Gps2, a Protein Partner for Human Papillomavirus E6 Proteins (original) (raw)
Abstract
We have used the yeast two-hybrid system to screen a cDNA library prepared from normal human epidermal keratinocytes and identified protein partners for human papilloma virus (HPV) E6 proteins. A clone that encoded Gps2 interacted with E6 proteins from HPVs of high and low oncogenic risk. The specificity of these reactions was verified and the regions of E6 that were required for interaction were mapped. Steady-state and pulse-chase analyses of cells cotransfected with DNAs expressing E6 from either HPV6 or HPV18 and Gps2 demonstrated that the E6 proteins induced the degradation of Gps2 in vivo but not in vitro. Gps2 exhibited transcriptional activation activity, and high-risk E6 suppressed this activity.
Human papillomaviruses (HPVs) are small DNA tumor viruses. Over 90 different HPVs have been identified. These viruses are classified into subtypes based on their ability to infect cutaneous or mucosal epithelia (81). Mucosal HPVs are further divided into two subgroups. The low-risk HPVs, including types 6 and 11, are associated with benign lesions such as condyloma acuminata (22), whereas the high-risk HPVs, such as types 16 and 18, are associated with lesions that can progress to cancer (80). High-risk HPVs are the causative agent of at least 90% of cervical cancers (6, 80). In vitro experiments support the association of the high-risk HPVs with malignant tumors, as DNA from these viruses can immortalize human keratinocytes and transform rodent cell lines (36, 46, 55, 62, 77, 78). Even though both high-risk and low-risk HPVs cause cellular proliferation in cervical mucosa, low-risk HPVs have limited transforming activity (52, 62). However, in rare cases, HPV6 and HPV11 can transform primary rodent cells (68). Although HPV6 is rarely found in cervical tumors, tumors at other sites have been reported to harbor HPV6 more frequently (5). These observations suggest that HPV6, and possibly HPV11, also possesses some oncogenic potential.
E6 and E7 are the major transforming proteins of HPVs (74). Differences in the transforming potential between high- and low-risk HPVs are reflected in the intrinsic differences between their respective E6 and E7 proteins (4). High- but not low-risk E6 can cooperate with high-risk E7 to immortalize human keratinocytes (4, 26, 27, 47). However, the E6 proteins from both high- and low-risk HPVs can immortalize human mammary epithelial cells (3). In one instance, weak immortalization activity of E6 genes from low-risk HPV types was reported when they were inserted in human epithelial cells in conjunction with E7 from high-risk types (25).
The transforming properties of HPV E6 and E7 result in part from their ability to complex with and modulate the action of critical host proteins that regulate cell growth and differentiation. Both E6 and E7 from high-risk HPV types have been found to interact with important cell cycle regulators. High-risk E7 proteins interact with the Rb family of proteins, which are negative cell cycle regulators involved in the control of the G1/S check point (14, 48). High-risk HPV E6 proteins interact with and facilitate the degradation of p53, a multifunctional protein involved in many cellular processes, including proliferation, differentiation, DNA repair, and apoptosis (60, 75). The interaction between p53 and HPV E6 is mediated by E6-AP, an E3-like ubiquitin ligase (28, 29, 31, 45). Low-risk HPV E6 proteins bind p53 with a reduced affinity and do not direct its degradation. The ability of mucosal HPV types to immortalize human keratinocytes and degrade p53 can be correlated with their association with human malignancy. However, p53 degradation alone may not account for the transforming activities of the HPV E6 proteins (49, 63). Other high-risk HPV E6 proteins such as E6 from HPV8 (8E6) and the more distantly related cottontail rabbit papillomavirus and bovine papillomavirus (BPV) E6 proteins all possess transforming activities; however, none of them complexes with p53 (37, 39, 61, 67).
Other protein partners for HPV E6s have been identified. However, most of these interactions have only been shown with high-risk HPV E6s. E6 binding protein, Drosophila discs large tumor suppressor, paxillin, interferon regulatory factor 3, E6-targeted protein, Tyk2, and protein kinase N were all shown to interact with E6 proteins from high-risk HPVs (11, 17, 18, 20, 38, 44, 54, 57, 71). Both high-risk and low-risk E6 proteins are able to bind McM7, the multicopy maintenance protein 7 subunit of replication licensing factor M (41, 42). Recently, 16E6 was shown to interact with the C/H1, C/H3, and C-terminal domains of p300, whereas 6E6 interacts weakly only with the amino-proximal C/H1 domain of p300 (51, 79). The CBP/p300 family of coactivators stimulate transcription from various promoters by interacting with different transcription factors (32). The combined effect of their action is cell cycle inhibition and tumor suppression (15, 21, 64). Despite the fact that high-risk HPV E6 binds E6-AP, protein destabilization does not seem to be a common strategy for E6 interactions. For example, other than p53, HPV E6 degrades only E6TP1 and hDLG of the proteins mentioned above (18, 20, 54). Both high- and low-risk mucosal HPV E6 proteins interact with Bak, a Bcl-2 family protein involved in regulating apoptosis. They also induce degradation of Bak in vivo, though with different efficiencies (69, 70). It is apparent that HPV E6 proteins can regulate multiple cellular processes through their interaction with different cellular proteins.
Despite differences in their transforming potential, all HPV E6 proteins have a strikingly similar primary structure and cause proliferative changes in human cells (72). It is conceivable that the different HPV E6 proteins affect overlapping cellular processes involved in proliferation and differentiation and are therefore likely to share certain cellular protein partners. Here we report that Gps2 (AMF-1), a protein that interacts with the BPV 1E2 protein (9), also interacts with high- and low-risk HPV E6 proteins from both mucosal and cutaneous HPVs.
MATERIALS AND METHODS
Plasmids. (i) HPV E6 plasmids.
The E6 open reading frame (ORF) from a cloned HPV6 genome was amplified by PCR using the primers 5′-TTTAGCAAACGAGGCACCAT-3′ and 5′-TGTAACCCTCCATGGTCTGG-3′ and T/A cloned into the pCRII vector (Invitrogen, Carlsbad, Calif.) to construct 6E6-pCRII. 18E6-pCRII was cloned as above using the primers 5′-AAACACACCACAATACCA-3′ and 5′-CCTTAGGCCCATGGATAC-3′. _Nco_I fragments containing full-length 6E6 or 18E6 from their pCRII constructs were fused in frame to the Gal4 DNA-binding domain (Gal4-BD) in pAS2-1 (Clontech, Palo Alto, Calif.) to create 6E6-Gal4-BD and 18E6-Gal4-BD for use as baits in library screening. The E6 ORF from HPV8 was cloned using the 5′ primer 5′-GGGAATTCATGGACGGGCAGGACAAG-3′ and the 3′ primer 5′-CCGGATCCTTACCAATCATGATACAA-3′ as an _Eco_RI-_Bam_HI fragment into pACTII (Clontech) to create a Gal4 activation domain (Gal4-AD) fusion designated 8E6-Gal4-AD. 1E6-Gal4-AD was generated the same way from cloned HPV1 genomic DNA using the primers 5′-GGGAATTCATGGCGACACCAATCCGG-3′ and 5′-CCGGATCCTTATATAGCATACAAGCG-3′. The primers for generating the 11E6 ORF were 5′-GGGAATTCATGGAAAGTAAAGATGCC-3′ and 5′-CCGGATCCTTAGGGTAACAAGTCTTC-3′. The primers for generating the 16E6 ORF were 5′-GGGAATTCATGCACCAAAAGAGAACT-3′ and 5′-CCGGATCCTTACAGCTGGGTTTCTCT-3′. Cloning the _Nco_I fragment from 6E6-pCRII into the pACTII vector generated a 6E6-Gal4-AD fusion. To create deletions of 6E6, full-length 6E6 was released from 6E6-pCRII as a _Bam_HI-_Eco_RV fragment, and the purified fragment was digested with _Bsm_I, _Apo_I, _Mbo_II, or _Rsa_I. The 3′ restriction site from each N-terminal fragment was filled with Klenow enzyme and then digested with _Nco_I for cloning into pAS2-1 between the _Nco_I and _Sma_I sites. The corresponding fragments were subsequently released from pAS2-1 with _Nco_I and _Bam_HI and subcloned into pACTII to produce Gal4-AD fusions with the 6E6 DNA sequences encoding the following amino acids; 6E6-1-36, 6E6-1-68, 6E6-1-94, and 6E6-1-131. The larger fragment corresponding to the C-terminal half from _Bsm_I digestion of the full-length 6E6 fragment was first filled with Klenow and then digested with _Eco_RI for cloning into pET21 (Novagen, Madison, Wis.) prepared by digesting with _Bam_HI, end-filling with Klenow, and then digesting with _Eco_RI. This fragment was subsequently released from pET21 with _Bam_HI and _Sal_I and subcloned into pAS2-1 to place the _Nco_I site in frame. The resulting construct, Bsm-C-pAS2-1, was digested with _Nco_I and _Sal_I, and the released insert was purified and then digested with _Rsa_I for cloning into the _Nco_I and _Sma_I sites of pACTII to generate the deletion 6E6-36-131, which contained the 6E6 fragment from the _Bsm_I site to the _Rsa_I site. Two other deletion mutants, 6E6-68-131 and 6E6-101-131, were generated by PCR amplification of the corresponding fragment from 6E6-pCRII, digestion of the resulting PCR product with _Bam_HI and _Eco_RI, and cloning into pACTII. The 5′ and 3′ primers for 6E6-68-131 were 5′-GCGCGTGGATCCTAGAATTTCATGGAAAAATC-3′ and 5′-GTAGGCAGAATTCCTTCCACGTACAATTTAGC-3′. The 5′ primer for 6E6-101-131 was 5′-GACGTGCGGATCCGGTGCTACCTGTGTCAC-3′, and the 3′ primer was the one used to create 6E6-68-131.
For glutathione-_S_-transferase (GST) fusion proteins, full-length 6E6 from the 6E6-pCRII construct was cloned into pGEX-2T (Pharmacia, Piscataway, N.J.) in the _Eco_RI site. Full-length 18E6 was released from 18E6-pCRII with _Nco_I, and the ends were filled with Klenow prior to ligation with the end-filled _Bam_HI site in pGEX-2T. The sequences encoding the HPV 1E6, 8E6, 11E6, and 16E6 ORFs were cloned into pALEX (50) between a filled _Sal_I site and a _Hin_dIII site to generate GST fusion proteins.
For in vitro translation, _Nco_I fragments of 6E6 and 18E6 from their pCRII constructs were cloned into the pET21 vector. The 6E6 deletion mutants 1-94 and 1-131 were cloned into pET21 between the _Nco_I and _Sal_I sites, while the deletion mutant 36-131 was cloned into pET21 between the _Nco_I and _Xho_I sites.
For transfection of eukaryotic cells, 6E6 and 18E6 were amplified by PCR from their respective pCRII constructs and cloned into the pHANE vector (65). The resulting E6-hemagglutinin (HA) constructs were in frame with an HA tag placed at the N terminus. The 5′ primer for 6E6-HA was 5′-AACGAGGAATTCATGGAAAGTGCAAATGCCTCCAC-3′ and the 3′ primer was 5′-CAATATGGATCCGGGTAACATGTCTTCCATGC-3′. This ORF was cloned as an _Eco_RI-_Bam_HI fragment into pHANE. The primers for 18E6-HA have _Eco_RI sites 5′ primer, 5′-CACCACGAATTCATGGCGCGCTTTGAGGATCC-3′; 3′ primer, 5′-TACTTAGAATTCTACTTGTGTTTCTCTGCGTCG-3′, and the ORF was cloned as an _Eco_RI fragment into pHANE. The _Nco_I fragments of 6E6 and 18E6, from their respective pCRII constructs, were cloned into the _Pme_I site of pmycpl.1 (7) to create 6E6-Myc and 18E6-Myc, respectively.
(ii) Gps2 constructs.
The Gps2 cDNA clone Gps2-Gal4-AD was acquired from a normal human foreskin keratinocyte cDNA two-hybrid library (Clontech) by screening with a 6E6-Gal4-BD bait and subsequently used to construct other Gps2 clones. Gps2-Gal4-AD contains the entire sequence of Gps2 plus 60 bp at the N terminus fused in frame to the Gal4-AD in the vector pGAD10 (Clontech). Cloning the _Eco_RI fragment from Gps2-Gal4-AD into pBTM116 (73) generated a Gps2-LexA DNA-binding domain fusion protein. This same fragment was cloned into pET21a to construct Gps2-pET for in vitro translation, into pALEX to construct GST-Gps2, and into the Flag vector pCF2H (56) to construct Gps2-Flag for in vivo expression in eukaryotic cells.
(iii) Other constructs.
The protein kinase C (PKC)-Flag construct was from Jae-Woe Soh (Department of Genetics, Columbia University, New York, N.Y.). The GST-p300 1-347 fusion protein was a generous gift from Yuan Chang and Patrick Moore (33). A fragment from the zyxin gene that contains three LIM domains was amplified from a full-length zyxin cDNA using the primers 5′-GGCAGACCATGGCTGTCAACGAACTCTGC-3′ and 5′-GGGCCTGAATTCACTCAGGTCTGGGCTCTAG-3′ and cloned into pET21 between the _Nco_I and _Eco_RI sites to create LIM for use in the coupled in vitro transcription-translation system. The plasmid containing the serum response element (SRE)-Luc cassette was purchased from Stratagene (La Jolla, Calif.). The 2X-TRE-Luc plasmid was a gift from Audrey Minden (12). RL-TK has a Renilla luciferase gene under the control of a basic thymidine kinase promoter and was purchased from Promega (Madison, Wis.).
Yeast two-hybrid library screen.
A human foreskin keratinocyte cDNA library was purchased from Clontech. It contains 5 × 106 independent clones that were constructed using both oligo(dT) and random priming and cloned in pGAD10 to create Gal4-AD fusions. The average size of the cDNA inserts was 1.4 kb. Saccharomyces cerevisiae strain YGH1 (35) was used for the library screen. The screening procedure was performed as described in the Clontech Matchmaker System manual. Briefly, 1 mg of bait DNA, 500 μg of library DNA, and 20 mg of herring sperm DNA were mixed with 8 ml of competent yeast cells for transformation. The transformed cells were plated onto plates lacking leucine, tryptophan, and histidine Leu− Trp− His−. For 18E6 transformations, the plates also contained 2.5 mM 3-amino-1,2,4-triazole to reduce background on His− selection. β-Galactosidase (β-gal) lift assays were performed on the transformants, and positive colonies were streaked three times to separate the plasmids. The darkest blue colonies were picked each time the β-gal lift assay was performed. Positive clones were streaked twice on Leu− plates and grown in Leu− liquid medium to select for loss of the bait DNA. Yeast DNA was isolated and transformed into Escherichia coli strain Top10. Plasmid DNAs from Top10 transformants were transformed into YGH1 with the appropriate bait to confirm their interaction. The DNA from the clones that turned blue only with the bait and not with the vector or a laminin-Gal4-BD fusion clone was sequenced and characterized further.
(i) Yeast strains and β-gal assays.
Yeast strains YGH1 and L40 (73) were used for transformation of Gal4-BD fusion proteins and LexA fusion proteins, respectively. Strains Y187 and Y190 were from Clontech. All strains were maintained at 30°C on YPD plates. Transformation was performed as described in the Clontech Matchmaker System manual. Briefly, a stationary-phase yeast culture was diluted into 300 ml of YPD medium to an optical density at 600 nm (OD600) of between 0.2 and 0.3 and grown for 3 h at 30°C. To prepare competent yeast cells, cells were collected by centrifugation at 1,000 × g for 5 min at room temperature, washed once with H2O, and resuspended in 1.5 ml of 1× TE-LiAc (10× TE is 0.1 M Tris-HCl plus 10 mM EDTA [pH 7.5]; 10× LiAc is 1 M LiAc [pH 7.5]). For small-scale cotransformation, 0.5 μg of each DNA and 100 μg of herring sperm carrier DNA were mixed with 100 μl of competent yeast cells. After adding 600 μl of a polyethylene glycol (PEG)-LiAc solution (1× LiAc, 1× TE, 40% PEG 4000), the mix was incubated at 30°C for 0.5 h. Cells were then added to 70 μl of dimethyl sulfoxide and incubated at 42°C for 15 min. The cells were then pelleted and suspended in 1× TE for selection on Leu− Trp− SD plates.
(ii) Filter lift assay for β-gal activity.
Four to six days after transformation, the yeast colonies were lifted onto nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.), and the cells were lysed by freezing at −80°C for 20 min and thawing at room temperature. The filter disks were placed onto Whatman paper soaked in 2 ml of Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 0.3% β-mercaptoethanol) containing 0.33 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml and incubated at 37°C for 1 to 3 h.
(iii) Liquid β-gal assay.
Individual yeast colonies were picked into 3 ml of Leu− Trp− SD medium and grown to stationary phase. They were then diluted 1:30 in medium and grown to OD600 of between 0.4 and 0.6. The cells were collected by centrifugation at 1,500 × g for 5 min at 4°C and washed once with Z-buffer. The cell pellets were resuspended in 250 μl of Z-buffer, and the cells were lysed following addition of 200 μl of ice-cold acid-washed glass beads by vortexing three times for 1 min each. The cell lysates were clarified by centrifugation at 3,000 × g for 5 min at 4°C. Total protein concentrations were determined by the method of Bradford (8). For β-gal assays, 200 μl of 4-mg/ml _o_-nitrophenyl-β-d-galactopyranoside (ONPG) was added to 150 μl of cell extract diluted in 800 μl of Z-buffer, and the mixture was incubated at 30°C until a pale yellow color developed. The reaction was stopped by adding 500 μl of 1 M Na2CO3, and the OD420 was determined. Z-buffer alone incubated with ONPG was used as a control for measuring OD420. The β-gal units were calculated as (1,000 × OD420)/(t × mg), where t is the reaction time in minutes and mg is the total amount of lysate protein in milligrams that was added to the reaction (2).
In vitro transcription and translation.
pET21 clones were subjected to in vitro transcription and translation using TNT coupled reticulocyte lysate systems for Gps2 or TNT coupled wheat germ systems for E6 proteins as described by the manufacturer (Promega). For HPV E6 and LIM clones, [35S]cysteine (Amersham Pharmacia Biotech, Piscataway, N.J.) was used for labeling, while Gps2 was labeled with [35S]methionine (Amersham Pharmacia Biotech).
GST protein purification and GST pulldown assays. (i) GST protein purification.
E. coli strain BL21(DE3) was the host for production of GST fusion proteins. GST-p300 cultures were grown at 30°C, while all other cultures were grown at 37°C. Cell culture (500 ml) was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) when the OD600 reached 0.5 to 0.8. After 3 to 4 more hours, cells were harvested and resuspended in 20 ml of cold phosphate-buffered saline (PBS) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 mM dithiothreitol (DTT), and 0.2 mg of lysozyme per ml. All subsequent steps were performed at 4°C. Cells were lysed by addition of Triton X-100 to 1% and sonicated three times for 20 s each in a Branson Sonifier 450 (Branson, Danbury, Conn.). The cell lysate was clarified by centrifugation at 20,000 × g for 15 min. Then, 600 μl of preswollen glutathione-agarose beads (Sigma, St. Louis, Mo.) was added to the lysate and mixed at 4°C for 2 h. The beads were then washed four times with 45 ml of PBS plus 1% Triton X-100.
(ii) GST pulldown assays.
Forty microliters of a 50% suspension of glutathione-agarose beads containing 3 to 5 μg of GST fusion protein was blocked at 4°C for 0.5 h in 500 μl of binding buffer (20 mM Tris-HCl [pH 7.5], 50 mM KCl, 0.5 mM EDTA, 3% bovine serum albumin, 1% NP-40). The beads were pelleted at 7,000 rpm for 20 s in a microcentrifuge and resuspended in 100 μl of binding buffer. Then 5 μl of 35S-labeled in vitro translation product was added to the mix, and binding was performed at 4°C for 1 h. The beads were then washed six times with 1 ml of binding buffer and boiled for 5 min in 15 μl of 5× sodium dodecyl sulfate (SDS) loading buffer prior to analysis by SDS-polyacrylamide gel electrophoresis (PAGE) (43). After electrophoresis, the bound 35S-labeled protein was detected by autoradiography.
Cell culture and transfections.
NIH 3T3 cells were grown and maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, N.Y.) containing 10% bovine calf serum (HyClone Laboratories Inc., Logan, Utah.). 293T and C33A cells were grown in DMEM containing 10% fetal bovine serum (HyClone). The media were supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) (Gibco-BRL) unless otherwise noted.
Coimmunoprecipitation.
For coimmunoprecipitation assays, 293T cells were transfected by the calcium phosphate precipitation method as previously described (76). For other studies, NIH 3T3 and C33A cells were transfected with Lipofectamine Plus reagents from Gibco-BRL following the manufacturer's protocol. 293T cells in 10-cm dishes were transfected with 20 μg of Gps2-Flag, PKC-Flag, or vector DNA. At 36 h after transfection, cells were collected into ice-cold PBS and pelleted at 1,700 × g for 5 min at 4°C. All of the following steps were performed on ice. The cells were resuspended in 1 ml of lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 100 mM NaF, 10% glycerol, 200 μM Na3VO4, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1 mM PMSF) and sonicated for 1 min. After centrifugation at 14,000 rpm in a microcentrifuge for 5 min, the cell lysate was precleared by incubation with 50 μl of a 10% suspension of IgGsorb (The Enzyme Center, Malden, Mass.) in lysis buffer for 15 min, and then the beads were pelleted. The supernatant was transferred to a fresh tube, and the total protein concentration was determined. One milligram of cellular protein, in a total volume of 500 μl of lysis buffer, was mixed with 10 μl of [35S]cysteine-labeled in vitro-translated E6 proteins for 2 h to overnight at 4°C. Then 100 μl of anti-flag M2 affinity gel (Kodak, New Haven, Conn.) was added to the mix, and incubation was continued for another hour. The beads were harvested and washed five times with 1 ml of lysis buffer per wash by rotating at 4°C for 5 min and then centrifuging the beads in a microcentrifuge at 7,000 rpm for 20 s. After the final wash, the beads were boiled for 5 min in SDS- loading buffer and subjected to SDS-PAGE. The coprecipitated E6 proteins were visualized by autoradiography.
Coimmunoprecipitation in vivo was performed on extracts from 293T cells that were transfected with 5 μg of a Gps2-Flag construct and 15 μg of Myc-tagged E6 constructs. At 36 h after transfection, cells were labeled with 35S-Translabel (ICN, Irvine, Calif.) for 3 h. Cell lysates were prepared as described above except that the concentration of NP-40 in the lysis buffer was 0.1% and the pH of the solution was 8.0. Gps2-Flag protein was immunoprecipitated as above, and the coprecipitated proteins were separated on a 4 to 12% Bis-Tris gel (Novex, San Diego, Calif.) and visualized by autoradiography.
In vivo degradation assays. (i) Steady-state analysis.
C33A or NIH 3T3 cells (2 × 105) were plated into six-well plates the night before transfection. The next day, 0.25 μg of Gps2-Flag plasmid was cotransfected with 2.5 μg of plasmid 6E6-HA, 18E6-HA, or the pHANE vector and 50 ng of a cytomegalovirus (CMV)-Luc reporter as an internal control to monitor the efficiency of transformation. After 36 h, cells were washed with PBS and lysed with the lysis buffer from the dual luciferase reporter assay system kit from Promega. After centrifugation at 14,000 rpm in a microcentrifuge at 4°C, the total protein concentration of each clarified lysate was determined. Lysates were diluted in lysis buffer to make the total protein concentration of each sample equal. Then 25 μl of 5× SDS loading buffer was added to 100 μl of each lysate, and the samples were boiled for 5 min. Thirty microliters from each sample was subjected to SDS-PAGE and subsequently to Western blot analysis with anti-Flag antibody (Kodak) to detect Gps2.
(ii) Pulse-chase degradation assay.
NIH 3T3 cells were transfected with 0.25 μg of the Gps2-Flag construct in the presence of 2.5 μg of plasmid 6E6-HA, 18E6-HA, or pHANE. At 36 h after transfection, the cells were washed twice with Met− Cys− DMEM (Specialty Media, Lavalette, N.J.) and incubated for 15 min at 37°C in the same medium. Then 200 μCi of 35S-Translabel in 1 ml of Met− Cys− DMEM with 1% dialyzed calf serum was added to each monolayer, and the cells were incubated for 30 min. At the end of the labeling period, cells were washed twice in complete medium containing 10% serum and incubated for 1 or 3 h prior to harvesting. Cell lysates were prepared as described in the section on coimmunoprecipitation, and identical amounts of protein from each sample were immunoprecipitated for 2 h at 4°C with 50 μl of anti-Flag M2 affinity gel (Kodak). The beads were then washed and boiled in SDS loading buffer to release bound Gps2. The eluate was subjected to SDS-PAGE, and the 35S-labeled protein was visualized with a PhosphorImager.
In vitro degradation assay.
The E6 degradation assay was performed as described by Gao et al. (18). E6 proteins and Gps2 or p53 were transcribed and translated in vitro as described above. Two microliters from each translation reaction was subjected to SDS-PAGE, and the intensity of the bands was quantitated by PhosphorImager to calculate the relative concentration of each translated product. p53 (2 μl) or an equal amount of Gps2 protein was mixed with twice that amount of each E6 protein diluted in degradation buffer (25 mM Tris [pH 7.5], 100 mM NaCl, 3 mM DTT) in a total volume of 25 μl and incubated at 30°C for 4 h. Then 25 μl of 2× SDS sample buffer was added at the end of the incubation to stop the reaction, and the samples were boiled for 5 min. Ten microliters from each reaction was subjected to SDS-PAGE. The bands for Gps2 and p53 were visualized using a PhosphorImager.
Luciferase assay.
Transfected NIH 3T3 cells in six-well plates were harvested 36 h after transfection into 300 μl of 1× lysis buffer per well (Dual luciferase reporter assay system; Promega). The luciferase activities from both firefly and Renilla luciferase constructs were quantified with a Berthold Lumat LB9501 luminometer (Wallac Inc., Gaithersburg, Md.) using the reagents and protocol provided by the manufacturer.
RESULTS
Gps2 interacts with E6.
To identify novel proteins that might interact with HPV E6 proteins, DNAs encoding 18E6 and 6E6 were fused in frame to the Gal4-BD and used as baits to screen a yeast two hybrid library containing cDNAs from normal human foreskin keratinocytes fused to the Gal4-AD. Several criteria were used to define a positively interacting clone. When cotransformed with either bait, the colonies expressed β-gal in lift assays. The colonies did not express β-gal when they were cotransformed with the vector pAS2-1 or with a plasmid expressing Gal4-BD-laminin. The positive interactions remained positive in three different yeast strains tested: YGH1, used for library screening, and two other yeast strains, Y187 and Y190. A clone encoding Gps2 (34) was identified by using either 6E6-Gal4-BD or 18E6-Gal4-BD in the yeast two-hybrid screen. Gps2 is a 37-kDa nuclear protein with no significant homology to other known proteins. Gps2 was first identified as a protein that could suppress the lethal G protein subunit-activating mutations in the pheromone response pathway in S. cerevisiae (66). Subsequently, this protein was also found to interact with the BPV 1E2 protein and the human T-cell lymphotrophic virus type 1 (HTLV-1) transforming protein Tax (9, 34).
Gps2 binds to E6 proteins in vitro.
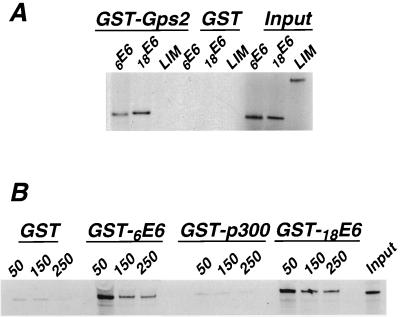
The interactions between Gps2 and HPV E6 proteins were verified by an in vitro binding assay. RNAs encoding 6E6 and 18E6 were translated in a rabbit reticulocyte lysate and labeled with [35S]cysteine. Labeled proteins were mixed with GST-Gps2 fusion protein that was purified from bacteria. Neither 6E6 nor 18E6 was bound by the GST protein. However, the GST-Gps2 fusion protein bound both proteins (Fig. 1A). The specificity of binding was confirmed in two ways; first we used the double zinc finger LIM domain from zyxin and demonstrated that it did not bind Gps2 (Fig. 1A). In the other approach, GST-E6 fusions produced in bacteria were used to capture 35S-labeled in vitro-translated Gps2. The complexes bound to agarose beads were subjected to washes with increasing salt concentrations and to SDS-PAGE. Gps2 was able to bind both GST-6E6 and GST-18E6, and these interactions were resistant to 250 mM salt (Fig. 1B). Gps2 was unable to bind GST or a GST-p300 fusion that contained the first 347 amino acids of p300, which do not include the C/H1 domain that is required for interaction with Gps2 (53; unpublished observation). These results support our findings using the yeast two-hybrid system and confirm that Gps2 is a protein partner for HPV E6 proteins. Furthermore, the interactions are specific, as demonstrated by their stability at physiologic and higher salt concentrations.
FIG. 1.
E6 proteins binding to Gps2. (A) In vitro-translated and 35S-labeled 6E6, 18E6, and the negative control LIM domain from zyxin were incubated with GST or GST-Gps2 fusion proteins bound to agarose beads. E6 proteins that interacted with the bound baits were eluted from the beads and subjected to SDS-PAGE analysis followed by autoradiography. (B) In vitro-translated and 35S-labeled Gps2 was allowed to interact with equal amounts of purified GST, GST-E6, or GST-p300 fusion proteins. After binding at 50 mM salt, the agarose beads were subjected to washes with buffer containing increasing concentrations of salt (millimolar) as indicated above the lanes. After extensive washes, the bound Gps2 was eluted from the beads and subjected to SDS-PAGE analysis followed by autoradiography. The lanes marked input contain 10% of the reacting material.
Coimmunoprecipitation of E6 with Gps2.
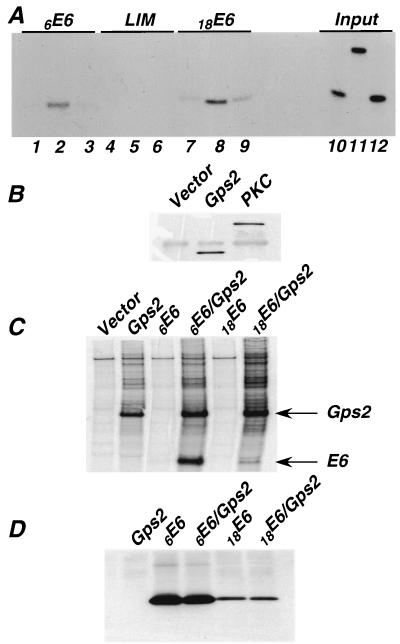
We next tested whether interaction between these proteins could be detected in a coimmunoprecipitation assay. A modified assay was used because HPV E6 proteins are expressed at only very low levels in animal cells and are relatively insoluble. 293T cells were transfected with constructs expressing either the Flag vector alone, Flag-tagged Gps2, or Flag-tagged PKC, which served as a negative control. At 36 to 48 h after transfection, cell lysates were prepared. These lysates were mixed with in vitro-translated, 35S-labeled 6E6, 18E6, or LIM before the Flag-tagged proteins were immunoprecipitated. The immunoprecipitates were subjected to SDS-PAGE, and E6 proteins in the complex were detected by autoradiography. Both 18E6 and 6E6 coprecipitated with Gps2 but not with PKC or the Flag vector alone (Fig. 2A). The LIM control did not coprecipitate with either of the Flag-tagged proteins. Figure 2B shows that the PKC-Flag and Gps2-Flag proteins were retained on the Sepharose beads after immunoprecipitation with anti-Flag antibody.
FIG. 2.
Coimmunoprecipitation of E6 with Gps2. (A) 293T cells were transfected with 20 μg of the Flag vector alone (lanes 1, 4, and 7), Gps2-Flag (lanes 2, 5, and 8), or PKC-Flag (lanes 3, 6, and 9). Cell lysates were prepared 36 h after transfection. Equal amounts of total protein were mixed with 10 μl of in vitro-translated, 35S-labeled 6E6, 18E6, or LIM and then immunoprecipitated with anti-Flag antibody. Coprecipitated proteins were subjected to SDS-PAGE and analyzed by autoradiography. Lanes 10, 11, and 12 contain 10% of the 6E6, LIM, or 18E6 input material, respectively. (B) After immunoprecipitation with anti-Flag antibody, Sepharose beads were boiled in SDS loading buffer and subjected to SDS-PAGE analysis. The retention of PKC-Flag and Gps2-Flag proteins was monitored by Western blot analysis with an anti-Flag antibody. (C) 293T cells were transfected with a plasmid expressing Gsp2-Flag, Myc-tagged E6, or both plasmids. At 36 h after transfection, cells were labeled with 35S-Translabel for 3 h, and then whole-cell lysates were prepared. Equal amounts of protein from each lysate were subjected to immunoprecipitation with anti-Flag affinity gel, and the coprecipitated proteins were subjected to SDS-PAGE and autoradiography. The bands corresponding to E6 and Gps2 are indicated alongside the gel. (D) E6 expression in each of the above cotransfections was measured by Western analysis using an anti-Myc antibody.
To demonstrate that these interactions can occur in vivo, 293T cells were cotransfected with plasmids encoding Gps2-Flag and Myc-tagged 6E6 or 18E6. The E6 proteins were expressed to high levels because the E6 ORFs were placed in a vector that contained the simian virus 40 origin of DNA replication (7). At 36 h after transfection, cell proteins were labeled with 35S for 3 h, and whole-cell lysates were prepared. Following immunoprecipitation with anti-Flag antibody affinity gel, the beads were examined for the presence of E6 proteins. Figure 3C demonstrates that both 6E6-Myc and 18E6-Myc were coimmunoprecipitated with Gps2. The level of E6 protein expressed in each transfected cell lysate was detected by Western analysis using an anti-Myc antibody (Fig. 3D). Note that coexpression of Gps2 does not have a substantive effect on the level of accumulation of either of these E6 proteins during the 3-h course of labeling. These data demonstrate that Gps2 can specifically interact with E6 proteins from both high- and low-risk mucosal HPVs both in vivo and in vitro.
FIG. 3.
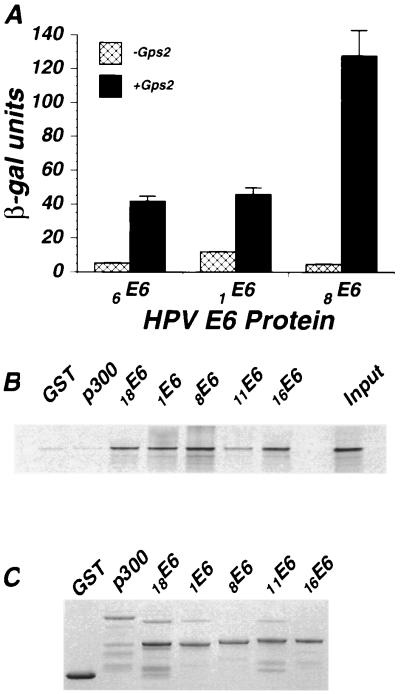
Interaction between cutaneous HPV E6 and Gps2. (A) HPV E6–Gal4-AD fusions were cotransformed with a Gps2-LexA fusion or the LexA vector into yeast strain L40. Liquid β-gal assays were performed on the cotransformed colonies as described in Materials and Methods. The β-gal values are averages of three separate experiments. (B) In vitro-translated and 35S-labeled Gps2 was allowed to interact with equal amounts of purified GST or GST-E6 fusion proteins bound to agarose beads. Captured Gps2 was assayed by SDS-PAGE and autoradiography after boiling the beads in SDS loading buffer. (C) The amount of GST fusion protein used in each binding assay was determined by Coomassie brilliant blue staining of the SDS-PAGE gel shown in panel B. Interaction with GST-p300 was assayed as an additional negative control.
Interaction of Gps2 with E6 proteins from cutaneous-type HPVs.
Although E6 proteins from cutaneous and mucosal HPVs are evolutionarily distantly related, their overall structure and a certain degree of homology are conserved. Protein interactions for cutaneous HPV E6s have not been well characterized, and E6 proteins from high-risk cutaneous HPVs do not interact with p53 (37, 39, 67). Because Gps2 interacts with both high- and low-risk E6 proteins from mucosal HPVs, we next asked if E6 proteins from cutaneous-type HPVs also interacted with Gps2. 1E6 and 8E6 were chosen as examples of low- and high-risk cutaneous-type HPVs, respectively. When these two proteins were fused to the Gal4-BD, they exhibited strong self-activating activity (data not shown). To circumvent this problem, sequences encoding 1E6 and 8E6 were fused to the Gal4-AD, and Gps2 was expressed as a LexA fusion protein. 1E6-Gal4-AD or 8E6-Gal4-AD and Gps2-LexA constructs were cotransformed into the yeast strain L40, and the β-gal activity was determined on logarithmically growing cultures to quantitate the relative interaction between Gps2 and these HPV E6 proteins. In this assay, both cutaneous E6 proteins interacted with Gps2. The 8E6-Gps2 interaction resulted in a threefold-higher level of β-gal activity than the 1E6-Gps2 interaction (Fig. 3A). To verify that activation resulted from E6-Gps2 interactions, mucosal and cutaneous HPV E6 proteins were produced as GST fusions and mixed with 35S-labeled, in vitro-translated Gps2. The GST-E6 proteins were captured on beads and analyzed for the presence of Gps2 by SDS-PAGE. The results of this analysis reveal that each of the E6 proteins tested bound 5 to 10% of the input Gps2 protein (Fig. 3B). The level of E6 protein used in each assay was similar (Fig. 3C). These findings indicate that Gps2 is a protein partner for both cutaneous and mucosal HPV E6 proteins.
Identification of the regions of E6 that are required for interaction with Gps2.
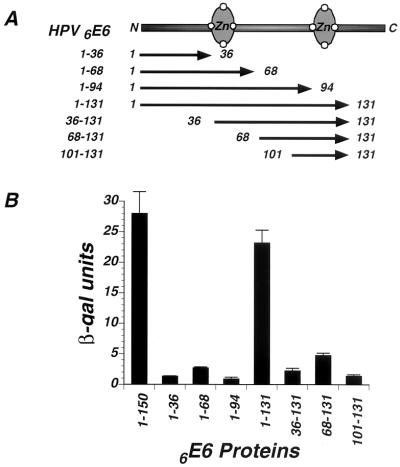
To identify the region(s) of E6 that is required for binding to Gps2, constructs containing 5′ and 3′ truncations of the 6E6 ORF fused to Gal4-AD were cotransformed into the yeast strain L40 with a Gps2-LexA construct. The constructs examined were 6E6-1-36, 6E6-1-68, 6E6-1-94, and 6E6-1-131 (Fig. 4A). Cotransformants were analyzed for interaction by measuring the β-gal activity that accumulated in these cells. Among these deletion mutants, only 6E6-1-131 interacted with Gps2 (Fig. 4B). Therefore, amino acids 94 to 131 are required for 6E6 to bind to Gps2, as the 94-amino-acid construct 6E6-1-94 was unable to interact. Starting from the deletion mutant 6E6-1-131, further deletions were made from the N terminus. None of these mutants (6E6-36-131, 6E6-68-131, or 6E6-101-131) interacted with Gps2, as determined by analysis of β-gal activity in the yeast two-hybrid assay (Fig. 4B). This result suggests that amino acids 1 to 36 are also required for the Gps2-E6 interaction to occur. However, these experiments do not differentiate between a requirement for the identified amino acids to facilitate protein folding and their actual involvement in binding to Gps2. To ensure that the failure to interact was not a result of lack of protein expression, Western analysis was used to demonstrate that E6 accumulated to similar levels in yeast cells transformed with the various deletion mutants (data not shown). This analysis suggests that at least two regions of 6E6 are required for E6 to interact with Gps2. One region contains the first 36 amino acids of the protein, while the other is within amino acids 94 to 131, which encodes the second zinc finger.
FIG. 4.
Identification of Gps2-interactive domains in E6. (A) Schematic representation of the 6E6 ORF. Deletions of 6E6 were fused in frame to Gal4-AD in the yeast vector pACTII for Gps2 binding studies in yeast cells. The numbers of the starting and ending amino acids in 6E6 define the deletion mutants. (B) Yeast strain L40 was cotransformed with Gal4-AD fusions of 6E6 deletions and Gps2-LexA. Liquid β-gal assays were performed on the contransformants as described in Materials and Methods. The values are averages from three separate experiments.
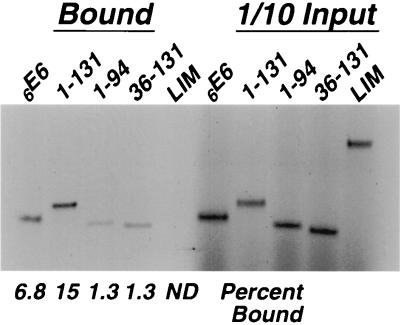
However, these studies are insufficient by themselves to define the interaction domain. Therefore, full-length and truncated 6E6 proteins were labeled with 35S by in vitro translation and examined for their ability to bind to GST-Gps2. There is at least a fivefold difference in binding between the proteins 6E6-1-94 and 6E6-36-131 compared to 6E6-1-131 (Fig. 5). Of the three E6 variants examined, only the 6E6-1-131 protein was bound to the same degree as the wild-type E6 protein (Fig. 5). These results corroborate the results of the yeast two-hybrid studies. All of the proteins expressed from the truncated E6 clones terminate at a stop codon in the cloning vector that is 38 codons downstream of the insert, while full-length E6 utilizes its own stop codon. Further analyses will be required to determine what regions are physically involved in binding or if these regions merely play a role in directing protein folding and rendering E6 available for interactions with Gps2.
FIG. 5.
Interaction of truncated HPV E6 proteins with GST-Gps2. In vitro-translated and 35S-labeled 6E6 proteins containing the amino acids specified above the lanes and LIM were allowed to interact with GST-Gps2. The bound proteins were separated by SDS-PAGE and visualized by autoradiography. The percentage of input material bound is indicated below the lanes. ND, not detectable.
Gps2 is degraded by 18E6 and 6E6 in vivo but not in vitro.
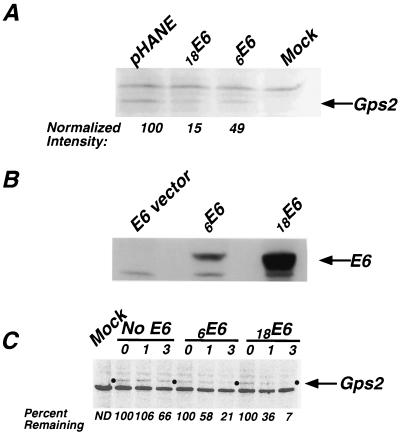
E6 proteins from high-risk HPVs induce the degradation of p53 (60). Because Gps2 interacted with high-risk 18E6, we asked if Gps2 was degraded in the presence of 18E6. Gps2-Flag and HA-tagged E6 constructs from HPV6 and HPV18 or the vector alone were cotransfected into NIH 3T3 cells. Equal amounts of protein from the transfected cell lysates were subjected to SDS-PAGE and then to Western blot analysis using an anti-Flag antibody to detect Gps2. When cells cotransfected with the E6 vector and Gps2 were compared to cells cotransfected with 18E6 and Gps2, the accumulation of Gps2 was significantly reduced in the presence of E6 (Fig. 6A). Cotransfection with 6E6 also resulted in a decrease in the level of Gps2, although to a much lesser extent than 18E6 when the band intensities were normalized to the level of luciferase activity expressed from the same promoter in the extract (Fig. 6A). However, when the level of E6 expression in the cotransfected cell lysates was assayed by Western blot, we demonstrated that less 6E6 was available to interact with Gps2 and effect degradation (Fig. 6B). The same results were found when C33A, a cervical cancer cell line, was used as the host (data not shown). These findings suggest that coexpression with HPV E6 proteins results in degradation of Gps2 in vivo.
FIG. 6.
Degradation of Gps2 in vivo. Gps2-Flag construct (250 ng) was cotransfected with 2.5 μg of 6E6-HA, 18E6-HA, or HA vector pHANE and 50 ng of a CMV-Luc reporter into NIH 3T3 cells. The lane labeled Mock contained only the Flag and HA vector DNAs. (A) Thirty-six hours posttransfection, cell lysates were collected, and equal amounts of total protein were subjected to SDS-PAGE and Western blot analysis using an anti-Flag antibody to detect the protein level of Gps2. The arrow points to the bands corresponding to Gps2. The normalized intensities of the Gps2 bands, as displayed below the lanes, were determined by dividing the absolute intensity of the respective bands by the level of luciferase expressed by the cotransfected reporter control. (B) The levels of E6 expressed by the 6E6-HA and 18E6-HA constructs were assayed by probing a Western blot prepared using extracts from the experiment described above with anti-HA antibody. (C) Cultures of cotransfected NIH 3T3 cells (without the CMV-Luc internal control) were pulse-labeled and chased as described in Materials and Methods, and cell lysates were prepared directly after the pulse (time zero) and after 1 and 3 h. The lysates with the same amounts of protein were immunoprecipitated with an anti-Flag antibody and subjected to SDS-PAGE, and the labeled Gps2 was visualized and quantified with a PhosphorImager. The percentage of Gps2 remaining at each time point is presented below the lanes. ND, not detectable.
To rule out the possibility that the decreased accumulation of Gps2 resulted from lower levels of expression, the stability of de novo-synthesized Gps2 in cotransfected NIH 3T3 cells was analyzed in a pulse-chase experiment. The results of this analysis demonstrate that newly synthesized Gps2 is degraded within 3 h after synthesis in cells cotransfected with either 18E6 or 6E6, whereas the majority of Gps2 in cells cotransfected with the E6 vector is stable (Fig. 6C). Thus, two different experimental approaches reveal that Gps2 degradation is accelerated in the presence of E6.
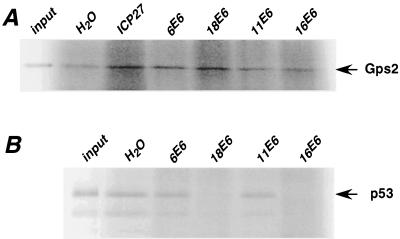
We then asked if high-risk HPV E6s could induce degradation of Gps2 in vitro, as was demonstrated for p53 (60). In vitro-synthesized Gps2 and E6 proteins were mixed and incubated at 30°C for 4 h. As a positive control, in vitro-translated p53 was subjected to the same degradation assay. As shown in Fig. 7, under these conditions p53 was almost completely degraded when incubated in the presence of 18E6 and 16E6 (Fig. 7B). In contrast, Gps2 was not degraded under this condition by E6 from either high- or low-risk HPVs (Fig. 7A). This indicates that, unlike p53, E6 proteins from high-risk HPVs degrade Gps2 in vivo but not in vitro.
FIG. 7.
Assay for degradation of Gps2 in vitro. In vitro-translated and 35S-labeled Gps2 or p53 was mixed with in vitro-translated HPV E6 proteins and analyzed for degradation as described in Materials and Methods. An unprimed reticulocyte lysate and in vitro-translated herpes simplex virus ICP27 protein were used as negative controls. One-fifth of the total reaction was subjected to SDS-PAGE, and the bands for Gps2 and p53 were visualized using a PhosphorImager.
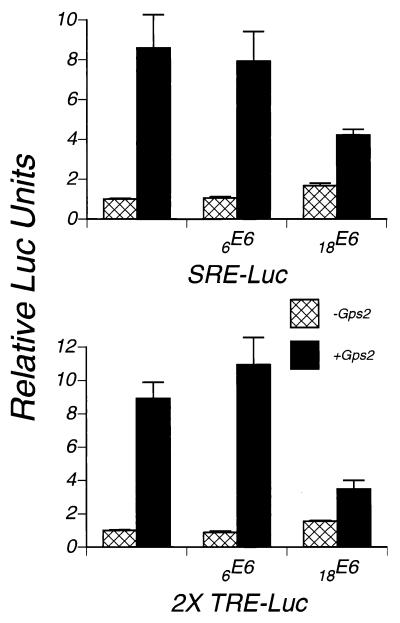
Transactivation by Gps2 is suppressed by high-risk HPV E6.
Gps2 has been reported to suppress a G protein-mediated signaling pathway and c-Jun N-terminal kinase activity (34, 66) and to stimulate transcriptional activation by both BPV 1E2 and c-Jun-AD (9). To test the effect of Gps2 on transcriptional activation, we cotransfected cells with luciferase reporters driven by promoters that contain enhancers found in HPV promoters and a plasmid expressing Gps2. The SRE-Luc reporter construct contains a basic TATA promoter and five repeats of the SRE site, whereas the 2X-TRE-Luc reporter contains a basic TATA promoter and two tissue plasminogen activator response element (TRE) sites. Gps2 enhanced transcription from both of these promoters (Fig. 8 and Table 1). Inclusion of an 18E6 in the transfection assays resulted in suppression of transactivation by Gps2 despite the ability of 18E6 alone to slightly activate each of these promoters above its baseline level of activity (Fig. 8 and Table 1). Cotransfection of 6E6 with Gps2 had little discernable effect on either reporter (Table 1). While the ability of 18E6 to suppress Gps2-mediated activation correlated with its ability to induce the degradation of Gps2 (Fig. 6), there was no such result with 6E6, probably because 6E6 is less efficient at inducing degradation because of its low level of expression (Fig. 6B).
FIG. 8.
Transactivation by Gps2 and the effect of E6. Luciferase reporters driven by the indicated promoters were transfected into NIH 3T3 cells with or without Gps2 or HPV E6s. The amount of DNA for the luciferase reporter, Gps2, and E6 in the transfections was 100, 500, and 1,500 ng, respectively. The vectors for Gps2 and HPV E6 constructs were also added to keep the total amount of DNA in each transfection constant. RL-TK DNA was added to each transfection as a control for transfection efficiency. Cell lysates were collected at 36 h after transfection, and the firefly and Renilla luciferase activities were quantitated as described in Materials and Methods. The ratio of firefly luciferase to Renilla luciferase in cells transfected with only these constructs was set at 1. For each of the other transfections, the ratio of the firefly luciferase to Renilla luciferase was normalized to the ratio described above and plotted as relative luciferase units. Each point shown is the average of two separate experiments.
TABLE 1.
Effect of E6 proteins on Gps2-induced gene activation
| Promoter element | Fold enhancement | ||
|---|---|---|---|
| Gps2 alonea | Gps2 + 6E6b | Gps2 + 18E6b | |
| SRE | 8.59 | 7.52 | 2.53 |
| 2×-TRE | 8.92 | 12.56 | 2.24 |
DISCUSSION
Small DNA tumor viruses have adopted different strategies to promote cellular proliferation. One strategy involves the interaction of viral proteins with cell proteins to interfere with the normal cellular processes. HPVs seem to have evolved a similar strategy. The HPV E6 protein has several cellular protein partners (reviewed in references 10 and 40). However, most of the known interactions are limited to high-risk HPV E6 proteins, and little is known about the protein partners for low-risk HPV E6s. Yet, even for high-risk HPV E6s, the observed protein interactions cannot fully explain their transforming activities. Low-risk HPVs also cause proliferative changes in cells, presumably through the action of their E6 and E7 proteins. Identification of novel protein partners for low-risk HPV E6 may increase our understanding of the mechanism of the cellular changes caused by these viruses. These partners also have the potential to provide drug targets for the prevention and treatment of genital warts. We used the two-hybrid library screening approach to identify a novel protein partner that interacts with both high- and low-risk E6s as well as with cutaneous-type HPV E6 proteins. To our knowledge, this is the first such cellular protein with this property.
Gps2 is a 37-kDa protein with no homology to other known proteins (9, 34). It is ubiquitously expressed in different tissues and cell types (34), which is consistent with its ability to interact with both mucosal and cutaneous HPV E6 proteins. The subcellular localization of Gps2 was reported to be nuclear, although it was excluded from the nucleoli (9). However, we also observed punctate staining of Gps2 in the cytoplasm and perinuclear region, although this signal was not as strong as the nuclear one (data not shown). HPV E6 proteins locate in the nuclear and membranous compartments (1, 23). Thus, it would be possible for Gps2 and HPV E6 proteins to colocalize. The N terminus of Gps2 has the potential to form a coiled-coil structure that could be involved in protein interactions (9, 34). This might explain why both Gps2 clones found in the two-hybrid screen encoded the entire ORF as well as a poly(A) tail.
Through deletion analysis of 6E6, we identified two regions that were necessary for efficient binding of Gps2. One encompassed the first 36 amino acids, while the other contained amino acids 95 to 131 (Fig. 4 and 5). Interestingly, these two regions have significant homology with p300, another Gps2-binding protein (24), and are highly conserved between mucosal and cutaneous HPV types. Recently, Gps2 was shown to bind p300 and facilitate its recruitment into complexes with BPV 1E2 (53). Amino acids 1 to 595 in p300 are necessary and sufficient for Gps2 binding. The regions of p300 that interact with Gps2 contain amino acid sequences that are homologous to the regions of E6 (24) that we have identified as being necessary for binding to Gps2. This sequence conservation raised the possibility that Gps2 binds to both E6 and p300 at these two homology regions.
E6-induced protein degradation has been reported for p53, E6TP1, hDLG, and Bak (18, 20, 54, 60, 69). Degradation of p53, hDLG, and E6TP1 occurs both in vivo and in vitro (18, 20, 60). However, degradation of Gps2 in the presence of E6 was only observed in vivo (Fig. 6 and 7). This is consistent with what was seen when HPV E6 proteins bind Bak, a proapoptotic Bcl-2 family protein (69, 70). Like Gps2, Bak also interacts with and is degraded by both high-risk and low-risk E6 proteins, and the E6-induced degradation of Bak was observed in vivo but not in vitro. Degradation of p53 by E6 is mediated by E6-AP, an E3-like ubiquitin ligase (30, 58, 59). Degradation of Bak seems to be mediated through E6-AP and is normally regulated by E6-AP in the absence of HPV E6 (70). However, it is not clear if E6-AP plays a role in the degradation of Gps2. The fact that we did not observe degradation in vitro suggests a degradation pathway that is different from that used for p53. Pim et al. (54) demonstrated that high-risk E6 mutants that could not interact with E6-AP still retained the ability to induce the degradation of hDLG and that low-risk E6 could also effect degradation if it was rendered able to bind hDLG. This result also suggests that there are alternatives to the E6-AP ubiquitin pathway. On the other hand, in vitro degradation assays do not always reflect what is happening in vivo. There are clear examples of mutants of both p53 and E6 that are defective in in vitro degradation assays but active in vivo (13, 16, 19).
The HTLV Tax protein also interacts with and degrades Gps2 in vivo (34). It would be interesting to determine if Tax- and 18E6-induced degradation of Gps2 uses the same pathway. The observation that two viral transforming proteins have evolved to degrade the same host cell protein suggests a role for Gps2 in protecting cells from transformation.
The normal function of Gps2 is still unclear. We tested the effect of Gps2 on transactivation because Gps2 enhances the transcriptional activation function of both BPV 1E2 and c-Jun (9). The ability to complex with the general transcriptional coactivator p300 (53) and its nuclear localization suggest a role for Gps2 in transcriptional regulation. We discovered that overexpression of Gps2 alone was sufficient to activate transcription from several promoters (Fig. 8). There are several different mechanisms by which Gps2 could enhance transcription. Peng et al. (53) have demonstrated that Gps2 can bring p300 and its histone acetylase activity to the promoter. This might result in chromatin remodeling.
18E6 suppressed transactivation by Gps2 (Table 1). This suppression probably reflects the E6-induced degradation of Gps2, as the degree of suppression by 18E6 correlated with its ability to induce degradation (Table 1 and Fig. 6). However, at this time we cannot explain the apparent contradictory result that we obtained with 6E6. 6E6 can induce degradation of Gps2, yet in cotransfection experiments it fails to suppress Gps2-mediated transactivation (Table 1). One possibility is that the level of degradation induced by 6E6 was insufficient for us to observe an effect on Gps2 transcriptional activation. Alternatively, transactivation by Gps2 and degradation of Gps2 are two different processes. In measuring degradation of Gps2, we are detecting the protein level of Gps2. However, when measuring transactivation, we are determining the level of expression of a reporter, which is influenced by both the protein level of Gps2 and the effect of the contransfected E6 protein. E6 is known to have nonspecific effects on transcription. Because 18E6-HA was expressed at a much higher level than 6E6-HA (Fig. 6B) and because degradation of Gps2 by 18E6 is more efficient than by 6E6 (probably related to higher levels of 18E6), this dual effect might result in more pronounced suppression of Gps2 transactivation by 18E6 than by 6E6.
How might binding of Gps2 contribute to the growth-promoting effect of HPVs? The p300 binding and general transcription activation properties of Gps2 suggest a positive role for it in p300-mediated transcriptional regulation, the end result of which is the suppression of cell proliferation. HPV E6 proteins might attenuate the functions of p300 by direct interaction (51) or by suppressing the function of Gps2. The interaction between Gps2 and HPV E6 offers a novel target for designing drugs to inhibit both cutaneous and mucosal HPVs.
REFERENCES
- 1.Androphy E J, Hubbert N L, Schiller J T, Lowy D R. Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J. 1987;6:989–992. doi: 10.1002/j.1460-2075.1987.tb04849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacharach E, Goff S P. Binding of the human immunodeficiency virus type 1 Gag protein to the viral RNA encapsidation signal in the yeast three-hybrid system. J Virol. 1998;72:6944–6949. doi: 10.1128/jvi.72.8.6944-6949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Band V, Dalal S, Delmolino L, Androphy E J. Enhanced degradation of p53 protein in HPV-6 and BPV-1 E6-immortalized human mammary epithelial cells. EMBO J. 1993;12:1847–1852. doi: 10.1002/j.1460-2075.1993.tb05833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbosa M S, Vass W C, Lowy D R, Schiller J T. In vitro biological activities of the E6 and E7 genes vary among human papillomaviruses of different oncogenic potential. J Virol. 1991;65:292–298. doi: 10.1128/jvi.65.1.292-298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann A M, Daling J R, Sherman K J, Maden C, Miller B A, Coates R J, Kiviat N B, Myerson D, Weiss N S, Hislop T G, et al. Human papillomavirus infection and anal cancer. Int J Cancer. 1989;43:1042–1049. doi: 10.1002/ijc.2910430615. [DOI] [PubMed] [Google Scholar]
- 6.Bosch F X, Manos M M, Munoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 7.Boyer J L, Ketner G. Genetic analysis of a potential zinc-binding domain of the adenovirus E4 34k protein. J Biol Chem. 2000;275:14969–14978. doi: 10.1074/jbc.M000566200. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 9.Breiding D E, Sverdrup F, Grossel M J, Moscufo N, Boonchai W, Androphy E J. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Mol Cell Biol. 1997;17:7208–7219. doi: 10.1128/mcb.17.12.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campo M S. HPV and cancer: the story unfolds. Trends Microbiol. 1998;6:424–426. doi: 10.1016/s0966-842x(98)01385-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen J J, Reid C E, Band V, Androphy E J. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- 12.Collins L R, Minden A, Karin M, Brown J H. Galpha12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J Biol Chem. 1996;271:17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- 13.Crook T, Ludwig R L, Marston N J, Willkomm D, Vousden K H. Sensitivity of p53 lysine mutants to ubiquitin-directed degradation targeted by human papillomavirus E6. Virology. 1996;217:285–292. [Google Scholar]
- 14.Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckner R. p300 and CBP as transcriptional regulators and targets of oncogenic events. Biol Chem. 1996;377:685–688. [PubMed] [Google Scholar]
- 16.Foster S A, Demers G W, Etscheid B G, Galloway D A. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J Virol. 1994;68:5698–5705. doi: 10.1128/jvi.68.9.5698-5705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Kumar A, Srinivasan S, Singh L, Mukai H, Ono Y, Wazer D E, Band V. PKN binds and phosphorylates human papillomavirus E6 oncoprotein. J Biol Chem. 2000;275:14824–14830. doi: 10.1074/jbc.275.20.14824. [DOI] [PubMed] [Google Scholar]
- 18.Gao Q, Srinivasan S, Boyer S N, Wazer D E, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–744. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardiol D, Banks L. Comparison of human papillomavirus type 18 (HPV-18) E6-mediated degradation of p53 in vitro and in vivo reveals significant differences based on p53 structure and cell type but little difference with respect to mutants of HPV-18 E6. J Gen Virol. 1998;79:1963–1970. doi: 10.1099/0022-1317-79-8-1963. [DOI] [PubMed] [Google Scholar]
- 20.Gardiol D, Kuhne C, Glaunsinger B, Lee S S, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18:5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- 21.Giles R H, Peters D J, Breuning M H. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 1998;14:178–183. doi: 10.1016/s0168-9525(98)01438-3. [DOI] [PubMed] [Google Scholar]
- 22.Gissmann L, deVilliers E M, zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982;29:143–146. doi: 10.1002/ijc.2910290205. [DOI] [PubMed] [Google Scholar]
- 23.Grossman S R, Mora R, Laimins L A. Intracellular localization and DNA-binding properties of human papillomavirus type 18 E6 protein expressed with a baculovirus vector. J Virol. 1989;63:366–374. doi: 10.1128/jvi.63.1.366-374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossman S R, Perez M, Kung A L, Joseph M, Mansur C, Xiao Z X, Kumar S, Howley P M, Livingston D M. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell. 1998;2:405–415. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 25.Halbert C L, Demers G W, Galloway D A. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawley-Nelson P, Vousden K H, Hubbert N L, Lowy D R, Schiller J T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson J B, Bedell M A, McCance D J, Laimins L A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64:519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huibregtse J M, Scheffner M, Howley P M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huibregtse J M, Scheffner M, Howley P M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huibregtse J M, Scheffner M, Howley P M. E6-AP directs the HPV E6-dependent inactivation of p53 and is representative of a family of structurally and functionally related proteins. Cold Spring Harb Symp Quant Biol. 1994;59:237–245. doi: 10.1101/sqb.1994.059.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Huibregtse J M, Scheffner M, Howley P M. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janknecht R, Hunter T. Versatile molecular glue: transcriptional control. Curr Biol. 1996;6:951–954. doi: 10.1016/s0960-9822(02)00636-x. [DOI] [PubMed] [Google Scholar]
- 33.Jayachandra S, Low K G, Thlick A E, Yu J, Ling P D, Chang Y, Moore P S. Three unrelated viral transforming proteins (vIRF, EBNA2, and E1A) induce the myc oncogene through the interferon-responsive PRF element by using different transcription coadaptors. Proc Natl Acad Sci USA. 1999;96:11566–11571. doi: 10.1073/pnas.96.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Jin D Y, Teramoto H, Giam C Z, Chun R F, Gutkind J S, Jeang K T. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem. 1997;272:25816–25823. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- 35.Kasof G M, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;19:4390–4404. doi: 10.1128/mcb.19.6.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur P, McDougall J K. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988;62:1917–1924. doi: 10.1128/jvi.62.6.1917-1924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keen N, Elston R, Crawford L. Interaction of the E6 protein of human papillomavirus with cellular proteins. Oncogene. 1994;9:1493–1499. [PubMed] [Google Scholar]
- 38.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiyono T, Hiraiwa A, Ishii S, Takahashi T, Ishibashi M. Inhibition of p53-mediated transactivation by E6 of type 1, but not type 5, 8, or 47, human papillomavirus of cutaneous origin. J Virol. 1994;68:4656–4661. doi: 10.1128/jvi.68.7.4656-4661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubbutat M H, Vousden K H. New HPV E6 binding proteins: dangerous liaisons? Trends Microbiol. 1998;6:173–175. doi: 10.1016/s0966-842x(98)01267-0. [DOI] [PubMed] [Google Scholar]
- 41.Kuhne C, Banks L. E3-ubiquitin ligase/E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G box. J Biol Chem. 1998;273:34302–34309. doi: 10.1074/jbc.273.51.34302. [DOI] [PubMed] [Google Scholar]
- 42.Kukimoto I, Aihara S, Yoshiike K, Kanda T. Human papillomavirus oncoprotein E6 binds to the C-terminal region of human minichromosome maintenance 7 protein. Biochem Biophys Res Commun. 1998;249:258–262. doi: 10.1006/bbrc.1998.9066. [DOI] [PubMed] [Google Scholar]
- 43.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Labrecque S, Gauzzi M C, Cuddihy A R, Wong A H, Pellegrini S, Matlashewski G J, Koromilas A E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18:5727–5737. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- 45.Maki C G, Huibregtse J M, Howley P M. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- 46.Matlashewski G, Schneider J, Banks L, Jones N, Murray A, Crawford L. Human papillomavirus type 16 DNA cooperates with activated Ras in transforming primary cells. EMBO J. 1987;6:1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munger K, Phelps W C, Bubb V, Howley P M, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munger K, Werness B A, Dyson N, Phelps W C, Harlow E, Howley P M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa S, Watanabe S, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Mutational analysis of human papillomavirus type 16 E6 protein: transforming function for human cells and degradation of p53 in vitro. Virology. 1995;212:535–542. doi: 10.1006/viro.1995.1511. [DOI] [PubMed] [Google Scholar]
- 50.Panagiotidis C, Silverstein S. pAlex, a dual-tag prokaryotic expression vector for the purification of full-length proteins. Gene. 1995;164:45–47. doi: 10.1016/0378-1119(95)00417-5. [DOI] [PubMed] [Google Scholar]
- 51.Patel D, Huang S M, Baglia L A, McCance D J. The E6 protein of human papillomavirus type 16 binds to and inhibits coactivation by CBP and p300. EMBO J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pecoraro G, Morgan D, Defendi V. Differential effects of human papillomavirus type 6, 16, and 18 DNAs on immortalization and transformation of human cervical epithelial cells. Proc Natl Acad Sci USA. 1989;86:563–567. doi: 10.1073/pnas.86.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng Y C, Breiding D E, Sverdrup F, Richard J, Androphy E J. Amf-1/Gps2 binds p300 and enhances its interaction with papillomavirus E2 proteins. J Virol. 2000;74:5872–5879. doi: 10.1128/jvi.74.13.5872-5879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pim D, Thomas M, Javier R, Gardiol D, Banks L. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene. 2000;19:719–725. doi: 10.1038/sj.onc.1203374. [DOI] [PubMed] [Google Scholar]
- 55.Pirisi L, Yasumoto S, Feller M, Doniger J, DiPaolo J A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987;61:1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Relaix F, Wei X J, Wu X, Sassoon D A. Peg3/Pw1 is an imprinted gene involved in the TNF-NFκB signal transduction pathway. Nat Genet. 1998;18:287–291. doi: 10.1038/ng0398-287. [DOI] [PubMed] [Google Scholar]
- 57.Ronco L V, Karpova A Y, Vidal M, Howley P M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheffner M. Ubiquitin, E6-AP, and their role in p53 inactivation. Pharmacol Ther. 1998;78:129–139. doi: 10.1016/s0163-7258(98)00003-5. [DOI] [PubMed] [Google Scholar]
- 59.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 60.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 61.Schiller J T, Vass W C, Lowy D R. Identification of a second transforming region in bovine papillomavirus DNA. Proc Natl Acad Sci USA. 1984;81:7880–7884. doi: 10.1073/pnas.81.24.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlegel R, Phelps W C, Zhang Y L, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988;7:3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sedman S A, Hubbert N L, Vass W C, Lowy D R, Schiller J T. Mutant p53 can substitute for human papillomavirus type 16 E6 in immortalization of human keratinocytes but does not have E6-associated trans-activation or transforming activity. J Virol. 1992;66:4201–4208. doi: 10.1128/jvi.66.7.4201-4208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snowden A W, Perkins N D. Cell cycle regulation of the transcriptional coactivators p300 and CREB binding protein. Biochem Pharmacol. 1998;55:1947–1954. doi: 10.1016/s0006-2952(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 65.Soh J W, Lee E H, Prywes R, Weinstein I B. Novel roles of specific isoforms of protein kinase C in activation of the c-Fos serum response element. Mol Cell Biol. 1999;19:1313–1324. doi: 10.1128/mcb.19.2.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spain B H, Bowdish K S, Pacal A R, Staub S F, Koo D, Chang C Y, Xie W, Colicelli J. Two human cDNAs, including a homolog of Arabidopsis FUS6 (COP11), suppress G-protein- and mitogen-activated protein kinase-mediated signal transduction in yeast and mammalian cells. Mol Cell Biol. 1996;16:6698–6706. doi: 10.1128/mcb.16.12.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steger G, Pfister H. In vitro expressed HPV 8 E6 protein does not bind p53. Arch Virol. 1992;125:355–360. doi: 10.1007/BF01309654. [DOI] [PubMed] [Google Scholar]
- 68.Storey A, Osborn K, Crawford L. Co-transformation by human papillomavirus types 6 and 11. J Gen Virol. 1990;71:165–171. doi: 10.1099/0022-1317-71-1-165. [DOI] [PubMed] [Google Scholar]
- 69.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999;80:1513–1517. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 70.Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17:2943–2954. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- 71.Tong X, Howley P M. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc Natl Acad Sci USA. 1997;94:4412–4417. doi: 10.1073/pnas.94.9.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turek L P, Smith E M. The genetic program of genital human papillomaviruses in infection and cancer. Obstet Gynecol Clin N Am. 1996;23:735–758. doi: 10.1016/s0889-8545(05)70275-8. [DOI] [PubMed] [Google Scholar]
- 73.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 74.Vousden K H. Human papillomavirus oncoproteins. Semin Cancer Biol. 1990;1:415–424. [PubMed] [Google Scholar]
- 75.Werness B A, Levine A J, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 76.Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woodworth C D, Doniger J, DiPaolo J A. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol. 1989;63:159–164. doi: 10.1128/jvi.63.1.159-164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yasumoto S, Burkhardt A L, Doniger J, DiPaolo J A. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986;57:572–577. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmermann H, Degenkolbe R, Bernard H U, O'Connor M J. The Human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- 81.zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]