Inflammatory cytokine production by immunological and foreign body multinucleated giant cells (original) (raw)
Abstract
Multinucleated giant cells (MGC) are a common feature of granulomas. The mechanism of their formation has been studied extensively, but their function has not been completely characterized. A new method for the in vivo production of MGC was developed involving subcutaneous injection of microscopic nitrocellulose particles with adsorbed mycobacterial antigens into the footpads of sensitized BALB/c mice (immune [I]-MGC), or by nitrocellulose administration to non-sensitized mice (foreign body [FB]-MGC). The development of granulomas with a highly enriched MGC population was observed 2 weeks after the nitrocellulose injection. MGC were larger with a greater number of nuclei in I-MGC than in FB-MGC. From days 7–28 after nitrocellulose administration, the production of interleukin-1α (IL-1α) and tumour necrosis factor-α (TNF-α) was demonstrated in both MGC types by in situ reverse transcription–polymerase chain reaction (RT–PCR) and immunohistochemistry. After 2 months, the MGC had ceased production of IL-1α and TNF-α, but the expression of transforming growth factor-β (TGF-β) was very high, occurring together with extensive fibrosis. These results suggest that MGC are an active source of inflammatory cytokines, which can contribute to the initiation, maintenance and down-regulation of granulomatous inflammation induced by immunological and inert substances.
Introduction
Multinucleated giant cells (MGC) are highly stimulated cells of macrophage (Mφ) lineage at a terminal stage of differentiation.1, 2 They are common constituents of granulomas induced by physical and biological stimuli. 3 MGC are produced by cell–cell fusion induced by different cytokines, such as interferon-γ (IFN-γ), interleukin (IL)-1, -3, -4 and -6, and granulocyte–macrophage colony-stimulating factor (GM-CSF). 4–7 In this formation process different factors, such as calcium and integrins, participate.6, 8
MGC are a common cellular component of granulomatous inflammation. 3 Granulomas are focal, chronic, predominantly mononuclear inflammatory reactions evoked by persistent poorly biodegradable tissue irritants. 9 The composition of the irritant and its degradability and immunogenicity are crucial factors in the aetiology and development of these lesions. In fact, according to the nature of the irritant, granulomas can be divided in two types: immunological and non-immunological (or foreign body) granulomas. Immunological granulomas are usually produced by infectious agents and involve cell-mediated immunity (delayed-type hypersensitivity). Non-immunological (or foreign body) granulomas are induced by inert, non-biodegradable material. Although the cellular bulk of both types of granulomas are Mφ and their derivatives, the cell populations of these lesions differ with respect to several metabolic parameters. 9 MGC are a common feature of both types of granulomas. However, their specific functions are not completely known. Some experimental results, particularly in vitro, have indicated that MGC represent an adaptation for enhanced phagocytic activity and serve a useful purpose in degrading or eliminating tissue irritants that are resistant to elimination by isolated Mφ. 10 Other investigators have suggested that MGC are a useless constituent of granulomas, and consider these cells as a desperate manifestation of inefficiency in the elimination of poorly biodegradable material. 11
Considerable evidence suggests that MGC are formed by the fusion of monocytes and/or Mφ. 3 Several important Mφ functions performed by MGC, such as phagocytosis, 12 superoxide production, 11 antigen presentation1 and nitric oxide production, 13 have been demonstrated that indicate this origin. As another important Mφ function is the production and secretion of a large group of important molecules, particularly cytokines, with essential functions in the activation and regulation of the immune response and inflammation, we postulated that MGC could produce inflammatory cytokines. In order to explore this hypothesis, we designed an in vivo experimental model of immune MGC (I-MGC) and foreign body MGC (FB-MGC) formation, involving subcutaneous (s.c.) inoculation of mycobacterial antigens embedded in nitrocellulose microscopic particles into footpads of presensitized BALB/c mice (I-MGC), or by injecting nitrocellulose into non-sensitized animals (FB-MGC).
Our immunohistochemistry, immunoelectronmicroscopy and in situ reverse transcription–polumerase chain reaction (RT–PCR) results showed that both I-MGC and FB-MGC were able to produce IL-1α (IL-1α), tumour necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β), according to the age of the lesions; however, they were unable to produce Mφ inflammatory proteins α and β (MIP-α and MIP-β). This is a novel and important function of MGC, considering the pleiotropic and crucial activities of these cytokines in physiological and pathological situations.
Materials and methods
Experimental model of I-MGC and FB-MGC production in mice
Male BALB/c mice were used at 6–8 weeks of age. Virulent Mycobacterium tuberculosis H37Rv was cultured in Youmann's modification of Proskauer and Beck's medium. After 5–6 weeks, the culture medium was filtered to eliminate bacilli and the culture filtrate proteins (CFP) were precipitated with 45% (NH4)2SO4. Phenylmethylsulphonyl fluoride (PMSF) (Sigma Chemical Co., St. Louis, MO) was added as enzymatic inhibitor before dialysis in phosphate-buffered saline (PBS). Twenty micrograms of CFP was adsorbed in fragments of 5-mm nitrocellulose. Nitrocellulose was converted to microscopic particles according to the method previously described. 14
In order to induce I-MGC, mice were sensitized with two s.c. and intraperitoneal (i.p.) injections of CFP, 1 week apart. For the first immunization, 50 µg of CFP was emulsified in 100 µl of Freund's complete adjuvant, and for the second immunization, 25 µg of CFP was dissolved in saline. Ten days after the second immunization, I-MGC were induced by the s.c. injection (into both footpads) of 10 µg of CFP embedded in microscopic nitrocellulose particles suspended in 40 µl of PBS. FB-MGC were induced by injection of the same amount of plain nitrocellulose into non-sensitized animals.
Preparation of tissue for histology and immunohistochemistry
Groups of four mice in two different experiments were killed by exsanguination at 7, 14, 21, 28, 45 and 60 days after the s.c. injection of nitrocellulose. For histological and immunohistochemical analyses, the tissue was prepared as described previously. 15 Briefly, footpad tissue was dissected and fixed immediately by immersion in absolute ethanol. After being embedded in paraffin, sections were stained with haematoxylin and eosin, and Masson trichromic. In each section, at least 25 giant cells were randomly selected, and the number of nuclei and the surface area of the cells and nuclei were determined, in µm2, using a Zidas Zeiss image analyser. Analysis of variance (anova) was used to determine statistical significance in these morphometric parameters between I-MGC and FB-MGC; a _P_-value of < 0·05 was considered significant.
For immunohistochemistry, after the peroxidase was inactivated with H2O2, the sections were incubated for 3 hr with rabbit-specific polyclonal antibodies against mouse IL-1α and TNF-α (Genzyme, Boston, MA), diluted 1:50 in PBS for 2 hr, with MIP-1α and MIP-1β diluted 1:20 in PBS (R & D Systems, Minneapolis, MN) and overnight at 4° with rabbit polyclonal panspecific TGF-β (R & D Systems) diluted 1:50 in PBS. Bound antibodies were detected with goat anti-rabbit immunoglobulin G (IgG) labelled with peroxidase (Dako, Carpinteria, CA) diluted 1:500 in PBS and diaminobenzidine. Control negative slides consisted of incubating the tissue with PBS instead of with primary antibody.
Preparation of tissue for conventional electron microscopy and immunoelectron microscopy
For ultrastructural studies the tissue was prepared as previously described. 15 Briefly, after the mice were killed, footpads were dissected, the s.c. tissue was separated, fragmented into small pieces and fixed by immersion for 2 hr at 4° in 4% (vol/vol) glutaraldehyde dissolved in 0·001 m cacodylate buffer, pH 7·4, postfixed in 2% (vol/vol) osmium tetraoxide and embedded in epoxy resin. Sections of 1 µm were stained with toluidine blue and examined using a light microscope to select representative areas, from which ultrathin sections were obtained, contrasted and examined with a Zeiss M-10 electron microscope. For immunoelectron microscopy, the tissue was fixed by immersion in 4% paraformaldehyde dissolved in Sörensen buffer, pH 7·4, for 2 hr at 4° and embedded in LR-White hydrosoluble resin. Thin sections from 70 to 90 nm were mounted on nickel grids and incubated overnight at 4° with specific polyclonal rabbit anti-mouse antibodies to IL-1α, TNF-α and TGF-β, diluted 1:20. Bound antibodies were detected using goat anti-rabbit IgG conjugated to 10-nm gold particles (Sigma) diluted 1:20. The grids were contrasted with uranium salts and analysed using an electron microscope.
Preparation of tissue for in situ RT–PCR
The same alcohol-fixed tissues embedded in paraffin were used for cytokine mRNA expression determined by in situ RT–PCR. Footpad sections were mounted in silane cover slides, deparaffinized for 18 hr at 60° and sequentially immersed in xylene (30 min at 37°), absolute ethanol, 50% ethanol and water. Cells were rendered permeable by incubation for 10 min at room temperature in 0·02% HCl, followed by a 90-second incubation with 0·01% Triton-X-100 and then a 30-min incubation at 37° with 1 µg/ml of proteinase K (Gibco BRL, Gaithersburgh, MD). The DNA was then removed by incubation for 15 min at 37° with DNAse, 3 U per tissue section (Gibco BRL). Reverse transcription was performed at 37° for 1 hr, incubating the tissue in 50 µl of reverse transcriptase buffer (1×, Gibco BRL), dithiothreitol (DTT) 0·01 m, dNTP mix 0·05 mm (Gibco BRL), 1·25 µg of oligo dT and 400 U of Maloney murine leukaemia virus reverse transcriptase (Gibco BRL). The sections were sealed using the Assembly tool (Perkin-Elmer, Branchburg, NJ). The RNA was then removed by incubation with 3 U of RNAse and fixed again by immersion with 4% paraformaldehyde in Sorensen buffer for 3 min at 4°. PCR was performed, incubating the tissue sections with 50 µl of 1× reaction buffer (Gibco BRL), 1·5 U of Taq polymerase, 2 mm HgCl and dNTPs labelled with digoxigenin (Boehringer Mannheim, Mannheim, Germany). The slides were sealed again and placed in the thermocycler (Touch Down; Hybaid, Middlesex, UK). After an initial denaturation at 94° for 4 min, 35 cycles were performed of annealing at 60° for 1 min and extension at 72° for 1 min. The probe sequences for murine IL-1α, TNF-α and TGF-β have been described previously. 16, 17 PCR products were detected with monoclonal mouse antidigoxigenin antibodies coupled to alkaline phosphatase and tetrazolium nitroblue (Boehringer Mannheim), and counterstained using nuclear fast red. Expression of actin was used as a positive control, and the negative control consisted of performing the whole procedure without the cytokine primers.
Results
Histopathological, morphometric and immunohistochemical features
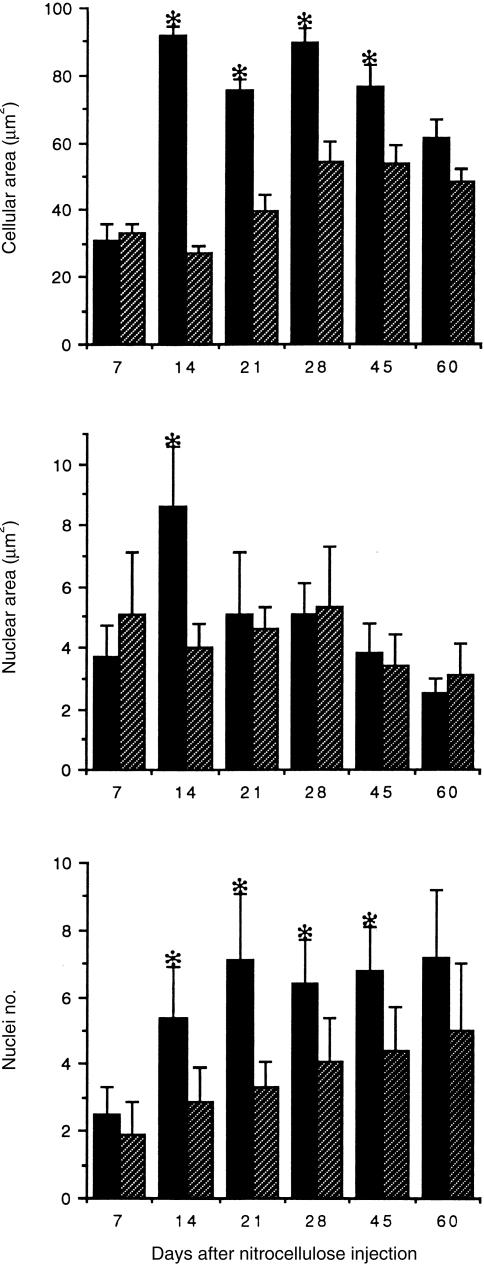
Seven days after inoculation of nitrocellulose into the footpad, an extensive inflammatory reaction was observed in the s.c. tissue (Fig. 1a). This inflammatory infiltrate comprised neutro-phils, lymphocytes, Mφ and few MGC. Both I-MGC and FB-MGC were small (31–33 µm2) with two or three nuclei (Fig. 1a, Fig. 2). Although relatively small, both types of MGC showed immunoreactivity for IL-1α and TNF-α (Fig. 1b), but they were negative for TGF-β, MIP-1α and MIP-1β. Fourteen days after nitrocellulose inoculation, the s.c. inflammatory infiltrate comprised predominantly MGC and some Mφ. MGC were observed intermixed with Mφ or in isolated small groups of four or five cells. At this time-point, the I-MGC cellular size had increased threefold, and their nuclei number and area had doubled. In contrast, the FB-MGC did not increase in size (Fig. 2). However, both MGC types showed strong IL-1α and TNF-α immunostaining, which was stronger in I-MGC. Three and 4 weeks after nitrocellulose injection, the inflammatory infiltrate comprised almost exclusively MGC (Fig. 1c). These cells were organized into nodules of 15–20 cells surrounded by thin collagen fibrous septa in which some lymphocytes or Mφ were located. The I-MGC cellular and nuclei size were slightly smaller than at the previous time-point, but their IL-1α and TNF-α immunostaining was the strongest (Fig. 1d), with negative TGF-β immunostaining (Fig. 1f). In contrast, the FB-MGC doubled their cellular size and slightly increased their nuclei number and nuclear volume (Fig. 2). They also showed the strongest IL-1α and TNF-α immunostaining at this time-point. At the 45- and 60-day time-points after nitrocellulose administration, the inflammatory infiltrate had decreased. However, there were still many MGC distributed diffusely in the s.c. areas, surrounded by thick collagen fibres, which produced some nerve and skin gland deformation (Fig. 1g). At these time-points, morphometric analysis showed that I-MGC had decreased their cellular size by 30–35%; the nuclei showed condensed chromatin and their number did not change but their size decreased by 50% (Fig. 2). Interestingly, the IL-1α and TNF-α immunostaining was very faint or undetectable (Fig. 1h), but the TGF-β immunoreactivity was strong (Fig. 1e). At this late inflammatory stage, FB-MGC was also the predominant cell type; their cellular size and nuclei volume had decreased only by 8–10% and the mean number of nuclei did not change (Fig. 2). As observed in I-MGC, these FB-GMC were also negative when immunostained for IL-1α and TNF-α, but they were strongly positive for TGF-β.
Figure 1.
Representative light microscopy features during the evolution and formation of multinucleate giant cells (MGC) and their cytokine production. (a) Footpad subcutaneous (s.c.) tissue 7 days after injection of mycobacterial antigens embedded in nitrocellulose microscopic particles. There is an acute and chronic inflammation with few MGC (arrowheads). (b) In this inflammatory infiltrate, there are many interleukin-1α (IL-1α)-immunostained macrophages (Mφ) (arrows). MGC are also positive (arrowheads). (c) Twenty-one days after injection of mycobacterial antigens embedded in nitrocellulose particles, the cellular inflammatory response is constituted predominantly by MGC. (d) In the same lesion shown in Fig. 1(c), all MGC are immunoreactive to IL-1α. (e) The in situ polymerase chain reaction (PCR) analysis shows a high level of transcription of the IL-1α gene in these immune (I)-MGC formed 21 days after nitrocellulose injection. (f) In contrast, at this time-point there is no I-MGC immunostaining to transforming growth factor-β (TGF-β). (g) Sixty days after nitrocellulose injection, there are many MGC-forming nodules surrounded by collagen fibres (arrowheads). (h) In the same lesion as Fig. 1(g), there is no MGC TNF-α immunostaining, but there is TGF-β immunoreactivity (i).
Figure 2.
Morphometric analysis of immune (black bars) and foreign body (hatched bars) multinucleated giant cells (MGC). From days 14–45 after subcutaneous (s.c.) injection of nitrocellulose, immune (I)-MGC were significantly larger and had a greater number of nuclei than foreign body (FB)-MGC. The nuclear areas were similar, except at day 14 (*P < 0·05).
Electronmicroscopy and immunoelectron microscopy observations
In lesions produced 21 days after nitrocellulose injection, the cytoplasm of I-MGC showed numerous lamellopodia, primary and secondary lysosomes, as well as large vacuoles with fibrillar material that corresponded to fragments of nitrocellulose particles. In the central cytoplasmic area there were few mitochondria and numerous cisterns of rough endoplasmic reticulum (Fig. 3a). The immunogold-staining technique showed the presence of TNF-α and IL-1α in the inner space of the rough endoplasmic reticulum (Fig. 3b), and in small- and medium-size vacuoles below the cell membrane as well as diffusely distributed in the intervacuolar cytoplasm (Fig. 3c). The nuclei showed chromatin clumps associated with the nuclear membrane and nucleolus. A similar subcellular structure was observed in the FB-MGC, but with apparently fewer lamellopodia, lysosomes and rough endoplasmic reticulum. In both MGC types developed 45 days after nitrocellulose injection, the presence of shrinkage nuclei owing to condensed chromatin, together with well-preserved organelles, was common (Fig. 3d). Thus, some of these MGC in chronic inflammatory infiltrate exhibited clear apoptotic changes.
Figure 3.
Representative electronmicroscopy and immunoelectron microscopy micrographs of multinucleated giant cells (MGC) induced by mycobacterial antigens embedded in nitrocellulose particles. (a) Subcellular structure of MGC 21 days after nitrocellulose injection. The nuclei (N) have condensed chromatin associated with the nuclear membrane, and numerous vacuoles (V) are surrounded by well-developed rough endoplasmic reticulum (arrows). Primary lysosome (L) and long lamellopodia (arrowheads) are also shown. (b) The inner space of the rough endoplasmic reticulum has interleukin-1α (IL-1α) immunoreactivity (arrows), as well as in small- and medium-size vacuoles (*). (c) Near the cell membrane, the cytoplasm and medium-size vacuoles (*) show strong tumour necrosis factor-α (TNF-α) immunolabelling. (d) Subcellular structure of MGC 45 days after nitrocellulose injection. The cytoplasm is well preserved. There are two nuclei: one has condensed chromatin associated with the nuclear membrane (black asterisk) and the other one shows total condensed chromatin (white asterisk), which is a typical apoptotic cell feature.
Cytokine gene expression determined by in situ RT–PCR
MGC are derived from Mφ, so their strong cytokine immunostaining could be a result of their endocytic functions. For this reason, it was necessary to demonstrate cytokine gene transcription directly. The results obtained using in situ RT–PCR showed a high cytokine gene transcription in both types of MGC, with good correlation with the cytokine kinetics determined by immunohistochemistry. In fact, particularly in I-MGC, a high gene expression of IL-1α and TNF-α was observed, particularly at days 21 and 28 after the nitrocellulose injection (Fig. 1e). By days 45 and 65 the expression of both cytokines had decreased and transcription of TGF-β was very high. Both MGC types were negative for MIP transcripts.
Discussion
Although MGC were first reported in tuberculous granulomas by Rockitansky 18 and Langhans 19 over a century ago, their specific functional significance has still not been completely defined. In recent years research on the MGC biology has focused on the development, metabolic and morphological attributes, among others. 1–8 All these investigations were performed in in vitro models. We have developed a novel method for the generation of MGC in vivo. Indeed, our results show that a highly enriched MGC population can be produced 2 weeks after s.c injection of nitrocellulose particles into mouse footpad.
MGC are recognized as a common feature of chronic inflammation induced by immunological and non-immunological stimuli. In this experimental system, it was possible to induce FB-MGC after the administration of plain nitrocellulose or I-MGC after the inoculation of specific antigens embedded in nitrocellulose particles into mice previously sensitized with the same antigen. As M. tuberculosis is the typical MGC-inducing micro-organism, 20 we used an antigen mixture derived from the liquid culture medium of the virulent M. tuberculosis strain, H37Rv, to induce I-MGC, with the specific intention of studying inflammatory cytokine production by these cells. In both FB-MGC and I-MGC induction, an extensive acute and chronic inflammatory reaction was produced during the first week after nitrocellulose inoculation, being higher in I-MGC. Interestingly, even at this early time-point some small MGC were produced. Three weeks and at subsequent time-points after nitrocellulose administration, > 90% of the inflammatory cells were MGC. However, I-MGC were significantly larger than FB-MGC, particularly 14–28 days after the s.c. injection. At the ultrastructural level, I-MGC showed longer pseudopodia and a greater develop-ment of rough endoplasmic reticulum than FB-GMC, indicating higher metabolic activity in the former cell type. Moreover, the immunogold study showed strong IL-1α and TNF-α labelling in the inner space of the rough endoplasmic reticulum, suggesting that there is an active synthesis of these cytokines by both MGC types.
There are recent reports that demonstrate the presence of inflammatory cytokines in MGC by immunohistochemistry. 15, 21 The detection of cytokine transcripts by RT–PCR has also been shown, but only in an indirect manner using in vitro models. 6, 22 The demonstration of cytokine gene transcription is important, considering that the cytokine presence demonstrated by immunohistology can be caused by the phagocytic and endocytic activity that these cells normally manifest, particularly in an inflammatory microenvironment with a high production of inflammatory cytokines. In this regard, we have previously shown strong MGC immunostaining to IL-1α, TNF-α and TGF-β in the lungs of tuberculous mice. 15 The results of the present study, using another experimental model of MGC formation, corroborate and extend these observations. In fact, to our knowledge, this study is the first to show the presence of these cytokine transcripts and proteins in MGC using an in vivo model and in situ. Moreover, the kinetic expression of cytokines changed during the course of the inflammatory process, with IL-1α and TNF-α synthesized at a high level by MGC from the start until the maturation of the chronic inflammatory process. Considering that MGC are a common cellular element of granulomatous inflammation, and IL-1α and TNF-α are significant cytokines involved in the recruitment and activation of inflammatory cells as well as in the granuloma formation, 23, 24 it is reasonable to propose that MGC contribute to the initiation and maintenance of the inflammatory process. Interestingly, the chemokines MIP-1α and MIP-1β, which are also important factors in the recruitment of inflammatory cells, were not produced by MGC, at least in this experimental model. Thus, not all the cytokines produced by Mφ are also produced by MGC.
Two months after nitrocellulose inoculation, a pure population of MGC, organized in nodules, were observed. These MGC nests were surrounded by fibrous tissue. At this time, many MGC showed abundant gene transcription and translation of TGF-β, but not IL-1α and TNF-α. As TGF-β is a potent fibrogenic and anti-inflammatory cytokine, 25 it is plausible to propose that MGC may also be involved in the down-regulation of inflammation and in the induction of the fibrotic or cicatrization process.
There is little information available in regard to the mechanisms involved in MGC elimination. Interestingly, 45 days after nitrocellulose inoculation, we found, at the ultrastructural level, many MGC with apoptotic features. Thus, it is possible that this death cell mechanism could be involved in the normal elimination of MGC in tissues.
In conclusion, our results showed that a highly enriched MGC population can be induced by s.c. injection of microscopic nitrocellulose particles into mouse footpad. In this in vivo system, both I-MGC and FB-MGC were important sources of proinflammatory and anti-inflammatory cytokines, which were preferentially produced depending on the phase of the inflammatory process. This experimental in vivo model of MGC formation may contribute to future studies of the biological attributes and functions of these cells.
Acknowledgments
This work was supported by the Mexican National Council for Research and Technology CONACyT, contract no-28700 M.
References
- 1.Papadimitriou JM, Van Bruggen Y. Evidence that multinucleate giant cells are examples of mononuclear phagocytic differentiation. J Pathol. 1986;148:149. doi: 10.1002/path.1711480205. [DOI] [PubMed] [Google Scholar]
- 2.Kreipe H, Radzun HJ, Rudolph P, et al. Multinucleated giant cells generated in vitro. Terminally differentiated macrophages with down regulated c-fms expression. Am J Pathol. 1988;130:232. [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers TJ. Multinucleated giant cells. J Pathol. 1978;126:125. doi: 10.1002/path.1711260302. [DOI] [PubMed] [Google Scholar]
- 4.Fais S, Burgio VL, Silvestri M, Capobianchi A, Pallone F. Multinucleated giant generation induced by interferon γ: changes in the expression and distribution of the intercellular adhesion molecule 1 during macrophage fusion and multinucleated giant cell formation. Lab Invest. 1994;71:737. [PubMed] [Google Scholar]
- 5.McInnes A, Rennick DM. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemaire Y, Yang H, Lafont V, Dornand J, Commes T, Cantine MF. Differential effects of macrophage and granulocyte-macrophage colony-stimulating factors on cytokine gene expression during rat alveolar macrophage differentiation into multinucleated giant cells. J Immunol. 1996;157:5118. [PubMed] [Google Scholar]
- 7.Enelow RI, Sullivan GW, Carper HT, Mandell GL. Induction of multinucleated giant cell formation from in vitro culture of human monocytes with interleukin 3 and interferon γ: comparison with other stimulating factors. J Respir Cell Mol Biol. 1992;6:57. doi: 10.1165/ajrcmb/6.1.57. [DOI] [PubMed] [Google Scholar]
- 8.Orentas JR, Reinlib L, Hildreth JEK. Anti-class II MHC antibody induces multinucleated giant cell formation from peripheral blood monocytes. J Leukoc Biol. 1992;51:199. doi: 10.1002/jlb.51.3.199. [DOI] [PubMed] [Google Scholar]
- 9.Boros DL. Granulomatous inflammations. Prog Allergy. 1978;24:183. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- 10.Enelow RI, Sullivan GW, Carper HT, Mandell GL. Cytokine-induced human multinucleated giant cells have enhanced candidacidal activity and oxidative capacity compared with macrophages. J Infect Dis. 1992;166:664. doi: 10.1093/infdis/166.3.664. [DOI] [PubMed] [Google Scholar]
- 11.Mariano M, Spector WG. The formation and properties of macrophages polykaryons (inflammatory giant cells) J Pathol. 1974;113:1. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- 12.Papadimitriou JM, Robertson TA, Walters M. An analysis of the phagocytic potential of multinucleate foreign body giant cells. Am J Pathol. 1975;78:343. [PMC free article] [PubMed] [Google Scholar]
- 13.Facchetti F, Vermi W, Fiorentini S, et al. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am J Pathol. 1999;154:145. doi: 10.1016/S0002-9440(10)65261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abou Zeid C, Filley E, Steele J, Rook GAW. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands into antigen-bearing particles. J Immunol Methods. 1987;98:5. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez Pando R, Orozco EH, Arriaga AK, et al. Analysis of the local kinetics and localization of interleukin 1a, tumor necrosis factor a and transforming growth factor b, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray LJ, Lee R, Martens C. In vitro expression in T cell subsets of the autoimmune MRL/Mp-Ipr/Ipr mouse. Eur J Immunol. 1990;20:163. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 17.Reiner SL, Zheng S, Corry DB, Locksley RM. Constructing polycompetitor cDNA for quantitative PCR. J Immunol Methods. 1993;165:37. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 18.Rokitansky C. Lehrbuch der Pathologischem Anatomic. 3. Vienna: Braumuller; 1855. [Google Scholar]
- 19.Langhans T. Ueber riesenzellen mit wandstandingen kernen in tuberkeln und die fibrose form des tuberkels. Virchows Arch Pathol Anat Physiol Klin Med. 1868;42:382. [Google Scholar]
- 20.Gasseries A, Most J. Generation of multinucleated giant cells in vitro by culture of human monocytes with Mycobacterium bovis BCG in combination with cytokine-containing supernatants. Infect Immun. 1999;67:395. doi: 10.1128/iai.67.1.395-402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Graaf J, Tamminga RYJ, Dam-Meiring A, Kamps WA, Timens W. The presence of cytokines in Langerhans' cell histiocytosis. J Pathol. 1996;180:400. doi: 10.1002/(SICI)1096-9896(199612)180:4<400::AID-PATH701>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Seitzer U, Schell Toellner D, Toellner KM, et al. Properties of multinucleated giant cells in a new in vitro model for human granuloma formation. J Pathol. 1997;182:99. doi: 10.1002/(SICI)1096-9896(199705)182:1<99::AID-PATH807>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Junming L, Vilcer J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987;56:234. [PubMed] [Google Scholar]
- 24.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassali P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 25.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]