-deficient 8.3-CTLs could kill NOD β cells in vivo, we transferred NRP-differentiated 8.3-CD8+ CTLs from 8.3-NOD.PO–/– or 8.3-NOD.PO+/+ mice into RIP-B7.1–transgenic NOD.scid mice and observed these mice for development of diabetes. We did these experiments in RIP-B7.1-NOD.scid mice because they constitutively express the costimulatory molecule B7.1 on β cells. This enables a faster readout of CD8+ T cell–induced diabetes than when using NOD.scid mice as hosts. As indicated in Table 1, the incidence and age at onset of diabetes in mice transferred with perforin-deficient 8.3-CTLs were similar to the incidence and age at onset of diabetes in mice transferred with perforin-expressing 8.3-CTLs (5/7 vs. 7/7 mice; 8 ± 10 vs. 11 ± 8 days). Therefore, 8.3-CTLs do not require perforin to effect β-cell damage in vivo.
Table 1.
Diabetogenicity of antigen-activated 8.3-CD8+ T-cell lines in RIP-B7-NOD.scid mice
Discussion
This study has investigated whether diabetogenic CD8+ T cells bearing a representative NOD mouse–derived, β cell–reactive, and H-2Kd–restricted TCR (8.3-TCR) require perforin and/or Fas to effect β-cell damage. This TCR is representative of the MHC class I–restricted TCRs that are putatively involved in the initiation and progression of autoimmune diabetes, for several reasons. First, it uses a TCRα rearrangement that is also used by a large fraction of the CD8+ T cells that infiltrate islets at the earliest stages of insulitis (34). Second, it targets the same peptide/H-2Kd complex on β cells as ∼50% of the islet-associated CD8+ T cells in acutely diabetic nontransgenic NOD mice (Anderson, B. et al., manuscript submitted for publication). Third, it is highly diabetogenic when expressed as a transgene in NOD mice (33, 37). Finally, it can initiate diabetogenesis in RAG-2–/– 8.3-NOD mice, which bear a monoclonal TCR repertoire (33). We have shown that, in vitro, CD8+ CTLs expressing this TCR can use both perforin and FasL to lyse antigenic peptide–pulsed non–β-cell targets but can only kill NOD β cells via Fas. In agreement with these results, abrogation of perforin expression in 8.3-NOD mice did not protect these mice from diabetes and actually increased the penetrance of the disease through the population. These data provide one solution to the paradoxical need for both Fas (30, 32) and perforin (29, 31) for the development of autoimmune diabetes: unlike the CD8+ T cells involved in the progression of autoimmune diabetes, the CD8+ T cells involved in the initiation of the disease process would lyse β cells in a Fas-dependent and perforin-independent manner.
Previous studies in a virus-induced model of autoimmune diabetes had argued for perforin as the major mechanism of CTL-induced damage of β cells in autoimmune diabetes. CD8+ CTLs specific for a transgenic viral neoantigen (glycoprotein from lymphocytic choriomeningitis virus [LCMV-GP]) required perforin to cause diabetes in LCMV-infected RIP-LCMV-GP–transgenic C57BL/6 mice (29). In contrast, adoptive-transfer studies of an NOD mouse–derived, highly diabetogenic, and perforin-positive CD8+ CTL clone into NOD.lpr/lpr mice supported the alternative view that CD8+ T cell–mediated lysis of β cells in NOD mice is entirely dependent on the expression of Fas (30). Studies of perforin- or Fas-deficient NOD mice also yielded apparently contradictory results: perforin-deficient NOD mice developed severe insulitis but rarely became diabetic despite expressing Fas (31), and NOD.lpr/lpr mice developed neither diabetes nor insulitis despite expressing perforin (32).
We have shown that 8.3-NOD.PO–/– mice develop diabetes even more frequently than 8.3-NOD.PO+/– mice. The mechanisms underlying the increased incidence of diabetes in 8.3-NOD.PO–/– mice are unclear but may be related to the hyperreactivity of perforin-deficient 8.3-CD8+ T cells in response to antigen stimulation. Alternatively, the absence of perforin in 8.3-NOD.PO–/– mice may induce the upregulation of FasL expression on 8.3-CD8+ CTLs and thus enhance their ability to lyse β cells via Fas. We favor the former possibility, as analyses of Fas and FasL expression on CTLs from RAG-2–/– 8.3-NOD and RAG-2–/– 8.3-NOD.PO–/– mice did not reveal any differences (our unpublished data). Whatever the mechanisms, the increased diabetes susceptibility of 8.3-NOD.PO–/– mice demonstrate that 8.3-CTLs do not require perforin to kill NOD β cells in vivo.
Several arguments could be made to explain the different outcome of the present study when compared with the outcomes of some of these previous studies. The dramatic differences in the diabetes susceptibility of perforin-deficient RIP-LCMV-GP–transgenic B6 mice upon LCMV infection compared with perforin-deficient 8.3-NOD mice could conceivably be due to differences in the kinetics of the disease in these two models, to differences between the genetic backgrounds of the mice under study (NOD vs. B6), and/or to differences in the molecular nature (and, hence, timing and expression) of the corresponding target β-cell autoantigens (transgenic vs. endogenous). Unlike spontaneous autoimmune diabetes in the NOD mouse, the virus-induced form of diabetes in the LCMV model is acute and is not preceded by chronic inflammation of the pancreas by mononuclear cells (39). The powerful antigenic stimulus associated with LCMV infection in these mice might promote the use of the perforin pathway by CD8+ CTLs to kill infected cells as a way to ensure the rapid clearance of the virus. It is also possible that the choice between Fas and perforin pathways by CTL clonotypes maturing in NOD or B6 mice is influenced by one or several of the numerous MHC-linked and non–MHC-linked genetic differences between these two backgrounds (40). These effects would be analogous to those underlying differences in the requirement for IFN-γ expression for the development of insulitis and diabetes in RIP-LCMV-GP–transgenic B6 mice compared with NOD mice: IFN-γ–deficient RIP-LCMV-GP–transgenic B6 mice develop neither diabetes nor insulitis, whereas IFN-γ–deficient NOD mice develop severe insulitis and diabetes, albeit in a decelerated form (41, 42). The nature of the target autoantigen is another important consideration in the interpretation of the results of studies in different models. It has been shown that Fas-mediated cytotoxicity by CD8+ CTLs can be triggered independently of perforin-mediated cytotoxicity, depending on the structure of the triggering antigenic peptide/MHC complex (43). In this regard, it is worth noting that 8.3-CTLs could not lyse NOD β cells from NOD.lpr/lpr mice despite the fact that these CTLs had cytotoxic granules and expressed perforin. Furthermore, perforin-deficient 8.3-CTLs could efficiently transfer diabetes into RIP-B7.1–transgenic NOD.scid mice. It is therefore likely that some of the β-cell autoantigens targeted by CD8+ CTLs in NOD mice can only elicit Fas-mediated cytotoxicity.
Any interpretation of the available data, however, cannot ignore the fact that both perforin-deficient NOD mice and NOD.lpr/lpr mice are resistant to diabetes (31, 32). At first glance, this seems paradoxical because it suggests that both perforin and Fas, which subserve a somewhat redundant role in cell-mediated cytotoxicity, are necessary for the development of diabetes. Although it is possible that the diabetes resistance of the perforin-deficient NOD mice of Kagi et al. (31) was due to factors independent of the absence of perforin in CD8+ CTL, we think this is unlikely for several reasons. Like the perforin-deficient NOD mice (intercrossed at the N7 backcross) of Kagi et al., our perforin-deficient NOD mice (intercrossed at the N10 backcross) are also highly resistant to diabetes development. Because there are no known diabetes-susceptibility or -resistance genes near the perforin-locus on murine chromosome 10 (40), it is unlikely that the diabetes resistance of these mice resulted from introduction of a putative, B6-derived, diabetes-resistant allele into the NOD background. We also found a gene dosage effect of the perforin mutation on diabetes incidence, although this effect was significantly greater in our colony than in the colony of Kagi et al. (31). Perforin gene dosage effects have been observed in diabetes-unrelated experiments (22) and are not too surprising, as perforin mRNA and protein are not abundant in perforin-expressing CTLs in vivo (44). This gene dosage effect suggests that perforin+/– CD8+ CTLs express limiting levels of perforin; this is compatible with the view that diabetes development in nontransgenic NOD mice requires the involvement of perforin+ CTLs. Nevertheless, this gene dosage effect is equally compatible with the existence of an unknown, perforin-linked Idd locus. Thus, this possibility cannot be excluded at present.
But if perforin is the major mechanism of β-cell cytotoxicity, why do perforin-competent NOD.lpr/lpr mice not develop diabetes? The lack of diabetes-susceptibility loci near the lpr locus on chromosome 19 also makes it highly unlikely that the reported diabetes resistance of NOD.lpr/lpr mice (intercrossed at the N6 backcross) was due to cointroduction of diabetes resistance genes into the NOD background along with the lpr gene. It could be argued, however, that the diabetes resistance of Fas-deficient NOD mice resulted from pleiotropic effects of the Fas deficiency on T-cell development and/or function, rather than from the absence of Fas on the β-cell surface. Unlike perforin-deficient mice, NOD.lpr/lpr mice exhibit severe defects in lymphocyte homeostasis, including massive lymphadenopathy, accumulation of CD4–CD8–B220+ T cells, and constitutive upregulation of FasL on T cells (32). Nevertheless, human and murine β cells can express Fas (30, 45–47), and neither T cells from diabetic NOD mice (32) nor NOD islet–derived diabetogenic CD8+ CTL clones (30) can transfer diabetes into NOD.lpr/lpr mice. It is thus likely that diabetes is a Fas-dependent process as well.
Our observation that a CD8+ CTL specificity representative of those CTLs putatively involved in the initiation of spontaneous diabetes in NOD mice does not require perforin to effect β-cell damage in vitro and in vivo, and kills NOD β cells via Fas exclusively, provides one solution to the seemingly paradoxical need for both perforin and Fas for the development of diabetes. Previous studies have suggested that initiation of insulitis in NOD mice is mediated by a cytopathic effect of certain CD8+ CTLs on β cells, leading to the release of sequestered β-cell autoantigens and the subsequent activation of diabetogenic CD4+ T cells and other CD8+ CTLs (16–19). We therefore propose that CD8+ CTL clonotypes involved in the initiation of autoimmune diabetes effect the initial β-cell insult via Fas exclusively and that those recruited later in the disease process kill β cells primarily via perforin. This would explain why NOD.lpr/lpr mice do not even develop insulitis (32) and why perforin-deficient NOD mice develop severe inflammation of islets by CD4+ T cells and yet remain diabetes resistant (31).
In conclusion, we have shown that CD8+ T cells bearing a highly diabetogenic, β cell–specific, H-2Kd–restricted TCR that is representative of the TCRs expressed by islet-associated CD8+ CTLs propagated from the earliest insulitic lesions of NOD mice do not require perforin to trigger diabetes and that they can only kill NOD β cells via Fas. These results provide an explanation for the insulitis and diabetes resistance of Fas-deficient NOD mice and suggest that the initial CD8+ CTL–mediated β-cell insult in spontaneous autoimmune diabetes is effected via Fas. Future studies with TCRs from additional CD8+ CTL clones should provide further insights on the role of Fas and perforin during early and late stages of diabetes development.
Acknowledgments
We thank M. Bevan, P. Goldstein, B. Wipke, W. Clark, D. Serreze, and R. Flavell for providing reagents and mice. We also thank S. Culp and K. Rouleau for excellent technical assistance, and L. Bryant for flow cytometry. This work was supported by the Juvenile Diabetes Foundation International and the Medical Research Council of Canada. J. Verdaguer is supported by a fellowship from the Canadian Diabetes Association. P. Santamaria is a Senior Scholar of the Alberta Heritage Foundation for Medical Research.
References
- 1.Delovitch T, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune disregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 2.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. J Exp Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller BJ, Appel MC, O’Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in non-obese diabetic mice. J Immunol. 1988;140:52–58. [PubMed] [Google Scholar]
- 5.Yagi H, et al. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 6.Christianson S, Shultz L, Leiter E. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T cells from diabetic versus prediabetic NOD.NON-thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 7.Reich EP, Sherwin RS, Kanagawa O, Janeway CA., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989;341:326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- 8.Haskins K, McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet–specific T cell clone. Science. 1990;249:1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 9.Nakano N, Kikutani H, Nishimoto H, Kishimoto T. T cell receptor V gene usage of islet beta cell–reactive T cells is not restricted in non-obese diabetic mice. J Exp Med. 1991;173:1091–1097. doi: 10.1084/jem.173.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley B, Haskins K, Rosa FL, Lafferty K. CD8 T cells are not required for islet destruction induced by a CD4+ islet–specific T-cell clone. Diabetes. 1992;41:1603–1608. doi: 10.2337/diab.41.12.1603. [DOI] [PubMed] [Google Scholar]
- 11.Daniel D, Gill R, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 12.Katz J, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 13.Peterson J, Haskins K. Transfer of diabetes in the NOD-scid mouse by CD4 T cell clones. Differential requirement for CD8 T cells. Diabetes. 1996;45:328–336. doi: 10.2337/diab.45.3.328. [DOI] [PubMed] [Google Scholar]
- 14.Serreze D, Leiter E. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr Opin Immunol. 1994;6:900–906. doi: 10.1016/0952-7915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 15.Wong FS, Visintin I, Wen L, Flavell R, Janeway CA., Jr CD8 T cell clones from young NOD islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 T cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the generation of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 17.Wicker L, et al. β2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 18.Serreze D, Leiter E, Christianson G, Greiner D, Roopenian D. Major histocompatibility complex class I–deficient NOD.β2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–508. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 20.Kay T, Parker J, Stephens L, Thomas H, Allison J. RIP-β2-microglobulin transgene expression restores insulitis, but not diabetes, in β2-microglobulinnull nonobese diabetic mice. J Immunol. 1996;157:3688–3693. [PubMed] [Google Scholar]
- 21.Serreze D, et al. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol. 1997;157:3978–3986. [PubMed] [Google Scholar]
- 22.Kagi D, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 23.Kagi D, et al. Fas and perforin pathways as major mechanisms of T cell–mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 24.Kojima H, et al. Two distinct pathways of specific killing revealed by perforin-mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 25.Hanabuchi S, et al. Fas and its ligand in a general mechanism of T-cell mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:4930–4934. doi: 10.1073/pnas.91.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte– and natural killer cell–mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh C, et al. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 29.Kagi D, Odermatt B, Ohashi P, Zinkernagel R, Hengartner H. Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity. J Exp Med. 1996;183:2143–2152. doi: 10.1084/jem.183.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chervonsky A, et al. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 31.Kagi D, et al. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J Exp Med. 1997;186:989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itoh N, et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1997;186:613–618. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiLorenzo T, et al. MHC class I-restricted T cells are required for all but end stages of diabetes development and utilize a prevalent T cell receptor α chain gene rearrangement. Proc Natl Acad Sci USA. 1998;95:12538–12542. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong FS, et al. The role of lymphocyte subsets in accelerated diabetes in nonobese diabetic-rat insulin promoter-B7.1 (NOD-RIP-B7-1) mice. J Exp Med. 1998;187:1985–1993. doi: 10.1084/jem.187.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallny H, et al. Identification and quantitation of a naturally presented peptide as recognized by cytotoxic T lymphocytes specific for animmunogenic tumor variant. Int Immunol. 1992;4:1085–1090. doi: 10.1093/intimm/4.10.1085. [DOI] [PubMed] [Google Scholar]
- 37.Verdaguer J, et al. Acceleration of spontaneous diabetes in TCRβ-transgenic nonobese diabetic mice by beta cell-cytotoxic CD8+ T cells expressing identical endogenous TCRα chains. J Immunol. 1996;157:4726–4735. [PubMed] [Google Scholar]
- 38.Santamaria P, et al. Beta cell cytotoxic CD8+ T cells from non-obese diabetic mice use highly homologous T cell receptor alpha chain CDR3 sequences. J Immunol. 1995;154:2494–2503. [PubMed] [Google Scholar]
- 39.Ohashi P, et al. Ablation of tolerance and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 40.Wicker L, Todd J, Peterson L. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 41.Hultgren B, Huang X, Dybal N, Stewart T. Genetic absence of γ-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 42.Herrath MV, Oldstone M. Interferon γ is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1996;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao W, Tykodi S, Esser M, Braciale V, Braciale T. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 44.Muller C, et al. Detection of perforin and granzyme A mRNAs in infiltrating cells during LCMV infection of mice. Eur J Immunol. 1989;19:1253–1259. doi: 10.1002/eji.1830190716. [DOI] [PubMed] [Google Scholar]
- 45.Leithäuser F, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 46.Stassi G, et al. Expression of apoptosis-inducing CD95 (Fas/Apo-1) on human beta cells sorted by flow cytometry and cultured in vitro. Transplant Proc. 1995;27:3271–3275. [PubMed] [Google Scholar]
- 47.Yamada K, Takane-Gyotoku N, Ichikawa F, Inada C, Nokada K. Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia. 1996;39:1306–1312. doi: 10.1007/s001250050574. [DOI] [PubMed] [Google Scholar]