The Role of the Hairless (hr) Gene in the Regulation of Hair Follicle Catagen Transformation (original) (raw)
Abstract
Mice that carry a mutation at the hairless (hr) locus develop seemingly normal hair follicles (HF) but shed their hairs completely soon after birth. Histologically, their HFs degenerate into characteristic utriculi and dermal cysts shortly after the entry of the HF into the first regression phase (catagen), during the initiation of HF cycling. Here, we show that at least nine distinct stages of HF disintegration can be distinguished in hr/hr mice. Toward the end of HF morphogenesis (day 15 postpartum) the proximal hair bulb in hr/hr skin undergoes premature and massive apoptosis. This is associated with a dyscoordination of cell proliferation in defined HF compartments, malpositioning of the proximal inner root sheath, striking atrophy of outer root sheath, and failure of trichilemmal keratinization in the developing club hair. Rather than undergoing their normal catagen-associated involution, the hair bulb and central outer root sheath disintegrate into separate cell clusters, thus disrupting all epithelial contact with the dermal papilla. Dermal papilla fibroblasts fail to migrate upward, and break up into clusters of shrunken cells stranded in the reticular dermis as dermal cyst precursors, while the upper HF epithelium transforms into utriculi. Some dermal papilla cells, which normally never undergo apoptosis, also become TUNEL+ in hr/hr skin, and their normally high expression of a key adhesion molecule, neural cell adhesion molecule, declines. Thus, loss of a functional hr gene product (a putative zinc finger transcription factor) initiates a premature, highly dysregulated catagen, which results in the destruction of the normal HF architecture and abrogates the HF’s ability to cycle. This provides new insights into the pathobiology of the hr mutation, and suggests that the normal hr gene product is a crucial element of catagen control.
The hair follicle (HF) is a dynamic structure that generates hair through complex and exquisitely regulated cycles of growth and remodeling. However, little is understood about the exact nature, sequence, and interplay of the molecular signals that govern the cyclic transformations of the HF between rest (telogen), growth (anagen), and regression (catagen). 1-4 Several lines of evidence point to the hairless (hr) gene as one potentially important regulatory molecule in hair cycle control. An autosomal recessive mutation of the hairless (hr) gene in mice evokes severe, irreversible HF abnormalities. Specifically, this mutation abrogates the ability of the HF to anchor the hair shaft and to cycle, leading to complete hair loss during the third week of postnatal life in homozygous (hr/hr) mutants. 5,6 The hairless phenotype in mice is strikingly similar to the human disease known as papular atrichia 7 (MIM #209500), that displays complete hair loss after shedding of the first hairs generated in the course of a seemingly normal HF morphogenesis. 8,9 These HF abnormalities suggest a functionally important role of the hr gene product in normal skin physiology, namely in the initiation of HF cycling upon entry into the first catagen. 2,10 This was recently confirmed by identification of the human homolog of the hr gene, in which a recessively inherited mutation leads to complete loss of body hairs in affected individuals with papular atrichia. 9 The human gene is highly homologous to the mouse and rat hairless genes (84% and 83% respectively 9 ), suggesting high conservation and thus functional significance of the hr gene among different mammalian taxons.
Though the structure of the hr gene in human and mouse has been clarified, the biological function(s) of the corresponding protein remain to be elucidated. 10,11 The hr gene encodes a putative single zinc-finger transcription factor, which is expressed in the HF, epidermis, and brain. 9,12,13 This locus maps to mouse Chromosome 14 14 and to human Chromosome 8p, in a region syntenic with mouse Chromosome 14. 9 The hairless mutation in HRS/J mice is caused by stable integration of a modified polytropic retrovirus into intron 6 of the hr gene, and subsequent abnormal splicing. 15 In humans, the recessively inherited type of complete hair loss over the entire body is associated with a variety of missense, nonsense, and deletion mutations in the hr gene. 9,16-18
Our previous study of the fully developed hairless phenotype in adult 3- to 4-month-old HRS/J hr/hr mice had suggested that the hr mutation disrupts the integrity of selected functional tissue units in most HF compartments, including the isthmus and hair bulb region as well as the dermal papilla. This prompted us to speculate that HF disintegration in hr/hr mice might result from a disturbance in apoptosis and/or an impairment of cell adhesion, 10,19 which remains to be demonstrated. Also, these studies did not address the earliest stages of hr phenotype development in neonatal hr/hr mice undergoing their first synchronized, spontaneous HF regression (catagen). 1,3,20,21
Therefore, in the current study, we have systematically characterized the chronology of HF transformation in HRS/J hr/hr hairless mutant mice by routine histology, histochemistry, and immunohistology throughout the period of postnatal skin development when the macroscopic phenotype is first evident and when the hair shafts are shed (ie, during days 14–20 postpartum). This was compared to age-matched, heterozygous +/hr mice, which develop and maintain an apparently normal fur coat. We demonstrate that the development of the hr/hr phenotype is an exquisitely regulated process, whose precise temporal and cell-type-specific sequence of events strongly suggests critical roles for the hr gene product in normal HF regression, namely in the control of intrafollicular apoptosis and tissue integrity during catagen.
Materials and Methods
Animals and Tissue Collection
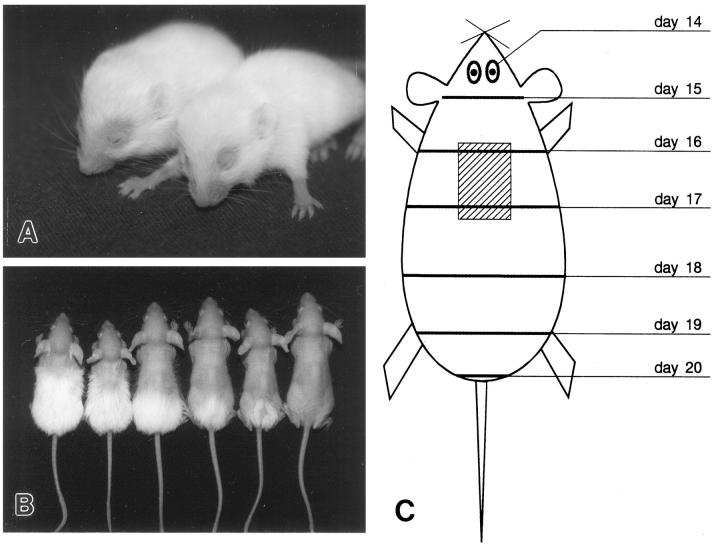
HRS/J hairless mice were purchased from the Laboratory of Biomodels (Moscow District, Russia) and were bred at the animal department of the Severtsov Institute, Moscow. Mice were housed in community cages (1 male and 3 females) under standard conditions (12-hour light periods, water and mouse chow ad libitum). The pups were obtained by mating homozygous (hr/hr) males to heterozygous (+/hr) females, because homozygous (hr/hr) females are poor mothers. 11,22 After day 14 postpartum (pp) (ie, the onset of periorbital hair loss), hr/hr and +/hr pups were selected by their unequivocal phenotypic differences in macroscopic fur appearance (Figure 1A) ▶ , which were also confirmed by routine histology.
Figure 1.
Progression of hair loss in hr/hr mice. A: Initial stage of hair loss in hr/hr pups (day 14 pp). Note the alopecic skin around the eyes and on the forelimbs. B: hr/hr pups at different stages of hair shedding (days 16–21 pp). C: Scheme of progression of the wave of hair shedding in hr/hr mice. The reference area examined (hatched) was located on the upper back. All stages of HF transformation were observed in this area during days 15–18 of mouse postnatal development.
Our study encompassed the entire period of hair loss in young hr/hr mice, from its onset at day 14 of postnatal development (periorbital alopecia) up to day 20 pp (complete alopecia) (Figure 1B) ▶ . Gross observations were performed daily to document the hair loss. We generated a tissue bank of skin samples including seven time points (daily sampling) with 6 homozygous (hr/hr) and 4 heterozygous (+/hr) age-matched mice of both sexes, derived from 3–4 different litters, for each time point. Because the changes in HF structure during the hair loss occur very rapidly, and to characterize the sequence of pathological events in hr/hr mice more precisely, all skin samples were harvested from the same defined region (1.0 × 1.2 cm) of upper back skin (Figure 1C) ▶ . The following statements on the chronology of hair loss and on the corresponding stages of HF transformation in hr/hr refer to this region of the integument. Because heterozygous mutants also display some HF abnormalities later in postnatal life, including spontaneous patches of alopecia after the third month of life (Panteleyev and Paus, unpublished observation), only those parameters of the first catagen transition of the +/hr mice were used for comparison with hr/hr mice, which were histologically identical to normal catagen development in C57BL/6J mice. 18,23
All skin samples were harvested parallel to the dorsal midline to obtain longitudinal sections through the HF 24 and were processed for routine histology, immunohistochemistry, TUNEL stain, and histochemistry as described below. Skin samples of +/hr mice of corresponding ages were used for comparison, after shaving their hair-bearing skin with a cordless trimmer.
Skin Sections, Histochemistry, and Immunohistology
Skin samples were frozen in liquid nitrogen immediately after harvesting and were embedded in Tissue-Tek (Miles, Elkhart, IN) medium for storage at −70°C as described. 21 Air-dried 6-μm cryostat sections were collected on silane-coated slides, fixed in cold acetone (−20°C) for 10 minutes, and then processed for neural cell adhesion molecule (NCAM) 19,25 and interleukin-1 receptor type 1 (IL-1R1) 26 immunohistochemistry. Alkaline phosphatase (AP) activity was visualized according to the staining protocol routinely used in AP or APAAP immunohistochemistry, but without the customary blocking of endogenous AP by levamisole. 27 Both AP activity and NCAM expression were used as sensitive markers for dermal papilla (DP) fibroblasts. 25,27
TUNEL/Hoechst 33242/Ki-67 Triple Stain
TdT-mediated dUTP-digoxigenin DNA nick end-labeling was performed using the commercially available ApopTag In Situ Apoptosis Detection Kit (Oncor, Gaithersburg, MD) on formalin-fixed (10%) 10-μm cryostat sections of skin samples according to the manufacturer’s protocol. This was combined with Ki-67 immunodetection as a proliferation marker, using a rabbit antiserum (1:100; Dianova, Hamburg, Germany), followed by an incubation with goat-anti-rabbit TRITC-labeled IgG (Jackson Immunoresearch, West Grove, PA; 1:200, 37°C, 1 hour), and a consequent nuclear counterstain by Hoechst 33342 dye (10 μg/ml) (Sigma, St. Louis, MO) as previously described. 23 TUNEL-positive (apoptotic) and Ki-67+ (proliferating) cells were detected with a fluorescence microscope (Zeiss), using the appropriate filters.
Results
Macroscopic Evaluation
Visible hair loss in hr/hr mutant pups began at day 14 pp in the periorbital region and on the forelimbs, while the rest of the integument still showed a normal hair pattern (Figure 1A) ▶ . One day later, two zones of naked skin around the eyes fused and formed the front of hair shedding that subsequently progressed in a cranio-caudal direction (Figure 1, B and C) ▶ . This alopecia front was sharply demarcated, and there was no zone of diffuse alopecia interspersed between the completely naked skin and the macroscopically still normal fur coat. At the age of 3 weeks, hr/hr animals lost their first hair coat completely, with the notable exception of the vibrissae (see Figure 1B ▶ ), whereas +/hr mice retained a normal-appearing hair coat.
Histological Changes in hr Mouse Skin
Histology revealed that the most dynamic and most striking HF transformations in the selected reference area (dorsal thoracic midline; Figure 1C ▶ ) of hr/hr skin occurred during only two days of postnatal development (on days 15–16). Some of these HF transformations proceeded within hours and in a very narrow zone of the progressing wave of hair shedding.
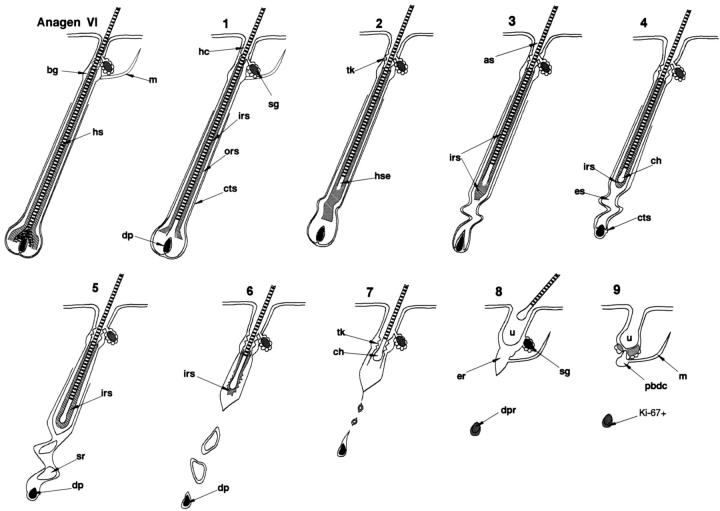
The key steps in the microscopic development of the hairless phenotype could be divided into nine distinct, successive stages of HF transformation and disintegration (Figure 2) ▶ , which underlie the macroscopic development of alopecia (Figure 1B) ▶ . These stages were of different length but were characterized by clearly defined, substantial changes in HF morphology and in the other parameters defined below. It is important to note that these HF transformations in hr/hr skin differed from normal HF regression patterns at this stage of postnatal mouse life, which transform anagen VI HF via catagen I-VIII into telogen HF within about 2 days. 20,21 Stage 1 of the hairless phenotype formation was noted in the reference skin area of hr/hr mice on day 14 pp, stages 2–4 on day 15, stages 4–7 on day 16, stages 6–8 on day 17, and stages 7–9 on day 18 (Figures 1B, 1C, and 2) ▶ ▶ . By day 20, hr/hr mice had developed total truncal alopecia (with the exception of the vibrissae), with the characteristic presence of utriculi, numerous epithelial cell clusters in the deep dermis (dermal cyst precursors), and the disappearance of normal HF structures that is observed in adult hr/hr mice. 19 The individual pathological HF transformations in hr skin are described below in detail and are summarized schematically in Figure 2 ▶ .
Figure 2.
Schematic representation of HF disintegration in hr/hr skin. The apparently normal HF at anagen VI stage (day 14 pp) is shown. Its subsequent transformation can be divided into 9 successive stages, as outlined below. 1: The HF in hr/hr skin appear normal. However, the hair canals (hc) are significantly widened as compared to +/hr littermates. 2: The hair shaft (hse) looses its connection with the hair bulb. The follicle above the hair bulb narrows and becomes curved, lacking its normal hair shaft content. 3: The hair bulb shrinks abruptly. The proximal inner root sheath (irs) does not shorten, and therefore terminates below the end of the hair shaft. The hair canals gradually widen further and form ampulliform structures (as). 4: The hair bulb disappears and the dermal papilla (DP) is nearly devoid of a surrounding epithelium, however, it is still encased by the perifollicular connective tissue sheath (cts). The HF portion between the DP and the proximal end of the hair shaft forms a narrow, curved strand of epithelial cells (es). The proximal part of the IRS coalesces around the end of the hair shaft. The club hair (ch) starts to form, however, it displays an abnormally rounded and irregular shape. 5: The epithelial strand begins to break up into separate cell clusters (sr), surrounded by a thin layer of connective tissue sheath. The DP loses its connection with the proximal HF epithelium. A thick and prominent IRS retains the hair shaft in its follicular mooring. 6: The IRS abruptly disintegrates, resulting in rapid upward movement of the hair shaft. The DP remains in the deep dermis, surrounded by a few epithelial cells. 7: The abnormally large and bulbous club hair (ch) that lacks normal spiculae is mechanically retained in the zone of trichilemmal keratinization (tk). 8: Further widening of the pilary canal and complete regression of the ORS allows the hair club to pass through the zone of the trichilemmal comb, and the hair shaft falls out. The characteristic utriculi (u) of hr/hr skin form. 9: Only a small cluster of epithelial cells (putative bulge-derived cells, pbdc) remains in contact with the utricle and arrector pili muscle (m). The sebaceous gland gradually surrounds PBDC clusters to form a circular structure. The epithelial cells that remain in contact with dermal papilla start to proliferate (Ki-67 positivity). as, ampulliform dilatation of hair canal; bg, bulge; ch, club hair; cts, connective tissue sheath; dp, dermal papilla; dpr, dermal papilla remnant; er, epithelial remnant of ORS; es, epithelial strand; hc, hair canal; hs, hair shaft; hse, hair shaft end; irs, inner root sheath; m, arrector pili muscle; ors, outer root sheath; pbdc, putative bulge-derived cells; sg, sebaceous gland; sr, strand remnants; tk trichilemmal keratinization; u, utricle.
Stages of hr HF Transformation
Stage 1
Pelage HF in hr/hr skin appear normal and are largely indistinguishable from normal HF in their final stage of postnatal development (resembling mature anagen VI HF). 28 However, the hair canals in hr/hr skin are significantly widened (Figure 3A) ▶ compared to +/hr littermates (Figure 3B) ▶ . During subsequent stages of HF transformation, hair canals gradually widen further, spreading downward from the upper outer root sheath (ORS) portion adjacent to the epidermis toward the orifice of the sebaceous gland (SG) duct (Figure 3C) ▶ .
Figure 3.
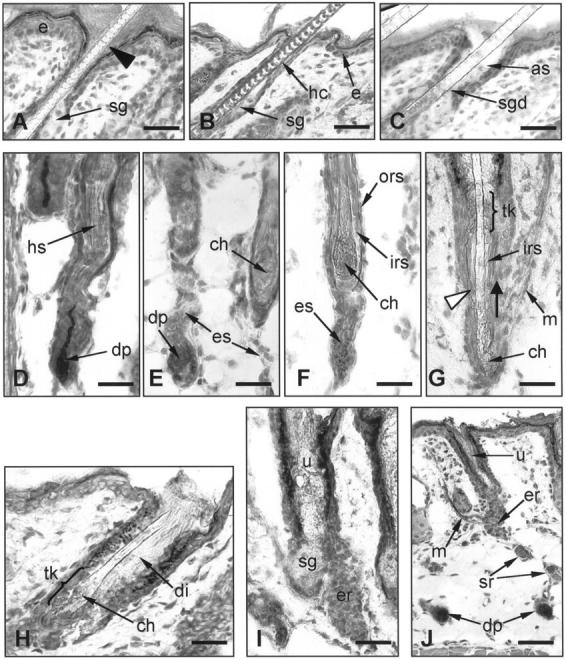
The process of HF disintegration in hr/hr skin. A: The HF infundibulum in hr/hr skin at stage 1 of hairless phenotype formation (day 14 pp). The dilation of the HF infundibulum starts from its most distal portion (arrowhead) adjacent to the epidermis (e). B: The HF infundibulum in +/hr skin (control littermates) at day 15 pp. The hair canal (hc) above the sebaceous gland (sg) is narrow. C: At the stage 3 of hairless phenotype formation (day 15 pp) the dilation of HF infundibulum progresses downward and reaches the entrance of the sebaceous gland duct (sgd). The characteristic ampulliform structure is formed (as). D: hr/hr skin at day 15 pp (stage 2). The hair shaft (hs) loses its connection with the hair bulb and becomes loose. The IRS does not undergo normal shortening. The ORS portion just above the hair bulb narrows, and lacks its normal hair shaft content. E: hr/hr skin at day 15 pp (stages 3–5). The hair bulb shrinks abruptly. The HF portion between the DP and the proximal end of the hair shaft forms a narrow and curved strand of epithelial cells (es) which begins to disintegrate. The proximal part of the IRS coalesces around the end of the hair shaft. A rounded, abnormally large and bulbous club hair (ch) is formed. F: The IRS around the abnormal club hair (ch) prevents any possibility of direct contact with the ORS. G: The IRS abruptly disintegrates (stage 6) starting from its most proximal portion (white arrowhead) . Lacking any anchoring, the hair shaft starts to slip upward (bold arrow). H: Abnormal club hair is mechanically retained by a comb-like ORS structure in the zone of trichilemmal keratinization (tk). The dilation of the HF infundibulum (di) reaches its maximum. I: The shedding of the hair shaft results in formation of a utricle (u). The rest of the epithelial sheath disintegrates rapidly and only a small epithelial remnant (er) is visible close to the sebaceous gland (sg). J: The dermal papillae (dp) remain stranded in the deep dermis. The epithelial HF remnant (er) which contains the putative bulge derived cells is still associated with the arrector pili muscle (m). e, epidermis; sg, sebaceous gland; sgd, sebaceous gland duct; hs, hair shaft; dp, dermal papilla; ch, club hair; es, epithelial strand; irs, inner root sheath; ors, outer root sheath; tk, zone of trichilemmal keratinization; m, arrector pili muscle; uc, utricular cavity; er, epithelial HF remnants; sr, remnants of disintegrated epithelial strand. Scale bars: A-I, 23 μm; J, 50 μm. D and J, AP histochemistry; all others, H&E.
Stage 2
The bulb of hr/hr HF is approximately of equal size and shape as in stage 1. However, the hair shaft looses its connection with the hair bulb. The follicle above the hair bulb narrows and becomes curved, lacking its normal hair shaft content (Figure 3D) ▶ .
Stage 3
The hair bulb shrinks abruptly and the proximal end of the hair shaft (near the subcutis) becomes loose. Unlike during normal catagen development, 20,23 the proximal inner root sheath (IRS) does not shorten and therefore terminates below the end of the hair shaft.
Stage 4
The hair bulb gradually disappears and the DP has almost no surrounding epithelium. However, the DP is still ensheathed by the perifollicular connective tissue sheath. In contrast to normal catagen development in +/hr mice (Figure 4A) ▶ , the proximal end of the IRS in hr/hr skin does not shorten. Instead, it coalesces around the end of the hair shaft, thereby obstructing any direct contact between the hair shaft and the ORS (Figure 3F) ▶ . The club hair starts to form, but displays an abnormally rounded and irregular shape (Figure 3, E and F) ▶ while the normal club hairs in +/hr mice have a serrated appearance at the corresponding stage of HF regression (day 18–20 pp) (Figure 4A) ▶ . The HF portion between the DP and the proximal end of the hair shaft in hr/hr skin forms a narrow, curved strand of epithelial cells (Figure 3E) ▶ . In striking contrast to the HF of +/hr mice at the comparable stage of catagen development (catagen VI-VIII of the normal mouse hair cycle 20 ), this strand of the epithelial cells in hr/hr skin fails to shorten (Figures 3, D and F, and 4B) ▶ ▶ .
Figure 4.
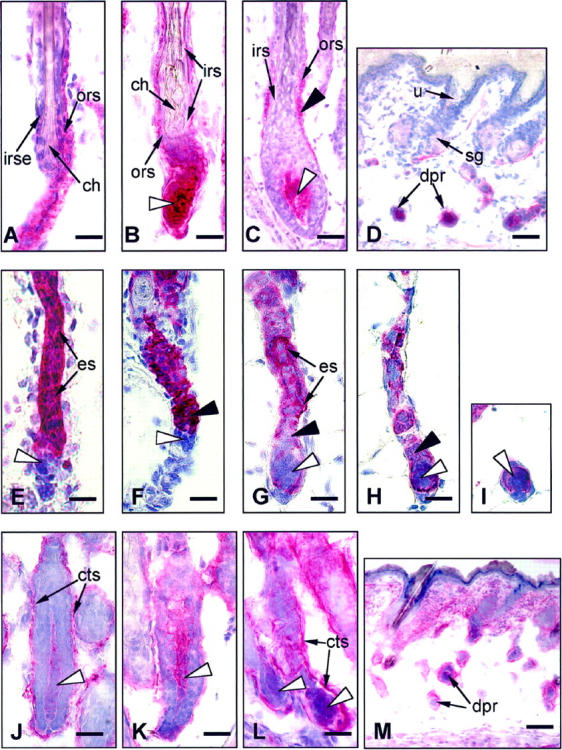
Alkaline phosphatase (A-D), IL-1R1 (E-I), and NCAM (J-M) reactivity in the skin of HRS/J hr/hr mice. A: In +/hr control skin at the middle catagen stage of HF regression (day 18 pp), the proximal IRS is significantly shortened, thus allowing direct contact between the ORS and the club hair (ch). The club hair has a serrated appearance, which provides the proper anchoring of the hair shaft in the epithelial mooring. B: hr/hr skin at day 15 pp (stage 3). The proximal part of the IRS coalesces around the end of the hair shaft. A rounded, abnormal club hair is formed. Dermal papilla (white arrowhead) is constantly AP-positive. C: The AP reactivity (black arrowhead) in the proximal ORS during initiation of hr/hr HF transformation (day 14 pp). Dermal papilla, white arrowhead. D: Dermal papilla remnants (dpr) remain stranded in the dermis after completion of HF disintegration and retain their strong AP-positivity. E: In the skin of heterozygous +/hr mice, strong IL-1R1 immunoreactivity was seen in the epithelial cells of the regressing ORS (es) throughout catagen phase (dermal papilla, white arrowhead). F: With progression of epithelial strand contraction in +/hr skin, the most intense Il-1R1 expression is seen in the portion of the epithelial strand (black arrowhead) directly adjacent to the distal pole of the dermal papilla (white arrowhead), which was always IL-1R1-negative. G: In the skin of homozygous hr/hr mice, the HF entry into hr transformation was associated with a prominent decline of IL-1R1 expression in the epithelial strand (es). The portion adjacent to the dermal papilla displays weak IL-1R1 positivity or negativity (black arrowhead). H: At stages 4 and 5 of hr/hr HF transformation, the keratinocytes of the epithelial strand located just above the dermal papilla become completely IL-1R1-negative (black arrowhead), while the dermal papilla (white arrowhead) retains the thin IL-1R1-positive epithelial sheath more prominently around its proximal pole. I: On days 19–20 of hr/hr HF transformation, when dermal papilla remnants turn into the amorphous ball-shaped structures, the associated IL-1R1 immunoreactivity lost its peripheral localization and was also seen inside these keratinocyte and fibroblast-containing cell clusters (white arrowhead). J: NCAM expression in the dermal papilla (white arrowhead) of hr/hr skin at day 14 pp (stage 1) is weak, while NCAM immunoreactivity in the perifollicular connective tissue sheath (cts) is prominent. K: In +/hr control skin during early catagen, NCAM immunoreactivity in the dermal papilla is more prominent compared to the corresponding stage of HF transformation in hr/hr skin (stage 1). L: At later stages of hr/hr HF transformation (stages 4–5), NCAM expression in the DP remains low, while in the connective tissue sheath (cts) it is very prominent. M: The dermal papilla remnants (dpr) stranded in the deep dermis after the completion of hairless phenotype formation are surrounded by an NCAM-positive connective tissue sheath. irse, inner root sheath end; cts, perifollicular connective tissue sheath; other legends same as Figure 3 ▶ . Scale bars: A-C, E-L, 23 μm; D and M, 50 μm.
Stage 5
The epithelial strand begins to break up into separate cell clusters, surrounded by a thin layer of connective tissue sheath (Figure 4H) ▶ . The DP loses its connection with the proximal HF epithelium. Almost no ORS is left in the middle HF portion, and only a thin single layer of ORS remnant surrounds a thick and prominent IRS as the only structure to retain the hair shaft in its follicular mooring.
Stage 6
The IRS abruptly disintegrates, starting from its lower end (Figure 3G) ▶ . The hair shaft moves rapidly upward because it is no longer anchored by the IRS. The DP remains in the deep dermis, surrounded by a few epithelial cells (Figures 3J and 4I) ▶ ▶ .
Stage 7
The abnormally large and bulbous hair club that lacks normal spiculae is mechanically retained in the distal HF portion (more distant from the subcutis) by a keratinous, comb-like ORS structure in the zone of trichilemmal keratinization which tightly embraces the hair shaft (Figure 3, G and H) ▶ .
Stage 8
Further widening of the pilary canal and regression of the ORS allows the hair club to pass through the zone of the trichilemmal comb, and the hair shaft falls out. In this stage, the characteristic utriculi of hr/hr skin 5,19 form (Figure 3, I and J) ▶ , which do not develop in +/hr skin. The remainder of the HF epithelial sheath forms a gradually disintegrating mass of epithelial cells positioned on one side of the utricle’s proximal pole. The SG, which retains its normal structure, is positioned on the opposite side of the utricle (Figure 3I) ▶ .
Stage 9
Only a small cluster of keratinocytes from the disintegrated HF remains in contact with the proximal portion of the utricle (Figure 3J) ▶ . Because of the structure and position of these cell clusters, they are referred to as the putative bulge-derived cells (PBDC), previously described in the skin of adult hr/hr mice. 19 At stage 9, the lower portion of these PBDC clusters remains in contact with the arrector pili muscle, which changes neither its shape nor its position despite the dramatic changes of HF morphology in hr/hr skin (Figure 3J) ▶ . As the PBDC become separated from the degenerating ORS and are located on the lower utricular pole, the SG gradually surrounds the PBDC clusters to form a circular structure. Some ORS and DP cell remnants are present in the dermis as separate, ball-shaped spheres, composed of epithelial and fibroblast-like cells (Figures 3J and 4D) ▶ ▶ .
Alkaline Phosphatase (AP) Activity
In addition to the arrector pili muscle, which is positive for AP activity in normal mouse skin, 27 positive AP staining was consistently observed in hr/hr DP during all stages of HF regression and disintegration (Figure 4, B ▶ -D). Starting at stages 1–2, weak AP positivity is also seen in the central ORS (Figure 4C) ▶ , where it gradually increases until the onset of ORS disintegration (stage 5). At stages 5–7, this AP reactivity is seen in epithelial cell clusters originating from the middle ORS portion, but disappears thereafter (note that the central ORS of normal mouse HF is never AP-positive 27 ). DP remnants stranded in the deep dermis after completion of HF disintegration are histochemically recognizable by their strong and consistent AP activity (Figure 4D) ▶ .
IL-1R1 Expression
We have previously used IL-1R1 expression as a reliable marker for ORS and hair bulb keratinocytes. 26 In heterozygous +/hr mice with apparently normal hairs, strong IL-1R1 immunoreactivity is seen in the epithelial cells of the regressing ORS throughout the entire catagen phase (Figure 4E) ▶ with the highest intensity of expression in the portion of the regressing epithelial strand directly adjacent to the distal (upper) pole of the DP (Figure 4F) ▶ . The DP itself is always IL-1R1-negative. This corresponds to the IL-1R1 expression pattern observed in normal mouse HF. 26 In homozygous hr/hr mice, however, the HF entry into hr transformation is associated with a prominent decline in the intensity and distribution of IL-1R1 immunoreactivity in the proximal ORS, and consequently, in the epithelial strand (Figure 4G) ▶ , as compared to +/hr or to normal C57BL/6 mice. 26 At stage 4 of hairless phenotype development, the keratinocytes of the epithelial strand located just above the DP exhibit a prominent decline in IL-1R1 expression (Figure 4, G and H) ▶ , whereas the DP retains a thin, IL-1R1-positive epithelial sheath that is most prominent around its proximal pole, near the subcutis (Figure 4H) ▶ . Later (on days 19–20 pp), when the DP remnants in hr/hr skin become isolated ball-shaped structures (stage 9), the associated IL-1R1 immunoreactivity loses its peripheral localization and is also seen inside these keratinocyte- and fibroblast-containing cell clusters (Figure 4I) ▶ . At this point, it becomes difficult to distinguish epithelium and mesenchyme in these structures using this marker.
NCAM Expression
In addition to NCAM-positive nerve fibers of mouse skin, 25,29 at stage 1 of hr/hr HF transformation (day 14 pp) NCAM immunoreactivity is also seen in the DP. A thin layer of the connective tissue sheath is also NCAM-positive. This corresponds well with the NCAM expression patterns reported for normal mouse HF. 25,30 However, by stage 2, NCAM immunoreactivity declines in the DP (Figure 4J) ▶ of hr/hr HF compared to +/hr DP (Figure 4K) ▶ , where it remains stable during the entire catagen-associated HF transformation. Whereas the NCAM expression in DP of hr/hr skin nearly ceased by stage 4 of HF transformation, the perifollicular connective tissue sheath of hr/hr HF exhibited a stable NCAM immunoreactivity (Figure 4L) ▶ . This expression pattern was seen not only around the epithelial strand (stages 3–4), but also around remnants of the disintegrating ORS, until the end of hairless phenotype development (stages 6–9). DP fibroblasts stranded in the dermis were always surrounded by a thin NCAM-positive connective tissue layer (Figure 4M) ▶ .
Patterns of Proliferation and Apoptosis
The patterns of intrafollicular proliferation and apoptosis during HF transformation were compared between hr/hr and +/hr mice using a TUNEL/Hoechst33343/Ki-67 triple stain. At day 14 pp, all follicles in the skin of hr/hr and +/hr pups had completed HF morphogenesis and displayed the morphological signs of a mature anagen VI follicle. 28,31 In accordance with this finding, strong Ki-67 staining in the hair matrix indicated the presence of normal-appearing proliferative activity in the HF of both genotypes (not shown). However, TUNEL stains revealed strikingly different patterns of apoptosis between hr/hr and +/hr mice. Notably, hr/hr mutants at this stage of HF transformation (stage 1) displayed TUNEL-positive cells in the hair matrix of approximately 7% of HF (1–3 positive cells in every TUNEL-positive hair bulb) while no TUNEL-positive cells were seen in the hair matrix of +/hr skin. On day 14 pp, the DP was TUNEL-negative in both hr/hr and hr/+ mice. Note that mature anagen VI hair bulbs of adolescent C57BL/6J mice are devoid of TUNEL-positive cells, and that like in +/hr mice, normal C57BL/6 mice never display TUNEL-positive cells in the hair matrix at day 14 pp. 23
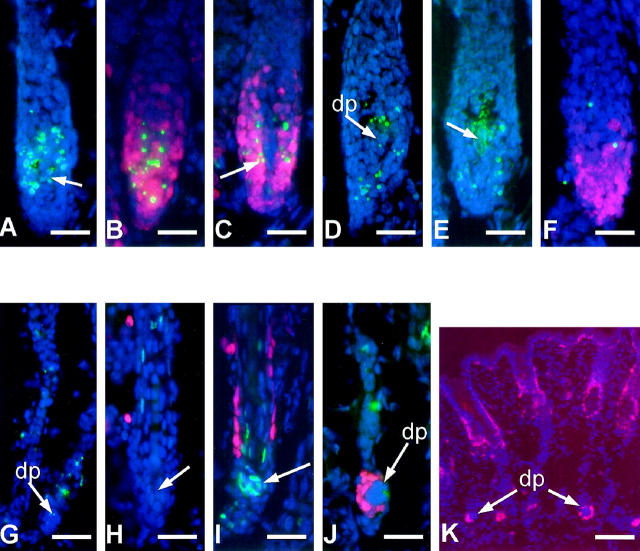
On day 15 pp, no changes in TUNEL or Ki-67 expression were found in +/hr skin compared to the preceding day. In contrast, hr/hr skin displayed a massive, premature apoptosis in the bulb region of nearly all HF (about 90%) at stage 2 of hairless phenotype development (Figure 5A) ▶ . Strikingly, this burst of programmed epithelial cell death in the hr/hr hair bulb was accompanied by a high rate of cell proliferation in the same HF region (Figure 5B) ▶ . In every follicle, on average about 20% (range, 10–50%) of matrix cells were TUNEL-positive. In individual cells, a colocalization of TUNEL-positive apoptotic bodies and Ki-67 IR was observed (Figure 5C) ▶ . Interestingly, on day 15 pp, some TUNEL-positive DP cells were observed as well (Figure 5, A and D) ▶ . In a minority of HF, even massive DP apoptosis was detected (Figure 5E) ▶ . In the skin of +/hr pups, DP fibroblasts were always TUNEL-negative, exactly as we had shown throughout normal mouse catagen development. 23
Figure 5.
TUNEL stain and Ki-67 immunoreactivity in hr/hr and +/hr HF. A: Premature and excessive apoptosis (apoptotic bodies in green color) in the hair matrix of hr/hr skin at day 15 pp (stage 1 of hr/hr HF transformation). Dermal papilla, white arrow. B: The apoptosis takes place in the same region of the hair matrix where the high level of proliferation is detected using Ki-67 immunostaining (red color) as a proliferation marker. C: In some cells, the TUNEL positivity colocalizes with Ki-67 expression (arrow). D: On day 15 pp, some TUNEL-positive DP cells are observed as well. E: In a minority of HF, massive apoptosis was seen in the DP (arrow). F: In +/hr control skin, the first TUNEL-positive cells in the proximal HF were detected at day 17 pp, after the prominent decrease of proliferative activity. This TUNEL-positivity was never as prominent as during the early stages of hr/hr HF transformation. G: Stages 4–5 of HF transformation in hr/hr skin. The DP is Ki-67- and TUNEL-negative. Some TUNEL-positive cells are present in the epithelial strand indicating the normal wave of intrafollicular apoptosis. H: There is no TUNEL positivity in the hair club region of hr/hr skin during HF disintegration. I: TUNEL-positive cells (arrow) in the hair club of +/hr HF in the corresponding stage of catagen transformation. J: At stage 6 of hr/hr HF transformation, the epithelial cells adjacent to the DP remnants start to proliferate. K: In the final stages (8–9) of hr/hr HF transformation, all epithelial cells that remain associated with the detached dermal papilla are proliferating, as confirmed by their active Ki-67 expression. Scale bars: A-H, 23 μm; I-J, 35 μm; K, 50 μm.
In contrast to hr/hr skin, +/hr follicles displayed the first catagen-associated, occasional TUNEL-positive cells only after a prominent decline of Ki-67 expression in the distal hair matrix cells (Figure 5F) ▶ . These normal TUNEL-positive cells later spread over the involuting proximal +/hr HF portion, much like during normal catagen development in C57BL/6J mice, 23 but never became as abundant (not more than 5–8 TUNEL-positive cells per follicle) as during the premature, excessive, and ectopic apoptosis observed in hr/hr skin. Because the onset of apoptosis-driven HF regression is normally preceded by and associated with a sharp decline in proliferative activity in the hair bulb, 4 (Paus et al, unpublished observations) this indicates a dramatic failure of cell cycle and apoptosis coordination in hair bulb keratinocytes of hr/hr mice.
The switch from stage 2 to stage 3 of hairless phenotype development in hr/hr skin around day 15 pp occurs extremely rapidly, and was accompanied by a significant decline in the number of TUNEL-positive cells in the hair matrix. Ki-67 immunoreactivity in the hair matrix of hr/hr HF declined as well, though not quite as abruptly. Some Ki-67-positive cells were detected in the remnants of the involuting hair matrix of hr/hr HF even at stage 3 of HF transformation (not shown).
At stage 4 of hairless phenotype development (day 16 pp), after a short period of virtual absence, TUNEL-positive cells reappeared in the strand of epithelial cells located between the DP and the distal portion of hr/hr HF (Figure 5G) ▶ . On the one hand, these cells suggested the presence of a second wave of intrafollicular apoptosis in hr/hr skin which corresponded well to the expected pattern and location of normal, catagen-associated apoptosis in this HF region. 23,32 On the other hand, however, hr/hr skin failed to display any TUNEL-positive cells in the region of club hair formation (Figure 5H) ▶ . This is in striking contrast to catagen HF in +/hr (Figure 5I) ▶ or C57BL/6 mice, 23 and further demonstrates that the absence of a functional hr gene product causes severe disturbances in normal catagen-associated HF transformation. 23,32,33
During the final stage of hr/hr HF transformation (stage 9), the epithelial cells that remained associated with the detached DP remnants were Ki-67-positive, suggesting a high proliferative activity in this ectopic epithelial compartment of hr/hr skin (Figure 5, J and K) ▶ . The epithelial nature of these cells was confirmed by their expression of IL-1R1 (Figure 4I) ▶ , which is a useful and reliable marker for distinguishing HF keratinocytes from the follicle-associated mesenchyme. 26
Discussion
This study provides a detailed analysis of the hairless mutation in mice as a model to study the role of the hr gene product in normal catagen control. We show that the development of the hr/hr phenotype (summarized in Figure 2 ▶ ) is heralded by a premature, massive, and ectopic up-regulation of intrafollicular apoptosis in defined HF compartments, accompanied by a loss of coordination with cell proliferation. This may lead to malpositioning of the proximal IRS, ORS, and hair cortex and consequent failure of trichilemmal keratinization in the club hair zone. The epithelial strand of hr/hr skin is not able to undergo its normal contraction during late catagen, and instead disintegrates into separate cell clusters, while DP fibroblasts remain stranded in the dermis (Figures 3J and 4 ▶ ▶ , D, H, and M). Thus, by the time the hr/hr HF should initiate its lifelong cycling activity by entry into the first catagen, it has already been essentially destroyed.
We demonstrate that widening of the pilary canal is the first morphological difference between hr/hr and +/hr infantile mice. This widening starts from the most distal (adjacent to the epidermis) region of the suprainfundibular ORS (Figure 3A) ▶ , which exhibits an epidermal type of keratinization. 34 This may reflect hr mutation-related abnormalities in the interfollicular epidermis rather than in the HF itself, which actually displays normal morphological features at that time. hr mRNA expression has been reported not only in the HF, but also in the interfollicular epidermis 12 .
The very brief yet dramatic up-regulation of apoptosis in the hair matrix of hr/hr mutant skin reported here (Figure 5, A and B) ▶ offers a completely novel and profound insight into hr biology. The onset of normal catagen is preceded by a cessation of mitotic activity in the hair matrix; 20,35 therefore, stage 2 of hr/hr HF transformation, which is characterized by very high proliferative activity of matrix cells, cannot be accurately referred to as an equivalent of early catagen. Although the first TUNEL-positive cells in the +/hr hair matrix were observed only after the cessation of proliferative activity (after the onset of catagen), in the hair matrix of hr/hr skin, massive apoptosis coincides with proliferation, and thus occurs prematurely in a late anagen VI-like stage.
Furthermore, in several matrix cells, the co-localization of Ki-67 IR and TUNEL-positive apoptotic bodies was evident (Figure 5C) ▶ , suggesting that some follicle keratinocytes have begun apoptosis during or immediately after cell division, without entering into the G0 phase of the cell cycle. This might imply that hr is involved in coordinating the expression of genes required for the gradual switch from proliferation to apoptosis in defined matrix cell populations. Inactivation of the hr gene due to a mutation in the hr locus may invoke a dysregulation in this apparently tightly controlled system. 1-4 Thus, the normal hr gene product may not be a catagen repressor, but rather an essential regulator of the earliest events associated with normal HF regression, during which the stepwise down-regulation of matrix cell proliferation must be carefully coordinated with an up-regulation of apoptosis in selected matrix cells.
The presence of TUNEL-positive cells (Figure 5, A, D, and E) ▶ and of declining NCAM expression (Figure 4, J and L) ▶ in the DP of hr/hr skin at the early stages of hairless phenotype development is very unusual. Normal DP cells never show signs of apoptosis during any hair cycle stage, not even after chemotherapy, 23 and they express NCAM protein permanently. 25,30,36 In fact, NCAM expression may be a key topobiological feature linking DP fibroblasts to a unique mesenchymal cell population with special secretory properties. 37 Thus, DP cells are also severely affected by the hr mutation, possibly as the result of alterations in the local signaling milieu that controls apoptosis and cell adhesion in the DP. This could result a decline in an as yet elusive secretory DP activities that may be important for the orderly development of HF regression, thus further disrupting the controls of HF apoptosis and topobiology operative during normal catagen.
The formation of an excessively large and bulbous hair club without spiculae that cannot anchor the hair shaft in hr/hr HF is associated with malpositioning of the proximal inner root sheath (IRS). Specific, spicula-like anchoring structures are formed by the interdigitation of club cells and transformed ORS keratinocytes, 35 which undergo trichilemmal keratinization. 38 This type of keratinization is also characteristic for the isthmus portion of the HF, where it forms specific comb-like structures resembling the serrated surface of the hair club. 38,39 The IRS is able to suppress the trichilemmal keratinization of ORS cells in the isthmus region where this type of keratinization occurs only above the point where the IRS sloughs into the pilary canal. 38 It is reasonable to invoke this model of IRS-ORS interactions in the isthmus for the process of hair club formation, where trichilemmal keratinization is also the main mechanism. The interdigitation of club cells and ORS keratinocytes in normal mouse HF is most likely regulated by hair club-ORS intersignaling that may be interrupted in the skin of hr/hr mice, where the IRS collapsed around the proximal end of the hair shaft.
During early catagen in apparently normal HRS/J +/hr mice, the proximal IRS loses its connection with the hair matrix and is significantly shortened before the termination of hair shaft production, thus allowing for direct contact between the most proximal hair cortex (which is even free of the cuticle at this stage of catagen 35 ) and surrounding ORS cells. This may initiate the trichilemmal keratinization in the zone of contact (Figure 4A) ▶ . In contrast, in hr/hr skin, hair shaft production is terminated much earlier than IRS formation (Figure 3D) ▶ , resulting in IRS malpositioning and its consequent coalescence around the hair shaft end (Figures 3F and 4B) ▶ ▶ . This temporal disturbance may be attributed to abnormal and premature apoptosis in the hair matrix of hr/hr skin described above.
This suggestion is supported by observations that the pharmacological suppression of matrix cell proliferation during the late anagen stage with colchicine results in IRS malposition, formation of abnormal hair club, and hair loss in normally haired C57BL/6J inbred mice. 6 Thus, our findings not only illuminate one reason for the failure of trichilemmal keratinization during catagen in hr/hr HF, but also provide further insight into the normal mechanisms of club hair formation.
That the DP becomes stranded in the reticular dermis of hr skin is one of the most intriguing consequences of the hairless mutation. 5,19 The mechanisms of the upward DP movement during normal catagen remain largely unknown. 40,41 Yet this is clearly a key process to ensure the perpetuation of mesenchymal-epithelial contact and signaling during the hair cycle. Our observation in hr/hr mice suggests that the absence of proper hair germ-hair club signals, and/or apoptosis and cell adhesion abnormalities in the DP itself, may be related to the failure of the DP to move upwards. The epithelial strand portion directly adjacent to the DP and characterized by elevated level of IL-1R1 expression during catagen (may in normally haired +/hr skin) also be involved in retaining the DPs association with the HF, since in hr/hr mice this portion of epithelial strand is characterized by suppression of IL-1R1 immunoreactivity (Figure 4, G and H) ▶ . IL-1R1 is known as a potent regulator of ICAM-1, 42 which in turn is implicated in regulation of adhesion and proliferation of skin lymphocytes and follicular keratinocytes 43,44 and is associated with some HF pathological conditions in humans, such as alopecia areata. 45,46 These results further support the idea that hairless mice are an attractive model for elucidating the molecular controls of normal and pathological catagen-associated DP movement.
The Ki-67 antigen expression detected in the epithelial cells which remained associated with the DP remnants at stages 7–8 of hr/hr HF transformation suggests that these epithelial cell clusters begin to proliferate (Figure 5J) ▶ despite the disintegration of hr/hr HF at this stage. It is of interest that other isolated remnants of the proximal ORS, which did not contain DP fibroblasts, displayed no proliferative activity. These fibroblast-associated epithelial cell clusters are most likely the source of AP-positive dermal cysts in mature hr/hr skin. 19 They may also be involved in the induction of the second wave of hair growth, which takes place in hr/hr mice during the 6th and 7th weeks of life 47 and results in sparse and abnormal hairs. The proliferative capacity of remnants of the follicular epithelium that remain associated with DP-derived fibroblasts further supports an inductive role of the DP in the stimulation of epithelial cell proliferation 41,48 and underscores the utility of hr/hr mice as a unique model for dissecting the factors governing the control of HF cycling. 10,19
The current studies confirm and extend our previous hypothesis that the hr mutation in mice is associated with total disintegration of the ORS below the SG duct, including the isthmus region. 19 Only a small cluster of ORS epithelial cells retains its integrity. Most likely, this represents a remnant of the bulge, believed to be one site of epithelial stem cells in the HF, 49 based on their location (association with arrector pili muscle (Figure 3J) ▶ and palisade nerve fibers), keratin 17 expression, and their ability to produce columnar epithelial outgrowths. 19
Previously, several mechanisms have been proposed as the immediate cause of hair shedding in hr/hr mice, including widening of the pilary canal, 50 improper hair club formation, 6,8 and disintegration of the permanent (isthmus) portion of the ORS. 19 According to our current findings, each of these mechanisms seems to participate in the shedding of hairs in hr/hr skin, as the end result of a long chain of events that begins with a dramatic and premature up-regulation of apoptosis in the hair matrix, a failure to coordinate HF keratinocyte proliferation and apoptosis, and the disruption of the normal adhesion milieu of the HF. This is followed by IRS malpositioning and improper formation of club hairs, which are not sufficiently anchored in the ORS, but simply embedded into a poorly-organized, degenerating IRS that fails to undergo its normal shortening. 20,21
Despite the evidence that the lower 3/4 of the HF involute in a stringently controlled manner during catagen, 21,35,51 it is still unclear exactly how this process is regulated. The HF degeneration in mice carrying a partial loss-of-function mutation in the hr locus provides an attractive model for further dissection of cellular and molecular controls of HF cycling, especially the transcriptional controls of catagen, in which the hr gene product appears to play a pivotal role. 10,19 It is reasonable to predict that a deeper understanding of exactly how this putative transcription factor regulates apoptosis, proliferation, and cell adhesion changes during each normal HF regression will facilitate the development of novel therapies that target the control of catagen as the most relevant clinical problem of hair biology. 1,2
Acknowledgments
We thank Evelin Hagen and Ruth Pliet for excellent technical assistance and Carina van der Veen for help with manuscript preparation.
Footnotes
Address reprint requests to Ralf Paus, M.D., Dept. of Dermatology, University Hospital Eppendorf, University of Hamburg Martinistrasse 52, D-20246, Hamburg, Germany. E-mail: paus@uke.uni-hamburg.de.
Supported in part by grants from Wella AG Darmstadt and from Deutsche Forschungsgemeinschaft (Pa 345/8–1, to R. P.), the National Cancer Institute (CA 34196, to J. P. S.), and the National Alopecia Areata Foundation (to A. M. C.). A. A. P. also is grateful to Drs. L. Kligman and A. M. Kligman (Foundation for Basic Cutaneous Research) for their partial financial support of his work.
References
- 1.Paus R: Control of the hair cycle and hair diseases as cycling disorders. Curr Opin Dermatol 1996, 3:248-258 [Google Scholar]
- 2.Paus R: Principles of hair cycle control. J Dermatol 1998, 25:793-802 [DOI] [PubMed] [Google Scholar]
- 3.Stenn KS, Combates NJ, Eilertsen KJ, Gordon JS, Pardinas JR, Parimoo S, Prouty SM: Hair follicle growth controls. Dermatol Clinics 1996, 14:543-558 [DOI] [PubMed] [Google Scholar]
- 4.Stenn K, Parimoo S, Prouty S: Growth of the hair follicle: a cycling and regenerating biological system. Chuong C-M eds. Molecular basis of epithelial appendage morphogenesis. 1998, :pp 111-130 RG Landes Company, Austin, TX, [Google Scholar]
- 5.Montagna W, Chase HB, Melaragno HP: The skin of hairless mice. I. The formation of cysts and the distribution of lipids. J Invest Dermatol 1952, 19:83-94 [DOI] [PubMed] [Google Scholar]
- 6.Mann SJ: Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec 1971, 170:485-500 [DOI] [PubMed] [Google Scholar]
- 7.Sundberg JP, Dunstan RW, Compton JG: Hairless mouse. HRS/J hr/hr. Monographs on Pathology of Laboratory Animals: Integument and Mammary Glands. Edited by TC Jones, U Mohr, R Hunt. Heidelberg, Springer-Verlag, 1989, pp 192–197
- 8.David LT: Studies on the expression of genetic hairlessness in the house mouse (Mus musculus). J Exp Zool 1934, 68:501-518 [Google Scholar]
- 9.Ahmad W, ul Haque MF, Brancolini V, Tsou HC, ul Haque S, Lam HM, Aita VM, Owen J, deBlaquire M, Frank J, Cserhalmi-Friedman PB, Leask A, McGrath JA, Peacocke M, Ahmad M, Ott J, Christiano AM: Alopecia universalis associated with a mutation in the human hairless gene. Science 1998, 279:720-724 [DOI] [PubMed] [Google Scholar]
- 10.Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM: Molecular and functional aspects of the hairless (hr) gene in laboratory rodents and humans. Exp Dermatol 1998, 7:249-267 [DOI] [PubMed] [Google Scholar]
- 11.Sundberg JP: The hairless and rhino mutations, chromosome 14. Sundberg JP eds. Handbook of Mouse Mutations with Skin and Hair Abnormalities. 1994, :pp 291-312 CRC Press, Boca Raton [Google Scholar]
- 12.Cachon-Gonzalez MB, Fenner S, Coffin JM, Moran C, Best S, Stoye JP: Structure and expression of the hairless gene of mice. Proc Natl Acad Sci USA 1994, 91:7717-7721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson CC: Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci 1996, 16:7832-7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snell GD: Inheritance in the house mouse, the linkage relations of short-ear, hairless, and naked. Genetics 1931, 16:42-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoye JP, Fenner S, Greenoak GE, Moran C, Coffin JM: Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell 1988, 54:383-391 [DOI] [PubMed] [Google Scholar]
- 16.Ahmad W, Irvine AD, Lam H, Buckley C, Bingham EA, Panteleyev AA, Ahmad M, McGrath JA, Christiano AM: A missense mutation in the zinc-finger domain of the human hairless gene underlies congenital atrichia in a family of Irish travellers. Am J Hum Genet 1998, 63:984-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zlotogorski A, Ahmad W, Christiano AM: Congenital atrichia in five Arab Palestinian families resulting from a deletion mutation in the human hairless gene. Hum Genet 1998, 103:400-404 [DOI] [PubMed] [Google Scholar]
- 18.Ahmad W, Zlotogorski A, Panteleyev AA, Lam HM, Ahmad M, ul Haque MF, Abdallah HM, Dragan L, Christiano AM: Genomic organization of the human gene (HR) and identification of a mutation underlying congenital atrichia in an Arab Palestinian family. Genomics 1999, 56:141-148 [DOI] [PubMed] [Google Scholar]
- 19.Panteleyev AA, van der Veen C, Rosenbach T, Sokolov VE, Müller-Röver S, Paus R: Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol 1998, 110:902-907 [DOI] [PubMed] [Google Scholar]
- 20.Straile WE, Chase HB, Arsenault C: Growth and differentiation of hair follicles between periods of activity and quiescence. J Exp Zool 1961, 148:205-216 [DOI] [PubMed] [Google Scholar]
- 21.Paus R, Handjiski B, Czarnetzki BM, Eichmüller S: A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol 1994, 103:143-147 [DOI] [PubMed] [Google Scholar]
- 22.Howard A: “Rhino”, an allele of hairless in the mouse. J Hered 1940, 31:467-470 [Google Scholar]
- 23.Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R: Analysis of apoptosis during hair follicle regression. Am J Pathol 1997, 151:1601-1617 [PMC free article] [PubMed] [Google Scholar]
- 24.Paus R, Hofmann U, Eichmüller S, Czarnetzki BM: Distribution and changing density of γ-delta T cells in murine skin during the induced hair cycle. Br J Dermatol 1994, 130:281-289 [DOI] [PubMed] [Google Scholar]
- 25.Müller-Röver S, Peters EJM, Botchkarev VA, Panteleyev AA, Paus R: Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J Histochem Cytochem 1998, 46:1-9 [DOI] [PubMed] [Google Scholar]
- 26.Eichmüller S, van der Veen C, Moll I, Hermes B, Hofmann U, Müller-Röver S, Paus R: Clusters of perifollicular macrophages in normal murine skin: physiological degeneration of selected hair follicles by programmed organ deletion. J Histochem Cytochem 1998, 46:361-370 [DOI] [PubMed] [Google Scholar]
- 27.Handjiski B, Eichmüller S, Hofmann U, Czarnetzki BM, Paus R: Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol 1994, 131:303-310 [DOI] [PubMed] [Google Scholar]
- 28.Chase HB: Growth of the hair. Physiol Rev 1954, 34:113-126 [DOI] [PubMed] [Google Scholar]
- 29.Botchkarev VA, Eichmüller S, Johansson O, Paus R: Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J Comp Neurol 1997, 386:379-395 [DOI] [PubMed] [Google Scholar]
- 30.Combates NJ, Chuong C-M, Stenn KS, Prouty SM: Expression of two Ig family adhesion molecules in the murine hair cycle: DDC in the bulge epithelia and NCAM in the follicular papilla. J Invest Dermatol 1997, 109:672-678 [DOI] [PubMed] [Google Scholar]
- 31.Philpott M, Paus R: Principles of hair follicle morphogenesis. Chuong C-M eds. Molecular Basis of Epithelial Appendage Morphogenesis. 1998, :pp 75-110 RG Landes Company, Austin, TX, [Google Scholar]
- 32.Cotsarelis G: The hair follicle: dying for attention. Am J Pathol 1997, 151:1505-1509 [PMC free article] [PubMed] [Google Scholar]
- 33.Paus R, Rosenbach T, Haas N, Czarnetzki BM: Patterns of cell death: the significance of apoptosis for dermatology. Exp Dermatol 1993, 2:3-11 [DOI] [PubMed] [Google Scholar]
- 34.Kopan R, Fuchs E: A new look into an old problem: keratins as tools to investigate determination, morphogenesis, and differentiation in skin. Genes Dev 1989, 3:1-15 [DOI] [PubMed] [Google Scholar]
- 35.Parakkal PF: Morphogenesis of the hair follicle during catagen. Z Zellforsch 1970, 107:174-186 [DOI] [PubMed] [Google Scholar]
- 36.Hardy MH, Vielkind U: Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 1. Follicle morphogenesis in wild-type mice. Acta Anat (Basel) 1996, 157:169-182 [DOI] [PubMed] [Google Scholar]
- 37.Müller-Röver S, Paus R: Topobiology of the hair follicle: adhesion molecules as morphoregulatory signals during hair follicle morphogenesis. Chuong C-M eds. Molecular Basis of Epithelial Appendage Morphogenesis. 1998, :pp 283-314 RG Landes Company, Austin, TX, [Google Scholar]
- 38.Pinkus H, Iwasaki T, Mishima Y: Outer root sheath keratinization in anagen and catagen of the mammalian hair follicle: a seventh distinct type of keratinization in the hair follicle, trichilemmal keratinization. J Anat 1981, 133:19-35 [PMC free article] [PubMed] [Google Scholar]
- 39.Panteleyev AA, Paus R, Wanner R, Nürnberg W, Eichmüller S, Thiel R, Zhang J, Henz BM, Rosenbach T: Keratin 17 gene expression during the murine hair cycle. J Invest Dermatol 1997, 108:324-329 [DOI] [PubMed] [Google Scholar]
- 40.Commo S, Bernard BA: Immunohistochemical analysis of tissue remodeling during the anagen-catagen transition of the human follicle. Br J Dermatol 1997, 137:31-38 [PubMed] [Google Scholar]
- 41.Jahoda CA, Reynolds AJ: Dermal-epidermal interactions: adult follicle-derived cell populations and hair growth. Dermatol Clin 1996, 14:573-583 [DOI] [PubMed] [Google Scholar]
- 42.Hong L, Imeri L, Opp MR, Postlethwaite AE, Seyer JM, Krueger JM: Intercellular adhesion molecule-1 expression induced by interleukin (IL)-1b or an IL-1b fragment is blocked by an IL-1 receptor antagonist and a soluble IL-1 receptor. J Neuroimmunol 1993, 44:163-170 [DOI] [PubMed] [Google Scholar]
- 43.Kaplan ED, Holbrook KA: Dynamic expression patterns of tenascin, proteoglycans, and cell adhesion molecules during human hair follicle morphogenesis. Dev Dyn 1994, 199:141-155 [DOI] [PubMed] [Google Scholar]
- 44.Limat A, Wyss-Coray T, Hunziker T, Braaten LR: Comparative analysis of surface antigens in cultured human outer root sheath cells and epidermal keratinocytes: persistence of low expression of class I MHC antigens in outer root sheath cells in vitro. Br J Dermatol 1994, 131:184-190 [DOI] [PubMed] [Google Scholar]
- 45.Nickoloff BJ, Griffiths CE: aberrant intercellular adhesion molecule-1 (ICAM-1) expression by hair follicle epithelial cells and endothelial leukocyte adhesion molecule-1 (ELAM-1) by vascular cells are important adhesion-molecule alterations in alopecia areata. J Invest Dermatol 1991, 96:91S-92S [DOI] [PubMed] [Google Scholar]
- 46.Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS: Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest 1998, 101:62-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mann SJ, Straile WE: New observation on hair loss in the hairless mouse. Anat Rec 1961, 140:97-101 [Google Scholar]
- 48.Jahoda CA, Horne KA, Oliver RF: Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311:560-562 [DOI] [PubMed] [Google Scholar]
- 49.Cotsarelis G, Sun TT, Lavker RM: Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61:1329-1337 [DOI] [PubMed] [Google Scholar]
- 50.Fraser F: The expression and interaction of hereditary factors producing hypotrichosis in the mouse: histology and experimental results. Can J Res 1946, 24D:10-25 [DOI] [PubMed] [Google Scholar]
- 51.Weedon D, Strutton G: Apoptosis as the mechanism of the involution of hair follicles in catagen transformation. Acta Derm Venereol 1981, 61:335-339 [PubMed] [Google Scholar]