Dual Signaling Role of the Protein Tyrosine Phosphatase SHP-2 in Regulating Expression of Acute-Phase Plasma Proteins by Interleukin-6 Cytokine Receptors in Hepatic Cells (original) (raw)
Abstract
One of the major actions of interleukin-6 (IL-6) is the transcriptional activation of acute-phase plasma proteins (APP) genes in liver cells. Signaling by the IL-6 receptor is mediated through the signal transducing subunit gp130 and involves the activation of Janus-associated kinases (JAKs), signal transducer and activator of transcription 3 (STAT3), and mitogen-activated protein (MAP) kinase. Functional analysis of gp130 in rat hepatoma cells by using transduced chimeric G-CSFR-gp130 receptor constructs demonstrates that SHP-2, the Src homology 2 (SH2) domain-containing protein tyrosine phosphatase, acts as a negative regulator of the JAK/STAT signaling in part by downregulating JAK activity, thereby indirectly moderating the induction of STAT3-dependent APP genes. This study shows that in hepatoma cells, the recruitment and tyrosine phosphorylation of SHP-2, but not SHC, is the primary signaling event associated with the activation of MAP kinases (ERK1/2) by gp130. Overexpression of truncated SHP-2 that lacks Grb2-interacting sites, but not the full-length catalytically inactive SHP-2, reduces ERK activation by IL-6, confirming the signal-mediating role of SHP-2. Activation of ERK1/2 is correlated with induction of the immediate-early response genes. Stimulation of the c-fos, c-jun, and egr-1 genes is essentially absent in cells expressing gp130 with a Y759F mutation, which is unable to recruit SHP-2. Interestingly, both JAK/STAT and SHP-2 pathways regulate the induction of the junB gene. Moreover, disengagement of SHP-2 from gp130 signaling not only enhances APP gene induction but also further reduces cell proliferation, in part correlated with the attenuated expression of immediate-early response genes. These results suggest that IL-6 regulation of APP genes is affected by SHP-2 in two ways: SHP-2 acts as a phosphatase on the JAK/STAT pathway and serves as linker to the MAP kinase pathway, which in turn moderates APP production.
The Src homology 2 (SH2) domain-containing protein tyrosine phosphatase, SHP-2, interacts with many proteins by recognizing the tyrosine-phosphorylated Y(I/V)X(L/V/I) motifs through its amino-terminal SH2 domain (for a review, see reference 53). This protein-protein interaction enhances the tyrosine phosphatase activity of SHP-2 by relieving the inhibitory intramolecular interaction between the amino-terminal SH2 domain and the catalytic phosphatase domain (26). Upon tyrosine phosphorylation, several growth factor receptors are detected in association with SHP-2 (receptors for platelet-derived growth factor [PDGF], epidermal growth factor [EGF], fibroblast growth factor, and insulin) (30, 32, 68, 69), cytokine receptors (receptors for interleukin-2 [IL-2], IL-3, IL-6, and interferon) (1, 13, 17, 64, 77), and adapter molecules (insulin receptor substrate [IRS], daughter of sevenless [DOS], SHP substrate 1/signal-regulatory protein [SHPS-1/SIRP/BIT], and platelet/endothelial cell adhesion molecule 1 [PECAM-1]) (18, 24, 31, 40, 54, 58, 67). Based on cell biological data and genetic evidence from Drosophila, Caenorhabditis elegans, and mice, SHP-2 is a positive regulator of cell proliferation (20, 24, 59, 79). Invariably, SHP-2 has been linked to the process of mitogen-activated protein (MAP) kinase activation (45, 68). Two different mechanisms have been suggested by which SHP-2 activates MAP kinases (ERK1/2). One mechanism, which appears not to depend on the phosphatase activity of SHP-2, is through tyrosine phosphorylation of SHP-2 as observed in response to PDGF, IL-3, and IL-6-type cytokines (19, 45, 77). Among the possible tyrosine phosphorylation sites that reside primarily in the C-terminal half of SHP-2, which also harbors the phosphatase domain, are four sites with the YXNX motifs known to serve as docking element for Grb2 (growth factor receptor binding protein 2). Grb2 itself is constitutively associated with SOS (son of sevenless), the GTP exchange factor for Ras. Activation of Ras by the SHP-2–Grb2–SOS route induces the phosphorylation and activation of Raf-1/MEK-1/MAP kinases. The second mechanism is dependent on the substrate binding and/or phosphatase activity of SHP-2 (47, 53). In the examples of insulin and EGF signaling, it has been proposed that the phosphatase activity of SHP-2 is important in the activation of the MAP kinase pathway by removing inhibitory phosphates in receptor or adapter molecules. In these cases, overexpression of the catalytically inactive SHP-2 mutant suppresses the activation of MAP kinases (32, 72).
Initiation of signaling by IL-6R results in a rapid tyrosine phosphorylation of Janus-associated kinases (JAKs), signal transducers and activators of transcription (STATs), and SHP-2. Activation of JAKs and STAT3 is essential for the several biological activities of IL-6, in particular for stimulation or inhibition of proliferation (19, 46, 49, 63, 80) and induction of acute-phase plasma protein (APP) genes (42). Mutational studies of gp130, the signal-transducing receptor subunit for IL-6 cytokines, demonstrates that tyrosine residues Y769, Y814, Y905, and Y915, which are part of the YXXQ motif, upon phosphorylation, are docking sites for STAT3 or STAT1 (23, 63), whereas Y759 is the site of SHP-2 interaction (19, 34). Although the role of SHP-2 in activation of the MAP kinase pathway is recognized, a connection of this pathway with induction of genes such as the APP genes has not been demonstrated.
Our previous studies suggested that SHP-2 downregulates gp130-mediated signaling by associating with the phosphorylated Y759 of gp130 and exerting tyrosine phosphatase activity, possibly onto JAK (34). By preventing recruitment of SHP-2 by the Y759F mutation in gp130, a prolonged activation of JAK and STAT3 and correspondingly enhanced and more sensitive gene induction of APP was obtained. However, these studies could not demonstrate the relative contribution of the SHP-2-dependent downstream signaling pathways to modulated gene induction.
This report shows that recruitment of SHP-2 by gp130 is primarily responsible for the activation of ERK1 and ERK2 in rat hepatoma cells. Moreover, we demonstrate that gp130, through SHP-2 and ERKs, induces a subset of immediate-early response genes. Enhanced ERK activity did not affect immediate induction of APP genes by IL-6, but during long-term treatment it influenced APP expression indirectly by attenuating the inhibitory effect of IL-6 on cell proliferation.
MATERIALS AND METHODS
Cell lines and cytokines.
Rat hepatoma H-35 cells (clone T-7-18 [4]), the epidermal growth factor receptor-positive clone 86-6 (74) of human HepG2 cells (36), were cultured in Dulbecco’s minimal essential medium supplemented with 10% fetal calf serum. In general, cell cultures used for analyzing signaling were maintained for 24 h in serum-free medium prior to extraction. Cells were treated in serum-free minimal essential medium containing 50 ng of human recombinant IL-6 (Genetics Institute, Cambridge, Mass.) per ml, 50 ng of granulocyte colony-stimulating factor (G-CSF; Immunex, Seattle, Wash.) per ml, 500 ng of insulin (Sigma, St. Louis, Mo.) per ml, 100 ng of EGF (Calbiochem, San Diego, Calif.) per ml, or PD98059 (Calbiochem) at 25 μM (long term) or 75 μM (short term).
Plasmid constructs and antibodies.
The cDNAs to mouse SHP-2 (_Eco_RI-_Not_I fragment from pREP4-SHP-2), catalytically inactive SHP-2C463S (from pREP4-SHP-2CS) (54; generously provided by H. Ohnishi), and mouse SHP-2 (positions 1 to 552) with a variant C-terminal 33-residue extension (lacking the most C-terminal Grb2 binding site) (16; generously provided by G.-S. Feng) were inserted as _Not_I fragments into the expression vector pDC302 (50), resulting in pDC-SHP-2, pDC-SHP-2CS, and pDC-SHP-2var, respectively. The corresponding epitope-tagged constructs, containing the Myc epitope added to the N terminus of SHP-2, SHP-2CS, and the C-terminally truncated SHP-2ΔC (positions 1 to 545; lacking the two potential C-terminal Grb2 binding sites), as well as the Myc epitope added to the C terminus of the 214-residue amino-terminal segment of SHP-2 (containing the two tandem SH-2 domains but lacking the entire catalytic phosphatase domain including the four potential Grb2 binding sites), SHP-2Δ, were generated by PCR, verified by sequencing, and inserted into pDC302. The chimeric receptors containing the extracellular domains of human G-CSF receptor (G-CSFR) and transmembrane and full-length wild-type or Y259F (=Y2F) cytoplasmic domain of gp130 with the C-terminal FLAG epitope and termed G-gp130(WT) and G-gp130(Y2F), respectively, have been described previously (34). The equivalent FLAG epitope (DYKDDDDK) was also added to the C terminus of truncated versions of G-gp130(133) (42), yielding G-gp130(133)WT-FLAG and G-gp130(133)Y2F-FLAG. The chimeric receptor constructs were cloned into the retroviral vector MINV (22) and used to generate stably transduced H-35 cells (34). Transduced cells were selected in medium containing 2 mg of G-418 per ml. Primary clones were screened for the level of chimeric receptor protein by Western blotting, G-CSF binding, and G-CSF-specific stimulation of _sis_-inducible element binding activity of STAT3; expression of haptoglobin, α2-macroglobulin, and thiostatin genes; and attenuated cell proliferation. Based on the observation that the cell responses generally correlated with the level of receptor protein, most of the characterization of signaling has been carried out with two representative lines of H-35 cells expressing equal amounts of G-gp130 receptor protein. The responsiveness of the pool of transduced cells and separate primary clones is shown in Fig. 6A and 8B, respectively. Anti-FLAG monoclonal (M2) antibodies were purchased from Kodak (Rochester, N.Y.); polyclonal antibodies against FLAG, SHP-2, gp130, and Grb2 (C-23) and monoclonal antibodies against Myc (9E10) were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.); and polyclonal antibodies against Myc and the N-terminal epitope of SHP-2 were obtained from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Phosphorylation-specific anti-STAT3 and anti-ERK1/2 antibodies were purchased from New England Biolabs (Beverly, Mass.). Phosphotyrosine antibodies (PY20), and anti-SHC antibodies were purchased from Transduction Laboratory (Lexington, Ky.).
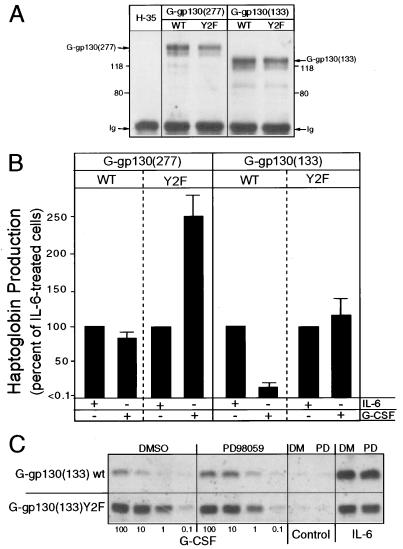
FIG. 6.
Suppression of ERK activation enhances Hp production. (A) Lysates of parental H-35 cells and H-35 cells stably transduced with the G-gp130 constructs were reacted with anti-FLAG antibodies. Immunoprecipitated proteins were immunoblotted with anti-FLAG antibodies. (B) Cell cultures transduced with the indicated receptors were treated for 48 h with IL-6 or G-CSF. The amount of Hp secreted during the second 24-h period was determined by immunoelectrophoresis, normalized to the cell number, and expressed relative to the values of the IL-6-treated cultures in each series (mean and standard deviation; n = 3 to 6). (C) G-gp130(133)WT and G-gp130(133)(Y2F) cultures were treated for 48 h with IL-6 or increasing doses of G-CSF in the presence of dimethyl sulfoxide (DMSO, DM) or 25 μM PD98059 (PD). Hp produced during the second 24-h treatment period was determined by Western blotting of 5 μl of culture medium. The band represents the immunoreactive Hp β subunit.
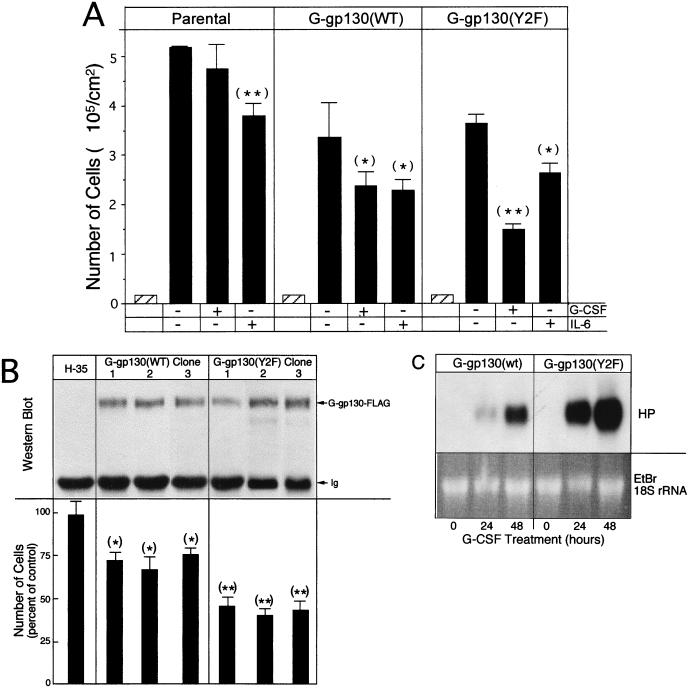
FIG. 8.
Proliferation is reduced by the action of gp130. (A) Cells (∼2 × 104/cm2) were cultured in six-well dishes for 48 h in serum-free medium. Then they were treated with medium containing 10% serum alone or G-CSF or IL-6 in addition. After 2 days, the treatment media were replaced by fresh media. Two days later, cell numbers were determined and calculated per 10 cm2 of culture area (mean and standard deviation; n = 3). The cell number at the onset of the experiment is indicated by a hatched bar. (B) (Top) Extracts of parental H-35 cells and cultures of three independent clonal lines of G-gp130(WT) and G-gp130(Y2F) cells were immunoprecipitated with anti-FLAG antibodies and analyzed by Western blotting for anti-FLAG reactive proteins. (Bottom) The same cells were analyzed for proliferation in response to G-CSF treatment as the cells in panel A. In each series, the values for the treated cell cultures were calculated relative to the mean cell number determined for the medium control cultures (set to 100%) (mean and standard deviation; n = 3). ∗, P < 0.05; ∗∗, P < 0.005. (C) G-gp130(WT) and G-gp130(Y2F) cells in six-well dishes were cultured for 0, 24, and 48 h in medium containing 1% serum in the presence of G-CSF. Total cellular RNA (10 μg) were analyzed by Northern blot hybridization for haptoglobin mRNA. An autoradiogram after a 6-h exposure is shown. EtBr, ethidium bromide.
Isolation of transiently transfected HepG2 cells by FACS.
HepG2 cells in 15-cm-diameter dishes (4 × 106 cells/dish) were transfected by the calcium phosphate method (33) with a total of 20 μg of DNA per ml including 1 μg of pEGFP(N1) (Upstate Biotechnology, Inc.) per ml and expression vectors for G-gp130(WT) (0.5 to 5 μg/ml) or SHP-2 forms (5 μg/ml). At 36 h after transfection, the cells were released by trypsin, dispersed into a single-cell suspension (7.5 × 106 cells/ml), and subjected to sterile high-speed fluorescence-activated cell sorting (FACS) (7.5 × 104 cells/sec) in a Vantage instrument with TurboSort option (Becton Dickinson). Green fluorescent protein (GFP)-positive (∼5% of total cell population) and -negative (control) cells were collected. Post-sort analysis of GFP-positive cell population by FACScan indicated that >90% of cells had the gated phenotype. Cells were replated into 24- or 96-well culture plates and cultured for 24 h prior to analysis.
Immunoprecipitation and Western blotting.
Cells were lysed in 50 mM Tris-HCl (pH 7.5)–1% Nonidet P-40–150 mM sodium chloride–0.25% sodium lauroyl sarcosine–1 mM activated sodium orthovanadate–1 mM sodium fluoride–10% glycerol–protease inhibitors (34). Cell lysates were incubated with 1 to 2 μg of antibodies for 2 h. Immune complexes were recovered by binding to protein G-Sepharose beads. Immunoprecipitated proteins were electrophoresed on sodium dodecyl sulfate–7.5 or 10% polyacrylamide gels, electrotransferred onto a polyvinylidene difluoride membrane (Schleicher & Schuell, Keene, N.H.), and reacted with primary antibodies and secondary antibodies (horseradish peroxidase-conjugated anti-mouse or anti-rat goat antibodies [Cappel, West Chester, Pa.]) in 20 mM Tris-HCl (pH 7.5)–150 mM NaCl–0.1% Tween 20 containing 4% milk or 1% albumin. Immune complexes were visualized by enhanced chemiluminescence (ECL; Amersham, Piscataway, N.J.). To perform additional antibody reactions, membranes were treated with 0.1 M glycine (pH 2.7) in 0.1 M sodium chloride for 16 h.
Northern hybridization.
Total cellular RNAs were extracted by either the Trizol method (Life Technology, Grand Island, N.Y.) or the guanidine chloride-CsCl method (12). mRNAs were purified on mini-oligo(dT) cellulose spin column. The RNAs were separated on a 1.5% formaldehyde–agarose gel, transferred onto a nylon membrane (Schleicher & Schuell), and reacted with 32P-labeled cDNA probes for egr-1, junB, c-jun, c-fos, haptoglobin, and triosephosphate isomerase (3).
Thymidine incorporation assay.
Cells were seeded into 96-well plates (2.5 × 104 cells/well) and cultured for 24 h. They were then treated for 8 h with serum-free medium followed by the same medium with or without cytokines (six to eight separate wells per treatment). After 16 h, 0.4 μCi of [3H]thymidine was added to the cultures and incubation was continued for 8 h. The cells were washed, trypsinized, and collected onto filter paper with a cell harvester. Incorporation of 3H was measured with a liquid scintillation counter (Wallac, Gaithersburg, Md.). Statistical evaluation of the data was performed by Student’s t test.
RESULTS
The G-gp130(Y2F) receptor is deficient in signaling to MAP kinase.
To study the signaling of gp130 toward APP genes in hepatic cells that contain endogenous gp130, we resorted to the use of the G-CSFR–gp130 chimeric receptor, in which the extracellular domain of G-CSFR was recombined with the transmembrane and the cytoplasmic domain of gp130 (5). This receptor undergoes a G-CSF-mediated dimerization (27), thereby mimicking IL-6-induced dimerization of the gp130 cytoplasmic domain and initiation of signaling identical to IL-6R (76). We established H-35 cells stably transduced with FLAG epitope-tagged G-CSFR–gp130 wild type [termed G-gp130(WT)] or G-CSFR–gp130Y759F that contains a mutant SHP-2 docking site [termed G-gp130(Y2F)] (34). Four independently transduced cultures indicated that G-gp130(WT) was consistently two to four times more highly expressed than G-gp130(Y2F) (34; see the example in Fig. 6A). To assess the proposed role of SHP-2 in connecting gp130 to the MAP kinase pathway and to identify the effects of MAP kinase on APP regulation, we selected clonal lines that express equivalent amounts of chimeric receptors, as determined in a whole-cell extract by immunoblotting with anti-FLAG polyclonal antibodies and shown for two representative lines in Fig. 1A. The two cell lines also displayed comparable 125I-G-CSF binding activity, indicating approximately 1,500 ligand binding sites per cell (data not shown). Brief treatment of the cells with G-CSF led to similar levels of tyrosine phosphorylation of the chimeric receptors recovered by immunoprecipitation (Fig. 1B). Phosphorylation of the chimeric receptor was also in a similar range to that for the endogenous gp130 activated by IL-6 (Fig. 1C, top). As expected, G-gp130(Y2F) cells failed to recruit SHP-2 to the chimeric receptors (Fig. 1C, right). Moreover, the analysis demonstrated that activated G-gp130(WT) and endogenous gp130 appeared to interact with SHP-2 (as shown by coimmunoprecipitation) and mediated its tyrosine phosphorylation (Fig. 1C, top) but that nonappreciable amounts of tyrosine-phosphorylated SHP-2, in contrast to non-tyrosine-phosphorylated SHP-2, were found in association with G-gp130 (Fig. 1B and C, bottom).
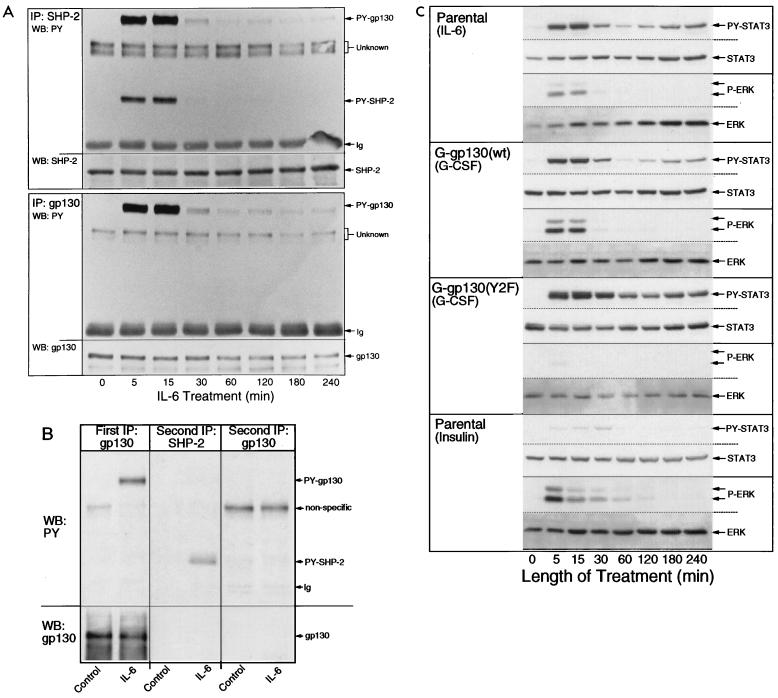
FIG. 1.
Expression of G-gp130(WT) and G-gp130(Y2F) in H-35 cells. (A) Aliquots of total-cell extracts (30 μg of protein) from parental H-35 cells, G-gp130(WT) cells, and G-gp130(Y2F) cells were separated on 7.5% polyacrylamide gels. After being transferred to a membrane, the proteins were reacted with anti-FLAG polyclonal antibodies. (B) Confluent monolayers of parental H-35 cells, G-gp130(WT) cells, and G-gp130(Y2F) cells in 10-cm-diameter dishes were treated for 10 min with G-CSF and then lysed. Proteins were immunoprecipitated (IP) with anti-FLAG monoclonal antibodies and analyzed on Western blots (WB) first with antiphosphotyrosine (PY) antibodies and then with anti-FLAG polyclonal antibodies. Ig, immunoglobulin. (C) G-gp130(WT) and G-gp130(Y2F) cells were treated for 10 min with G-CSF or IL-6. Half of the cell lysate was reacted with anti-SHP-2, and the other half was reacted with anti-FLAG. Immunoprecipitated proteins were analyzed by Western blotting first with antiphosphotyrosine and then with anti-SHP-2 antibodies.
Since signaling by gp130 cytoplasmic domains is a function of cytokine treatment, we established the kinetics of the action by the endogenous gp130 in parental H-35 cells as a standard for comparison, by determining the phosphorylation of gp130 and SHP-2 (Fig. 2A and B) and of STAT3 and ERK1/2 (Fig. 2C). IL-6 treatment elicited a temporally coordinated tyrosine phosphorylation of gp130 and SHP-2, with maximum phosphorylation after 5 to 15 min, which returned to close to the basal level by 30 min (Fig. 2A). Of note is that a low-to-trace-level tyrosine phosphorylation of both gp130 and SHP-2 persisted over the subsequent 4-h treatment period. Moreover, the results in Fig. 2A suggested that phosphorylated gp130 interacted with SHP-2 but not with tyrosine-phosphorylated SHP-2, forming a complex that would remain intact under the conditions of the immunoprecipitation procedure. Sequential reactions of cell lysates with antibodies against gp130 and SHP-2 confirmed that gp130 immunoprecipitation led to a representative recovery of the cellular receptor subunit (Fig. 2B).
FIG. 2.
Time course of STAT3 and ERK activation. (A) H-35 cells in 10-cm-diameter dishes were treated with IL-6 for the times indicated and then lysed. Half of the lysate was reacted with anti-SHP-2 antibodies, and the other half was reacted with anti-gp130 antibodies. The two series of immunoprecipitates (IP) were analyzed under identical conditions by immunoblotting with antiphosphotyrosine (PY) antibodies and subsequently with anti-SHP-2 antibodies (top) or anti-gp130 (bottom). (B) Lysates from control and IL-6-treated culture were immunoprecipitated (First IP) with anti-gp130 antibodies. The supernatant lysate fractions were divided into two. Half was immunoprecipitated (Second IP) with anti-SHP-2 antibodies, and the other half was immunoprecipitated with anti-gp130 antibodies. In the latter immunoprecipitation, after binding to protein G-Sepharose, the resin was not washed but immediately boiled in sodium dodecyl sulfate buffer. Half of the first and all of the two second immunoprecipitates were separated on one sodium dodecyl sulfate–7.5% polyacrylamide gel. The blotted proteins were reacted first with antiphosphotyrosine and then with anti-gp130 antibodies. (C) Parental H-35, G-gp130(WT), and G-gp130(Y2F) cells in six-well culture plates were treated with IL-6, G-CSF, or insulin for the indicated times. Aliquots of whole-cell lysate (30 μg protein) were analyzed by immunoblotting for tyrosine-phosphorylated STAT3, STAT3, phosphorylated ERK1/2, and ERK in the same membrane.
ERK1/2 and STAT3 were activated in response to IL-6 with a kinetics that was in part comparable to the phosphorylation of gp130 (Fig. 2C, top). A notable difference was that the level of phosphotyrosine STAT3 was elevated longer than that of phosphorylated ERK1/2, as seen after the 30-min treatment. Interestingly, the kinetics of ERK1/2 activation correlated closely with that of tyrosine phosphorylation of SHP-2 and gp130 (Fig. 2A). The very transient ERK1/2 activation appears to be characteristic to IL-6, because treatment of H-35 cells with insulin produced a significantly prolonged activation of ERKs with minimal effect on STAT3 (Fig. 2C, bottom).
Since both SHP-2 and SHC have been suggested to be signaling molecules connecting gp130 with the MAP kinase pathway (41, 55), we examined the contribution of SHP-2 in G-gp130 cell lines (Fig. 2C). In separate experiments (results not shown), we established that both G-gp130 and G-gp130(Y2F) cells responded to IL-6 by an activation of ERK1/2 that was practically indistinguishable from the parental cells. Treatment with G-CSF elicited an appreciable activation of ERK1/2 only in G-gp130(WT) cells. G-gp130(Y2F) cells essentially failed to activate ERK1/2 (Fig. 2C). The strong phosphorylation of STAT3 in both cell lines attested to the comparable signaling capabilities of each chimeric receptor through the JAK/STAT pathway. The activation of ERK1/2 and STAT3 by G-CSF in G-gp130(WT) cells showed essentially the same kinetics as did activation by IL-6. In contrast, and in agreement with previous data (34), G-CSF treatment of G-gp130(Y2F) cells produced a prolonged STAT3 activation.
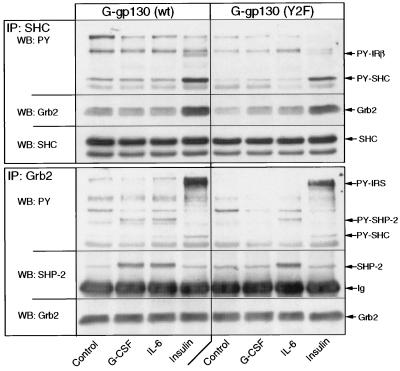
To assess the potential involvement of SHC in the gp130 signaling process, we measured gp130-dependent tyrosine phosphorylation of immunodetectable SHC in both G-gp130(WT) and gp130(Y2F) cells (Fig. 3). Insulin treatment served as a positive control of SHC activation. IL-6 and G-CSF treatments did not detectably enhance the phosphorylation of SHC. In contrast, insulin treatment led to a prominent tyrosine phosphorylation of SHC (52-kDa isoform) and corresponding association of SHC with Grb2 (Fig. 3, top). The complementary analysis of immunoprecipitated Grb2 demonstrated the recovery of tyrosine-phosphorylated SHP-2 from IL-6- or G-CSF-treated G-gp130(WT) cells and from only IL-6-treated G-gp130(Y2F) cells (Fig. 3, bottom). In contrast, Grb2 immunoprecipitates from the insulin-treated cells yielded tyrosine-phosphorylated SHC and IRS protein but a negligible amount of SHP-2. The results suggested that the activation of the STAT pathway and the MAP kinase pathway by gp130 is separable and that SHP-2 may function as a major mediator of the gp130 signal to ERK1/2.
FIG. 3.
Interaction of Grb2 with SHP-2 but not with SHC by gp130 signaling. G-gp130(WT) cells and G-gp130(Y2F) cells in 10-cm-diameter dishes were treated for 10 min with medium alone, G-CSF, IL-6, or insulin. Half of the cell lysate was immunoprecipitated (IP) with anti-SHC antibodies, and the other half was immunoprecipitated with anti-Grb2 antibodies. Immunoprecipitated proteins were separated on a sodium dodecyl sulfate–10% polyacrylamide gel, and immunoblots were reacted with antiphosphotyrosine (PY) and then anti-Grb2 and anti-SHC (top) or antiphosphotyrosine, anti-SHP-2, and anti-Grb2 (bottom). Note that in H-35 cells the 52-kDa isoform of SHC predominates and the 66-kDa isoform is undetectable.
Role of SHP-2 in activation of MAP kinases.
We sought an independent demonstration of the suggested role of SHP-2 in connecting gp130 to the MAP kinase pathway. We reasoned that the amino-terminal segment of SHP-2, containing the two SH2 domains but lacking the phosphorylation domain and the four potential Grb2 binding sites (SHP-2Δ), would be sufficient to bind to phosphorylated Y759 of gp130 but would abort subsequent signal propagation in a dominant negative fashion because of the absence of its phosphorylation sites acting as Grb2 docking elements. On the other hand, the enzymatically active SHC-2 variant that lacks the C-terminal binding sequence for Grb2 (16) or the catalytically inactive SHP-2CS should support the ERK activation process if only presentation of Grb2 docking sites is required of SHP-2. Moreover, the SHP-2CS could maintain a wild-type-like signal-transducing role through the process of substrate trapping (53).
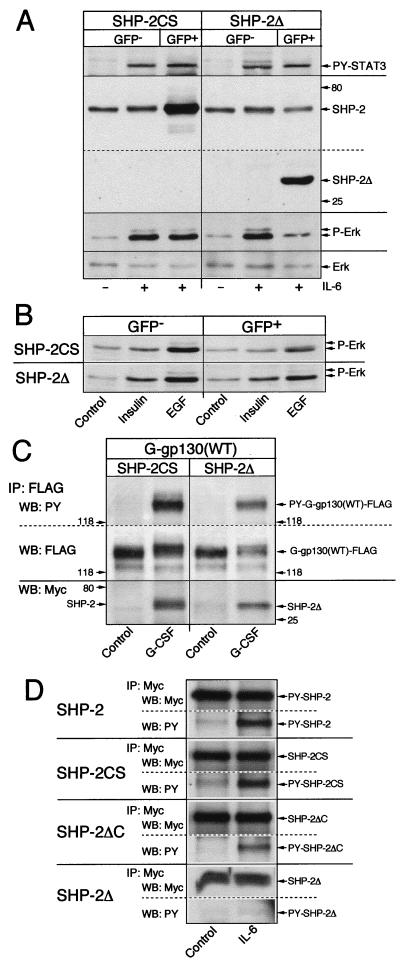
Overexpressed SHP-2Δ (33a), like SHP-2CS (34, 66), in transiently transfected hepatoma cells yielded a minor enhancing activity on IL-6-mediated induction of cotransfected IL-6RE chloramphenicol acetyltransferase reporter constructs, probably by preventing endogenous wild-type SHP-2 from acting as a phosphatase on the JAK/STAT pathway. However, it was not possible to determine whether these SHP-2 mutants would also modify activation of the ERK pathway. Our attempts to establish, by transfection or retroviral transduction, H-35 cells with stable expression of SHP-2 mutants at a level effective in suppressing the endogenous SHP-2 action were unsuccessful. Therefore, we resorted to an alternative approach. We overexpressed SHP-2CS or SHP-2Δ in transiently transfected HepG2 cells, which, unlike H-35 cells, have the ability to take up and express plasmid constructs at relatively high levels. The transfected cells in the culture were isolated by FACS with cotransfected GFP as marker. Coselected GFP-negative cells served as experimental controls. IL-6 treatment of the GFP-positive cells overexpressing SHP-2CS resulted in an ERK activation as seen with GFP-negative control cells, whereas the cells overexpressing SHP-2Δ had a significantly suppressed activation (Fig. 4A). The equal tyrosine phosphorylation of STAT3 in both cell types attested to the proper signaling function of gp130 towards the JAK-STAT pathway.
FIG. 4.
Effect of overexpressed SHP-2 mutants on the activation of MAP kinases. HepG2 cells were transfected with expression vectors for GFP and SHP-2CS or SHP-2Δ. (A) GFP-negative and GFP-positive cells were selected by FACS and, after a 24-h reculture period, treated with IL-6 for 15 min. Equal amounts of cell lysate (30 μg protein) were separated on one sodium dodecyl sulfate–10% polyacrylamide gel. After protein transfer, the membrane was cut along the 60-kDa position (dotted line), the top part was reacted with antiphosphotyrosine (PY) STAT3, and the bottom part was reacted with antiphosphotyrosine ERK. Then both sections were reacted with anti-SHP-2 recognizing the N-terminal epitope, and the bottom section was reacted with anti-ERK. (B) HepG2 cells were transfected with expression vectors for GFP and SHP-2CS or SHP-2Δ. FACS-selected GFP-negative and GFP-positive cells (2 × 105 per well in 24-well culture plates) were cultured overnight. After incubation for 8 h in serum-free medium, the cells were stimulated for 10 min with medium alone or with insulin or EGF. Equal amounts of whole-cell lysate were analyzed by Western blotting for phosphorylated ERK. (C) HepG2 cells in 15-cm-diameter dishes were transfected with expression vector for G-gp130(WT) (5 μg/ml) and Myc SHP-2CS or SHP-2Δ-Myc (5 μg/ml). After the cultures were divided into two and allowed to recover for 36 h, the cells were treated for 15 min with medium alone (control) or G-CSF. G-gp130 protein was immunoprecipitated (IP) with anti-FLAG antibodies and analyzed by Western blotting (WB). The membrane section with proteins of >100 kDa was reacted first with antiphosphotyrosine (PY) and then with anti-FLAG antibodies. The membrane section with proteins of <100 kDa was reacted with anti-Myc antibodies. The same ECL exposure for each section is reproduced; however, the portions showing SHP-2CS and SHP-2Δ have been rearranged due to the size difference of the proteins. (D) HepG2 cells in 10-cm-diameter dishes were transfected with the expression vectors for the Myc epitope-tagged versions of the indicated SHP-2 proteins (5 μg/ml). Subcultures were treated for 15 min with medium alone (control) or with IL-6. Proteins were immunoprecipitated with anti-Myc antibodies (IP:Myc) and analyzed by Western blotting first for reaction with antiphosphotyrosine antibodies (WB:PY) and then for reaction with anti-Myc antibodies (WB:Myc). The composite shows only the sections of the Myc-reacting proteins. Due to the high level of expressed proteins, the ECL reaction for anti-Myc is much shorter than for antiphosphotyrosine.
To identify the specificity of SHP-2 mutants to interfere with receptor signaling toward the MAP kinase pathway, we applied the same experimental approach to measure the effects of transiently overexpressed SHP-2CS or SHP-2Δ on activation of ERKs by insulin and EGF. In the EGFR+ HepG2 cell line 86-6, insulin and EGF activated ERK1/2 to a somewhat lower level than IL-6 did (compare Figs. 4A and B). Overexpressed SHP-2CS and SHP-2Δ did not prevent ERK activation by growth factors, but both mutant forms appeared to reduce the magnitude of immunodetectable phosphorylated ERKs (Fig. 4B). Notable was that SHP-2Δ did not exert as prominent a suppressive action on signaling by insulin or EGF as on signaling by IL-6. This suggests that the growth factor receptors engage alternative pathways, such as through SHC-Grb2 (Fig. 3 for insulin), which activate MAP kinase in hepatoma cells independently of SHP-2.
In separate sets of experiments (data not presented), we determined that the SHP-2 proteins, with a variant C-terminal sequence (SHP-2var) or with a C-terminal truncation (SHP-2ΔC) overexpressed in HepG2 cells, did not produce a significantly impaired activation of ERK1/2 by IL-6 treatment and did not produce a deregulated, transdominant positive ERK activation. The difference in action of these SHP-2 mutants, as well as SHP-2CS, from SHP-2Δ was tentatively attributed to the Grb2 recruiting capability retained by the former SHP-2 proteins. However, the results do not rule out the possibility that different degrees of substrate trapping (53) and/or catalytic action by the overexpressed SHP constructs did contribute to the observed “normal” IL-6 response. Alternatively, the prominent inhibitory effect of SHP-2Δ but not of the other SHP-2 mutants could be related to substantially different binding activities of the SHP-2 proteins to gp130, thereby determining the degree of inhibited ERK activation through termination of signal communication.
Two separate analyses identified gp130 interaction and activation of SHP-2. Transient overexpression of FLAG-tagged G-gp130(WT) and Myc-tagged SHP-2 proteins in HepG2 cells permitted the detection of comparable ligand-induced interaction of G-gp130 with SHP-2CS or SHP-2Δ by coimmunoprecipitation (Fig. 4C). Since tyrosine phosphorylation of receptor-associated SHP-2 proteins was not detectable (wild-type SHP-2 is shown in Fig. 1C and 2A), we determined in separate transfection experiments the recovery of SHP-2 proteins with enhanced tyrosine phosphorylation by the action of the endogenous IL-6R (Fig. 4D). The results indicate that wild-type SHP-2, SHP-2SC, and the C-terminally truncated SHP-2ΔC, which all contain potential Grb2 binding sites, are sensitive to gp130-dependent tyrosine phosphorylation. In contrast, no significant modification of SHP-2Δ protein was detectable (Fig. 4D, bottom), even though SHP-2Δ protein was observed in physical association with ligand-activated gp130 (Fig. 4C). Collectively, the results suggest that the gp130-recruited SHP-2 serves as a major mediator to the ERK pathway and that this function requires the carboxy-terminal half but not the phosphatase activity of the SHP-2 protein.
Modified pattern of gene activation by G-gp130(Y2F) cells.
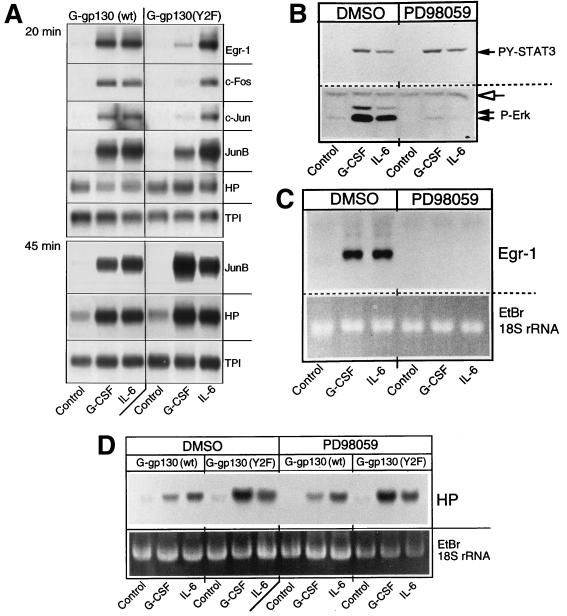
A number of immediate-growth-response genes, such as egr-1, c-fos, c-jun, and junB, are controlled by the ERK-sensitive serum response factor/ternary complex factor (SRF/TCF) and/or AP-1 components. This would explain, in part, why these immediate-response genes are induced in cells treated with IL-6 cytokines (71, 73). Since the role of the gp130-controlled ERK pathway for induction of immediate-response genes has not been demonstrated in hepatic cells or related to the induction of APP genes, the stable G-gp130 H-35 cell lines appeared well suited to define this connection. Short-term treatment of the cells with IL-6 indicated an increase in production of mRNAs for Egr-1, c-Fos, and c-Jun, which was maximal after 20 min of treatment (Fig. 5A), and a return to the basal level by 45 min (data not shown). In contrast, JunB mRNA, which also was immediately induced, reached its maximal level by 45 min (Fig. 5A). In response to G-CSF, G-gp130(WT) cells exhibited essentially the same induction profile of immediate-early response genes as seen with IL-6. G-gp130(Y2F) cells, however, showed a minor induction of Egr-1, c-Fos, JunB, and c-Jun mRNAs after 20 min of G-CSF treatment. Surprisingly, the level of JunB mRNA rose dramatically during the subsequent 30 min of G-CSF treatment, exceeding that achieved by IL-6 treatment. The regulation of the immediate-response gene differed from that of the APP genes in that mRNA expression for the latter genes, such as for the haptoglobin (Hp) gene, was induced with slower kinetics. After 20 min of G-CSF or IL-6 treatment, only minimal changes relative to the control cells, if any, were seen. By 45 min of treatment, the enhanced Hp mRNA was apparent, with the higher relative expression already detectable in G-CSF-treated G-gp130(Y2F) cells (Fig. 5A).
FIG. 5.
Activation of immediate-response genes. (A) G-gp130(WT) and G-gp130(Y2F) cells were cultured in 15-cm-diameter dishes. After a 24-h serum deprivation, the cells were treated for 20 or 45 min with medium alone, G-CSF, or IL-6. Polyadenylated RNA (5 μg) was analyzed by Northern blot hybridization with the indicated probes. (B to D) G-gp130(WT) cells were pretreated with dimethyl sulfoxide (DMSO) or PD98059 (75 μM) and treated for 10 min (B), 20 min (C), or 2 h (D) with medium alone (Control) or medium containing G-CSF or IL-6 in the presence of dimethyl sulfoxide or PD98059. (B) Cell extracts were analyzed by immunoblotting for active STAT3 and ERK1/2 on a single membrane. The open arrow indicates the nonspecific band that demonstrates equal amounts of protein loading. (C) Total cellular RNA (5 μg) was subjected to Northern blot hybridization with the Egr-1 probe. (D) Total RNA (10 μg) was analyzed by Northern blot hybridization with the haptoglobin (HP) probe. EtBr, ethidium bromide.
The mediator role of the MAP kinase pathway in regulating the expression of immediate-response genes also could be demonstrated by treating G-gp130(WT) cells with G-CSF or IL-6 in the presence or absence of the MEK-1 inhibitor PD98059 (14). Whereas activation of ERK1/2 was prominently suppressed, tyrosine phosphorylation of STAT3 remained unaffected (Fig. 5B). If gp130 induction of the immediate-early response genes is mediated primarily through MAP kinases, we would expect that PD98059-inhibited ERK activation would also result in a loss of immediate-response gene regulation. Such an inhibition was indeed observed, as demonstrated by the minimal Egr-1 mRNA accumulation (Fig. 5C). In contrast, treatment of G-gp130(WT) and G-gp130(Y2F) cells for 2 h with G-CSF or IL-6, in the presence or absence of PD98059, did not significantly alter the induction of haptoglobin mRNA (Fig. 5D). This result suggests that the transcriptional activation process acting on the Hp gene is not critically dependent on a PD98059-sensitive pathway.
ERK has the potential to moderate APP gene expression.
Since the Hp gene is responsive to STAT3, and gp130 signaling activates STAT3 more prominently than it activates ERK1/2 (Fig. 2C), a potential long-term effect of ERK on Hp or other APP genes may not be readily apparent. Hence, to assess the effect of gp130-controlled ERK on APP gene induction, we used an alternative approach. We noted that by removing the three distal Box3 motifs from the cytoplasmic domain of gp130, as achieved by the truncation to 133 residues (42), the level of STAT activation and therefore Hp induction is reduced, but SHP-2 and ERK activation is retained at the normal level (43). Because of the altered ratio of STAT to ERK activation, the signaling by G-gp130(133) constructs with and without SHP-2 recruitment should more prominently indicate the contribution of ERK to Hp regulation. We established H-35 cells that were stably transduced with FLAG-tagged G-gp130(133)WT or G-gp130(133)Y2F. The noncloned cultures expressed the truncated receptors at a slightly higher level than did the cultures transduced with full-length G-gp130 constructs (Fig. 6A). These cells responded to G-CSF by increasing their Hp production (Fig. 6B). However, compared to the response to IL-6, a greater quantitative difference in the Hp regulation between the wild-type and Y2F mutant receptors was observed. Induction of Hp by G-gp130(133)WT was approximately 15% of that by IL-6R, whereas induction by G-gp130(133)Y2F exceeded that by IL-6R. G-CSF treatment in the presence of PD98059 revealed that by attenuating ERK activation, G-gp130(133)WT but not the Y2F mutant produced a threefold-enhanced Hp induction (Fig. 6C). Due to the limitation imposed by the use of a chemical inhibitor, the long-term treatment of cells with PD98059 could not be as effective as the Y2F mutation. The results nevertheless suggest that gp130-activated ERK1/2 has a moderating effect on APP expression controlled by the JAK-STAT pathways and that the manifestation of this effect is dependent on the magnitude and duration of STAT activation. Moreover, the data indicate that the regulation of immediate-early genes and that of APP genes is the result of two separable gp130 signals.
Enhanced inhibition of cell proliferation by G-gp130(Y2F).
IL-6 cytokines have been associated with stimulation or inhibition of proliferation, depending upon the cell type (19, 49). Observing gp130-mediated activation of early response genes in H-35 cells, we asked whether a corresponding effect on DNA synthesis and proliferation was detectable. We determined [3H]thymidine incorporation into parental H-35 cells, G-gp130(WT) cells, and G-gp130(Y2F) cells in response to IL-6 or G-CSF under two separate sets of culture conditions (Fig. 7A). The cells were treated either with serum-free medium, to reduce the potential influence of serum growth factor, or with medium containing 10% serum, to avoid complications due to loss of survival factors, if these were required for maintaining full viability of the culture. Under both culture conditions, IL-6 lowered, thymidine incorporation by about 50% in each cell type. G-CSF had a similar inhibitory effect in G-gp130(WT) cells to that of IL-6 but was somewhat more effective than IL-6 in G-gp130(Y2F) cells.
FIG. 7.
Effect of gp130 signals on thymidine incorporation. (A) Cells in 96-well plates were maintained for 8 h in serum-free medium and then treated for 16 h with serum-free medium containing no additives, G-CSF, or IL-6 followed by 8 h with [3H]thymidine. Values for 3H incorporations (cpm/culture) are shown (mean and standard deviation; n = 5). (B) HepG2 cells were transfected with expression vector for GFP and G-gp130(WT) or G-gp130(Y2F) (1 μg/ml each) and plasmid DNA carrier (18 μg/ml). GFP-positive and GFP-negative cells were selected by FACS. From each preparation, an aliquot of 105 cells was plated in one well of 24-well plates and the remaining cells were distributed in aliquots of 2.5 × 104 cells in 96-well cluster plates. After overnight recovery, the cells in the 24-well plates were lysed and equal amounts of whole-cell lysate were analyzed by Western blotting for anti-FLAG-reactive proteins (top). The cells in the 96-well plates were treated with medium alone or with G-CSF or IL-6 and processed for [3H]thymidine incorporation as in panel A. The values determined in each culture were expressed relative to the mean value calculated for the medium control in each series (set to 100%) (mean and standard deviation; n = 3 to 5). ∗, P < 0.05; ∗∗, P < 0.005.
To verify that the inhibitory effect of G-gp130(WT) and G-gp130(Y2F) on DNA synthesis, as suggested by the response of H-35 cell clones, was not cell line restricted, expression vectors for the same receptors, together with the GFP marker, were transfected into HepG2 cells. FACS-sorted GFP-positive cells showed a prominent expression of the introduced receptor proteins (Fig. 7B. top) and a G-CSF-specific inhibition of thymidine incorporation in the range observed for the endogenous IL-6R (Fig. 7B, bottom). As noted for H-35 cells, G-gp130(Y2F) was also a more effective inhibitor than G-gp130(WT) in HepG2 cells.
To confirm that the inhibitory action of these cytokines on thymidine incorporation is also manifested at the level of cell proliferation, H-35 cells were cultured first for 48 h in serum-free medium, and then for 4 days in complete medium containing either G-CSF or IL-6. The number of cells determined after the treatment period indicated that IL-6 caused a uniform 30% reduction in the number of cells compared to the control treated cultures (Fig. 8A). In response to G-CSF, the G-gp130(WT) cell culture had a similar 30% reduced cell count whereas the G-gp130(Y2F) cell culture was reduced by 60% (Fig. 8A). Of note is that despite the reduced cell proliferation in the presence of G-CSF or IL-6, each of the cultures exhibited a net increase in cell number during the treatment period. As is apparent in Fig. 8A, there is considerable variability in proliferation rates among the cultures. Therefore, to rule out clonal variations as major factors determining the proliferative response, we analyzed additional clonal lines of receptor-transduced H-35 cells (Fig. 8B). Clones were chosen that expressed approximately equal levels of immunodetectable receptor proteins to those in the lines used for this study (Fig. 8B, top). Each group of clonal lines showed a comparable G-CSF-sensitive reduction in proliferation, which ranged around 30% for G-gp130(WT) cells and 55% for G-gp130(Y2F) cells (Fig. 8B, bottom). Although proliferation was reduced during the long-term treatment, no adverse effect on APP gene expression was detectable. In fact, the induction of mRNA for haptoglobin (Fig. 8C) and other APPs (data not shown) was much more prominent in the more strongly growth-inhibited G-gp130(Y2F) cells than in G-gp130(WT) cells.
DISCUSSION
In hepatic cells the cytoplasmic domain of gp130 engages two separate signaling pathways, both of which are influenced by SHP-2. As shown previously (34), gp130-recruited SHP-2 attenuates the activity of the JAK/STAT pathways, thereby affecting efficacy and duration of signaling towards induction of APP genes. On the other hand, as shown above, SHP-2 mobilizes the MAP kinase pathway, which stimulates immediate-early response genes, influences proliferation of the cells, and moderates APP production.
Depending upon the experimental model, gp130 signaling has been characterized in terms of regulated transcription of genes, such as APP in liver cells (42), or proliferation and differentiation, such as in lymphoid and myeloid cells (19, 49, 79). Structure-function analysis of gp130 indicated separate regions within the cytoplasmic domain that are critical for mediating these processes. The definition of gp130 signaling has focused on the JAK-STAT and SHP-2/MAPK pathways (29, 42, 60, 80). Other pathways, which involve members of the Tec, Src and Fes family protein tyrosine kinases, have been proposed (for a review, see reference 25), but none of these have been recognized as being critical for mediating APP induction in hepatoma cells (75a). Data from the different models suggest that the various gp130-regulated responses are not all dependent on an identical array of signals. Most prominently, proliferation, as well as differentiation, requires activation of both STAT and MAP kinase (19, 49, 80) whereas induction of APPs, but not the tissue inhibitor for metalloproteinase-1 (7) or collagen (2), is maximal with activation of STAT3 in the absence of activated MAP kinase.
gp130 recruitment of SHP-2 correlates with activation of the MAP kinase pathway and is necessary for obtaining proliferation control (19, 60). SHP-2 is similarly implicated in mediating a proliferative signal by other receptor systems, such as for insulin, EGF, and PDGF (45). The functional role of SHP-2 in signal transduction in hepatoma cells has been assessed indirectly by preventing recruitment of SHP-2 to gp130 or by overexpressing SHP-2 mutants. The data indicated that in hepatic cells SHP-2 exerts a signal-communicating role toward MAP kinase that is more prominent for gp130 than for EGF receptor and insulin, suggesting that gp130 does not engage as broad a range of alternative signaling pathways as do the growth factor receptors. The results also document the relevance of the phosphatase domain, but not the catalytic function, of SHP-2 in associating with MAP kinase activation (Fig. 4). The extent to which substrate trapping or failure to recruit Grb2 mechanistically contributes to this regulatory phenotype remains to be defined. Even though gp130 signaling to both the SHP-2/ERK and JAK/STAT pathways are evident in hepatoma cells (Fig. 2), a growth-inhibitory rather than growth-stimulatory activity is registered for IL-6 treatment (Fig. 8). The SHP-2-controlled mechanism appears in part to restrain inhibition, explaining why gp130 without SHP-2 engagement [i.e., gp130(Y2F)] exerts a stronger antiproliferative effect (Figs. 7 and 8). The observation that the very same receptor subunit is more effective in STAT3 activation suggests that the STAT3-dependent pathways in H-35 cells may have antiproliferative functions, which may also include modulated expression of cyclin-dependent kinase inhibitors. Cha et al. (11) have observed a growth inhibition of hepatoma cells following dexamethasone treatment that correlated with increased expression of the cyclin-dependent kinase inhibitor p21_cip/WAF-1_. The IL-6-suppressed proliferation of osteoblastic cells has been similarly attributed to an enhanced expression of p21_cip/WAF-1_, in part by gp130-triggered activation of STAT3 and STAT3-sensitive induction of transcription of the p21_cip/WAF-1_ gene (6). Our preliminary immunoblot analysis of H-35 cells (33a) indicated, however, that p21_cip/WAF-1_ protein expression is not appreciably affected by gp130 signaling and that only a minor increase in the level of p27_kip1_ protein was detected after 24 to 48 h of treatment with IL-6 or G-CSF. The molecular mechanism responsible for attenuated proliferation in cytokine-treated hepatoma cells is still unknown.
The precise mode by which SHP-2 restricts STAT3 activation is unclear. As suggested by studies on other hematopoietin receptors, the receptor-recruited and activated protein tyrosine phosphatase, either SHP-1 (28, 35) (gp130 does not interact with SHP-1 [34]) or SHP-2 (34, 66), may desensitize the action of the receptor such as by dephosphorylation of JAK, receptor subunits, or other receptor-associated proteins. However, direct interactions of SHP-2 with JAKs or STATs have not been consistently seen (66, 81). The effects observed with the phosphatase-inactive SHP-2CS (66) and truncated SHP2Δ support the notion that phosphatase activity contributes to the promotion of proliferation (56) and the moderation of gp130 signaling toward gene induction (62, 66). Whether the loss of catalytic activity alone or also the loss of substrate binding activity of SHP-2 assists in enhancing the APP regulation in G-gp130(Y2F) cells remains to be clarified. The experimental approach involving overexpression of the phosphatase-inactive SHP-2CS or SHP-2Δ by transient transfection in hepatoma cells proved inconclusive. Both SHP-2 forms cause a similarly enhanced gp130 signaling toward transfected APP constructs (33a, 34). In contrast, overexpressed SHP-2var and SHP-2ΔC, like wild-type SHP-2, had minimal modulatory effects on IL-6 regulation of APPs (33a).
The components that establish the link of SHP-2 to the MAP kinase pathway at the plasma membrane site still remain to be determined. A number of SHP-2-associated proteins which are considered to be necessary in orchestrating SHP-2-dependent signal communication have been described (31, 32, 54). Particular attention has been paid to the members of the signal regulatory protein (SIRP) family which, in part, are defined by their interaction with SHP-2 and control the activation of MAP kinases (31, 67). Analysis of H-35 cells, however, indicated that these cells, in contrast to normal liver cells, have low levels of SHP-2-interacting proteins and have no appreciable cytokine-activated association with SHP-2, as defined by their ability to coprecipitate with SHP-2 under the conditions used in the experiments in Fig. 1C, 2A, and 2B and by immunoblotting with broad-specificity anti-SIRP antibodies (33a).
Studies on gp130 signaling in hepatoma cells have focused on the induction of type 2 APP genes. The role of STAT3 in mediating the induction of several of such APP genes containing STAT binding elements has been experimentally confirmed (33, 37, 42, 75, 82). In addition, the level of sustained STAT3 DNA binding activity may correlate with APP gene expression (57). The observation that APP gene expression is maintained elevated at maximal level for days in chronically IL-6-treated hepatoma cells argues against an effective negative-feedback signaling system, e.g., by using members of the suppressor-of-cytokine-signaling (SOCS) family as described for other cell types (15, 51, 65). The phosphatase action of SHP-2 moderates the STAT3 activation, as seen immediately following signal initiation (Fig. 2C) (34). During long-term IL-6 treatment, the moderating role of the phosphatase activity of SHP-2 is less evident. Our experimental analysis was unable to detect quantitative differences in phosphotyrosine STAT3 or DNA binding activity of STAT3 at 24 h and later time points during G-CSF treatment in G-gp130(WT) and G-gp130(Y2F) cells (33a).
A separate mode by which SHP-2 affects APP gene regulation is suggested. SHP-2, through the activation of ERK1/2 and the subsequent stimulation of immediate-early genes, might moderate the JAK/STAT-mediated growth inhibitory signal, and the cell proliferation indirectly lowers APP gene induction. Indeed, several lines of evidence indicate an inverse relationship between proliferation and APP gene expression. A higher expression of APP genes was measured in IL-6-treated cultures of H-35 and HepG2 cells, following a prolonged period of serum deprivation (10). Similarly, enhanced growth and reduced APP expression is found in liver during regeneration following partial hepatectomy (44, 48, 61). Growth factors, such as EGF or insulin, that are recognized to stimulate the proliferative potential of hepatic cells exert inhibitory action on APP gene induction in cultured liver cells (10, 74). The prominent ERK activation by these factors through transcription factors such as AP-1, SRF, and Ets-related factors may not only control immediate growth response genes but also modify the transcription rate of APP genes that harbor binding sites for such transcription factors (21). Although ERK-sensitive transcription factors appear to be stimulatory on certain genes (TIMP-1) (9, 38), inhibitory action on others, such as rat α-, β-, and γ-fibrinogen and thiostatin, is also detected (33a, 74). Moreover, it is likely that ERKs also indirectly affect APP expression by their ability to phosphorylate signal-transducing proteins such as STAT3 (78, 83), C/EBP (52), or glucocorticoid receptors (39), which have target sequences in certain APP promoters. Even though those ERK-dependent changes may be relatively minor and remain largely undetectable by our biochemical analysis of G-gp130(WT) versus G-gp130(Y2F) cells, their combined effects on APP gene transcription, accumulation of APP mRNA, and production of APP appear to be substantial (Fig. 8C).
ACKNOWLEDGMENTS
We are greatly indebted to Immunex Corporation and Genetics Institute for generously providing cytokines; H. Ohnishi and G.-S. Feng for providing various SHP-2 constructs; S. Pruitt for providing probes for Egr-1, JunB, and c-Jun; A. Ullrich for providing anti-SIRP antibodies; Y. Wang and Erin Kinzie for providing assistance in experimental work; R. G. Hawley for generating recombinant G-gp130 retrovirus; C. Stewart and D. C. Sheedy for performing flow cytometric work; C.-F. Lai and O. Robledo for giving helpful advice; and L. Scere and M. Held for performing secretarial assistance.
This work was supported by NIH grant CA26122 to H.B. and Roswell Park grant CA16056.
REFERENCES
- 1.Adachi M, Ishino M, Torigoe T, Minami Y, Matozaki T, Miyazaki T, Taniguchi T, Hinoda Y, Imai K. Interleukin-2 induces tyrosine phosphorylation of SHP-2 through IL-2 receptor beta chain. Oncogene. 1997;14:1629–1633. doi: 10.1038/sj.onc.1200981. [DOI] [PubMed] [Google Scholar]
- 2.Auble D T, Brinckerhoff C E. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. 1991;30:4629–4635. doi: 10.1021/bi00232a039. [DOI] [PubMed] [Google Scholar]
- 3.Baumann H, Hill R E, Sauder D N, Jahreis G P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986;102:370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann H, Prowse K R, Marinokovic S, Won K-A, Jahreis G P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280–295. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumann H, Gearing D, Ziegler S F. Signaling by the cytoplasmic domain of hematopoietin receptors involves two distinguishable mechanisms in hepatic cells. J Biol Chem. 1994;269:16297–16304. [PubMed] [Google Scholar]
- 6.Bellido T, O’Brien C A, Roberson P K, Manolagas S C. Transcriptional activation of the p21(WAF1,CIP1,SDI1) gene by interleukin-6 type cytokines. J Biol Chem. 1998;273:21137–21144. doi: 10.1074/jbc.273.33.21137. [DOI] [PubMed] [Google Scholar]
- 7.Botelho F M, Edwards D R, Richards C D. Oncostatin M stimulates c-Fos to bind a transcriptionally responsive AP-1 element within the tissue inhibitor of metalloproteinase-1 promoter. J Biol Chem. 1998;273:5211–5218. doi: 10.1074/jbc.273.9.5211. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg J F, Horvath C M, Besser D, Lathem W W, Darnell J E., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugno M, Graeve L, Gatsios P, Koj A, Heinrich P C, Travis J, Kordula T. Identification of the interleukin-6/oncostatin M response element in the rat tissue inhibitor of metalloproteinases-1 (TIMP-1) promoter. Nucleic Acids Res. 1995;23:5041–5047. doi: 10.1093/nar/23.24.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos S P, Wang Y, Baumann H. Insulin modulates STAT3 protein activation and gene transcription in hepatic cells. J Biol Chem. 1996;271:24418–24424. doi: 10.1074/jbc.271.40.24418. [DOI] [PubMed] [Google Scholar]
- 11.Cha H H, Cram E J, Wang E C, Huang A J, Kasler H G, Firestone G L. Glucocorticoids stimulate p21 gene expression by targeting multiple transcriptional elements within a steroid responsive region of the p21waf1/cip1 promoter in rat hepatoma cells. J Biol Chem. 1998;273:1998–2007. doi: 10.1074/jbc.273.4.1998. [DOI] [PubMed] [Google Scholar]
- 12.Chirgwin J M, Przybyia A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 13.David M, Zhou G, Pine R, Dixon J E, Larner A C. The SH2 domain-containing tyrosine phosphatase PTP1D is required for interferon alpha/beta-induced gene expression. J Biol Chem. 1996;271:15862–15865. doi: 10.1074/jbc.271.27.15862. [DOI] [PubMed] [Google Scholar]
- 14.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 16.Feng G-S, Hui C-C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 17.Fuhrer D K, Feng G S, Yang Y C. Syp associates with gp130 and Janus kinase 2 in response to interleukin-11 in 3T3-L1 mouse preadipocytes. J Biol Chem. 1995;270:24826–24830. doi: 10.1074/jbc.270.42.24826. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 20.Gutch M J, Flint A J, Keller J, Tonks N K, Hengartner M O. The Caenorhabditis elegans SH2 domain-containing protein tyrosine phosphatase PTP-2 participates in signal transduction during oogenesis and vulval development. Genes Dev. 1998;12:571–585. doi: 10.1101/gad.12.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattori M, Tugores A, Westwick J K, Veloz L, Leffert H L, Karin M, Brenner D A. Activation of activating protein 1 during hepatic acute phase response. Am J Physiol. 1993;264:G95–G103. doi: 10.1152/ajpgi.1993.264.1.G95. [DOI] [PubMed] [Google Scholar]
- 22.Hawley R G, Lieu F H, Fong A Z, Goldman S J, Leonard J P, Hawley T S. Retroviral vectors for production of interleukin-12 in the bone marrow to induce a graft-versus-leukemia effect. Ann. N.Y., Acad. Sci. 1996;795:341–345. doi: 10.1111/j.1749-6632.1996.tb52687.x. [DOI] [PubMed] [Google Scholar]
- 23.Hemmann U, Gerhartz C, Heesel B, Sasse J, Kurapkat G, Grotzinger J, Wollmer A, Zhong Z, Darnell J E, Jr, Graeve L, Heinrich P C, Horn F. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. J Biol Chem. 1996;271:12999–3007. doi: 10.1074/jbc.271.22.12999. [DOI] [PubMed] [Google Scholar]
- 24.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 25.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 26.Hof P, Pluskey S, Dhe-Paganon S, Eck M J, Shoelson S E. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 27.Horan T, Wen J, Narhi L, Parker V, Garcia A, Arakawa T, Philo J. Dimerization of the extracellular domain of granulocyte-colony stimulating factor receptor by ligand binding: a monovalent ligand induces 2:2 complexes. Biochemistry. 1996;35:4886–4896. doi: 10.1021/bi9525841. [DOI] [PubMed] [Google Scholar]
- 28.Jiao H, Berrada K, Yang W, Tabrizi M, Platanias L C, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol. 1996;16:6985–6992. doi: 10.1128/mcb.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem. 1996;271:5961–5964. doi: 10.1074/jbc.271.11.5961. [DOI] [PubMed] [Google Scholar]
- 30.Kazlauskas A, Feng G S, Pawson T, Valius M. The 64-kDa protein that associates with the platelet-derived growth factor receptor beta subunit via Tyr-1009 is the SH2-containing phosphotyrosine phosphatase Syp. Proc Natl Acad Sci USA. 1993;90:6939–6943. doi: 10.1073/pnas.90.15.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 32.Kharitonenkov A, Schnekenburger J, Chen Z, Knyazev P, Ali S, Zwick E, White M, Ullrich A. Adapter function of protein-tyrosine phosphatase 1D in insulin receptor/insulin receptor substrate-1 interaction. J Biol Chem. 1995;270:29189–29193. doi: 10.1074/jbc.270.49.29189. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Baumann H. The carboxyl-terminal region of STAT3 controls gene induction by the mouse haptoglobin promoter. J Biol Chem. 1997;272:14571–14579. doi: 10.1074/jbc.272.23.14571. [DOI] [PubMed] [Google Scholar]
- 33a.Kim, H., and H. Baumann. Unpublished observation.
- 34.Kim H, Hawley T S, Hawley R G, Baumann H. Protein tyrosine phosphatase 2 (SHP-2) moderates signaling by gp130 but is not required for the induction of acute-phase plasma protein genes in hepatic cells. Mol Cell Biol. 1998;18:1525–1533. doi: 10.1128/mcb.18.3.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klingmuller U, Lorenz U, Cantley L C, Neel B G, Lodish H F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 36.Knowles B B, Howe C C, Aden D P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 37.Kordula T, Ripperger J, Morella K M, Travis J, Baumann H. Two separate signal transducer and activator of transcription proteins regulate transcription of the serine proteinase inhibitor-3 gene in hepatic cells. J Biol Chem. 1996;271:6752–6757. doi: 10.1074/jbc.271.12.6752. [DOI] [PubMed] [Google Scholar]
- 38.Korzus E, Nagase H, Rydell R, Travis J. The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. J Biol Chem. 1997;272:1188–1196. doi: 10.1074/jbc.272.2.1188. [DOI] [PubMed] [Google Scholar]
- 39.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhne M R, Pawson T, Lienhard G E, Feng G-S. The insulin receptor substrate 1 associates with the SH-2 containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- 41.Kumar G, Gupta S, Wang S, Nel A E. Involvement of Janus kinases, p52shc, Raf-1, and MEK-1 in the IL-6 induced mitogen-activated protein kinase cascade of growth responsive B cell line. J Immunol. 1994;152:4436–4447. [PubMed] [Google Scholar]
- 42.Lai C F, Ripperger J, Morella K K, Wang Y, Gearing D P, Fey G H, Baumann H. Separate signaling mechanisms are involved in the control of STAT protein activation and gene regulation via the interleukin 6 response element by the box 3 motif of gp130. J Biol Chem. 1995;270:14847–14850. doi: 10.1074/jbc.270.25.14847. [DOI] [PubMed] [Google Scholar]
- 43.Lai C-F, Ripperger J, Wang Y, Kim H, Hawley R B, Baumann H. The STAT3-independent signaling pathway by glycoprotein 130 in hepatic cells. J Biol Chem, 1999;274:7793–7802. doi: 10.1074/jbc.274.12.7793. [DOI] [PubMed] [Google Scholar]
- 44.Leffert H L, Koch K S, Lu X P, Brenner D A, Karin M, Skelly H F, Rippe R A, Fey G, Chojkier M. Cellular and molecular biology of hepatocyte growth, regeneration and gene expression. Adv Second Messenger Phosphoprotein Res. 1990;24:352–358. [PubMed] [Google Scholar]
- 45.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutticken C, Wegenka U M, Yuan J, Buschmann J, Schindler C, Ziemiecki A, Harpur A G, Wilks A F, Yasukawa K, Taga T, Kishimoto T, Barbieri G, Pellegrini S, Sendtner M, Cotteinrich P, Horn F. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- 47.Milarski K L, Saltiel A R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 48.Milland J, Tsykin A, Thomas T, Aldred A R, Cole T, Schreiber G. Gene expression in regenerating and acute-phase rat liver. Am J Physiol. 1990;259:G340–G347. doi: 10.1152/ajpgi.1990.259.3.G340. [DOI] [PubMed] [Google Scholar]
- 49.Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, Akira S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA. 1996;93:3963–3966. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosley B, Beckmann M P, March C J, Idzerda R L, Gimpel S D, Vanden Bos T, Friend D, Alpert A, Anderson D, Jackson J, Wignall J M, Smith C, Gallis B, Sims J E, Urdal D, Widmer M B, Cosman D. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- 51.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci USA. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 54.Ohnishi H, Kubota M, Ohtake A, Sato K, Sano S. Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J Biol Chem. 1996;271:25569–25574. doi: 10.1074/jbc.271.41.25569. [DOI] [PubMed] [Google Scholar]
- 55.Qiu Y, Ravi L, Kung H J. Requirement of ErbB2 for signaling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393:83–85. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 56.Qu C K, Feng G S. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–439. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- 57.Ripperger J A, Fritz S, Richter K, Hocke G M, Lottspeich F, Fey G H. Transcription factors Stat3 and Stat5b are present in rat liver nuclei late in an acute phase response and bind interleukin-6 response elements. J Biol Chem. 1995;270:29998–30006. doi: 10.1074/jbc.270.50.29998. [DOI] [PubMed] [Google Scholar]
- 58.Sagawa K, Kimura T, Swieter M, Siraganian R P. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31) J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 59.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiemann W P, Bartoe J L, Nathanson N M. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. J Biol Chem. 1997;272:16631–16636. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- 61.Scotte M, Masson S, Lyoumi S, Hiron M, Teniere P, Lebreton J P, Daveau M. Cytokine gene expression in liver following minor or major hepatectomy in rat. Cytokine. 1997;9:859–867. doi: 10.1006/cyto.1997.0273. [DOI] [PubMed] [Google Scholar]
- 62.Servidei T, Aoki Y, Lewis S E, Symes A, Fink J S, Reeves S A. Coordinate regulation of STAT signaling and c-fos expression by the tyrosine phosphatase SHP-2. J Biol Chem. 1998;273:6233–6241. doi: 10.1074/jbc.273.11.6233. [DOI] [PubMed] [Google Scholar]
- 63.Stahl N, Boulton T G, Farruggella T, Ip N Y, Davis S, Witthuhn B A, Quelle F W, Silvennoinen O, Barbieri G, Pellegrini S, Ihle J N, Yancopoulos G D. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 64.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 65.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 66.Symes A, Stahl N, Reeves S A, Farruggella T, Servidei T, Gearan T, Yancopoulos G, Fink J S. The protein tyrosine phosphatase SHP-2 negatively regulates ciliary neurotrophic factor induction of gene expression. Curr Biol. 1997;7:697–700. doi: 10.1016/s0960-9822(06)00298-3. [DOI] [PubMed] [Google Scholar]
- 67.Takada T, Matozaki T, Takeda H, Fukunaga K, Noguchi T, Fujioka Y, Okazaki I, Tsuda M, Yamao T, Ochi F, Kasuga M. Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J Biol Chem. 1998;273:9234–9242. doi: 10.1074/jbc.273.15.9234. [DOI] [PubMed] [Google Scholar]
- 68.Tang T L, Freeman R M, Jr, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 69.Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, Bohmer F D. Association of SH2 domain protein tyrosine phosphatase with the epidermal growth factor receptor in human tumor cells. J Biol Chem. 1995;270:21277–21284. doi: 10.1074/jbc.270.36.21277. [DOI] [PubMed] [Google Scholar]
- 70.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 71.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 72.Ugi S, Maegawa H, Kashiwagi A, Adachi M, Olefsky J M, Kikkawa R. Expression of dominant negative mutant SHPTP2 attenuates phosphatidylinositol 3′-kinase activity via modulation of phosphorylation of insulin receptor substrate-1. J Biol Chem. 1996;271:12595–12602. doi: 10.1074/jbc.271.21.12595. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Fuller G M. Interleukin-6 and ciliary neurotrophic factor trigger Janus kinase activation and early gene response in rat hepatocytes. Gene. 1995;162:258–289. doi: 10.1016/0378-1119(95)00295-h. [DOI] [PubMed] [Google Scholar]
- 74.Wang, Y., J. Ripperger, G. H. Fey, D. Samols, T. Kordula, M. Wetzler, R. A. Van Etten, and H. Baumann. Regulation of hepatic acute phase genes by epidermal growth factor and Src protein tyrosine kinases. Submitted for publication. [DOI] [PubMed]
- 75.Wang Y, Morella K K, Ripperger J, Lai C F, Gearing D P, Fey G H, Campos S P, Baumann H. Receptors for interleukin-3 (IL-3) and growth hormone mediate an IL-6-type transcriptional induction in the presence of JAK2 or STAT3. Blood. 1995;86:1671–1679. [PubMed] [Google Scholar]
- 75a.Wang, Y., C. Zhu, and H. Baumann. Unpublished results.
- 76.Ward L D, Hammacher A, Howlett G J, Matthews J M, Fabri L, Moritz R L, Nice E C, Weinstock J, Simpson R J. Influence of interleukin-6 (IL-6) dimerization on formation of the high affinity hexameric IL-6.receptor complex. J Biol Chem. 1996;271:20138–20144. doi: 10.1074/jbc.271.33.20138. [DOI] [PubMed] [Google Scholar]
- 77.Welham M J, Dechert U, Leslie K B, Jirik F, Schrader J W. Interleukin (IL)-3 and granulocyte/macrophage colony-stimulating factor, but not IL-4, induce tyrosine phosphorylation, activation, and association of SHPTP2 with Grb2 and phosphatidylinositol 3′-kinase. J Biol Chem. 1994;269:23764–23768. [PubMed] [Google Scholar]
- 78.Wen Z, Zhong Z, Darnell J E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 79.Xia S, Rose D W, Sasuoka T, Maegawa H, Burke T R, Roller P P, Shoelson S E, Olefsky J M. SYP(SH-PTP2) is a positive mediator of growth factor-stimulated signal transduction. J Biol Chem. 1994;269:21244–21248. [PubMed] [Google Scholar]
- 80.Yamanaka Y, Nakajima K, Fukada T, Hibi M, Hirano T. Differentiation and growth arrest signals are generated through the cytoplasmic region of gp130 that is essential for Stat3 activation. EMBO J. 1996;15:1557–1565. [PMC free article] [PubMed] [Google Scholar]
- 81.Yin T, Shen R, Feng G-S, Yang Y-C. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J Biol Chem. 1997;272:1032–1037. doi: 10.1074/jbc.272.2.1032. [DOI] [PubMed] [Google Scholar]
- 82.Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem. 1996;271:9503–9509. doi: 10.1074/jbc.271.16.9503. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Blenis J, Li H C, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]