Gut-Derived Sepsis Occurs When the Right Pathogen With the Right Virulence Genes Meets the Right Host: Evidence for In Vivo Virulence Expression in Pseudomonas aeruginosa (original) (raw)
Abstract
Objective
To define the putative role of the PA-I lectin/adhesin, a binding protein of Pseudomonas aeruginosa, on lethal gut-derived sepsis after surgical stress, and to determine if this protein is expressed in vivo in response to physical and chemical changes in the local microenvironment of the intestinal tract after surgical stress.
Summary Background Data
Previous work from the authors’ laboratory has established that lethal gut-derived sepsis can be induced after the introduction of P. aeruginosa into the cecum of mice after a 30% hepatectomy. This effect does not occur when P. aeruginosa is introduced into the cecum of sham operated control mice. Previous experiments further established that the mechanism of this effect is due to the presence of the PA-I lectin/adhesin of P. aeruginosa, which induces a permeability defect to a lethal cytotoxin of P. aeruginosa, exotoxin A.
Methods
Three strains of P. aeruginosa, one lacking functional PA-I, were tested in two complementary systems to assess virulence. Strains were tested for their ability to adhere to and alter the permeability of cultured human colon epithelial cells, and for their ability to induce mortality when injected into the cecum of mice after a 30% hepatectomy. To determine if PA-I is “in vivo expressed” when present in the cecal environment after hepatectomy, strains were retrieved from the cecum of sham-operated and hepatectomy-treated mice 24 and 48 hours after their introduction into the cecum and their PA-I expression was assessed.
Results
Results indicated that PA-I plays a putative role in lethal gut-derived sepsis in the mouse, because strains lacking functional PA-I had an attenuated effect on cultured human epithelial cells, and were nonlethal when injected into the cecum of mice after 30% surgical hepatectomy. Furthermore, surgical stress in the form of hepatectomy significantly altered the intestinal microenvironment, resulting in an increase in luminal norepinephrine associated with an increase in PA-I expression in retrieved strains of P. aeruginosa. Co-incubation of P. aeruginosa with norepinephrine increased PA-I expression in vitro, suggesting that norepinephrine plays a role in the observed response in vivo.
Conclusions
Lethal gut-derived sepsis may occur when intestinal pathogens express virulence determinants in response to environmental signals indicating host stress. In this regard, the PA-I lectin/adhesin of P. aeruginosa appears to be a specific example of in vivo virulence expression in colonizing pathogens in the intestinal tract in response to surgical stress.
Pseudomonas aeruginosa is the one of the most common organisms cultured from critically ill surgical patients and is associated with the highest mortality rate. 1 The mere presence of P. aeruginosa in the intestinal tract of critically ill surgical patients is associated with a 70% mortality rate—a three-fold increase over physiologically matched critically ill patients who culture negative for this organism. 2 Similar findings are seen in critically ill infants, in whom the presence of P. aeruginosa in feces is associated with a five-fold increase in the development of sepsis syndrome. 3 While it is tempting to dismiss these high mortality rates as simply reflective of the poor immunologic status of these patients, it is possible that P. aeruginosa acts as a primary pathogen in the intestinal tract while remaining clinically undetectable. 4
Previous work from our laboratory has established that P. aeruginosa introduced into the cecum of mice can induce a state of gut-derived sepsis after a 30% surgical hepatectomy, whereas it has no effect in sham-operated control mice. 5 We further established that this effect appears to be due to the presence of a key binding protein of P. aeruginosa, the PA-I lectin/adhesin, which induces mortality in this model by disrupting the tight junctional barrier to lethal cytotoxins of this organism. Mortality in this model is completely independent of bacteremia. In fact, at similar doses, intravenous or intraperitoneal administration of this organism is not lethal to the mouse, whereas its introduction into the lung or gut results in 100% mortality at 48 hours. 5–7 These findings beg a more complete understanding of the pathogenesis of this organism, which carries a high degree of prevalence and polyclonality in the intestinal tract of critically ill patients. 8
Insight into the mechanisms of lethal gut-derived sepsis due to P. aeruginosa may be gained from basic observations on the molecular regulation of bacterial virulence. All bacteria regulate gene expression in response to different environmental signals, a property that is crucial to their ability to survive and compete with other organisms. 9 For example, invasive pathogens may need one set of genes to adhere to the host in a particular situation, such as the intestinal epithelial lining, and must immediately switch off this gene in transit through the tissues to avoid adhering to a hostile macrophage or neutrophil. In this situation, virulence structures such as adherence appendages may be helpful one moment and a liability the next. The environmental cues that act as signals for the control of virulence in animal pathogens are generally simple physical and chemical factors such as temperature, pH, osmolality, nutrient availability, and as most recently described, norepinephrine concentration. 10 Variability in the virulence phenotype of P. aeruginosa in response to alterations in the intestinal environment may influence the lethal effect of this organism in a given host. Virulence phenotype transformation in response to host stress may explain in part the observation that human volunteers ingesting large doses of the organism remain healthy despite persistent fecal shedding of the bacteria for up to 7 days, whereas its presence in the intestinal tract of a critically ill patient is associated with a high mortality rate. 11,12
The purpose of the present study was three-fold. First, we sought to establish the putative role of the PA-I lectin/adhesin of P. aeruginosa on the intestinal epithelial barrier and mortality in mice after surgical stress (30% hepatectomy) using a strain of P. aeruginosa lacking functionally expressed PA-I. Second, we sought to determine if environmental cues known to affect bacterial virulence, such as pH, redox state, or norepinephrine concentration, are altered in the intestinal tract of mice after surgical stress in the form of a 30% hepatectomy. Finally, we determined if strains of P. aeruginosa retrieved from the intestinal tracts of mice after hepatectomy have an increase in the functional expression of PA-I, thereby implicating that it is “in vivo expressed” by the effects of surgical stress.
MATERIALS AND METHODS
Experimental Design
All experiments were approved by the Animal Care and Use Committee at the University of Chicago. Inbred mice of the Balb/c (Jackson Laboratories) background weighing between 20 and 25 g were used for all experiments. Mice were housed in individual wire bottom cages to limit coprophagy during the entire experimental period. Previous work using this mouse model demonstrated that only the combination of hepatectomy and starvation (48 hours of water only) resulted in significant perturbations in mucosal barrier function as a direct result of its microbial flora. 13,14P. aeruginosa injected into the cecum of mice after hepatectomy and starvation results in a 100% mortality rate 5; in contrast, cecal injection of live P. aeruginosa after sham laparotomy with 48 hours of starvation (water only) results in no mortality and the mice appear healthy. We focused our work on the cecum because it is the area of greatest adherence of bacteria, and the area with the greatest permeability defect and ICAM expression as a direct result of its bacterial flora. 15,16 Three experimental protocols were used to address the specific aims.
Experiment I
To determine if surgical stress alters the physical microenvironment of the cecum, groups of mice underwent 30% surgical hepatectomy and were allowed water only for the duration of the study period. Mice in the control group underwent sham laparotomy and were allowed access to water and chow ad libitum. Mice were killed after 48 hours, and pH, redox state, and norepinephrine concentration determined in cecal contents.
Experiment II
To determine the putative role of the PA-I lectin of P. aeruginosa on lethal gut-derived sepsis in this model, groups of mice (n = 5) underwent surgical hepatectomy and cecal injection of one of three strains of P. aeruginosa. P. aeruginosa strain ATCC 27853 was used as positive control because it formed the basis of our previous experiments with this model. Two other strains, ATCC 33347 and ATCC 33347–66, were also injected into the cecum after hepatectomy; ATCC 33347 has been previously reported to contain a very high concentration of PA-I, whereas its mutant 33347–66 contains no detectable PA-I. Each strain was assayed for its PA-I content and toxin production, and was also tested for its ability to adhere to and alter the permeability of cultured intestinal epithelial cells (Caco-2). After injection into the mouse cecum, the lethal effect of each strain was determined by assessing mortality at 48 hours.
Experiment III
To determine whether the functional expression of PA-I is increased as a result of the altered microenvironment of the mouse cecum after hepatectomy, P. aeruginosa was retrieved from the cecum of mice by culturing feces on Pseudomonas isolation agar at 24 and 48 hours after hepatectomy and cecal injection of P. aeruginosa. P. aeruginosa was similarly isolated from the cecum of sham-operated controls at 24 and 48 hours. Both groups of mice were allowed water only during the study period. Strains of P. aeruginosa were also exposed to 0.01% norepinephrine for 45 minutes in standard growth media (tryptic soy agar) and PA-I content was determined.
Mouse Model of Endogenous P. aeruginosa Sepsis
The mouse model we developed involves injection of live strains of P. aeruginosa grown overnight in tryptic soy agar and washed and resuspended in PBS as described. 5 Two hundred microliters of ∼ 2×106 cfu/gm of P. aeruginosa is injected into the cecum based on previously published dose-response data. 5 Animals were anesthetized (ketamine 100mg/kg, xylazine 10 mg/kg, and atropine 0.04 mg/kg intraperitoneally). Through a midline incision, the floppy left lobe of the liver is cauterized using electrocautery, and weighed to insure uniformity in the resection. Before closure, a puncture into the cecum with a 27-g needle is used to inject bacteria. The cecal puncture site is tied off with a 4–0 silk suture and swabbed with alcohol, and the abdomen is closed. We have had no episodes of peritonitis or spillage, and have performed subsequent cultures of the peritoneum that have been consistently sterile. This model results in 100% mortality at 48 hours after the procedure; animals die a typical septic death with features of chromodacryorrhea, lethargy, scant diarrhea, and ruffled fur. Animals are sacrificed when they are moribund. Sham-operated control animals injected with identical doses of P. aeruginosa have 100% survival and do not appear ill.
Measurement of pH, Redox State, and Norepinephrine in Cecal Contents (Feces)
Two groups of mice were studied. Mice subjected to sham laparotomy fed chow ad libitum were compared to mice undergoing a 30% surgical hepatectomy and allowed water only for the duration of the study period. At 48 hours, mice were killed and cecal contents harvested by placing the surgically excised cecum into a vial of 1 mL of 0.9% saline, which was vortexed and the cecal tissue removed. Oxidation/reduction potential (redox state) and pH was measured in suspended cecal contents using separate probes of the ORP/pH electrode system (Orion Research, Cambridge, MA). Norepinephrine and its immediate precursor, dopamine, were measured by high-pressure liquid chromatography in both cecal contents and cecal tissues. 17
Bacterial Strains and Toxin Characterization Studies
Three strains of P. aeruginosa were used for the studies outlined below. ATCC strain 27853 was obtained from the American Type Culture Collection (Manassas, VA) and was originally isolated from a clinical blood culture of a septic patient. P. aeruginosa ATCC 27853 is the prototype strain for antibiotic sensitivity in most clinical hospital laboratories and its clinical and laboratory behavior is well characterized. 18 The PA-I negative strain of P. aeruginosa ATCC 33347–66, and ATCC 33347, were obtained as a generous gift from Dr. Nachem C. Garber (Department of Life Sciences, Bar-Ilan University, Israel). ATCC 33347 contains the highest concentration of PA-I of all strains reported to date. Strains were assayed in duplicate for exotoxin A, elastase (LasB), LasA protease activity, azocasein protease, pyoverdine, pyocyanin, and phospholipase C, using both Western blot analysis and enzymatic activity assays when indicated. 19 Extracellular virulence factor production was expressed in relative scale from + to +++++.
PA-I Assay
Bacterial strains were lysed by sonication and assayed for PA-I by erythrocyte agglutination assay, and by Western blot analysis using specific polyclonal antibody as previously described. 20,21 Antibody was generously provided by Dr. N.C. Garber. Because PA-I can be in the “in” (intracytoplasmic) or “out” (extracellular-fimbrial) position, depending on the growth conditions of the media, erythrocytes agglutination assays were performed on both bacterial cell sonicates and whole bacterial cells. In ideal growth media, most PA-I is intracytoplasmic. 21 To determine the amount of PA-I that is surface expressed, and therefore constitutively available for adherence to intestinal epithelia, whole bacteria cells were assayed for PA-I using the erythrocyte agglutination assay. Human O erythrocytes specifically agglutinate PA-I, an effect that can be inhibited by n-acetyl D-galactosamine (GalNAc), an oligosaccharide that specifically binds PA-I. Assays were performed in the presence and absence of GalNAc. For PA-I mRNA, P. aeruginosa was grown in tryptic soy broth, and 1 mL was washed and resuspended in 2 mL of Trizol (Gibco, Rockville, MD) and sonicated. The RNA pellet was resuspended in RNase free water and digested using RNase free Dnase (Promega, Madison, WI). RNA samples were reextracted with phenol and chloroform. The RNA pellet was resuspended in RNase free water and quantitated spectrophotometrically. RNA (10μg) was separated by gel electrophoresis and transferred to nylon membranes (Amersham, Arlington Heights, IL). After cross linking, membranes were prehybridized in XOTCH solution (composition: 7% wt/vol SDS, 1% wt/vol BSA, 200 mM NaH2PO4, 10 mM EDTA, with 15% vol/vol deionized formamide). A 400 bp cDNA probe specific for PA-I was radiolabeled with a 32P-dCTP (cytidine triphosphate) using a random primer labeling kit (DECA primer II, Ambion, Austin, TX). 22 Membranes were then incubated with labelled probes for 18 hours. Membranes were then washed twice in 2×SSC/0.1% SDS for 15 minutes and then three times in 0.1×SSC/0.1% SDS at 65°C. Bands were quantified by laser densitometry.
Effect of P.
aeruginosa on Adherence to and Alteration of the Intestinal Epithelial Barrier
The various stains of P. aeruginosa were assessed for their ability to adhere to Caco-2 cells by incubating bacteria with dispersed cells for 45 minutes at 28°C and then performing quantitative culture as previously described. 5 Bacterial strains were also screened for their ability to decrease the transepithelial electrical resistance (TEER) of monolayers of Caco-2 cells grown to confluence on collagen coated transwells as described. 5 Transepithelial electrical resistance is a sensitive measure of tight junctional barrier function and has been previously shown by our lab to be profoundly affected by the PA-I lectin of P. aeruginosa. 5 Based on previously published dose-response curves, ∼approximately 106 to 107 cfu/mL of live P.aeruginosa will decrease TEER by 80% at 4 hours after apical exposure. 5 Briefly, 50 μL of live Pseudomonas in nonantibiotic cell culture media were added to the apical side of the cell monolayer at a final concentration of ∼1 × 107 cfu/mL and TEER measured at 2 and 4 hours.
Statistical Analysis
Data were loaded onto the SigmaStat (Jandel Corporation, San Rafael, CA) program and tested for significance using a one-way ANOVA and Newman-Keuls post hoc testing where appropriate. For nonparametric data involving percent (incidence) of mortality, the Fisher exact test was used. P values of <.05 were accepted for statistical significance.
RESULTS
Effect of Hepatectomy and Starvation on the Cecal Microenvironment
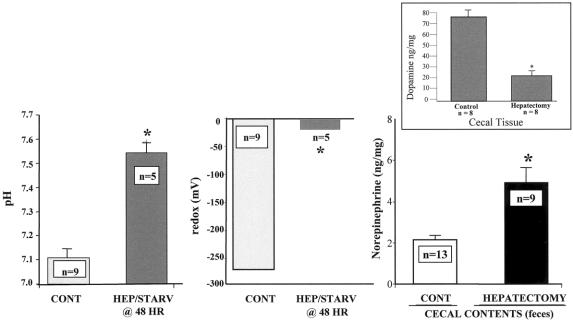
The pH, oxidation reduction potential (redox state), dopamine, and norepinephrine concentration in cecal contents after hepatectomy are summarized in Figure 1. A significant alteration in the cecal microenvironment was observed after hepatectomy and starvation. A statistically significant increase in norepinephrine concentration was observed in cecal contents from mice after hepatectomy. This occurred in the presence of a decrease in the concentration of cecal mucosal dopamine, the immediate precursor of norepinephrine. These findings suggest that the increase in fecal norepinephrine may be derived from the cecal tissue, and is further suggested by the observation that two metabolites of dopamine, HVA or DOPAC, were not increased in cecal tissues (data not shown).
Figure 1. Effect of hepatectomy and starvation on pH, redox state, and norepinephrine concentration in the cecum of mice. Mice in the control group underwent sham laparotomy and were allowed access to chow and water ad libitum (Control/fed). Mice in the hepatectomy group underwent a 30% surgical hepatectomy and were allowed water ad libitum only (Hepatectomy/starved). Results demonstrate that a statistically significant increase in pH, redox state, and norepinephrine concentration was observed in cecal contents of mice after hepatectomy and 48 hours of starvation (*P < .001). Norepinephrine concentration was increased in the cecal contents only, while cecal tissue levels were not statistically different between groups (data not shown). The small graph represents the cecal tissue levels of dopamine, the immediate precursor of norepinephrine, between groups. A statistically significant decrease (*P < .001) in dopamine was observed in mice after hepatectomy, suggesting that the elevated luminal concentration of norepinephrine is derived from the cecal tissues.
PA-I Alters the Barrier Function of Cultured Intestinal Epithelia and Plays a Key Role in Lethal Gut-Derived Sepsis in the Mouse
Figure 2 displays the PA-I profile of the different strains of P. aeruginosa and summarizes their effects on Caco-2 cells and mouse mortality when introduced into the cecum after hepatectomy. Figure 3 summarizes the extracellular virulence factor profile of the different strains. Although the PA-I negative strain was not identical to ATCC 33347, it had significantly increased amounts of exotoxin A.
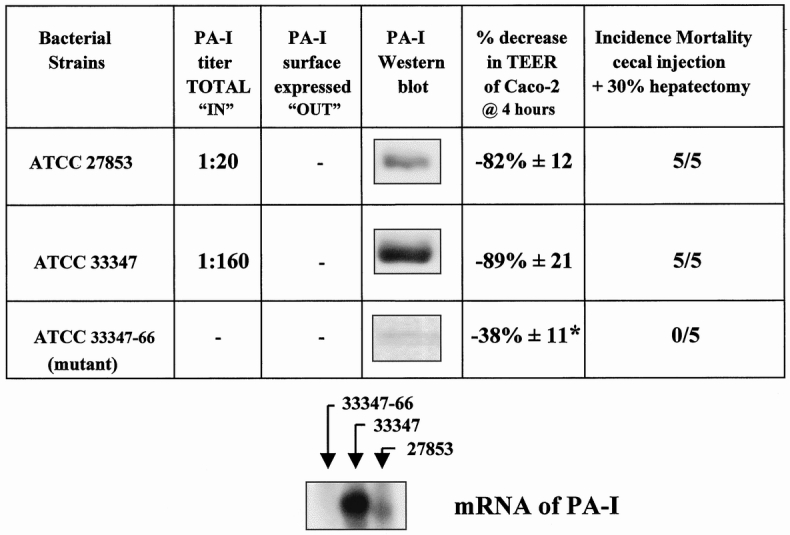
Figure 2. Characterization of strains of Pseudomonas aeruginosa for PA-I concentration and their effects on the barrier function of Caco-2 cells and mouse mortality after introduction into mouse cecum after a 30% hepatectomy. Strain 33347 demonstrated a high degree of PA-I expression by both Western blot analysis and erythrocyte agglutination activity. None of the strains had surface expressed PA-I, as assessed by erythrocyte agglutination activity using whole bacterial cells. PA-I mRNA was not detectable in 33347–66. PA-I mRNA correlated to protein expression in 33347 and 27853. The PA-I negative strain ATCC 33347–66 (mutant) had an attenuated effect on the adherence to Caco-2 cells and did not decrease transepithelial electrical resistance to the same degree as PA-I positive strains (*P < .001). The PA-I negative strain (33347–66) was completely nonlethal when introduced into the cecum of mice after hepatectomy.
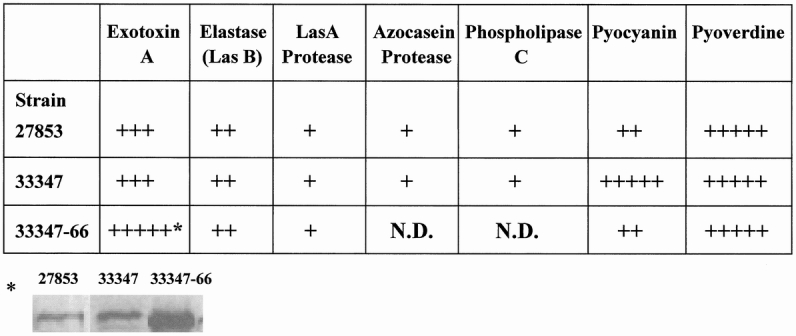
Figure 3. Extracellular virulence factor profile of strains of Pseudomonas aeruginosa. Extracellular virulence factor concentration is expressed in relative scale from + to +++++. Strains differed significantly in extracellular virulence factor concentrations. While strain 33347–66 had a considerable increase in the amount of exotoxin A compared to 33347, it did not induce mortality in mice (see Fig. 2).
Effect of the Cecal Microenvironment on PA-I Expression In Vivo
Figure 4 summarizes the effect of norepinephrine on the functional expression of PA-I as measured by ability of whole bacterial cells to agglutinate human erythrocytes. Figure 4 also demonstrates that strains retrieved from the cecum of mice undergoing hepatectomy had functionally expressed PA-I whereas those from control mice did not.
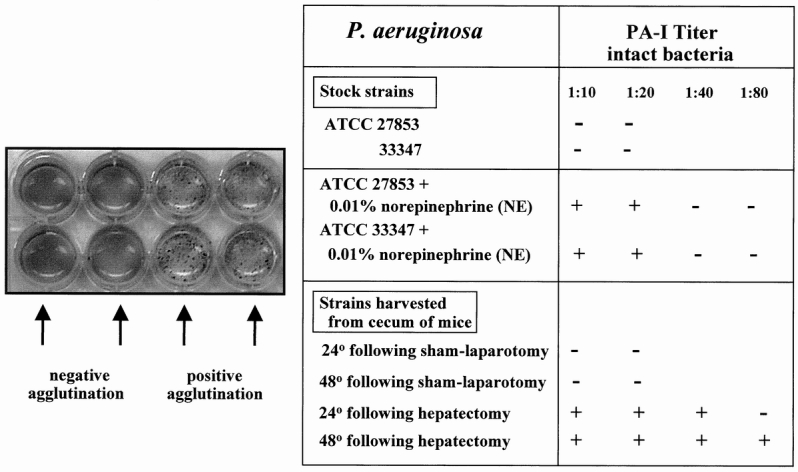
Figure 4. Erythrocyte agglutination assays of the various strains of Pseudomonas aeruginosa exposed to norepinephrine in vitro or harvested from the cecum of sham-operated control or 30% surgical hepatectomy mice. Results demonstrate that while neither 27853 nor 33347 had functionally (surface) expressed PA-I, both strains expressed PA-I when coincubated with 0.01% norepinephrine. This effect was GalNAc-inhibitable. Strains were harvested and assayed for PA-I 24 and 48 hours after cecal injection of strain 27853 into the cecum of sham operated control and 30% surgical hepatectomy. Only strains harvested from the cecum of mice undergoing 30% surgical hepatectomy displayed evidence of functionally expressed PA-I. The agglutination titer of PA-I after 48 hours in the mouse cecum after hepatectomy (1:80) was higher than its total PA-I titer at baseline in vitro (1:20; see Fig. 2), suggesting that the cecal environment after 30% hepatectomy may increases the synthesis as well as the surface expression of PA-I as a result of the in vivo condition.
DISCUSSION
Microbial geneticists have long appreciated that bacteria change their virulence characteristics in response to changes in their physical microenvironment. 23 These “environmental cues” act as sensory input signals to the molecular machinery of bacteria through complex signal transduction pathways. Many of the genes that regulate the phenotypic capability of an organism do so in a cell-density–dependent manner using a well-established quorum-sensing signaling system. 24 This system allows intercommunication between bacteria to sense their population density and regulate gene expression accordingly, presumably in the amount necessary to overcome the host. Bacterial populations, such as those that exist in biofilms, have been described as multicellular organisms that are able to “sense” environmental changes so as to act in their own best interest using analogous systems such as smell, taste, and feel. 25
The ability of bacteria to regulate gene expression in response to environmental cues is crucial to their ability to survive and compete with other organisms. In order to remain metabolically economical, however, gene products that express survival function predominate during ideal growth conditions, while virulence genes are, in general, “turned off.” While certain bacteria and bacterial populations may be uniquely opportunistic, harm to the host is usually an unanticipated and inadvertent consequence of its virulence repertoire, as bacteria usually depend on the host to survive. Therefore the expression of a lethal phenotype in a given bacterial population may be governed by extraordinary provocation of its virulence genes at a time of unique host susceptibility.
Data from the present study suggest that surgical stress alters the intestinal microenvironment characterized by altered pH, redox state, and norepinephrine release. Each of these parameters has been demonstrated in in vitro studies to alter the virulence phenotype of bacteria. 7 We focused our in vitro experiments on the effects of norepinephrine because it has been recently shown to promote the adherence of commensal Escherichia coli to the intestinal mucosa in a rat model of chronic endogenous norepinephrine release. 26 We have previously shown that hepatectomy and starvation in mice shifts the composition of intestinal E. coli to that of a more adherent strain capable of altering the permeability of cultured mouse colon cells. 27E. coli has been reported to increase its adherence phenotype in the presence of norepinephrine via signaling molecules involved in the quorum-sensing system. 28 Data from the present study suggest that luminal norepinephrine release may also play a role in increasing the adhesive capacity and lethal effect of P. aeruginosa introduced into the intestinal tract of mice after hepatectomy. The further finding that norepinephrine increased the surface expression of PA-I in P. aeruginosa may demonstrate a general strategy among colonizing intestinal microbes to increase their adherence phenotype in response to their perception of host stress. Because the intestinal tract is disproportionately vasoconstricted during catabolic stress and is among the organs with the most abundant concentration of norepinephrine, catabolic stress such as occurs after surgery and short-term starvation, may create a unique local microenvironment whereby bacteria sense extreme host stress via such signals as luminal norepinephrine. 29 Further experiments are underway to address the additive or synergistic effects of pH and redox state on P. aeruginosa virulence in this model.
We have previously established that injection of P. aeruginosa into the cecum of mice can induce a state of lethal gut-derived sepsis similar to that seen in severe and prolonged catabolic stress. 5 Animals appear septic throughout the course of the study, yet do not display evidence of histologic breakdown in the intestinal tissues. The lethal effect of P. aeruginosa after its introduction into the cecum of mice after hepatectomy appears to be mediated via a PA-I lectin/adhesin-induced permeability defect to its cytotoxin, exotoxin A. 5 The injection of the combination of purified PA-I and exotoxin A into the cecum of mice after hepatectomy is lethal, whereas each alone has no effect in this model. Furthermore, pretreatment of P. aeruginosa with GalNAc, a specific binder of PA-I, completely prevents mortality in this model. 5P. aeruginosa injected into the cecum of sham-operated controls does not result in mortality despite an equal level of translocation to the liver and blood. 5 Although it is possible that the lethal effect of P. aeruginosa injected into the cecum of mice after hepatectomy may be a result of abnormal immune function imposed by hepatectomy, it is also possible that the surgical stress itself alters the virulence phenotype of intestinal bacteria in these animals, in part due to norepinephrine release into the intestinal tract. The findings of alterations in pH, redox state, and norepinephrine concentrations in the intestinal tract of mice after hepatectomy suggest the potential of surgical stress on the local microenvironment itself to shift the virulence phenotype of the intestinal microflora.
Results from the present study confirm that PA-I is a key factor in P. aeruginosa gut-derived sepsis in mice after a typical yet recoverable surgical stress. The behavior of P. aeruginosa in the Caco-2 cell system is similar to other intestinal pathogens in that it readily adheres to and alters their epithelial barrier function. Studies in cultured human intestinal epithelial cells demonstrate that intestinal pathogens known to cause disease in man, such as Salmonella and enteropathogenic E. coli, induce a contact-dependent defect in epithelial barrier function via dysregulation of tight junctional proteins. 30,31 In fact, it is proposed that the ability of the epithelium to maintain tight junction integrity is a major defense against bacterial invasion and cytotoxicity. 32 If defined by its ability to adhere to and alter the TEER of cultured human epithelial cells, P. aeruginosa induces the most rapid and severe permeability defect of any pathogen reported to date. The finding that the strain of P. aeruginosa lacking PA-I had an attenuated effect in the Caco-2 system and failed to induce mortality when injected into the cecum of mice after hepatectomy suggests a putative role for PA-I in the mortality demonstrated in this model. The PA-I negative strain contained a very high concentration of exotoxin A and therefore had significant potential to induce mortality (Fig. 3). The PA-I positive and negative strains used in this study are not identical, however, and therefore mortality could be due to an as-yet-unidentified virulence determinant (Fig. 3). Our previous studies with this model have established that exotoxin A alone does not alter the permeability of the intestinal epithelium and does not cause mortality when injected into the cecum of mice after hepatectomy, yet is lethal when injected systemically. 5 On the other hand, purified PA-I results in a profound alteration in the permeability of the intestinal epithelium equal to that of the whole organism, yet is completely innocuous when injected systemically. When the combination of PA-I and exotoxin A is injected into the cecum of mice undergoing hepatectomy, however, mortality is 100%, similar to injection of the whole organism. Taken together, these studies suggest that the mortality in this model is likely due to a PA-I–induced permeability defect to potent cytotoxins of P. aeruginosa such as exotoxin A. 5
The mechanism by which norepinephrine increases the functional expression of PA-I remains to be clarified. Data from the present study suggest that the observed increase in PA-I in strains retrieved from the cecum of mice after hepatectomy is both through an increase in total synthesis of PA-I and its relocation to the outer surface. PA-I can be in the intracellular (intracytoplasmic—“in”) position or in the extracellular (fimbrial—“out”) position, depending on the culture media or local environment. Previous studies have demonstrated that under ideal growth conditions, the majority (>80%) of PA-I is intracytoplasmic. 21 To discriminate for position, PA-I was assayed using both lysed and intact bacterial cells. Agglutination assays with intact bacterial cells assess surface or functionally expressed PA-I. Because the baseline titer of total PA-I for ATCC 27853 was 1:20 and resulted in no agglutination with intact cells, the large increase in the agglutination titer of PA-I to 1:80, using intact bacterial strains harvested from the cecum of mice after hepatectomy, would suggest an increase in both surface expression and total concentration. A similar increase above baseline values for total PA-I was found when strains were coincubated with norepinephrine (Fig. 4).
In summary, the PA-I lectin/adhesin of P. aeruginosa plays a key role in experimental gut-derived sepsis in the mouse. The finding that the PA-I lectin/adhesin of P. aeruginosa is “in vivo” expressed in the intestinal tract of mice after surgical hepatectomy is the first example to suggest that clinically relevant and potentially lethal pathogens may sense host stress and change their virulence phenotype accordingly. Thus, the mere presence of P. aeruginosa in the intestinal tract of critically ill patients may be of etiopathogenic importance in the development of sepsis syndrome and multiple organ failure, and its true virulence potential may remain elusive if examined under ideal growth conditions only.
Discussion
Dr. J. Wesley Alexander (Cincinnati, Ohio): As most people realize, translocation is an easy phenomenon to produce in experimental animals. A lot of studies have been done in mice, and it has been shown that the degree of translocation is clearly related to the occurrence of death in those animals.
In man, studies have shown that at least 11% of people coming to a surgical operation where the abdomen is open have translocation of live bacteria to the mesenteric lymph nodes or to the serosa of the bowel wall. Other studies using more sensitive and sophisticated techniques have shown that about 50% of people in ICUs have evidence of translocation by detecting bacterial DNA in the blood of those patients as measured by PCR. There is a lot of controversy now as to whether this is of any consequence in man, i.e., whether the translocation is an epiphenomenon or whether it really contributes to morbidity. Recent observations of positive blood cultures in patients in the surgical ICU do not correlate with outcome as much as they do with severity of illness. There are several important points, Dr. Alverdy, about which I would like to ask you to comment.
It would seem that the up-regulations of these virulence factors in Pseudomonas is an important cause of mortality in your experimental animals. Do you have any evidence that this same phenomenon occurs in humans? There is certainly an increase in intestinal bacteria, particularly Pseudomonas, in patients who are critically ill, and it is this group that is likely to have high levels of norepinephrines.
Dr. Nicolas V. Christou (Montreal, Quebec, Canada): We all make the assumption that host–parasite interactions are uniform and that the outcome is predictable. For example, bacteria given access to a susceptible host will result in a septic response and it will have an adverse outcome. That is a basic assumption, but clinically it doesn’t always follow. There are good examples in the literature to which you have added with this work. If one is homogeneous for TNF-1 allele, for example, and one gets challenged by bacteria infection, one will produce very little TNF and a minimal septic response without an adverse outcome. If one carries the type 2 allele homozygosity, one will produce massive amounts of TNF and a marked septic response and most likely die when challenged by the same infection. You have now shown that the bacteria and the types of genes that they express are also important. For this reason, the work is very, very significant. Pseudomonas is unique in its ability not only to up-regulate the gene but also to have these exotoxins such as toxin A and elastases, which can contribute to the detriment of the host. They have the ability, with PA-I up-regulation, to deliver these in areas that can be harmful. Therefore, I would like to ask you some questions related to your paper.
What kills the host? Is it the actual exotoxin A and elastases that get access to invasive tissue? Or is it the systemic inflammatory response that is generated by the absorption of these toxins, perhaps the whole bacteria?
As Dr. Alexander asked, what else translocates in your model? Lipopolysaccharide is in very high concentration in the small bowel and in the cecum of both mice and humans, and it only takes a few molecules to get across into the blood to turn on a massive systemic inflammatory response. Do you have any evidence that this might be occurring? Given this, what therapeutic strategies would you recommend for us to follow to take advantage of the fine work you presented to us today?
Presenter Dr. John C. Alverdy (Chicago, Illinois): In terms of human correlates of this work, to our knowledge there are no studies that have demonstrated that bacteria change their virulence phenotype as a result of surgical stress or the critically ill state. The main reason for this is that before the molecular biology explosion, we have been concerned with only two aspects of bacteria in our patients, what is their species and which antibiotics are they susceptible to in terms of microbicidal activity. A recent study (Am J Respir Crit Care Med 1999;160:1212) has demonstrated that P. aeruginosa is highly prevalent in the feces of critically ill patients and carries a high degree of polyclonality. This study suggests that in critically ill patients, not all P. aeruginosa are alike. The experiments we have presented today are an extension of our previous work with E. coli and are the first to demonstrate that bacteria can be transformed to a more virulent state when present in the environment of the intestine after surgical stress. Previous studies such as these have not been performed because they are methodologically difficult and have problematic observational indeterminancies. While we attempted to use proper and carefully selected controls, one must keep in mind that we have examined these bacteria after they have been isolated and grown in ideal growth conditions, thus removing them from the effect of the very environment in which we wish to examine them. Despite this, they seem to retain their transformed virulence phenotype. These types of studies will be extremely difficult to perform in critically ill humans and will be subject to the Heisenberg uncertainty principle of physics, which states that in a changing environment, certain measurements (position and momentum) are fleeting phenomenon.
Regarding Dr. Christou’s questions as to the cause of mortality in this model, we now have compelling evidence to suggest that P. aeruginosa alters epithelial permeability to a number of its known cytotoxins such as exotoxin A and elastase. We have previously published data using this model which demonstrate that translocation of whole bacteria and bacteremia of P. aeruginosa are irrelevant events in the mortality associated with this model. In fact, intravenous or intraperitoneal injection of similar doses of P. aeruginosa does not result in mortality in this model. Our previous data demonstrate that it is the PA-I–induced epithelial permeability defect to exotoxin A that is the cause of mortality in this model. We believe that use of the PA-I negative strain in the present study confirms our previous studies. Regarding the role of lipopolysaccharide (LPS) and endotoxin in this model, while there has been much enthusiasm for gut-derived LPS as a cause of sepsis syndrome in animal models, neither in vitro or in vivo studies demonstrate that lethal quantities of this product leak across the gut epithelium, nor does LPS injection into the intestine result in mortality. In fact, recent studies suggest that E. coli LPS does not leak across the gut after catabolic stress in mice, despite the demonstration of a significant epithelial permeability defect (Shock 1998;10:43).
Dr. Philip S. Barie (New York, New York): I have two questions that relate to potential clinical translation of these observations.
First, many studies now suggest that norepinephrine, as opposed to dopamine or other vasopressors, may be the first choice of vasopressor when necessary for clinical therapy of septic shock. Your studies, however, would suggest that one would need to be careful about the clinical administration of norepinephrine in therapeutic doses for the treatment of shock. Have you any data looking at norepinephrine administered systemically in these animals rather than locally into the cecum in terms of its effect on your model?
Second, we now recognize that nosocomial pneumonia is becoming more common than surgical site infection as the number one nosocomial infection afflicting surgical patients. The number two pathogen for nosocomial pneumonia, as you are aware, is Pseudomonas aeruginosa. One of the major virulence factors for Pseudomonas in the pathogenesis of nosocomial pneumonia is believed to be its propensity for adherence to oropharyngeal mucosal cells. Are there any correlates in your data with the adherence phenomena identified for Pseudomonas and oropharyngeal mucosal cells, or any evidence that there may be a systemic response that affects clones of Pseudomonas elsewhere in the body once the local gut phenomenon has developed?
Dr. Alverdy: The use of systemic norepinephrine for cardiopulmonary support can be lifesaving. It is not known whether systemic norepinephrine administration results in significant tissue accumulation of the drug in the intestinal mucosa or lumen. This is doubtful given the short half-life of the drug. Lyte and colleagues have used a rat model in which endogenous norepinephrine release was increased using a prodrug, and this effect did result an increase in E. coli adherence to the intestinal mucosa and therefore the potential for increasing bacterial virulence via systemic norepinephrine does exist.
However, this model may not be applicable to the clinical use of norepinephrine. Regarding extrapolation of the data from the present experiments to the lung, Dr. Dara Frank and colleagues have recently published a model in which lung instillation of P. aeruginosa was lethal to the mouse whereas its intravenous administration had no effect. Similar to our model, the lethal effect was dependent on the ability of the organism to deliver its toxins systemically, which in this case required the well-described type III secretion system of P. aeruginosa.
Dr. Andrew M. Munster (Baltimore, Maryland): I just have a single question. I am not sure that you have answered, at least to my satisfaction, Dr. Christou’s question about what kills the patient. To that end, I would like to ask if it would be possible that the translocation induces local cytokine induction in the cecal wall or adjacent lymph nodes? If so, did you measure the induction of any other inflammatory cytokines? Second, is it possible that this exotoxin allows the translocation of endotoxin? If so, did you measure endotoxin in the portal vein or in the liver?
Dr. Alverdy: While it is certainly possible and likely that cytokines play a role in the mortality observed in this model, I do not believe they are the inciting event. One proposed theory of sepsis syndrome and multiple organ failure is that there is discordance or pathologic amounts of cytokines that initiate and perpetuate the septic response. I do not believe we should discard the possibility that it is the pathogens themselves that are running the show. Our data clearly show that surgical stress itself can alter the virulence phenotype of P. aeruginosa and that this organism can induce a state of gut derived sepsis that is lethal in a vulnerable host. While the cytokine response to infection is important, it is downstream of the most proximate point in the infection process, namely the point at which bacteria adhere to mammalian cells. Bacterial adherence to host cells has been established to be the crucial initiating event for infection. We are focusing our efforts to understand exactly how surgical stress is unique in its ability to initiate this process, which perhaps lies in its effect on the molecular machinery of the microbe.
Footnotes
Correspondence: John C. Alverdy, MD, FACS, Dept. of Surgery, Section of General Surgery, University of Chicago, 5841 S. Maryland, MC 6090, Chicago, IL 606037.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
Supported by NIH grants DK-38510 and DK-47722 (to E.C.) and Digestive Disease Center Grant DK-42086.
E-mail:jalverdy@surgery.bsd.uchicago.edu
Accepted for publication April 2000.
References
- 1.Kaufman DF, Haas CE, Edinger R, Hollick G. Antibiotic susceptibility in the surgical intensive care unit compared with the hospital-wide antibiogram. Arch Surg 1998; 133: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract: the “undrained abscess of multiple organ failure. Ann Surg 1993; 218: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierro A, van Saene HK, Jones MO, et al. Clinical impact of abnormal gut flora in infants receiving parenteral nutrition. Ann Surg 1988; 227: 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buret A, Cripps AW. The immunoevasive activities of Pseudomonas aeruginosa. Am Rev Respir Dis 1993; 148: 793–805. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin RS, Musch MW, Hollbrook CJ, et al. The key role of Pseudomonas aeruginosa PA-I lectin on experimental gut-derived sepsis. Ann Surg (in press). [DOI] [PMC free article] [PubMed]
- 6.Schook LB, Carrick L, Berk JS. Murine gastrointestinal tract as a portal of entry in experimental Pseudomonas aeruginosa infections. Infect Immun 1976; 14: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurahashi K, Kajikawa O, Sawa T, et al. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 1999; 104: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonten MJ, Bergmans DC, Speijer H, Stobberingh EE. Characteristics of polyclonal endemicity of Pseudomonas aeruginosa colonization in intensive care units: implications for infection control. Am J Respir Crit Care Med 1999; 160: 1212–9. [DOI] [PubMed] [Google Scholar]
- 9.Guiney DG. Regulation of bacterial virulence gene expression by the host environment. J Clin Invest 1997; 99: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freestone PP, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol Lett 1999; 172: 53–60. [DOI] [PubMed] [Google Scholar]
- 11.Griffith SJ, Nathan C, Selander RK. The epidemiology of Pseudomonas aeruginosa in oncology patients in a general hospital. J Infect Dis 1989; 160: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 12.Buck AC, Cooke EM. The fate of ingested Pseudomonas aeruginosa in normal persons. J Med Microbiol 1969; 2: 521–525. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson BA, Guo J, Laughlin RJ, Alverdy JC. Increased type 1 fimbrial expression among commensal Escherichia coli in the murine cecum following catabolic stress. Infect Immun 1998; 67: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alverdy JC, Hendrickson B, Guandalini SS, et al. Perturbed bioelectrical properties of the mouse cecum following hepatectomy and starvation: the role of bacterial adherence. Shock 1999; 12: 235–241. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu S, Panes J, Grisham MB, et al. Effects of intestinal stasis on intercellular adhesion molecule 1 expression in the rat: role of enteric bacteria. Gastroenterology 1997; 112: 1971–8. [DOI] [PubMed] [Google Scholar]
- 16.Spitz J, Hecht, G, Taveras M, et al. The effect of dexamethasone on intestinal permeability: the role of bacterial adherence. Gastroenterology 1994; 106: 35–41. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann BM, Seiden LS, Landis CA, et al. Sleep deprivation in the rat, XVIII: Regional brain levels of monoamines and their metabolites. Sleep 1994; 17: 583–9. [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Cerniglia CE. Detection of Pseudomonas aeruginosa from clinical and environmental samples by amplification of the exotoxin A gene using PCR. Appl Environ Microbiol 1994; 60: 3739–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh SJ, Silo-Suh L, Woods DE, et al. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol 1999; 181: 3890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajolet-Laudinat O, Girod-de Bentzmann S, Tournier JM, et al. Cytotoxicity of Pseudomonas aeruginosa internal lectin PA-I to respiratory epithelial cells in primary culture. Infect Immun 1994; 62: 4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glick J, Garber NC. The intracellular localization of P aeruginosa lectins. J Gen Microbiol 1983; 129: 3085–3090. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg AP, Vogelstein BA. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Analyt Biochem 1984; 137: 266–267. [DOI] [PubMed] [Google Scholar]
- 23.Guiney DG. Regulation of bacterial virulence gene expression by the host environment. J Clin Invest 1997; 99: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 1999; 23:96: 13904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro JA. Thinking about bacterial populations as multicellular organisms. Ann Rev Microbiol 1998; 52: 81–104. [DOI] [PubMed] [Google Scholar]
- 26.Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res 1997; 70: 195–201. [DOI] [PubMed] [Google Scholar]
- 27.Rocha F, Laughlin R, Musch M, et al. Surgical stress alters epithelial barrier dysfunction by affecting the virulence phenotype of intestinal E. coli. Submitted for publication.
- 28.Freestone PP, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol Lett 1999; 172: 53–60. [DOI] [PubMed] [Google Scholar]
- 29.Chen LQ, Shepherd AP. Role of H+, alpha 2-receptors in escape from sympathetic vasoconstriction. Am J Physiol 1991; 261 (3Pt2):H868–73. [DOI] [PubMed] [Google Scholar]
- 30.McCormick BA, Colgan SP, Delp-Archer C, et al. Salmonella typhimurium attachment to human intestinal epithelial monolayer: transcellular signalling to subepithelial neutrophils. J Cell Biol 1993; 123: 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitz JC, Joutsouris A, Alverdy JC, Hecht G. Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol 1995; 31: 635–639. [DOI] [PubMed] [Google Scholar]
- 32.Lee A, Chow D, Haus B, et al. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am J Physiol 1999; 277 (1Pt1):L204–L217. [DOI] [PubMed] [Google Scholar]