Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole (original) (raw)
Abstract
Rapid discharge of secretory organelles called rhoptries is tightly coupled with host cell entry by the protozoan parasite Toxoplasma gondii. Rhoptry contents were deposited in clusters of vesicles within the host cell cytosol and within the parasitophorous vacuole. To examine the fate of these rhoptry-derived secretory vesicles, we utilized cytochalasin D to prevent invasion, leading to accumulation of protein-rich vesicles in the host cell cytosol. These vesicles lack an internal parasite and are hence termed evacuoles. Like the mature parasite-containing vacuole, evacuoles became intimately associated with host cell mitochondria and endoplasmic reticulum, while remaining completely resistant to fusion with host cell endosomes and lysosomes. In contrast, evacuoles were recruited to pre-existing, parasite-containing vacuoles and were capable of fusing and delivering their contents to these compartments. Our findings indicate that a two-step process involving direct rhoptry secretion into the host cell cytoplasm followed by incorporation into the vacuole generates the parasitophorous vacuole occupied by Toxoplasma. The characteristic properties of the mature vacuole are likely to be determined by this early delivery of rhoptry components.
Keywords: exocytosis/mitochondria/parasitophorous vacuole/rhoptry/trafficking
Introduction
Toxoplasma gondii is a widespread, obligate intracellular parasite capable of infecting virtually all types of nucleated mammalian and avian cells. As an opportunistic human pathogen, Toxoplasma is an important cause of disease in immunocompromised individuals (Luft et al., 1993) and in neonates following congenital infection (Wong and Remington, 1994). Toxoplasma belongs to a large phylum of related parasites, called the Apicomplexa, which also includes the human pathogens Plasmodium (malaria) and Cryptosporidium, as well as numerous animal pathogens (Dubey, 1977). These parasites are unified by common anterior specializations that are involved in cell entry. While they infect a variety of different cell types in a range of different hosts, all are faced with similar complications in establishing a specialized compartment within the host cell where they reside. In the case of Toxoplasma, the parasitophorous vacuole has been shown to resist fusion with the endocytic and exocytic pathways of the host cell (Mordue and Sibley, 1997; Mordue et al., 1999a). This unique compartment is also endowed with the capacity to interact with host cell mitochondria and endoplasmic reticulum (ER) (Jones et al., 1972; Endo et al., 1981; Sinai et al., 1997). Avoidance of endocytic fusion and selective recruitment of host cell organelles such as mitochondria and ER are traits shared by several intracellular bacterial pathogens such as Chlamydia (Matsumoto et al., 1991) and Legionella (Horwitz, 1983).
Unlike phagocytic uptake or entry of bacterial pathogens, host cell invasion by Toxoplasma occurs by active penetration of the host cell (Dobrowolski and Sibley, 1996; Dobrowolski et al., 1997a). The process of invasion is accompanied by the sequential discharge of three separate sets of secretory organelles termed micronemes, rhoptries and dense granules (Dubremetz et al., 1993; Carruthers and Sibley, 1997). The initial discharge of micronemal proteins from the anterior end of the parasite is associated with attachment to the host cell via secreted adhesins (Fourmaux et al., 1996; Carruthers et al., 2000; Garcia-Reguet et al., 2000; Brecht et al., 2001). Following this directional attachment, the anterior end of the parasite forms a tight junction with the host cell plasma membrane. Subsequently, club-shaped organelles called rhoptries release their contents from the anterior end of the parasite (Nichols et al., 1983; Porchet-Hennere and Nicolas, 1983; Carruthers and Sibley, 1997), discharging a family of proteins called ROPs. Once the parasite is within the vacuole, the exocytosis of dense granules releases GRA proteins that are involved in modifying the intracellular compartment occupied by the parasite (Dubremetz et al., 1993; Carruthers and Sibley, 1997). While the parasitophorous vacuole is uniquely adapted for intra cellular survival, the role of specific parasite proteins in establishing the characteristics of this compartment is not understood.
Previous studies have proposed two opposing models for generating the membrane that surrounds the intracellular parasite. The first model postulates that invasion is accompanied by discharge of proteins and membranous material from the rhoptries to form the parasitophorous vacuole de novo. This secretion-based model is supported by the observations that the vacuolar membrane is devoid of host cell markers (Mordue et al., 1999b), and the vacuole contains lamellar profiles that resemble internal rhoptry structures in both Toxoplasma and Plasmodium (Nichols and O’Connor, 1981; Nichols et al., 1983; Stewart et al., 1985; Bannister et al., 1986). Several lines of evidence also support an alternative model, that the parasitophorous vacuole forms by direct invagination of the host cell plasma membrane. First, capacitance measurements of patch-clamped host cells indicate that the majority of the vacuolar membrane (>85%) surrounding intracellular Toxoplasma comes from the host cell plasma membrane (Suss-Toby et al., 1996). Secondly, fluorescent lipid tracers placed selectively in the host cell plasma membrane are readily internalized during cell invasion by both Toxoplasma (Mordue et al., 1999b) and Plasmodium (Ward et al., 1993). Resolving these alternative models and determining the role of specific parasite components in formation of the vacuole have been complicated by the extremely rapid process of cell invasion, which is completed within 15–20 s (Morisaki et al., 1995).
When the invasion of Toxoplasma or Plasmodium is prevented by treatment with cytochalasins (Cyt), which are potent inhibitors of actin polymerization, the parasite still efficiently accomplishes many of the early interactions with its host cell. For example, Cyt-arrested parasites still secrete adhesins from the micronemes, form a tight junction with the host cell and discharge the contents of rhoptries, resulting in the generation of nascent parasitophorous vacuoles within the host cell cytoplasm (Aikawa et al., 1981; Carruthers and Sibley, 1997). These nascent parasitophorous vacuoles formed under cytochalasin D (CytD) treatment contain rhoptry proteins but lack both micronemal and dense granule proteins (Carruthers and Sibley, 1997). In the present study, we have used treatment with CytD, which selectively uncouples protein secretion from invasion, to examine the contribution of rhoptry proteins to the biogenesis of the parasitophorous vacuole in the absence of an occupying parasite.
Results
Secretion from the rhoptries results in formation of discrete vesicles within the host cell cytosol
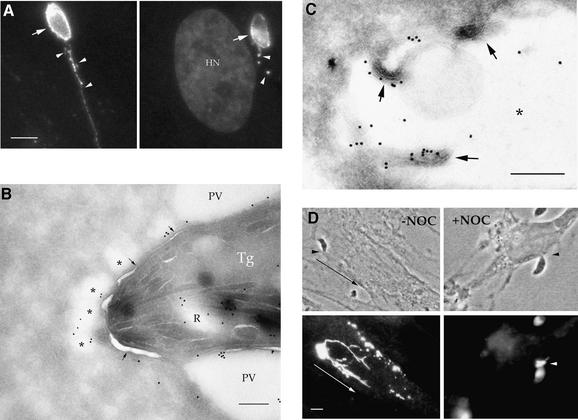
To examine the fate of ROP proteins during invasion, human foreskin fibroblast (HFF) monolayers were pulsed with parasites for short intervals (5–10 min), fixed and examined by indirect immunofluorescence microscopy. As markers for rhoptry discharge, we examined the rhoptry proteins ROP1, a protein of predicted mass 60 kDa (Saffer et al., 1992), and ROP2, a protein of predicted mass 55 kDa (Beckers et al., 1994). ROP proteins were discharged both into the forming parasitophorous vacuole and into numerous small, satellite vesicles that often extended in clusters out away from the vacuole (Figure 1A). Double immunofluorescence labeling revealed that the majority of parasite-containing vacuoles had adjacent satellite vesicles (typical of those seen in Figure 1A) that were ROP2-positive but GRA1-negative (data not shown). The fate of the satellite vesicles was ascertained by allowing parasites to invade HFF cells during a brief pulse (5 min) followed by extensive washing to remove extracellular parasites and return to culture in fresh medium. While the satellite vesicles were initially detected in close proximity to 86% of parasite-containing vacuoles, their abundance dropped to 72% after 30 min and to 45% after 60 min (based on counting 100 parasite-containing vacuoles per time point).
Fig. 1. Distribution of rhoptry-derived secretory vesicles formed by Toxoplasma in HFF cells. (A) IF staining of rhoptry proteins discharged during normal invasion. Release of rhoptry proteins into the parasitophorous vacuole resulted in staining of the vacuolar membrane (arrows) and clusters of small vesicles within the host cell cytosol (arrowheads). Cells were fixed, permeabilized and stained with mAb Tg49 anti-ROP1 followed by fluorescently conjugated goat anti-mouse IgG. Counter-staining with DAPI reveals the host cell nucleus (HN) in proximity to the parasitophorous vacuole. Scale bar = 5 µm. (B) CryoimmunoEM of rhoptry-derived secretory vesicles formed during normal invasion. Secretory vesicles that contain ROP proteins (*) lie adjacent to the parasitophorous vacuole (PV) but are outside the limiting membrane of this compartment (arrows). Stained with mAb Tg49 to ROP1 followed by goat anti-mouse IgG conjugated to 18 nm gold. A partially discharged rhoptry (R), which contains ROP1, is located near the anterior end of the parasite (Tg). Scale bar = 250 nm. (C) Higher magnification of a ROP2-positive satellite vesicle formed in the vicinity of a parasite-containing vacuole (not shown) during invasion. The limiting membrane of the rhoptry-derived secretory vacuole is not readily apparent, yet it contains internal membranous structures (arrows). The lumen of the secretory vesicle is marked by *. ROP2 was visualized by staining with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 18 nm gold. Scale bar = 250 nm. (D) IF of rhoptry-derived secretory vesicles formed under CytD block. ROP1-positive vesicles were formed within the host cell cytosol, emanating from the site of parasite attachment (arrowheads). In the absence of nocodazole (–NOC), migration away from the site of injection led to ribbons of vesicles stretched out within the host cell cytosol. In the presence of NOC, migration was blocked and vesicles remain concentrated at the site of injection (arrowhead in lower right panel). Cells were fixed, permeabilized and stained for the parasite protein ROP1 using mAb Tg49 followed by BODIPY-conjugated goat anti-mouse IgG. Scale bar = 5 µm.
When examined by cryoimmunoEM, these rhoptry-derived secretory vesicles were often clustered within the host cell cytosol near the anterior end of the parasite (* in Figure 1B), yet they remained separated from the parasitophorous vacuole membrane (indicated by the arrows in Figure 1B). The limiting membrane of the rhoptry-derived vesicles was typically not well defined, yet they often contained internal membranous structures (Figure 1C). These features are reminiscent of the early parasitophorous vacuole, which often lacks a distinct, electron-dense membrane bilayer and contains internal membrane whorls (Nichols et al., 1983; Bannister et al., 1986). The abundance, close proximity and gradual disappearance over time of these rhoptry-derived secretory vesicles suggested that they might contribute to biogenesis of the vacuole. However, the fate of the satellite vesicles formed under these conditions could not be determined due to the fact that they contain many of the same protein markers comprising the mature parasitophorous vacuole membrane.
Generation of empty, rhoptry-derived secretory vesicles
To investigate the fate of rhoptry-derived secretory vesicles within host cells, we took advantage of our earlier findings that while CytD blocks parasite invasion, it does not prevent attachment or rhoptry secretion (Carruthers and Sibley, 1997). When monolayers were challenged with parasites in the presence of CytD for brief intervals (5–10 min), rhoptry proteins were discharged into a cluster of vesicles that accumulated beneath the point of parasite contact with the host cell (Figure 1D). Treatment with CytD did not cause spontaneous release of the rhoptries by extracellular parasites (data not shown), and formation of the rhoptry-derived secretory vesicles required intimate attachment of the anterior end of the parasite with the host cell membrane. To determine the frequency of ROP-positive vacuoles, monolayers were challenged with equal numbers of parasites in the presence or absence of CytD, fixed and co-stained for ROP2 and GRA1 by indirect immunofluorescence labeling. Approximately 67% of cell-associated parasites produced ROP2-positive and GRA1-negative vesicles within the host cell cytosol, beneath the point of attachment. This value is similar to the frequency of rhoptry-derived satellite vesicles that formed in the vicinity of normal parasite-containing vacuoles in the absence of CytD (86%, see above). Thus, the vesicles formed by secretion in the presence of CytD closely resemble the satellite vesicles produced during normal invasion, yet they lack an internal parasite and hence they are referred to here as evacuoles from the Greek ‘evacuo’, meaning empty.
To follow the fate of evacuoles formed in the cytosol of host cells, HFF cells were challenged with Toxoplasma in the presence of CytD for a brief pulse of 5 min, then washed to remove extracellular parasites and returned to fresh culture medium containing CytD. Incubation of monolayers for up to 1 h resulted in migration of the evacuoles away from the initial site of parasite attachment to form ribbon-like structures within the host cell cytosol (Figure 1D). The relatively ordered migratory pattern of the evacuoles suggested that the host cell cytoskeleton might be involved as a scaffold. Since this pattern was observed in the presence of CytD, which not only blocks parasite invasion but also depolymerizes the host cell actin microfilaments, we investigated the possible involvement of the host cell microtubule network in migration using the destabilizing drug nocodazole (NOC). Treatment of host cells with NOC abolished the migration of the evacuoles, resulting in their remaining concentrated at the site of formation (Figure 1D). The vesicular nature of the evacuoles led us to explore further whether they are formed by an endocytic event and whether they interact with the endosomal/lysosomal systems of the host cell.
Evacuoles do not originate from the host cell plasma membrane
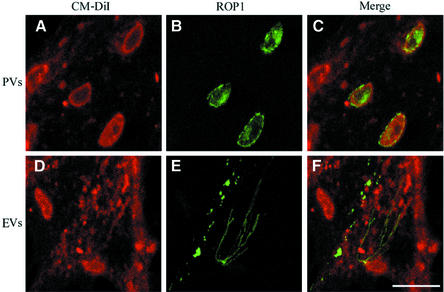
During normal parasite invasion, the host cell plasma membrane invaginates to form the parasitophorous vacuole and this process can be visualized by following internalization of the membrane lipid dye DiIC16 placed in the host cell membrane prior to invasion (Mordue et al., 1999a,b). To determine whether evacuoles also form by internalization of the host cell plasma membrane, we examined the redistribution of DiIC16 that was placed in the host cell plasma membrane prior to formation of evacuoles in HFF cells. In parallel, we examined the formation of parasitophorous vacuoles in DiIC16-labeled host cells. As expected, DiIC16 was readily incorporated into parasitophorous vacuoles formed by normal invasion as shown by the colocalization of the lipid label with ROP1 discharged into the parasitophorous vacuole (Figure 2A–C). In contrast, DiIC16 was not detected in association with ROP1-positive evacuoles that were formed by challenge of HFF cells with parasites in the presence of CytD (Figure 2D–F). These results indicate that evacuoles do not originate from the host cell plasma membrane but rather result from direct secretion of rhoptry contents into the host cell cytosol.
Fig. 2. Confocal micrographs showing uptake of the lipophilic dye CM-DiIC16 from the host cell plasma membrane into parasitophorous vacuoles (PVs) (top panels) but not into evacuoles (EVs) (bottom panels). Pre-labeled HFF monolayers were challenged with parasites to allow formation of parasite-containing vacuoles (A–C) or challenged with parasites in the presence of 1 µM CytD to form evacuoles (D–F). Monolayers were fixed, permeabilized and stained for the parasite rhoptry protein ROP1 using mAb Tg49 followed by BODIPY-conjugated goat anti-mouse IgG to visualize either parasitophorous vacuoles (B) or evacuoles (E). Scale bar = 10 µm.
Evacuoles avoid fusion with host cell endocytic organelles
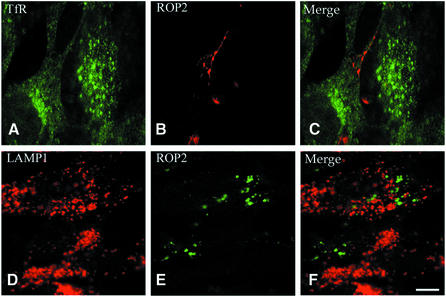
The parasitophorous vacuole avoids fusion with host cell endocytic compartments, while phagosomes containing dead or antibody-opsonized Toxoplasma readily fuse with host cell endosomes and lysosomes (Mordue and Sibley, 1997; Mordue et al., 1999a). To investigate whether vacuolar non-fusigenicity was also a trait of evacuoles, the distribution of host endosomal markers was examined in conjunction with that of the parasite protein ROP2 by double immunofluorescence staining. We examined the distribution of the early endocytic marker transferrin receptor (TfR) at 15 min post-infection and the late-endosomal/lysosomal marker lysosome-associated membrane protein 1 (LAMP1) at 60 min post-infection, as described previously (Mordue and Sibley, 1997; Mordue et al., 1999a). No colocalization of evacuoles with TfR or LAMP1 was detected in samples analyzed by laser scanning confocal (CF) microscopy (Figure 3). Because actin has been reported to be involved in endocytosis in some systems (Qualmann et al., 2000), we wanted to test whether the decreased fusigenicity of evacuoles was due to the disruption of actin microfilaments by the presence of CytD. A similar absence of fusion was observed when evacuoles were allowed to form in the presence of CytD and then the drug was washed out and cells were cultured in fresh medium prior to analysis (data not shown). Therefore, evacuoles are inherently non-fusigenic with the endocytic system of the host cell, consistent with their being formed by direct secretion into the cytosol.
Fig. 3. Confocal immunofluorescence micrographs showing the absence of fusion of early and late endocytic vesicles with evacuoles. Mouse 3T3 cells containing evacuoles (A–C) were fixed after 15 min, permeabilized and stained with anti-TfR mAb R17.217.13 followed by FITC-conjugated goat anti-rat IgG. HFF cells containing evacuoles (D–F) were fixed after 60 min, permeabilized and incubated with anti-LAMP1 mAb H4A3 followed by Texas Red-conjugated goat anti-mouse IgG. Evacuoles were visualized by staining for the parasite rhoptry protein ROP2 using a specific rabbit serum followed by Texas Red (B)- or BODIPY (E)-conjugated goat anti-rabbit IgG. Scale bar = 10 µm.
To confirm the non-fusigenicity of evacuoles, co-staining with host endosomal markers was examined by immunoEM. In parallel, we examined the association of these endosomal markers with mature parasitophorous vacuoles containing intact parasites. As expected, the vast majority of parasite-containing vacuoles were devoid of TfR or LAMP1 staining (Table I and data not shown). Additionally, even when TfR- or LAMP1-positive endocytic organelles were observed in the vicinity of parasite-containing vacuoles, their respective membranes were not in close proximity (data not shown). Similarly, when we examined the distribution of evacuoles, detected by staining for ROP2, they were also largely devoid of host TfR and LAMP1 labeling and were not found in close proximity to endocytic compartments (Table I and data not shown). These data conclusively demonstrate that, like the mature parasite-containing vacuole, evacuoles remain segregated from the host cell endocytic system.
Table I. Colocalization of host cell mitochondria, ER and endosomal markers with parasitophorous vacuoles and evacuoles as determined by cryoimmunoEM.
| | Parasitophorous vacuoles (%) | Evacuoles (%) | | | ------------------------------- | ------------- | ----- | | Mitochondrial associationa | 73.3b | 51.7b | | ER (PDI-positive) associationc | 66.7b | 52.2b | | TfR-positived | 4.7e | 3.3e | | LAMP1-positived | 5.2f | 0.0f |
Evacuoles colocalize with host cell mitochondria and ER
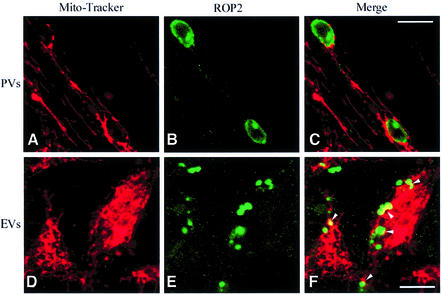
Mature _Toxoplasma_-containing parasitophorous vacuoles have been shown to associate intimately with host cell mitochondria (Jones et al., 1972; Sinai et al., 1997). To investigate whether evacuoles have a similar propensity, we examined their fate in HFF cell monolayers that had been labeled with MitoTracker Red to visualize host cell mitochondria. In parallel, we monitored the fate of parasitophorous vacuoles formed by normal parasite invasion. Minimal interaction between parasitophorous vacuoles or evacuoles and host cell mitochondria was observed in HFF monolayers that were examined after a 15 min pulse for infection (data not shown). However, when infected monolayers were incubated for an additional 45 min chase period, significant interactions with host cell mitochondria were detected (Figure 4). Mitochondria were found associated with the perimeter of parasitophorous vacuoles, detected by their content of ROP2, where they typically appeared as a beaded rim of red fluorescence (Figure 4A–C). Following the 45 min chase period, evacuoles were also found in association with host cell mitochondria as detected by the colocalization between MitoTracker Red and ROP2 (arrowheads in Figure 4F).
Fig. 4. Confocal immunofluorescence micrographs showing association of host cell mitochondria with parasitophorous vacuoles (PVs) and evacuoles (EVs). HFF cells were labeled with the mitochondrial vital dye MitoTracker Red CMXRos and challenged with parasites to form parasitophorous vacuoles in the absence of drug (A–C) or challenged with parasites in the presence of 1 µM CytD to form evacuoles (D–F). Following a 45 min chase, monolayers were fixed, permeabilized and stained for the parasite rhoptry protein ROP2 using a specific rabbit serum followed by BODIPY-conjugated goat anti-rabbit IgG to visualize the parasitophorous vacuoles (B) or evacuoles (E). Scale bars = 10 µm.
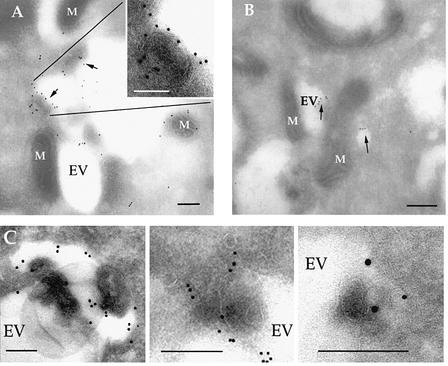
Because the resolution of light microscopy proved insufficient to evaluate whether evacuoles were intimately associated with or merely in the proximity of mitochondria, we utilized immunoEM to further examine their interaction with host organelles. Following challenge of HFF cells with Toxoplasma in the presence of CytD, clusters of evacuoles were detected by their content of ROP1 (Figure 5A) or ROP2 (Figure 5B). Evacuoles occurred as clusters within the host cell cytosol and were often associated with mitochondria. Like the rhoptry-derived vesicles formed during normal invasion (Figure 1C), evacuoles had a poorly defined limiting membrane and often contained internal membrane profiles (Figure 5A insert and 5C). The association of the parasitophorous vacuole with host cell mitochondria has been described previously as involving close apposition of their membranes (Sinai et al., 1997), which is in contrast to the normal repulsion of host cell organelles. Quantitative analyses of EM colocalization studies indicated that ∼50% of evacuoles and >70% of parasitophorous vacuoles were intimately associated with host cell mitochondria by 60 min post-formation (Table I).
Fig. 5. CryoimmunoEM localization of rhoptry proteins within evacuoles formed in host cells. (A) Immunogold staining for the parasite rhoptry protein ROP1 (detected with mAb Tg49 followed by goat anti-mouse IgG conjugated to 18 nm gold). A large cluster of evacuoles (EV), which contain internal electron-dense profiles (arrows), is closely associated with host cell mitochondria (M). Insert shows an enlarged region containing a multilamellar structure. (B) Immunogold staining for the parasite rhoptry protein ROP2 (detected with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 12 nm gold) reveals smaller clusters of evacuoles (EV) in close association with mitochondria (M). (C) Evacuoles often contained electron-dense membranous structures protruding into the lumen. Examples from samples stained with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 18 nm gold. Scale bars = 250 µm.
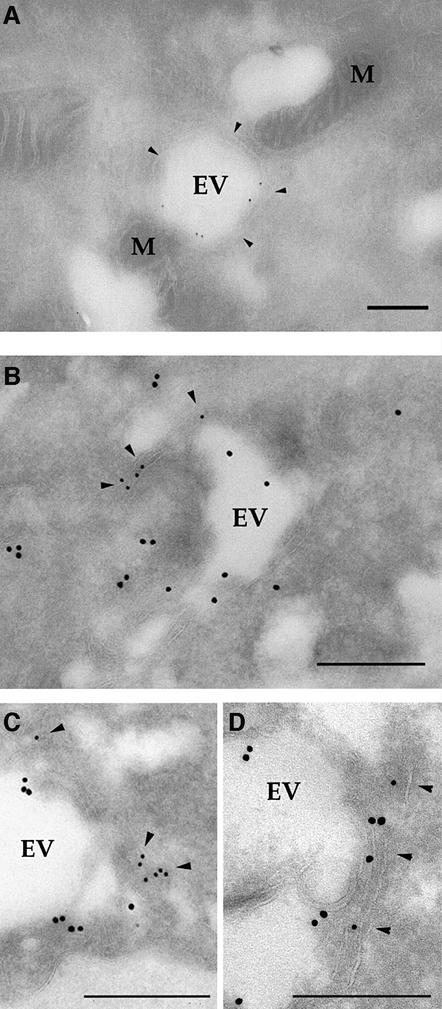
Evacuoles were also observed to form tight associations with host membranes that resembled the ER (Figure 6A). Double immunoEM labeling of ROP2-positive evacuoles with antibodies against the ER marker protein disulfide isomerase (PDI) confirmed that these enveloping membranes were components of the host cell ER (Figure 6B). Individual evacuoles were often enveloped with strands of ER, detectable as membrane cisternae that were positively stained for PDI (Figure 6C and D). Quantitative analyses of EM colocalization studies revealed that 52% of evacuoles and 66% of parasitophorous vacuoles were surrounded by PDI-positive membranes by 60 min post-infection (Table I). Thus, like the mature parasite-containing vacuole (Jones et al., 1972; Sinai et al., 1997), evacuoles are endowed with the capacity to intimately associate with host mitochondria and ER.
Fig. 6. CryoimmunoEM of evacuoles associated with host ER membranes. (A) Immunogold staining for the parasite rhoptry protein ROP2 (detected with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 12 nm gold) reveals an evacuole wrapped with a double layer of host cell membranes that resembles ER (arrowheads). M, mitochondria. (B) Colocalization of ROP2 (detected with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 18 nm gold) with the host ER marker, PDI (detected with mAb RL77 followed by goat anti-mouse IgG conjugated to 12 nm gold). Host membranes that stain positively for PDI (arrowheads) surround a prominent evacuole (EV). Scale bars = 250 nm. (C and D) Examples of evacuoles that were closely associated with ER membranes as shown by colocalization of ROP2 (18 nm gold) and PDI (12 nm gold). Staining as in (B). Scale bars = 250 nm.
Evacuoles cosediment and associate physically with host cell mitochondria
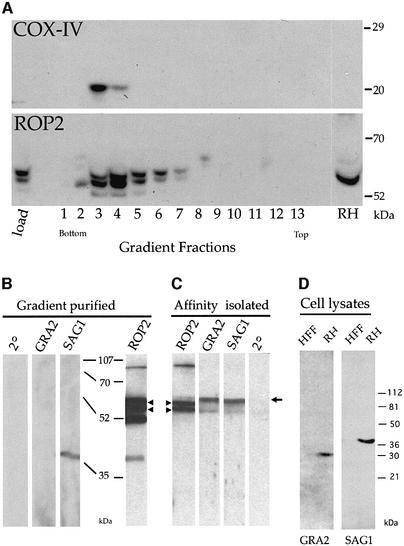
The intimate apposition of evacuoles and host cell mitochondria suggested that rhoptry-derived secretory vesicles are capable of associating physically with host organelles. To examine this interaction further, mitochondria were isolated from HFF cells harboring evacuoles. The mitochondrial pellet was resuspended in the self-forming density gradient material iodixanol and subjected to ultracentrifugation. Immunoblot analysis of the gradient fractions using a monoclonal antibody against the host cell mitochondrial protein cytochrome oxidase subunit IV (COX-IV) demonstrated that mitochondria were concentrated into two fractions near the bottom of the gradient (Figure 7A, COX-IV panel). The fractionation profile of evacuoles along the gradient was revealed by immunoblot analysis of the parasite rhoptry protein ROP2 (Beckers et al., 1994). The migration of ROP2 as a doublet, with several smaller bands in some samples, is likely to be due to proteolytic degradation during sample processing, as reported previously (Beckers et al., 1994). The distribution of ROP2 coincided with the mitochondrial fractions (3 and 4) and extended into neighboring lighter fractions (Figure 7A, ROP2 panel). The heterogeneous migration of ROP-positive vacuoles is consistent with their appearance by EM, which indicates that they are variable in size and contain different amounts of internal membranous material (see Figures 1C and 5C). Notably, the heaviest fraction of ROP-positive vesicles comigrates with mitochondria (fraction 4), suggesting that this association may change the density of evacuoles or that the association selects for a sub-class of evacuoles.
Fig. 7. Western blot analyses demonstrating co-isolation of evacuoles with host cell mitochondria. (A) Host cell mitochondria and evacuoles cosedimented on density gradients. The distribution of the host mitochondria and evacuoles along the gradient was established by probing replicate blots with mAb 1A12-A12 against the mitochondrial marker COX-IV (top) or with rabbit polyclonal antibody against the parasite rhoptry protein ROP2 (bottom) followed by the appropriate HRP-conjugated secondary antibodies. (B) Immunoblot analysis of parasite proteins that cofractionate with host cell mitochondria. Proteins in the combined fractions 3 and 4 from (A) were resolved by SDS–PAGE, blotted onto nitrocellulose and probed with rabbit polyclonal antibodies followed by HRP-conjugated goat anti-rabbit IgG. Evacuoles co-sedimented with mitochondria, as shown by the presence of the parasite rhoptry protein ROP2. A small amount of the parasite surface protein SAG1 was detected while GRA_2_ was absent; (2°) secondary antibody alone. (C) Evacuoles physically associated with host cell mitochondria. Mitochondria were affinity isolated from the gradient fractions shown in (B) using mAb II-14-10 against the host cell mitochondrial protein prohibitin and subjected to SDS–PAGE and immunoblot analysis as described above. The parasite rhoptry protein ROP2 was specifically pulled down with mitochondria, while the parasite cell surface protein SAG1, also found in the starting fraction, was not. Arrowheads indicate the position of ROP2 (the lower bands represent typical degradation products); the arrow to the right identifies a non-specific, cross-reacting protein recognized by the rabbit sera. The relative migrations of molecular weight standards are indicated in kDa. (D) Control western blots showing the relative migration of GRA2 (28 kDa) and SAG1 (36 kDa when reduced). HFF, lysates of host cells; RH, parasite lysate. Probed with mono-specific, rabbit polyclonal sera followed by HRP-conjugated secondary goat anti-rabbit IgG.
To evaluate the specificity of this co-fractionation, gradient fractions containing mitochondria were assayed by immunoblot analysis for the presence of ROP2 as well as several non-rhoptry-associated parasite proteins. As controls for non-specific binding, we used antibodies to the major cell surface protein SAG1 (Kasper et al., 1983) and to a major dense granule constituent, GRA2 (Mercier et al., 1998b and Figure 7D), which are not found in evacuoles (Carruthers and Sibley, 1997). ROP2 was abundant in the combined mitochondrial fraction, while SAG1 was only weakly detected and GRA2 was absent (Figure 7B). Thus, while evacuoles are enriched in the mitochondrial fraction of the gradient, neither dense granules nor intact parasites are found there as contaminants.
The presence of ROP2 in the mitochondrial fractions of the gradient provided only indirect evidence that evacuoles associate with host cell mitochondria and did not rule out the possibility that they merely comigrated due to a similar density. Therefore, the association of ROP2 with mitochondria was examined by immunoblot analysis following affinity isolation using a monoclonal antibody against the mitochondrial protein prohibitin. ROP2 was efficiently copurified in association with affinity-isolated mitochondria (Figure 7C), a result confirmed by immunoEM (data not shown). In contrast, neither GRA2 nor SAG1 were detected in association with affinity-isolated mitochondria (Figure 7C), even after long exposure times (data not shown). Collectively, these findings indicate that evacuoles are capable of forming a tight and specific interaction with host cell mitochondria.
Evacuoles are capable of fusing with parasite-containing vacuoles
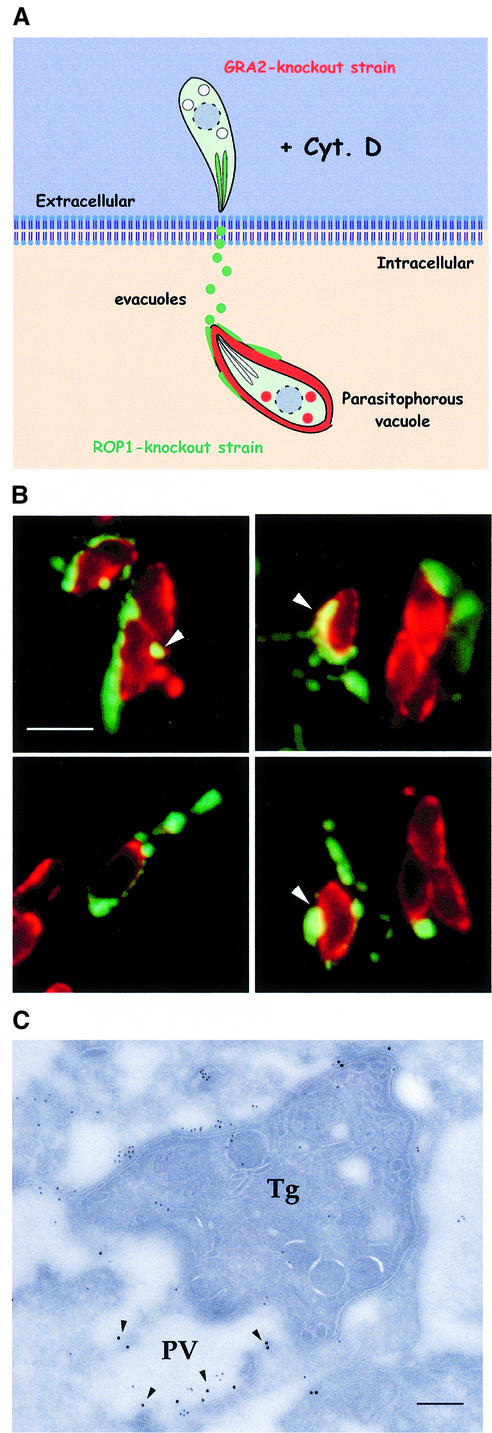
Our earlier observations indicated that evacuoles are abundantly produced during normal invasion and thus may contribute to formation of the parasitophorous vacuole. To investigate whether evacuoles are capable of interacting with previously established parasite-containing parasitophorous vacuoles, a sequential infection protocol was designed to separately discriminate the fate of these two compartments (see model, Figure 8A). To identify unambiguously the two types of vacuoles, we used two knockout strains of Toxoplasma that form fully functional parasitophorous vacuoles and support normal in vitro growth. First, a Toxoplasma mutant completely lacking the rop1 gene (Kim et al., 1993) was allowed to infect HFF monolayers and form parasite-containing vacuoles. Following extensive rinsing of the infected host cells, they were challenged anew with a gra2 knockout mutant of Toxoplasma (Mercier et al., 1998a) in the presence of CytD to prevent invasion and promote evacuole formation. Sequential infection by this scheme produced parasite-containing vacuoles devoid of ROP1 but positive for GRA2 (phenotypically referred to as Rop1–/Gra2+) and evacuoles containing the ROP1 protein as a marker.
Fig. 8. Evacuoles associate and fuse with parasitophorous vacuoles. (A) Model depicting the double mutant strategy used to track the association of evacuoles with parasitophorous vacuoles. HFF cell monolayers were challenged with a rop1 knockout strain to form parasitophorous vacuoles, rinsed thoroughly and challenged anew with a gra2 knockout strain in the presence of 1 µM CytD to form evacuoles. Parasite-containing vacuoles that are formed by the first wave of parasites are Rop1–/Gra2+. In contrast, only evacuoles formed by the second wave of parasites are Rop1+. (B) Examples of evacuoles that formed tight associations with pre-existing parasitophorous vacuoles. Evacuoles often enveloped portions of the parasitophorous vacuole and formed ribbons along the surface of the parasite-containing vacuole. Prominent colocalization was detected as a yellow fluorescence signal (arrowheads). Doubly challenged monolayers were fixed, permeabilized and stained for ROP1 using the mAb Tg49 followed by BODIPY-conjugated goat anti-mouse IgG and rabbit anti-GRA2 followed by Texas Red-conjugated goat anti-rabbit IgG. Scale bar = 10 µm. (C) Doubly challenged HFF cell monolayers processed for cryoimmunoEM. Fusion of evacuoles with pre-existing parasitophorous vacuoles (PV) resulted in colocalization of ROP1 (18 nm gold) and GRA2 (12 nm gold) in the same vacuole (arrowheads). Evacuoles were visualized by staining for ROP1 using mAb Tg49 followed by goat anti-mouse IgG conjugated to 18 nm gold. Parasite-containing vacuoles were detected by staining with rabbit anti-GRA2 followed by goat anti-rabbit conjugated to 12 nm gold. Tg denotes Toxoplasma cell. Scale bar = 250 nm.
Doubly challenged monolayers were immunostained using antibodies against the parasite secretory proteins ROP1 and GRA2 to recognize evacuoles and parasitophorous vacuoles, respectively. Indirect immunofluorescence (IF) microscopy analyses revealed numerous examples of Rop1+ evacuoles, formed by the second challenge, which had migrated into close proximity with Rop1–/Gra2+ parasitophorous vacuoles formed by the first round of infection (Figure 8B). In several instances, Rop1+ vacuoles had evidently fused with Gra2+ parasitophorous vacuoles, resulting in colocalization of signals (Figure 8B, arrowheads). To examine this interaction further, we analyzed similar doubly challenged monolayers by cryoimmunoEM. Evacuoles, detected by their content of Rop1+, were frequently observed in close apposition to pre-existing parasitophorous vacuoles distinguished by their content of Gra2+ (data not shown). The presence of the ROP1 protein within parasitophorous vacuoles labeled with GRA2 was taken as definitive evidence for delivery of evacuoles to pre-existing parasitophorous vacuoles, as this combination of markers can only arise by fusion (Figure 8C). Collectively, these observations indicate that evacuoles are endowed with the capacity to associate with and fuse with separately formed parasitophorous vacuoles.
Discussion
We demonstrate here that direct discharge of rhoptries into the host cell cytosol leads to formation of vesicles that exhibit many of the hallmark features of the mature parasite-containing vacuole. The fate of rhoptry-derived secretory vesicles, termed evacuoles, was examined in the absence of the parasite itself by exploiting the ability of the actin microfilament-destabilizing drug CytD to block parasite invasion but not junction formation or rhoptry secretion. Evacuoles formed under these conditions avoided recognition and fusion with host cell endosomes and lysosomes, while intimately associating with host cell mitochondria and ER. Similar rhoptry-derived secretory vesicles were abundantly produced during normal invasion by Toxoplasma and were capable of fusing with pre-existing parasite-containing parasitophorous vacuoles. Our results indicate that direct discharge of rhoptry components into the host cell cytosol is an important first step in the formation of the parasite-containing vacuole and that the fundamental characteristics of the vacuole are established by this early secretory event.
In contrast to the parasite-containing vacuole, which forms in part by internalization of the host cell plasma membrane, evacuoles appear to form by direct injection into the cytoplasm as shown by their failure to internalize the membrane lipid DiIC16. The secretion of rhoptry proteins by the parasite is analogous to the delivery of proteins into eukaryotic cells by bacterial type III secretion systems, which form an intimate contact with the host cell and inject bacterial proteins directly into the host cell cytoplasm (Rosqvist et al., 1995; Galan and Collmer, 1999). While type III secretion systems have been described in bacterial pathogens of plants and animals, they have not been found in eukaryotic parasites such as Toxoplasma, which instead uses an elaborate apical complex to direct protein secretion into the host cell during invasion. The importance of direct injection of proteins into the host cell cytosol during parasite invasion has not previously been recognized, in part due to the rapid nature of this event, which is tightly coupled with the invasion process. A similar process of rhoptry secretion and vacuole formation may also be important during the invasion of other apicomplexan parasites, including the human malarial parasite Plasmodium.
Previous kinetic measurements have indicated that rhoptry discharge is fully complete within the time frame necessary for parasite entry into the vacuole, which occurs in ∼15–20 s (Carruthers and Sibley, 1997). Rhoptry discharge is closely tied to the initial attachment to the host cell by the anterior end of the parasite and may underlie the abrupt spike in membrane capacitance that is detected on initial binding of the parasite to the host cell (Suss-Toby et al., 1996). Thus, rhoptry discharge apparently occurs in a forcible burst, resulting in direct injection of materials into the host cell cytosol. Evacuoles were found to contain large amounts of the rhoptry proteins ROP1 and ROP2 and they presumably also contain other rhoptry components discharged during invasion (Nichols et al., 1983; Sadak et al., 1988; Saffer et al., 1992).
Interestingly, both the rhoptry-derived secretory vesicles formed during invasion and evacuoles formed in the presence of CytD lack a clearly defined limiting membrane but contain abundant internal membranes that often appear as whorls or flattened sheets. The basis for the multilamellar morphology of the evacuoles is not precisely known but this appearance indicates that they also contain lipid that is organized into membrane sheets or bilayers. The absence of host plasma membrane or endocytic markers suggests that the lipid component of evacuoles is derived from secretion by the parasite. Rhoptry secretion has previously been associated with discharge of membranous material within the parasitophorous vacuole of both T.gondii (Nichols et al., 1983) and Plasmodium spp. (Stewart et al., 1985; Bannister et al., 1986). Consistent with this, a rhoptry-enriched fraction of Toxoplasma was found to be rich in phosphatidylcholine and cholesterol (Foussard et al., 1991). Thus, discharge of rhoptries may release preformed lipids that form the membranous structures observed in evacuoles. The subsequent insertion of rhoptry-derived lipids into a hybrid parasitophorous vacuole would be expected to significantly alter its properties and may explain the selectively fusigenic nature of this compartment. Attempts to specifically label rhoptries with fluorescent lipids, and thereby track their release in live cells, have not met with success. Future studies using purified evacuoles may allow biochemical characterization of their contents and testing of this important hypothesis.
The non-fusigenic nature of the Toxoplasma parasitophorous vacuole is believed to result from the parasite’s active mechanism of entry into the host cell. The moving junction that forms between the apical end of the parasite and the host cell plasma membrane during entry is responsible for actively excluding host proteins from entering the parasitophorous vacuole (Mordue et al., 1999a). Thus, the parasitophorous vacuole membrane lacks host cell components such as clathrin and AP-2 (Mordue and Sibley, 1997) that are normally involved in active endocytic/phagocytic uptake by the host cell (Sorkin, 2000; Tjelle et al., 2000). The parasitophorous vacuole membrane also fails to recruit host cell proteins instrumental to promoting fusion along the endocytic pathway such as small GTP-binding proteins of the Rab family and _N_-ethyl-maleamide-sensitive factor (NSF) (Mordue and Sibley, 1997). We show here that evacuoles also lack markers for host cell endocytic compartments and avoid fusion with the endocytic network of the host cell.
Concurrently with the lack of interaction with endocytic and exocytic vesicles of the host cell (Mordue et al., 1999a), the Toxoplasma parasitophorous vacuole has been shown to interact intimately with host cell mitochondria and ER (Jones and Hirsch, 1972; Endo et al., 1981; Sinai et al., 1997). Evacuoles were observed in tight association with mitochondria by EM and their membranes were generally separated by <20 nm, in contrast to the >50 nm distances that normally separate host cell organelles (Sinai et al., 1997). Evacuoles were also shown to copurify in density gradients and were co-immunoprecipitated with host cell mitochondria. These results indicate that a tight interaction forms between evacuoles and mitochondria, similar to that described previously for parasitophorous vacuoles (Sinai et al., 1997). Evacuoles did not remain at the site of formation but migrated in a process dependent on host cell microtubules. Previous studies have shown that the association of host cell mitochondria with the parasite-containing vacuole depends in part on the host cell microtubular cytoskeleton (Sinai et al., 1997). The physiological reason for this host organelle association is not known but it has been suggested to play a role in scavenging lipids for the growing parasitophorous vacuole and/or the parasites residing therein (Sinai et al., 1997). There is a precedent for acquisition of phosphatidylserine by host cell mitochondria from a specialized compartment of the ER (Chiao et al., 1995), suggesting that a similar mechanism may be exploited by the parasite. The ability of evacuoles to associate with host cell mitochondria and ER indicates that this feature of the Toxoplasma parasitophorous vacuole is mediated by rhoptry secretory components.
Evacuoles were highly enriched in the host cell cytosol when challenged with parasites under CytD arrest, and these vesicles were shown to be capable of associating and fusing with pre-existing parasitophorous vacuoles. The mechanism that drives this homotypic fusion is unknown; however, it stands in marked contrast to the resistance to fusion with host cell endocytic organelles that is characteristic of both parasite-derived compartments. Evacuoles were also formed under natural conditions of invasion and were readily detected as small vesicles staining for rhoptry proteins in the vicinity of a parasite-containing parasitophorous vacuole. Fusion of these satellite vesicles containing rhoptry proteins with the parasitophorous vacuole membrane may be important for delivery of proteins in their proper configuration to the vacuolar membrane. For example, this process may facilitate delivery of proteins like ROP2, which is inserted into the parasitophorous vacuole as a transmembrane protein with its N-terminus protruding into the host cell cytosol (Beckers et al., 1994). Furthermore, the delivery of rhoptry components to the parasitophorous vacuole may impart it with its unique ability to avoid endocytic fusion and recruit host cell organelles, inasmuch as these traits are characteristic of the evacuoles themselves.
Our findings provide a new model for biogenesis of the parasitophorous vacuole by a two-step process that resolves alternative and controversial models for its formation. During an initial burst of secretion, the contents of rhoptries are discharged directly into the cytosol of the host cell where they coalesce as multilamellar vesicles. Invagination of the host cell plasma membrane leads to formation of the vacuole, which is subsequently modified by the fusion of these rhoptry-derived secretory vesicles. The resulting hybrid vacuole resists fusion with endocytic compartments while recruiting host ER and mitochondria. This model reconciles previous studies, suggesting that parasite secretion is primarily responsible for formation of the vacuole (Nichols and O’Connor, 1981; Nichols et al., 1983; Bannister et al., 1986) with findings that the host cell plasma membrane is the primary source of the vacuolar membrane (Suss-Toby et al., 1996; Mordue et al., 1999a,b). In addition, this model may explain the phenomenon that the vacuole expands considerably during the first 30–60 min after formation (Sibley et al., 1986), despite the absence of delivery from the host cell plasma membrane, endocytic and exocytic compartments to the vacuole (Mordue et al., 1999a). The similar interactions of evacuoles and the mature parasite-containing parasitophorous vacuoles with host cell organelles suggest that the crucial characteristics of the vacuole are determined by delivery of components discharged from the rhoptries. These observations narrow the search for the parasite factors responsible for the avoidance of fusion with endocytic vesicles and for host cell organelle association with those components released from the parasite rhoptries.
Materials and methods
Chemicals and antisera
CytD (Sigma) and NOC (ICN) were dissolved in dimethylsulfoxide (DMSO) at 1 mM and stored at 4°C. CM-DiIC16 (Molecular Probes) was dissolved in ethanol at 1 mg/ml and stored at –20°C. 4-(2-aminoethyl) benzenesulfonyl fluoride, HCl (AEBSF) (Calbiochem) was dissolved in water at 100 mM and stored at –20°C. Fluorescently conjugated secondary antibodies were obtained from either Jackson ImmunoResearch Laboratoriess Inc. or Molecular Probes. Antibodies to host proteins were obtained from the Hopkins Hybridoma Center, the American Type Culture Collection or commercial sources as indicated.
Cell culture and invasion assays
Tachyzoites of the RH strain of T.gondii or genetically engineered mutant strains were maintained by serial passage in HFF grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 mM HEPES and 10% (v/v) fetal bovine serum (FBS) as described previously (Morisaki et al., 1995). Parasites were harvested by passage though 3.0 µm pore polycarbonate filters (Nucleopore) and washed in Hanks’ balanced salt solution containing 0.1 mM EGTA and 10 mM HEPES (Morisaki et al., 1995). HFF cell monolayers grown on 12 mm glass coverslips were challenged with ∼107 parasites/ml in MEM containing 3% (v/v) FBS for 5 min at 37°C. Infected monolayers were washed in phosphate-buffered saline (PBS) and returned to culture at 37°C in DMEM containing 10% (v/v) FBS for defined time periods prior to fixation. To allow formation of evacuoles, HFF monolayers were challenged with 108 parasites/ml that had been pretreated with 1 µM CytD for 10 min at room temperature. Following challenge for intervals of 10–60 min at 37°C in the presence of 1 µM CytD, the monolayers were rinsed in PBS prior to fixation.
Indirect immunofluorescence and confocal microscopy
For IF microscopy, infected monolayers were fixed in 2.5% (w/v) formaldehyde dissolved in PBS for 30 min at room temperature and permeabilized by incubation at 37°C for 15 min in PBS containing 0.05% (w/v) saponin, 5% (v/v) FBS and 5% (v/v) normal goat serum. The parasite protein ROP1 was detected using mAb Tg49 (Saffer et al., 1992) (provided by Joe Schwartzman, Dartmouth Medical School) and ROP2 was detected using a monospecific, polyclonal rabbit antiserum described previously (Beckers et al., 1994) (provided by Con Beckers, University of Alabama, Birmingham, AL). Control monolayers were incubated with hyperimmune rabbit sera to glutathione _S_-transferase as described previously (Dobrowolski et al., 1997b). Following incubation in primary antibodies, monolayers were rinsed in PBS containing 1% (v/v) FBS and incubated with fluorescently conjugated (FITC, BODIPY or Texas Red) goat anti-mouse IgG or goat anti-rabbit IgG diluted in PBS containing 1% (v/v) FBS. Host cell endocytic compartments were stained with mAb R17.217.13 (obtained from ATCC) to detect TfRs or using mAb 1D4B to detect LAMP1 (obtained from Hopkins Hybridoma Center) followed by goat anti-mouse IgG conjugated to Texas Red or BODIPY as described previously (Mordue et al., 1999a). After rinsing in PBS, the slides were mounted in Prolong Antifade (Molecular Probes) and examined using a Zeiss Axioplan fluorescence microscope equipped with a Zeiss 63× 1.4 NA plan-apochromat objective. CF microscopy was performed using a Bio-Rad MRC 1024 laser scanning confocal microscope equipped with a krypton–argon laser and outfitted as appropriate with Texas Red and fluorescein epifluorescence filter sets. Images were imported directly into Adobe Photoshop and processed using similar settings for control and experimental cells.
DiIC16 surface labeling of host cells
HFF cells grown on glass coverslips were labeled by incubation for 5 min at 15°C with 1 µg/ml of the fluorescent lipophilic dye CM-DiIC16 resuspended in 10 mM HEPES containing 300 mM sorbitol. Labeled monolayers were washed and incubated for 10 min at 37°C with either untreated parasites to allow formation of parasitophorous vacuoles or with parasites pretreated with 1 µM CytD in order to promote formation of evacuoles. Following fixation, monolayers were processed for IF as described above.
MitoTracker Red labeling of host cell mitochondria
MitoTracker Red CMXRos (Molecular Probes) was dissolved in DMSO at a final concentration of 1 mM. HFF cells grown on glass coverslips were labeled by incubation for 30 min at 37°C with 0.1 µM MitoTracker Red CMXRos in culture medium, washed in PBS and returned to culture medium for 60 min at 37°C. Labeled monolayers were incubated for 10 min at 37°C with either untreated parasites to allow formation of parasitophorous vacuoles or with parasites pretreated with 1 µM CytD in order to promote formation of evacuoles. Monolayers were washed in PBS to remove unattached parasites and incubated at 37°C for a further 50 min in medium with or without CytD. Following fixation, monolayers were processed for IF as described above.
Electron microscopy
HFF monolayers were challenged for 15 min at 37°C with untreated parasites to allow formation of parasitophorous vacuoles or challenged with parasites pretreated with CytD to promote formation of evacuoles. Monolayers were either processed immediately or after rinsing in PBS to remove extracellular parasites and incubation in complete medium at 37°C for an additional 45 min. Cells were removed from the substrate by trypsinization and pelleted by centrifugation and fixed in 4% (w/v) formaldehyde and 0.5% (v/v) glutaraldehyde in HEPES-buffered saline (30 mM HEPES, 100 mM NaCl, 0.5 mM CaCl2, 0.5 mM MgCl2 pH 7.0) on ice for 1 h. Fixed cells were pelleted, embedded in gelatin and infiltrated in PBS containing 2.3 M sucrose containing 20% (w/v) polyvinylpyrrolidone. Ultrathin sections were incubated with dilutions of mAb Tg49 to ROP1 or polyclonal rabbit antisera to ROP2 followed by goat anti-rabbit IgG or goat anti-mouse IgG conjugated to 18 or 12 nm gold particles, respectively (Amersham). Host cell endosomes were stained with mAb R17 to TfR, or mAb 1D4B to LAMP1, followed by goat anti-mouse IgG conjugated to gold as described previously (Mordue et al., 1999a). Host cell ER compartments were labeled with the mAb RL77 (Affinity Bioreagents), which recognizes PDI. For quantitative immunoEM analysis, counts were made from 10 or more negatives (magnification ×12 000–32 000) from each of four separate experiments.
Sequential infections
Sequential formation of parasitophorus vacuoles and evacuoles in the same cells was generated as follows: the rop1 knockout clone 4R2 (Kim et al., 1993) was used to infect HFF monolayers by challenge for 15 min at 37°C to form parasitophorous vacuoles as described above. Following extensive rinsing in PBS at room temperature, the same monolayers were challenged with the gra2 knockout clone B2 (Mercier et al., 1998a), which had been pretreated with 1 µM CytD for 10 min at room temperature. Following incubation for 15 min at 37°C in the presence of CytD, monolayers were again rinsed in PBS, fixed and immunostained as described above. In similar experiments, HFF monolayers were sequentially challenged with Rop1-deficient and Gra2-deficient parasites as described above and processed for cryoimmunoEM.
Mitochondrial preparation from host cells containing evacuoles
To examine the association of evacuoles with mitochondria, HFF cells were biochemically fractionated using a modification of published procedures (Enriquez and Ataardi, 1996). Briefly, parasites were resuspended in DMEM containing 3% (v/v) FBS, pretreated with 1 µM CytD for 10 min at room temperature and used to challenge HFF monolayers. Following a 1 h incubation at 37°C, excess parasites were removed by rinsing with PBS and HFF cells were removed from the substrate by gentle scraping in prechilled buffer A (1 mM Tris–HCl pH 7.0, 0.13 M NaCl, 5 mM KCl, 7 mM MgCl2 and 1 mM AEBSF). Cells were pelleted by centrifugation at 1000 g at 4°C and resuspended in 5 ml of prechilled buffer B (10 mM Tris–HCl pH 6.7, 10 mM KCl, 0.15 mM MgCl2, 1 mM AEBSF) containing 2 µM CytD and 10 µM NOC. Cells were incubated on ice for 10 min to allow the microtubule and microfilament networks to disassemble, and then mechanically disrupted by sequential passage through 20, 23, 25 and 27 gauge needles followed by homogenization using a Dounce homogenizer. Sucrose was added to a final concentration of 0.25 M and the suspension was subjected to centrifugation at 1600 g. The post-nuclear supernatant was collected and subjected to centrifugation at 8100 g for 10 min at 4°C to generate a mitochondrial pellet. The pellet was washed in 10 mM Tris–HCl pH 7.1, 30 mM EDTA, 0.25 M sucrose, resuspended in buffer B and mixed with iodixanol (Nycodenz) to generate a 20% (w/v) solution. The solution was loaded into a Quickseal ultracentrifuge tube (Beckman) and subjected to ultracentrifugation in a TLN100 near-vertical rotor (Beckman) at 180 000 g for 4 h at 4°C to generate an isopycnic gradient. The tubes were pierced with a 23 gauge needle and 150 µl fractions collected in a dropwise fashion from the bottom of the tube.
Analyses of the mitochondrial fractions
Individual fractions were analyzed by immunoblot analysis to determine the distribution of the parasite rhoptry protein ROP2 using polyclonal rabbit antibody and the host mitochondrial proteins prohibitin using mAb II-14-10 (Labvision) or COX-IV using mAb IA12-A12 (Molecular Probes). The fractions containing both ROP2 and prohibitin were pooled and assayed by immunoblot analysis for the presence of parasite proteins using specific rabbit antibodies to the surface protein SAG1(p30) (obtained from Lloyd Kasper, Dartmouth Medical College) (Kasper et al., 1983) and the dense granule secretory protein GRA2 (Mercier et al., 1998b).
To affinity purify mitochondria, anti-mouse IgG-coated magnetic beads (Dynal) were incubated with anti-prohibitin antibodies, rinsed several times with PBS containing 0.1% (w/v) bovine serum albumin (BSA) and resuspended in 400 µl of PBS/BSA containing 0.2% (v/v) Triton X-100. Portions of the mitochondrial-positive fractions were added to the antibody-coated beads, agitated overnight at 4°C and washed to remove unbound material. Specifically absorbed proteins were released from the beads by boiling for 5 min in SDS–PAGE loading buffer containing 50 mM dithiothreitol. The association of ROP2, GRA2 and SAG1(p30) with affinity-purified mitochondria was assayed by immunoblot analysis.
SDS–PAGE and immunoblot analysis
Protein samples were resolved on 7.5–12.5% SDS–PAGE gels (Laemmli, 1970) and transferred to nitrocellulose membranes by electrophoretic transfer. Immunoblots were rinsed briefly in 20 mM Tris–HCl pH 7.4 containing 150 mM NaCl and 0.1% (v/v) Tween-20 (TBST) for 1 h at room temperature, pre-incubated with 3% newborn calf serum in TBST (TBST–NCS) for 30 min at room temperature to block non-specific binding sites, and rinsed with TBST containing 500 mM NaCl. Polyclonal antisera or monoclonal ascites diluted in TBST–NCS were used to probe blots for 1 h at room temperature. Membranes were washed with TBST (one 15 min wash followed by two 5 min washes), incubated for 1 h with an horseradish peroxidase (HRP)-conjugated secondary antibody diluted in TBST–NCS and washed as before with TBST. Antibody binding was detected using enhanced chemiluminescent substrates (Pierce).
Acknowledgments
Acknowledgements
We acknowledge Con Beckers, Jean-François Dubremetz, Lloyd Kasper and Joseph Schwartzman for generous gifts of antibodies and John Boothroyd for providing the rop1 mutant used here. We are grateful for the critical review of the manuscript provided by Dan Goldberg, Joe Vogel and Hans Wolf-Watz. We thank Wandy Beatty, Marilyn Levy and Jaime Dant for expert cryoimmunoEM, and Olivia Giddings and Stacie Gooch for technical assistance. Supported in part by a grant from NIH (AI 34036) to L.D.S. S.H. was supported by the Wenner-Gren Foundation, and a grant from the MFR, Sweden. L.D.S. was partially supported by a Scholar Award in Molecular Parasitology from the Burroughs Wellcome Fund.
References
- Aikawa M., Miller,L.H., Rabbege,J.R. and Epstein,N. (1981) Freeze-fracture study on the erythrocyte membrane during malaria invasion. J. Cell Biol., 91, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister L.H., Mitchell,G.H., Butcher,G.A. and Dennis,E.D. (1986) Lamellar membranes associated with rhoptries in erythrocytic merozoites of Plasmodium knowlesi: a clue to the mechanism of invasion. Parasitology, 92, 291–303. [DOI] [PubMed] [Google Scholar]
- Beckers C.J.M., Dubremetz,J.F., Mercereau-Puijalon,O. and Joiner,K.A. (1994) The Toxoplasma gondii rhoptry protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracelluar parasite, and is exposed to the host cell cytoplasm. J. Cell Biol., 127, 947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht S., Carruthers,V.B., Ferguson,D.J., Giddings,O.K., Wang,G., Jaekle,U., Harper,J.M., Sibley,L.D. and Soldati,D. (2001) The Toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J. Biol. Chem., 276, 4119–4127. [DOI] [PubMed] [Google Scholar]
- Carruthers V.B. and Sibley,L.D. (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol., 73, 114–123. [PubMed] [Google Scholar]
- Carruthers V.B., Sherman,G.D. and Sibley,L.D. (2000) The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem., 275, 14346–14353. [DOI] [PubMed] [Google Scholar]
- Chiao Y.J., Lupo,G. and Vance,J.E. (1995) Evidence that phosphatidyl serine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidyl ethanolamine is derived from decarboxylation of phosphatidylserine. J. Biol. Chem., 270, 11190–11198. [DOI] [PubMed] [Google Scholar]
- Dobrowolski J.M. and Sibley,L.D. (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell, 84, 933–939. [DOI] [PubMed] [Google Scholar]
- Dobrowolski J.M., Carruthers,V.B. and Sibley,L.D. (1997a) Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol., 26, 163–173. [DOI] [PubMed] [Google Scholar]
- Dobrowolski J.M., Niesman,I.R. and Sibley,L.D. (1997b) Actin in Toxoplasma gondii is encoded by a single-copy gene, ACT1 and exists primarily in a globular form. Cell Motil. Cytoskeleton, 37, 253–262. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. (1977) Toxoplasma, Hammondia, Besniotia, Sarcocystis, and other tissue cyst-forming coccidia of man and animals. In Kreier,J.P. (ed.), Parasitic Protozoa. Academic Press, New York, NY, pp. 101–237.
- Dubremetz J.F., Achbarou,A., Bermudes,D. and Joiner,K.A. (1993) Kinetics and pattern of organelle exocytosis during Toxoplasma gondii host-cell interaction. Parsitol. Res., 79, 402–408. [DOI] [PubMed] [Google Scholar]
- Endo T., Pelster,B. and Piekarski,G. (1981) Infection of murine peritoneal macrophages with Toxoplasma gondii exposed to ultraviolet light. Z. Parasitenk., 65, 121–129. [DOI] [PubMed] [Google Scholar]
- Enriquez J.A. and Ataardi,G. (1996) Analysis of aminoacylation of human mitochondrial tRNAs. Methods Enzymol., 264, 183–194. [DOI] [PubMed] [Google Scholar]
- Fourmaux M.N., Achbarou,A., Mercereau-Puijalon,O., Bderre,C., Brache,I., Loyens,A., Odberg-Ferragut,C., Camus,D. and Dubremetz,J.F. (1996) The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol. Biochem. Parasitol., 83, 201–210. [DOI] [PubMed] [Google Scholar]
- Foussard F., Leriche,M.A. and Dubremetz,J.F. (1991) Characterization of the lipid content of Toxoplasma gondii rhoptries. Parasitology, 102, 367–370. [DOI] [PubMed] [Google Scholar]
- Galan J.E. and Collmer,A. (1999) Type III secretion machines: Bacterial devices for protein delivery into host cells. Science, 284, 1322–1328. [DOI] [PubMed] [Google Scholar]
- Garcia-Reguet N. et al. (2000) The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell Microbiol., 2, 353–364. [DOI] [PubMed] [Google Scholar]
- Horwitz M.A. (1983) The Legionaires’ disease bacterium (L. pneumophila) inhibits phagosome–lysosome fusion in human monocytes. J. Exp. Med., 158, 2108–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.C. and Hirsch,J.G. (1972) The interaction of Toxoplasma gondii and mammalian cells. II The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J. Exp. Med., 136, 1173–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.C., Yeh,S. and Hirsch,J.G. (1972) The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J. Exp. Med., 136, 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L.H., Crabb,J.H. and Pfefferkorn,E.R. (1983) Purification of the major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J. Immunol., 130, 2407–2412. [PubMed] [Google Scholar]
- Kim K., Soldati,D. and Boothroyd,J.C. (1993) Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science, 262, 911–914. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond.), 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Luft B.J. et al. (1993) Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med., 329, 995–1000. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Bessho,I., Uehira,K. and Suda,T. (1991) Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions. J. Electron Microsc., 40, 356–363. [PubMed] [Google Scholar]
- Mercier C., Howe,D.K., Mordue,D., Lingnau,M. and Sibley,L.D. (1998a) Targeted disruption of the GRA2 locus in Toxoplasma gondii decreases acute virulence in mice. Infect. Immun., 66, 4176–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C.M., Cesbron-Delauw,M.F. and Sibley,L.D. (1998b) The amphipathic α-helices of the toxoplasma protein GRA2 mediate post-secretory membrane association. J. Cell Sci., 111, 2171–2180. [DOI] [PubMed] [Google Scholar]
- Mordue D.G. and Sibley,L.D. (1997) Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol., 159, 4452–4459. [PubMed] [Google Scholar]
- Mordue D., Håkansson,S., Niesman,I. and Sibley,L.D. (1999a) Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol., 92, 87–99. [DOI] [PubMed] [Google Scholar]
- Mordue D.G., Desai,N., Dustin,M. and Sibley,L.D. (1999b) Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med., 190, 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki J.H., Heuser,J.E. and Sibley,L.D. (1995) Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci., 108, 2457–2464. [DOI] [PubMed] [Google Scholar]
- Nichols B.A. and O’Connor,R.G. (1981) Penetration of mouse peritoneal macrophages by the protozoan Toxoplasma gondii. Lab. Invest., 44, 324–335. [PubMed] [Google Scholar]
- Nichols B.A., Chiappino,M.L. and O’Connor,G.R. (1983) Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J. Ultrastruct. Res., 83, 85–98. [DOI] [PubMed] [Google Scholar]
- Ossorio P.N., Schwartzman,J.D. and Boothroyd,J.C. (1992) A Toxoplasma gondii rhoptry protein asscoiated with host cell penetration has an unusual charge asymmetry. Mol. Biochem. Parasitol., 50, 1–16. [DOI] [PubMed] [Google Scholar]
- Porchet-Hennere E. and Nicolas,G. (1983) Are rhoptries really extrusomes? J. Ultrastruct. Res., 84, 194–203. [DOI] [PubMed] [Google Scholar]
- Qualmann B., Kessels,M.M. and Kelly,R.B. (2000) Molecular links betwen endocytosis and the actin cytoskeleton. J. Cell Biol., 150, F111–F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Håkansson,S., Forsberg,Å. and Wolf-Watz,H. (1995) Functional conservation of the secretion and translocation machinery for virulence of yersiniae, salmonellae and shigellae. EMBO J., 14, 4187–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadak A., Taghy,Z., Fortier,B. and Dubremetz,J.F. (1988) Characteriza tion of a family of rhoptry proteins of Toxoplasma gondii. Mol. Biochem. Parasitol., 29, 203–211. [DOI] [PubMed] [Google Scholar]
- Saffer L.D., Mercereau-Puijalon,O., Dubermetz,J.F. and Schwartzman, J.D. (1992) Localization of a Toxoplasma gondii rhoptry protein by immunoelectron microscopy during and after host cell penetration. J. Protozool., 39, 526–530. [DOI] [PubMed] [Google Scholar]
- Sibley L.D., Krahenbuhl,J.L., Adams,G.M.W. and Weidner,E. (1986) Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J. Cell Biol., 103, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai A.P., Webster,P. and Joiner,K.A. (1997) Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci., 110, 2117–2128. [DOI] [PubMed] [Google Scholar]
- Sorkin A. (2000) The endocytosis machinery. J. Cell Sci., 113, 4375–4376. [DOI] [PubMed] [Google Scholar]
- Stewart M.J., Schulman,S. and Vanderberg,J.P. (1985) Rhoptry secretion of membranous whorls by Plasmodium berghei sporozoites. J. Protozool., 32, 280–283. [DOI] [PubMed] [Google Scholar]
- Suss-Toby E., Zimmerberg,J. and Ward,G.E. (1996) Toxoplasma invasion: The parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc. Natl Acad. Sci. USA, 93, 8413–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjelle T.E., Lovdal,T. and Berg,T. (2000) Phagosome dynamics and function. BioEssays, 22, 255–263. [DOI] [PubMed] [Google Scholar]
- Ward G.E., Miller,L.H. and Dvorak,J.A. (1993) The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci., 106, 237–248. [DOI] [PubMed] [Google Scholar]
- Wong S. and Remington,J.S. (1994) Toxoplasmosis in pregnancy. Clin. Infect. Dis., 18, 853–862. [DOI] [PubMed] [Google Scholar]