Murine Oncostatin M Stimulates Mouse Synovial Fibroblasts in Vitro and Induces Inflammation and Destruction in Mouse Joints in Vivo (original) (raw)
Abstract
Oncostatin M (OSM) is a multifunctional cytokine, a member of the interleukin-6/leukemia inhibitory factor (IL-6/LIF) family, that can regulate a number of connective-tissue cell types in vitro including cartilage and synovial tissue-derived fibroblasts, however its role in joint inflammation in vivo is not clear. We have analyzed murine OSM (muOSM) activity in vitro and in vivo in mouse joint tissue, to determine the potential role of this cytokine in local joint inflammation and pathology. The effects of muOSM and other IL-6/LIF cytokines on mouse synovial fibroblast cultures were assessed in vitro and showed induction of monocyte chemotactic protein-1, interleukin-6, and tissue inhibitor metalloproteinase-1, as well as enhancement of colony growth in soft agarose culture. Other IL-6/LIF cytokines including IL-6, LIF, or cardiotrophin-1, did not have such effects when tested at relatively high concentrations (20 ng/ml). To assess effects of muOSM in articular joints in vivo, we used recombinant adenovirus expressing muOSM cDNA (AdmuOSM) and injected purified recombinant virus (106 to 108 pfu) intra-articularly into the knees of various mouse strains. Histological analysis revealed dramatic alterations in the synovium but not in synovium of knees treated with the control virus Ad-dl70 or knees treated with Adm-IL-6 encoding biologically active murine IL-6. AdmuOSM effects were characterized by increases in the synovial cell proliferation, infiltration of mononuclear cells, and increases in extracellular matrix deposition that were evident at day 4, but much more marked at days 7, 14, and 21 after administration. The synovium took on characteristics similar to pannus and appeared to contact and invade cartilage. Collectively, these results provide good evidence that OSM regulates synovial fibroblast function differently than other IL-6-type cytokines, and can induce a proliferative invasive phenotype of synovium in vivo in mice on overexpression. We suggest that OSM may contribute to pathology in arthritis.
The process of inflammatory arthritis involves a number of different cell types including resident connective tissue cells of the joint such as synoviocytes, endothelial cells, and chondrocytes, and their interaction with infiltrating cells including macrophages/monocytes, T lymphocytes, B cells, as well as polymorphonuclear cells. Such interactions are mediated in large part by cytokines and particular cytokines secreted by monocyte/macrophages, ie, interleukin-1 (IL-1) and tumor necrosis factor (TNF), are thought to drive effector functions of the local connective tissue cells. 1-4 Such effects include activation of synoviocyte proliferation and secretion of matrix metalloproteinases as well as other cytokines and chemokines, and activation of chondrocyte metabolism resulting in cartilage breakdown. 1,4 However, other cytokines likely participate in this process and may in fact dominate certain aspects of these complex chronic inflammatory processes.
The 28-kd cytokine oncostatin M (OSM) is another product of macrophages and of activated T cells, 5,6 and is found at enhanced levels in synovial fluids of rheumatoid arthritis patients. 7-9 OSM can affect a number of cell types including hepatocytes, 10 vascular smooth muscle cells, 11 Kaposi’s sarcoma cells, 12,13 as well as melanoma and carcinoma cell lines. 5 Human OSM induces responses by connective tissue cells such as human synovial fibroblasts, 7,14-17 chondrocytes, 18,19 and osteoblasts, 20 in addition to endothelial cells 21,22 and epithelial cells. 23,24 The regulation of TIMP-1, MMP-1, as well as monocyte chemotactic protein-1 (MCP-1) by OSM in synovial fibroblasts in vitro 7 suggest an important role for this cytokine in chronic joint inflammation. Human OSM binds and activates human receptors characterized as type I complex, which is identical to the leukemia inhibitory factor (LIF) receptor, and type II complex (specific for OSM) 25 and induces intracellular signaling pathways involving JAK-1, JAK-2, and TYK-2 kinases and activation of the STAT transcriptional activator proteins STAT-1 and STAT-3. 26-28 However, the precise role that OSM plays in chronic inflammation, particularly in comparison to other members of its family of cytokines, and in context of other proinflammatory cytokines, is still not clear.
To determine the effects of OSM in vivo, the use of animal models is necessary. The murine analogue of OSM has been recently identified 29 and is expressed at high levels in mouse bone marrow and at lower levels in thymus and spleen. Mouse OSM can regulate proliferation of a number of different cell types in vitro, 30 supports expansion of multipotential hematopoietic progenitors 31 and Sertoli cells, 32 and enhances hepatocyte differentiation. 33 Murine OSM (muOSM) binds its own specific receptor but, unlike human OSM, does not bind the LIF receptor. 30,34,35 Furthermore, it is clear that human OSM cannot interact with the mouse OSM receptor to induce responses. 30,36 We have previously shown that muOSM can stimulate TIMP-1 mRNA expression in mouse and rat fibroblast cell lines, whereas human OSM cannot. 36 In this investigation of mouse OSM and its regulation of connective tissue cells of joints, we have used recombinant cytokines to study in vitro responses of synovial fibroblasts derived from mouse synovium, and have used an adenovirus vector system to overexpress OSM in vivo in mouse joint tissues on local intra-articular administration. Our results suggest that muOSM is a potent stimulator of mouse synovial fibroblast responses in vitro, and that overexpression of OSM results in dramatic phenotypic changes in the synovium that are consistent with synovial cell proliferation, matrix remodeling, and infiltration of mononuclear cells, and thus results in major physiological alterations to the tissue.
Materials and Methods
Reagents
Recombinant mouse cytokines OSM, IL-6, LIF, and IL-1β (derived from Escherichia coli) were purchased from R&D Systems (Minneapolis, MN). Recombinant mCT-1, expressed in mammalian cells and purified as a fusion protein 37 was obtained from D. Pennica (Genentech, South San Francisco, CA). Enzyme-linked immunosorbent assay (ELISA) kits for mouse MCP-1 (MCAF/JE) were purchased from Biosource (Camarillo, CA).
Cells
Primary mouse synovial fibroblasts were derived from explants of finely minced synovial tissue from normal C57BL/6 mice, 10 to 12 weeks old (Charles River Laboratories, Ottawa, Canada), and passaged in Dulbecco’s modified minimal essential medium (F-15) media. Two separate primary cell cultures of fibroblasts, each derived from pooled mouse synovium (10 animals/preparation) have been analyzed. These were passaged several times before use, show a typical synovial fibroblast morphology, and tested positive for VCAM-1 expression (by immunohistochemistry). Primary rat fibroblasts were derived from explants of Sprague-Dawley rat synovium as above and cultured in Dulbecco’s modified minimal essential medium. All fibroblast cell lines were supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1.5 μg/ml Fungizone (Bristol-Myers Squibb Canada, Montreal, PQ).
Assays for IL-6 and mMCP-1 Production
Cultures of synovial fibroblasts were plated in 24-well Corning plates (Cambridge, MA). Fibroblasts were allowed to recover for 24 hours, and were then refed with medium containing 2% serum and stimulated, in triplicate, with the indicated cytokines. Supernatants were collected after 24 hours and stored at −20°C until analysis. Statistical analysis was completed with one-way analysis of variance using pairwise multiple comparison procedures (Fisher least significant difference method) provided by Sigmastat software.
IL-6 content of supernatants was assayed using a modified B9 hybridoma proliferation assay. 38 The IL-6 dependent B9 cells were plated into 96-well microtiter plates at 2.5 × 10 3 cells/well in 25 μl of RPMI containing 5% fetal calf serum, 1% penicillin/streptomycin, and 2-mercaptoethanol. Seventy-five μl of various dilutions of test sample supernatant was added. Cells were incubated for 3 days at 37°C, 5% CO2, and 100% humidity. B9 cellular proliferation was measured using the 3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay as described by Mossman et al. 39 Briefly, 10 μl of 5 mg/ml MTT (Sigma, St Louis, MO) was added to each of the wells and the cells were incubated for 4 hours at 37°C. Fifty μl of 10% Triton X-100 and 0.5 mol/L of HCl were added to each well to lyse the cells. Plates were incubated overnight in the dark and then absorbances read at 540 nm using a Labsystems Multiskan MCC/340 ELISA plate reader (Labsystems, Helsinki, Finland). IL-6 activity was compared to a standard preparation of human recombinant IL-6 with the limit of detection being 10 pg/ml. Anti-mouse IL-6 (R&D Systems) completely inhibited IL-6 activity of mouse synovial fibroblast (MSF) supernatants whereas a control antibody had no effect. Cell supernatants were analyzed for mMCP-1 content using a commercial ELISA kit (Biosource Cytoscreen, Camarillo, CA) according to the manufacturer’s instructions. The limit of detection was 9 pg/ml.
Proliferation in Anchorage-Independent Conditions
Growth in anchorage-independent conditions was assessed by suspending single cell suspensions of synovial fibroblasts in 0.4% agarose in Dulbecco’s modified minimal essential medium containing 20% fetal bovine serum at a concentration of 1 × 10 3 cells/ml in 24-well plates. The usage of mouse serum in place of fetal bovine serum did not alter results. Cytokines were used at a concentration of 10 ng/ml, and all treatments were performed in triplicate or quadruplicate. Plates were incubated at 37°C, 100% humidity for 14 to 17 days before being optically graded for colony growth and size. Our studies show that the diameter of our cell/colony is correlated with cell number, in that a colony of >20 μm has a cell number of ∼10 or greater. Recently, published work by Imamura et al 40 defines a human synoviocyte colony as five cells or greater.
RNA Isolation and Analysis
Subconfluent fibroblast cultures were stimulated with the indicated cytokines in medium containing 2% serum and incubated for 18 to 24 hours. RNA was isolated from cultures by the method of Chomczynski and Sacchi. 41 Cells were lysed in guanidium isothiocyanate. RNA was extracted with a 1:1 ratio of phenol-chloroform and then precipitated overnight in isopropanol. RNA was washed in 70% ethanol then dried under vacuum. Northern analysis was performed by running 5 to 15 μg of total RNA on a 1.2% agarose formaldehyde gel. After transfer to nylon membrane (ICN, Biotrans, NY), blots were probed with 32P-labeled antisense oligonucleotides corresponding to bp 164 to 191 of the mouse TIMP-1 cDNA sequence (5′-CTTATAACGCTGGTATAAGGTGGTCTCG-3) (Mobix, McMaster University) and bp 213 to 238 of the mouse MCP-1 sequence 42 (5′-TGATCCCAATGAGTAGGCTGGAGAGC-3′) (Mobix, McMaster University). Regulation of mTIMP-3, mIL-6 and β-actin mRNA was examined using 32P-labeled cDNA fragments to mTIMP-3, rat IL-6, and human β-actin, respectively. RNA levels for NIH 3T3 fibroblasts, mouse synovial fibroblasts, and lung tissue from C57BL/6 mice injected with recombinant muOSM were analyzed by densitometry and normalized to β-actin.
β-Galactosidase (lac Z) Expression
Mouse synovial fibroblasts were infected with recombinant adenovirus expressing muOSM (AdmuOSM) and AdmCMVlacZ at multiplicity of infection of 10 and 50. After 24 hours, the cell supernatant was removed and the cells were fixed in 0.5% glutaraldehyde for 10 minutes at room temperature. The fixative was aspirated, and then staining solution containing 50 mmol/L phosphate buffer, pH 7.4, 5 mmol/L K4Fe(CN)6, 5 mmol/L K3Fe3(CN)6, 2 mmol/L MgCl2, 0.05% Triton X-100 (BioRad, Hercules, CA) and 0.5 mg/ml of 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-gal) (Boehringer Mannheim, Mannheim, Germany) diluted from a 20-mg/ml stock solution of X-gal dissolved in dimethylformamide, was added. Color was allowed to develop for 45 minutes. The stained fibroblasts were visualized under a microscope and photographs were taken using a Zeiss inverted microscope with attached camera (Zeiss, Jena, Germany).
Animals and Delivery of Cytokines and Adenovirus Vectors
C3H/Hej, MRL/++, MRL/lpr, and C57BL/6 mouse strains (Charles River Laboratories, Ottawa, Canada) were housed until 14 to 16 weeks old. Animal studies were conducted in compliance with the Canadian Council on Animal Care and approved by the Animal Research Ethics Board at McMaster University, Canada. Mice were injected intra-articularly with adenovirus vectors (1 × 10 6 to 5 × 10 7 pfu/joint) or phosphate-buffered saline (PBS). Mice were maintained under isofluorane anesthesia, knees were swabbed with 70% ethanol, and a 5-μl volume (treatment) was injected into the synovial space using a 30-gauge needle. The contralateral knee was left uninjected or treated with PBS. Animals were sacrificed at various times after administration. Joints were dissected away from the limbs, fixed in buffered formalin, decalcified, then trimmed and processed for histology by routine paraffin embedding, sectioning, and staining using hematoxylin and eosin (H&E) and Elastic van Geison (EVG) techniques.
Histopathology Score
Joint tissues were graded based on a pathological scoring system used by Gong et al 43 with minor additions. Tissues were given scores ranging from 0 to 2 in the following categories: synovial inflammation, synovial hyperplasia, pannus formation and cartilage erosion, bone destruction, and synovitis with or without cellular effusion into the joint space. Cumulative pathological scores were obtained by totaling the scores obtained in each category. Statistical analysis was again completed with one-way analysis of variance using pairwise multiple comparison procedures (Fisher LSD Method) provided by Sigmastat software.
Results
muOSM Regulation of IL-6 and MCP-1 Expression
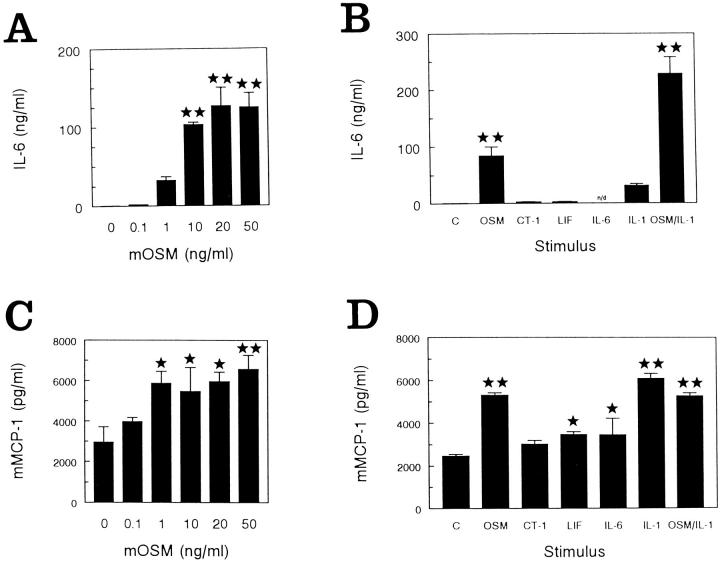
Initial experiments sought to establish the effects of mouse OSM on mouse synovial fibroblasts. To assess the regulation of IL-6 and MCP-1 production by muOSM, MSF were stimulated with muOSM, other gp130 cytokines, or mIL-1β for 24 hours. IL-6 production was studied using the B9 hybridoma proliferation assay whereas the production of MCP-1 protein was examined by ELISA. In studies of MSF in cell culture and anchorage-independent growth, two separate cell lines (each from pooled knee synovium of 10 mice) were analyzed. Both cell lines responded with identical trends in each of three separate experiments per cell line. Data shown represents one of three experiments. Stimulation with muOSM resulted in a dose-dependent increase in IL-6 protein detected in supernatants. Marked increases were noted at 1 ng/ml (50 pmol/L) of muOSM and maximal levels were reached at 20 ng/ml (1 nmol/L) of stimulus, resulting in >300-fold increases over control (Figure 1A) ▶ . Mouse CT-1 (1 nmol/L) or LIF (1 nmol/L) were ineffective when tested at 20 ng/ml (Figure 1B) ▶ . mIL-1β induced 100-fold increases in IL-6 production whereas co-stimulation with muOSM resulted in a synergistic increase in IL-6 of >800 times that of control levels. OSM had a modest influence on the amount of mMCP-1 protein detected in the same supernatants of mouse synovial fibroblasts, which showed very high constitutive levels of mMCP-1 protein (∼3 ng/ml) (Figure 1C) ▶ and express mMCP-1 mRNA constitutively (Figure 2) ▶ . Concentrations of muOSM (10 to 50 ng/ml) that were able to stimulate >300-fold increases in IL-6, induced increases in MCP-1 protein of approximately twofold (statistically significant), whereas mCT-1, mLIF, or mIL-6 had less effect (Figure 1D) ▶ . mIL-1β also enhanced the levels of mMCP-1 protein by twofold.
Figure 1.
muOSM stimulates IL-6 and MCP-1 protein production by primary mouse synovial fibroblasts. Primary mouse synovial fibroblasts were cultured in 24-well dishes and stimulated with increasing concentrations of muOSM (0 to 50 ng/ml) (A and B). Supernatants were collected after 24 hours, stored at −20°C and then analyzed for IL-6 by the B9 hybridoma proliferation assay (A) and for mMCP-1 by ELISA (B). In addition, activity was compared to other IL-6/LIF cytokines and IL-1 in separate cell cultures (C and D), stimulated as indicated with muOSM (OSM), mIL-6 (IL-6), mLIF (LIF), or mCT-1 (CT-1) at 20 ng/ml and/or mIL-1β at 5 ng/ml. Supernatants were collected and analyzed as above. Data shows the mean and SD of triplicate treatments in one of two separate experiments that gave similar results. One star indicates significant difference from control (P < 0.05), whereas two stars indicate significance at P < 0.01, assessed by analysis of variance as in the Methods section. n.d., (not determined). IL-6 levels were not determined in supernatants from cells stimulated with IL-6.
Figure 2.
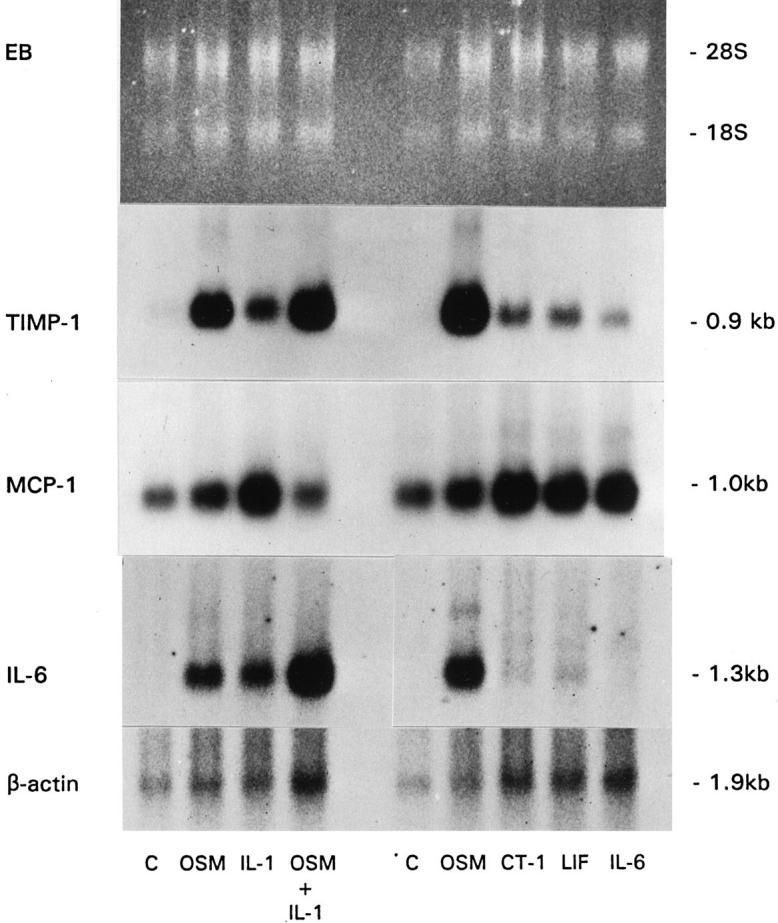
Regulation of mRNA in primary mouse synovial fibroblast cells. Primary mouse synovial fibroblast cells were stimulated for 22 hours with the indicated cytokines in two separate experiments (left and right panels). Total RNA was extracted and Northern blots were probed with mTIMP-1 and mMCP-1/JE oligonucleotides as well as a cDNA probe to rat IL-6. β-actin mRNA levels served as a control for RNA loading. Cytokine concentrations were 20 ng/ml muOSM (OSM), mCT-1 (CT-1), mLIF (LIF), and mIL-6 (IL-6) and 5 ng/ml mIL-1β (IL-1).
To assess levels of IL-6, MCP-1, and TIMP-1 mRNA expression, Northern analysis (Figure 2) ▶ was completed after MSF stimulation for 18 hours with muOSM, mIL-1β, and the combination of muOSM and mIL-1β (left panel), or with other IL-6/LIF cytokines (right panel). muOSM was found to be a potent inducer of IL-6 mRNA in these cells. Densitometry analysis from both experiments determined this to be ninefold and 20-fold increases in IL-6 transcripts, respectively (Figure 2) ▶ . None of the other IL-6/LIF cytokines tested induced increases exceeding twofold. The combination of muOSM and mIL-1β had an additive effect on IL-6 expression (9.1- and 8.3-fold increases induced by muOSM and mIL-1β, respectively, with the combination of muOSM and mIL-1β resulting in a 13.8-fold increase). Similar trends were seen at the protein level (Figure 1) ▶ .
Neither muOSM nor any of the other gp130 cytokines were able to alter the 18-hour level of mMCP-1 mRNA after normalization to β-actin. Basal expression of this chemokine mRNA was very high, consistent with protein levels (Figure 1, C and D) ▶ . Murine IL-1β enhanced MCP-1 expression, although only a twofold increase in MCP-1 mRNA levels was observed by densitometry. The combination of muOSM and mIL-1β resulted in a significant decrease in the amount of mMCP-1 mRNA after normalization to β-actin, which was also noted at the protein level (Figure 1C) ▶ . Stimulation with muOSM resulted in marked increases in mTIMP-1 mRNA (estimated as 42-fold when analyzed by densitometry and normalized to β-actin), whereas mCT-1 and mLIF induced modest increases in TIMP-1 message (approximately fourfold) and mIL-6 gave only a slight increase of twofold (Figure 2 ▶ , right). mIL-1β resulted in a sevenfold increase in TIMP-1 message and the combination of mIL-1β and muOSM was similar to OSM alone in ability to regulate TIMP-1 mRNA.
Proliferation in Anchorage-Independent Conditions
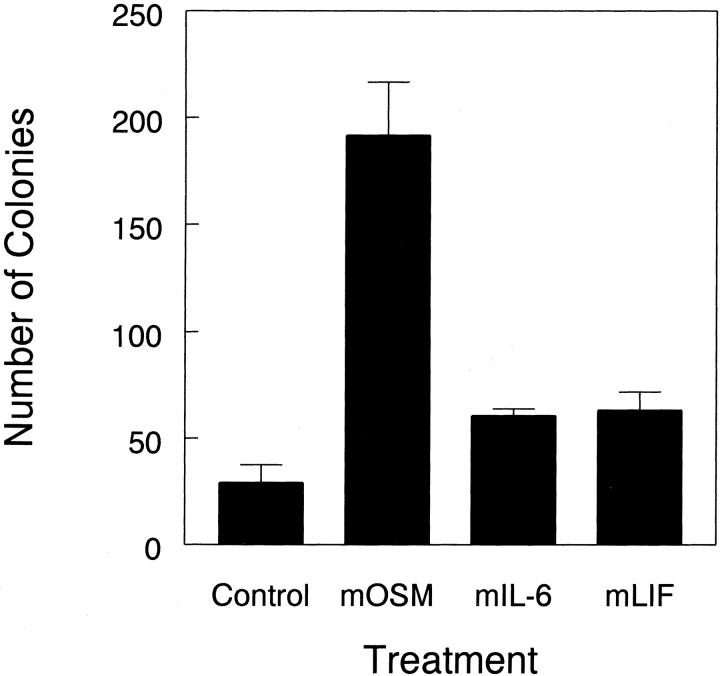
Proliferation and colony formation by synovial fibroblasts in anchorage-independent conditions has been considered an indicator of the ability of synoviocytes to contribute to the aggressive and destructive pannus tissue seen in rheumatoid arthritis patients. We examined the response of mouse and rat synovial fibroblasts to muOSM, mIL-6, and mLIF in soft agarose cultures for the ability to induce the formation of cell colonies. Colonies smaller than 20 μm represent single cells or clusters of only a few cells, whereas colonies of 20 μm or larger represent true, thriving colonies. Although mIL-6 and mLIF induced minimal increases in colonies 20 μm in diameter or larger, muOSM induced sixfold increases in the formation of colonies by MSF (Figure 3) ▶ . In standard anchorage-dependent proliferation studies (not shown), we did not observe significant regulation of proliferation by OSM as measured by MTT incorporation. Therefore anchorage-independent growth is unlikely to simply reflect standard proliferation. Cultures of rat synovial fibroblasts, derived in a similar manner to MSF cultures, also showed induction of IL-6 production and an increase (11-fold) in colony growth assays in response to muOSM (not shown). Thus, muOSM is able to induce the anchorage-independent growth of synovial fibroblasts in vitro, under conditions that are suggestive of the behavior of synovial fibroblasts in the inflamed joint in vivo.
Figure 3.
OSM stimulates anchorage-independent growth. Single cell suspensions of mouse synovial fibroblasts were suspended in 0.4% agarose in Dulbecco’s modified minimal essential medium containing 20% fetal bovine serum and the indicated cytokines at a concentration of 10 ng/ml. All treatments were completed in triplicate. After 14 to 17 days, the resultant cell colonies were optically graded for size as being 0 to 20 μm in diameter (single cells or small groups, white bars), of >20 μm (true colonies, black bars). Data shown is representative of two separate experiments.
Adenovirus Vectors in Vitro and in Vivo
The responses of mouse synovial cells to OSM in vitro suggest they could be a major target of OSM in vivo. To assess the potential of OSM to regulate synovial tissue responses in vivo, we have used adenovirus vectors administered locally. The use of Ad vectors in expressing genes in synovium of rabbits and mice has been reported. 44,45 We have constructed and characterized an adenovirus vector that expresses mouse OSM in vitro and in vivo, on administration to mice. 46 Intramuscular administration resulted in induction of IL-6 and acute-phase protein levels in serum, and local administration resulted in induction of TIMP-1 in lungs on intratracheal treatment. 46 To ensure that mouse synovial cells and tissue can be infected and express adenovirus vector inserts, we first assessed in vitro infection of MSF and in vivo administration of an Ad vector expressing lacZ. Mouse synovial fibroblasts infected in vitro with AdmCMVlacZ (AdlacZ) at a multiplicity of infection of 10 and 50 showed significant expression of the virus as noted by the colorimetric conversion of X-gal to a blue product (Figure 4A) ▶ demonstrating that this primary synovial fibroblast cell line is infectable with adenovirus constructs. Both AdmuOSM-infected and uninfected control cells were negative for X-gal staining. Intra-articular administration of AdlacZ to mice resulted in detection of lacZ activity primarily in the lining cells of the synovium at day 1 after administration (Figure 4B) ▶ and was detectable in synovium of animals for up to 21 days after treatment (not shown).
Figure 4.
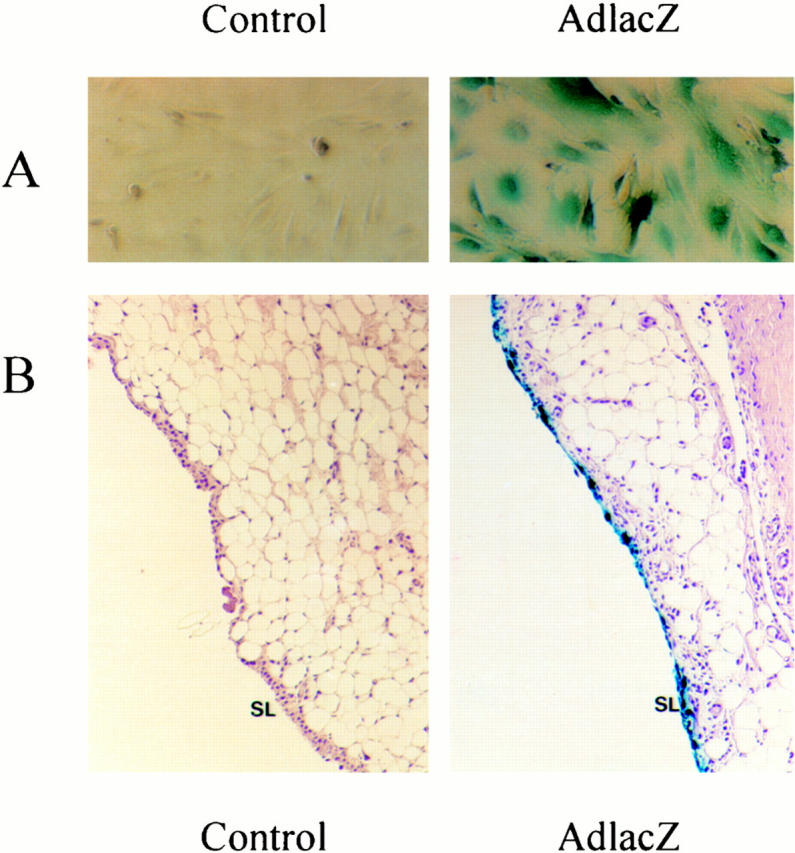
AdlacZ infection of synovial cells in vitro and in vivo. A: Primary mouse synovial fibroblasts were untreated or infected with AdmCMVlacZ or AdmuOSM at multiplicity of infection of 10 and 5. Fibroblasts were stained for lacZ expression with X-gal. The blue staining demonstrates expression of lacZ. B: Mice were treated with intra-articular AdlacZ (5 × 10 7 pfu) and sacrificed 24 hours later. Joints were removed, fixed, decalcified, and stained for lacZ expression. Blue staining was consistently observed in synovial lining cells.
The effects of the Ad vectors expressing muOSM, lacZ, and mIL-6 on the histology of joint tissue were then examined for up to 21 days after administration. Results shown in Figure 5 ▶ display typical tissue histological analysis representative of at least five joints per treatment whereas the contralateral knees of C3H/Hej mice were untreated. Particle calculation shows that doses of AdmOSM particles administered (as opposed to pfu administered) were similar or less than particle administrations into knees treated with Add170 or AdmIL-6. Therefore the effects of AdmOSM are not because of particle number alone, but because of the specific properties of the mOSM-expressing recombinant virus. Sagittal sections of the contralateral knees (Figure 5A) ▶ of any of the treatments showed no differences from untreated animals. Joints treated with AdlacZ, or with empty vector Addl70 (Figure 5B) ▶ showed little or no evidence of inflammatory changes at day 7, which also reflects earlier time points (not shown) using these doses of Ad vector. Joints from animals treated with AdmuOSM, however, showed a progressive inflammatory response that began to be evident by day 4 (Figure 5C) ▶ . This included increased numbers of infiltrating cells that were mainly mononuclear with some PMNs, and increased numbers of cells with synovial fibroblast-like morphology that contributed to increased thickness of the synovial lining. At day 7 after AdmuOSM treatment (Figure 5, D and E) ▶ the amount of infiltration and hyperplasia of the synovium was enhanced with an apparent decrease in subsynovial adipocytes. Increased matrix deposition was evident throughout the synovial tissue, and hyperplastic synovial lining was found to adhere in places to the cartilage surface, particularly at the margins. Figure 5D ▶ shows marked degradation of the outer surface of the meniscal cartilage (arrowhead) again adjacent to the hyperplastic synovial tissue. Furthermore, some sections showed that the hyperplastic synovium invaded cartilage and protruded through to bone marrow (Figure 5G) ▶ . EVG staining showed a re-organization of collagen deposition toward a more diffuse distribution of collagen fibrils (Figure 5H) ▶ . Emperical observation during dissection consistently showed swelling evident in the AdmuOSM-treated joints but not in other treatments or contralateral knees. Joints examined at day 14 after treatment showed a further increase in fibroblast-like cells and ongoing infiltration, resulting in a substantial synovial lesion.
Figure 5.
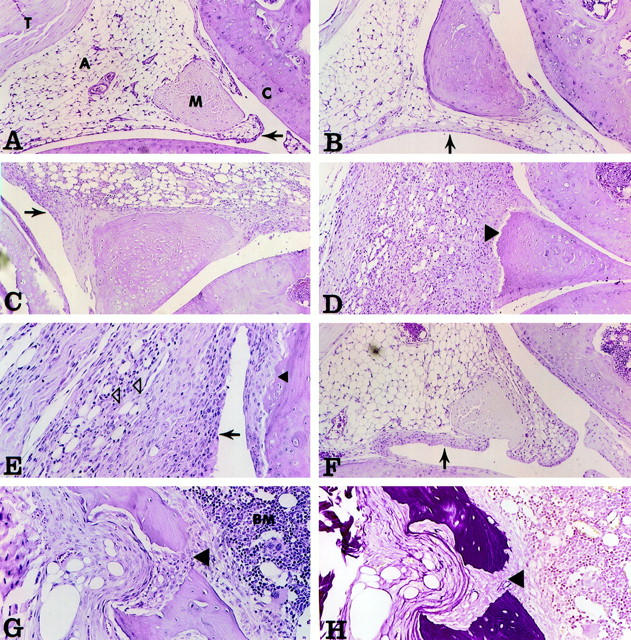
Histopathology of mouse knee joints. Adenovirus vectors were injected into the knee joints of mice, and the animals were sacrificed at various times following administration. Knee joints were fixed, decalcified, paraffin-embedded, and stained using H&E and EVG techniques. Tissues were assessed using pathological criteria as shown in Table 1 ▶ . Original magnification: ×100 (A–D and F); ×200 (E, G, and H). A: Contralateral joint of AdmuOSM, day 7. A, Adipocytes; M, meniscus; C, articular cartilage; T, tendon. Note the normal appearance and thickness of the synovial lining (arrow). B: Addl70, day 7. The synovial lining shows little if any thickening (arrow). C: AdmuOSM, day 4. Note the substantial increase in the thickness of the synovial lining (arrow). D: AdmuOSM, day 7. The synovial lining is extensively thickened and the entire synovium is infiltrated with mononuclear and some polymorphonuclear cells (PMN), with a concomitant decrease in adipocyte presence. Note the eroded miniscal outer surface (arrow). E: AdmuOSM, day 7. Higher magnification shows infiltration of the synovium, the PMN being clearly visible (open arrowheads). Note also the erosion of the articular cartilage adjacent to synovial tissue (closed arrowhead). F: AdmIL-6, day 7. The synovial lining shows a minor increase in thickness (arrow). G and H: AdmuOSM, day 7, showing matching fields stained with H&E and EVG, respectively. The synovial tissue has eroded the articular cartilage and underlying bone, and has penetrated the bone marrow (BM) (large arrowhead).
The lesions were still evident at day 21, at which time synovial hyperplasia appeared to be no less abundant, but the degree of infiltration was less extensive than days 7 and 14. The results were similar on administration of AdmuOSM to other strains of mice including C57BL/6 and MRL/++. Joints of MRL/lpr mice treated with AdmuOSM showed a more rapid response to AdmuOSM, such that synovial hyperplasia and tissue remodeling was extensive at day 7 and day 14. Thus, dramatic connective tissue changes were evident in AdOSM treated joints. In our hands the pannus-like tissue in treated mice does not yield sufficient quantities for RNA Northern analysis. We have been able to detect transgene mRNA by Northern blots in lungs of animals treated intratracheally. 46. Development of reagents to measure muOSM protein specifically will enable future studies that determine the expression level of transgene as well as endogenous OSM in mouse joint tissues.
Joints of C3H/HeJ mice treated with AdmIL-6 showed a mild increase in hyperplasia at day 7 (Figure 5F) ▶ which was not sustained. This construct was previously shown to induce various effects in lungs of rats treated intratracheally. 47 However, 5 × 10 7 pfu of AdmIL-6, a dose 10-fold greater than was shown to be effective for AdmuOSM (5 × 10 6 pfu), did not result in inflammatory responses in C3H/HeJ mice.
To quantify the response of mouse joints to the adenoviral vectors, a rating of inflammatory indices of day 7-treated joints in shown in Table 1 ▶ . For each treatment, a minimum of three and a maximum of nine joints were assessed. The total pathological score is shown in Table 1 ▶ . Assessment of contralateral knee joints in AdmOSM treated animals by histopathology indices showed no difference from untreated joints (not shown), indicating intra-articular administration did not result in general effects at other joints. For every measurement of joint pathology, AdmuOSM consistently demonstrated effects not seen by administration of either the control Addl70 vector, or AdmIL-6.
Table 1.
Inflammatory Indices of Day 7-Treated Joints
| Score (±SD) | ||||
|---|---|---|---|---|
| Add170 | AdOSM | AdIL-6 | ||
| Pathological index | Synovial inflammation | 0.0 (0.0) | 1.7 (0.3)* | 0.3 (0.6) |
| Synovial hyperplasia | 0.2 (0.4) | 2.0 (0.0)* | 0.3 (0.6) | |
| Pannus formation cartilage erosion | 0.0 (0.0) | 1.6 (0.5)* | 0.3 (0.6) | |
| Bone destruction | 0.0 (0.0) | 1.3 (0.5)* | 0.3 (0.6) | |
| Synovitis | 0.0 (0.0) | 0.48 (0.5) | 0.0 (0.0) | |
| Pathological score | Total | 0.2 (0.4) | 7.0 (1.8)* | 1.2 (2.3) |
Discussion
OSM as well as other IL-6/LIF cytokines are found in elevated levels in synovial fluid of rheumatoid arthritic patients. 7-9 However, the precise role of OSM in human rheumatoid arthritis is not clear. Its ability to regulate responses in many connective-tissue cell types including synovial fibroblasts and chondrocytes suggests an important role in tissue remodeling processes. 7,9,14,16,18,48 The establishment of the actions of OSM in local inflammation in animal models will help to discern the role of OSM in human disease. Cloning of the mouse OSM homologue has been followed by studies that show similar, but not identical, effects in the mouse and human systems. Novel observations are described in the present study, suggesting that mouse OSM stimulates a chronic inflammatory response in mouse joints.
Mouse OSM regulates several responses in (MSF) in vitro. MSF produced high levels of IL-6 (30- to 300- fold) in response to muOSM (Figure 1) ▶ and this was consistent in several lines of MSF cells as well as fibroblast cultures derived from mouse lung or skin (not shown). In contrast, mLIF, mCT-1, or human OSM were not able to induce IL-6 expression. Marked induction of TIMP-1 mRNA by muOSM was evident at day 1 (Figure 2) ▶ and at day 3 (not shown) after treatment in vitro. This is consistent with other studies on mouse fibroblast NIH 3T3 cells 36 and in human systems using the human OSM protein. 15,17 Similar to expression of IL-6, TIMP-1 did not respond to LIF, CT-1, or IL-6 stimulation in MSF. This could be because of low receptor number on fibroblasts for these cytokines, however mLIF and mCT-1 can induce STAT-1/3 activation in MSF indicating receptor presence on these cells (Carl D. Richards, unpublished observations). Alternatively, additional signaling pathways induced by the specific OSMR complex may participate in the regulation of IL-6. The stimulation of IL-6 by OSM may contribute to the levels of IL-6 that are elevated in rheumatoid arthritis synovial fluid and tissue as established in the literature. 49,50
MCP-1 was also induced in vitro by muOSM, although to a much lesser extent (twofold induction) than IL-6. Basal mRNA levels and protein content in cell culture supernatants were high (3 ng/ml) possibly making it more difficult to detect induction. Analysis in human synovial fibroblasts showed lower basal levels of MCP-1 production and more marked MCP-1 responses to human OSM. 7 This may reflect species differences in levels of MCP-1 response, or possibly may reflect differences in the regulation of separate MCP factors. Mouse MCP-5 has recently been characterized and shows greater identity to human MCP-1 (66% amino acid identity) than mouse MCP-1 does (55% homologous). Whether OSM does indeed regulate other MCPs has yet to be determined. The stimulation of MCP-1 will contribute to the pool of chemotactic factors that control the infiltration of inflammatory cells. MCP-1 is primarily chemotactic for monocytes/macrophages and T cells and because MCP-1 is found in elevated levels in rheumatoid arthritis patient synovial fluid and tissue, the infiltration of such mononuclear cells in rheumatoid arthritis may thus be mediated in part by the OSM-mediated regulation of MCP-1.
The ability of synovial fibroblast like cells to grow in soft agarose has been described previously, and this has been noted as a reflection of the aggressive nature of the fibroblasts from synovium or pannus of rheumatoid arthritis patients. 51-53 This can be regulated in culture by a number of growth factors, including platelet-derived growth factor, transforming growth factor-β, and fibroblast growth factor. 52,54 Our data also shows that OSM can stimulate the formation of colonies in soft agarose, and therefore act as a stimulus of such phenotypic change. Collectively, this data suggests that muOSM is a potent stimulus of synovial cell responses in vitro, supporting a potentially important role in inflamed joints in vivo, unique from other IL-6/LIF cytokines.
Analysis of mouse joints treated with the AdmuOSM vector showed extensive synovial phenotypic change including hyperplasia, mononuclear cell infiltration, extracellular matrix remodeling, and cartilage damage. These effects are consistent with the activities of muOSM on MSF in vitro. Anchorage-independent growth stimulation may reflect the hyperplasia and formation of aggressive cells that overlay cartilage and penetrate the tissue, as has been suggested by others. 53,55-57 The presence of mononuclear cells may reflect in part the regulation of MCP-1 or other chemotactic factors by OSM. The development of inflammation is evident by day 4, but hyperplasia and extracellular matrix remodeling is most evident at day 7 and thereafter in this model. The expression of cytokines by this and other Ad vectors are typically for 3 to 7 days in other tissues, but it is not known at this time whether muOSM is expressed for a similar period in this model. Certainly the effects of a single administration are evident for 14 to 21 days, suggesting sustained phenotypic change has been induced characteristic of chronic inflammation. Neither the control vector (Add170) nor the AdmIL-6 vector produced such change, even though 10-fold greater doses of each have been tested. The lack of effects of AdmIL-6 (previously published to express high levels of IL-6 and induce lung and liver responses 46 ) would suggest that the effects of AdmuOSM are not mediated through IL-6, which is also consistent with the differences in responses of MSF to these cytokines. Whether the sustained effects of AdmuOSM depend on the recruitment of certain cell populations, and activation of certain cytokines, is the subject of further examination.
Recent data has shown that muOSM binds to gp130 and signaling requires the specific OSM receptor subunit. 34,35 In contrast to human OSM, muOSM interacts very weakly with the mouse LIF receptor complex and does not mediate responses in cells that express the LIF receptor only. 25,30,36 Furthermore, in vitro binding assay data clearly shows that human OSM does not act on the muOSM receptor complex, suggesting species specificity of this interaction. This suggests that recently published work examining the effect of human OSM administered to mice 58 reflects interaction of human OSM with receptors other the specific muOSM receptor complex. This is likely, but possibly not exclusively, the mouse LIF receptor. Since the work of Wallace et al 58 showed an inhibitory action by systemic administration of human OSM in models of chronic inflammation, this implies that such LIF receptor activation may result in anti-inflammatory effects in mice. We believe that OSM receptor activation induces chronic inflammation on local administration, and therefore suggest that elevated levels of OSM found in human rheumatoid arthritis tissue contribute to the pathology of the disease through the specific OSM receptor.
Acknowledgments
We thank Donna Gjomerac and Jane-Anne Schroeder for expert technical assistance, and Sara DeSilvio for secretarial assistance.
Footnotes
Address reprint requests to Dr. Carl D. Richards, Associate Professor, Department of Pathology and Molecular Medicine, McMaster University, HSC-4H17, 1200 Main St. West, Hamilton, ON Canada L8N 3Z5. E-mail: richards@fhs.mcmaster.ca.
Supported in part by The Arthritis Society (Canada), Medical Research Council of Canada, Hamilton Health Sciences Corporation and St. Joseph’s Hospital, Hamilton, Ontario, Canada.
C. D. R. is senior research scholar of the Arthritis Society.
References
- 1.Feldmann M, Brennan FM, Maini RN: Rheumatoid arthritis. Cell 1996, 85:307-310 [DOI] [PubMed] [Google Scholar]
- 2.Miossec P, van den Berg W: Th1/Th2 cytokine balance in arthritis. Arthritis Rheum 1997, 40:2105-2115 [DOI] [PubMed] [Google Scholar]
- 3.Arend WP, Dayer J: Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum 1995, 38:151-160 [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA: Biological basis for interleukin-1 in disease. Blood 1996, 87:2095-2147 [PubMed] [Google Scholar]
- 5.Bruce G, Linsley P, Rose T: Oncostatin M. Prog Growth Factor Res 1992, 4:157-170 [DOI] [PubMed] [Google Scholar]
- 6.Shoyab M, Malik N, Wallace PM: Oncostatin M. Cytokines, ch. 1998, :pp 401-414 R Thorpe. New York, Academic Press, 27. Edited by A Mire-Sluis [Google Scholar]
- 7.Langdon CM, Leith J, Smith F, Richards CD: Oncostatin M stimulates monocyte chemoattractant protein-1- and interleukin-1-induced matrix metalloproteinaise-1 production by human synovial fibroblasts in vitro. Arthritis Rheum 1997, 40:2139-2146 [DOI] [PubMed] [Google Scholar]
- 8.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z: The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor and oncostatin M in rheumatoid arthritis. Arthritis Rheum 1997, 40:1096-1105 [DOI] [PubMed] [Google Scholar]
- 9.Cawston TE, Curry VA, Summers CA, Clark IM, Riley GP, Life PF, Spaull JR, Goldring MB, Koshy PJT, Rowan AD, Shingleton WD: The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum 1998, 41:1760-1771 [DOI] [PubMed] [Google Scholar]
- 10.Richards CD, Brown TJ, Shoyab M, Baumann H, Gauldie J: Recombinant oncostatin-M stimulates the production of acute phase proteins in hepG2 cells and rat primary hepatocytes in vitro. J Immunol 1992, 148:1731-1736 [PubMed] [Google Scholar]
- 11.Grove RI, Eberhardt C, Abid S, Mazzucco C, Liu J, Kiener P, Todaro G, Shoyab M: Oncostatin M is a mitogen for rabbit vascular smooth muscle cells. Proc Natl Acad Sci USA 1993, 90:823-827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles SA, Martinez-Maza O, Rezai A, Magpantay L, Kishimoto T, Nakamura S, Radka SF, Linsley PS: Oncostatin M as a potent mitogen for AIDS-Kaposi’s sarcoma-derived cells. Science 1992, 255:1432-1434 [DOI] [PubMed] [Google Scholar]
- 13.Nair BC, DeVico AL, Nakamura S, Copeland TD, Chen Y, Patel A, O’Neil T, Oroszlan S, Gallo RC, Sarngadharan MG: Identification of a major growth factor for AIDS-Kaposi’s sarcoma cells as oncostatin M. Science 1992, 255:1430-1432 [DOI] [PubMed] [Google Scholar]
- 14.Richards C, Langdon C, Botelho F, Brown TJ, Agro A: Oncostatin M inhibits IL-1-induced expression of IL-8 and granulocyte-macrophage colony-stimulating factor by synovial and lung fibroblasts. J Immunol 1996, 156:343-349 [PubMed] [Google Scholar]
- 15.Richards CD, Agro A: Interaction between oncostatin M, interleukin 1 and prostaglandin E2 in induction of IL-6 expression in human fibroblasts. Cytokine 1994, 6:40-47 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton JA, Leizer T, Piccoli DS, Royston KM, Butler DM, Croatto M: Oncostatin M stimulates urokinase-type plasminogen activator activity in human synovial fibroblasts. Biochem Biophys Res Comm 1991, 180:652-659 [DOI] [PubMed] [Google Scholar]
- 17.Richards CD, Shoyab M, Brown TJ, Gauldie J: Selective regulation of metalloproteinases inhibitor (TIMP-1) by oncostatin M in fibroblasts in culture. J Immunol 1993, 150:5596-5603 [PubMed] [Google Scholar]
- 18.Cawston TE, Ellis AJ, Humm G, Lean E, Ward D, Curry V: Interleukin-1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Comm 1995, 215:377-385 [DOI] [PubMed] [Google Scholar]
- 19.Hui W, Bell M, Carroll G: Oncostatin M (OSM) stimulates resorption and inhibits synthesis of proteoglycan in porcine articular cartilage explants. Cytokine 1996, 8:495-500 [DOI] [PubMed] [Google Scholar]
- 20.Levy JB, Schindler C, Raz R, Levy DE, Baron R, Horowitz MC: Activation of the JAK-STAT signal transduction pathway by oncostatin-M in cultured human and mouse osteoblastic cells. Endocrinology 1996, 137:1159-1165 [DOI] [PubMed] [Google Scholar]
- 21.Brown TJ, Rowe JM, Liu J, Shoyab M: Regulation of interleukin-6 expression by oncostatin M. J Immunol 1991, 147:2175-2180 [PubMed] [Google Scholar]
- 22.Brown T, Liu J, Brashem-Stein C, Shoyab M: Regulation of G-CSF and GM-CSF Expression by oncostatin M. Blood 1993, 82:33-37 [PubMed] [Google Scholar]
- 23.Cichy J, Potempa J, Travis J: Biosynthesis of alpha-1-proteinase inhibitor by human lung-derived epithelial cells. J Biol Chem 1997, 272:8250-8255 [DOI] [PubMed] [Google Scholar]
- 24.Sallenave J-M, Tremblay GM, Gauldie J, Richards C: Oncostatin M, but not interleukin 6 or leukemia inhibitory factor, stimulates expression of alpha1-proteinase inhibitor in A549 human alveolar epithelial cells. J Interferon Cyt Res 1997, 17:337-346 [DOI] [PubMed] [Google Scholar]
- 25.Mosley B, Delmus C, Friend D, Boiani N, Thoma B, Park LS, Cosman D: Dual oncostatin-M (OSM) receptors. J Biol Chem 1996, 271:32635-32643 [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Modrell B, Aruffo A, Scharnowske S, Shoyab M: Interactions between oncostatin M and the IL-6 signal transducer, gp130. Cytokine 1994, 6:272-278 [DOI] [PubMed] [Google Scholar]
- 27.Botelho F, Edwards D, Richards CD: Oncostatin M stimulates c-Fos to bind a transcriptionally responsive AP-1 element within the tissue inhibitor of metalloproteinase-1 promoter. J Biol Chem 1998, 273:5211-5218 [DOI] [PubMed] [Google Scholar]
- 28.Auguste P, Guillet C, Fourcin M, Olivier C, Veziers J: Signaling of type II oncostatin M receptor. J Biol Chem 1997, 272:15760-15764 [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura A, Ichihara M, Kinjyo I, Moriyama M, Copeland NG, Gilbert DJ, Jenkins NA, Hara T, Miyajima A: Mouse oncostatin M: an immediate early gene induced by multiple cytokines through the JAK-STAT5 pathway. EMBO J 1996, 15:1055-1063 [PMC free article] [PubMed] [Google Scholar]
- 30.Ichihara M, Hara T, Kim H, Murate T, Miyajima A: Oncostatin M and leukemia inhibitory factor do not use the same functional receptor in mice. Blood 1997, 90:165-173 [PubMed] [Google Scholar]
- 31.Mukouyama Y, Hara T, Kim M, Kogo H, Miyajima A: In vitro expansion of murine multipotential hematopoietic progenitors from the embryonic aorta-gonad-mesonephros region. Immunity 1998, 8:105-114 [DOI] [PubMed] [Google Scholar]
- 32.Hara T, Tamura K, de Miguel M, Mukouyama Y-S, Kim H-J, Kogo H, Donovan PJ, Miyajima A: Distinct roles of oncostatin M and leukemia inhibitory factor in the development of primordial germ cells and sertoli cells in mice. Dev Biol 1998, 201:144-153 [DOI] [PubMed] [Google Scholar]
- 33.Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoti T, Miyajima A: Fetal liver development requires a paracrine action of oncostatin M through the gp 130 signal transducer. EMBO J 1999, 18(suppl 8):2127-2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka M, Hara T, Copeland NG, Gilbert DJ, Jenkins NA, Miyajima A: Reconstitution of the functional mouse oncostatin M (OSM) receptor: molecular cloning of the mouse OSM receptor β subunit. Blood 1999, 93:804-815 [PubMed] [Google Scholar]
- 35.Lindberg RA, Juan TSC, Welcher AA, Sun Y, Cupples R, Guthrie B, Fletcher FA: Cloning and characterization of a specific receptor for mouse oncostatin M. Mol Cell Biol 1998, 18:3357-3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards CD, Kerr C, Tanaka M, Hara T, Miyajima A, Pennica D, Botelho F, Langdon CM: Regulation of tissue inhibitor of metalloproteinase-1 in fibroblasts and acute phase proteins in hepatocytes in vitro by mouse oncostatin M, cardiotrophin-1, and IL-6. J Immunol 1997, 159:2431-2437 [PubMed] [Google Scholar]
- 37.Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh S, Darbonne WC, Knutzon DS, Yen R, Chien KR, Baker JB, Wood WI: Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA 1995, 92:1142-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordan RP, Richards CD, Gauldie J: Cytokines and their cellular receptors: basic protocol, measurement of interleukin 6. Current Protocols in Immunology, suppl. 1996, :pp 66.1-66.5 John Wiley & Sons, 17. Edited by JE Coligan. New York [DOI] [PubMed] [Google Scholar]
- 39.Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983, 65:55-63 [DOI] [PubMed] [Google Scholar]
- 40.Imamura F, Aono H, Hasunuma T, Sumida T, Tateishi H, Maruo S, Nishioka K: Monoclonal expansion of synoviocytes in rheumatoid arthritis. Arthritis Rheum 1998, 41:1979-1986 [DOI] [PubMed] [Google Scholar]
- 41.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analyt Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 42.Rollins BJ, Sunday ME: Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol 1991, 11:3125-3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong J, Ratkay LG, Waterfield JD, Clark-Lewis I: An antagonist of monocyte-chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med 1997, 186:131-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nita I, Ghivizzani SC, Galea-Lauri J, Bandara G, Georgescu HI, Robbins PD, Evans CH: Direct gene delivery to synovium an evaluation of potential vectors in vitro and in vivo. Arthritis Rheum 1996, 39:820-828 [DOI] [PubMed] [Google Scholar]
- 45.Sawchuk SJ, Boivin GP, Duwel LE, Ball W, Bove K, Trapnell B, Hirsch R: Anti-T cell receptor monoclonal antibody prolongs transgene expression following adenovirus-mediated in vivo gene transfer to mouse synovium. Hum Gene Ther 1996, 7:499-506 [DOI] [PubMed] [Google Scholar]
- 46.Kerr C, Langdon CM, Graham F, Gauldie J, Hara T, Richards CD: Adenovirus vector expressing mouse oncostatin M induces acute phase proteins and TIMP-1 expression in vivo in mice. J Interferon Cyt Res 1999, 19:1195-1205 [DOI] [PubMed] [Google Scholar]
- 47.Xing Z, Braciak T, Jordana M, Croitoru K, Graham FL, Gauldie J: Adenovirus-mediated cytokine gene transfer at tissue sites: overexpression of IL-6 induces lymphocytic hyperplasia in the lung. J Immunol 1994, 153:4059-4069 [PubMed] [Google Scholar]
- 48.Hui W, Bell MC, Carroll GJ, Layton MJ: Modulation of cartilage proteoglycan metabolism by LIF binding protein. Cytokine 1998, 10:220-226 [DOI] [PubMed] [Google Scholar]
- 49.Honssiau F, Bukasa K, Sindic C, Van Damme J, Van Snick J: Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum 1988, 31:784-788 [DOI] [PubMed] [Google Scholar]
- 50.Hirano T, Matsuda T, Turner M, Miyasaka N, Buchan G, Tang B, Sato K, Maini R, Feldmann M, Kishimoto T: Excessive production of interleukin-6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol 1988, 18:1797-1801 [DOI] [PubMed] [Google Scholar]
- 51.Yocum DE, Lafyatis R, Remmers EF, Schumacher HR, Wilder RL: Hyperplastic synoviocytes from rats with streptococcal cell wall-induced arthritis exhibit a transformed phenotype that is thymic-dependent and retinoid inhibitable. Am J Pathol 1988, 132:38-48 [PMC free article] [PubMed] [Google Scholar]
- 52.Lafyatis R, Remmers EF, Roberts AB, Yocum DE, Sporn MB, Wilder RL: Anchorage-independent growth of synoviocytes from arthritic and normal joints. J Clin Invest 1989, 83:1267-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton JA: Hypothesis: in vitro evidence for the invasive and tumor-like properties of the rheumatoid pannus. J Rheumatol 1983, 10:845-851 [PubMed] [Google Scholar]
- 54.Bucala R, Ritchlin C, Winchester R, Cerami A: Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med 1991, 173:569-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zvaifler NJ, Firestein GS: Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum 1994, 37:783-789 [DOI] [PubMed] [Google Scholar]
- 56.Imamura F, Aono H, Hasunuma T, Sumida T, Tateishi H, Maruo S, Nishioka K: Monoclonal expansion of synoviocytes in rheumatoid arthritis. Arthritis Rheum 1998, 41:1979-1986 [DOI] [PubMed] [Google Scholar]
- 57.Firestein GS: Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum 1996, 39:1781-1790 [DOI] [PubMed] [Google Scholar]
- 58.Wallace PM, MacMaster JF, Rouleau KA, Brown TJ, Loy JK, Donaldson KL, Wahl AF: Regulation of inflammatory responses by oncostatin M. J Immunol 1999, 162:5547-5555 [PubMed] [Google Scholar]