Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes (original) (raw)
Abstract
Highly active antiretroviral therapy (HAART) has been advocated for the management of primary HIV-1 infection without clear understanding of its immunological effects. Here, we demonstrate that early use of HAART during primary infection preserves HIV-specific CD8+ T cells physically and functionally while HIV-specific T cell help is sustained. We also show that even transient administration of HAART at seroconversion can preserve HIV-specific immunity. In contrast, delayed initiation of HAART is associated with a progressive loss of HIV-specific CD8+ T cells and absent HIV-specific T cell help. These results imply that HIV-specific T help is damaged during primary HIV-1 infection. Early drug treatment, which preserves this immunity, also preserves HIV-specific CD8+ T cells. These results have implications for understanding the early pathogenesis of HIV-1 infection and suggest that acute HIV infection should be treated aggressively and as early as possible.
Hallmarks of primary HIV-1 infection include an acute viral syndrome characterized by fever, rash, and lymphadenopathy in association with high levels of viral replication and dissemination of virus to lymphoid tissues (1, 2). Virus-specific CD8+ cytotoxic T lymphocyte (CTL) responses are first detected during this time and are thought to mediate the decline of the initial viremia (1–4). Because the outcome of HIV-1 infection is influenced by these early events (5–7), highly active antiretroviral therapy (HAART) has been recommended for acute HIV-1 infection (8). This therapeutic intervention may limit viral dissemination, minimizing harmful effects on cellular immune function and secondary lymphoid organ architecture. However, the effects of HAART on the interaction between T lymphocyte responses and HIV-1 soon after acquisition of the virus are uncertain. Some preliminary support for early use of HAART is based on three patients who were treated with combination antiretroviral therapy at seroconversion. The viral load was suppressed to undetectable levels and this correlated with measurable HIV-specific T lymphocyte helper function (9). There is increasing evidence that HIV-specific CTLs may play a crucial role in controlling viremia (10–12). Initiation of HAART during established infection is associated with decline of HIV-specific CTLs (13–17). This could reflect lack of HIV-specific T help for the maintenance of CD8+ T cell responses (18).
We hypothesized that the use of HAART for acute HIV-1 infection as a means of preserving HIV-specific CD4+ T cell responses might also preserve CD8+ T cell responses to the virus. This was tested in a group of eight patients with acute symptomatic primary HIV-1 infection. A detailed longitudinal analysis of HIV-specific CD8+ and CD4+ T lymphocyte immunity was performed. The identification of early dominant CTL responses directed against the same HLA-B8-restricted epitope within the HIV-1 Nef protein (FLKEKGGL) in six of the eight patients provided an unusual opportunity to study the influence of HAART on responses of identical specificity.
Materials and Methods
Study Population.
Patients (SC2, SC9, SC10, SC11, SC12, SC15, SC18, and SC19) with acute symptomatic HIV-1 infection were recruited from the Chelsea and Westminster Hospital (London). All were Caucasian homosexual men aged 25–37 yr with well-documented recent exposure to HIV-1 through high-risk unprotected sexual encounters, followed by typical seroconversion illness, which included fever, rash, and lymphadenopathy (Table 1). Additional clinical features included meningism in SC2, constipation in SC10, parasthesia and oral candidiasis in SC11, anorexia in SC12, and myalgia and arthralgia in SC15. The patient-reported start of the seroconversion illness was defined as onset of symptoms. Primary infection was defined as the presence of HIV-1 RNA or p24 antigen during acute symptomatic illness in the absence of HIV antibodies, or a typical viral syndrome following a negative HIV-1 antibody test 6 mo or less previously. Appropriate ethical approval was obtained.
Table 1.
Patient characterization
| Patient HLA | DFOSx | CD4/CD8, ×106/liter | ART |
|---|---|---|---|
| SC2 | 1 (−) | − | |
| A1 | 7 (+) | 384/2016 | − |
| B7/8 | 22 | 518/5994 | − |
| Cw0701 | 110 | − | |
| Cw0702 | 172 | 330/840 | − |
| DR4/53 | 253 | 325/− | − |
| DQ7 | 307 | 325/663 | − |
| 382 | 325/− | − | |
| 543 | 475/1143 | − | |
| 606 | 449/993 | Z/3/I | |
| 605 | 401/786 | Z/3/I | |
| 718 | 588/1013 | Z/3/I | |
| 760 | 532/835 | PC | |
| 1059 | 395/860 | − | |
| 1083 | 451/1246 | − | |
| 1163 | 351/680 | − | |
| 1340 | 465/1012 | Z/3/E | |
| SC9 | −180 (−) | − | |
| A1/2 | 20 (+) | 624/592 | − |
| B8/13 | 107 | 864/792 | − |
| Cw0 | 175 | 720/1056 | − |
| Cw0701 | 240 | 588/569 | − |
| DR2/11 | 287 | 716/913 | − |
| DR51/52 | 348 | 619/716 | − |
| DQ6/7 | 390 | d/3/I | |
| 421 | 647/633 | d/3/I | |
| 499 | 657/570 | d/3/I | |
| 560 | 879/956 | d/3/I | |
| 618 | 701/739 | d/3/I | |
| 713 | 687/587 | d/3/I | |
| 839 | 338/463 | PC | |
| 877 | 500/529 | PC | |
| 1006 | 524/518 | − | |
| 1146 | 531/508 | − | |
| SC10 | 1 (−) | − | |
| A1/3 | 36 (+) | − | |
| B8/35 | 49 | 598/− | d/3/I |
| DR1/8 | 71 | 528/592 | d/3/I |
| DQ4/5 | 85 | d/3/I | |
| 112 | 693/751 | d/3/I | |
| 153 | 913/977 | d/3/I | |
| 214 | 603/535 | d/3/I | |
| 250 | 658/596 | d/3/I | |
| 313 | 599/509 | d/3/I | |
| 376 | 805/769 | d/3/I | |
| 438 | 600/769 | d/3/I | |
| 500 | 858/529 | d/3/I | |
| 559 | 1168/771 | d/3/I | |
| 629 | 1431/1448 | d/3/I | |
| 691 | 1321/1277 | d/3/I | |
| 753 | 1429/1166 | d/3/I | |
| 791 | 1690/1759 | d/3/I | |
| 853 | 1640/1532 | d/3/I | |
| 875 | 1971/− | d/3/E | |
| 881 | 1223/1094 | d/3/E | |
| 959 | 1566/1386 | d/3/E | |
| 1029 | 741/734 | d/3/E | |
| 1079 | 1754/1841 | d/3/E | |
| SC11 | −180 (−) | ||
| A1 B8 | 24 (+) | 351/3042 | − |
| Cw0201 | 32 | d/3/R | |
| DR3/11 | 48 | 728/1248 | d/3/R |
| DR52 | 71 | − | |
| DQ2/7 | 233 | 427/1383 | − |
| 689 | 405/1221 | d/3/N | |
| 757 | 598/1220 | d/3/N | |
| 836 | 584/1194 | d/3/N | |
| SC12 | −139 (−) | − | |
| A1 | 23 (+) | − | |
| B8/39 | 30 | 589/1953 | − |
| Cw0701 | 35 | 3/I | |
| Cw0702 | 45 | Z/3/I | |
| DR2/3 | 51 | 952/1372 | Z/3/I |
| DR51/52 | 110 | 1071/1283 | Z/3/I |
| DQ2/6 | 156 | 1176/1502 | Z/3/I |
| 234 | 1324/1324 | Z/3/I | |
| 290 | 866/1009 | Z/3/I | |
| 361 | 928/1396 | d/3/I | |
| 487 | 1302/1261 | d/3/I | |
| 544 | 1179/1314 | d/3/I | |
| 656 | 1180/1265 | d/3/I | |
| 770 | 1526/1647 | d/3/I | |
| 815 | 2075/2752 | d/3/I | |
| 832 | − | ||
| 874 | 1544/3422 | − | |
| 977 | 1058/2673 | 3/I/R | |
| 1019 | 1050/1969 | 3/I/R | |
| SC15 | 1 (−) | 227/195 | − |
| A1/68 | 4 | Z/3/I | |
| B8/35 | 11 | 514/1104 | Z/3/I |
| Bw4/6 | 25 (+) | 429/− | Z/3/I |
| Cw4 | 39 | 477/1171 | 3/d/N |
| Cw0704 | 130 | 697/− | 3/d/N |
| 241 | 512/389 | 3/d/N | |
| 331 | 599/470 | 3/d/N | |
| SC18 | 1 (−) | − | |
| A2/11 | 8 | 619/631 | − |
| B50/58 | 12 (+) | 785/1170 | d/3/I |
| Bw6/4 | 19 | 696/721 | d/3/I |
| Cw0401 | 89 | 742/730 | − |
| Cw10 | 174 | 1009/788 | − |
| DR3/4 | 285 | 643/750 | − |
| DR52/53 | 552 | 853/1063 | − |
| DQ2/8 | 599 | 557/1248 | − |
| SC19 | −60 (−) | − | |
| A11/29 | 30 (+) | 525 | − |
| B8/44 | 60 | 333/873 | − |
| Cw06 | 75 | 310/868 | − |
| Cw0701 | 125 | 445/1714 | − |
| DR3/7 | 195 | 133/1041 | − |
| DR52/53 | 197 | 232/1010 | d/3/S |
| DQ2 | 212 | 335/1032 | d/3/S |
| 307 | 298/3918 | d/3/S | |
| 363 | 534/1958 | d/3/S |
HLA Typing.
The HLA class I and II genotype of each patient was determined by PCR using sequence-specific primers (19).
Determination of Viral Load.
Virus load was quantified from cryopreserved plasma by using the Amplicor Reverse Transcription–PCR Kit (Roche Diagnostics).
Assessment of Functional HIV-Specific CD8+ and CD4+ T Cell Responses.
Peripheral blood leukocytes (PBL) were separated from heparinized blood by Ficoll/Hypaque density gradient centrifugation. Bulk cultures were prepared by the addition of autologous phytohemagglutinin (PHA)-activated lymphocytes to PBL (20). Cytolytic activity was determined by using standard chromium release assays (20, 21). Synthetic peptides (Table 2, reference strain HIV IIIB) were used at a concentration of 2 μM in IFN-γ enzyme-linked immunospot (ELISPOT) assays to define CD8+ T cell responses directly ex vivo as previously described (22). PHA was always included as a positive control. Recombinant HIV-1-derived proteins [gp120, p24, p66 at 10 μg/ml; National Institute for Biological Standards and Control (NIBSC), Potters Bar, U.K.], two synthetic Tat peptides (Tat 32–72 and Tat 49–85 at 5 μg/ml; NIBSC), and overlapping pooled peptides spanning HIV-1 Nef protein (5 μg/ml; NIBSC) were used to determine HIV-specific CD4+ T cell responses in PBL depleted of CD8+ T cells with anti-CD8-conjugated Dynabeads (Dynal, Merseyside, U.K.) by IFN-γ ELISPOT assays with addition of 0.5 μg anti-CD28 mAb (Becton Dickinson) and by proliferation assays (22, 23). HIV-unrelated antigens streptokinase/streptodornase (200 units/ml) and PHA were used as positive controls. Results of proliferation assays are expressed as stimulation index (SI), which is defined as the ratio of [3H]thymidine incorporation with antigen/[3H]thymidine incorporation without antigen. All assays were performed in duplicate.
Table 2.
Peptides
| Name | Sequence | HLA | Epitope |
|---|---|---|---|
| GSE | GSEELRSLY | A1 | p17 71–79 |
| ILK | ILKEPVHGV | A2 | RT 476–484 |
| ACQ | ACQGVGGPGHK | A11 | p24 349–359 |
| AVD | AVDLSHFLK | A11 | Nef 84–92 |
| QVP | QVPLRPMTYK | A11 | Nef 73–82 |
| FNC | FNCGGEFFY | A29 | gp120 376–384 |
| DCK | DCKTILKAL | B8 | p24 329–337 |
| FLK | FLKEKGGL | B8 | Nef 92–99 |
| GEI | GEIYKRWII | B8 | p24 259–267 |
| GGK | GGKKKYKLK | B8 | p17 24–32 |
| GPK | GPKVKQWPL | B8 | RT 185–183 |
| SQR | SQRRQDILDLWIY- | B13 | Nef 103–127 |
| HTQGYFPDWQNY | |||
| RYP | RYPLTFGWCYK | B18 | Nef 134–144 |
| PPI | PPIPVGDIY | B35 | p24 260–268 |
| VPL | VPLRPMTY | B35 | Nef 74–81 |
| NAN | NANPDCKTI | B51 | p24 325–333 |
| TAF | TAFTIPSI | B51 | RT 295–302 |
| RAI | RAIEAQQHL | B51 | gp41 557–565 |
| HTQ | HTQGYFPDWQ | B57 | Nef 116–125 |
| ISP | ISPRTLNAW | B57 | p24 147–155 |
| KAF | KAFSPEVIPMF | B57 | p24 162–172 |
| PIV | PIVLPEKDSW | B57 | RT 410–419 |
| TST | TSTLQEQIGW | B57 | p24 240–249 |
Tetrameric Complexes.
Phycoerythrin-conjugated peptide/HLA-B8 tetrameric complexes were used to track antigen-specific responses physically, as previously described (24).
Cell Staining and FACS Analysis.
Cells were stained with tetramers at 37°C for 30 min, washed in PBS and 0.1% sodium azide, and then stained with anti-human CD8-Tricolor (Caltag, South San Francisco, CA) at 4°C for 20 min, washed again, and then fixed with 1% formaldehyde in PBS. Stained cells were analyzed by using a Becton Dickinson FACScan machine with cellquest software.
Proviral DNA Sequencing.
Genomic DNA was extracted directly from uncultured PBMC by using the Purgene DNA Isolation Kit (Gentra Systems). Full-length Nef was amplified by using nested PCR as described previously (21). p24 (amino acids 558–965) was amplified similarly using primers 5′-AGAACTTTAAATGCATGGGTAAAAGT-3′/5′-ACTCCCTGACATGCTGTCATCAT-3′ for the primary reaction (annealing at 53°C), and 5′-ATGCTAAACACAGTGGGGGGACA-3′/5′-CAACAAGGTTTCTGTCATCCAATTTTTTAC-3′ for the secondary reaction (annealing at 55°C). Products were cloned by the T-vector system (Invitrogen) and sequenced by dye terminator sequencing using M13F primer on an ABI 377 DNA Sequencer (Perkin–Elmer Applied Biosystems).
Results
Delayed Initiation of HAART.
We examined the influence of delayed initiation of HAART (more than 6 mo after seroconversion) on the breadth of specificities and on the function of HIV-specific CD8+ T cells for 1–4 yr after the onset of symptoms. Several studies have shown that initiation of HAART during chronic HIV infection results in a decay of HIV-specific CD8+ T cell numbers (14, 15) and function (16, 17). We corroborate these findings by observing a decline in tetramer-stainable HIV-specific CD8+ T cell populations and a decline in the functional response measured by IFN-γ ELISPOT. Furthermore, we found patients who started HAART more than 6 mo after seroconversion had no detectable HIV-1-specific CD4+ T cell responses.
SC2.
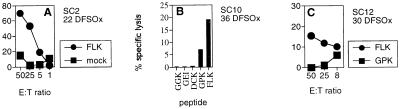
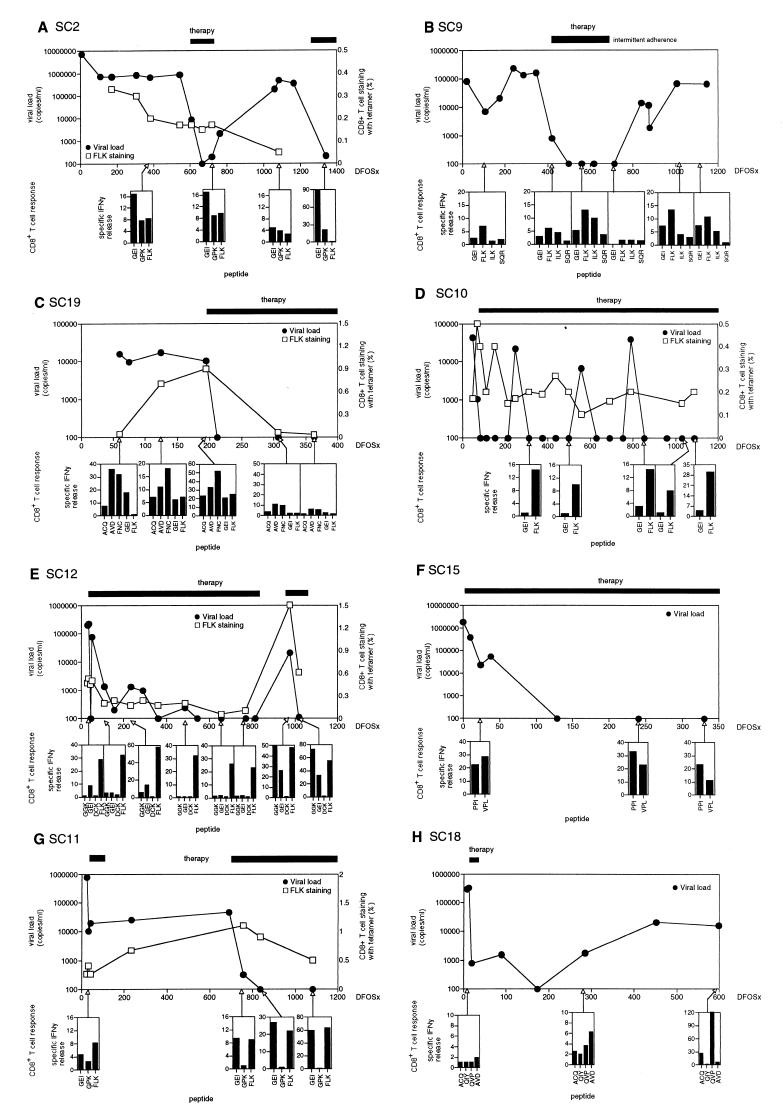
This patient declined HAART initially and remained untreated for 21 mo after seroconversion. The dominant CTL response at 22 DFOSx was directed against the HLA B8-restricted FLK epitope (21) (Fig. 1A).
Figure 1.
CTL activity of SC2, SC10, and SC12 soon after infection. Chromium release assays were performed with bulk-cultured PBL of SC2 at 22 DFOSx (A), SC10 at 36 DFOSx (B), and SC12 at 30 DFOSx (C). HLA B8-expressing target cells (B-LCL) were labeled with the indicated HLA B8-binding peptides, and bulk-cultured PBL were used at an effector to target ratio of 60:1 in B, and as indicated in A and C. Spontaneous release was below 20%.
At 382 DFOSx and 760 DFOSx, responses to the HLA B8-restricted GEI, GPK, and FLK epitopes were detected by IFN-γ ELISPOT. However, at 1,059 DFOSx, these responses were significantly reduced (Fig. 2A). At 1,340 DFOSx, strong GEI- and GPK-specific, but no FLK-specific, CD8+ T cell responses were detectable. The restoration of GEI- and GPK-specific T cell responses is likely to reflect stimulation by autologous virus during the period without treatment (780–1,340 DFOSx). Sequence analysis of the GEI and FLK epitopes at 1,083 DFOSx revealed that the majority of the GEI sequences (7/9 clones) encoded the index peptide epitope GEIYKRWII, whereas the majority of the FLK sequences (8/10 clones) encoded a CTL escape variant FLKENGGL, as described previously (21).
Figure 2.
The immune response during and after HIV infection. Thawed, uncultured PBL of SC2 (A), SC9 (B), SC19 (C), SC10 (D), SC12 (E), SC15 (F), SC11 (G), and SC18 (H) were studied. For viral load (●), data points on the baseline were below the limit of detection of the assay (<400 copies per ml). PBL were stained with tetramers specific for the HLA B8-restricted epitope FLK (□). Median values are shown and expressed as percentage of total CD8+ T cells. Initiation and duration of HAART is indicated by the black bar on top of the upper panel. IFN-γ-ELISPOT analysis is shown in the lower panels and the time of analysis is indicated by the arrows pointing to the time axis (x axis). Specific IFN-γ release indicated the number of spots induced by peptide stimulation (peptides are indicated at the x axis of the ELISPOT panels) divided by the number of spots observed without antigen stimulation. In each patient, 9–20 peptides were used to screen CD8+ T cell responses in each sample, but only those peptides that stimulated a response at some point in the study are shown. Consistently negative responses are not plotted.
Tetramer-positive FLK-specific CD8+ T cells decreased until HAART was started, despite a persistent viremia around 106 copies per ml of plasma (Fig. 2A). This decline of antigen-specific CTL coincided with the emergence of FLK-epitope variants that escaped recognition by autologous CTL (21).
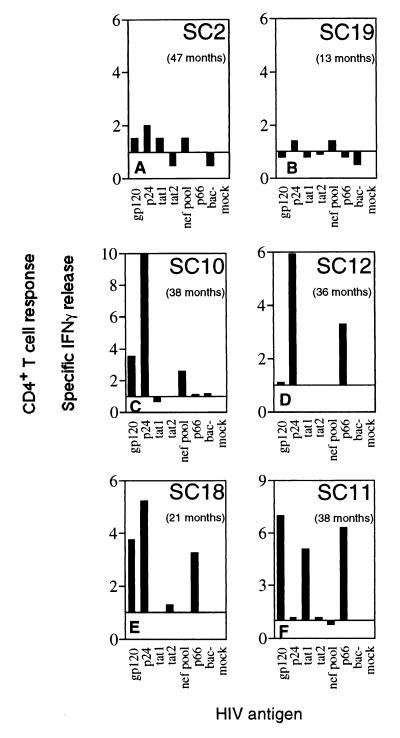
Assessment of HIV-specific CD4+ T cell responses from CD8-depleted PBL at 1,340 DFOSx revealed no significant IFN-γ ELISPOT response to any of the tested antigens (Fig. 3A). In addition, there were no CD4+ T cell proliferative responses at 543 DFOSx (not shown).
Figure 3.
HIV-specific CD4+ T cell responsiveness. CD8-depleted PBL of SC2 (A), SC19 (B), SC10 (C), SC12 (D), SC18 (E), and SC11 (F) were stimulated with the indicated HIV-derived antigens, and specific IFN-γ secretion is shown as the number of spots induced by antigen stimulation (antigens are indicated at the x axis of the ELISPOT panels) divided by the number of spots observed without peptide stimulation. Values in parentheses indicate the months following onset of symptoms when cells were sampled and the assay performed.
SC9.
This patient remained untreated for 400 DFOSx. When HAART was started, viremia cleared by 499 DFOSx. However, because treatment was stopped at 800 DFOSx, viremia reappeared at 839 DFOSx. The dominant CTL response at 107 DFOSx was directed against the HLA B8-restricted FLK epitope (Fig. 2B). At 421 DFOSx, CD8+ T cell responses against the FLK and the HLA-A2-restricted ILK epitope were observed and at 560 DFOSx, responses to the FLK, ILK, and GEI peptides were detected. However, at 713 DFOSx these responses were significantly reduced to almost undetectable levels (Fig. 2B). At 1,006 and 1,146 DFOSx—when viremia had reappeared because of poor compliance with therapy—GEI-, FLK-, and ILK-specific CD8+ T cell responses were again detectable. Sequence analysis of the GEI and FLK epitopes at 877 DFOSx revealed no variation within the GEI epitope (10/10 clones), and all FLK epitope sequences (10/10 clones) encoded the index FLKEKGGL sequence (not shown).
No CD4+ T cell proliferative responses were detected at 499 and 713 DFOSx (not shown).
SC19.
This patient started HAART at 197 DFOSx. At 60 DFOSx, a significant IFN-γ ELISPOT response was observed against several epitopes (ACQ, AVD, FNC, and GEI), but no FLK-specific response was observed, a finding consistent with absent tetramer staining for FLK-specific CD8+ T cells at this time (Fig. 2C). Later, at 125 and 195 DFOSx, the pattern of epitope responsiveness was maintained and increased in magnitude. In addition FLK-responsiveness was detected and correlated with positive FLK-tetramer staining (Fig. 2C). However, after initiation of HAART, responses to all epitopes were reduced compared with pretreatment levels; in particular, FLK responsiveness was abolished. This corresponded to absent tetramer staining of FLK-specific CD8+ T cells. Sequencing at 307 DFOSx revealed that all analyzed GEI sequences (10/10 clones) encoded the index peptide epitope GEIYKRWII and no sequence variation was observed within the FLK epitope (7/7 clones) (not shown).
No HIV-specific CD4+ T cell responses were detected in CD8-depleted PBL at 363 DFOSx (Fig. 3B).
Early Initiation of HAART.
We compared the breadth and specificity of functional HIV-specific T cell responses in patients in whom initiation of HAART was delayed with those who started treatment during seroconversion. We found that HIV-specific CD8+ and CD4+ T cell responses were maintained despite the absence of detectable antigen in patients who started HAART at seroconversion. Furthermore, we found evidence that a short course of HAART for 1–4 wk during primary infection can result in sustained HIV-specific CD4+ and CD8+ T cell responses.
Sustained Therapy. SC10.
This patient received HAART from 49 DFOSx. The dominant cytolytic response in the acute phase of infection was directed against the HLA-B8-restricted FLK epitope (Fig. 1B). Over the entire period of treatment, FLK-induced IFN-γ secretion was observed in direct ex vivo ELISPOT assays (Fig. 2D). The HLA-B8-restricted p24 Gag GEI epitope induced detectable IFN-γ secretion at 853 DFOSx. The pattern of peptide responsiveness remained remarkably constant during the 1,100 days of observation. Sequence analysis at 1,088 DFOSx revealed no sequence variation within the FLK epitope (9/9 clones; not shown).
A transient rise in FLK-specific tetramer-positive CD8+ T lymphocytes was detected after HAART was started, followed by a decline as virus load fell to undetectable levels. However, despite prolonged suppression of virus load, tetramer-positive CD8+ T cells specific for the FLK epitope were consistently detected at levels of 0.2–0.3% of CD8+ T cells (Fig. 2D).
The intermittent reappearance of viremia in the patient was due, on at least one occasion, to a brief period of noncompliance.
Assessment of HIV-specific CD4+ T cell responsiveness from CD8-depleted PBL at 1,088 DFOSx revealed a strong response to recombinant p24 antigen and less pronounced responses to recombinant gp120 antigen and to overlapping pooled Nef peptides (Fig. 3C).
SC12.
This patient received HAART from 30 DFOSx. The immunodominant cytolytic response during acute infection was directed against the HLA B8 FLK epitope (Fig. 1C). This correlated with IFN-γ ELISPOT analysis, where a dominant response to the FLK epitope and a less pronounced response to the GEI epitope was observed (Fig. 2E). The FLK response was maintained throughout the 800 days of HAART (Fig. 2E).
SC12 stopped therapy at 832 DFOSx, and this resulted in reappearance of viremia. Concomitantly, there was an increase in CTL responsiveness and in FLK-stainable CD8+ T cells from 0.1% before HAART was stopped to 1.5% afterward. Interestingly, not only FLK-responsive CD8+ T cells were boosted by the viral recrudescence, but other responses, inapparent during the treatment period and specific for the HLA B8-restricted GGK and GEI epitopes, became detectable. At 874 DFOSx, SC12 continued HAART. This resulted in control of viremia and a fall in FLK-stainable CD8+ T cell numbers to 0.6% of CD8+ T cells. Sequence analysis of the GEI and FLK epitopes at 1,050 DFOSx revealed that all GEI sequences (10/10 clones) encoded the index peptide epitope GEIYKRWII and, similarly, all FLK sequences (9/9 clones) encoded the index FLKEKGGL sequence (not shown).
Assessment of HIV-specific CD4+ T cell responsiveness from CD8-depleted PBL at 1,019 DFOSx revealed a strong response to recombinant p24 antigen and to recombinant p66 antigen (Fig. 3D). In addition, proliferative responses to recombinant p24 antigen (SI 6.2) and recombinant p66 antigen (SI 3.4) were observed at 656 DFOSx (not shown).
SC15.
This patient received HAART at seroconversion. IFN-γ ELISPOT analysis revealed that the dominant response was directed against the HLA B35-restricted epitopes PPI and VPL (Fig. 2F), and both responses were maintained throughout the 300 days of HAART (Fig. 2F).
A CD4+ T cell proliferative response against recombinant p66 antigen (SI 3) was observed at 129 DFOSx (not shown).
Limited-Course Therapy. SC11.
This patient received HAART from 32 DFOSx. IFN-γ ELISPOT analysis revealed that the dominant response was directed against the GEI and FLK (Fig. 2G). SC11 remained on HAART for 40 days and then stopped treatment until 640 DFOSx. After 40 days of treatment, the viremia fell by 100-fold. Reinitiation of treatment at 640 DFOSx resulted in clearance of viremia at 757 DFOSx. At 32, 757, 836, and 1,082 DFOSx, GEI- and FLK-specific CD8+ T cell responses were observed (Fig. 2G). FLK-specific responsiveness correlated with an FLK-positive tetramer-stainable CD8+ T cell population of 0.4%, 1.1%, 0.9%, and 0.5% at 32, 757, 836, and 1,082 DFOSx.
Analysis of HIV-specific CD4+ T cell responses from CD8-depleted PBL at 1,082 DFOSx revealed strong responses to recombinant gp120 and p66 antigen, and to the Tat peptide 32–72 (Fig. 3F). In addition, a strong proliferative CD4+ T cell response to recombinant p66 was measured at 48 DFOSx (SI 12.5; not shown).
SC18.
This patient received HAART from 12 DFOSx. IFN-γ ELISPOT analysis revealed that the dominant response was directed against the HLA A11-restricted epitopes ACQ, QVP, and AVD (Fig. 2H).
SC18 remained on HAART for 20 days. This period of treatment resulted in a fall of viremia of more than 100-fold and at 174 DFOSx to undetectable levels. However, viremia reappeared on 285 DFOSx. At 8 DFOSx, no significant CD8+ T cell response could be observed. At 285 DFOSx, a significant response against the QVP and AVD was detected, and, at 599 DFOSx, a strong ACQ- and QVP-specific response was observed.
At 599 DFOSx, strong CD4+ T cell responses to recombinant p24, gp120, and p66 antigens were detected (Fig. 3E). In addition, a proliferative response against recombinant p24 antigen (SI 6.6) was measured at 285 DFOSx (not shown).
Discussion
The immunological consequences of therapeutic intervention during primary HIV-1 infection are largely unknown. During the natural course of infection, there is good evidence that early CTL activity is a key factor in controlling the initial viremia (4, 21, 25, 26). The early interplay between viral replication and CTL activity influences the equilibrium viral set-point, which in turn is predictive of the subsequent rate of disease progression (5–7, 27). Intervention at early stages of infection could therefore dramatically influence clinical outcome of HIV infection.
We analyzed the induction and maintenance of HIV-specific CD8+ T cell responses in patients during acute HIV-1 infection, and then followed the evolution of these responses for up to 3 yr and compared the impact of early vs. delayed treatment on HIV-specific T cell responses. Initiation of HAART at seroconversion was associated with persistence of HIV-specific CTL defined by tetramer staining methods and functional assays, despite a substantial decay in viral load. This was associated with potent HIV-specific CD4+ T cell responses directed against multiple antigens. Interestingly, in two patients, despite discontinuation of early treatment after 2 and 4 wk, we still observed evidence of a beneficial effect on the maintenance of HIV-specific T cell responses. This observation is encouraging because prolonged treatment with HAART is expensive, toxic, and commonly associated with intermittent adherence.
In contrast, CTL responsiveness declined in patients in whom initiation of HAART was delayed (6–18 mo after seroconversion). This correlated with absent HIV-specific T cell help. These findings are comparable to other studies (14–17, 28) that reported a decline of HIV-specific CTLs after administration of HAART in chronically infected patients. Sequence analysis of CTL epitopes confirmed that decline in CTL responsiveness in late-treated patients was not the result of selection of epitope variants that might still emerge during HAART (29, 30). In the present study, the decline in T cell responsiveness was apparent only during effective treatment, i.e., in the absence of viremia. When two of three late-treated patients discontinued therapy and viremia reappeared, they regained HIV-specific CTL responses but not HIV-specific CD4+ T cell responses.
Although other factors may explain the different immunological effect of early as compared with late initiation of treatment, our findings suggest that preservation of HIV-specific T help plays an important role. In murine chronic viral infections, functional CD4+ T helper cells are required for the maintenance of sustained CTL responses (31–35). Furthermore, functional T help might be important for maintenance of memory CD8+ T cell responses after resolution of acute viral infection (36). Extrapolation of these findings to HIV-1 infection would suggest that sustained CTL responsiveness in the absence of detectable antigen will depend on functional HIV-specific T help. Evidence that these features are in turn associated with a favorable clinical outcome is based on studies of long-term nonprogressors (LTNP) who have both sustained CTL activity (11, 37) and HIV-specific T help (9, 38). Furthermore, a recent report demonstrated a correlation between HIV-p24-specific proliferative CD4+ T cell responses and Gag-specific CTL precursors (18).
In summary, these findings suggest that maintenance of sustained, functional CTL responsiveness depends on functional T cell help in HIV patients who receive effective antiretroviral therapy. Functional HIV-specific T cell help and CD8+ T cell responses are preserved in patients who are treated at seroconversion, but not in patients who start treatment later, long after the seroconversion illness has resolved. These findings have important implications for understanding the early pathogenesis of HIV infection and for the design of therapeutic protocols that aim to limit immune system damage during primary HIV-1 infection.
Acknowledgments
We thank Timothy A. Yap for sequencing assistance, and Anele Waters and the patients for their cooperation in this study. D.A.P., J.A.W., A.K.S., and R.E.P. are supported by the Wellcome Trust, U.K. A.O. is a European Molecular Biology Organization Fellow; A.D.K. is a Neil Hamilton Fairley Fellow.
Abbreviations
CTL
cytotoxic T lymphocyte
HAART
highly active antiretroviral therapy
PBL
peripheral blood leukocyte
PHA
phytohemagglutinin
ELISPOT
enzyme-linked immunospot
SI
stimulation index
DFOSx
days following onset of symptoms
References
- 1.Clark S J, Saag M S, Decker W D, Cambell H S, Roberson J L, Veldkamp P J, Kappes J C, Hahn B H, Shaw G M. N Engl J Med. 1991;324:954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 2.Daar E S, Moudgil T, Meyer R D, Ho D D. N Engl J Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 3.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantaleo G, Demarest J F, Schacker T, Vaccarezza M, Cohen O J, Daucher M, Graziosi C, Schnittman S S, Quinn T C, Shaw G M, et al. Proc Natl Acad Sci USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 7.Mellors J W, Kingsley L A, Rinaldo C R, Jr, Todd J A, Hoo B S, Kokka R P, Gupta P. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ho D D. N Engl J Med. 1995;333:450–451. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 10.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, et al. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 11.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinaldo C, Huang X L, Fan Z F, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, et al. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogg G S, McMichael A J. Immunol Lett. 1999;66:77–80. doi: 10.1016/s0165-2478(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 14.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, et al. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 16.Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunce M, O'Neill C M, Barnardo M C N M, Krausa P, Browning M J, Morris P J, Welsh K I. Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 20.Nixon D F, Townsend A R, Elvin J G, Rizza C R, Gallwey J, McMichael A J. Nature (London) 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 21.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelleher A D, Carr A, Zaunders J, Cooper D A. J Infect Dis. 1996;173:321–329. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 24.O'Callaghan C A, Byford M F, Wyer J R, Willcox B E, Jakobsen B K, McMichael A J, Bell J I. Anal Biochem. 1999;266:9–15. doi: 10.1006/abio.1998.2930. [DOI] [PubMed] [Google Scholar]
- 25.Koup R A, Safrit J T, Coa Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 27.Mellors J W, Munoz A, Giorgi J V, Margolick J B, Tassoni C J, Gupta P, Kingsley L A, Todd J A, Saah A J, Detels R, et al. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley J M, Talal A, Hurley A, Ji X, Chaudhry M R, Yaman M, et al. J Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, et al. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 30.Lewin S R, Vesanen M, Kostrikis L, Hurley A, Duran M, Zhang L, Ho D D, Markowitz M. J Virol. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matloubian M, Conception R J, Ahmed R. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasenkrug K J, Brooks D M, Dittmer U. J Virol. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J D, Suresh M, Altman J D, Ahmed R. J Exp Med. 1988;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Herrath M G, Yokoyama M, Dockter J, Oldstone M B A, Whitton J L. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein M R, Miedema F. Trends Microbiol. 1995;3:386–391. doi: 10.1016/s0966-842x(00)88984-2. [DOI] [PubMed] [Google Scholar]
- 38.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]