Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation (original) (raw)
Abstract
To assess whether heterozygosity of the donor cell genome was a general parameter crucial for long-term survival of cloned animals, we tested the ability of embryonic stem (ES) cells with either an inbred or F1 genetic background to generate cloned mice by nuclear transfer. Most clones derived from five F1 ES cell lines survived to adulthood. In contrast, clones from three inbred ES cell lines invariably died shortly after birth due to respiratory failure. Comparison of mice derived from nuclear cloning, in which a complete blastocyst is derived from a single ES cell, and tetraploid blastocyst complementation, in which only the inner cell mass is formed from a few injected ES cells, allows us to determine which phenotypes depend on the technique or on the characteristics of the ES cell line. Neonatal lethality also has been reported in mice entirely derived from inbred ES cells that had been injected into tetraploid blastocysts (ES cell-tetraploids). Like inbred clones, ES cell-tetraploid pups derived from inbred ES cell lines died shortly after delivery with signs of respiratory distress. In contrast, most ES cell-tetraploid neonates, derived from six F1 ES cell lines, developed into fertile adults. Cloned pups obtained from both inbred and F1 ES cell nuclei frequently displayed increased placental and birth weights whereas ES cell-tetraploid pups were of normal weight. The potency of F1 ES cells to generate live, fertile adults was not lost after either long-term in vitro culture or serial gene targeting events. We conclude that genetic heterozygosity is a crucial parameter for postnatal survival of mice that are entirely derived from ES cells by either nuclear cloning or tetraploid embryo complementation. In addition, our results demonstrate that tetraploid embryo complementation using F1 ES cells represents a simple, efficient procedure for deriving animals with complex genetic alterations without the need for a chimeric intermediate.
Cloned mammals have been generated through the transfer of embryonic and somatic nuclei into enucleated oocytes. However, a serious impediment to the general utility of the procedure is the low survival rate of cloned animals (1–9) as only 1–5% of reconstructed, nuclear transfer embryos develop into animals surviving to adulthood (1–9). In several species, including sheep (1, 2), cows (2–4), and mice (7–11), placental abnormalities, fetal overgrowth, and respiratory failure have been reported and often are associated with neonatal mortality. Factors that contribute to these abnormalities, also referred to as “large offspring syndrome” (2), as well as the parameters that affect the long-term survival of clones remain undefined.
Vertebrate development involves programmed changes in gene expression that promote the differentiation of totipotent embryonic cells into somatic cells that build the body of the animal. It has been proposed that the pattern of gene expression inherent to a differentiated somatic cell nucleus must be returned to a totipotent embryonic state, or “reprogrammed,” for nuclear clones to develop normally (12, 13). In support of this hypothesis, we recently have demonstrated that the inactive X chromosome in female somatic cells is reprogrammed after nuclear transfer to a transcriptional and epigenetic state appropriate for embryonic development (14). It has been suggested, however, that inefficient and faulty reprogramming may limit the long-term survival of clones or may be related to their abnormal phenotypes (12, 13). For example, conflicting evidence has been reported concerning the deregulation of telomere length in clones derived from somatic cell nuclei, where shortened (15), normal (16), and increased (17) telomere lengths all have been observed.
We previously have found that a significantly higher proportion of clones derived from embryonic stem (ES) cell nuclei than clones derived from somatic nuclei developed to term (10). This finding raised the question of whether the nucleus of an undifferentiated embryonic cell is easier to reprogram than that of a terminally differentiated somatic cell. In other words, is the potency of the ES cell nucleus to direct development of a clone intrinsically higher than that of a somatic nucleus? Alternatively, clone survival may be influenced by undefined parameters of the nuclear transfer procedure itself that may differentially affect survival or development of clones derived from ES cell as opposed to somatic nuclei. For example, it has been proposed that the cell cycle stage of the donor cell and recipient oocyte are critical for normal clone development and survival but no clear evidence is available (1, 3, 11). Also, in the direct injection method used for cloning the mouse (7), the isolation of the donor nucleus involves extensive physical manipulations, and it is possible that ES cell nuclei are inherently more resistant to mechanical damage then some somatic cells.
ES cells can be used as a model donor cell to determine whether the abnormal phenotypes of cloned animals are a consequence of the nuclear transfer technique itself or instead due to inherent properties of the donor cell nucleus. ES cells have the unique ability to autonomously direct embryonic development either after nuclear transfer (10, 11) or after injection into tetraploid host blastocysts (18–20). Tetraploid mouse blastocysts are not capable of completing normal development independently (21), but when complemented by the introduction of diploid ES cells, develop into conceptuses where the embryo proper (epiblast) is derived entirely from the ES cells and the extraembryonic lineages arise largely from the tetraploid host cells (18–20). Thus, introduction of ES cells into tetraploid embryos is another method, independent of nuclear cloning, for generating mice that are completely derived from ES cells. Comparing mice derived by these two methods from the same donor ES cell lines should yield insight into the causes of abnormal phenotypes observed in cloned animals. Phenotypes observed in both ES cell clones and ES cell-tetraploid mice are more likely caused by the intrinsic developmental properties of the donor ES cell nucleus whereas phenotypes observed only in nuclear clones must be caused by the nuclear transfer procedure itself.
Previously, we have shown that mice cloned from 129/Sv inbred ES cells developed to term but invariably died shortly after birth (10). In contrast, when a C57BL/6 × 129/Sv F1 ES cell line was used as a source of nuclear donors, all newborns survived to adulthood (10). These results raised the question of whether the difference in survival was due to anecdotal characteristics of the particular F1 and inbred ES lines used as donors or whether chromosomal heterozygosity, often referred to as “hybrid vigor,” was advantageous for long-term survival of clones.
In this study, we have compared the phenotypes of animals derived from F1 and inbred ES cells created by either tetraploid embryo complementation or nuclear cloning. We report that hybrid vigor is a general attribute beneficial for the survival of both types of ES cell-derived animals. These results suggest that the death of inbred ES cell clones at term is not a direct result of the nuclear transfer procedure itself but instead is caused by the limited developmental potential of inbred ES cells. In contrast, as fetal and placental overgrowth were observed only in cloned ES cell animals, our data indicate that the dramatic loss of neonatal growth control observed in cloned animals is a direct result of the nuclear transfer procedure itself.
Materials and Methods
Production of ES Cell Clones.
Nuclear transfer of ES cell nuclei into enucleated metaphase II oocytes was carried out as described (7–11). One to 3 h after nuclear transfer, oocytes were activated for 5 h with 10 mM Sr2+ in Ca2+ free media in the presence of 5 μg/ml of Cytochalasin B. Embryos were cultured_in vitro_ to the blastocyst stage and transferred to recipient mothers.
Embryo Culture.
All embryo culture was carried out in microdrops on standard bacterial Petri dishes (Falcon) under mineral oil (Squibb). Modified Chatot, Ziomek, Bavister (CZB) media (22) was used for embryo culture unless otherwise noted. Hepes-buffered CZB (22) was used for room temperature operations whereas long-term culture was carried out in bicarbonate-buffered CZB at 37°C with an atmosphere of 5% CO2 in air.
Preparation of Two Cell Embryos for Electrofusion.
B6D2F1 females were superovulated by i.p. injection of 7.5 units of pregnant mares' serum (Calbiochem) followed 46–50 h later with 7.5 units of human chorionic gonadotropin (Calbiochem). After administration of HCG, females were mated with B6D2F1 males. Fertilized zygotes were isolated from the oviduct 24 h later. Zygotes were left in Hepes-buffered CZB with 0.1% bovine testicular hyaluronidase for several minutes at room temperature to remove any remaining cumulus cells. After washing, zygotes were transferred to a new culture dish containing drops of bicarbonate-buffered CZB and placed at 37°C overnight to obtain two-cell embryos.
Preparation of Tetraploid Embryos by Electrofusion.
Forty hours post-human chorionic gonadotropin the blastomeres of two-cell embryos were electrofused to produce one-cell tetraploid embryos. Electrofusion was carried out on an inverted microscope using the lid of a Petri dish as a micromanipulation chamber. Platinum wires were used as both electrodes and micromanipulators to align two-cell embryos for fusion. A group of 15 two-cell embryos was placed on the stage in a 200-μl drop of M2 media (Sigma). Embryos were aligned with the interface between their two blastomeres perpendicular to the electrical field, and a single electrical pulse of 100 V with a duration of 100 μs was applied to each individually. Manipulation of a single group took less then 5 min. After electrofusion, embryos were returned to CZB media at 37°C. Embryos that had not undergone membrane fusion within 1 h were discarded.
Culture of ES Cells.
Derivation, culture, and targeted mutagenesis of ES cells were carried out as described (23) with ES cell lines derived from both inbred and F1 blastocysts. ES cells were cultured in DMEM with 15% FCS containing 1,000 units/ml leukocyte inhibiting factor on gamma-irradiated primary feeder fibroblasts. For blastocyst injection ES cells were trypsinized, resuspended in DMEM, and first preplated on a standard 10-cm tissue culture dish for 30 min to remove feeder cells and debris.
Piezo Micromanipulator Injection of Tetraploid Blastocyts.
For microinjection, 5–6 blastocysts were placed in a drop of DMEM with 15% FCS under mineral oil. A flat tip microinjection pipette with an internal diameter of 12–15 μm was used for ES cell injection. Fifty ES cells were picked up in the end of the injection pipette. The blastocyst to be injected was held in the vicinity of the inner-cell mass with a standard holding pipette. The injection pipette containing the ES cells was pressed against the zona opposite the inner-cell mass. A brief pulse of the Piezo (Primetech, Ibaraki, Japan) was applied, and the injection needle was simultaneously pushed through the zona and trophectoderm layer into the blastocoel cavity. About 10 ES cells then were expelled from the injection pipette and pushed against the inner-cell mass of the blastocyst. After injection of the entire group, blastocysts were returned to CZB media and placed at 37°C until transfer to recipient females.
Recipient Females and Caesarean Sections.
Ten injected blastocysts were transferred to each uterine horn of 2.5 days postcoitum pseudopregnant Swiss females that had mated with vasectomized males. Recipient mothers were killed at 19.5 days postcoitum, and pups were quickly removed from the uterus. After cleaning fluid from their air passages, pups were placed under a warming light and respiration was observed. Surviving pups were fostered to lactating BALB/c albino mothers.
Results
Nuclear Transfer with F1 and Inbred ES Cells.
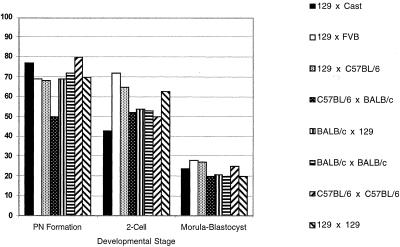
We compared donor nuclei derived from four different ES cell lines of three inbred backgrounds (129/Sv, C57BL/6, and BALB/c) with six different F1 lines (129/Sv × C57BL/6, C57BL/6 × 129/Sv, BALB/c × 129/Sv, 129/Sv ×Mus castaneus, C57BL/6 × BALB/c, and 129/Sv × FVB) in nuclear cloning experiments. Cells from each of these ES cell lines can contribute efficiently to the germ line after incorporation into chimeric animals (data not shown). We successfully reconstructed and activated 817 oocytes by using inbred ES cell donor nuclei and 783 oocytes by using F1 ES cell donor nuclei as judged by pronucleus (PN) formation. The efficiency of PN formation for all cell lines, inbred or F1, was ≈70% (Fig.1). Activated oocytes with a visible PN derived from either an inbred or F1 nucleus developed to the blastocyst stage with about 20% efficiency. The efficiency of cleavage-stage development was similar for clones derived from the four inbred and five F1 ES cell lines, indicating that neither genetic background nor genetic heterozygosity influence in vitro preimplantation development of ES cell clones (Fig. 1).
Figure 1.
In vitro preimplantation development of ES cell clones. Values are displayed as the percentage of embryos reaching each developmental stage. For PN formation rate, efficiency is expressed as the percent of total oocytes surviving reconstruction for each ES cell line. In the case of two-cell and blastocyst stage development, efficiency is expressed as the percent of embryos of all with PN. Data from two independent 129/Sv × 129/Sv ES cell lines were similar and therefore combined.
To assess full-term development of inbred and F1 ES cell clones, blastocysts were transferred to pseudopregnant recipient mothers. When delivered by caesarian section at embryonic day 19 of gestation, 15 of 182 cloned inbred blastocysts (8%) and 28 of 169 cloned F1 blastocysts (17%) were found to have developed to term. However, all inbred clones died within a few minutes after delivery of apparent respiratory failure (Table1). In striking contrast, 78% of clones (22 of 28 pups) derived from the various F1 ES cell donors initiated breathing and developed into healthy adults (Table 2). As all pups derived from inbred ES cells died at birth and the great majority of clones derived from F1 ES cell nuclei survived, these results confirm our previous conclusion (10) that heterozygosity of the donor cell genome is critical for the survival of ES cell clones. In addition, our results indicate that heterozygosity is of general, rather then anecdotal, importance in the survival of mice cloned from ES cells.
Table 1.
Survival of inbred ES cell clones
| ES cell line | Genotype | Total active PN | Blastocyst stage-ET (% PN) | Pups alive at term (% ET) | Pups surviving to adulthood |
|---|---|---|---|---|---|
| J1 | 129/Sv | 352 | 68 (19) | 6 (9) | 0 |
| V18.6 | 129/Sv | 178 | 40 (22) | 7 (18) | 0 |
| V26.2 | C57BL/6 | 164 | 40 (24) | 2 (5) | 0 |
| V39.7 | BALB/c | 123 | 34 (28) | 0 (0) | 0 |
| Total | — | 817 | 182 (22) | 15 (8) | 0 |
Table 2.
Survival of F1 ES cell clones
| ES cell line | Genotype | Total active PN | Blastocyst stage-ET (% PN) | Pups alive at term (% ET) | Pups surviving to adulthood (% alive) |
|---|---|---|---|---|---|
| V6.5* | C57B/6 × 129/Sv | 381 | 79 (21) | 18 (23) | 15 (80) |
| 129B6 | 129/Sv × C57BL/6 | 66 | 18 (27) | 3 (17) | 2 (67) |
| F1.2–3 | 129/Sv × M. cast | 143 | 27 (18) | 3 (11) | 2 (67) |
| V8.1 | 129/Sv × FVB | 69 | 19 (28) | 2 (11) | 2 (100) |
| V17.2 | BALB/c × 129/Sv | 99 | 21 (21) | 2 (10) | 1 (50) |
| V30.30 | C57BL/6 × BALB/c | 25 | 5 (20) | 0 | 0 |
| Total | 783 | 169 (22) | 28 (17) | 22 (78) |
Survival of ES Cell Animals Derived by Tetraploid Embryo Complementation.
To test whether heterozygosity of the donor ES cell genome was playing a role in the cloning process itself or instead influencing the developmental potency of the donor ES cell nucleus, we transferred cells from inbred and F1 ES lines into tetraploid blastocysts. Injection of ES cells into the blastocoel cavity of tetraploid blastocysts was aided by the use of a Piezo-driven micromanipulator. After micromanipulation, the resulting composite embryos were transferred to recipient females. We injected 312 tetraploid blastocysts with four different inbred ES cell lines. After transfer to recipient females, these embryos gave rise to 20 pups (6%) that were alive and active at caesarian section. However, 17 of the 20 newborns died of respiratory failure within 30 min. Of the three remaining pups, two were unable to sustain respiration and died within the next few hours (Table 3). Only one inbred ES cell-tetraploid pup was able to sustain respiration and developed to adulthood. The manifestation of respiratory failure was remarkably similar to that observed in neonatal, inbred ES cell clones. In contrast, of 344 tetraploid blastocysts injected with six different F1 ES cell lines, 60 (18%) developed to birth, 51 of which (85%) survived to adulthood (Table4). Therefore, genetic heterogeneity of the donor ES cells has a significant effect on long-term survival of both nuclear clones and ES cell-tetraploid pups. In addition, these results suggest that the death of inbred ES cell clones is not a consequence of the nuclear transfer procedure but is rather due to the decreased developmental potency of inbred ES cells as compared with their F1 counterparts.
Table 3.
Survival of inbred ES cell-tetraploid pups
| ES cell line | Genotype | 4N blasts injected | Pups alive at term (% Inj) | Pups respiring after C-section (% alive) | Pups surviving to adulthood (% alive) |
|---|---|---|---|---|---|
| J1 | 129/Sv | 120 | 9 (7.5) | 0 | 0 |
| V18.6 | 129/Sv | 48 | 5 (10) | 1 (20) | 0 |
| V26.2 | C57BL/6 | 72 | 3 (4) | 1 (33) | 0 |
| V39.7 | BALB/c | 72 | 3 (4) | 1 (33) | 1 (33) |
| Total | Inbred | 312 | 20 (6) | 3 (15) | 1 (5) |
Table 4.
Survival of F1 ES cell-tetraploid pups
| ES cell line | Genotype | 4N blasts injected | Pups alive at term (% Inj) | Pups respiring after C-section (% alive) | Pups surviving to adulthood (% alive) |
|---|---|---|---|---|---|
| V6.5 | C57BL/6 × 129/Sv | 72 | 18 (25) | 17 (94) | 16 (89) |
| V6.5* | C57BL/6 × 129/Sv | 60 | 11 (18) | 9 (81) | 9 (81) |
| V6.5† | C57BL/6 × 129/Sv | 20 | 1 (15) | 1 (100) | 1 (100) |
| 129B6 | 129/Sv × C57BL/6 | 48 | 2 (4) | 1 (50) | 1 (50) |
| F1.2–3 | 129/Sv × M. Cast. | 48 | 4 (8) | 3 (75) | 3 (75) |
| V8.1 | 129/Sv × FVB | 24 | 7 (30) | 7 (100) | 7 (100) |
| V17.2 | BALB/c × 129/Sv | 48 | 13 (27) | 12 (92) | 11 (85) |
| V30.11 | C57BL/6 × BALB/c | 24 | 4 (30) | 4 (100) | 3 (75) |
| Total | F1 | 344 | 60 (18) | 54 (90) | 51 (85) |
Inbred ES Cell-Derived Animals Die of Respiratory Failure.
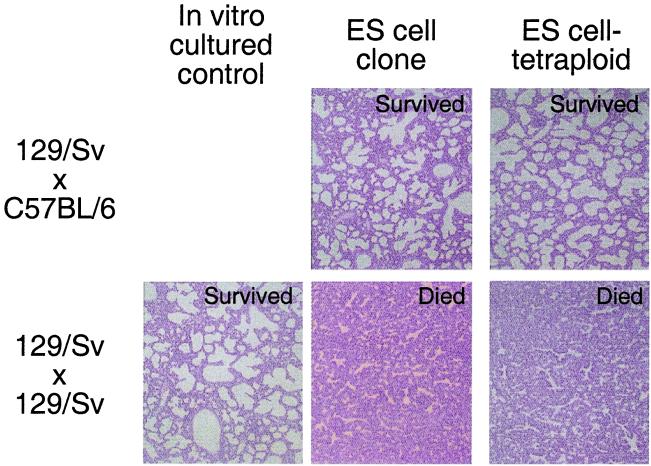
Both ES cell-tetraploid pups and clones derived from inbred ES cells appeared to suffer from respiratory distress after delivery. Because of this similarity, we performed histological analysis of both F1 and inbred completely ES cell-derived neonates. Examination of the lungs from inbred clones as well as from inbred ES cell-tetraploid pups revealed that the alveoli were not inflated. In contrast, the lungs of newborns derived from F1 ES cells were fully inflated and alveoli were expanded (Fig. 2). In addition, interstitial bleeding was seen often in inbred ES cell-derived mice of both types (data not shown). The extraembryonic tissues of the ES cell-tetraploid animals, such as the placenta, are largely derived from the tetraploid host blastocyst rather then the donor ES cells (18), as in ES cell clones. Therefore, our data suggest that the underlying defect leading to the common cause of neonatal lethality observed in both types of inbred ES cell-derived animals is likely localized to the embryonic lineages. Together, these observations suggest that the failure to initiate breathing and/or sustain normal circulation likely contributed to postnatal death of both inbred clones and inbred ES cell-tetraploid pups and that their death is not directly linked to placental malfunction.
Figure 2.
Hemotoxylin and eosin staining of lung sections from ES cell-derived and control neonatal mice. Neonatal mice delivered by cesarean section were observed for 45 min for respiration. After observation, lungs were removed and placed overnight in Bouin's fixative. After fixation, tissue was paraffin-embedded, sectioned, and stained. Magnification: ×40.
Embryonic and Placental Overgrowth in ES Cell-Derived Mice.
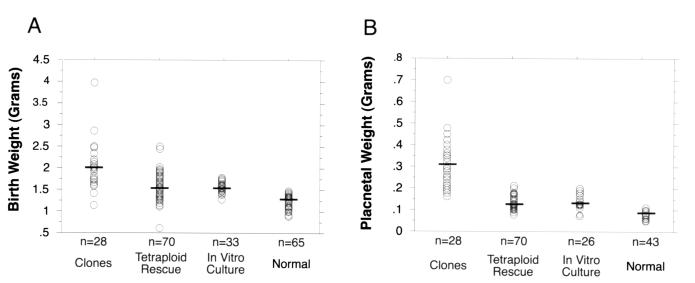
Embryonic and placental overgrowth and dysfunction have been suggested as potential causes of neonatal mortality in cloned livestock (2–4) and mice (7–8). This speculation led us to investigate the role of increased birth and placental weight in the survival of mice completely derived from ES cells. Neonatal mice cloned from ES cells were found to have a mean embryo weight of 2.1 g (Fig.3A) and a mean placental weight of 0.32 g (Fig. 3B). These weights were significantly higher than those of ES cell-tetraploid pups, normal pups, or pups derived from normal embryos in vitro cultured to the blastocyst stage (Fig. 3). Neither birth nor placental weights of ES cell-tetraploid pups were significantly different from neonates derived from in vitro cultured, control embryos (Fig. 3).
Figure 3.
Birth (A) and placental (B) weights of ES cell-derived and control neonatal mice. Birth and placental weights of clones were significantly higher than either ES cell-tetraploid mice (P < 0.0001 for both weights) or _in vitro_-cultured controls (_P_ < 0.0001 for both weights) in pair-wise comparisons. Neither birth nor placental weights of ES cell-tetraploid mice were significantly different from_in vitro_-cultured controls (_P_ > 0.05 for both weights). Birth and placenta weights of normal mice were significantly lower than cloned, ES cell-tetraploid or in vitro_-cultured pups (for all P < 0.004). Pairwise comparisons were performed by using the Student's_t test. Data from normal pups were recorded from litters with a size less than or equal to three. Cross-bars mark the mean weight for each data set. _In vitro_-cultured, control animals were generated by isolating two-cell stage embryos, culturing them to the blastocyst stage, and then transferring them to recipient females.
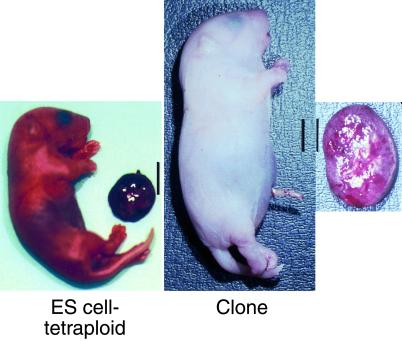
The increase in birth weight observed in ES cell clones was occasionally severe. A pup with extreme overgrowth is illustrated in Fig. 4. This figure compares the largest recovered clone (birth weight 4.0 g, placental weight 0.70 g) with the largest ES cell-tetraploid pup derived from the same ES cell line (birth weight 1.8 g, placental weight 0.15 g).
Figure 4.
ES cell clones display increased neonatal birth and placental weight. These two animals were derived from the same ES cell line, F1.2–3, one cloned by nuclear transfer, the other derived by tetraploid embryo complementation. Note the dramatic increase in both neonatal and placental size in the cloned pup. (Bars = 1 cm.)
Both extremely large and more normally sized embryos and placentas were observed in cloned conceptuses derived from both inbred and F1 ES cells. Significantly, whereas both large and more normal F1 ES cell clones survived postnatally, both large and more normal-sized inbred ES cell clones died. It has been previously suggested that neonatal and placental overgrowth might be related to neonatal lethality in cloned animals (2–4, 7, 8) but in our experiments no correlation between placental or embryonic overgrowth and neonatal survival was apparent.
Our data confirm other studies that suggest that either in vitro culture or transfer of embryos to pseudopregnant recipient mothers can cause increased placental and embryonic birth weight (2). Because placental and embryonic weights of ES cell clones were significantly higher than those of ES cell-tetraploid mice or other control mice, our data indicate that the loss of neonatal growth control is a direct consequence of the nuclear cloning procedure.
Survival of Mice After Long-Term In Vitro Culture and Gene Targeting of ES Cells.
It has been previously shown that prolonged passage of ES cells is detrimental to their developmental potency (19, 20). Survival of F1 ES cell pups seemed to be due to the superior developmental potency of F1 ES cells rather than the method by which they were produced. To investigate whether continuous in vitro culture would impair the survival of F1 ES cell-tetraploid pups, a 129Sv × C57BL/6 ES cell line (V6.5) was kept in culture for 25 passages. When these cells were injected into tetraploid blastocysts at either passage 15 or 25, live pups were generated at a frequency similar to that observed with low passage cells (data not shown). Similarly, ES cell-tetraploid mice were efficiently generated from ES cells that carried a targeted insertion at the Rosa26 locus and had been subjected to puromycin selection (Table 4). In addition, F1 ES cells were subjected to two consecutive rounds of drug selection. An F1 ES cell subclone carrying the insertion at the Rosa26 locus was transfected with a tet-inducible promoter driving expression of a hygromycin-thymidine kinase cassette, and subclones expressing the construct were isolated by hygromycin selection. Injection of these double-selected cells into 20 tetraploid blastocysts resulted in one full-term pup, which survived to adulthood (Table 4). Our results indicate that live, adult mice entirely derived from ES cells can be generated from F1 ES cells even after long-term passage of the cells in culture or after consecutive rounds of drug selection.
Discussion
In this study we compared development and long-term survival of mice derived by either nuclear cloning or tetraploid embryo complementation in an effort to understand some of the parameters that lead to abnormal development of cloned animals. We were interested to determine whether phenotypic abnormalities frequently seen in cloned mice such as loss of neonatal growth control, respiratory failure, and neonatal mortality are a consequence of the nuclear transfer procedure itself or are due to the intrinsic developmental potency of the nucleus. Our results indicate that respiratory competence and neonatal survival depend on the genetic makeup of the donor cell nucleus whereas neonatal overgrowth is a consequence of the nuclear transfer procedure.
Hybrid Vigor and the Survival of Completely ES Cell-Derived Mice.
We have demonstrated that genetic heterozygosity is a crucial parameter influencing postnatal survival of mice derived from ES cells by nuclear cloning or tetraploid embryo complementation. Pups derived from inbred ES cells by either method die perinatally with a similar phenotype of respiratory failure. In contrast, the great majority (80–85%) of pups derived from F1 ES cells by either procedure survived to adulthood. Nonetheless, a small fraction of the F1 ES cell clones died from respiratory failure, as also is observed in the cloning of noninbred farm animals.
Adult ES cell mice have been previously generated at a low frequency from early passage R1 ES cells by tetraploid embryo complementation (20) and nuclear transfer (11). The R1 ES cell line was derived from an intercross between two different 129 substrains (20), consistent with our observations that some level of heterozygosity in the donor cell genome is important for postnatal survival of ES cell pups. The common respiratory phenotype observed in both cloned and ES cell-tetraploid mice suggests that the neonatal lethality observed in ES cell clones is not due to the nuclear transfer procedure per se but more likely due to some inherent inability of inbred ES cells to maintain developmental potency. Our observation that heterozygosity of the donor ES cell genome rescues the neonatal lethality observed in both types of ES cell mice, even after long-term in vitro culture, is consistent with this conclusion.
Neonatal Overgrowth Is a Result of the Nuclear Transfer Procedure.
Increased placental and embryonic weights have been reported in cloned farm animals and mice (2, 7, 8). In our experiments increased birth and placental weights were observed only in ES cell clones and not in ES cell-tetraploid pups, suggesting that this phenotype must be a direct result of the nuclear transfer procedure. It is not clear why the different nuclear transfer procedures used for cloning of mice or farm animals all lead to neonatal overgrowth. It is widely assumed that the “reprogramming” of the genome from a state that is appropriate for the donor cell to one that is appropriate for an early embryonic state is crucial for successful nuclear cloning (12, 13). Faulty gene expression during development may result from failed epigenetic reprogramming or epigenetic instability in the donor cell population and may contribute to the phenotypes observed in mammalian clones (12,13). It will be of great interest to investigate whether normal expression of genes such as imprinted genes that are known to have an important impact on embryonic growth is affected in nuclear clones.
Practical Implications.
The possibility of deriving mice directly from ES cells without the production of a chimeric intermediate has great potential for facilitating the generation of animals with multiple genetic alterations. In conventional approaches, targeted ES cells are injected into diploid blastocysts to generate chimeric founders. The derivation of mice carrying the desired mutations or transgenes requires outcrossing of the chimeras with wild-type mice. Thus, the generation of compound animals that combine multiple desired mutations or transgenes in their genome entails time-consuming and expensive cycles of crossing mice derived from different chimeric founders. In contrast, the ES cell-tetraploid technology in combination with F1 ES cells allows assembling multiple genetic alterations in the same ES cell line by consecutive gene targeting cycles in vitro before generating mutant animals.
For example, the generation of a mouse with a homozygous, targeted mutation normally would require a single round of in vitro ES cell gene targeting, followed by production of a chimeric mouse. The chimeric founder then would be bred to generate heterozygous offspring that are interbred to generate mice homozygous for the desired mutation. The whole process requires a minimum of three mouse generations (or 9 months of breeding). If other mutations or transgenes are to be introduced into the mutant background, the targeted mice need to be crossed with the respective transgenic strain, which causes segregation of the alleles necessitating additional breeding cycles. In contrast, multiple genetic alterations could be introduced into F1 ES cells by consecutive targeting, each step requiring 2–4 weeks of tissue culture, followed by injection of the multiply targeted cells into tetraploid blastocysts. Unlike nuclear cloning technology, which has proven both difficult to master and transfer from laboratory to laboratory, the ES cell-tetraploid technology can easily be adapted by any laboratory experienced in the production of chimeric mice by ES cell blastocyst injection.
At present, the mechanisms that permit long-term survival of clones and ES cell-tetraploid pups derived from F1 but not from inbred ES cells are unclear. Although it is generally assumed that “hybrid vigor” is an important parameter in animal survival under various selective conditions, it is not apparent whether wide-ranging chromosomal heterozygosity or heterozygosity at only a few crucial modifier loci is required. Examining the potency of ES cells that have been derived from embryos generated by backcrossing F1 mice and their parental inbred strains may clarify this question.
Acknowledgments
This work was supported by the Victoria and Bradley Geist Foundation, the Kosasa Family Foundation, and the Harold Castle Foundation (to R.Y.) and by National Institutes of Health Grants 5-R35-CA44339 and RO1-CA84198 (to R.J.).
Abbreviations
CZB
Chatot, Ziomek, Bavister
ES
embryonic stem
PN
pseudopronucleus
Footnotes
See commentary on page 5949.
References
- 1.Wilmut I, Schnieke A E, McWhir J, Kind A J, Campbell K H S. Nature (London) 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 2.Young L E, Sinclair K D, Wilmut I. Rev Reprod. 1998;3:155–163. doi: 10.1530/ror.0.0030155. [DOI] [PubMed] [Google Scholar]
- 3.Cibelli J B, Stice S L, Golueke P J, Kane J J, Jerry J, Blackwell C, Ponce de Leon F A, Robl J M. Science. 1998;280:1256–1258. doi: 10.1126/science.280.5367.1256. [DOI] [PubMed] [Google Scholar]
- 4.Wells D N, Misica P M, Tervit R H. Biol Reprod. 1999;60:996–1005. doi: 10.1095/biolreprod60.4.996. [DOI] [PubMed] [Google Scholar]
- 5.Baguisi A, Behboodi E, Melican D T, Pollock J S, Destrempes M M, Cammuso C, Williams J L, Nims S D, Porter C A, Midura P, et al. Nat Biotechnol. 1999;17:456–461. doi: 10.1038/8632. [DOI] [PubMed] [Google Scholar]
- 6.Polejaeva I A, Chen S H, Vaught T D, Page R L, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares D L, et al. Nature (London) 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- 7.Wakayama T, Perry A C, Zuccotti M, Johnson K R, Yanagimachi R. Nature (London) 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 8.Wakayama T, Yanagimachi R. Nat Genet. 1999;22:127–128. doi: 10.1038/9632. [DOI] [PubMed] [Google Scholar]
- 9.Ogura A, Inoue K, Ogonuki N, Noguchi A, Takano K, Nagano R, Suzuki O, Lee J, Ishino F, Matsuda J. Biol Reprod. 2000;62:1579–1584. doi: 10.1095/biolreprod62.6.1579. [DOI] [PubMed] [Google Scholar]
- 10.Rideout W M, Wakayama T, Wutz A, Eggan K, Jackson-Grusby L, Dausman J, Yanagimachi R, Jaenisch R. Nat Genet. 2000;24:109–110. doi: 10.1038/72753. [DOI] [PubMed] [Google Scholar]
- 11.Wakayama T, Rodriguez I, Perry A C, Yanagimachi R, Mombaerts P. Proc Natl Acad Sci USA. 1999;96:14984–14989. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurdon J B, Colman A. Nature (London) 1999;402:743. doi: 10.1038/45429. [DOI] [PubMed] [Google Scholar]
- 13.Kikyo N, Wolffe A P. J Cell Sci. 2000;113:11–20. doi: 10.1242/jcs.113.1.11. [DOI] [PubMed] [Google Scholar]
- 14.Eggan K, Akutsu H, Hochedlinger K, Rideout W, Yanagimachi R, Jaenisch R. Science. 2000;290:1578–1581. doi: 10.1126/science.290.5496.1578. [DOI] [PubMed] [Google Scholar]
- 15.Shiels P G, Kind A J, Campbell K H, Waddington D, Wilmut I, Colman A, Schnieke A E. Nature (London) 1999;399:316–317. doi: 10.1038/20580. [DOI] [PubMed] [Google Scholar]
- 16.Lanza R P, Cibelli J B, Blackwell C, Cristofalo V J, Francis M K, Baerlocher G M, Mak J, Schertzer M, Chavez E A, Sawyer N, et al. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- 17.Betts D H, Bordignon V, Hill J R, Winger Q, Westhlusin M E, Smith L C, King W A. Proc Natl Acad Sci USA. 2001;98:1077–1082. doi: 10.1073/pnas.031559298. . (First Published January 16, 2001, 10.1073/pnas.031559298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy A, Gocza E, Diaz E M, Prideaux V R, Ivanyi E, Markkula M, Rossant J. Development (Cambridge, UK) 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z Q, Kiefer F, Urbaneck P, Wagner E F. Mech Dev. 1997;62:137–145. doi: 10.1016/s0925-4773(97)00655-2. [DOI] [PubMed] [Google Scholar]
- 20.Nagy A, Rossant J, Nagy R, Abramov-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman M H, Webb S. Development (Cambridge, UK) 1990;110:1121–1132. doi: 10.1242/dev.110.4.1121. [DOI] [PubMed] [Google Scholar]
- 22.Chatot C L, Lewis J L, Torres I, Ziomek C A. Biol Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- 23.Hogan B, Beddington R, Costantini F. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 253–289. [Google Scholar]