Autoregulation of the Human Liver X Receptor α Promoter (original) (raw)
Abstract
Previous work has implicated the nuclear receptors liver X receptor α (LXRα) and LXRβ in the regulation of macrophage gene expression in response to oxidized lipids. Macrophage lipid loading leads to ligand activation of LXRs and to induction of a pathway for cholesterol efflux involving the LXR target genes ABCA1 and apoE. We demonstrate here that autoregulation of the LXRα gene is an important component of this lipid-inducible efflux pathway in human macrophages. Oxidized low-density lipoprotein, oxysterols, and synthetic LXR ligands induce expression of LXRα mRNA in human monocyte-derived macrophages and human macrophage cell lines but not in murine peritoneal macrophages or cell lines. This is in contrast to peroxisome proliferator-activated receptor γ (PPARγ)-specific ligands, which stimulate LXRα expression in both human and murine macrophages. We further demonstrate that LXR and PPARγ ligands cooperate to induce LXRα expression in human but not murine macrophages. Analysis of the human LXRα promoter led to the identification of multiple LXR response elements. Interestingly, the previously identified PPAR response element (PPRE) in the murine LXRα gene is not conserved in humans; however, a different PPRE is present in the human LXR 5′-flanking region. These results have implications for cholesterol metabolism in human macrophages and its potential to be regulated by synthetic LXR and/or PPARγ ligands. The ability of LXRα to regulate its own promoter is likely to be an integral part of the macrophage physiologic response to lipid loading.
Oxidized lipid signaling in macrophages is central to the pathogenesis of atherosclerosis (20, 24). Exposure of macrophages and other vascular cells to oxidized low-density lipoprotein (oxLDL) leads to complex changes in gene expression that are collectively thought to influence the development of the atherosclerotic lesion. Mounting evidence suggests that nuclear receptor signaling pathways mediate many of the effects of oxidized lipids on cellular gene expression. Macrophage uptake of oxLDL has the potential to provide the cell with oxidized fatty acid ligands of peroxisome proliferator-activated receptor γ (PPARγ) as well as oxysterol ligands of liver X receptor α (LXRα) and LXRβ (8, 12, 13).
LXRα and LXRβ have been identified as key regulators of lipid homeostasis in multiple cell types (18). Targeted disruption of the Lxrα gene in mice uncovered roles for this receptor in the regulation of both hepatic bile acid synthesis and intestinal cholesterol absorption (16, 19). The observation that sterol regulatory element-binding protein 1-c is a target for LXRs suggests that LXRs may be involved in the control of lipogenesis (6, 17, 21). Recent work has also implicated LXRs in the control of gene expression in response to macrophage lipid loading. Multiple genes potentially involved in the cellular cholesterol efflux pathway, including the putative cholesterol/phospholipid transporter ABCA1 (5, 19, 22, 28), ABCG1 (29), and apolipoprotein E (apoE) (11), have been identified as transcriptional targets of LXR/retinoid X receptor (RXR) heterodimers. Moreover, ligand activation and/or retroviral expression of LXRα in macrophages and fibroblasts stimulates ABCA1-mediated cholesterol efflux to extracellular acceptors such as apoAI (22, 28). These observations suggest that the rate of cholesterol efflux in macrophages and other peripheral cells is controlled, at least in part, by LXR signaling pathways.
The mechanisms that control expression of the LXRα and LXRβ genes are not well understood. In mice, LXRα is expressed primarily in the liver, intestine, adipose tissue, and macrophages, whereas LXRβ is widely expressed (15, 30). Clearly, distinct _trans_-acting factors must be involved in the regulation of these genes. Liver expression of LXRα has been reported to be responsive to dietary fatty acids in mice (25). This led to the suggestion that the murine LXRα (mLXRα) gene may be a target for PPARα regulation in the liver; however, synthetic PPARα-selective ligands have not been shown to influence LXRα expression in liver cells. It has previously been shown that expression of LXRα, but not LXRβ, is induced by synthetic PPARγ ligands in both human and murine macrophages (2). As a consequence of this regulation, ligands for LXR and PPARγ additively promote cholesterol efflux from macrophages. We identified a functional PPAR response element (PPRE) in the promoter of the mLXRα gene and demonstrated that induction by synthetic PPARγ ligands is lost in PPARγ-deficient murine macrophages. Thus, expression of LXRα is not only tissue specific, but it is also likely to be regulated in response to certain metabolic signals.
We demonstrate here that expression of LXRα is highly induced in human macrophages in response to lipid loading. Moreover, we demonstrate that this lipid inducibility is likely to result from feedback induction of the LXRα gene by LXR/RXR heterodimers. The human LXRα gene (hLXRα) is a direct target for regulation by both LXR and PPARγ, and synthetic ligands for these receptors cooperatively induce its expression in macrophages. Interestingly, autoregulation of the LXRα promoter is not observed in murine macrophages. Consistent with this difference, we show that certain LXR target genes, such as apoE, are more highly regulated by LXR ligands in human versus murine macrophages. This species-specific difference in LXRα regulation may have implications for cholesterol metabolism and its potential to be regulated by synthetic LXR and/or PPARγ ligands.
MATERIALS AND METHODS
Reagents and plasmids.
pCMX expression plasmids for PPARγ, RXRα, LXRα, and LXRβ have been described (7, 28). pCMX-VP16-LXRα was a gift from Ron Evans (Salk Institute). GW7845, GW3965, and T0901317 were provided by Tim Willson (GlaxoSmithKline). LG268 was provided by Rich Heyman (Ligand Pharmaceuticals). Ligands were dissolved in ethanol or dimethyl sulfoxide prior to use in cell culture. The hLXRα promoter was cloned from the bacterial artificial chromosome (BAC) clone RP11-390K5 by PCR using the high-fidelity polymerase Pfu. A region spanning from −2625 to −1368 (relative to the transcription start site from exon 1A) was amplified by PCR from RP11-390K5 and cloned into the _Bam_HI site of pTK-Luciferase to create pTK-hLXRα(−2625)-Luc. Regions corresponding to bp −2625 to +375, −2210 to +375, −1383 to +375, and −560 to +375 of the hLXRα promoter were amplified by PCR and cloned into _Kpn_I/_Nhe_I-digested pGL3-Luciferase (Promega), creating pGL3-hLXRα(−2625)-Luc, pGL3-hLXRα(−2210)-Luc, pGL3-hLXRα(−1383)-Luc, and pGL3-hLXRα(−560)-Luc.
5′ RACE.
5′ rapid amplification of cDNA ends (RACE) was performed using the SMART RACE cDNA Amplification kit (Clontech). Briefly, first-strand cDNA was synthesized from total RNA derived from primary human macrophages or tetradecanoyl phorbol acetate-differentiated THP-1 cells using a poly(dT) primer and the SMART II oligonucleotide. 5′ RACE PCR was then performed using the Universal Primer mix and either of two gene-specific primers complementary to the hLXRα mRNA sequence [hLXRα(141), TGCCTCCCTGGGCCTGGCTGCTT, or hLXRα(391), TTGCAGCCCTCGCAGCTCAGAACAT]. Amplification was performed using touchdown PCR on a BioRad iCycler Thermal Cycler (BioRad). 5′ RACE products were cloned by TOPO TA Cloning (Invitrogen). Approximately 40 clones were sequenced.
Cell culture and transfections.
THP-1 and MonoMac-6 cells were cultured in RPMI medium containing 10% fetal bovine serum (FBS). NIH 3T3 and 3T3-F442A cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum, and HepG2 cells were grown in modified Eagle's medium (MEM) containing 10% FBS. Peritoneal macrophages were obtained from thioglycolate-injected mice as described (29) and cultured in DMEM containing 10% FBS. Human primary monocytes/macrophages were obtained as previously described (29) and maintained in Iscove's modified Dulbecco's medium containing 10% FBS. Human primary preadipocytes were obtained from ZenBio, Inc., and cultured in a Ham's F-12 medium–DMEM mixture (1:1). For ligand treatments, cells were cultured in RPMI medium, DMEM, Iscove's modified Dulbecco's medium, or Ham's F-12 medium supplemented with 10% lipoprotein-deficient serum (LPDS) (Intracel) and receptor ligands for 48 h. In some experiments, cells were sterol depleted by inclusion of 5 μM simvastatin and 100 μM mevalonic acid during the treatment period. Transient transfections of HepG2 cells were performed in triplicate in 48-well plates. Cells were transfected with reporter plasmid (100 ng/well), receptor plasmids (5 to 50 ng/well), pCMV–β-galactosidase (50 ng/well), and pTKCIII (to a total of 205 ng/well) using the MBS mammalian transfection kit (Stratagene). Following transfection, cells were incubated in MEM containing 10% LPDS and the indicated ligands or vehicle control for 24 h. Luciferase activity was normalized to β-galactosidase activity.
RNA analysis.
Total RNA was isolated using Trizol reagent (Life Technologies, Inc.). Northern analysis was performed as described (26) using radiolabeled cDNA probes. Blots were normalized using cDNA probes to 36B4 and quantitated by PhosphorImager (Molecular Dynamics) analysis. Real-time quantitative PCR assays were performed using an Applied Biosystems 7700 sequence detector. Briefly, 1 μg of total RNA was reverse transcribed with random hexamers using the Taqman Reverse Transcription Reagents kit (Applied Biosystems) according to the manufacturer's protocol. Each amplification mixture (50 μl) contained 50 ng of cDNA, 900 nM forward primer, 900 nM reverse primer, 100 nM dual-labeled fluorogenic probe (Applied Biosystems), and 25 μl of Universal PCR Master mix. PCR thermocycling parameters were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 and 60°C for 1 min. All samples were analyzed for β-actin (human) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (mouse) expression in parallel in the same run using probe and primers from predeveloped assays for β-actin and GAPDH (Applied Biosystems). Quantitative expression values were extrapolated from separate standard curves for β-actin or GAPDH and human- or mouse-generated expression with 10-fold dilutions of cDNA (in duplicate). Each sample was normalized to β-actin or GAPDH, and replicates were then averaged and fold induction was determined. The following human primers were used: hLXRα forward (F) (5′-AAGCCCTGCATGCCTACGT-3′), hLXRα reverse (R) (5′-TGCAGACGCAGTGCAAACA-3′), hLXRα Taqman probe (FAM-CCACCATCCCCATGACCGACTGAT-TAMRA), human apoE (hapoE) F (5′-CGCTGGGTGCAGACACTGT-3′), hapoE R (5′-GGCCTTCAACTCCTTCATGGT-3′), and hapoE probe (FAM-TCCATCAGCGCCCTCAGTTCCTG-TAMRA). The following murine primers were used: mLXRα F (5′-CAACAGTGTAACAGGCGCT-3′), mLXRα R (5′-TGCAATGGGCCAAGGC-3′), mLXRα Taqman probe (FAM-TCAGACCGCCTGCGCGTCA-TAMRA), murine apoE (mapoE) F (5′-GGAGGTGACAGATCAGCTCGA-3′), mapoE R (5′-TCCCAGAAGCGGTTCAGG-3′), and mapoE probe (FAM-CAAAGCAACCAACCCTGGGAGCAG-TAMRA).
Gel shift assays.
In vitro-translated RXRα, LXRα, and PPARγ were generated from pCMX-RXRα, pCMX-hLXRα, and pCMX-PPARγ plasmids using the TNT Quick Coupled Transcription/Translation system (Promega). Gel shift assays were performed as described (10) using in vitro-translated proteins and the following oligonucleotides (only one strand shown): hLXRα PPRE (GATCGGATTTTGAACTTTGTACTTGTTTCC), hLXRα DR4-A (GATCGGGTGGATCACTTGAGGTCAGGAG), hLXRα DR4-B (GATCAGATGGATCACTTGAGGTCAGGAG), hLXRα DR4-C (GATCGCTGAGGTTACTGCTGGTCATTCA), and CYP7A LXR response element (LXRE) (CCTTTGGTCACTCAAGTTCAAGTG).
RESULTS
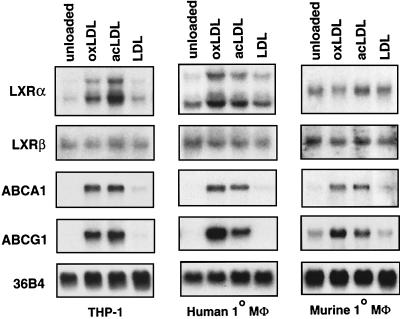
Previous work has demonstrated that macrophage expression of PPARγ is induced in response to oxLDL (27). We investigated whether expression of LXRα or LXRβ in macrophages might also be regulated by modified lipoproteins. The human monocytic cell line THP-1 was used as a model system. THP-1 cells were differentiated for 24 h with 40 ng of tetradecanoyl phorbol acetate per ml and then treated in the presence of LPDS for 48 h with either vehicle alone or 100 μg of (protein) LDL, oxLDL, or acetylated LDL (acLDL) per ml. In order to ensure maximal sterol depletion of the cells, treatments were carried out in the presence of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor simvastatin (5 μM) and mevalonic acid (100 μM). As shown in Fig. 1, the treatment of THP-1 macrophages with oxLDL or acLDL led to a significant induction of LXRα mRNA. In contrast, native LDL, which is not readily internalized by these cells, had no effect on LXRα expression. Expression of the related nuclear receptor LXRβ was not altered in response to native or modified LDL. Induction of LXRα mRNA in these experiments paralleled that of the known LXR target genes ABCA1 and ABCG1. Similar results were obtained with primary human monocyte-derived macrophages (Fig. 1). Surprisingly, while oxLDL and acLDL were effective inducers of ABCA1 and ABCG1 expression in murine macrophages, they had little or no effect on LXRα expression. Modified LDL also had no effect on LXRα expression in murine RAW264.7 macrophages (reference 28 and data not shown). These observations suggest that species-specific differences may exist in the mechanisms controlling LXRα expression.
FIG. 1.
Induction of LXRα expression in human macrophages in response to modified LDL loading. Differentiated THP-1 macrophages, primary human monocyte-derived macrophages (Mφ), or thioglycolate-elicited mouse peritoneal macrophages were incubated for 48 h in RPMI medium containing 10% LPDS, 5 μM simvastatin, and 100 μM mevalonic acid. Cells were treated with vehicle control or 100 μg (protein) of either LDL, oxLDL, or acLDL per ml as indicated. Total RNA (10 μg/lane) was electrophoresed through formaldehyde-containing gels, transferred to nylon, and hybridized to 32P-labeled cDNA probes. 36B4 was used as a control for loading and integrity of the RNA.
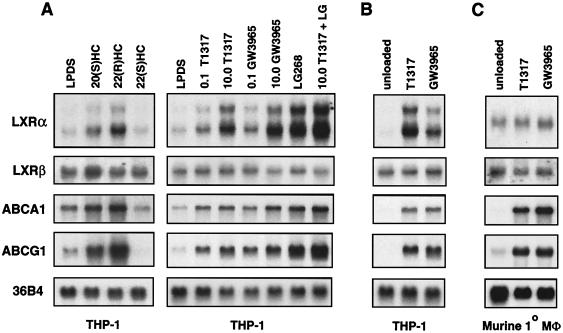
The ability of modified LDL to modulate LXRα gene expression led us to hypothesize that the LXRα gene might itself be a downstream target of the LXR signaling pathway in human macrophages. To address this possibility, we examined the ability of oxysterols and synthetic LXR ligands to regulate LXRα expression in various macrophage cell lines. As shown in Fig. 2A, the treatment of human THP-1 macrophages with the oxysterol LXR ligand 22(R)-hydroxycholesterol (2 μg/ml) or with either of two synthetic LXR agonists, T1317 (19, 21) and GW3965 (14), led to a prominent induction of LXRα mRNA expression. Treatment with the synthetic RXR-specific agonist LG268 (100 nM) also induced LXRα expression, and the combination of the LXR ligand and LG268 had an additive effect. 22(S)-hydroxycholesterol (2 μg/ml), which binds but does not activate LXRs (23), did not alter LXRα expression. Similar to the results obtained with modified lipoproteins (Fig. 1), induction of ABCA1 and ABCG1 expression by nuclear receptor ligands paralleled that of LXRα. Expression of LXRβ was not influenced by LXR or RXR ligands. An even more dramatic induction of LXRα expression by LXR ligands was observed when endogenous cholesterol synthesis was inhibited by treatment with simvastatin (Fig. 2B). Similar results were observed in primary human monocyte-derived macrophages and MonoMac-6 cells (Fig. 3 and data not shown). The reduced basal expression of LXRα observed in the presence of simvastatin suggests that endogenous oxysterol LXR ligands are required for tonic expression of LXRα. Interestingly, the sterol regulatory element-binding protein 1-c gene has been reported to be a target for LXR and to be similarly dependent on endogenous LXR ligands for its expression in liver cells (6, 17).
FIG. 2.
Oxysterols and synthetic LXR ligands stimulate LXRα expression in THP-1 macrophages. Differentiated THP-1 macrophages (A and B) or thioglycolate-elicited mouse peritoneal macrophages (Mφ) (C) were incubated for 48 h in RPMI medium plus 10% LPDS or RPMI medium plus 10% LPDS, 5 μM simvastatin, and 100 μM mevalonic acid (unloaded). Oxysterols [20(S)HC, 22(R)HC, or 22(S)HC, 2.0 μg/ml], synthetic LXR ligand (GW3965 or T1317, 0.1 to 10.0 μM), or RXR ligand (LG268, 50 nM) was included as indicated. Northern analysis was performed as described in the legend to Fig. 1.
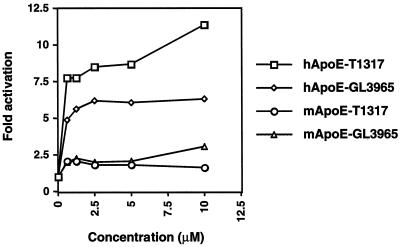
FIG. 3.
Differential induction of the LXR target gene apoE by LXR ligands in human and murine macrophages. Differentiated THP-1 macrophages or thioglycolate-elicited mouse peritoneal macrophages were incubated for 48 h in RPMI medium plus 10% LPDS, 5 μM simvastatin, and 100 μM mevalonic acid. Cells were treated with the indicated concentrations of either T1317 or GW3965. The expression of apoE mRNA was monitored by real-time quantitative PCR (Taqman) assays (see Materials and Methods).
In contrast to the results obtained with human macrophages, treatment of primary murine macrophages or the murine macrophage cell line RAW264.7 with LXR-selective ligands did not significantly alter LXRα expression (Fig. 2C and data not shown). The failure of LXRα to be induced in murine macrophages does not result from a general defect in the LXR signaling pathway, since the LXR target genes ABCA1 and ABCG1 are effectively induced in these cells (Fig. 2C). Rather, the ability of LXRs to regulate LXRα expression is apparently species specific. The possibility that the murine gene is responsive to LXR ligands in certain tissues or under certain conditions not tested here, however, cannot be excluded.
The species-specific difference in the ability of LXR ligands to induce LXRα receptor expression suggested the possibility that human macrophages may be more responsive than murine macrophages to LXR activation. Previous work has indicated that the LXR target gene apoE is particularly sensitive to the level of LXR present in the cell. Induction of apoE expression by LXR ligand is significantly reduced in macrophages from either LXRα−/− or LXRβ−/− mice, even in the presence of high concentrations of ligand (11). We therefore compared the dose response of the LXR target apoE to two synthetic LXR ligands in THP-1 cells and murine macrophages. As shown in Fig. 3, the induction of apoE was significantly higher in human cells in response to both GW3965 and T1317. This difference is consistent with the higher level of LXRα receptor expression in human cells in the presence of LXR ligand. Thus, the ability of the hLXRα gene to undergo autoregulation is likely to have implications for LXR target gene expression.
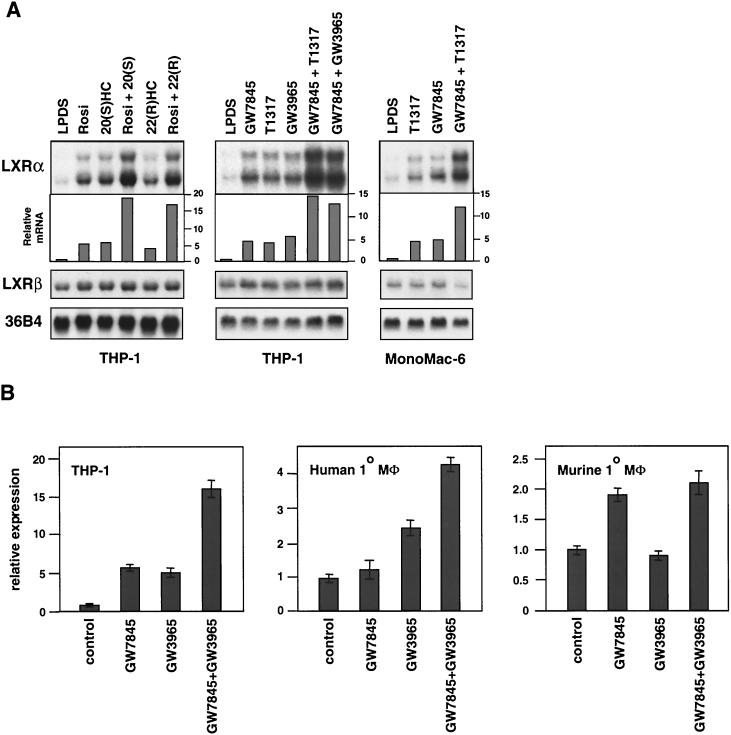
Previous work has shown that macrophage LXRα expression is also induced by PPARγ-specific ligands (2, 4). Accordingly, activation of PPARγ in THP-1 cells leads to the induction of primary LXR target genes such as ABCA1 and ABCG1. In contrast to LXR ligands, PPARγ ligands have been shown to promote LXRα mRNA expression in both human and murine macrophages. We therefore examined the effects of simultaneous activation of both the PPARγ and LXR pathways on macrophage gene expression. RNA expression was monitored by either Northern analysis or real-time quantitative PCR (Taqman) assays (see Materials and Methods). As shown in Fig. 4, the treatment of THP-1 macrophages with oxysterol LXR ligands, synthetic LXR ligands (T1317 or GW3965), or synthetic PPARγ ligands alone (rosiglitazone or GW7845) stimulated LXRα expression. The combination of an LXR ligand and a PPARγ ligand had an additive effect. Similar results were obtained with the human monocytic cell line MonoMac-6 (Fig. 4A) and human primary monocytes/macrophages (Fig. 4B). Interestingly, the response of LXRα to this combined treatment was much more prominent than that observed for ABCA1 or ABCG1 (reference 2 and data not shown). This observation suggested that the hLXRα gene might be a direct target of both LXR/RXR and PPARγ/RXR heterodimers, whereas ABCA1 and ABCG1 are likely to be direct targets of only LXR/RXR. Consistent with the results shown in Fig. 2C, LXR ligands failed to induce LXRα expression in murine macrophages, even when used in combination with a PPARγ ligand (Fig. 4B).
FIG. 4.
Ligands for LXR and PPARγ additively induce LXRα expression in human macrophages. Differentiated THP-1 macrophages, MonoMac-6 cells, human monocyte-derived macrophages (Mφ), or thioglycolate-elicited mouse peritoneal macrophages were incubated for 48 h in RPMI medium plus 10% LPDS. Oxysterols {20(S)HC [20 (S)] or 22(R)HC [22 (R)], 2.0 μg/ml}, synthetic LXR ligands (GW3965 or T1317, 5 μM), and/or PPARγ ligands (rosiglitazone [Rosi] or GW7845, 5 μM) were included as indicated. Northern blots (A) were quantitated by phosphorimager analysis and normalized to 36B4. The level of expression relative to LPDS control (fold induction) is indicated; real-time quantitative PCR assays (B) were performed in duplicate as described in Materials and Methods and normalized to 36B4 or β-actin. The level of mRNA expression relative to control (fold induction) is indicated.
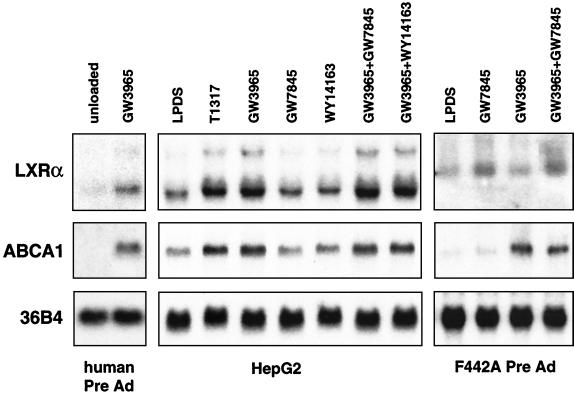
We further addressed whether the ability of LXRs and PPARs to regulate LXRα expression was specific to macrophages or whether it also occurred in other cell types. As shown in Fig. 5, autoregulation of the hLXRα gene was also observed in liver and adipose cell lines. Treatment with the LXR ligand GW3965 or T1317 induced LXRα expression in both human preadipocytes and the human hepatoma cell line HepG2. As in macrophages, induction of LXRα expression paralleled induction of ABCA1. In contrast, ligands for PPARγ (GW7845) or PPARα (WY14613) had no effect on LXRα expression in HepG2 cells. Consistent with the results obtained in macrophages, the mLXRα gene was induced by the PPARγ ligand but not by the LXR ligand in 3T3-F442A preadipocytes under similar conditions. In experiments not shown, we have observed induction of LXRα mRNA expression by synthetic PPARγ and LXR ligands in THP-1 cells in the presence of the protein synthesis inhibitor cycloheximide but not in the presence of the RNA polymerase inhibitor actinomycin D, consistent with direct transcriptional effects. Taken together, these results reveal a partially overlapping pattern of regulation of the mLXRα and hLXRα genes by nuclear receptors. Both the hLXRα and mLXRα genes are targets for PPARγ regulation in macrophages and adipocytes. The hLXRα gene, but not the mLXRα, is also regulated by LXR itself in multiple cell types, including macrophages, adipocytes, and hepatocytes.
FIG. 5.
Autoregulation of the hLXRα gene in preadipocytes and HepG2 cells. Primary human preadipocytes (Pre Ad), HepG2 cells, or 3T3-F442A murine preadipocytes were cultured for 48 h in media containing 10% LPDS and one or more of the following nuclear receptor ligands as indicated: GW3965 (5 μM), T1317 (5 μM), GW7845 (5 μM), and Wy14643 (50 μM).
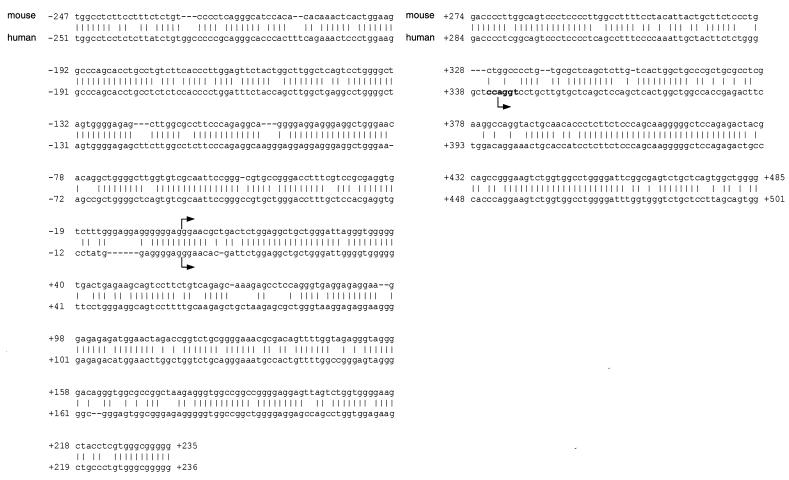
To investigate the molecular basis for the regulation of hLXRα expression by LXR and PPARγ ligands, we cloned and analyzed the 5′-flanking region from the hLXRα gene. The transcriptional start site of the hLXRα gene was mapped by 5′ RACE using RNA derived from primary human macrophages and THP-1 cells. Analysis of the 5′ RACE products revealed two distinct transcriptional start sites (Fig. 6). As a result of alternative splicing, these give rise to two alternatively utilized exon 1 sequences (Fig. 7A). The vast majority of the products of the 5′ RACE reactions corresponded to use of the downstream (exon 1B) start site, suggesting that this is the primary site utilized in human macrophages. The genomic sequence and organization of the hLXRα gene were determined by searching the human genome and the high throughput genomic sequence databases (National Center for Biotechnology Information) using the revised mRNA sequence for hLXRα. We identified a BAC clone (RP11-390K5) containing the entire hLXRα mRNA sequence and approximately 5 kb of the 5′-flanking sequence. Comparison of the hLXRα and mLXRα genomic sequences revealed a similar genomic structure, with each gene composed of 10 exons. Exon 1A from the hLXRα gene shows a high level of homology to the previously reported transcriptional start site for the mLXRα gene. In the human genomic sequence, exon 1B is located approximately 343 bp downstream of the exon 1A start site. This sequence is conserved in the murine genomic sequence, and a previous report suggested that this region might be utilized as an alternative start site in the mouse (1). In both humans and mice, exon 1 is comprised entirely of untranslated sequence; therefore, the use of alternative exon 1 sequences does not impact the protein product.
FIG. 6.
Sequence comparison of the hLXRα and mLXRα transcriptional start sites. The putative transcriptional start sites are marked by arrows. The initiator element is indicated in bold type. The previously published mouse transcriptional start site is designated as position +1 (1).
FIG. 7.
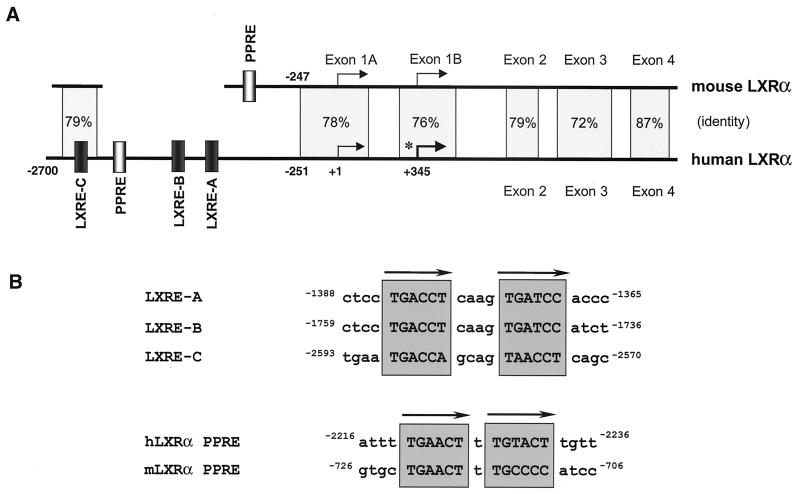
Comparison of the hLXRα and mLXRα promoter regions. (A) Schematic representation of the hLXRα and mLXRα genes. Transcription start sites, genomic structure, sequence identity, and nuclear receptor binding sites are indicated. The asterisk denotes the major start site in macrophages. The arrows indicate hormone response element half sites. (B) Sequences of the LXREs and PPREs from the hLXRα and mLXRα promoters.
The proximal 2.6 kb of the hLXRα promoter region from BAC clone RP11-390K5 was cloned and sequenced. Comparison with the mLXRα gene revealed conservation of the transcriptional start regions and similarity up to approximately 250 bp upstream of the exon 1A start site (79% identity) (Fig. 6 and 7). However, relatively poor conservation of the sequence was found further upstream. In particular, the previously identified PPRE located in the mLXRα gene (2) is not conserved in the human sequence. A potential PPRE (DR-1) was identified in the hLXRα 5′-flanking region that is not conserved in location or sequence in comparison to the mouse (Fig. 6). In addition, the hLXRα gene was found to contain three potential LXREs (DR-4). Only one of these potential LXREs (LXRE-C) was in a region conserved in the mouse promoter sequence; furthermore, the mouse DR4-C sequence differed from the human sequence within one half site.
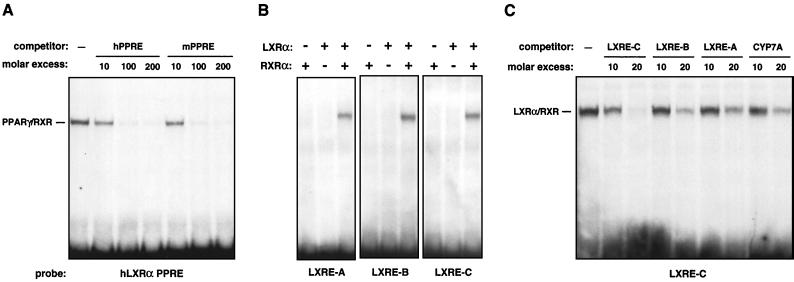
We next endeavored to determine whether the identified elements represented bona fide binding sites for LXR/RXR or PPARγ/RXR heterodimers. As shown in Fig. 8A, gel mobility shift analysis using in vitro-translated proteins and radiolabeled oligonucleotides confirmed that the putative PPRE from the hLXRα gene bound PPARγ/RXR heterodimers with affinity similar to that of the previously identified PPRE from the mLXRα gene (2). Furthermore, all three putative LXREs bound in vitro-translated LXRα/RXR (Fig. 8B). Competition assays indicated that the distal element (LXRE-C) bound LXRα/RXR with significantly higher affinity than LXRE-A, LXRE-B, or the LXRE from the murine CYP7A gene (Fig. 8C).
FIG. 8.
PPARγ and LXRα bind to response elements in the hLXRα promoter. Gel mobility shift assays were performed using in vitro-translated receptors and end-labeled oligonucleotide probes as described in Materials and Methods. (A) Direct binding of PPARγ/RXR heterodimers to a putative PPRE from the hLXRα promoter. (B) Direct binding of LXRα/RXR heterodimers to the LXRE-A, LXRE-B, and LXRE-C sites from the hLXRα promoter. (C) Competition for LXRα/RXR binding to LXRE-C. Unlabeled oligonucleotide was included in the binding reaction at the indicated molar excess.
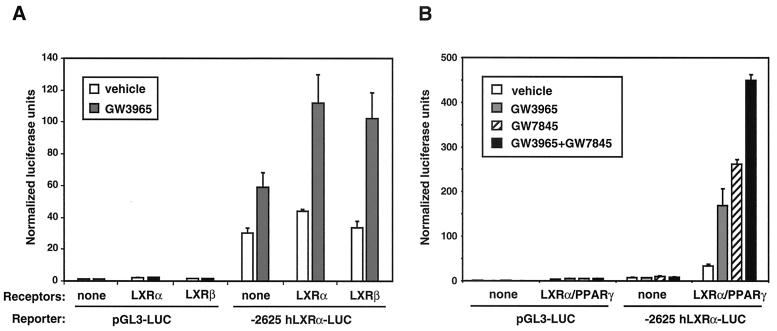
Finally, we analyzed the ability of PPARγ, LXRα, and LXRβ to regulate the hLXRα promoter in transient transfection assays. A pGL3-based luciferase reporter construct containing bp −2625 to +345 of the hLXRα promoter (−2625 hLXRα-luc) was transiently transfected into HepG2 cells along with pCMX expression vectors encoding LXRα, LXRβ, RXRα, and/or PPARγ. As shown in Fig. 9A, the LXRα/RXRα and LXRβ/RXRα heterodimers activated the hLXRα promoter in a ligand-dependent manner, but they had no effect on the control pGL3-luc reporter. Note that since HepG2 cells express endogenous LXRs, a background level of ligand-dependent induction of the reporter is seen in the absence of transfected receptor. When expression vectors for both LXRα and PPARγ were cotransfected with the hLXRα promoter, an additive effect of LXR-selective (GW3965) and PPARγ-selective (GW7845) ligands was observed (Fig. 9B). These results confirm that the LXR and PPAR binding sites identified above are in fact able to mediate activation of the hLXRα promoter by PPARγ/RXR, LXRα/RXR, and LXRβ/RXR heterodimers. Moreover, the additive effect of PPARγ and LXR activation on the hLXRα promoter in transient transfection assays is consistent with the ability of PPAR and LXR ligands to additively induce expression of the endogenous hLXRα gene.
FIG. 9.
LXRα, LXRβ, and PPARγ activate the hLXRα promoter. (A) LXRα/RXRα and LXRβ/RXR heterodimers activate the −2625-bp LXRα proximal promoter. HepG2 cells were transfected with either control pGL3-luc or -2625 LXRα-luc reporters with or without CMX-mLXRα/CMX-RXRα or CMX-mLXRβ/CMX-RXRα and CMV–β-galactosidase. Following transfection, cells were incubated for 24 h in MEM supplemented with 10% LPDS and 1 μM GW3965 or vehicle control. Luciferase activity was normalized for transfection efficiency using β-galactosidase activity. (B) Ligand activation of PPARγ and LXRα has an additive effect on the −2625-bp LXRα promoter. HepG2 cells were transfected as in panel A except that CMX-mPPARγ1 and GW7845 (1 μM) were included as indicated.
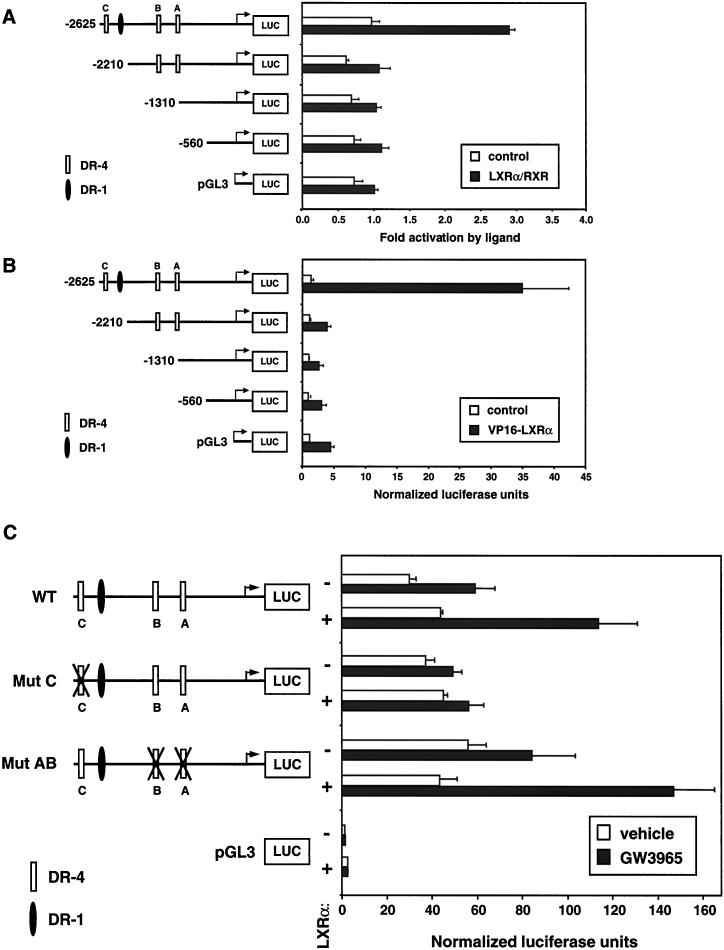
In order to address the relative importance of the individual nuclear receptor binding sites, deletion and mutation analyses were performed. We analyzed the ability of LXRα and RXR expression vectors to activate luciferase reporters containing bp −2625 to +345, −2210 to +345, −1310 to +345, or 560 to +345 of the hLXRα promoter. Surprisingly, deletion from bp −2625 to −2210, which deletes LXRE-C, resulted in the complete loss of LXR responsiveness (Fig. 10A). Similar results were obtained with an expression vector encoding a superactive VP16-LXRα fusion protein (11). The construct containing LXRE-C (bp −2625 to +345) was activated over 30-fold by VP16-LXRα, whereas those lacking this element were unresponsive (Fig. 10B). These observations suggested that the LXRE-C element is required for induction of the hLXRα promoter by LXR. To test this directly, we introduced specific mutations in each of the LXREs. As shown in Fig. 10, mutation of LXRE-C alone abolished promoter activation by LXRα, while simultaneous mutation of LXRE-A and LXRE-B had no effect. These results indicate that LXRE-C is the primary element mediating induction of the LXRα promoter by LXR/RXR heterodimers. The other potential response elements (LXRE-A and -B) apparently do not contribute to the induction of the hLXRα promoter despite their ability to bind LXR/RXR in vitro. This is consistent with the observation that LXRE-C has the highest affinity for LXR/RXR of the three sites (Fig. 8). Taken together, these results demonstrate that the hLXRα promoter is a direct target for regulation by both PPARγ/RXR and LXRα/RXR heterodimers.
FIG. 10.
Deletion and mutation analysis of the hLXRα proximal promoter. (A) Luciferase reporters containing bp −2625 to +345, −2210 to +345, −1310 to + 345, or 560 to +345 of the hLXRα promoter were transfected into HepG2 cells in the presence or absence of CMX-hLXRα, CMX-RXRα, and 5 μM T1317. Luciferase activity was normalized for transfection efficiency using β-galactosidase activity. The data are expressed as fold activations in the presence of the indicated ligand versus in the absence of ligand and represent the average of triplicate experiments. (B) hLXRα promoter constructs were cotransfected into HepG2 cells along with CMX vector or CMX-VP16-LXRα in the presence of 5 μM T1317. Data are expressed as relative luciferase activities normalized to β-galactosidase activities and represent the average of triplicate experiments. (C) Mutations were introduced into individual LXREs in the bp −2625 to +345 hLXRα promoter construct by site-directed mutagenesis. Wild-type (WT) and mutant (Mut) reporters were transfected into HepG2 cells along with CMX-hLXRα and CMX-RXRα in the presence or absence of 1 μM GW3965. Data are expressed as luciferase activities normalized to β-galactosidase activities and represent the averages of triplicate experiments.
DISCUSSION
Members of the nuclear receptor superfamily are now recognized to play a central role in the control of lipid-inducible gene expression. Both the PPAR and LXR subfamilies have been implicated in the regulation of gene expression and lipid metabolism in response to specific lipid ligands. PPARγ is expressed at high levels in a number of specialized cell types, including adipocytes, colonic epithelia, and macrophages. No high-affinity endogenous ligand for this receptor has been described; however, physiologic activators of PPARγ are likely to include native and oxidized polyunsaturated fatty acids (7, 9, 13). LXRα is expressed at high levels in many of the same tissues as PPARγ, including macrophages and adipose tissue, while LXRβ is ubiquitously expressed. Considerable evidence suggests that the physiologic ligands for LXRs are oxysterols such as 24(S)-hydroxycholesterol and 24(S),25-epoxycholesterol (8, 12, 23).
In macrophages, the PPAR and LXR families appear to coordinate a physiologic response to oxLDL uptake and lipid loading. A primary functional consequence of PPAR and LXR activation in macrophages is the induction of a pathway for cholesterol and phospholipid efflux. Ligands for either receptor additively promote cholesterol efflux from human macrophages (2, 28). The role of PPARγ in this response appears to be to induce expression of the scavenger receptor CD36, the HDL receptor SR-BI, and LXRα (2, 3, 27). The role of LXR in this response appears to be to regulate several genes that have been directly implicated in the cholesterol efflux pathway including ABCA1, ABCG1, and apoE (5, 11, 22, 28, 29). Ligands for PPARγ and LXR additively promote cholesterol efflux from macrophages, presumably as a consequence of the ability of PPARγ to control LXRα expression.
In the present study, we have shown that the hLXRα gene is itself induced in macrophages in response to cellular lipid loading. Moreover, we have shown that this induction is likely to be mediated by the direct binding of LXR/RXR heterodimers to the LXRα promoter. Interestingly, tonic expression of LXRα in human cells appears to be dependent on endogenous production of oxysterol intermediates in the cholesterol biosynthetic pathway. Inhibition of cholesterol synthesis by simvastatin led to a complete loss of LXRα expression in THP-1 cells. Surprisingly, the ability of LXRα to regulate its own promoter appears to be species specific. Oxysterol and synthetic ligands of LXRs induce LXRα expression in human macrophage cell lines and primary human macrophages but not in murine cell lines or primary murine macrophages. In humans, this induction is observed in multiple cell types, including macrophages, adipocytes, and hepatoma cells. Cloning and analysis of the human LXRα 5′-flanking region led to the identification of the critical LXRE that is likely to mediate lipid inducibility.
Previous work demonstrated that expression of the LXRα gene is induced in both human and murine macrophages by PPARγ-specific ligands (2, 4). A functional PPRE has been identified in the promoter of the mLXRα gene; however, the molecular basis for regulation of the hLXRα gene by PPARγ has not been explored. In the present work, we have shown that although the PPRE present in the murine proximal promoter is not conserved in the human gene, a functional PPRE is present in a different region of the hLXRα promoter. Thus, while the hLXRα gene is a target for both PPARγ and LXR, the murine gene appears to be a target only for PPARγ. The possibility that the murine gene is responsive to LXR in certain tissues or under certain conditions not tested here, however, cannot be excluded. At present, hLXRα is the only known common target gene for both PPARγ and LXRs. Tobin et al. have previously reported that liver LXRα expression was responsive to dietary fatty acids and have suggested that the mLXRα gene may be a target for PPARα regulation in liver (25). However, we have not observed regulation of LXRα mRNA by either PPARα- or PPARγ-specific ligands in liver cells (Fig. 5). Rather, our data suggest that the LXRα gene is a target for PPAR regulation only in certain tissues such as macrophages and adipocytes.
Substantial differences in lipid metabolism exist between mice and humans. The results presented here have implications for cholesterol metabolism in both species and its potential to be regulated by synthetic LXR and/or PPARγ ligands. We have outlined an unexpected species-specific difference in the regulation of LXRα expression by oxidized lipid ligands of LXRs. In human macrophages, the ability of LXRα to regulate its own promoter is likely to be an integral part of the physiologic response to lipid loading. The LXRα autoregulatory loop provides a mechanism whereby the cellular response to lipid loading can be amplified and maximized. The species-specific difference in the ability to amplify the LXR response raises the possibility that humans may be more responsive than mice to LXR agonists in general and to LXRα agonists in particular.
Several lines of evidence support the hypothesis that upregulation of LXRα expression can impact LXR target gene expression and cellular function, even though most cells also express significant levels of LXRβ. First, the phenotype of LXRα−/− mice clearly indicates that the two receptors are not entirely redundant (16). Second, although studies have shown that induction of ABCA1 and ABCG1 is preserved in LXRα−/− macrophages in the presence of maximal concentrations of ligands (19, 29), expression of apoE is reduced in either LXRα−/− or LXRβ−/− mice under identical conditions (11). Thus, some target genes are more sensitive than others to the absolute levels of LXR present in the cell. It is for this subset of genes that autoregulation of the LXRα promoter is likely to have the greatest impact. Third, studies have also shown that the level of LXRα expression is a key determinant of both the sensitivity of ABCA1 induction and the rate of cholesterol efflux. Retroviral expression of LXRα in cells that already express LXRβ shifts the dose response of ABCA1 to LXR ligands and dramatically stimulates cholesterol efflux (28). Finally, we have shown here that certain LXR target genes, such as apoE, are in fact significantly more responsive to LXR ligand in human macrophages than in murine macrophages (Fig. 3).
Our results also suggest that LXRα may play a more prominent role than LXRβ in certain human cell types, especially in the context of cellular lipid loading. In resting macrophages, for example, expression of LXRβ is more prominent than that of LXRα (Fig. 2 and data not shown). Upon lipid loading and LXR activation, however, the balance is shifted dramatically in favor of LXRα. This could have an important impact on gene expression and lipid metabolism if certain LXR target genes are preferentially activated by either LXRα or LXRβ. The development of selective ligands for either LXRα or LXRβ should shed light on this issue.
ACKNOWLEDGMENTS
We thank Tim Willson (GlaxoSmithKline) for GW3965, GW7845, and T0901317; Rich Heyman (Ligand Pharmaceuticals) for LG268; and Harleen Ahuja for help with real-time PCR assays. We also thank Peter Edwards, Matthew Kennedy, and Tim Willson for helpful discussions and Brenda Mueller for administrative support.
P.T. is an Assistant Investigator of the Howard Hughes Medical Institute at the University of California, Los Angeles.
REFERENCES
- 1.Alberti S, Steffensen K R, Gustafsson J A. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene. 2000;243:93–103. doi: 10.1016/s0378-1119(99)00555-7. [DOI] [PubMed] [Google Scholar]
- 2.Chawla A, Boisvert W A, Lee C, Laffitte B A, Barak Y, Joseph S B, Liao D, Nagy L, Edwards P A, Curtiss L K, Evans R M, Tontonoz P. A PPARgamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 3.Chinetti G, Gbaguidi F G, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapman J, Fruchart J C, Tedgui A, Najib-Fruchart J, Staels B. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation. 2000;101:2411–2417. doi: 10.1161/01.cir.101.20.2411. [DOI] [PubMed] [Google Scholar]
- 4.Chinetti G, Lestavel S, Bocher V, Remaley A T, Neve B, Torra I P, Teissier E, Minnich A, Jaye M, Duverger N, Brewer H B, Fruchart J C, Clavey V, Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 5.Costet P, Luo Y, Wang N, Tall A R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 6.DeBose-Boyd R A, Ou J, Goldstein J L, Brown M S. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc Natl Acad Sci USA. 2001;98:1477–1482. doi: 10.1073/pnas.98.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. 15-deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 8.Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 9.Kliewer S A, Lenhard J M, Wilson T M, Patel I, Morris D C, Lehmann J M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 10.Laffitte B A, Kast H R, Nguyen C M, Zavacki A M, Moore D D, Edwards P A. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 11.Laffitte B A, Repa J J, Joseph S B, Wilpitz D C, Kast H R, Mangelsdorf D J, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 13.Nagy L, Tontonoz P, Alvarez J G, Chen H, Evans R M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPAR gamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 14.Oliver W R, Shenk J L, Snaith M R, Russell C S, Plunket K D, Bodkin N L, Lewis M C, Winegar D A, Sznaidman M L, Lambert M H, Xu H E, Sternbach D D, Kliewer S A, Hansen B C, Willson T M. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peet D J, Janowski B A, Mangelsdorf D J. The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev. 1998;8:571–575. doi: 10.1016/s0959-437x(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 16.Peet D J, Turley S D, Ma W, Janowski B A, Lobaccaro J M, Hammer R E, Mangelsdorf D J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 17.Repa J J, Liang G, Ou J, Bashmakov Y, Lobaccaro J M, Shimomura I, Shan B, Brown M S, Goldstein J L, Mangelsdorf D J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repa J J, Mangelsdorf D J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 19.Repa J J, Turley S D, Lobaccaro J A, Medina J, Li L, Lustig K, Shan B, Heyman R A, Dietschy J M, Mangelsdorf D J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 20.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 21.Schultz J R, Tu H, Luk A, Repa J J, Medina J C, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf D J, Lustig K D, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz K, Lawn R M, Wade D P. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun. 2000;274:794–802. doi: 10.1006/bbrc.2000.3243. [DOI] [PubMed] [Google Scholar]
- 23.Spencer T A, Li D, Russel J S, Collins J L, Bledsoe R K, Consler T G, Moore L B, Galardi C M, McKee D D, Moore J T, Watson M A, Parks D J, Lambert M H, Willson T M. Pharmacophore analysis of the nuclear oxysterol receptor LXRa. J Med Chem. 2001;44:886–897. doi: 10.1021/jm0004749. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 25.Tobin K A, Steineger H H, Alberti S, Spydevold O, Auwerx J, Gustafsson J A, Nebb H I. Cross-talk between fatty acid and cholesterol metabolism mediated by liver X receptor-alpha. Mol Endocrinol. 2000;14:741–752. doi: 10.1210/mend.14.5.0459. [DOI] [PubMed] [Google Scholar]
- 26.Tontonoz P, Hu E, Spiegelman B M. Stimulation of adipogenesis in fibroblasts by PPARg2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 27.Tontonoz P, Nagy L, Alvarez J G, Thomazy V A, Evans R M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 28.Venkateswaran A, Laffitte B A, Joseph S B, Mak P A, Wilpitz D C, Edwards P A, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRalpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkateswaran A, Repa J J, Lobaccaro J-M A, Bronson A, Mangelsdorf D J, Edwards P A. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. J Biol Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 30.Willy P J, Umesono K, Ong E S, Evans R M, Heyman R A, Mangelsdorf D J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]