Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma (original) (raw)
Abstract
Glaucoma is an optic neuropathy with cupping of the optic disk, degeneration of retinal ganglion cells, and characteristic visual field loss. Because elevated intraocular pressure (IOP) is a major risk factor for progression of glaucoma, treatment has been based on lowering IOP. We previously demonstrated inducible nitric-oxide synthase (NOS-2) in the optic nerve heads from human glaucomatous eyes and from rat eyes with chronic, moderately elevated IOP. Using this rat model of unilateral glaucoma, we treated a group of animals for 6 months with aminoguanidine, a relatively specific inhibitor of NOS-2, and compared them with an untreated group. At 6 months, untreated animals had pallor and cupping of the optic disks in the eyes with elevated IOP. Eyes of aminoguanidine-treated animals with similar elevations of IOP appeared normal. We quantitated retinal ganglion cell loss by retrograde labeling with Fluoro-Gold. When compared with their contralateral control eyes with normal IOP, eyes with elevated IOP in the untreated group lost 36% of their retinal ganglion cells; the eyes with similarly elevated IOP in the aminoguanidine-treated group lost less than 10% of their retinal ganglion cells. Pharmacological neuroprotection by inhibition of NOS-2 may prove useful for the treatment of patients with glaucoma.
Glaucoma is characterized by excavation of the optic disk, loss of retinal ganglion cells, and a specific pattern of visual field loss (1, 2). Because elevated intraocular pressure (IOP) is a major risk factor for progression of this optic neuropathy, pharmacological and surgical treatment of glaucoma has been aimed at lowering IOP (3, 4). Recently, the cellular changes in the optic nerve and retina that can lead to retinal ganglion cell degeneration have been studied to determine the relevant mechanisms. A major objective of this work is to devise potential, therapeutic approaches to prevent retinal ganglion cell loss. Using laboratory models, including ischemia, optic nerve transection, optic nerve crush, and culture of retinal ganglion cells, various pharmacological agents have been tested as potential neuroprotective approaches (5–8). Although there have been no pharmacological studies in an animal model that closely resembles human glaucoma, these approaches have suggested that antagonism of excitotoxicity or supplementation of neurotrophic factors can protect, at least temporarily in animal models, retinal ganglion cells from degeneration.
To develop medical therapies for glaucoma based on pharmacological neuroprotection, our research has focused on an underlying mechanism that has been demonstrated in human glaucomatous tissue and that has the neurotoxic potential to cause glaucomatous optic nerve degeneration and loss of retinal ganglion cells. Our laboratory has demonstrated previously that the inducible isoform of nitric-oxide synthase (NOS-2) is present in astrocytes in the optic nerve heads from glaucomatous human eyes (9) and from rat eyes with chronic, moderately elevated IOP (10), but not in normal eyes. We have postulated that excessive nitric oxide causes neurodegeneration of the axons of the retinal ganglion cells in glaucoma. To test our hypothesis and to demonstrate the feasibility of using an inhibitor of NOS-2 as a pharmacological neuroprotective agent to treat glaucoma, we have tested aminoguanidine, a relatively specific inhibitor of NOS-2, in a rat model of glaucoma in which there is chronic, moderately elevated IOP and a slow, progressive loss of retinal ganglion cells.
MATERIALS AND METHODS
Rat Model of Chronic, Moderately Elevated IOP.
Adult, male Wistar rats weighing approximately 250 g at the beginning of the experiment were used. Animals were fed ad libitum and maintained in temperature-controlled rooms on a 12-hr light/12-hr dark cycle. Experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All surgical procedures were done under general anesthesia by using a mixture of 80 mg/kg ketamine (Fort Dodge Laboratories, Fort Dodge, IA) and 12 mg/kg xylazine (Butler, Columbus, OH), given i.p.
Chronic, moderately elevated IOP was produced unilaterally in 16 rats by cautery of three episcleral vessels (11–13); the contralateral eye served as the comparative control. To perform the cautery, sutures were placed in the lids to keep the eye open and in the bulbar conjunctiva to manipulate the globe. Three of the four to five major trunks formed by limbal-derived veins were exposed at the equator of the eye by incising the conjunctiva. Each vessel was lifted with a small muscle hook and cauterized by direct application of an ophthalmic, disposable cautery (Model RS201; Roboz Surgical Instrument Company, Rockville, MD) against the muscle hook. Immediate retraction and absence of bleeding of the cauterized ends of the vessels were noted as successful cauterization. After surgery, eyes were treated topically with bacitracin-neomycin-polymyxin (Pharmaderm, Melville, NY) for a few days during recovery.
Aminoguanidine Treatment and Measurements Taken During Treatment.
One group of eight animals was treated with aminoguanidine, a relatively selective inhibitor of NOS-2 (14–16), in the drinking water for 6 months; a second group of eight animals was untreated. At the time of cauterization, the rats were divided randomly into the two groups (drug-treated and untreated) and caged individually. To inhibit NOS-2 in one group, aminoguanidine (2.0 g/liter) was dissolved in their drinking water, which was made up and provided fresh three times per week. The control group was not treated but received fresh drinking water, from the same source, on the same schedule. At each refilling of the drinking bottle, total volume consumed was recorded. Once a week, each animal was weighed. Once a month, each animal was anesthetized (xylazine/ketamine) and IOP was determined bilaterally (13) by using the Mentor Pneumotonometer Model Classic 30 (Bio-Rad). The animals were awake within 15 min of the IOP measurement. On any given eye, three to five tonometer readings were taken and averaged. On a given day, mean ± SD was derived for all control and surgical (three vessel cautery) eyes. Significant differences between surgical and control eyes were determined by χ2 analysis with Student’s t test for independent means for each day on which measurements were performed.
Clinical Photography of Rat Fundus.
After 6 months of unilateral, chronic, moderately elevated IOP, photographs were taken of the optic disks of each eye of anesthetized rats with a fundus camera (50IA; Topcon, Tokyo, Japan) through a coverslip placed on the cornea with a drop of Goniosol (CIBAVision Ophthalmics, Atlanta, GA). Color photographs and red-free photographs for relief imaging were processed by using imagenet (Topcon).
Labeling and Counting of Retinal Ganglion Cells.
One week before sacrifice, Fluoro-Gold (Fluorochrome, Englewood, CO) was microinjected bilaterally into the superior colliculi of anesthetized rats immobilized in a stereotaxic apparatus (17). Fluoro-Gold is taken up by the axon terminals of the retinal ganglion cells and bilaterally transported retrogradely to the somas in the retina. The Fluoro-Gold labels approximately 95% of the retinal ganglion cells and persists for at least 3 weeks without significant fading or leakage. One week after Fluoro-Gold application, animals were sacrificed by overdose of the above anesthetic mixture and whole, flat-mounted retinas were assayed for retinal ganglion cell density. Rat eyes were enucleated and fixed in 4% paraformaldehyde for 30 min. Eyes were bisected at the equator, the lens was removed, and the posterior segments were prepared for flat mounts. Retinas were dissected from the underlying sclera, flattened by six radial cuts (largest cut superior for orientation), and mounted vitreal side up on gelatin-coated slides.
Labeled retinal ganglion cells were counted by using fluorescence microscopy in 12 identical-size fields of retina, as described previously (13). Noting retinal topography, six fields in two regional areas, approximately 1.0 (central) and 4.0 (peripheral) mm from the optic disk, were counted at ×125 magnification. We counted approximately 15% of the total retinal ganglion cells in each eye by using digital micrography (Spot; Diagnostic Instruments, Sterling Heights, MI) and thresholding black and white images and computer scanning for particle analysis by using optimas software (Optimas Corporation, Bothell, WA). Changes in retinal ganglion cell densities were expressed as percent loss of retinal ganglion cells comparing surgical and contralateral, control eyes from the same animal in the different retinal regions. The Wilcoxon signed-rank test was used to compare parameters between the untreated and aminoguanidine-treated groups.
Histology of Cross-Sections of Rat Optic Nerves.
Two of the eight animals in each group were used for histology. Segments of the myelinated portion of the optic nerves were fixed in 4% paraformaldehyde overnight and postfixed in 2% osmium tetroxide for 2 hr. Nerves then were dehydrated in alcohol, embedded in Epon, sectioned as 1-μm cross-sections, and stained with paraphenylene-diamine to identify myelin profiles.
RESULTS
Systemic Effects of Aminoguanidine.
Over the 6 months, there were no differences in body weights between groups. The starting weight (these and all values presented below are mean ± SD) in both groups was 255 ± 10 g; the weight at 6 months in both groups was 575 ± 15 g. In previous reports on the use of aminoguanidine in experimental animals, the drug did not affect body weight, cardiovascular functions, or kidney functions (16, 18, 19).
The two groups did consume different volumes of water. On a per day basis, the group that was not treated pharmacologically drank 41.2 ± 10.6 ml/day, whereas the group that was treated with aminoguanidine drank 30.2 ± 4.9 ml/day (P < 0.01). Given this volume of drinking, we calculate that the treated group received 60 mg aminoguanidine per day. Dosing was not increased as the animals gained weight during the 6 months of this experiment.
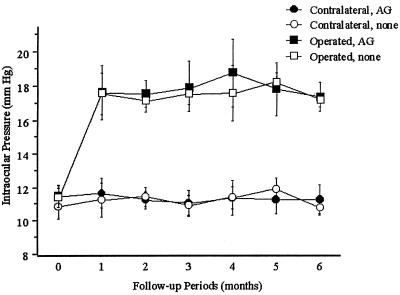
As demonstrated in Fig. 1, IOP was elevated in all eyes for 6 months after receiving three-vessel cautery (approximately 18 mmHg; 1 mmHg = 133 Pa) compared with the contralateral, control eyes (approximately 11.5 mmHg). Comparing animals that were not treated pharmacologically with animals treated with aminoguanidine, the elevated IOPs in the three vessel cautery eyes were the same. The IOPs in the contralateral eyes of these two groups were also the same. Thus, aminoguanidine did not affect IOP.
Figure 1.
Chronic, moderately elevated IOP in rat eyes that had three episcleral vessels cauterized, unilaterally (Operated), vs. the opposite eye (Contralateral). IOP was measured on anesthetized animals with a Mentor Classic 30 Pneumotonometer. In one group (n = 8), aminoguanidine (AG) was added to the drinking water; in the control group (n = 8), nothing was added to the drinking water (none). Throughout the 6-month follow-up, IOP was elevated in all Operated eyes compared with Contralateral eyes (P < 0.01). Elevated IOP was not different in the group treated with aminoguanidine vs. the group not treated pharmacologically (_P_ > 0.1). All values are mean ± SD.
Effects of Aminoguanidine on the Optic Nerve and Retina in Animals with Chronic, Moderately Elevated IOP.
After 6 months of unilateral, chronic, moderately elevated IOP, an experienced glaucoma specialist (B.B.) performed ophthalmoscopy on the rat eyes in a masked manner. By observing the optic disk, he correctly identified as glaucomatous the eyes with elevated IOP in animals that were not treated pharmacologically. He noted cupping and pallor of the optic disk and bending backward of the vessels over the edge of the cup as they progressed toward the center of the disk. In the contralateral eyes with normal IOP, there was no cup and the vessels were flat along the disk. In animals treated with aminoguanidine, he noted little or no differences in the appearance of the optic disks when comparing eyes with normal IOP and eyes with elevated IOP.
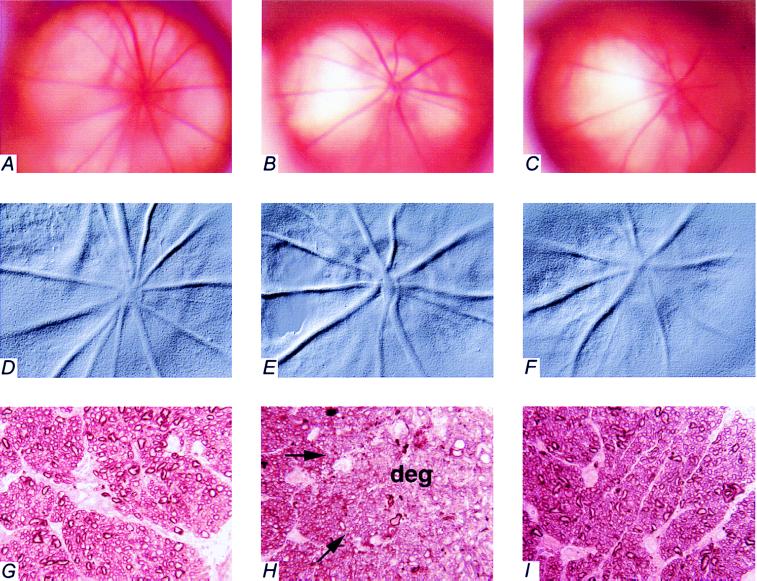
Fig. 2 shows the clinical appearances of the optic disks of the rats in vivo. In the eye with normal IOP (Fig_. 2 A_ in color and D in relief), the disk is pink and the vessels emerging from (arteries) and returning to (veins) the optic disk appear to run straight and flat from the center of the optic disk. In the eye with chronic, moderately elevated IOP for 6 months from an animal not treated pharmacologically (Fig. 2 B in color and E in relief), the optic disk is pale and cupped and the vessels, especially the veins, appear to dip over the rim of the cup. The projections of the vessels to the center of the optic disk are behind the plane of focus. In the eye with chronic, moderately elevated IOP for 6 months from an animal treated with aminoguanidine (Fig. 2 C in color and F in relief), there is no pallor or cupping and the vessels can be followed clearly to the center of the optic disk. These eyes look similar, clinically, to the eyes with normal IOP.
Figure 2.
Fundus photographs and histological cross-sections of optic nerves from eyes with normal IOP and chronic, moderately elevated IOP, with and without treating the animals with aminoguanidine for 6 months. Color fundus photographs (A–C) and red-free photographs for relief imaging (D–F) were taken on anesthetized animals. The highlights in B and C are reflections of light from the camera. Optic nerve cross-sections (G–I) were stained for myelin profiles (magnification, ×1,250). The peripheral edge of the optic nerve is to the right side of each photomicrograph, opposite the label (G–I). Arrows in H point to the approximate boundary between normal-appearing optic nerve tissue and degenerated (deg) tissue. Pictures from the same eyes are as follows: A, D, and G are from an eye with normal IOP and contralateral to B, E, and H, which are from an eye with chronic, moderately elevated IOP for 6 months from an animal not pharmacologically treated. C, F, and I are from an eye with chronic, moderately elevated IOP for 6 months from an animal treated with aminoguanidine.
Fig. 2 also shows cross-sections through the myelinated portion of the corresponding optic nerves. Comparing eyes with chronic, moderately elevated IOP with the contralateral eyes with normal IOP (Figs. 2G), axonal degeneration is apparent in peripheral regions of the optic nerve cross-section in the animal that was not treated pharmacologically (Fig. 2H) but is absent in the animal treated with aminoguanidine (Fig. 2I).
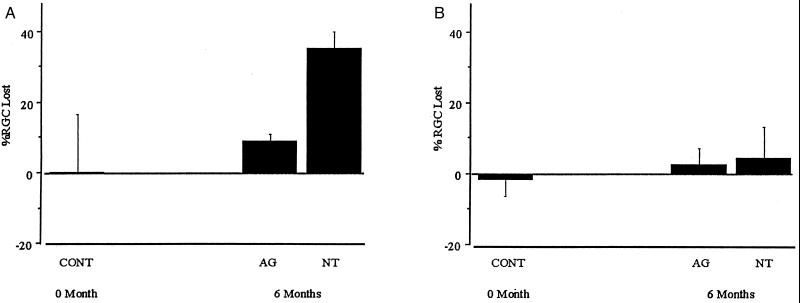
One week before sacrifice, the retinal ganglion cells of both eyes were labeled by bilateral injection of Fluoro-Gold into the superior colliculus. Upon enucleation, flat mounts of paired retinae were made and the retinal ganglion cells were counted in peripheral and central retinal areas. Fig. 3 demonstrates the loss of retinal ganglion cells by comparing the eye with elevated IOP to the contralateral, control eye in animals not treated pharmacologically and in animals treated with aminoguanidine. In the peripheral retina (Fig. 3A), retinal ganglion cell loss in eyes with chronic, moderately elevated IOP was 35.9 ± 5.7% at 6 months in untreated animals and 9.6 ± 3.5% at 6 months in animals treated with aminoguanidine (P < 0.01). In the peripheral retina, the number of retinal ganglion cells in eyes with chronic, moderately elevated IOP of animals not treated pharmacologically was significantly different than normal eyes (_P_ < 0.01). However, the number of retinal ganglion cells in eyes with chronic, moderately elevated IOP of animals treated with aminoguanidine was not significantly different than normal eyes (_P_ > 0.1). In the central retina, proximal to the optic disk, loss of retinal ganglion cells in eyes with chronic, moderately elevated IOP was not apparent (Fig. 3B). The loss of retinal ganglion cells in the peripheral retina is consistent with the peripheral areas of neurodegeneration in the optic nerve cross-sections (Fig. 2H).
Figure 3.
Percentage of retinal ganglion cells (RGC) lost at 6 months in rat eyes with chronic, moderately elevated IOP. One week after Fluoro-Gold labeling, retinal ganglion cells were counted in both eyes of each animal (n = 6 for the aminoguanidine-treated group; n = 6 for the untreated group). RGCs were counted in the peripheral retina (A), approximately 4.0 mm from the optic disk, and in the central retina (B), approximately 1.0 mm from the optic disk. To demonstrate uniform bilateral distribution of Fluoro-Gold in retinal ganglion cells after bilateral injection into the superior colliculus, a separate group of normal animals (n = 6) was studied. These animals had normal IOP bilaterally. The mean ± SD of the ratios of retinal ganglion cell counts for left and right eyes were 0.99 ± 0.16 in the peripheral retina and 1.02 ± 0.05 in the central retina. These values were used for the determination of percent RGC lost at “0 Month” (CONT); % RGC lost = 1 − [RGC density in eye with elevated IOP/RGC density in eye with normal IOP] × 100%. In peripheral retina at 6 months, percent RGC lost in eyes with elevated IOP from animals that were not treated pharmacologically (NT) is statistically significantly different than control (P < 0.01), whereas percent RGC lost in eyes with elevated IOP from animals that were treated with aminoguanidine (AG) is not statistically significantly different than control (_P_ > 0.1). All values are mean ± SD.
DISCUSSION
Because of the excessive nitric oxide produced by NOS-2, this isoform is cytodestructive in many tissues and neurotoxic in the central nervous system (20). We have hypothesized that excessive NO, released by reactive astrocytes, leads to the formation of peroxynitrite, which damages the axons of the retinal ganglion cells at the level of the lamina cribrosa in the optic nerve head (21). We interpret our current results to indicate that, by pharmacologically inhibiting NOS-2, we have protected the axons at the level of the optic nerve head from the neurodegeneration that leads to loss of retinal ganglion cells upon exposure to chronic, moderately elevated IOP. By using the rat model to demonstrate that NOS-2 is present in the target tissue and that inhibition of NOS-2 protects against the loss of retinal ganglion cells, our results support a cause-and-effect relationship between excessive NO and the optic neuropathy associated with chronic, moderately elevated IOP.
In the rat model that we have used, there is a slow, progressive optic nerve degeneration and loss of retinal ganglion cells that in many ways resembles the optic neuropathy of human glaucoma. Previously, the only experimental model that had been reported to have optic nerve changes similar to human glaucoma was the monkey model of laser-induced glaucoma (22). Our results demonstrate the utility of the rat model of chronic, moderately elevated IOP for testing pharmacological neuroprotective agents for the treatment of glaucoma.
These results demonstrate that pharmacological neuroprotection, using an inhibitor of NOS-2, may be a viable approach to treat glaucoma. This approach may be effective with or without a pharmacological agent to lower IOP. Inhibition of NOS-2 may be accomplished with an inhibitor of enzymatic activity, such as aminoguanidine, or by blocking induction and gene expression of the isoform. We emphasize that, in our experiments, pharmacological neuroprotection was accomplished without lowering the chronically elevated IOP.
The ability of aminoguanidine to inhibit NOS-2 was demonstrated after several years of use of the drug experimentally in diabetes models (23). Aminoguanidine also inhibits the formation of advanced glycation end products associated with diabetes (24, 25). There is no evidence to suggest that advanced glycation end products are relevant to the glaucomatous process. However, it is noteworthy for considering pharmacological neuroprotection for patients with glaucoma that aminoguanidine has been used in human clinical trials for complications related to diabetes.
Acknowledgments
With appreciation, we acknowledge Sucharita Das and Smita Vora for their assistance in the care and handling of the animals, Rhonda Curtis for performing the fundus photography, and Dr. M. Rosario Hernandez for assistance with the histology. This work was supported in part by grants from the National Institutes of Health (EY12017) and the Glaucoma Foundation and by National Institutes of Health Core Grant EY02687.
ABBREVIATIONS
IOP
intraocular pressure
NOS-2
inducible nitric-oxide synthase
Footnotes
A Commentary on this article begins on page 9455.
References
- 1.Armaly M F, Krueger D E, Maunder L, Becker B, Hetherington J, Jr, Kolker A E, Levene R Z, Maumenee A E, Pollack I P, Shaffer R N. Arch Ophthalmol. 1980;98:2163–2171. doi: 10.1001/archopht.1980.01020041015002. [DOI] [PubMed] [Google Scholar]
- 2.Quigley H A, Addicks E M, Green W R, Maumenee A E. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D R. Am J Ophthalmol. 1989;108:485–495. doi: 10.1016/0002-9394(89)90423-6. [DOI] [PubMed] [Google Scholar]
- 4.Alward W L. N Engl J Med. 1998;339:1298–1307. doi: 10.1056/NEJM199810293391808. [DOI] [PubMed] [Google Scholar]
- 5.Adachi K, Fujita Y, Morizane C, Akaike A, Ueda M, Satoh M, Masai H, Kashii S, Honda Y. Eur J Pharmacol. 1998;350:53–57. doi: 10.1016/s0014-2999(98)00317-3. [DOI] [PubMed] [Google Scholar]
- 6.Yoles E, Schwartz M. Arch Ophthalmol. 1998;116:906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- 7.Di Polo A, Aigner L J, Dunn R J, Bray G M, Aguayo A J. Proc Natl Acad Sci USA. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprioli J, Kitano S, Morgan J E. Invest Ophthalmol Visual Sci. 1996;37:2376–2381. [PubMed] [Google Scholar]
- 9.Neufeld A H, Hernandez M R, Gonzalez M. Arch Ophthalmol. 1997;115:497–503. doi: 10.1001/archopht.1997.01100150499009. [DOI] [PubMed] [Google Scholar]
- 10.Shareef, S. R., Sawada, A. & Neufeld, A. H. (1999) Invest. Ophthalmol. Visual Sci., in press. [PubMed]
- 11.Shareef S R, Garcia-Valenzuela E, Salierno A, Walsh J, Sharma S C. Exp Eye Res. 1995;61:379–382. doi: 10.1016/s0014-4835(05)80131-9. [DOI] [PubMed] [Google Scholar]
- 12.Laquis S, Chaudhary P, Sharma S C. Brain Res. 1998;784:100–104. doi: 10.1016/s0006-8993(97)01189-x. [DOI] [PubMed] [Google Scholar]
- 13.Sawada, A. & Neufeld, A. H. (1999) Exp. Eye Res., in press. [DOI] [PubMed]
- 14.Bryk R, Wolff D J. Biochemistry. 1998;37:4844–4852. doi: 10.1021/bi972065t. [DOI] [PubMed] [Google Scholar]
- 15.Brenner T, Brocke S, Szafer F, Sobel R A, Parkinson J F, Perez D H, Steinman L. J Immunol. 1997;158:2940–2946. [PubMed] [Google Scholar]
- 16.Waz W R, Van Liew J B, Feld L G. Kidney Blood Press Res. 1997;20:211–217. doi: 10.1159/000174148. [DOI] [PubMed] [Google Scholar]
- 17.Selles-Navarro I, Villegas-Perez M P, Salvador-Silva M, Ruiz-Gomez J M, Vidal-Sanz M. Invest Ophthalmol Visual Sci. 1996;37:2002–2014. [PubMed] [Google Scholar]
- 18.Turner C H, Owan I, Jacobs D S, McClintock R, Peacock M. Bone. 1997;21:487–490. doi: 10.1016/s8756-3282(97)00202-0. [DOI] [PubMed] [Google Scholar]
- 19.Soulis T, Cooper M E, Sastra S, Thallas V, Panagiotopoulos S, Bjerrum O J, Jerums G. Diabetologia. 1997;40:1141–1151. doi: 10.1007/s001250050799. [DOI] [PubMed] [Google Scholar]
- 20.Dawson V L, Dawson T M. Proc Soc Exp Biol Med. 1996;211:33–40. doi: 10.3181/00379727-211-43950e. [DOI] [PubMed] [Google Scholar]
- 21.Neufeld A H. J Glaucoma. 1998;7:434–438. [PubMed] [Google Scholar]
- 22.Quigley H A, Nickells R W, Kerrigan L A, Pease M E, Thibault D J, Zack D J. Invest Ophthalmol Visual Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 23.Corbett J A, Tilton R G, Chang K, Hasan K S, Ido Y, Wang J L, Sweetland M A, Lancaster J R, Jr, Williamson J R, McDaniel M L. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]