Absence of All Components of the Flagellar Export and Synthesis Machinery Differentially Alters Virulence of Salmonella enterica Serovar Typhimurium in Models of Typhoid Fever, Survival in Macrophages, Tissue Culture Invasiveness, and Calf Enterocolitis (original) (raw)
Abstract
In this study, we constructed an flhD (the master flagellar regulator gene) mutant of Salmonella enterica serovar Typhimurium and compared the virulence of the strain to that of the wild-type strain in a series of assays that included the mouse model of typhoid fever, the mouse macrophage survival assay, an intestinal epithelial cell adherence and invasion assay, and the calf model of enterocolitis. We found that the flhD mutant was more virulent than its parent in the mouse and displayed slightly faster net growth between 4 and 24 h of infection in mouse macrophages. Conversely, the flhD mutant exhibited diminished invasiveness for human and mouse intestinal epithelial cells, as well as a reduced capacity to induce fluid secretion and evoke a polymorphonuclear leukocyte response in the calf ligated-loop assay. These findings, taken with the results from virulence assessment assays done on an fljB fliC mutant of serovar Typhimurium that does not produce flagellin but does synthesize the flagellar secretory apparatus, indicate that neither the presence of flagella (as previously reported) nor the synthesis of the flagellar export machinery are necessary for pathogenicity of the organism in the mouse. Conversely, the presence of flagella is required for the full invasive potential of the bacterium in tissue culture and for the influx of polymorphonuclear leukocytes in the calf intestine, while the flagellar secretory components are also necessary for the induction of maximum fluid secretion in that enterocolitis model. A corollary to this conclusion is that, as has previously been surmised but not demonstrated in a comparative investigation of the same mutant strains, the mouse systemic infection and macrophage assays measure aspects of virulence different from those of the tissue culture invasion assay, and the latter is more predictive of findings in the calf enterocolitis model.
Over 40 genes are required for the structure, assembly, and function of flagella (25). These genes are categorized into three classes that are temporally expressed in a cascade-like manner. Class 1 genes include the master regulatory genes (flhD and flhC) which are required for the activation of transcription from class 2 promoters. Class 2 genes encode hook-basal body proteins (which make up the flagellar secretory apparatus), as well as the alternate sigma factor (FliA) which transcribes class 3 genes involved in motor and chemotaxis functions and filament structures. Regulation of flagellar synthesis is accomplished through an interaction between FliA and FlgM, an antisigma factor.
Previously, we and others demonstrated that flagella are not required for Salmonella enterica serovar Typhimurium virulence in the murine typhoid model (3, 23). Rather, we showed that some aspects of flagellar regulation, namely the FlgM-FliA regulatory system, are involved in the in vivo pathogenicity of serovar Typhimurium. Specifically, we found that the flgM gene, which encodes a negative regulator of flagellar synthesis (12), is required for the virulence of serovar Typhimurium in the mouse (33). We also reported that mutation of fliA, a locus which encodes the flagellin-specific sigma factor (ς28 [31]), restores virulence to a flgM mutant (33). Furthermore, we found that inactivation of fliC, the gene that encodes the phase-1 flagellin, but not fljB, the gene that encodes the phase-2 flagellin, reverses the attenuated phenotype of the flgM mutant (32). Based on this series of observations, we hypothesized that there is a link in serovar Typhimurium between the flagellar synthesis and regulation, but not flagella per se, and the virulence of the microbe for the mouse. One theoretical explanation for this association is that export of certain virulence-associated proteins may occur through the flagellar secretory components (13, 26), a suggestion based on the striking homologies between the flagellar secretory machinery and the type III virulence-associated export systems (9, 10, 13, 16, 21, 26, 35). However, in spite of the fact that several flagellar and virulence-related proteins are secreted by serovar Typhimurium (19), a role for the flagellar apparatus in the export of virulence proteins has not been demonstrated for Salmonella.
In this study, we sought to test the theory that a connection exists between the serovar Typhimurium flagellar synthesis apparatus and the virulence of the microbe. For that purpose, we constructed an flhD (the master flagellar regulatory gene) mutant of serovar Typhimurium that is incapable of producing any of the products of the flagellar cascade including regulatory elements, flagellar components, or flagellar export machinery. We also prepared an fljB fliC mutant of serovar Typhimurium that cannot produce flagellin and hence does not make flagella. We then compared the pathogenicities of the flhD mutant and the fljB fliC mutant with that of the wild-type strain (and in some cases a previously constructed fliA mutant) in a series of established virulence assays that included the mouse model of murine typhoid and the mouse macrophage survival assay, as well as in models thought to better reflect steps in the pathogenesis of gastroenteritis in humans, such as intestinal cell adherence and invasion assays and the calf loop model of enterocolitis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The following strains of Salmonella serovar Typhimurium were used in this study: the virulent wild-type strain SL3201 (15), KK2040 (flhD::Tn_10_, kind gift of K. Kutsukake [20]), SL3201 fliA::Tn_10_ (virulent, nonmotile [33]), and SL3201 fljB::Mud_J fliC_::Tn_10_ (32). Bacteria were grown at 37°C in Luria-Bertani (LB) broth (Difco Laboratories, Detroit, Mich.) with shaking or on LB agar, except where noted.
Transduction.
Bacteriophage P22HT_int_ was used as previously described (5) to transduce the flhD::Tn_10_ mutation from strain KK2040 into strain SL3201.
Motility assay.
Motility was assessed by stabbing motility agar with the bacterial strain and observing for migration away from the point of inoculation following incubation at 37°C for 18 h. Motility agar consisted of 10 g of tryptone per liter, 5 g of NaCl per liter, and 0.35% (wt/vol) agar, pH 7.4.
Assays for mouse virulence, kinetics of infection, and serology.
Mouse experiments were conducted according to the principles outlined by the National Institutes of Health (30). Virulence was assessed by 50% lethal dose (LD50) and determined with groups of 4 to 10 6- to 8-week-old female mice (C57BL/6J; Jackson Laboratories, Bar Harbor, Maine) inoculated orally with different doses of Salmonella. Food was removed from cages approximately 16 h before oral inoculation, and mice were given 25 μl of 2% NaHCO3 just prior to being fed the inoculum. Mice were monitored for death for up to 32 days, and the LD50 values were calculated by the Miller Tainter method of probit analysis (29) as detailed in reference 40. Virulence was also assessed by monitoring for death of C57BL/6J mice following intraperitoneal injection with approximately 700 CFU of organisms (33). This dose and this route of infection have been used previously to discern attenuated mutants from wild-type serovar Typhimurium strain SL3201 (33). The kinetics of infection were examined by orally infecting groups of C57BL/6J mice with approximately 107 CFU of bacteria per mouse. Five mice from each group were sacrificed at various times postinfection for the determination of CFU per spleen.
Survival in macrophages.
Survival in mouse resident peritoneal macrophages was measured as described previously (22, 33). Briefly, bacteria were grown overnight in LB broth. Bacteria were opsonized with normal mouse serum and allowed to infect resident mouse peritoneal macrophages at a multiplicity of infection (MOI) of approximately 5. Five wells per time point (T0 h, 4 h, and 24 h) were infected with each strain. After 50 min, the wells were washed three times. At 0 h, bacteria were enumerated from three wells for each strain, and the average number of macrophages was determined from the remaining two wells. Medium that contained gentamicin was added to the remaining wells. This procedure was repeated at 4 and 24 h postinfection. Controls included uninfected macrophages.
Assay for adherence and invasion of epithelial cells.
The flagellar regulatory mutants were tested in an in vitro model for the capacity to adhere to and invade two small intestinal epithelial cell lines: mouse MODE-K cells (36) and human Henle-407 cells (14). The bacteria were grown in a high salt concentration (0.3 M NaCl) for optimal adherence and invasion (34, 39). Assays were conducted with mid-log-phase to late log phase bacterial cells as described previously (7, 34). Briefly, bacteria were added to cells at an MOI of ∼20, and then the microtiter plates were gently centrifuged for 10 min at 2,000 × g. The purpose of this centrifugation step was to compensate for the lack of motility of some strains and to enhance physiological interactions of the organisms with the eucaryotic cells. The adherence assay was conducted for 90 min, and then the plates were washed. For the invasion assay, a subset of wells with washed infected cells was then incubated for an additional 90 min in the presence of media supplemented with 100 μg of gentamicin per ml. Results were calculated as mean percent adherence (number of bacteria after washes/number of bacteria in the initial inoculum × 100) or mean percent invasion (number of bacteria surviving gentamicin treatment/number of bacteria in initial inoculum × 100) from three to six samples.
Bovine ligated-ileal-loop assay for enteropathogenesis.
All bovine experiments were conducted according to the requirements of the Animal Scientific Procedures Act (United Kingdom, 1986). The assay has been described in detail elsewhere (38). Briefly, calves were terminally anesthetized with pentobarbitol, and intestinal loops were constructed in the ileum. Bacterial strains were grown overnight at 25°C, with shaking. The cultures were diluted approximately 1:3 in fresh LB medium and incubated at 37°C for 90 min, with shaking. The optical density at 600 nm was adjusted by addition of LB broth to give a concentration of approximately 3 × 108 CFU/ml. A total of 5 ml of this suspension was injected into each loop. The same volume of sterile LB broth was used as a negative control. All bacterial strains and controls were tested in three loops per animal. Polymorphonuclear leukocytes (PMNs) were isolated from 50 ml of blood removed from the calves, labeled with 111Indium (111In), and reinjected into the jugular vein. Twelve hours after inoculation, the anesthetized animals were humanely killed and all loops were exteriorized. Fluid secretion was measured as the ratio of volume of accumulated fluid to loop length. PMN influx was measured as the ratio of the 111In activity in test loops to that in control loops.
Statistical analyses.
For LD50 studies, probit analysis (29, 40) was used to calculate the standard error (SE) of the LD50. The 95% confidence limits (CL) of the LD50 were determined according to the following formula: LD50 ± 1.96 × SE of the LD50 (40). For studies of the number of Salmonella organisms per spleen over time, geometric mean (GM) values were determined for each group of five infected mice. The 95% confidence intervals about each GM were determined according to the following formula: GM ± _t_[95 for _n_-1] (2.78 for 5 animals) × SE of the GM. Means in these two sets of experiments for which 95% CL did not overlap were considered statistically significantly different at P = 0.05. For the number of Salmonella organisms per macrophage for which there were three assays per time point, the SE of the arithmetic mean was calculated, but the 95% confidence interval was not determined because of the small sample size. For data from tissue culture adherence and invasion assays that were presented as mean percentages, calculation of SE was deemed inappropriate. Rather, the range of the samples (n = 3 to 6) from which the mean percentile was calculated was given. For enteropathogenic responses induced by Salmonella in the calf loop model, Student's unpaired t test was used to compare means of groups.
RESULTS
Construction and characterization of a flagellar regulatory mutant.
The gene encoding the master regulator of flagella synthesis, flhD, was mutated in the virulent strain SL3201 by transduction of flhD::Tn_10_ from strain KK2040. As expected, SL3201 flhD::Tn_10_ was nonmotile in motility agar, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of cell lysates and supernatants from the flhD mutant showed no flagellin bands (data not shown). The in vitro growth rate of the flhD strain in rich medium was similar to that of SL3201 (data not shown).
Mouse virulence of flagellar mutants.
We compared the virulences of SL3201, the wild-type strain, SL3201 fljB::Mud_J fliC_::Tn_10_, a mutant capable of forming the basal body-hook complex but incapable of producing flagellin, and SL3201 flhD::Tn_10_, a mutant that lacks any products of the flagellar cascade including the flagellar secretory apparatus. As expected from previous studies (32), the virulence of the Fla− strain SL3201 fljB::Mud_J fliC_::Tn_10_ was very similar to that of the wild-type parent strain (Table 1). Mutation of the gene encoding the master regulator of flagellar synthesis, flhD, did not decrease the virulence of serovar Typhimurium. In fact, in each experiment the oral LD50 value of SL3201 flhD::Tn_10_ was at least 10-fold lower than that of SL3201, and the 95% CL of the mean oral LD50 value for the mice given SL3201 did not overlap with the CL of the mean oral LD50 for the mice given SL3201 flhD::Tn_10_. Therefore, the oral LD50 for the mice given SL3201 flhD::Tn_10_ was significantly lower than that of wild-type SL3201 (P = 0.05). These in vivo results suggest that products secreted solely by the serovar Typhimurium flagellar secretory apparatus are not required for invasion of the intestinal mucosa or persistence in tissues of mice.
TABLE 1.
Virulence of Salmonella enterica serovar Typhimurium flagellar mutants
| Bacterial strain | No. of dead mice/no. infecteda | Oral LD50b (CL)c |
|---|---|---|
| SL3201 | 10/10 | 1.5 × 105 (9 × 105–2.5 × 104) |
| SL3201 fljB::Mud_J fliC_::Tn_10_ | 10/10 | 1.0 × 105 (3.0 × 105–3.3 × 104) |
| SL3201 flhD::Tn_10_ | 10/10 | 1.4 × 104 (2.4 × 104–8.8 × 103) |
Kinetics of infection after oral inoculation.
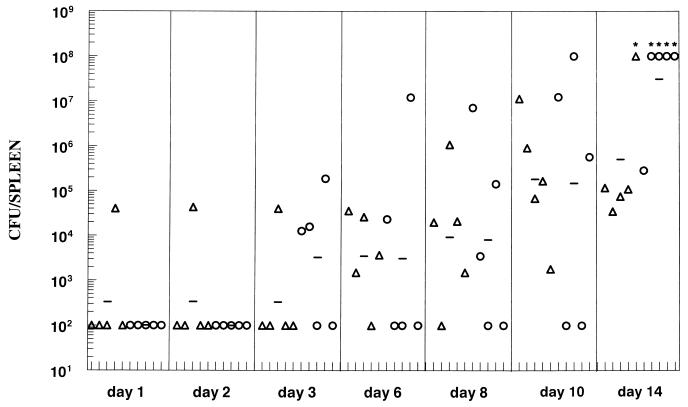
The capacities of the Fla− flhD and fljBfliC mutants to reach and multiply within the spleen were compared to those of the Fla+ wild-type strain. Mice were orally inoculated with approximately 107 CFU of SL3201, SL3201 flhD::Tn_10_, or SL3201 fljB::Mud_J fliC_::Tn_10_. By day 9 postinfection, slightly higher numbers of the flhD strain than of the wild-type strain were recovered from the spleens of infected mice, and two of five mice infected with the fljB fliC mutant had died (data not shown). We repeated and extended the kinetic study with the wild-type parent and the flhD mutant (Fig. 1). Groups of mice were infected and examined up to 14 days postinfection. Although the 95% CL of the GM of the two groups overlapped at all time points (see the legend to Fig. 2), certain trends were noted. First, Salmonella organisms were recovered from the spleens of only two mice during the first 2 days of infection. Second, the wild-type strain SL3201 was isolated from the spleens at earlier time points than was the flhD mutant (days 1 and 3). Third, four of the five _flhD_-infected mice died by day 14 of infection, compared to only one of the mice from the SL3201 group. All together, this pattern may indicate that the flhD mutant was less capable of reaching the spleen than was the wild-type strain, but once the organism reached the spleen it either replicated more rapidly or survived better than the wild-type strain.
FIG. 1.
Kinetic study of flagellar mutants recovered from the spleen following oral inoculation of mice. C57BL/6J mice were inoculated with SL3201 (triangles) or SL3201 flhD::Tn_10_ (circles) at a dose of approximately 107 CFU per mouse. At various times postinoculation up to 14 days, mice were sacrificed and the number of CFU per spleen was determined. Each symbol represents data from an individual animal. The horizontal line indicates the GM for the group of five mice. Asterisks indicate mice that had died; values of 108 CFU/spleen were assigned to these mice. The 95% CL of the GMs for the two groups overlapped at all time points including day 14 (data not shown). Limit of detection = 102 CFU/spleen.
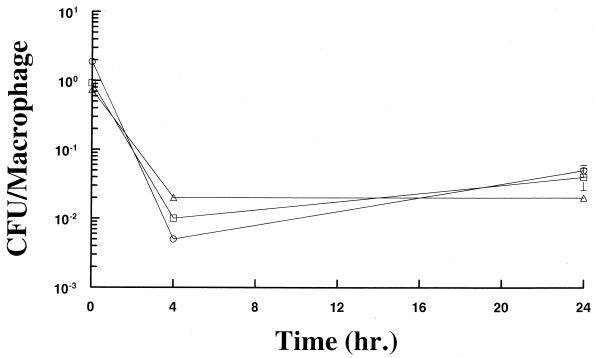
FIG. 2.
Survival in mouse resident peritoneal macrophages. Macrophages were infected in vitro with SL3201 (triangles), SL3201 flhD::Tn_10_ (circles), or SL3201 fljB::Mud_J fliC_::Tn_10_ (squares) at a MOI of approximately five opsonized bacteria per cell. The numbers of viable bacteria and macrophages per well were determined at 0, 4, and 24 h postinfection. The ratio of CFU/macrophage and one SE of the mean are shown. Note that the SE bars are covered by the symbols at 0 and 4 h postinfection.
Survival in mouse macrophages.
We have shown a correlation between the degree of survival of serovar Typhimurium in resident peritoneal macrophages and the rate of net growth of wild-type serovar Typhimurium in spleens (22). Therefore, we compared the survival rates of the flhD mutant, the fljB fliC mutant, and wild-type Salmonella in resident peritoneal macrophages. The CFU of all strains tested consistently decreased at 4 h postinfection of the macrophages (Fig. 2). However, between 4 and 24 h postinfection, the rate of net growth (difference between multiplication and death) of the flhD and fljB fliC mutants appeared to be higher than that of the wild-type strain, as revealed by the slopes of the lines between these time points which were steeper for the mutants than for the wild-type strain. By 24 h postinfection the numbers of all three bacterial strains were similar. No significant differences were seen in the numbers of macrophages present, regardless of the bacterial strain tested.
Adherence and invasion of intestinal epithelial cells.
The flagellar regulatory mutants were also tested in an in vitro model for the capacity to adhere to and invade mouse and human small intestinal epithelial cell lines (MODE-K and Henle-407). In these assays, bacteria were gently centrifuged onto the cell monolayers; this centrifugation step was included to compensate for the fact, which others have reported, that nonmotile strains of serovar Typhimurium are less invasive in vitro than their motile counterparts (8, 18). The mean percents (calculated from sample sizes of three to six) of Salmonella organisms that adhered to MODE-K cells were similar for all strains tested: for SL3201, the mean was 33% and the range was 23 to 48%; for SL3201 fliA::Tn_10_, the mean was 29% and the range was 20 to 36%; for SL3201 fljB::Mud_J fliC_::Tn_10_, the mean was 31% and the range was 16 to 47%; and for SL3201 flhD::Tn_10_, the mean was 32%, and the range was 29 to 35%. However, the wild-type serovar Typhimurium strain SL3201 invaded mouse MODE-K cells in greater numbers (mean, 20%; range, 19 to 29%) than did fliA (mean, 7%; range, 5 to 8%), fljB fliC (mean, 10%; range, 7 to 13%), and flhD flagellar mutants (mean, 6%; range, 5 to 8%), even following centrifugation of the bacteria onto the cells. The wild-type serovar Typhimurium strain SL3201 also invaded human Henle-407 cells in greater numbers (mean, 9.4%; range, 7.6 to 11%) than did the fliA (mean, 1.1%; range, 0.9 to 1.3%) and flhD (mean, 1.3%; range, 1.2 to 1.3%) flagellar mutants. These results are similar to those reported by Eichelberg and Galán (6), who found that fliA and flhD mutants of serovar Typhimurium invaded Henle-407 cells at reduced levels (∼35% of wild-type). These in vitro results suggest that the flagellar mutants are capable of binding to but are defective in penetrating the intestinal epithelial cells.
Induction of enteropathogenic responses.
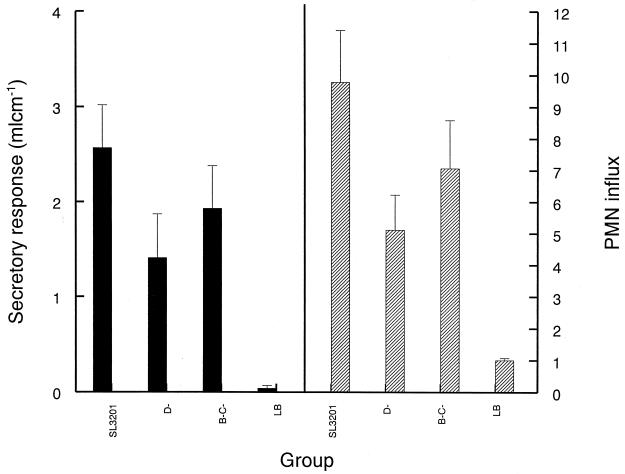
Salmonella infection can also cause enteritis in several animal hosts. Therefore, the contribution of serovar Typhimurium flagella or the flagellar secretory apparatus to enteropathogenesis was assessed in the well-established bovine ileal-loop model (Fig. 3). In that model, the secretory response induced by the flhD mutant was significantly less than that of the wild-type strain (P < 0.04 by Student's unpaired t test), as was the PMN influx evoked by the flhD mutant compared to that of the parent (P < 0.02). Moreover, the average fluid volume induced by the fljB fliC mutant and the average PMN influx in response to that mutant were reduced compared to what was observed with the parent strain, but these differences were not statistically significant.
FIG. 3.
Secretory response and PMN influx elicited by serovar Typhimurium strains in bovine intestinal loops. Approximately 1.5 × 109 CFU of the wild-type strain SL3201 (WT), SL3201 flhD::Tn_10_(D-) or SL3201 fljB::Mud_J fliC_::Tn_10_ (B-C-) was injected into each loop and left for 12 h before analysis. LB broth (LB) was also used as a negative control. The secretory response is shown as volume per length of loop. The PMN influx is a ratio of the PMN radioactivity within the infected loops to the PMN radioactivity within the control loops. Each mean is calculated from either nine loops in three calves (secretory response) or six loops in two of the three calves (PMN influx) and is presented with the SE.
DISCUSSION
In this investigation, flagellar mutants were tested in in vivo and in vitro assays to evaluate the importance of flagellar components in the virulence of serovar Typhimurium. An flhD mutant does not synthesize any flagellar components and therefore has no flagellar secretory apparatus. Both fliA and fljB fliC mutants produce functional flagellar secretory apparatuses, but the former is incapable of expressing certain class 3 gene products while the latter does not make flagellin. We and others have previously demonstrated that flagella are not necessary for the virulence of serovar Typhimurium in the mouse model of typhoid (23, 32). Consistent with the previous work, we found in this study that a mutation in the master regulator of flagellar synthesis (flhD) did not decrease virulence of the strain. The flhD mutant was virulent in mice whether administered intraperitoneally or orally (Table 1), which suggests that there are no factors secreted solely by the flagellar secretory apparatus necessary for virulence in the mouse model. In fact, the flhD mutant was slightly more virulent (the oral LD50 value was 10-fold lower than for the wild-type strain), and in kinetic studies (Fig. 1) more mice infected with the flhD mutant died than did those infected with the wild-type strain. Although the growth rate of the mutant was similar to that of the wild-type strain in rich media, it is possible that the slight increase in virulence by the flhD mutant may be due to the decreased metabolic burden resulting from the incapacity to synthesize any flagellar components. Consistent with this hypothesis is that both flagella-less mutants (flhD and fljB fliC) grew at a slightly faster net rate (flhD slightly faster than fljB fliC) in peritoneal macrophages between 4 and 24 h postinfection (Fig. 2).
All flagellar mutants tested (fliA, fljB fliC, and flhD) invaded MODE-K or Henle-407 cells less well than did the wild-type strain, although strains adhered to their target cells (MODE-K cells) similarly following centrifugation onto the cells. A possible explanation reported by others for the decreased invasion by fliA and flhD mutants is that the flagellar sigma factor (FliA) also affects the expression of invasion genes (6). Another possibility suggested by Lucas et al. (24) is that fliZ, rather than fliA, may be involved in invasion gene expression. These authors reasoned that fliZ is located downstream of fliA in the same operon. Moreover, the fliZ product activates hilA, and hilA is involved in the expression of invasion genes (1, 2). In any case, we found that the fliA (33) and flhD mutants were still virulent in susceptible mice. Therefore, we conclude that either the epithelial invasion assay does not reflect the interactions of the bacterium with the mouse intestine in the murine typhoid oral challenge model or the assay does mimic that step in the pathogenesis but the low number of flagellar mutant cells that invade epithelial cells is sufficient to cause systemic disease like the wild-type strain.
The difference between the flhD mutant and the wild-type strain in fluid secretion responses in bovine ligated ileal loops (Fig. 3) suggests that flagellar components of serovar Typhimurium are involved in enteropathogenesis. One possible explanation is that Salmonella secretes through the flagellar apparatus a factor(s) that affects fluid secretion. Type III secretion has been shown to be important in the enteropathogenesis of Salmonella infection (37). Salmonella pathogenicity island 1 (SPI-1) effectors that are secreted may be involved in cell invasion, PMN recruitment, and fluid secretion, although the correlation of cell invasion with enteropathogenesis is not clear. We speculate that the factor(s) responsible for fluid secretion that are exported by the type III secretory apparatus may also be secreted by the flagellar apparatus, or other factor(s) responsible for fluid secretion may be exported exclusively through the flagellar apparatus. Proteins secreted by the flagellar apparatus have been shown to affect virulence. For example, Yersinia enterocolitica secretes a phospholipase involved in virulence through the flagellar apparatus (42). Also, the anti-sigma factor (FlgM) regulates flagellar synthesis by secretion through the flagellar apparatus (17) and was shown to be involved in the virulence of serovar Typhimurium in the mouse model (33). However, the attenuated phenotype resulting from an flgM mutation can be reversed by a second mutation in fliA or fliC, a finding which suggests that unregulated expression of flagella attenuates the bacterial cell and not the lack of interaction of FlgM with the host cell (32, 33). Another explanation for the decreased fluid secretion response to an flhD mutant is that other flagellar proteins such as FliA or FliZ, which are not made in an flhD mutant, control expression of virulence determinants, as has been proposed by others (6, 24).
The PMN influx responses to both nonflagellated mutants (flhD and fljB fliC mutants) were lower than those of the wild-type strain (Fig. 3), suggesting that flagella or motility influences the recruitment of PMNs. Flagella of Salmonella are known to be potent inducers of cytokines (4, 41). In fact, Gewirtz et al. (11) recently reported that flagellin of serovar Typhimurium is responsible for induction of interleukin-8 (IL-8) production by intestinal epithelial cells exposed to wild-type organisms. As would be predicted from that observation, Gewirtz and colleagues also showed that serovar Typhimurium flhD and fljB fliC mutants (the same ones used in this study) failed to induce IL-8 secretion (11). IL-8 is a potent PMN attractant and is involved in PMN migration into the subepithelial and luminal compartments of the intestine by Salmonella (27, 28).
In conclusion, neither flagella nor components of the flagellar synthetic apparatus of serovar Typhimurium are required for the expression of virulence in the mouse model of typhoid fever or survival in mouse macrophages in vitro, but flagella are required for full virulence potential in tissue culture invasion assays and for the induction of a complete inflammatory response in the calf model of enteropathogenesis. In the latter model, the synthetic apparatus is also required for maximum fluid secretion in the calf intestine. The differential results obtained with these assays using the same mutant strains suggest that the mouse systemic infection and macrophage assays may measure aspects of virulence other than those measured by the tissue culture invasion assay and the calf enterocolitis model.
ACKNOWLEDGMENTS
We thank colleagues in the department and in the O'Brien laboratory for thoughtful discussions, suggestions, and critical reading of the manuscript. We also thank K. Kutsukake for strain KK2040 and S. Miller for suggestions.
The work performed in the United States was funded by Public Health Service grants from the National Institutes of Health AI 33525 (O'Brien) and AI 32951 (Metcalf) and by grant RO73FE (Metcalf) from the Uniformed Services University of the Health Sciences. The work performed at the Institute for Animal Health was jointly funded by the BBSRC, grant no. 201/S10274 (T.S.W.), the MAFF, grant no. OZ0308 (T.S.W.), and the EU, Fair 3 grant no. CT96-1743 (T.S.W.).
REFERENCES
- 1.Bajaj V, Hwang C, Lee C A. hilA is a novel ompRltoxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 3.Carsiotis M, Stocker B A D, Weinstein D L, O'Brien A D. A Salmonella typhimurium virulence gene linked to flg. Infect Immun. 1989;57:3276–3280. doi: 10.1128/iai.57.11.3276-3280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciacci-Woolwine F, Blomfield I C, Richardson S H, Mizel S B. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun. 1998;66:1127–1134. doi: 10.1128/iai.66.3.1127-1134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis R W, Botstein D, Roth J R, editors. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. pp. 13–16. [Google Scholar]
- 6.Eichelberg K, Galán J E. The flagellar sigma factor FliA (ς28) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect Immun. 2000;68:2735–2743. doi: 10.1128/iai.68.5.2735-2743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsinghorst E A, Baron L S, Kopecko D J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay B B, Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989;3:1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 9.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 10.Galán J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz A T, Simoin P O, Schmitt C K, Taylor L J, Hagedorn C H, O'Brien A D, Neish A N, Madera J L. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Investig. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillen K L, Hughes K T. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J Bacteriol. 1991;173:2301–2310. doi: 10.1128/jb.173.7.2301-2310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harshey R M, Toguchi A. Spinning tails: homologies among bacterial flagellar systems. Trends Microbiol. 1996;4:226–231. doi: 10.1016/0966-842X(96)10037-8. [DOI] [PubMed] [Google Scholar]
- 14.Henle G, Deinhardt F. The establishment of strains of human cells in tissue culture. J Immunol. 1957;79:54–59. [PubMed] [Google Scholar]
- 15.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature (London) 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 16.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 18.Jones G W, Richardson L A, Uhlman D. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J Gen Microbiol. 1981;127:351–360. doi: 10.1099/00221287-127-2-351. [DOI] [PubMed] [Google Scholar]
- 19.Komoriya K, Shibano N, Higano T, Azuma N, Yamaguchi S, Aizawa S-I. Flagellar proteins and type III-exported virulence factors are the predominant proteins secreted into the culture media of Salmonella typhimurium. Mol Microbiol. 1999;34:767–779. doi: 10.1046/j.1365-2958.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- 20.Kutsukake K, Iino T. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulation and flagellar formation in Salmonella typhimurium. J Bacteriol. 1994;176:3598–3605. doi: 10.1128/jb.176.12.3598-3605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lidell M C, Hutcheson S W. Characterization of the hrpJ and hrpU operons of Pseudomonas syringae pv. syringae Pss61: similarity with components of enteric bacteria involved in flagellar biogenesis and demonstration of their role in harpinPSS secretion. Mol Plant-Microbe Interact. 1994;7:488–497. doi: 10.1094/mpmi-7-0488. [DOI] [PubMed] [Google Scholar]
- 22.Lissner C R, Swanson R N, O'Brien A D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- 23.Lockman H A, Curtiss R., III Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990;58:137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas R L, Lostroh C P, DiRusso C C, Spector M P, Wanner B L, Lee C A. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:1872–1882. doi: 10.1128/jb.182.7.1872-1882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnab R M. Flagella and motility. In: Neidhardt F C, editor. Escherichia coli and Salmonella. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 26.Maurelli A T. Virulence protein export systems in Salmonella and Shigella: a new family or lost relatives? Trends Cell Biol. 1994;4:240–242. doi: 10.1016/0962-8924(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 27.McCormick B A, Miller S I, Carnes D, Madara J L. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick B A, Parkos C A, Colgan S P, Carnes D K, Madara J L. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 29.Miller L C, Tainter M L. Estimation of the E.D.50 and its error by means of logarithmic-probit paper. Proc Soc Exp Biol Med. 1944;57:261–264. [Google Scholar]
- 30.National Institutes of Health. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 85–23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 31.Ohnishi K, Kutsukake K, Suzuki H, Iino T. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet. 1990;221:139–147. doi: 10.1007/BF00261713. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt C K, Darnell S C, O'Brien A D. The attenuated phenotype of a Salmonella typhimurium flgM mutant is related to expression of FliC flagellin. J Bacteriol. 1996;178:2911–2915. doi: 10.1128/jb.178.10.2911-2915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt C K, Darnell S C, Tesh V L, Stocker B A D, O'Brien A D. Mutation of flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the attenuated phenotype. J Bacteriol. 1994;176:368–377. doi: 10.1128/jb.176.2.368-377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartera C, Metcalf E S. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Gijsegem F, Gough C, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S, Castello P, Boucher C. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 36.Vidal K, Grosjean I, Revillard J-P, Gespach C, Kaiserlian D. Immortalization of mouse epithelial cells by the SV40-large T gene. J Immunol Methods. 1993;166:63–73. doi: 10.1016/0022-1759(93)90329-6. [DOI] [PubMed] [Google Scholar]
- 37.Wallis T S, Galyov E E. Molecular basis of Salmonella-induced enteritis. Mol Microbiol. 2000;36:997–1005. doi: 10.1046/j.1365-2958.2000.01892.x. [DOI] [PubMed] [Google Scholar]
- 38.Wallis T S, Paulin S M, Plested J S, Watson P R, Jones P W. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect Immun. 1995;63:2755–2761. doi: 10.1128/iai.63.7.2755-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein D L, O'Neill B L, Hone D M, Metcalf E S. Differential early interactions between Salmonella enterica serovar Typhi and two other pathogenic Salmonella serovars with intestinal epithelial cells. Infect Immun. 1998;66:2310–2318. doi: 10.1128/iai.66.5.2310-2318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welkos S, O'Brien A. Determination of median lethal and infectious doses in animal model systems. In: Clark V L, Bavoil P M, editors. Methods in enzymology. 235. Bacterial pathogenesis, Part A. Identification and regulation of virulence factors. New York, N.Y: Academic Press; 1994. pp. 29–39. [DOI] [PubMed] [Google Scholar]
- 41.Wyant T L, Tanner M K, Sztein M B. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young G M, Schmiel D H, Miller V L. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc Natl Acad Sci USA. 1999;96:6456–6461. doi: 10.1073/pnas.96.11.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]