Stromelysin-1 Regulates Adipogenesis during Mammary Gland Involution (original) (raw)
Abstract
The matrix metalloproteinase MMP-3/stromelysin-1 (Str1) is highly expressed during mammary gland involution induced by weaning. During involution, programmed cell death of the secretory epithelium takes place concomitant with the repopulation of the mammary fat pad with adipocytes. In this study, we have used a genetic approach to determine the role of Str1 during mammary involution. Although Str1 has been shown to induce unscheduled apoptosis when expressed ectopically during late pregnancy (Alexander, C.M., E.W. Howard, M.J. Bissell, and Z. Werb. 1996. J. Cell Biol. 135:1669–1677), we found that during post-lactational involution, mammary glands from transgenic mice that overexpress the tissue inhibitor of metalloproteinases, TIMP-1 (TO), or mice carrying a targeted mutation in Str1 showed accelerated differentiation and hypertrophy of adipocytes, while epithelial apoptosis was unaffected. These data suggest that matrix metalloproteinases (MMPs) do not induce unscheduled epithelial cell death after weaning, but instead alter the stromal microenvironment. We used adipogenic 3T3-L1 cells as a cell culture model to test the function of MMPs during adipocyte differentiation. Fibroblastic 3T3-L1 progenitor cells expressed very low levels of MMPs or TIMPs. The transcription of a number of MMP and TIMP mRNAs [Str1, MT1-MMP, (MMP-14) collagenase-3 (MMP-13), gelatinase A (MMP-2), and TIMP-1, -2 and -3] was induced in committed preadipocytes, but only differentiated adipocytes expressed an activated MMP, gelatinase A. The addition of MMP inhibitors (GM 6001 and TIMP-1) dramatically accelerated the accumulation of lipid during differentiation. We conclude that MMPs, especially Str1, determine the rate of adipocyte differentiation during involutive mammary gland remodeling.
Keywords: transgenic mouse, mammary involution, MMP-3, TIMP-1, 3T3-L1 adipocytes
Introduction
When pups are removed from a lactating mouse, the mammary glands involute, losing the expression of the differentiated gene products associated with milk production and resulting in the loss of at least 90% of the lactating mammary epithelial cell number. Using this model of induced apoptosis in adult mice, we can determine physiologically relevant factors that control epithelial cell death. Mammary gland involution is a two-phase process. The first phase is characterized by the onset of epithelial apoptosis, which is p53 dependent (Jerry et al. 1998), and the second phase by upregulation of proteinases, repopulation of the mammary stroma by adipocytes, and completion of epithelial programmed cell death in a p53-independent manner (Li et al. 1996; Lund et al. 1996).
In cultured mammary epithelial cells, epithelial apoptosis is induced by expression of matrix metalloproteinases (MMPs), by the inhibition of integrin-mediated adhesion, or by altering cell cycle progression (Boudreau et al. 1995, Boudreau et al. 1996; Wang et al. 1994; Wiesen and Werb 2000). When stromelysin-1 (Str1) is misexpressed in mammary gland by transgenic means, mice show precocious growth of virgin gland to a mid-pregnant equivalent (Sympson et al. 1994), stromal alteration, and unscheduled apoptosis during pregnancy (Boudreau et al. 1995; Alexander et al. 1996; Thomasset et al. 1998) and tumor formation (Sternlicht et al. 1999). Therefore, we hypothesize that the response of mammary epithelial cells to ectopic Str1 reflects normal roles for this enzyme.
The prototype MMP, collagenase, was isolated as a collagenolytic activity specifically induced during involution of the tadpole tail (Gross 1966). Since the description of this activity, other MMPs have been shown to be induced during physiological remodeling reactions and to cleave structural proteins important to maintaining basement membrane integrity (Sternlicht and Werb 1999; Sternlicht et al. 1999; Vu and Werb 2000). One such MMP, Str1, is expressed and regulated during mammary gland development and involution (Witty et al. 1995). Str1 cleaves many basement membrane proteins (Mayer et al. 1993; Werb 1997), and can autoactivate pro-Str1 and other pro-MMPs to initiate a proteolytic cascade (Nagase 1997). It is synthesized and secreted by a subpopulation of stromal cells in mammary gland (Witty et al. 1995). Targets for proteinase activity are not limited to extracellular matrix (ECM) molecules. Cell surface molecules such as E-cadherin can be cleaved in cultured mammary epithelial cells transfected with an inducible Str1 cDNA (Lochter et al. 1997; Werb 1997; Sternlicht and Werb 2000). Other substrates include extracellular growth factors and cell-surface molecules (Werb 1997).
To test the importance of MMPs in involution in the present study, we have used two genetic approaches. We have investigated mammary gland involution in mice overexpressing the tissue inhibitor of matrix metalloproteinases, human TIMP-1 (TO) (Alexander et al. 1996), and in mice carrying a null mutation in Str1 (Str1_−/−)_ (Mudgett et al. 1998). Both of these transgenic mice have no overt defects and are able to complete pregnancy and lactate. Analysis of mammary glands from TO mice shows that the transgene is expressed and active (Alexander et al. 1996). By combining the information from these two strains, we can identify processes that require Str1 activity (if both TO and Str1_−/_− transgenic mice share the same phenotype) and those that require TIMP-1–inhibitable MMP activity (if TO but not Str1_−/_− mice show a specific phenotype). We show here that a Str1 deficiency specifically accelerates the differentiation of adipocytes during active remodeling, and that epithelial cell death is unaffected in either transgenic strain.
Materials and Methods
Materials
Ultraspec RNA isolation solution was from Biotecx. Enhanced chemiluminescence reagents were from Amersham Pharmacia Biotech. Immobilon P was from Millipore. Sources of antibodies were as follows: rabbit polyclonal anti–laminin antibody was from Collaborative Research (No. 40023); rat monoclonal anti–entactin (nidogen-1) antibody was from Upstate Biotechnology Inc. (No. 05-208); HRP-conjugated anti–rabbit (NA 9340) or anti–rat IgC (NA 9320) were from Amersham Pharmacia Biotech. Duralon-UV membrane was from Stratagene. The source of mouse cDNA probes was as follows: stromelysin-1 (Ostrowski et al. 1988), MT-1 MMP (Sato et al. 1994), pPARγ (Tontonoz et al. 1995), TIMP-1 (Gewert et al. 1987), TIMP-2 (a 360-bp coding sequence made by reverse transcription–PCR from published sequence using oligonucleotide sequences GGTCTCGCTGGACATTGGAGGAAAG and GGGTCCTCGATGTCGAGAAACTCCTG), TIMP-3 (Leco et al. 1994), TIMP-4 (Leco et al. 1997), vimentin (Capetanaki et al. 1990), and cytokeratin 18 (Kulesh and Oshima 1988). 3T3-L1 cells were from the American Type Culture Collection. GM6001 [3-(_N_-hydroxycarbamoyl-2(R)–isobutyl propionyl-l-tryptophan methylamide] was a gift of Dr. Richard Galardy (Glycomed Inc., Alameda, CA). Recombinant human TIMP-1 (rhTIMP-1) was a gift of Synergen Corp.; human TIMP-1 (hTIMP-1) purified from transfected BHK cells was a gift of Dr. Joni Mott (Lawrence Berkeley National Laboratory, Berkeley, CA).
Transgenic Mice
The derivation and characteristics of the transgenic mice expressing a human TIMP-1 transgene (TO mice) under the control of the β-actin promoter have been described (Alexander et al. 1996). In brief, these mice have no gross phenotype and express ∼50 ng/ml circulating human TIMP-1 and ∼20 ng/ml of tissue lysate from pregnant mammary gland. Mice carrying this transgene were compared with nontransgenic sibs or CD-1 control mice (Charles River Laboratories). Mice carrying a targeted null mutation in the Str1 gene (_Strl_−/−) were made by homologous recombination using the AB2.1 ES cell line from 129 mice (Mudgett et al. 1998), and were compared with a control line (Str1+/+) on the 129 background. These mice were also grossly normal. To standardize lactation from mouse to mouse, litters were adjusted to six pups per dam, and mothers were housed individually before weaning. For time points during pregnancy, 0.5 days post coitum (dpc) is assumed to be noon of the day of observation of the vaginal plug. Lactation is timed from the time of parturition.
Histology and Immunochemistry
For histologic evaluation, pieces of mammary gland were fixed in 4% paraformaldehyde overnight at 4°C, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E) according to standard techniques. For immunostaining of specific ECM molecules, 5 – 10 mm pieces of fresh mammary gland were infiltrated with 20% sucrose in 1 mM EDTA, 50 mM Tris pH 7.5 (4°C) and frozen in OCT for cryosectioning. Sections (6–10 μm) were cut and immediately fixed in 4% freshly prepared paraformaldehyde for 15 min, blocked in 0.1 M glycine (3 × 1 min in PBS), and then 10% sheep serum in PBS for 30 min at room temperature. Anti–entactin antibody (diluted 1:1,000 in 3% bovine serum albumin in Tris-buffered saline) was incubated on sections overnight at 4°C, washed three times in PBS/0.1% Tween for 5 min, and incubated with secondary antibody (HRP-conjugated anti–rat IgG, diluted 1:100 into 20% Carnation skim milk powder in PBS) for 60 min. The wash protocol was repeated and the sections were counterstained lightly with methylene blue.
Gel Electrophoresis of Mammary Gland Protein Lysates
Pieces of mammary gland tissue were homogenized in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0) at 0.25 mg wet weight/ml. Lysates were spun for 15 min at 4°C in a microcentrifuge, and insoluble fractions (ECM-enriched fractions) were washed once in RIPA buffer and boiled into sample buffer containing 5% SDS as described previously (Alexander et al. 1996). Extracts equivalent to 8 mg wet weight tissue were separated on 6% SDS-PAGE gels, and either stained with Coomassie blue or silver stain, or transferred to Immobilon P for immunoblotting of specific ECM constituents.
Assay of Apoptotic DNA Laddering
Fragments of mammary tissue (∼100 mg) were digested in lysis buffer (0.2 mg/ml proteinase K in 50 mM Tris-HCl, 0.1 M NaCl, 0.1 M EDTA, 1% SDS, pH 8.0) overnight at 60°C. The crude DNA preparations were extracted twice with phenol/chloroform and precipitated with propanol for 3 min at room temperature. After one rinse with 70% ethanol, DNA pellets were redissolved in TE with RNase A at 60°C for 20 min and digested for 30 min at 37°C. 10 μg of DNA was analyzed by electrophoresis on 2% agarose gels as described previously (Alexander et al. 1996).
Northern Blotting
Total RNA was isolated by homogenization (using an Omni 2000 Polytron) of pieces (100–200 mg) of mammary tissue or from 3T3-L1 cultures in Ultraspec solution according to the manufacturer's instructions. 10 μg of RNA was separated by standard formaldehyde gel electrophoresis, and transferred to Duralon membrane for hybridization with specific probes.
Culture and Differentiation of 3T3-L1 Cells
3T3-L1 fibroblasts (obtained from American Type Culture Collection) were routinely grown at subconfluence in 10% FCS/DME-H21. To initiate differentiation, cells were grown to confluence in 10-cm diameter dishes or 6- or 96-well tissue culture plates. At day 0, a differentiation-inducing mix was added (DM; 0.22 mM insulin, 0.6 μM dexamethasone, and 0.5 mM methylisobutylxanthine in culture medium) (Bernlohr et al. 1984) with or without MMP inhibitors (10 μM GM 6001 or 250 nM rhTIMP-1 or hTIMP-1 isolated for cell cultures). After 2 d, medium was replaced with fresh culture medium with or without inhibitors. To evaluate RNA expression in differentiating cultures, actively proliferating cultures (preadipocytes; pre), confluent cultures before DM administration (committed; com), and differentiating adipocytes after treatment with DM were scraped into Ultraspec solution, and RNA was purified according to the manufacturer's instructions. Cultures were evaluated for lipid accumulation at 4 d using an Oil Red O stain as follows: cells were fixed in 10% formalin in PBS and stained for 2 h with Oil Red O (Ramirez-Zacarias et al. 1992), washing well with water before and after staining. For quantification of lipid accumulation, cells were grown in 96-well plates, the Oil Red O dye was extracted into 100 μl isopropanol, and the absorbance of the solution was read at 510 nm.
Preparation of Nuclear Extracts and Western Blotting for C/EBPβ
Nuclear extracts were performed essentially as described by Finbloom et al_._ (1994). 3T3-L1 cells after various treatments were scraped from the dish and collected by centrifugation at 3,000 g for 1 min. Cell pellets were homogenized in Buffer A (20 mM Hepes, pH 7, 10 mM KCl, 10 mM MgCl2, 20% glycerol, 0.1% NP-40, 0.5 mM DTT, 0.25 mM PMSF). Nuclei were collected by centrifugation at 3,000 g for 5 min, the pellet was resuspended in Buffer A, layered onto a sucrose cushion (35% sucrose, 100 mM Hepes, pH 7, 20 mM MgCl2) and spun at 3,000 g for 15 min. The pellet was resuspended in Buffer A with 0.3 M NaCl and centrifuged at 10,000 g for 5 min. The resulting supernatant (containing the nuclear extract) was analyzed by Western blotting, using a polyclonal rabbit anti–rat C/EBPβ carboxy-terminal peptide antiserum from Santa Cruz Biotechnology, Inc. (SC-150).
Results
Stromelysin-1 Is Highly Upregulated during Mammary Gland Involution
The endogenous expression of Str1 mRNA is regulated during mammary gland development. Str1 expression was low in virgin glands, but it was induced during early pregnancy (3 dpc), continued to increase to 9 dpc, and was not detectable after 12 d of pregnancy or during lactation (Fig. 1 a).
Figure 1.
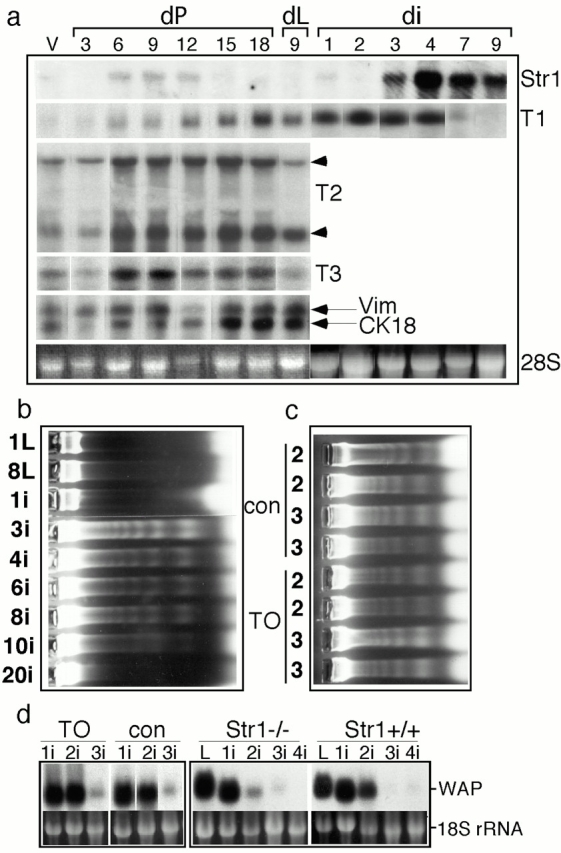
Apoptosis and epithelial function during gland involution in transgenic mice. (a) Total RNA was extracted from mammary glands at various time points, separated by agarose gel electrophoresis (10 μg/track), transferred to membranes, and probed with Str1, TIMP-1, -2 (2 mRNA species), TIMP-3, vimentin (Vim; an internal standard related to mesenchymal cell number), or cytokeratin 18 (CK18; an internal standard related to epithelial cell number) cDNAs. The ethidium bromide–stained 28S rRNA band is also shown for comparison. V, virgin; dP, days pregnant; dL, days lactating; di, days after weaning of pups. _(_b) To measure the onset of programmed cell death, DNA was extracted from glands at various stages and analyzed by agarose gel electrophoresis for evidence of laddering. Epithelial cells began to die between 1 and 3 d after weaning of pups. 1L and 8L, 1 and 8 d lactation; 1i–20i: 1 – 20 d involution after removal of pups after 8 d of lactation. (c) DNA samples from key time points, 2 and 3 d after weaning, are shown for two each of TO and control mice. The onset of apoptosis is normal in TO mice. (d) Differentiated epithelial function was assayed by measuring the expression of mRNA for the milk protein WAP. RNA was extracted from glands at various stages of involution (L, lactating; 1i–4i, 1–4 d of involution after removal of pups after 8 d of lactation) from TO and control mice, and from Str1_−/_− and Str1+/+ mice, and was analyzed by hybridizing Northern blots with a WAP cDNA probe. Ethidium bromide–stained gel tracks before membrane transfer are shown for comparison (18S rRNA). No significant differences were observed in either transgenic line.
Expression of a Str1 transgene during late pregnancy induces unscheduled apoptosis in dividing mammary epithelial cells (Boudreau et al. 1995; Alexander et al. 1996). Interestingly, the expression of endogenous Str1 mRNA is not detectable during late pregnancy, presumably protecting cells from Str1-induced cell death. Str1 mRNA was highly induced during involution, when the secretory epithelium undergoes apoptosis (Talhouk et al. 1992; Li et al. 1994; Lund et al. 1996), to ∼50-fold that typical of pregnant glands.
During mammary development induced by pregnancy, the expression of mRNA for the matrix metalloproteinase inhibitor, TIMP-1, is induced beginning at 6 dpc, continues throughout lactation, and declines sharply on the same day that stromelysin-1 mRNA is maximally induced (Fig. 1 a; Talhouk et al. 1992). Transgenic huTIMP-1 is expressed in parallel with actin mRNA. TIMP-2 and -3 are expressed throughout mammary development, decreasing during lactation (Fig. 1 a).
TIMP-1 Overexpression and Stromelysin-1 Deficiency Do Not Affect Apoptosis or Loss of Mammary Epithelial Function during Mammary Gland Involution
To determine whether Str1 has a role in inducing epithelial cell apoptosis during mammary gland involution after weaning, we measured DNA laddering in tissue extracts from wild-type, TO transgenic, and Str1_−/_− mice. In wild-type mice, ladders of DNA appeared between 1 and 3 d after weaning (Fig. 1b and Fig. c), correlating with the appearance of residual cell bodies in alveolar lumens in histological samples (data not shown; also see Lund et al. 1996). The peak of cell death was at 3 d, and cell death was significant for 8–10 d. After 10 d, resorption of the epithelium (>90% of epithelial cells) was complete. DNA laddering was also evident 2 and 3 d after the removal of pups (Fig. 1 c) from TO mice, indicating that the onset of epithelial involution was normal. To measure the decline of differentiated epithelial cell function that occurs during weaning, we assayed the expression of the mRNA for the milk protein, whey acidic protein (WAP). Surprisingly, both TO and Str1_−/_− mice showed the wild-type pattern of declining expression between days 2 and 3 postweaning (Fig. 1 d). These data indicate that Str1 and other TIMP-1–inhibitable MMPs do not play a significant role in apoptosis of secretory epithelial cells. Why then is the expression of Str1 so dramatically induced during mammary involution?
Mammary Adipogenesis Is Accelerated in Mice Deficient in MMP Function
We observed a striking alteration in the morphology of MMP-deficient glands during involution (Fig. 2). At first glance, these glands appeared to involute more rapidly because the area occupied by epithelial ducts and alveoli decreased. However, from morphological data, we deduced that this was due to accelerated repopulation of the gland with adipocytes. After weaning of wild-type mice allowed to lactate for 8 d, there is a delay of ∼4 d before significant recolonization of the mammary gland with differentiated adipocytes. In TO mice, differentiated hypertrophic adipocytes appeared in greater number than wild type as early as 2 d after weaning (Fig. 2, a and b). By histomorphometry of H&E-stained paraffin sections, mammary glands of TO mice harvested 4 d after weaning contained between 40 and 50% more unilocular adipocytes (Table , and Fig. 2c and Fig. d).
Figure 2.
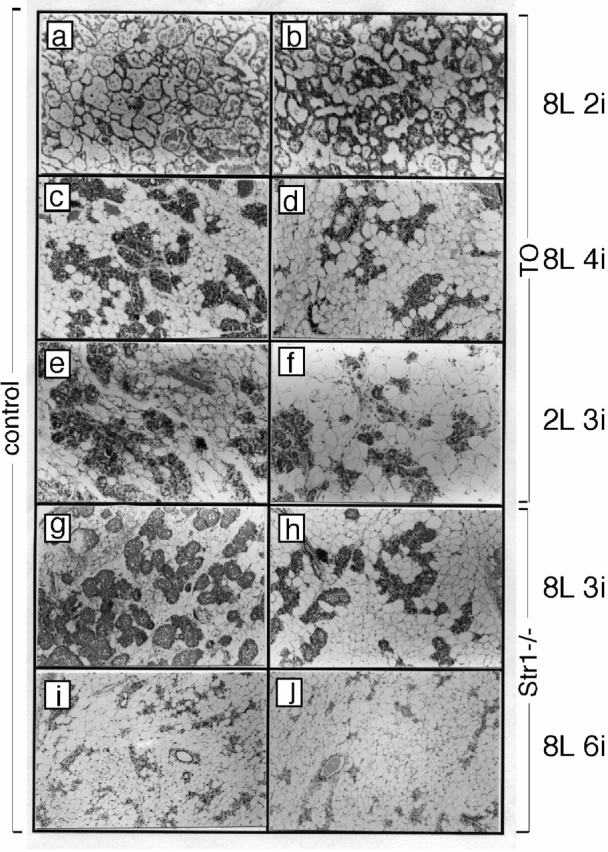
Inhibition of MMP activity affects the dynamics of the remodeling involuting mammary gland. H&E-stained sections of wild type (a, c, and e), TO (b, d, and f), Str+/+ (g and i), and Str1_−/_− (h and j) at mammary glands at 8 d lactation and 2 d involution (e and f), 8 d lactation and 4 d involution (c and d), 2 d lactation and 3 d involution (e and f), 8 d lactation and 3 d involution (g, h), and 8 d lactation and 6 d involution (i and j). Glands deficient in MMP activity show increased numbers of hypertrophic adipocytes during mammary gland involution.
Table 1.
Altered Adipogenesis and PECAM Expression in Involuting Glands of Str1−/− and TO Mice
| Mouse strain | Alveolar density | Adipocytes No./field | Adipocytes area/field | PECAM-1 mRNA/actin mRNA |
|---|---|---|---|---|
| Pixels | % | |||
| Str1+/+ | 9890 ± 604 | 650 ± 39 | 72 | 0.65 ± 0.06 |
| Str1−/− | 6824 ± 550 | 842 ± 55 | 91 | 0.95 ± 0.04 |
| Percent _Str1_−/− / Str1+/+ | 69 ± 8 | 129 ± 7 | 126 ± 2.2 | 150 ± 4 |
| Control | 15980 ± 785 | 310 ± 21 | 56 ± 4 | ND |
| TO | 10120 ± 802 | 420 ± 29 | 79 ± 7 | ND |
| Percent TO/control | 63 ± 8 | 145 ± 7 | 141 ± 8 | ND |
Next we altered the time course of mammary involution to determine whether this effect upon adipocyte differentiation was maintained. When pups are removed after 2 instead of 8 d of lactation, complete alveolar development is prevented, the epithelium regresses more rapidly, and cell death is complete by day 4 (Talhouk et al. 1992). Using this protocol, we found that the process of adipocyte colonization was accelerated in wild-type mice, so that the relative timing of adipocyte expansion and epithelial cell regression is maintained (Fig. 2 e). When TO females were weaned after 2 d of lactation, the differentiation of adipocytes was increased relative to controls (Fig. 2 f).
Histomorphometric analysis of involuting glands from Str1_−/_− mice at 3 d (Fig. 2g and Fig. h) and 6 d (Fig. 2i and Fig. j) after weaning revealed changes of adipocyte colonization that resembled those seen in TO mice. Thus, the number of adipocytes/microscopic field and the area that they occupied increased by 30% 6 d after weaning Str1_−/_− mice, and by 40% 4 d after weaning TO mice (Table ). Note that glands are scored when adipocyte hypertrophy is maximal, and this is different depending upon the mouse strain (TO mice and Str1_−/_− mice are on CD1 and 129 strain backgrounds, respectively). These data indicate that TIMP-1–sensitive MMPs, including Str1, regulate mammary adipogenesis.
Str1 Upregulation Parallels Mammary Gland Adipogenesis, Angiogenesis, and Remodeling of Stromal Matrix during Involution
Why does the absence of Str1 affect the rate of adipocyte differentiation? Previous studies using in situ hybridization have shown that Str1 mRNA is expressed by fibroblastic cells, some of which may be preadipocytes (Lund et al. 1996). Str1 protein is frequently associated with blood vessels (Talhouk et al. 1992). Detailed analysis of the time course of expression of Str1 mRNA, compared with other markers of cell function (such as WAP mRNA expression) showed that Str1 was induced only after the loss of differentiated epithelial cell function, and after the majority of epithelial cell death. Notably, Str1 was induced in parallel with markers usually associated with active remodeling and morphogenesis (Fig. 3).
Figure 3.
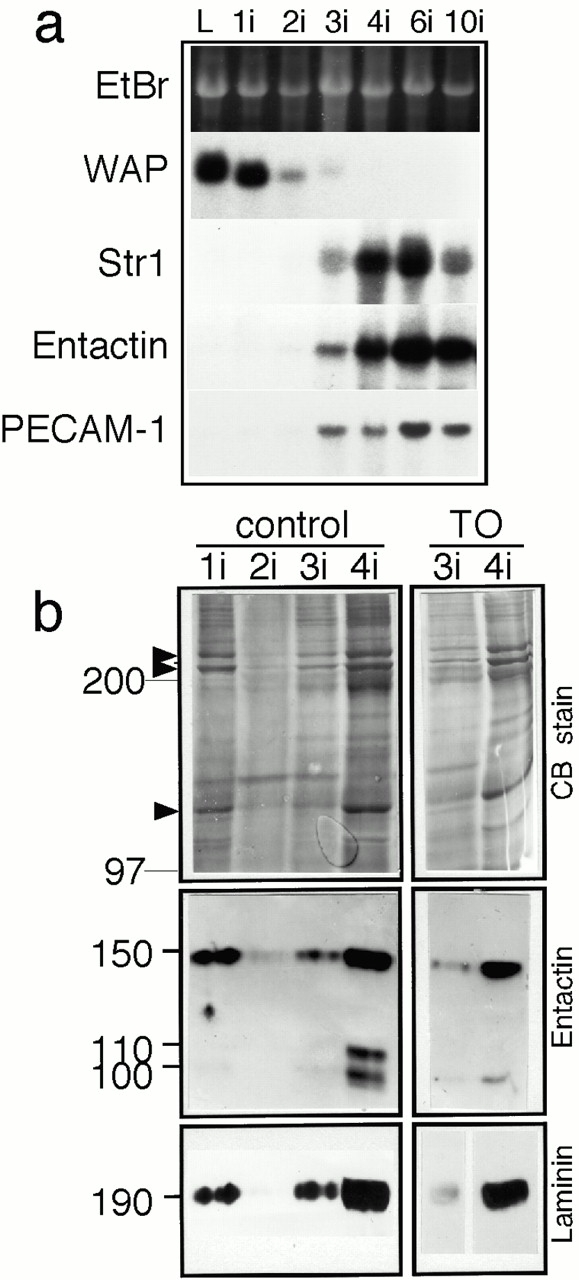
Upregulation of Str1 mRNA expression during stromal remodeling of involuting mammary gland. (a) mRNA was extracted from mammary glands at various time points (L, lactating; 1i–10i, 1–10 d of involution after weaning pups), Northern blots were prepared and probed with cDNAs encoding the milk protein WAP, Str1, the basement membrane protein entactin, and the endothelial cell surface adhesion molecule PECAM-1. (b) ECM-enriched extracts of 8 mg of mammary tissue from control and TO mice 1–4 d after weaning (1i–4i) were analyzed by SDS-PAGE; gels were either Coomassie blue stained (CB) or transferred to membranes for incubation with antisera to either entactin or laminin. Molecular weights of proteins are indicated at the left-hand side. Entactin migrates at 150 kD, with specific MMP-derived cleavage fragments migrating at 100 and 110 kD. The ECM-enriched fraction changes dramatically during the involutive phase of mammary gland remodeling (2–4 d after weaning). The collagens (arrowheads) and basement membrane proteins (entactin and laminin) are substantially reduced.
Repopulation of the mammary gland by differentiated adipocytes requires replacement of the interstitial ECM around the fibroblast-like preadipocytes, which is rich in fibrillar collagens and fibronectin (data not shown), by the basement membranes that surround differentiated adipocytes (Smas and Sul 1995). Concomitantly, the vasculature of the fat pad is remodeled so that there is a dense weave of capillaries in intimate contact with adipocytes (Crandall et al. 1997). mRNA for nidogen-1/entactin, an ECM molecule that is a prominent component of adipocyte basement membranes (see Fig. 4), was induced at 3 d, at the same time as Str1 (Fig. 3, a and b). We observed that the expression of PECAM-1 mRNA, a cell adhesion molecule specific to endothelial cells, was induced at 3 d, and peaked at 6 d after weaning. Interestingly, PECAM-1 mRNA was expressed at a higher level in involuting glands from Str1_−/_− mice (Table ). These data lead us to conclude that the timing of induction of Str1 mRNA is consistent with its expression during angiogenesis and remodeling by the stromal compartment.
Figure 4.
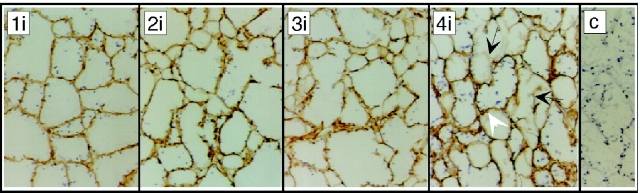
Immunostaining of entactin shows that epithelial basement membranes are retained during involution. Fixed cryosections from normal glands 1–4 d after weaning were stained for entactin. During gland involution (1i–4i), basement membranes around epithelial alveoli stained positively for entactin (brown HRP-linked product; arrowheads). 4 d after weaning (4i), entactin also appears around hypertrophying adipocytes (arrows). Immunostaining experiments using a subsaturating, diluted primary antibody (1:2,000), aimed at ensuring that this assay was quantitative for glands 2–3 d after weaning, did not show the dramatic loss of entactin observed biochemically (Fig. 3). Control sections (incubated with rat antiserum in place of primary antibody) are blank except for methylene blue–stained nuclei (c).
Mammary Gland Involution Is Characterized by a Biosynthetic Phase
We next verified that there was a switch in ECM at this time in involution. The activation of a biosynthetic stromal compartment was reflected in changes in the ECM of involuting glands. We observed dramatic changes in the protein profiles by SDS-PAGE analysis of ECM-enriched extracts in response to weaning. At 1 d after weaning, the mammary gland extracts contained collagens (Fig. 3 b, arrowheads), entactin, and laminin. These protein profiles resembled extracts from pregnant and lactating glands (Alexander et al. 1996; and data not shown). Coincident with the induction of epithelial apoptosis at 2 d after weaning, most basement membrane proteins, including basement membranes entactin and laminin, disappeared from the ECM-enriched fraction (Fig. 3 b). Immunoreactive entactin and laminin began to reappear after 3 d of involution (Fig. 3 b), when their mRNA transcripts were upregulated (Fig. 3 a, and data not shown) and were present in high amounts after 4 d.
To better define the cellular events that lead to these striking changes, we stained sections of involuting gland with antibodies to basement membrane proteins. Surprisingly, 2 and 3 d after weaning, the amount of entactin in basement membranes around epithelial alveoli appeared similar to that during lactation (Fig. 3 and Fig. 4). We conclude that during the initiation phase of apoptosis in involuting gland, ECM proteins normally SDS soluble become insoluble, leaving their antigenicity unaffected and the morphology of the basement membranes (at the light microscopic level) unchanged. We suggest that these biochemical changes are the result of extracellular cross linking by tissue transglutaminase, an enzyme known to be induced during apoptosis of other cell types (see Discussion). The increase of entactin protein measured biochemically by SDS-PAGE analysis of glands 4 d after weaning (Fig. 3) paralleled the increase of entactin observed by immunostaining around hypertrophying adipocytes in sections of similar glands (Fig. 4, Fig. 4i). During the biosynthetic phase (arrows), entactin protein localized not only to the regressing, insoluble epithelial basement membranes, but to the assembling, soluble basement membranes that surround hypertrophying adipocytes.
We then sought evidence to verify that the upregulation of Str1 had functional consequences by examining the integrity of one of its substrates in vivo, entactin/nidogen-1. The coinduction of Str1 and entactin during the biosynthetic phase of involution led to a characteristic pattern of entactin fragmentation (Fig. 3 b, Entactin), the result of Str1 cleavage of the 150-kD entactin molecule between the G1 and G2 domains (Alexander et al. 1996). This proteolysis was almost completely inhibited in parallel samples from TO mice. This result shows that the TIMP-1 transgene was not only highly expressed during involution, but that it was also an effective MMP inhibitor.
MMPs Regulate Adipogenesis in 3T3-L1 Cells in Culture
The results of the genetic experiments in the mice described above suggest that the rate of adipocyte hypertrophy in the mammary gland is enhanced in the absence of Str1. However, adipogenesis could be either a direct or an indirect target of MMPs in vivo. To examine whether there are direct effects of MMPs, we used a model system of adipogenic differentiation, namely cultured 3T3-L1 cells. These fibroblastic cells are not adipogenic in subconfluent cultures. At confluence, the cells become committed preadipocytes. Treatment of confluent cultures with a differentiation-inducing mix (DM; dexamethasone, insulin, and methylisobutylxanthine) induces the expression of proteins associated with mature adipocytes and the accumulation of lipids (Bernlohr et al. 1984).
We first determined the expression of MMPs and TIMPs in 3T3-L1 cells. Str1 expression was developmentally regulated in differentiating 3T3-L1 cells. Str1 mRNA was highly induced in confluent, committed preadipocytes, and expression continued in differentiating cultures (Fig. 5 a). We used the expression of two transcription factors that are expressed by differentiated adipocytes (peroxisome proliferator-activated receptor-γ (pPARγ) mRNA, a nuclear hormone receptor, and C/EBPβ) to monitor the differentiation reaction. Thus, C/EBPβ was highly induced after 2 d of treatment with DM (Fig. 6 a), and pPARγ after 4 d (Fig. 5 a) in parallel with lipid accumulation (Fig. 6b and Fig. c). Since Str1 can activate other MMPs, leading to a cascade of MMP-dependent proteolysis, we determined the expression of other MMPs. mRNA for collagenase-3 (MMP-13) was induced in parallel with Str1. mRNA for the cell-surface bound MT1-MMP (MMP-14), was also induced in committed cells, and increased during differentiation. mRNA for matrilysin (MMP-7) and collagenase (MMP-1) were not detected (data not shown).
Figure 5.
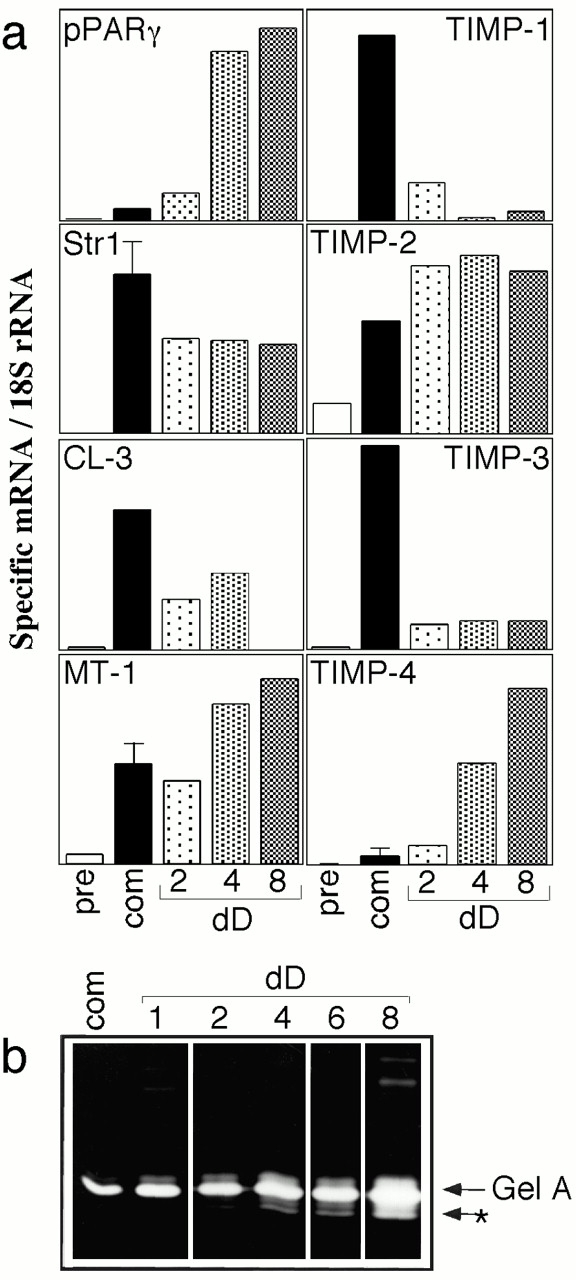
Expression of MMPs and TIMPs during adipocyte differentiation. (a) RNA was extracted from cultures of 3T3-L1 cells at various stages of differentiation. pre, subconfluent fibroblastic precursor; com, confluent, committed stage at day 0 of differentiation; 2–8 dD, 2–8 d differentiation after administration of DM. RNA was separated by agarose gel electrophoresis and transferred to membranes for analysis of expression of specific MMP and inhibitor mRNAs. Autoradiograms were scanned and the relative expression of specific mRNAs is a ratio of EtBr-stained 18S rRNA was quantified. (b) Enzymes secreted by adipocytes into supernatant media were analyzed by gelatin zymography. Gelatinase A, identified by comparison with mouse enzyme standards, was progressively upregulated and activated (*) as 3T3-L1 cells differentiated.
Figure 6.
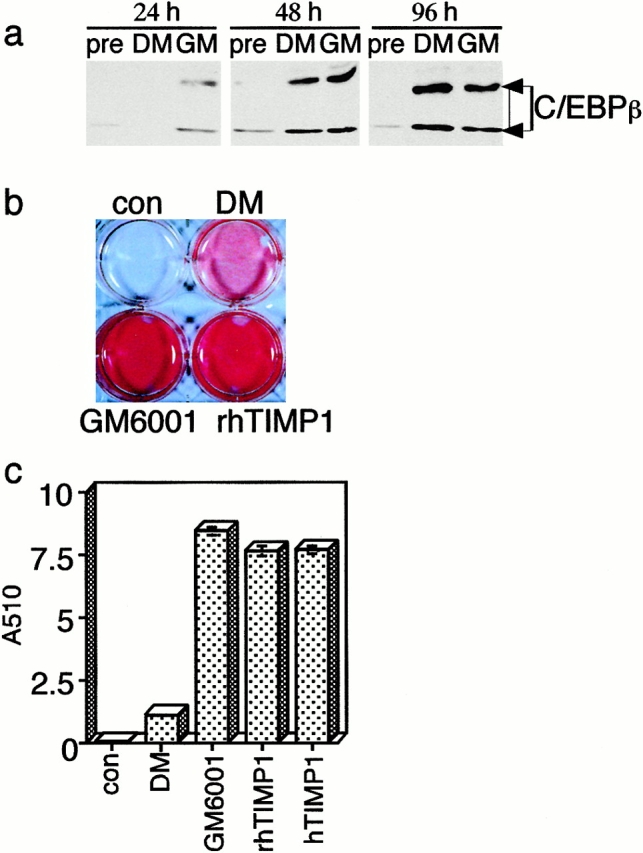
Inhibition of MMP activity accelerates lipogenesis in 3T3-L1 cultures. Confluent cultures of 3T3-L1 cells were induced to differentiate by adding DM, either in the presence or absence of the hydroxamate inhibitor GM6001, recombinant human TIMP-1 (rhTIMP1), or purified human TIMP-1 (hTIMP1). (a) In the presence of GM6001, nuclear C/EBPβ protein appears just 24 h after DM administration, earlier than control cultures. (b) After 4 d, the accumulation of lipid was assessed by Oil red O staining of tissue culture dishes containing 3T3-L1 cells. (c) To quantify Oil red O staining, the cultures were extracted in propanol and the absorbance at 510 nm was measured. Control cultures (con) were untreated with DM. Results shown are the average of six determinations and the bars indicate standard deviations. Cells treated with DM alone were normalized to one to reveal the fold induction of lipogenesis in the presence of MMP inhibitors. P values were determined using the Student's t test.
The expression of the proteolytic activity of MMPs is regulated by TIMPs. All four TIMPs were expressed in adipocytes, as they are in vivo in the mammary fat pat of mice during puberty (Fata et al. 1999). TIMP-1 and -3 were highly induced in committed cells, but showed little expression in differentiated adipocytes. TIMP-4 was expressed specifically by differentiated adipocytes, increasing in parallel with pPARγ. TIMP-2 expression was characteristic of committed and differentiated cells. We conclude that differentiated cells express a higher ratio of mRNAs for MMPs compared with TIMPs than committed cells.
We found that the relative increase in MMP mRNA expression was accompanied by an induction of proteolytic activity specific to the differentiation phase of 3T3-L1 development. Gelatinase A (MMP-2) was the major MMP identified by zymography of enzymes secreted into the media of induced 3T3-L1 cells (Fig. 5 b). MT1-MMP activates gelatinase A in a TIMP-2–dependent fashion (Will et al. 1996; Holmbeck et al. 1999; Caterina et al. 2000; Wang et al. 2000; Zhou et al. 2000). We observed significant activation of gelatinase A after 4 d of differentiation, and further induction and activation after 8 d. Thus, as inhibitor expression declined and MT1-MMP expression increased during adipocyte differentiation, this proteinase was activated.
In vivo, we found that ectopic expression of TIMP-1 expression increased the rate of adipocyte differentiation. If this effect is mediated by a direct effect on adipocytes, we would expect that the addition of an MMP inhibitor would increase the rate of adipogenesis during the differentiation of adipocytes in vitro. To test this hypothesis, we added three different MMP inhibitors [a synthetic hydroxamate inhibitor (GM6001; 10 μM), recombinant human TIMP-1 (250 nM), or natural human TIMP-1 purified from transfected BHK cells (250 nM)] to cultures of committed 3T3-L1 cells concomitant with the differentiation-inducing mix. An accelerated rate of differentiation with increased C/EBPβ expression was evident at day one of differentiation (Fig. 6 a). All three inhibitors stimulated lipogenesis by cells 4 d after induction by more than sevenfold (Fig. 6b and Fig. c). We conclude that Str1 determines the rate of hypertrophy and lipogenesis in differentiating adipocytes and that, in its absence, differentiation is accelerated.
Discussion
Stromelysin-1 Does Not Regulate Mammary Epithelial Cell Death during Involution Caused by Weaning
Basement membranes control the morphogenesis and survival of epithelial cells (Weaver and Bissell 1999) and are continuously remodeled by processes that are poorly understood. For example, the amount of specific basement membrane proteins is cyclically regulated during the division and death of mammary epithelium typical of human breast (Ferguson et al. 1992). By using two strains of transgenic mice with low endogenous MMP activity, we tested whether MMPs induced during involution contribute to the process. Our data indicate that Str1 regulates the phenotypic expression of stromal cells rather than mammary epithelial apoptosis during mammary gland involution. It is evident from earlier studies that overexpression of Str1 induces an altered stromal phenotype, with increases in collagen deposition during pregnancy (Thomasset et al. 1998). However, two lines of evidence from previous work implicated MMPs in mammary epithelial cell death during weaning. First, Elvax pellets containing TIMP-1, implanted at the onset of involution, protect proximal epithelial cells from death (Talhouk et al. 1992). Second, ectopic Str1 induces unscheduled programmed cell death during late pregnancy in transgenic mice and also induces death in vitro in cultured mammary epithelial cells (Sympson et al. 1994; Boudreau et al. 1995). An identical phenotype is induced by inhibitors of β1 integrin, suggesting that Str1 and β1 integrin operate in the same pathway to induce cell death, and that Str1 cleaves a β1 integrin ligand (Boudreau et al. 1995, Boudreau et al. 1996; Faraldo et al. 1998; Klinowska et al. 1999).
In fact, Str1 is not induced early enough to be the natural initiator of cell death. It appears later during the biosynthetic wave. It is not surprising, therefore, that epithelial apoptosis was unaffected in Str1_−/_− mice. However, the lack of effect in TO mice suggests that other TIMP-1–sensitive MMPs are not implicated either. One hypothesis that could reconcile the inhibition of mammary epithelial cell death caused by implants of TIMP-1 (Talhouk et al. 1992) with the lack of inhibition observed in this genetic analysis of TO and Str1_−/_− mice is that inflammatory cytokines, induced by surgery, are known to protect mammary epithelial cells from cell death (Lund et al. 1996). Another explanation could be that the TIMP-1 concentration was likely much higher in the affected zone proximal to the implanted pellets containing 10 μg of TIMP-1 (as high as 1–5 mM). In TO mice, concentrations of TIMP-1 were 0.1–2 nM in plasma and tissues (30 ng/ml tissue lysate of mammary gland 1 d after weaning; Alexander et al. 1996), sufficient to inhibit entactin proteolysis. For full efficacy, TIMP-1 was added to 3T3-L1 cell cultures at 250 nM, and the peptide hydroxamate GM 6001 inhibitor at 10 μM. At these levels, MTI-MMP and other metalloproteinases, such as ADAM-TS aggrecanase-1 (Arner et al. 1999), or other ADAMs might be inhibited. These enzymes are responsible for cleavage and shedding of many cell-surface proteins that regulate cell function and death (Amour et al. 1998).
Mice with null mutations other than MMPs have been tested for defects of involutive processes: uterine involution is normal in mice with a null mutation in matrilysin/MMP-7, possibly due to compensatory expression of other MMPs (Rudolph-Owen et al. 1997). On the other hand, apoptosis of chondrocytes during the ossification of growth plates in juvenile bone is inhibited by the absence of gelatinase-B/MMP-9 (Vu et al. 1998). This effect is not mediated by cleavage of ECM components but instead by reduced bioavailability of the growth factor, VEGF (Gerber et al. 1999). If MMPs do not mediate epithelial cell apoptosis during mammary gland involution, are proteinases required at all? Certainly, mice lacking the serine proteinase plasminogen show reduced apoptosis during mammary involution (Lund et al. 2000). Interestingly, in contrast to MMPs, the serine proteinases positively regulate adipogenesis (Selvarajan et al. 2001).
Stromelysin-1 Expression Occurs during a Biosynthetic Wave that Remodels the Mammary Gland during Involution
Using light microscopy and immunohistochemistry, we found no substantial change in amount, distribution, or appearance of specific components of the epithelial basement membranes during early involution. However, biochemically, the properties of these basement membrane proteins is dramatically affected between 1 and 2 d after weaning. Most become detergent insoluble and disappear from protein profiles of gland extracts. Soluble ECM components are almost completely absent during the onset of apoptosis. Using high-resolution techniques, some investigators have reported the apparent thickening of basement membranes during this phase (Strange et al. 1992; Warburton et al. 1982).
The induction of the enzyme tissue transglutaminase may explain these alterations of basement membrane morphology and biochemistry. This enzyme is associated with the onset of apoptosis in a number of cell types (Melino and Piacentini 1998), and is induced in early involuting mammary gland (Strange et al. 1992; Guenette et al. 1994). By cross linking many extracellular proteins, including basement membrane proteins such as laminin, collagen IV, and entactin, this enzyme is likely to be responsible for the biochemical alterations we observed in the ECM fractions at the onset of apoptosis. The stimulus for mammary involution clearly involves withdrawal of lactogenic hormones, and may include local stimuli such as milk edema (Lascelles and Lee 1978; Tenniswood et al. 1992).
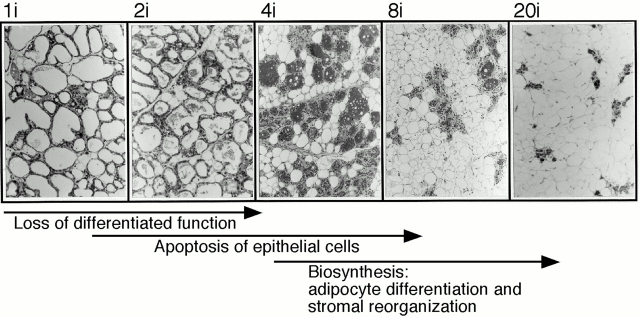
The histological data, together with the transcriptional activation of gene products typically associated with biosynthesis, describe an anabolic process that follows, and is closely coordinated with, the catabolic loss of epithelial cell function (Fig. 7). Taking advantage of the ability to manipulate the absolute timing of epithelial involution by altering the number of pups suckled and the length of lactation, we observed that the biosynthetic phase begins when WAP expression is reduced to <10% lactating levels and the number of live differentiated epithelial cells is reduced by 50%. During late pregnancy and immediately after parturition, the number of differentiated adipocytes in the fat pad (depending upon the mouse strain) is reduced to almost zero, and the majority of the gland becomes epithelial (Elias et al. 1972). Differentiated adipocytes collapse, but persist as thin, elongated undifferentiated cells during lactation (Ailhaud et al. 1992; Neville et al. 1998), which resemble the mammary fibroblasts surrounding the ductal network in the resting gland (Cunha and Hom 1996). During involution, adipocytes recolonize the interalveolar spaces, differentiate, and accumulate lipid until a fat pad of approximately virgin size is reconstituted. Clearly, adipocytes and epithelial cells interact to maintain a relative balance of these two cell types in the gland. Although several growth and transcription factors affect apoptosis or survival in mammary glands (Furth 1999; Song et al. 2000), the specific factors involved in local reciprocal control of adipocyte and epithelial involution are not yet understood.
Figure 7.
Mammary involution is divided into two interactive phases for epithelial and stromal remodeling. This scheme summarizes the relative time course of the catabolic events associated with epithelial involution, namely the loss of milk protein synthesis (differentiated function), together with apoptosis and regression of the majority of the epithelial cells, and the anabolic events responsible for reexpanding the fat pad.
Stromelysin-1 Accelerates Adipocyte Differentiation both in a Culture Model and In Vivo during Fat Pad Expansion
Str1_−/_− and TO mice showed unusual patterns of adipocyte colonization in involuting glands. Although the activation of the biosynthetic phase is not changed in these mice, the differentiation and hypertrophy of adipocytes is enhanced by 30–40% during active recolonization. Since Str1 mRNA is highly induced during fat cell differentiation, and fat cell differentiation is accelerated in glands with a null mutation, we hypothesize that Str1 normally inhibits adipocyte lipogenesis.
In support of this notion, the addition of MMP inhibitors to a culture model of differentiating adipocytes (3T3-L1 cells) accelerated lipogenesis sevenfold. 3T3-L1 cells were originally isolated as a lipogenic substrain of 3T3 fibroblasts (Green and Meuth 1974), and have been validated as a model of adipogenesis (Cornelius et al. 1994). Confluent precursor cells express markers specific to pre-adipocytes. After stimulation with inducers of cAMP accumulation, cells accumulate triglycerides, express many markers of terminal differentiation, and resemble multilocular adipocytes. Our data analyzing expression of an array of MMPs and their inhibitors suggest that high expression of Str-1, collagenase-3 (MMP-13), and MT-1 (MMP-14), and of the inhibitors TIMP-1, -2, and -3, parallel the commitment of undifferentiated 3T3-L1 fibroblast precursors to the adipocyte lineage. This coinduction of enzymes and inhibitors generates a low proteolytic index (proteinases/inhibitors), associated with the appearance of secreted latent gelatinase A (MMP-2). Other groups have shown that the amount of MMP-2 increases and TIMP-1 decreases during adipocyte differentiation (Johnson et al. 1994; Brown et al. 1997). An increase in the proteolytic index accompanies the transition from preadipocyte to terminally differentiated fat cells in culture. Specifically, the expression of mRNA for all three enzymes continues at high level, whereas that for TIMP-1 and -3 decreases. Concomitant with these changes, active MMP-2 appears in the medium. The expression of TIMP-2 mRNA is unaffected by differentiation. This inhibitor has a dual regulatory function for MMP-2, catalyzing the formation of a ternary activating complex for MT1 MMP at the cell surface, and inhibiting soluble enzyme activity (Gomez et al. 1997). TIMP-3 is an ECM-bound inhibitor that has a highly distinct inhibitor profile, inhibiting TACE and other membrane-bound disintegrin metalloproteinases (Amour et al. 1998). Interestingly, we found that TIMP-4 (Gomez et al. 1997) is induced in parallel with classic markers of terminal differentiation (Ailhaud et al. 1992), such as pPARγ. TIMP-4 may be the endogenous mediator that promotes terminal differentiation in adipocytes and may be a useful, novel adipocyte marker. By adding MMP inhibitors to maintain a low proteolytic index experimentally during the onset of differentiation in these cultures, lipogenesis is strikingly accelerated, mimicking the result observed in vivo.
There are several plausible mechanisms by which inhibition of MMPs could accelerate adipogenesis. The bioavailability of positive differentiation factors, such as IGF-1/IGF–binding protein (Rajkumar et al. 1999), and negative differentiation factors, such as Wnts (Cunha and Hom 1996; Ross et al. 2000), may be regulated by MMP action. Alternatively, MMPs may regulate the assembly of basement membrane per se. We favor the latter mechanism because adipocyte differentiation is characterized by the dramatic upregulation of synthesis of basement membrane proteins (Aratani and Kitagawa 1988). Accumulation of basement membrane distinguishes the adipocyte from its mesenchymal precursor, and may initiate terminal differentiation by stabilizing the adipocyte cell surface and generating specific intracellular signals. In support of this, Kawaguchi et al. 1998 found that subcutaneous injection of basement membrane (together with bFGF) induced the formation of stable fat pads in mice. Assembly of ECM is likely to be rate limiting for the differentiation of these cells; therefore, by coexpressing Str1 during differentiation, adipocytes may limit their own development. Indeed, Str1 cleaves entactin/nidogen-1 in vivo (Alexander et al. 1996), and entactin fragments inhibit the rate of basement membrane assembly (Pujuguet et al. 2000). Using primary rat adipocytes, Brown et al. 1997 found that the accumulation of ECM by differentiating clusters of cells was facilitated by the zinc chelator, 1,10-phenanthroline. In the present study, we observed that, under conditions inhibiting MMP function, entactin fragmentation ceases and adipocyte differentiation increases.
If MMPs are important in adipogenesis, then Str1_−/_− and TO mice could show increased adipogenesis in other tissues. MMPs may be rate limiting for differentiation only under situations where adipogenesis is very rapid, as it is during mammary involution. Fat pads from Str1_−/_− females on a 129 background were normal. However, the Str1_−/_− mice backcrossed onto an FVB/N background become obese with increasing age, reaching weights of up to 60 g (our unpublished observations). It may be relevant that humans with an allele that decreases Str1 promoter function to 25–50% show accelerated progression of their atherosclerotic lesions (Ye et al. 1996).
In conclusion, we have revealed a novel physiological role for MMPs as negative regulators of adipocyte metabolism and differentiation. The mechanisms by which epithelial death and basement membrane remodeling are regulated still remain elusive.
Acknowledgments
We are grateful to Dr. Rabih Talhouk for performing the Str1 Northern blot. We thank Dr. Lisa Coussens for bringing to our attention the obesity in FVB/N-Str1_−/_− mice.
This work was supported by grants from the National Cancer Institute (CA 57621 and CA 72006 to Z. Werb) and an Arthritis Foundation Investigator award (to C.M. Alexander).
Footnotes
Abbreviations used in this paper: dpc, days post coitum; ECM, extracellular matrix; H&E, hematoxylin and eosin; MMP, matrix metalloproteinase; Str1, stromelysin-1/MMP-3; TIMP, tissue inhibitor of metalloproteinases; TO, TIMP overexpressing transgenic mouse; WAP, whey acidic protein.
References
- Ailhaud G., Grimaldi P., Negrel R. Cellular and molecular aspects of adipose tissue development. Annu. Rev. Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Alexander C.M., Howard E.W., Bissell M.J., Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a TIMP-1 transgene. J. Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour A., Slocombe P.M., Webster A., Butler M., Knight C.G., Smith B.J., Stephens P.E., Shelley C., Hutton M., Knauper V., Docherty A.J., Murphy G. TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- Aratani Y., Kitagawa Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J. Biol. Chem. 1988;263:16163–16169. [PubMed] [Google Scholar]
- Arner E.C., Pratta M.A., Trzaskos J.M., Decicco C.P., Tortorella M.D. Generation and characterization of aggrecanase. A soluble cartilage-derived aggrecan-degrading activity. J. Biol. Chem. 1999;274:6594–6601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- Bernlohr D.A., Angus C.W., Lane M.D., Bolanowski M.A., Kelly T.J. Expression of specific mRNAs during adipocyte differentiationidentification of an mRNA encoding a homologue of myelin P2 protein. Proc. Natl. Acad. Sci. USA. 1984;81:5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N., Sympson C.J., Werb Z., Bissell M.J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N., Werb Z., Bissell M.J. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.M., Fox H.L., Hazen S.A., LaNoue K.F., Rannels S.R., Lynch C.J. Role of the matrixin MMP-2 in multicellular organization of adipocytes cultured in basement membrane components. Am. J. Pathol. 1997;272:C937–C949. doi: 10.1152/ajpcell.1997.272.3.C937. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y., Kuisk I., Rothblum K., Starnes S. Mouse vimentinstructural relationship to fos, jun, CREB and tpr. Oncogene. 1990;5:645–654. [PubMed] [Google Scholar]
- Caterina J.J., Yamada S., Caterina N.C., Longenecker G., Holmback K., Shi J., Yermovsky A.E., Engler J.A., Birkedal-Hansen H. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2 (Timp-2) gene alters ProMMP-2 activation. J. Biol. Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- Cornelius P., MacDougald O.A., Lane M.D. Regulation of adipocyte development. Annu. Rev. Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- Crandall D.L., Hausmann G.J., Kral J.G. A review of the microcirculation of adipose tissueanatomic, metabolic and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- Cunha G.R., Hom Y.K. Role of mesenchymal-epithelial interactions in mammary gland development. J. Mammary Gland Biol. Neoplasia. 1996;1:21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- Elias J.J., Pitelka D.R., Armstrong R.C. Changes in fat cell morphology during lactation in the mouse. Anat. Rec. 1972;177:533–548. doi: 10.1002/ar.1091770407. [DOI] [PubMed] [Google Scholar]
- Faraldo M.M., Deugnier M.-A., Lukashev M., Thiery J.P., Glukhova M.A. Perturbation of β1-integrin function alters the development of murine mammary gland. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J.E., Leco K.J., Moorehead R.A., Martin D.C., Khokha R. Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev. Biol. 1999;211:238–254. doi: 10.1006/dbio.1999.9313. [DOI] [PubMed] [Google Scholar]
- Ferguson J.E., Schor A.M., Howell A., Ferguson M.W.J. Changes in the extracellular matrix of the normal human breast during the menstrual cycle. Cell Tissue Res. 1992;268:167–177. doi: 10.1007/BF00338066. [DOI] [PubMed] [Google Scholar]
- Finbloom D.S., Petricoin E.F., III, Hackett R.H., David M., Feldman G.M., Igarashi K., Fibach E., Weber M.J., Thorner M.O., Silva C.M. Growth hormone and erythropoietin differentially activate DNA-binding proteins by tyrosine phosphorylation. Mol. Cell. Biol. 1994;14:2113–2118. doi: 10.1128/mcb.14.3.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth P.A. Mammary gland involution and apoptosis of mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 1999;4:123–127. doi: 10.1023/a:1018764922082. [DOI] [PubMed] [Google Scholar]
- Gerber H.-P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Gewert D.R., Coulombe B., Castelino M., Skup D., Williams B.R.G. Characterization and expression of a murine gene homologous to human EPA/TIMPa virus-induced gene in the mouse. EMBO (Eur. Mol. Biol. Organ.) J. 1987;6:651–657. doi: 10.1002/j.1460-2075.1987.tb04804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D.E., Alonso D.F., Yoshiji H., Thorgeirsson U.P. TIMPsstructure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipocyte cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Gross J. How tadpoles lose their tails. J. Invest. Derm. 1966;47:274–277. [PubMed] [Google Scholar]
- Guenette R.S., Corbeil H.B., Leger J., Wong K., Mezl V., Mooibroek M., Tenniswood M. Induction of gene expression during involution of the lactating mammary gland of the rat. J. Mol. Endocrinol. 1994;12:47–60. doi: 10.1677/jme.0.0120047. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S.A., Mankani M., Robey P.G., Poole A.R., Pidoux I. MT1-MMP–deficient mice develop dwarfism, osteopenia, arthritis, connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Jerry D.J., Kuperwasser C., Downing S.R., Pinkus J., He C., Dickinson E., Marconi S., Naber S.P. Delayed involution of the mammary epithelium in BALB/c-p53 null mice. Oncogene. 1998;17:2305–2312. doi: 10.1038/sj.onc.1202157. [DOI] [PubMed] [Google Scholar]
- Johnson M.D., Kim H.R., Chesler L., Tsao-Wu G., Bouck N., Polverini P.J. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J. Cell Physiol. 1994;160:194–202. doi: 10.1002/jcp.1041600122. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N., Toriyama K., Nicodemou-Lena E., Inou K., Torii S., Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D.A., Oshima R.G. Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol. Cell. Biol. 1988;8:1540–1550. doi: 10.1128/mcb.8.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska T.C., Soriano J.V., Edwards G.M., Oliver J.M., Valentijn A.J., Montesano R., Streuli C.H. Laminin and β1 integrins are crucial for normal mammary gland development in the mouse. Dev. Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- Lascelles A.K., Lee C.S. Involution of the mammary gland. In: Larson B.L., Smith V.R., editors. LactationA Comprehensive Treatise. Volume 4. Academic Press, Inc.; New York, NY: 1978. pp. 115–177. [Google Scholar]
- Leco K.J., Apte S.S., Taniguchi G.T., Hawkes S.P., Khokha R., Schultz G.A., Edwards D.A. Murine tissue inhibitor of metalloproteinase-4 (TIMP-4)cDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997;401:213–217. doi: 10.1016/s0014-5793(96)01474-3. [DOI] [PubMed] [Google Scholar]
- Leco K.J., Khokha R., Pavloff N., Hawkes S.P., Edwards D.R. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J. Biol. Chem. 1994;269:9352–9360. [PubMed] [Google Scholar]
- Li M., Hu J., Heermeier K., Hennighausen L., Furth P.A. Apoptosis and remodeling of mammary gland tissue during involution proceeds through p53-independent pathways. Cell Growth Differ. 1996;7:13–20. [PubMed] [Google Scholar]
- Li F., Strange R., Friis R.R., Djonov V., Altermatt H.-J., Saurer S., Niemann H., Andres A.-C. Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse. Int. J. Cancer. 1994;59:560–568. doi: 10.1002/ijc.2910590421. [DOI] [PubMed] [Google Scholar]
- Lochter A., Galosy S., Muschler J., Freedman N., Werb Z., Bissell M.J. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J. Cell Biol. 1997;1139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L.R., Bjørn S.F., Sternlicht M.D., Nielsen B.S., Solberg H., Autzen P., Østerby R., Christensen I.J., Bugge T.H., Stephens R.W. Lactational development and involution of the mammary gland requires plasminogen. Development. 2000;127:4481–4492. doi: 10.1242/dev.127.20.4481. [DOI] [PubMed] [Google Scholar]
- Lund L.R., Romer J., Thomasset N., Solber H., Pyke C., Bissell M.J., Dano K., Werb Z. Two distinct phases of apoptosis in mammary gland involutionproteinase-independent and -dependent pathways. Development (Camb.) 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Mann K., Timpl R., Murphy G. Sites of nidogen cleavage by proteases involved in tissue homeostasis and remodelling. Eur. J. Biochem. 1993;217:877–884. doi: 10.1111/j.1432-1033.1993.tb18316.x. [DOI] [PubMed] [Google Scholar]
- Melino G., Piacentini M. Tissue transglutaminase in cell deatha downstream or a multifunctional upstream effector? FEBS Lett. 1998;430:59–63. doi: 10.1016/s0014-5793(98)00521-3. [DOI] [PubMed] [Google Scholar]
- Mudgett J.S., Hutchinson N.I., Chartrain N.A., Forsyth A.J., McDonnell J., Singer I.I., Bayned E.K., Flanagan J., Kawka D., Shen C.F. Susceptibility of stromelysin-1–deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nagase H. Activation of matrix metalloproteinases. Biol. Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- Neville M.C., Medina D., Monks J., Hovey R.C. The mammary fat pad. J. Mammary Gland Biol. Neoplasia. 1998;3:109–115. doi: 10.1023/a:1018786604818. [DOI] [PubMed] [Google Scholar]
- Ostrowski L.E., Finch J., Krieg P., Matrisian L., Patskan G., O'Connell J.F., Phillips J., Slaga T.J., Breathnach R., Bowden G.T. Expression pattern of a gene for a secreted metalloproteinase during late stages of tumor progression. Mol. Carcinog. 1988;1:13–19. doi: 10.1002/mc.2940010106. [DOI] [PubMed] [Google Scholar]
- Pujuguet P., Simian M., Liaw J., Timpl R., Werb Z., Bissell M.J. Nidogen-1 regulates laminin-1–dependent mammary-specific gene expression. J. Cell Sci. 2000;113:849–858. doi: 10.1242/jcs.113.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar K., Modric T., Murphy L.J. Impaired adipogenesis in insulin-like growth factor binding protein-1 transgenic mice. J. Endocrinol. 1999;162:457–465. doi: 10.1677/joe.0.1620457. [DOI] [PubMed] [Google Scholar]
- Ramirez-Zacarias J.L., Castro-Munozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil Red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., MacDougald O.A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Rudolph-Owen L.A., Hulboy D.L., Wilson C.L., Mudgett J., Matrisian L.M. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1–deficient mice. Endocrinology. 1997;138:4902–4911. doi: 10.1210/endo.138.11.5478. [DOI] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Selvarajan S., Lund L.R., Takeuchi T., Craik C.S., Werb Z. A plasminogen cascade dependent on plasma kallikrein is required for adipocyte differentiation. Nat. Cell Biol. 2001;In press doi: 10.1038/35060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Sapi E., Brow W., Nilsen J., Tartaro K., Kacinski B.M., Craft J., Naftolin F., Mor G. Roles of Fas and Fas ligand during mammary gland remodeling. J. Clin. Invest. 2000;106:1209–1220. doi: 10.1172/JCI10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smas C.M., Sul H.S. Control of adipocyte differentiation. Biochem. J. 1995;309:697–710. doi: 10.1042/bj3090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M.D., Werb Z. ECM proteinases. In: Kreis T., Vale R., editors. Guidebook to the Extracellular Matrix and Adhesion Proteins. Oxford University Press; New York: 1999. pp. 503–562. [Google Scholar]
- Sternlicht M.D., Lochter A., Sympson C.J., Huey B., Rougier J.-P., Gray J.W., Pinkel D., Bissell M.J., Werb Z. The stromal proteinase MMP3/Stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R.R. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development (Camb.) 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Sympson C.J., Talhouk R.S., Alexander C.M., Chin J.R., Clift S.M., Bissell M.J., Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R.S., Bissell M.J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenniswood M.P., Guenette R.S., Lakins J., Mooibroek M., Wong P., Welsh J.-E. Active cell death in hormone-dependent tissues. Cancer Metastasis Rev. 1992;11:197–220. doi: 10.1007/BF00048064. [DOI] [PubMed] [Google Scholar]
- Thomasset N., Lochter A., Sympson C.J., Lund L.R., Williams D.R., Behrendtsen O., Werb Z., Bissell M.J. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am. J. Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B.M. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr. Opin. Genet. Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Vu T.H., Shipley J.M., Bergers G., Berger J.E., Helms J.A., Hanahan D., Shapiro S.D., Senior R.M., Werb Z. MMP-9/Gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T.H., Werb Z. Matrix metalloproteinaseseffectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Wang T.C., Cardiff R.D., Zukerberg L., Lees E., Arnold A., Schmidt E.V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Wang Z., Juttermann R., Soloway P.D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M.J., Mitchell D., Ormerod E.J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating and involuting rat mammary gland. J. Histochem. Cytochem. 1982;30:667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Weaver V.M., Bissell M.J. Functional culture models to study mechanisms governing apoptosis in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 1999;4:193–201. doi: 10.1023/a:1018781325716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysisregulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Wiesen J.F., Werb Z. Proteinases, cell cycle regulation and apoptosis during development and involution of the mammary gland. Mol. Reprod. Dev. 2000;56:534–540. doi: 10.1002/1098-2795(200008)56:4<534::AID-MRD12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Will H., Atkinson S.J., Butler G.S., Smith B., Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J. Biol. Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- Witty J.P., Wright J.H., Matrisian L.M. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol. Biol. Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Eriksson P., Hamsten A., Kurkinen M., Humphries S.E., Henney A.M. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter, which results in reduced gene expression. J. Biol. Chem. 1996;271:13055–13060. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Apte S.S., Soininen R., Cao R., Baaklini G.Y., Rauser R.W., Wang J., Cao Y., Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]