Dbf4p, an Essential S Phase-Promoting Factor, Is Targeted for Degradation by the Anaphase-Promoting Complex (original) (raw)
Abstract
The Dbf4p/Cdc7p protein kinase is essential for the activation of replication origins during S phase. The catalytic subunit, Cdc7p, is present at constant levels throughout the cell cycle. In contrast, we show here that the levels of the regulatory subunit, Dbf4p, oscillate during the cell cycle. Dbf4p is absent from cells during G1 and accumulates during the S and G2 phases. Dbf4p is rapidly degraded at the time of chromosome segregation and remains highly unstable during pre-Start G1 phase. The rapid degradation of Dbf4p during G1 requires a functional anaphase-promoting complex (APC). Mutation of a sequence in the N terminus of Dbf4p which resembles the cyclin destruction box eliminates this APC-dependent degradation of Dbf4p. We suggest that the coupling of Dbf4p degradation to chromosome separation may play a redundant role in ensuring that prereplicative complexes, which assemble after chromosome segregation, do not immediately refire.
In budding yeast, entry into S phase is brought about by the action of two protein kinases, whose catalytic subunits are encoded by the CDC28 and CDC7 genes. Both of these kinases require association with regulatory subunits to become fully activated. For Cdc28p, the regulatory subunits are a family of related proteins known as the cyclins (1). As their name suggests, cyclins are often present during only part of the cell cycle (18). The B-type cyclins (Clbs) are rapidly degraded in mitosis and remain highly unstable during pre-Start G1. This cell cycle-regulated, ubiquitin-mediated degradation is brought about by the action of the multisubunit anaphase-promoting complex (APC), also known as the cyclosome (36).
It is thought that a single protein, Dbf4p, activates the Cdc7 protein kinase (37, 50). Cdc7p and Dbf4p physically associate with each other and show a number of genetic interactions (4, 16, 20, 27, 31). Cdc7p was shown to be inactive in extracts from either dbf4 or cdc7 mutants; however, active kinase could be reconstituted by mixing these extracts (27). These experiments strongly support the idea that Dbf4p interacts with and activates Cdc7p.
In addition to interacting with Cdc7p, Dbf4p interacts with replication origins in vivo (16, 20). This interaction requires the essential ARS consensus sequence, which serves in vitro and in vivo as the binding site for the origin recognition complex (16). Analysis of Dbf4p deletions indicated that the origin-interacting domain of Dbf4p could be separated from the Cdc7p-interacting domain, which led to the proposal that in addition to activating Cdc7p, Dbf4p plays an essential role in recruiting the active kinase to replication origins (16). This model is consistent with recent experiments showing that Cdc7p is not simply a global regulator of the G1-S transition but, instead, is required throughout S phase for the firing of individual origins (3, 14). Several lines of evidence suggest that at least some of the targets of Cdc7p are members of the Mcm2 to Mcm7 family of proteins (19, 32) which are essential components of prereplicative complexes (pre-RCs) at replication origins (2, 15, 33, 48). Together, these results suggest that Cdc7p acts directly on pre-RCs to trigger initiation. Since the Dbf4p-Cdc7p appears to have been conserved in evolution (4, 25, 29, 34, 43), understanding the regulation of this kinase is important in understanding how the initiation of DNA replication is regulated in eukaryotes.
The DBF4 gene is expressed throughout the cell cycle (47); however, DBF4 transcript levels may show a modest increase at the end of G1 (5). To date, Dbf4p levels have not been examined in detail. Here we show that Dbf4p levels fluctuate during the cell cycle and that part of this fluctuation is due to cell cycle-regulated proteolysis of Dbf4p. The cell cycle-regulated degradation of Dbf4p requires the APC. Thus, not only is Dbf4p similar to cyclins in activating a protein kinase with an essential role in cell cycle progression but also its degradation occurs by a related mechanism.
MATERIALS AND METHODS
Plasmids and strains.
For epitope tagging Dbf4p, the 3′ end of the DBF4 gene was amplified by PCR with two oligonucleotides (5′AAAAAAGGATCCAAATGCAAATAACTCAATTTTTTG3′ and 5′AAAAAAGGATCCCAATATTTGAAATCTGAG3′). After _Bam_HI digestion, the PCR product was cloned in frame with the c-Myc epitope and 9 histidine residues in pMHT (15, 35). The plasmid was linearized with _Bsu_36I and transformed into W303-1a. This yields a full-length, epitope-tagged DBF4 gene under the control of its own promoter and a nonfunctional, untagged fragment of the 3′ end of the gene as previously described (35). For constructing epitope-tagged Gal1,10-driven Dbf4p, the 5′ end of the DBF4 gene was amplified by PCR (primers 5′AAAAAAGGATCCAATGGTTTCTCCAACG3′ and 5′AAAAAAGGATCCTATGTATTTAATGTAAGAAAC3′). After _Bam_HI digestion the PCR product was cloned into pMHTGal, a derivative of pMHT in which the Gal1,10 promoter was inserted between the _Eco_RI and _Bam_HI sites. The plasmid was linearized prior to transformation on galactose-containing plates. This results in the full-length DBF4 gene under the control of the Gal1,10 promoter and a truncated, nonfunctional 5′ fragment of the DBF4 gene. The resulting strains can grow on galactose-containing medium but cannot grow on glucose-containing medium.
To construct the DBF4 R62A L65A mutant, we cloned the full-length DBF4 gene (the PCR product of primers 5′AAAAAAGGATCCATGGTTTCTCCAACGAAAATG3′ and 5′AAAAAAGTCGACTATTTGAAATCTGAGATTTTC3′) into the pMHTgal vector. The resulting plasmid was used as the template for a single round of site-directed mutagenesis with the PCR primers 5′AGATCTCTTGAGGCCCTCGAGGCCCAACAGCAGC3′ and 5′GCTGCTGTTGGGCCTCGAGGGCCTCAAGAGATCT3′ as described in the QuikChange site-directed mutagenesis kit (Stratagene).
The yeast strains used are listed in Table 1.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| W303-1a | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 | 49 |
| yMIG02 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cdc16-123 | 17 |
| yMIG03 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cdc23-1 | 17 |
| yMIG07 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 DBF4::DBF4 myc URA3 | This study |
| yMIG08 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 DBF4::pGAL1-10-mycDBF4 URA3 | This study |
| yMIG10 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cdc16-123 DBF4::pGAL1-10-mycDBF4 URA3 | This study |
| yMIG11 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cdc23-1, DBF4::pGAL1-10-mycDBF4 URA3 | This study |
| yMIG12 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ura3-1::URA3 pGAL1-10-mycDBF4 R62A L65A | This study |
| yMIG13 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cdc16-123 ura3-1::URA3 pGAL1-10-mycDBF4 R62A L65A | This study |
| 2032 | MATa ade2-1 ura3-1 his3-11,15 leu2-3,112 can1-100 cdc7-1 | S. Piatti and K. Nasmyth |
| 1993 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cdc15-2 | 7 |
| KKY021 | MATa msd2-1::URA3, ura3 leu2, trp1 ade5 | L. Johnston |
| 15DAUcdc14-1 | MATa bar1::URA3, ura3 leu2 trp1 his2, cdc14-1 | A. Bueno |
Media and reagents.
Yeast cultures were grown in YP medium containing the required carbon source (2% glucose or 2% galactose–2% raffinose). Cell cycle blocks were performed as previously with α factor at 5 μg/ml, hydroxyurea (Sigma) at 0.2 M, and nocodazole (Sigma) at 5 μg/ml (13).
iImmunoblotting.
Yeast protein extracts were prepared by vortexing with glass beads as previously described (17); extracts were run on sodium dodecyl sulfate–7.5% polyacrylamide gels and electroblotted onto a Hybond ECL membrane (Amersham). After electrophoretic transfer, the blots were stained with Ponceau S to assess the loading and quality of transfer. The primary antibodies used were the 9E10 monoclonal antibody against the c-myc epitope (2 μg/ml) and the rabbit polyclonal anti-Dbf4p 36 (1:10 dilution) raised against the bacterially expressed N-terminal 163 amino acids of Dbf4p. Conditions for Cdc6p and DNA polymerase α immunoblots have been described previously (11, 17). Antiactin antibodies came from Sigma and were used as specified by the manufacturer. The secondary antibodies used were horseradish peroxidase-conjugated horse anti-mouse immunoglobulin G (1:4,000 dilution) (Vector Laboratories) and protein A (1:20,000 dilution) (Amersham). Proteins were detected with the enhanced chemiluminescence system (Amersham). For quantification, autoradiograms were scanned and analyzed in NIH Image. After quantification, the membranes were stained with amido black and destained exactly as described previously (21). The destaining in methanol causes some warping of the membrane.
RESULTS
Dbf4p is a phosphoprotein which is absent during G1.
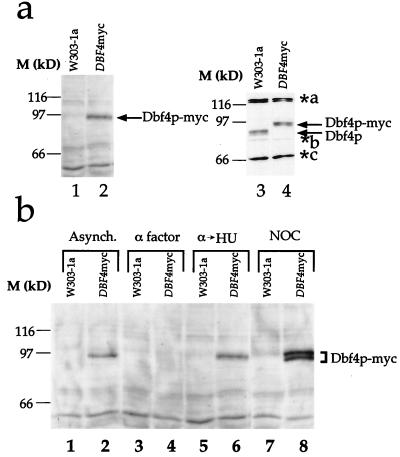
To detect Dbf4p in crude yeast extracts, we used two approaches. First, we constructed yeast strains in which the DBF4 gene was tagged so that the C terminus of the encoded protein, still expressed under the control of the DBF4 promoter, is fused to a short peptide which contains both an epitope from the c-myc gene recognized by the 9E10 monoclonal antibody and 9 histidine residues. Second, we generated polyclonal antibodies to recombinant Dbf4p. Figure 1a shows that we can detect Dbf4p in logarithmically growing cells by using both approaches. In Fig. 1a, lane 2, a polypeptide of approximately 94 kDa was recognized by 9E10 monoclonal antibody in whole-cell extracts. Although this is larger than the predicted size of tagged Dbf4p (84.1 kDa), this protein could be detected in an extract from a tagged (lane 2) but not an untagged (lane 1) yeast strain. By using the polyclonal antiserum, a polypeptide of the same molecular mass was recognized in extracts from the tagged strain (lane 4). In extracts from the untagged strain, this 94-kDa band was absent but a faster-migrating band, not present in extracts from the tagged strain, was now seen. Additional bands were also detected by the polyclonal antiserum. Since these were not detected by the 9E10 monoclonal antibody and since the migration of these polypeptides was not affected by the epitope tag, we conclude that these are nonspecific cross-reacting proteins. We conclude from these experiments that we can detect endogenous Dbf4p in whole-cell extracts without overexpression. We also conclude that the epitope tag does not significantly change the levels of Dbf4p.
FIG. 1.
Immunodetection of Dbf4p. (a) Detection of Dbf4p in whole-cell extracts. Extracts prepared from asynchronous populations of either W303-1a or the epitope-tagged derivative (yMIG07-DBF4myc) were probed with the 9E10 monoclonal antibody (lanes 1 and 2, respectively). In lanes 3 and 4, the same extracts were probed with polyclonal antibody to Dbf4p. Arrows indicate the positions of Dbf4p and Dbf4p-myc. The positions of nonspecific cross-reacting polypeptides are designated a, b, and c (asterisks). The relative levels of these background bands are variable due to differences in the times of incubation with primary antibody. (b) Dbf4p is absent from α factor-arrested cells. Cultures of cells harboring the epitope-tagged Dbf4p (DBF4myc) and the parental strain (W303-1a) were arrested in G1 (by α factor), S (by hydroxyurea [α→HU]) and in G2/M (by nocodazole [NOC]), and Dbf4p-myc was detected by immunoblotting with the 9E10 monoclonal antibody.
To begin to address the possibility that Dbf4p levels are cell cycle regulated, we examined Dbf4p in cells blocked at different points in the cell cycle. Figure 1b shows that while the tag-specific polypeptide could be detected in logarithmically growing cells (lanes 1 and 2), it could not be detected in cells blocked in G1 with the mating pheromone α factor (lanes 3 and 4). Tag-specific bands could, however, be detected in cells which were released from the α factor block into the ribonucleotide reductase inhibitor hydroxyurea (lanes 5 and 6), which arrests cells in early S phase (3, 42) or in cells blocked in G2/M with the microtubule inhibitor nocodazole (lanes 7 and 8). To confirm that this is not due to the epitope tag, we examined Dbf4p levels in an untagged strain. By using the polyclonal antibody, we showed that the untagged Dbf4p is absent from α factor-blocked cells and present in nocodazole-blocked cells (Fig. 2a).
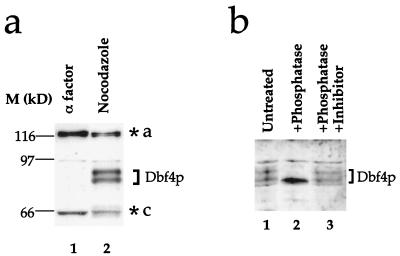
FIG. 2.
Dbf4p is a phosphoprotein. Absence of Dbf4p from α factor-blocked cells is independent of the epitope tag. Cultures of W303-1a were arrested in G1 (α factor) or G2/M (Noc) and extracts were probed with the polyclonal antibody to Dbf4p. (b) Dbf4p is phosphorylated in nocodazole-blocked cells. Extracts from nocodazole-blocked cells were treated with potato acid phosphatase as described previously (51) in either the absence (lane 2) or presence (lane 3) of phosphatase inhibitor (50 mM NaF) prior to electrophoresis and immunoblotting with anti-Dbf4p polyclonal antibody. Background bands are designated as in Fig. 1.
In extracts from both tagged and untagged strains, Dbf4p appears as a doublet in nocodazole-blocked cells (Fig. 1b and 2a). Figure 2b shows that treatment of extract from nocodazole-blocked cells with potato acid phosphatase converts this doublet into a faster-migrating single band (lanes 1 and 2), which is blocked by inclusion of phosphatase inhibitor (lane 3). This shows that Dbf4p is a phosphoprotein in nocodazole-blocked cells.
Dbf4p accumulates during S phase and is degraded at the onset of anaphase.
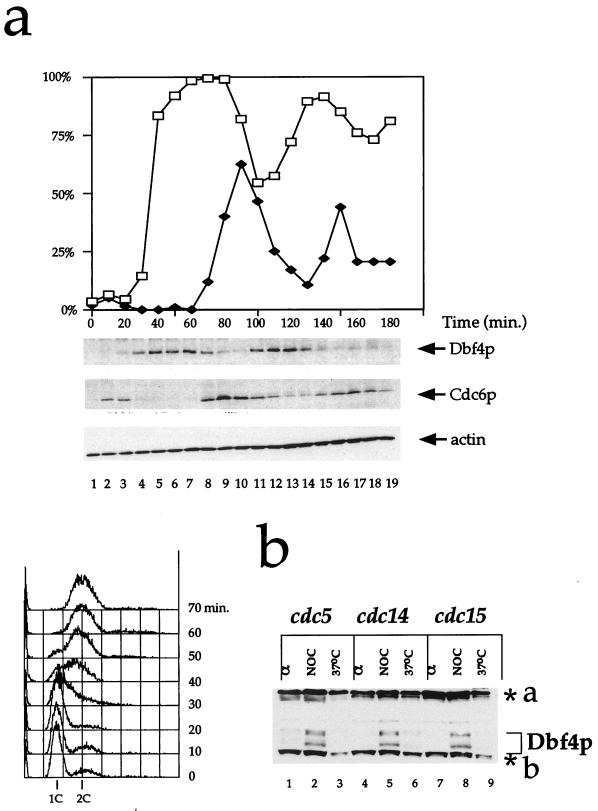
To further examine the regulation of Dbf4p levels during the cell cycle, we synchronized cells in G1 with α factor and released them from the α factor block. In this experiment, cells underwent two reasonably synchronous cell cycles after release as judged by budding index (Fig. 3a). Consistent with the results in Fig. 1, Dbf4p was absent from cells blocked in α factor (Fig. 3a, lane 1). Dbf4p was first detectable 20 min after release, approximately when the buds first emerged. This was just at or before the onset of DNA replication as determined by flow cytometry (see below). Dbf4p levels continued to rise from 20 to 60 min after release and began to rapidly decline at approximately 70 min after release. Figure 3a shows that this sudden drop in the Dbf4p level corresponds to the first appearance of binucleate cells. This pattern is repeated in the second cell cycle. Thus, Dbf4p is first synthesized in late G1/early S phase and is destroyed approximately at the onset of anaphase.
FIG. 3.
Kinetics of Dbf4p expression during the cell cycle. (a) Dbf4p levels after α factor block and release. Cells expressing Dbf4p-myc under its endogenous promoter (yMIG07) were grown to mid-log phase and blocked by α factor. The cells were harvested and washed several times to remove α factor and returned to fresh medium to allow cell cycle progression. Samples were taken every 10 min, and individual protein levels were determined by immunoblotting with the 9E10 monoclonal antibody (Dbf4p), 9H8/5 (Cdc6p), and anti-actin antibody. In addition, the Dbf4p blot was stained with amido black after autoradiography (21). The region between 66 and 116 kDa is shown. At each time point, the percentage of budded cells (□) and binucleate cells (⧫) was assessed by microscopy of 4′,6-diamidino-2-phenylindole (DAPI)-stained cells. Samples were taken and processed for fluorescence-activated cell sorting. (b) Dbf4p is absent from cells that have undergone anaphase. Cultures of _cdc5_ts, _cdc14_ts, and _cdc15_ts mutants grown to logarithmic phase were arrested in G1 (α) and in G2/M (NOC), both at the permissive temperatures, and a third aliquot was arrested at the restrictive temperature (37°C). Dbf4p was detected in extracts by immunoblotting with polyclonal antibody against Dbf4p. Background bands are designated as in Fig. 1.
This contrasts strongly with the results for Cdc6p. Figure 3a shows that there was a short burst of Cdc6p expression after release from α factor but that the protein had disappeared by 30 min after release. It reappeared at 70 min after the release, just as Dbf4p levels declined and as the chromosomes separated. This near mutual exclusivity of Dbf4p and Cdc6p nicely illustrates the states of cell competence for pre-RC assembly and origin firing (12).
The disappearance of Dbf4p occurred at the time of chromosome separation and was already complete by the time cytokinesis occurred. To investigate this further, we examined levels of Dbf4p in mutants which arrested in mitosis after the metaphase to anaphase transition. Figure 3b shows that while Dbf4p could be detected in all mutants arrested in G2/M with nocodazole, it was absent from all of these mutants when arrested at the restrictive temperature. None of these mutants have pre-RCs assembled at origins at this point (7, 13). From these results, we conclude that Dbf4p is degraded during mitosis at approximately the time of the metaphase-to-anaphase transition.
Dbf4p degradation in G1 is impaired when APC activity is compromised.
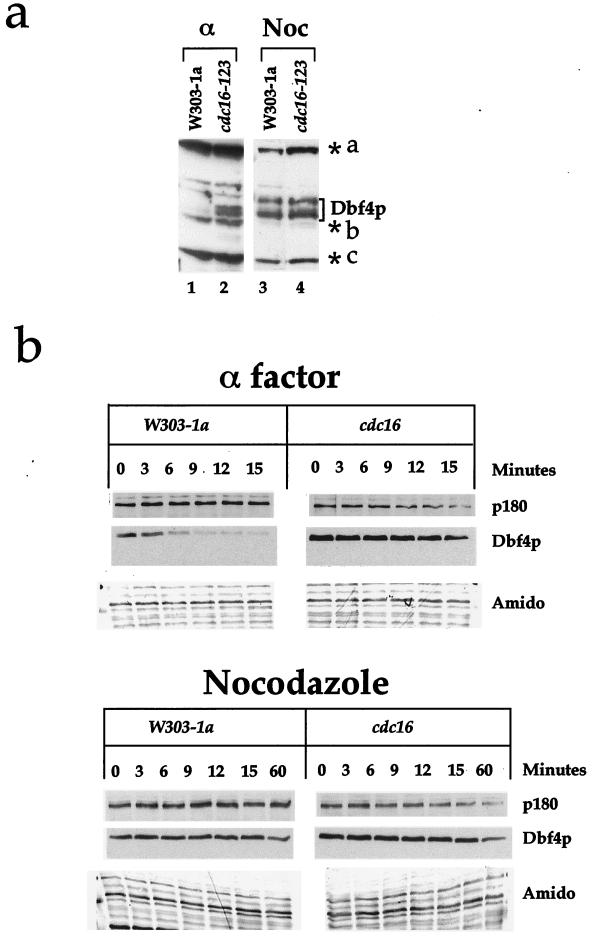
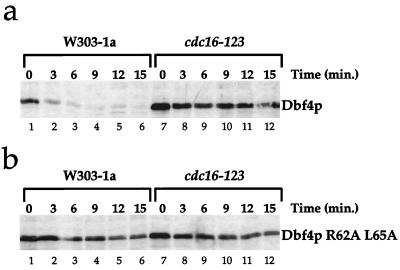
The precipitous decline in Dbf4p levels at the onset of anaphase suggested to us that Dbf4p may be targeted for degradation by the APC. This possibility was supported by the fact that although Dbf4p could not be detected in wild-type cells blocked in G1 with α factor (Fig. 1b, 2a, and 3a; Fig. 4a, lane 1), it could readily be detected in cdc16 mutant cells blocked with α factor even at the permissive temperature (Fig. 4a, lane 2). We note that this stabilized form of Dbf4p appears as a doublet. The significance of this is unknown. Cdc16p is a component of the APC, and previous experiments have shown that cdc16 mutants are defective in degradation of other mitotic substrates including Clbs, Pds1p, Ase1p, Cdc5p, and Cdc20p (6, 8, 26, 30, 40, 46). To test the possible involvement of the APC, we examined the rate of Dbf4p degradation during G1 in wild-type cells and a cdc16 mutant. Figure 4b shows that in α factor-arrested cells, Dbf4p is degraded rapidly in wild-type cells but is considerably more stable in the cdc16 mutant. Figure 4b also shows that even in wild-type cells, Dbf4p is more stable in nocodazole-arrested cells. Moreover, its degradation in nocodazole-arrested cells is unaffected in the cdc16 mutant. Very similar results were obtained with a cdc23 mutant (data not shown). These experiments show that Dbf4p is rapidly degraded during pre-Start G1 and that this rapid degradation requires the APC.
FIG. 4.
Dbf4p is targeted for destruction by APC. (a) Dbf4p is present in _cdc16_ts mutants arrested in G1 at the permissive temperature. Cultures of W303-1a and _cdc16_ts mutants grown at 24°C were arrested in G1 (α) and in G2/M (Noc). Dbf4p was detected by immunoblotting with polyclonal antibodies to Dbf4p. Background bands are designated as in Fig. 1. (b) Dbf4p degradation during G1 requires the APC. W303-1a and _cdc16_ts cell cultures harboring tagged Dbf4p under the GAL1-10 promoter were grown in galactose medium and arrested in G1 with α factor or in G2/M with nocodazole at the permissive temperature. The cells were then incubated at the restrictive temperature for 30 min, after which the promoter was repressed by harvesting the cells and releasing them into glucose medium containing cycloheximide. Cell aliquots were taken prior to changing the carbon source (0 min) and at different times points thereafter. Dbf4p levels were investigated on immunoblots with the 9E10 monoclonal antibody or the anti-polymerase α monoclonal antibody. The Dbf4p blot was stained with amido black as described in the legend to Fig. 3. The apparent distortion in the amido black pattern (Amido) is due to warping of the membrane during destaining (see Materials and Methods).
Dbf4p contains a destruction box-like sequence required for its degradation in G1.
The APC-dependent degradation of Dbf4p could be due to direct action on Dbf4p. Alternatively, the effects on Dbf4p degradation could be an indirect consequence of stabilization of some other protein(s) such as cyclins during G1 in the apc mutants. One common feature of many APC substrates is the presence of a “destruction box” near the N terminus. The destruction box contains two highly conserved amino acid residues (RXXL) and several other residues which are more moderately conserved. Within the entire Dbf4p, there are six RXXL motifs. Two of these are located within the N-terminal 70 amino acids. Preliminary analysis indicated that deletion of the first 14 amino acids, which removes the first motif, did not affect the APC-dependent degradation of Dbf4p in G1 while deletion of the first 67 amino acids, which removes both the first and second motifs, eliminated APC-dependent degradation of Dbf4p (M. G. Ferreira and J. D. Diffley, unpublished data). This suggested that the second motif plays a critical role in Dbf4p degradation. To analyze this more precisely, we constructed a double point mutation in which both the arginine and leucine residues within box 2 were converted to alanine. Figure 5a shows again that the wild-type Dbf4p is rapidly degraded in an APC-dependent manner. Figure 5b shows that the double point mutant is no longer capable of being targeted for APC-dependent degradation during G1. This stable mutant could still complement a dbf4 temperature-sensitive mutant, indicating that it is a functional protein. Furthermore, overproduction of Dbf4p R62A L65A did not arrest cell growth or result in any detectable rereplication as determined by flow cytometry (data not shown).
FIG. 5.
Dbf4p possesses a destruction box sequence responsible for APC-dependent degradation in G1. An experiment identical to that performed with wild-type Dbf4 in Fig. 4 was performed with wild-type Dbf4p (a) and the Dbf4p R62A L65A mutant (b).
DISCUSSION
Previous work has shown that Dbf4p associates with and activates Cdc7p analogous to the activation of cyclin-dependent kinases by the binding of cyclins. The analogy to cyclins is extended in this work with the demonstration that Dbf4p is present during only part of the cell cycle. This is because Dbf4p is targeted for proteolysis by the APC, which is also required to target the B cyclins for proteolysis during mitosis and G1. Thus, although Dbf4p is not related to the cyclins in its primary amino acid sequence, it performs an analogous function and is regulated very similarly to the cyclins.
Why is Dbf4p targeted for degradation by the APC at anaphase onset? Partial rereplication of the genome or the inappropriate activation of some or all origins prior to Start could be a dangerous, even lethal event. Consequently, cells appear to utilize multiple mechanisms to ensure that replication origins fire on schedule and just once in each cell cycle. The Cdc6 protein, which plays an essential role in DNA replication by loading the Mcm proteins into prereplication complexes, is degraded before S phase begins (17), and the Mcm proteins are present in the nucleus only during G1 and early S phases (10, 24, 53). In addition to pre-RCs, other components of the replication machinery appear to be similarly regulated. For example, like the Mcm proteins, DNA polymerase α-primase binds to chromatin during G1 and S phases. However, unlike the Mcm proteins, this chromatin association is independent of Cdc6p and therefore of pre-RC assembly (11).
The window of opportunity when pre-RCs can assemble and DNA polymerase α-primase loads onto chromatin coincides with the period when Cdc28/Clb kinase is inactive (9, 13, 38). Considerable evidence now indicates that Cdc28/Clb kinase blocks both pre-RC assembly and DNA polymerase α-primase loading (9, 11). Since Cdc28/Clb kinase is also essential for origin firing (45), origin firing and resetting of the replication machinery are mutually exclusive processes.
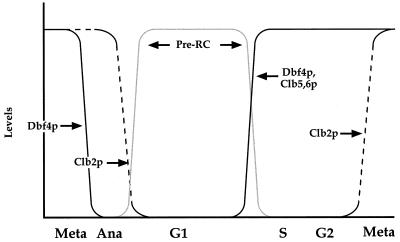
This mutual exclusivity ensures that replication origins do not fire more than once in any cell cycle. However, the transition from high kinase to low kinase activity at the end of mitosis is a potentially dangerous time when, at intermediate kinase levels, newly formed pre-RCs might be immediately and accidentally activated. We suggest that Dbf4p degradation provides a second, perhaps redundant, mechanism whereby newly formed pre-RCs cannot immediately refire. The results presented in this paper indicate that Dbf4p is targeted for degradation by the APC at the metaphase-to-anaphase transition and that Dbf4p is absent from cells blocked in anaphase by using a number of different temperature-sensitive mutants (Fig. 3b and 6). Clb2 is still present at this time in these mutants (28). In addition, pre-RCs have not yet assembled at this time in any of these mutants (7, 13). These results argue that Dbf4p degradation occurs before Clb2 and, as a consequence, also occurs prior to assembly of pre-RCs. Therefore, when pre-RCs finally assemble at the end of mitosis, they will be unable to immediately refire because these cells lack not only Clbs but also Dbf4p. The degradation of Dbf4p at the time of sister chromatid segregation ensures that this essential S phase-promoting signal can be completely erased before pre-RC assembly even begins.
FIG. 6.
Dbf4p degradation in the cell cycle. The details of this model are discussed in the text. Dbf4p levels (solid black lines) decrease at the metaphase (Meta)-anaphase (Ana) transition as a result of APC activation. After this, the APC targets Clb2p (dotted black lines) for degradation, which allows pre-RCs (solid grey lines) to assemble. Passage through Start inactivates the APC and allows Dbf4 as well as the S phase-promoting Clb5 and Clb6 to reaccumulate. This triggers the activation of pre-RCs at individual origins during S phase.
Inactivation of the APC has been reported to induce rereplication in budding yeast (22, 23). Although this is currently a controversial point (39), it is interesting to consider the possibility that the inability to degrade Dbf4p contributes to the rereplication phenotype. This is unlikely to be the sole explanation for rereplication in the apc mutants, since constitutive expression of the Dbf4p destruction box mutant does not cause spontaneous rereplication.
We note that the timing of Dbf4p degradation is similar to that of other APC substrates including the anaphase inhibitor Pds1 (8). Two WD-40 repeat proteins, Cdc20p and Hct1/Cdh1, have recently been implicated in activating the APC toward specific substrates; Cdc20 targets Pds1p, while Hct1/Cdh1 targets Clb2 (44, 52). At present, we do not know which, if any, of these targeting factors is required for Dbf4p degradation; however, the similarity in timing to Pds1 suggests that Dbf4p may be targeted by Cdc20. Further experiments are required to test this hypothesis.
The evidence presented in this paper indicates that a second, APC-independent mode of degradation is revealed when the APC-mediated degradation of Dbf4p is eliminated by either conditional inactivation of APC subunits or mutation of the Dbf4p destruction box. This APC-independent degradation does not appear to be cell cycle regulated, since the half-life of Dbf4p in G2/M is similar to that seen in G1-arrested cells in either the cdc16 mutant or the Dbf4p destruction box mutant. We do not know the significance of this degradation. At present, we also do not know how this second mode of degradation works, except that it does not require the PEST sequence (41) near the C terminus of Dbf4p (data not shown). The existence of this second pathway complicates any interpretation of the fact that the destruction box mutant appears functional, since this mutant is still degraded by this second pathway. Characterization of this pathway should help to address this issue.
ACKNOWLEDGMENTS
We thank Tamara Tugal for advice on phosphatase treatment and Hiro Yamano and Tim Hunt for discussions.
M.G.F. gratefully acknowledges the support of the Gulbenkian Ph.D. Program in Biology and Medicine.
ADDENDUM IN PROOF
While this paper was under consideration, two papers (L. Cheng et al., Mol. Cell. Biol. **19:**4270–4278, 1999, and G. Oshiro et al., Mol. Cell. Biol. **19:**4888–4896, 1999) showing APC-dependent degradation of Dbf4p have been published.
REFERENCES
- 1.Andrews B, Measday V. The cyclin family of budding yeast: abundant use of a good idea. Trends Genet. 1998;14:66–72. doi: 10.1016/s0168-9525(97)01322-x. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio O M, Weinstein D M, Bell S P. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM complexes and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 3.Bousset K, Diffley J F X. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 1998;12:480–490. doi: 10.1101/gad.12.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown G W, Kelly T J. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J Biol Chem. 1998;273:22083–22090. doi: 10.1074/jbc.273.34.22083. [DOI] [PubMed] [Google Scholar]
- 5.Chapman J W, Johnston L H. The yeast gene, DBF4, essential for entry into S phase is cell cycle regulated. Exp Cell Res. 1989;180:419–428. doi: 10.1016/0014-4827(89)90068-2. [DOI] [PubMed] [Google Scholar]
- 6.Charles J F, Jaspersen S L, Tinker-Kulberg R L, Hwang L, Szidon A, Morgan D O. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- 7.Cocker J H, Piatti S, Santocanale C, Nasmyth K, Diffley J F X. An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature. 1996;379:180–182. doi: 10.1038/379180a0. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Fix O, Peters J M, Kirschner M W, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 9.Dahmann C, Diffley J F X, Nasmyth K A. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 10.Dalton S, Whitbread L. Cell-cycle-regulated nuclear import and export of Cdc47, a protein essential for initiation of DNA-replication in budding yeast. Proc Natl Acad Sci USA. 1995;92:2514–2518. doi: 10.1073/pnas.92.7.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desdouets C, Santocanale C, Drury L S, Perkins G, Foiani M, Plevani P, Diffley J F X. Evidence for a Cdc6p-independent mitotic resetting event involving DNA polymerase α. EMBO J. 1998;17:4139–4146. doi: 10.1093/emboj/17.14.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diffley J F X. Once and only once upon a time: specifying and regulating origins of DNA replication in eukaryotic cells. Genes Dev. 1996;10:2819–2830. doi: 10.1101/gad.10.22.2819. [DOI] [PubMed] [Google Scholar]
- 13.Diffley J F X, Cocker J H, Dowell S J, Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson A D, Fangman W L, Brewer B J. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 1998;12:491–501. doi: 10.1101/gad.12.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donovan S, Harwood J, Drury L S, Diffley J F X. Cdc6-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci USA. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowell S J, Romanowski P, Diffley J F X. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science. 1994;265:1243–1246. doi: 10.1126/science.8066465. [DOI] [PubMed] [Google Scholar]
- 17.Drury L S, Perkins G, Diffley J F X. The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast. EMBO J. 1997;16:5966–5976. doi: 10.1093/emboj/16.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans T, Rosenthal E T, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 19.Hardy C F, Dryga O, Seematter S, Pahl P M, Sclafani R A. Mcm5/Cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc Natl Acad Sci USA. 1997;94:3151–3155. doi: 10.1073/pnas.94.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy C F J, Pautz A. A novel role for Cdc5p in DNA replication. Mol Cell Biol. 1996;16:6775–6782. doi: 10.1128/mcb.16.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 22.Heichman K A, Roberts J M. CDC16 controls initiation at chromosome replication origins. Mol Cell. 1998;1:457–463. doi: 10.1016/s1097-2765(00)80046-5. [DOI] [PubMed] [Google Scholar]
- 23.Heichman K A, Roberts J M. The yeast CDC16 and CDC27 genes restrict DNA replication to once per cell cycle. Cell. 1996;85:39–48. doi: 10.1016/s0092-8674(00)81080-6. [DOI] [PubMed] [Google Scholar]
- 24.Hennessy K M, Clark C D, Botstein D. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 1990;4:2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- 25.Hess G F, Drong R F, Weiland K L, Slightom J L, Sclafani R A, Hollingsworth R E. A human homolog of the yeast CDC7 gene is overexpressed in some tumors and transformed cell lines. Gene. 1998;211:133–140. doi: 10.1016/s0378-1119(98)00094-8. [DOI] [PubMed] [Google Scholar]
- 26.Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 27.Jackson A L, Pahl P M, Harrison K, Rosamond J, Sclafani R A. Cell cycle regulation of the yeast CDC7 protein kinase by association with the DBF4 protein. Mol Cell Biol. 1993;13:2899–2908. doi: 10.1128/mcb.13.5.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaspersen S L, Charles J F, Tinker-Kulberg R L, Morgan D O. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang W, Hunter T. Identification and characterization of a human protein kinase related to budding yeast Cdc7p. Proc Natl Acad Sci USA. 1997;94:14320–14325. doi: 10.1073/pnas.94.26.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juang Y L, Huang J, Peters J M, McLaughlin M E, Tai C Y, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- 31.Kitada K, Johnston L H, Sugino T, Sugino A. Temperature-sensitive cdc7 mutations of Saccharomyces cerevisiae are suppressed by the DBF4 gene, which is required for the G1/S cell cycle transition. Genetics. 1992;131:21–29. doi: 10.1093/genetics/131.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei M, Kawasaki Y, Young M R, Kihara M, Sugino A, Tye B K. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev. 1997;11:3365–3374. doi: 10.1101/gad.11.24.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang C, Stillman B. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 1997;11:3375–3386. doi: 10.1101/gad.11.24.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masai H, Miyake T, Arai K-I. hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J. 1995;14:3094–3104. doi: 10.1002/j.1460-2075.1995.tb07312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micklem G, Rowley A, Harwood J, Nasmyth K, Diffley J F X. Yeast origin recognition complex is involved in DNA replication and transcriptional silencing. Nature. 1993;366:87–89. doi: 10.1038/366087a0. [DOI] [PubMed] [Google Scholar]
- 36.Peters J M. SCF and APC: the yin and yang of cell cycle regulated proteolysis. Curr Opin Cell Biol. 1998;10:759–768. doi: 10.1016/s0955-0674(98)80119-1. [DOI] [PubMed] [Google Scholar]
- 37.Piatti S. Cell cycle regulation of S phase entry in Saccharomyces cerevisiae. Prog Cell Cycle Res. 1997;3:143–156. doi: 10.1007/978-1-4615-5371-7_12. [DOI] [PubMed] [Google Scholar]
- 38.Piatti S, Bohm T, Cocker J H, Diffley J F X, Nasmyth K. Activation of S-phase promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 1996;10:1516–1531. doi: 10.1101/gad.10.12.1516. [DOI] [PubMed] [Google Scholar]
- 39.Pichler S, Piatti S, Nasmyth K. Is the yeast anaphase promoting complex needed to prevent re-replication during G2 and M phases? EMBO J. 1997;16:5988–5997. doi: 10.1093/emboj/16.19.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prinz S, Hwang E S, Visintin R, Amon A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol. 1998;8:750–760. doi: 10.1016/s0960-9822(98)70298-2. [DOI] [PubMed] [Google Scholar]
- 41.Rechsteiner M, Rogers S W. Pest sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 42.Santocanale C, Diffley J F X. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- 43.Sato N, Arai K, Masai H. Human and Xenopus cDNAs encoding budding yeast cdc7-related kinases—in-vitro phosphorylation of mcm subunits by a putative human homolog of cdc7. EMBO J. 1997;16:4340–4351. doi: 10.1093/emboj/16.14.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab M, Annegret S L, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- 45.Schwob E, Bohm T, Mendenhall M D, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 46.Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spellman P T, Sherlock G, Zhang M Q, Iyer V R, Anders K, Eisen M B, Brown P O, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka T, Knapp D, Nasmyth K. Loading of an Mcm protein onto DNA-replication origins is regulated by Cdc6p and CDKs. Cell. 1997;90:649–660. doi: 10.1016/s0092-8674(00)80526-7. [DOI] [PubMed] [Google Scholar]
- 49.Thomas B J, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 50.Toone W M, Aerne B L, Morgan B A, Johnston L H. Getting started: regulating the initiation of DNA replication in yeast. Annu Rev Microbiol. 1997;51:125–149. doi: 10.1146/annurev.micro.51.1.125. [DOI] [PubMed] [Google Scholar]
- 51.Tugal T, Zou-Yang X H, Pappin D, Canas B, Kobayashi R, Hunt T, Stillman B. The Orc4p and Orc5p subunits of the Xenopus and human origin recognition complex are related to Orc1p and Cdc6p. J Biol Chem. 1998;273:32421–32429. doi: 10.1074/jbc.273.49.32421. [DOI] [PubMed] [Google Scholar]
- 52.Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 53.Yan H, Merchant A M, Tye B-K. Cell cycle-regulated nuclear localisation of MCM2 and MCM3, which are required for the initiation of DNA synthesis at chromosomal replication origins in yeast. Genes Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]