Roles of the C-Terminal End of SecY in Protein Translocation and Viability of Escherichia coli (original) (raw)
Abstract
SecY, a central component of the membrane-embedded sector of protein translocase, contains six cytosolic domains. Here, we examined the importance of the C-terminal cytosolic region of SecY by systematically shortening the C-terminal end and examining the functional consequences of these mutations in vivo and in vitro. It was indicated that the C-terminal five residues are dispensable without any appreciable functional defects in SecY. Mutants missing the C-terminal six to seven residues were partially compromised, especially at low temperature or in the absence of SecG. In vitro analyses indicated that the initial phase of the translocation reaction, in which the signal sequence region of the preprotein is inserted into the membrane, was affected by the lack of the C-terminal residues. SecA binding was normal, but SecA insertion in response to ATP and a preprotein was impaired. It is suggested that the C-terminal SecY residues are required for SecA-dependent translocation initiation.
Translocation of newly synthesized Escherichia coli proteins from the cytosol across the cytoplasmic membrane is facilitated by concerted actions of the SecA ATPase and the SecYEG integral membrane protein complex (for reviews, see references 14 and 22). Translocation is initiated by membrane insertion of the signal peptide and the following mature segment, leading to periplasmic exposure of the leader peptidase cleavage site. While initiation is triggered by ATP binding to SecA, continuation of translocation requires repeated hydrolysis cycles of ATP and/or the proton motive force (PMF) across the membrane. These processes appear to be closely related to the insertion/deinsertion cycle of SecA (5, 6). In this cycle, an ATP- and preprotein-dependent conformational change of the membrane-bound form of SecA results in its insertion into the membrane, which is followed by ATP hydrolysis-dependent release of the preprotein and deinsertion of SecA. These dynamic functions of SecA occur in its close interaction with the SecYEG components (5, 15, 22).
SecY is the central subunit of the SecYEG complex and contains 10 transmembrane segments (TM1 to TM10), five periplasmic regions (P1 to P5), and six cytosolic domains (C1 to C6). The SecYEG complex is believed to provide a channel-like pathway for protein translocation, probably by its oligomeric superassembly (4, 13). We isolated and characterized a number of cold-sensitive secY mutants in which protein export and cell growth were retarded at 20°C (22). In vivo and in vitro studies using these and the dominant-negative secY mutations suggested that the C-terminal cytosolic domains, C5 and C6, are particularly important for SecY functions (21, 27). Mutations in these domains significantly compromise the SecA-activating functions of SecY (33). In particular, a mutation (secY205) in C6 that changed Tyr429 (the 15th residue from the C terminus) to Asp was found to impair the SecA insertion reaction (15).
To further assess the importance of the C-terminal residues of SecY, we carried out systematic studies by constructing a series of C-terminal truncations of SecY, the results of which are reported in this paper.
MATERIALS AND METHODS
E. coli strains and plasmids.
The E. coli K-12 strains and plasmids used in this study are summarized in Table 1 and Table 2, respectively. Strains CSH26 and MC4100 were described previously (3, 20). P1 transduction was carried out according to the standard procedures (20). EM106 was a zhd-33::Tn_10_ transductant of KI308 (34). KC5 (secY_Δ_5), KC6 (secY_Δ_6), and KC7 (secY_Δ_7) were constructed by the plasmid integration and marker exchange procedures using a polA strain (7). EM106 (polA) was transformed with pCJ5, pCJ6, pCJ7, and pCJ9 to chloramphenicol resistance. These plasmid integrates were then grown in the absence of chloramphenicol, and segregants that had lost the drug resistance were screened by replica platings. By amplifying and sequencing a chromosomal segment from the pCJ5, pCJ6, and pCJ7 segregants, those retaining the respective secY allele were identified. However, no secY_Δ_9 allele was identified from the pCJ9 segregants. Finally, each chromosomal mutation was introduced into TW155 using zhd-33::Tn_10_ as a selective marker and by screening secY alleles directly by sequence determinations.
TABLE 1.
E. coli strains
| Strain | Genotype | Reference |
|---|---|---|
| CU101 | CSH26, polAI zhf::Tn_5_/F′ lacI_q_lacPL8 lacZ+Y+A+ pro+ | 34 |
| EM106 | CSH26, polA1 zhf::Tn_5 zhd-33_::Tn_10_ | Matsuo et al., unpublished |
| KI297 | MC4100, rpsE secY24 zhd-33::Tn_10_/F′ lacI_q_lacPL8 lacZ+Y+A+ pro+ | 10 |
| TW155 | MC4100, ompT::kan Δ_(uncB-uncC)_ | 15 |
| TW156 | MC4100, ompT::kan zhd-33::Tn_10_ Δ_(uncB-uncC)_ | 15 |
| GN5 | MC4100, ompT::kan zhd-33::Tn_10 secY205_ Δ_(uncB-uncC)_ | 15 |
| GN31 | MC4100, ompT::kan secY39 Tets | 17 |
| GN78 | MC4100, secG::kan | This study |
| KC5 | MC4100, ompT::kan zhd-33::Tn_10 secY_Δ_5_ Δ_(uncB-uncC)_ | This study |
| KC6 | MC4100, ompT::kan zhd-33::Tn_10 secY_Δ_6_ Δ_(uncB-uncC)_ | This study |
| KC7 | MC4100, ompT::kan zhd-33::Tn_10 secY_Δ_7_ Δ_(uncB-uncC)_ | This study |
| KC9 | MC4100, secG::kan zhd-33::Tn_10 secY+_/p secG+/pSTD342 | This study |
| KC10 | MC4100, secG::kan zhd-33::Tn_10 secY205_/p secG+/pSTD342 | This study |
| KC11 | MC4100, secG::kan zhd-33::Tn_10 secY_Δ_5_/p secG+/pSTD342 | This study |
| KC12 | MC4100, secG::kan zhd-33::Tn_10 secY_Δ_6_/p secG+/pSTD342 | This study |
| KC13 | MC4100, secG::kan zhd-33::Tn_10 secY_Δ_7_/p secG+/pSTD342 | This study |
TABLE 2.
Plasmids
| Plasmid | Cloned genesa | Replicon | Reference |
|---|---|---|---|
| pCJ1 | rpmD-rplO- **secYΔ1**-rpmJ | pACYC184 | This study |
| pCJ2 | rpmD-rplO- **secYΔ2**-rpmJ | pACYC184 | This study |
| pCJ3 | rpmD-rplO- **secYΔ3**-rpmJ | pACYC184 | This study |
| pCJ4 | rpmD-rplO- **secYΔ4**-rpmJ | pACYC184 | This study |
| pCJ5 | rpmD-rplO- **secYΔ5**-rpmJ | pACYC184 | This study |
| pCJ6 | rpmD-rplO- **secYΔ6**-rpmJ | pACYC184 | This study |
| pCJ7 | rpmD-rplO- **secYΔ7**-rpmJ | pACYC184 | This study |
| pCJ8 | rpmD-rplO- **secYΔ8**-rpmJ | pACYC184 | This study |
| pCJ9 | rpmD-rplO- **secYΔ9**-rpmJ | pACYC184 | This study |
| pCJ10 | rpmD-rplO- **secYΔ10**-rpmJ | pACYC184 | This study |
| pCJ11 | rpmD-rplO- **secYΔ11**-rpmJ | pACYC184 | This study |
| pCJ12 | rpmD-rplO- **secYΔ12**-rpmJ | pACYC184 | This study |
| pCJ13 | rpmD-rplO- **secYΔ13**-rpmJ | pACYC184 | This study |
| pCJ14 | rpmD-rplO- **secYΔ14**-rpmJ | pACYC184 | This study |
| pCJ15 | rpmD-rplO- **secYΔ15**-rpmJ | pACYC184 | This study |
| pCM10 | _rpmD-rplO_-**secY+**-rpmJ | pACYC184 | 18 |
| pCM56 | syd | pBR322 | Matsuo et al., unpublished |
| pSTV28 | Vector | pACYC184 | 8 |
| pSTD342 | _lacI_q | pACYC184 | Akiyama et al., unpublished |
| p secG+ | secG+ | pBR322 | Matsumoto et al., unpublished |
GN78 is a secG::kan (23) transductant of MC4100. It was transformed successively with pSTD342 (lacIq) and p_secG+. The respective secY allele was introduced by P1 transduction into the resulting transformant, using zhd-33::Tn_10 as a selective marker in the presence of IPTG (isopropylthiogalactopyranoside). Cotransduction was examined directly by sequence determination of the secY segment.
Plasmids pCJ1 to pCJ15 were derivatives of pCM10 with the indicated chain-terminating mutations in secY. These secY mutations were introduced by site-directed mutagenesis using the QuikChange kit (Stratagene) and the mutagenic primers shown in Table 3 as well as their complementary strands. A 1.3-kb _Sma_I-_Hin_dIII fragment of confirmed sequence was excised from each of the primary products of the mutagenesis and cloned back into the original vector.
TABLE 3.
Primers used for site-directed mutagenesis
| secY mutation | Sequencea |
|---|---|
| secY_Δ_1 | 5′ CTGAAAGGCTACGGCTAATAATTGGTCGCCCG3′ |
| secY_Δ_2 | 5′ CTGAAAGGCTACTGACGATAATTGGTC3′ |
| secY_Δ_3 | 5′ GAACCTGAAAGGCTAAGGCCGATAATTGGTC3′ |
| secY_Δ_4 | 5′ GGCGAACCTGAAATGATACGGCCGATAATTG3′ |
| secY_Δ_5 | 5′ GAAGGCGAACCTGTAAGGCTACGGCCG3′ |
| secY_Δ_6 | 5′ GAAGAAGGCGAACTAGAAAGGCTACGGCC3′ |
| secY_Δ_7 | 5′ CATTGAAGAAGGCGTAACTGAAAGGCTACGGC3′ |
| secY_Δ_8 | 5′ GCATTGAAGAAGTAGAACCTGAAAGGC3′ |
| secY_Δ_9 | 5′ GTCTGCATTGAAGTAGGCGAACCTGAAAG3′ |
| secY_Δ_10 | 5′ GAGTCTGCATTGTAGAAGGCGAACCTG3′ |
| secY_Δ_11 | 5′ GTATGAGTCTGCATAGAAGAAGGCGAACC3′ |
| secY_Δ_12 | 5′ CAGTATGAGTCTTAATTGAAGAAGGCG3′ |
| secY_Δ_13 | 5′ CCAGTCAGTATGAGTAAGCATTGAAGAAGGC3′ |
| secY_Δ_14 | 5′ GTCCAGTCAGTATTAGTCTGCATTGAAGAAG3′ |
| secY_Δ_15 | 5′ GATGTCCAGTCAGTAAGAGTCTGCATTGAAG3′ |
Media.
L medium (20), peptone medium (9), M9 medium (20), and ME medium (35) were used. Ampicillin, chloramphenicol, and tetracycline were added at concentrations of 50 μg/ml, 20 μg/ml, and 25 μg/ml, respectively, for selection in transformation or transduction. IPTG was added at a concentration of 1 mM for induction of lac promoter-directed transcription.
Immunoprecipitation and immunoblotting.
Export of OmpA (an outer membrane protein) and maltose-binding protein (MBP, a periplasmic protein) was examined by pulse-chase experiments (15). Intracellular accumulation of SecY and SecG was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole-cell proteins and their staining with the respective antibodies (15, 28). Visualization and quantification of electrophoretically separated proteins were done by means of a Fuji BAS1800 phosphor imager and a Fuji LAS1000 lumino imager, respectively, for pulse-chase and immunoblotting experiments.
In vitro translocation reactions.
The reaction system for in vitro protein translocation into inverted membrane vesicles (IMVs) was essentially as described previously (15), except that IMVs without the urea wash step were used for the translocation assays. The translocation reaction mixture contained 50 mM Tris-HCl (pH 7.5), 5 mM MgSO4, 0.1 mg of bovine serum albumin per ml, IMV (0.25 mg protein/ml), 15 μg of SecA per ml, 15 μg of SecB per ml, 1 mM ATP, and its regeneration system (18 mM phosphocreatine and 25 μg of creatine kinase per ml). Reaction at 37°C or at 20°C for 5 min was initiated by addition of [35S]pro-OmpA and terminated by chilling on ice.
To examine the extent of signal peptide processing, samples were directly treated with trichloroacetic acid (final concentration, 5%). To examine the extent of translocation, the reaction mixture was incubated further with 0.1 mg of proteinase K per ml at 0°C for 20 min. Trichloroacetic acid was then added to terminate proteolysis and to precipitate proteins. Labeled proteins were visualized by SDS-PAGE and exposed to a Fuji BAS1800 phosphor imager to determine radioactivities associated with unprocessed and mature OmpA species.
SecA insertion assay.
Purified SecA protein was iodinated with 125I, and its insertion into 4 M urea-washed IMVs was assayed essentially as described previously (6, 15), except that 1 mg of proteinase K per ml was used to digest the accessible portions of SecA.
RESULTS
Complementation abilities of SecY variants with C-terminal truncations.
We introduced a series of chain-terminating mutations into the 3′ region of secY. These secY alleles are named by the number of amino acid residues that are missing due to the introduced stop codon; for instance, secYΔ3 encodes SecYΔ3, lacking three C-terminal residues. The functionality of the C-terminally truncated proteins was examined by a variety of complementation tests, in which they were expressed in appropriate secY mutant strains. When the mutant proteins were overexpressed together with SecE (note that SecY per se is unstable in the absence of corresponding amounts of SecE [32]), all the mutant proteins overaccumulated, confirming that the C-terminal truncations did not significantly destabilize the protein (data not shown). That they can be stabilized by SecE suggests that the mutant proteins are properly integrated into the membrane.
The ability to support growth of a cold-sensitive secY39 mutant (1) at 20°C was found to be retained for the Δ1, Δ2, Δ3, Δ4, and Δ5 variants of SecY. In contrast, removal of six or more residues from the C terminus eliminated the complementation ability (Table 4). The protein export abilities of the plasmid-bearing secY39 cells were examined at 20°C by pulse-labeling MBP and OmpA and their immunoprecipitation. Whereas those expressing SecYΔ1 to -Δ5 were nearly as proficient as the wild-type cells in protein export, SecY variants missing six or more residues were distinctly less effective in supporting protein export (data not shown). Similar results were obtained when complementation activities were examined against another cold-sensitive mutant, secY205 (data not shown).
TABLE 4.
Complementation abilities of the C-terminally truncated SecY variantsa
| secY on plasmid | Relative efficiency of plating | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| secY39b (20°C) | secY24/sydc | polA (chromosomal integration)d | |||||||
| 20°C | 30°C | 37°C | 42°C | 20°C | 30°C | 37°C | 42°C | ||
| Wild type | + | + | + | + | + | ||||
| None (vector) | − | − | − | − | − | ||||
| Δ1 | + | ||||||||
| Δ2 | + | ||||||||
| Δ3 | + | + | + | + | + | ||||
| Δ4 | + | ||||||||
| Δ5 | + | + | + | + | + | + | + | + | + |
| Δ6 | − | (+) | + | + | + | − | + | + | + |
| Δ7 | − | − | − | + | + | ||||
| Δ8 | − | − | − | + | + | ||||
| Δ9 | − | − | (−) | + | + | − | − | + | + |
| Δ10 | − | − | − | − | (−) | ||||
| Δ11 | − | − | − | − | (+) | − | − | − | − |
| Δ12 | − | ||||||||
| Δ13 | − | − | − | − | (−) | − | − | − | − |
| Δ14 | − | − | − | − | |||||
| Δ15 | − | − | − | − |
Complementation tests using the secY24(Ts) mutant (2) indicated that some of the C-terminally truncated SecY variants missing more than five residues complemented the growth defect of this mutant at 42°C (data not shown). This raised the possibility that the requirement for the C-terminal region may be alleviated at elevated temperatures. We then examined the abilities of the SecY variants to complement other conditional defects of SecY at different temperatures. secY24 mutant cells are extremely sensitive to overproduction of Syd, a SecY-interacting protein. In the presence of excess amounts of Syd, the mutationally altered SecY24-SecE channel seems to be destroyed with respect to the high-affinity binding of SecA, and this phenotype can be observed at any temperature (18, 19, 28).
The SecYΔC plasmids were introduced into secY24 mutant cells that carried another compatible plasmid with the lac promoter-controlled syd. Without any complementing secY plasmid, this strain was unable to grow in the presence of IPTG, which lowered the efficiency of plating at least 104-fold (Table 4). Again, the Δ5 variant exhibited a wild-type level of complementation. The Δ6 variant exhibited reduced complementation ability at 20°C but not at 30°C or above. Even the Δ9 variant complemented the growth defect at 37°C and above. Further truncations inactivated the activity at any temperature.
While the lack of a _secY_-deleted chromosomal mutant precluded a simple functional assessment of the mutant proteins, we constructed strains in which a mutated secY had been integrated into the chromosome and the original secY+ had been placed under lac promoter control. This was achieved by plasmid integration into a polA strain (34). The growth phenotypes of the plasmid-integrated strains were examined in the absence of IPTG. Overall, the growth phenotypes were similar, if not identical, to what had been observed with the complemented secY24 syd cells (Table 4). Thus, the Δ6, Δ7, and Δ8 variants failed to support cell growth at low temperatures, whereas Δ10, Δ11, and Δ12 were nonfunctional at any temperature examined.
Phenotypic consequences of chromosomal mutations.
To construct more straightforward mutants with single chromosomal secY mutations, plasmid-integrated strains similar to those described above were subjected to excision of the plasmid sequence by growing them in the absence of antibiotic selection. Depending on the positions of the two successive recombinations, either the wild-type or the mutant allele will be left on the chromosome of the resulting strains (7). We were able to obtain chromosomal secY_Δ_5, secY_Δ_6, and secY_Δ_7 mutations. In spite of repeated trials, however, a secY_Δ_9 single mutant was not obtained.
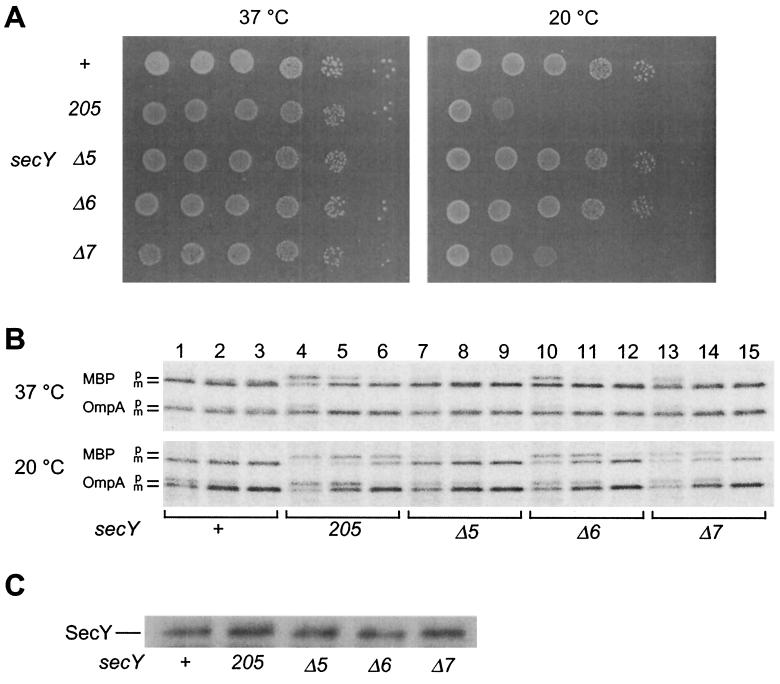
Immunoblotting experiments showed that the mutant forms SecYΔ5, SecYΔ6, and SecYΔ7 all accumulated normally in the respective chromosomal mutant strains (Fig. 1C). Thus, it was confirmed that these C-terminal truncations did not affect the stability of SecY. On agar plates, the secY_Δ_5 and secY_Δ_6 mutants formed colonies at wild-type efficiencies at both 37 and 20°C. It was noted that the above result with Δ6 was different from those obtained in the complementation tests, in which it did not complement the growth defect at 20°C. Growth of the secY_Δ_7 mutant was significantly compromised at 20°C (Fig. 1A). At the protein export level, Δ5 was normal (Fig. 1B, compare lanes 7 to 9 with lanes 1 to 3), but both the Δ6 and the Δ7 mutants showed slight retardation in MBP export at 37°C and more pronounced defects in export of both MBP and OmpA at 20°C (Fig. 1B, lanes 10 to 15).
FIG. 1.
Growth and protein export phenotypes of chromosomal secY mutants. (A) Growth at 37 and 20°C. Strains TW156 (secY+), GN5 (secY205), KC5 (secY_Δ_5), KC6 (secY_Δ_6), and KC7 (secY_Δ_7) were cultured at 37°C until mid-log phase. Cells were serially diluted with 0.9% NaCl solution (10-fold dilutions from left to right), and 2 μl of each was spotted on L-agar plates, which were photographed after 12 h at 37°C (left panel) or after 48 h at 20°C (right panel). (B) Protein export phenotypes. Cells were grown at 37°C (upper panel) and then at 20°C for 30 min (lower panel), followed by pulse-labeling with [35S]methionine for 30 s at 37°C or for 1 min at 20°C and chase with unlabeled methionine for 12 s (lanes 1, 4, 7, 10, and 13), 1 min (lanes 2, 5, 8, 11, and 14), and 5 min (lanes 3, 6, 9, 12, and 15). MBP and OmpA were immunoprecipitated and subjected to SDS-PAGE and phosphor imager visualization. (C) Cellular accumulation of the mutant SecY proteins. Whole-cell proteins from a fixed number of cells of mid-log-phase M9 cultures were subjected to SDS-PAGE and anti-SecY immunoblotting.
SecG is not essential but is required in vivo for full activity of protein export. Disruption of secG (Δ_secG_) shows synthetic lethality with a secA mutation (29) as well as with a number of secY mutations (G. Matsumoto et al., unpublished results). We examined what happens when the secY C-terminal chain-terminating mutations were combined with the Δ_secG_ mutation. The secY mutations were introduced by P1 transduction into the Δ_secG_ strain that carried a plasmid with secG placed under lac promoter control. Whereas viability of the secY_Δ_5 Δ_secG_ double mutant strain was IPTG independent, both the secY_Δ_6 Δ_secG_ and secY_Δ_7 Δ_secG_ double mutant strains showed IPTG-dependent growth (Fig. 2). Depletion of SecG in the absence of IPTG was confirmed in liquid cultures (data not shown). Thus, the Δ5 mutant was also normal with respect to the dispensability of SecG. In contrast, both the Δ6 and Δ7 mutants were functionally compromised, such that the assistance from SecG was essential.
FIG. 2.
SecG dependence of secY mutants. Strains KC9 (secY+ Δ_secG_::kan), KC10 (secY205 Δ_secG_::kan), KC11 (secY_Δ_5 Δ_secG_::kan), KC12 (secY_Δ_6 Δ_secG_::kan), and KC13 (secY_Δ_7 Δ_secG_::kan), all of which carried p_secG+_ (p_lac_-secG), were grown in peptone-IPTG medium at 37°C until mid-log phase. Then 2 μl of a 100-fold diluted culture was spotted onto peptone-IPTG agar (+) or L-agar (−) and incubated at 37°C for 12 h.
Mutational effects on in vitro translocase activities.
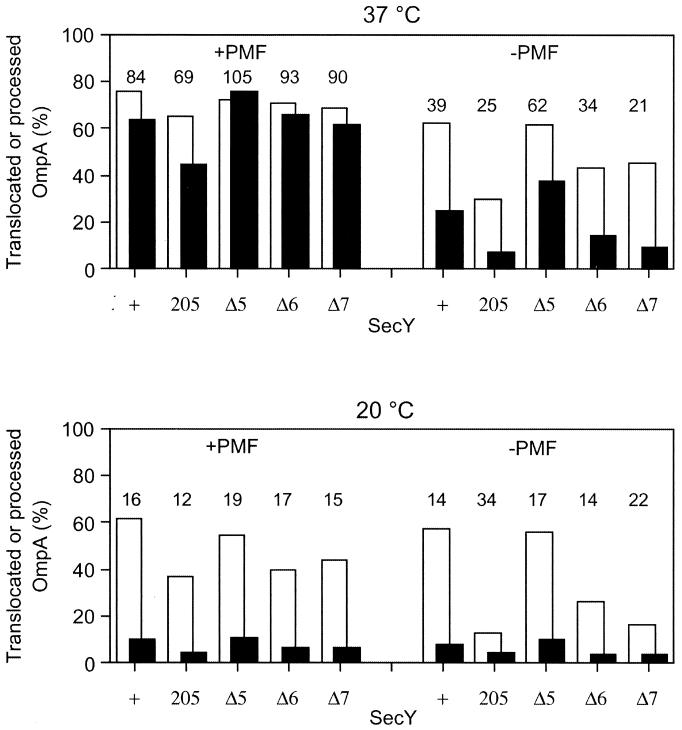
IMVs were prepared from the secY+ cells as well as from the Δ5, Δ6, and Δ7 mutant strains that additionally carried Δ_ompT_ and Δ_uncBC_ mutations (15). Using these IMV preparations and purified SecA, translocation of in vitro-synthesized and 35S-labeled pro-OmpA was examined. Reactions were allowed under conditions in which PMF was generated by the addition of the respiratory substrate succinate as well as under conditions in which PMF was dissipated by carbonylcyanide _m_-chlorophenylhydrazone. Translocated (pro)OmpA was determined after digestion with proteinase K (Fig. 3, solid columns). We also assayed the extents of signal sequence processing by direct gel electrophoresis (Fig. 3, open columns). The latter assay will provide information on the initial phase of the translocation reaction, in which the signal sequence and the following N-terminal segment of the precursor molecule are inserted into the membrane to expose the leader peptidase processing site to the periplasmic side.
FIG. 3.
In vitro translocation of pro-OmpA into IMVs prepared from secY mutants. IMVs were prepared from strains TW156 (secY+), GN5 (secY205), KC5 (secY_Δ_5), KC6 (secY_Δ_6), and KC7 (secY_Δ_7). They were incubated at 37°C (upper panel) or at 20°C (lower panel) for 5 min in the presence of SecA, SecB, ATP, the ATP regeneration system, and 35S-labeled pro-OmpA. PMF was imposed or dissipated, as indicated. Extents of translocation (solid columns) and signal peptide cleavage (open columns) were assayed. Values represent percentages of radioactivities associated with translocated (solid columns) and processed (open columns) molecules after appropriate corrections for the distribution of methionine residues. The number above each pair of columns indicates the percentage of translocated component in the processed protein.
The results shown in Fig. 3 indicate that the in vitro translocation activities of the Δ5 IMV were indistinguishable from those of the wild-type SecY IMV under any conditions examined. In contrast, the Δ6 and Δ7 variants had significant defects in translocation activity. Although Δ7 was slightly less active than Δ6, their activities were similarly lower compared to the wild-type or the Δ5 variant. Whereas these mutant IMVs were almost as active as the wild-type IMV at 37°C in the presence of PMF, their translocation activities were more significantly lowered in the absence of PMF, at low temperature, and when these conditions were combined. At 37°C, the translocation efficiencies and the processing efficiencies in the presence of PMF were close to each other; the translocation-to-processing ratios were more than 85%, except for the secY205 IMV (about 70%). In contrast, values for translocation were far below those for processing under more unfavorable reaction conditions; the ratios of translocation to processing efficiencies with the wild-type IMV were 39% in the absence of PMF at 37°C, and these values dropped to about 15% at 20°C irrespective of the PMF status. These results suggest that the initiation phase of the reaction was less affected by the unfavorable conditions than the translocation of the main body of the precursor protein, which was particularly lowered at the low temperature.
It is important to note that the translocation/processing ratios for the mutant IMVs were not particularly lower than the wild-type values under each condition (Fig. 3). Thus, apparently, the Δ6 and Δ7 mutations retarded the two steps to similar extents. Assuming that these two events occur successively, the mutational effects can then be explained solely by the retardation of the earlier event. Thus, it is suggested that the mutational effects are exerted at the stage that precedes processing of the signal peptide. The lack of more than five C-terminal residues of SecY compromises the initiation phase of the translocation process.
Mutational effects on SecA functions.
The translocation reaction is initiated upon binding of ATP to the SecA ATPase at the SecYEG integral membrane complex. A key reaction here is the ATP- and preprotein-dependent insertion of SecA into the SecYEG-containing membrane (5, 6, 15, 22). Our results that the initiation phase of translocation was affected by the C-terminal truncations of SecY could be attributed to a defect in SecA binding to the membrane. Membrane binding of SecA was examined by mixing IMVs with 125I-labeled SecA and pelleting the membrane-bound SecA molecules. IMVs from Δ5, Δ6, and Δ7 mutants exhibited essentially unaltered abilities to bind SecA compared to wild-type IMV (data not shown).
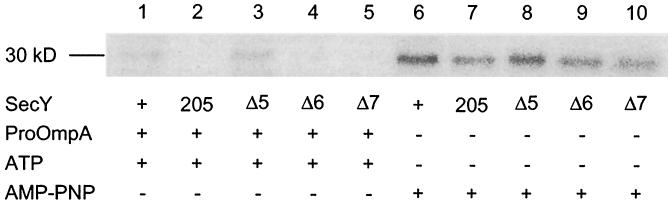
We then examined the SecA insertion reaction, using the [125I]SecA preparation, which was incubated with IMVs in the presence of ATP and pro-OmpA. IMVs from wild-type cells and the Δ5 cells gave the protease-protected 30-kDa SecA “inserted” fragment (Fig. 4, lanes 1 and 3) (6). In contrast, IMVs from the Δ6 and Δ7 mutants failed to support ATP- and pro-OmpA-dependent SecA insertion (“productive” insertion; Fig. 4, lanes 4 and 5), as had been shown previously for the secY205 mutant (Fig. 4, lane 2) (15). Preprotein-independent SecA insertion in the presence of a nonhydrolyzable ATP analog, AMP-PNP (idling insertion), was observed for all the IMV preparations (lanes 6 to 10). The ability to stimulate the translocation ATPase activities of SecA (12) was again normal for Δ5 but impaired for Δ6 and Δ7 (data not shown). These results argue against the possibility that the defective SecA insertion observed with the mutant IMVs was due to an enhancement in the deinsertion reaction of SecA, which should have been accompanied by hydrolysis of ATP (6). Our in vitro analyses of the mutant IMVs indicate that the C-terminal region of SecY is important in supporting the SecA insertion reaction, although the C-terminal five residues of SecY are dispensable.
FIG. 4.
Abilities of mutant IMVs to support insertion of the C-terminal SecA segment. 125I-labeled SecA (2 μg) was bound to 4 M urea-washed IMVs (5 μg of proteins in a final volume of 200 μl) at 0°C for 30 min, and the complexes were isolated by centrifugation. The standard insertion reaction mixture (15) contained the SecA-IMV complex, pro-OmpA, and ATP to measure productive insertion (lanes 1 to 5). To measure the futile mode of insertion, pro-OmpA was omitted and AMP-PNP was included instead of ATP (lanes 6 to 10). Reactions were allowed at 37°C for 20 min, followed by proteinase K treatment. The membrane-protected 30-kDa fragment of SecA was visualized by SDS-PAGE and phosphor imager exposure.
DISCUSSION
In this study we systematically shortened the C-terminal region of SecY and characterized the consequences of the truncations in vivo and in vitro. By all the criteria examined, SecYΔ5 is functionally identical to the wild-type SecY protein. In contrast, the absence of six or more residues from the SecY C terminus leads to significant loss of functions. Both the Δ6 and Δ7 mutants were significantly defective in protein export in vivo. They both required the presence of SecG to support cell viability (Fig. 2). In vitro, these mutant proteins exhibited similar decreases in SecA-dependent protein translocation activities (Fig. 3 and 4). Whereas SecYΔ6 and SecYΔ7, when expressed from a plasmid, failed to complement the chromosomal secY205 or secY39 mutation at 20°C (Table 4), only the latter mutation resulted in cold sensitivity in the chromosomal single-mutant configuration (Fig. 1). The basis for this apparent discrepancy is not known. Perhaps a subtle difference in the genetic background in the strains involved may have accounted for the lack of cold sensitivity of the Δ6 single mutant. It is also conceivable that SecYΔ6 was less functional in the presence of other mutated forms of SecY; this possibility should be considered, since an active translocation channel might be formed by oligomeric superassembly of SecYEG (4, 13).
We obtained peculiar complementation results at higher temperatures. At 37 and 42°C, SecY variants lacking as many as nine residues showed significant complementation ability against different conditional SecY defects (Table 4). Thus, the mutant protein may retain some activity at the high temperature, which could be manifested under the conditional experimental settings. This could be understood if the Sec function requirement is alleviated at higher temperatures. It was reported that the E. coli cellular physiology may change drastically at elevated temperatures, such that some Sec machinery-independent translocation of newly synthesized proteins may occur (36). In addition, the translocation system includes some intrinsically cold-sensitive processes (25).
In vitro translocation assays revealed that SecYΔ6 and SecYΔ7 had significantly lowered activities under “unfavorable” reaction conditions, such as at low temperature (20°C) and in the absence of PMF (Fig. 3). Under such conditions, the bulk translocation was preferentially lowered compared to the initial phase of the reaction leading to signal peptide processing. However, the mutational defects were observed equally for both the earlier and later phases of reaction, suggesting that the C-terminal truncations affect primarily the former stage. This was well corroborated by the observation that the SecA insertion reaction was impaired by these mutations (Fig. 4). Since a role of SecG may be to assist in the SecA reaction cycles (16, 24, 29), the increased dependence on SecG of the C-terminal truncation mutants can be taken as an in vivo piece of supporting evidence for the involvement of this SecY region in the SecA-dependent initiation of translocation.
Our results indicate that the C-terminal five residues are dispensable in SecY. The ClustalW program indicates that the C-terminal cytosolic domain is well conserved among the SecY homologues from E. coli relatives α-, β-, and γ-proteobacteria (Fig. 5), whereas those from ɛ proteobacteria (e.g., Helicobacter pylori and Campylobacter jejuni) are much different (not shown). Within the α, β, and γ groups, the SecY homologues from Rickettsia prowazekii and Buchenera aphidicola lack three and four C-terminal residues, respectively, compared with E. coli SecY. It was noted, however, that not more than five residues are missing from this group of organisms, and the last residue of SecYΔ5, leucine, is well conserved among them. On the other hand, SecY homologues from the gram-positive bacteria Bacillus subtilis and Staphylococcus carnosus contain C-terminal domains that are much shorter than that in E. coli SecY. Conceivably, SecYΔ5 is the minimum unit required for the life of _E. coli_-related organisms.
FIG. 5.
Sequence conservation at the C-terminal ends in SecY homologs from some bacterial species. Amino acid sequences of the most C-terminal cytosolic domains were aligned using the ClustalW program. The amino acid numbers of the E. coli protein are shown at the top. Conserved residues are shown in boldface.
A SecA segment encompassing residues 517 to 545 (in E. coli) is characteristically conserved among SecA homologues from gram-negative bacteria (26). A number of secA mutations that allele-specifically suppress the secY205 translocase defect have been mapped within or near this segment (17). This region was also characterized as a region accessible from the periplasm (26). Taken together with the observations that SecA is not effectively interchangeable between the gram-negative and gram-positive bacterial kingdoms (11, 30, 31), it is tempting to speculate that the two regions, the C terminus of SecY and the region around residue 530 of SecA, interact with each other. This interaction may contribute to the productive insertion of SecA into the SecYEG channel, thereby initiating translocation of a preprotein. The results reported here and the associated discussion will guide further mechanistic and structural understanding of protein translocase.
Acknowledgments
We thank Yoshinori Akiyama for discussion, Ei-ichi Matsuo and Gen Matsumoto for strains and plasmids, and Kiyoko Mochizuki, Toshiki Yabe, Yusuke Shimizu, Mikihiro Yamada, and Michiyo Sano for technical assistance.
This work was supported by grants from CREST, JST (Japan Science and Technology Corporation), and the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Baba, T., A. Jacq, E. Brickman, J. Beckwith, T. Taura, C. Ueguchi, Y. Akiyama, and K. Ito. 1990. Characterization of cold-sensitive secY mutants of Escherichia coli. J. Bacteriol. 172**:**7005-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, T., T. Taura, T. Shimoike, Y. Akiyama, T. Yoshihisa, and K. Ito. 1994. A cytoplasmic domain is important for the formation of a SecY-SecE translocator complex. Proc. Natl. Acad. Sci. USA 91**:**4539-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104**:**541-555. [DOI] [PubMed] [Google Scholar]
- 4.Collinson, I., C. Breyton, F. Duong, C. Tziatzios, D. Schubert, E. Or, T. Rapoport, and W. Kühlbrandt. 2001. Projection structure and oligomeric properties of a bacterial core protein translocase. EMBO J. 10**:**2462-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong, F., J. Eichler, A. Price, M. R. Leonard, and W. Wickner. 1997. Biogenesis of the gram-negative bacterial envelope. Cell 91**:**567-573. [DOI] [PubMed] [Google Scholar]
- 6.Economou, A., J. A. Pogliano, J. Beckwith, D. B. Oliver, and W. Wickner. 1995. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 83**:**1171-1181. [DOI] [PubMed] [Google Scholar]
- 7.Gutterson, N. I., and D. E. Koshland, Jr. 1983. Replacement and amplification of bacterial genes with sequences altered in vitro. Proc. Natl. Acad. Sci. USA 80**:**4894-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homma, T., T. Yoshihisa, and K. Ito. 1997. Subunit interactions in the Escherichia coli protein translocase: SecE and SecG associate independently with SecY. FEBS Lett. 408**:**11-15.9180258 [Google Scholar]
- 9.Ito, K., M. Wittekind, M. Nomura, K. Shiba, T. Yura, A. Miura, and H. Nashimoto. 1983. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell 32**:**789-797. [DOI] [PubMed] [Google Scholar]
- 10.Ito, K., and Y. Akiyama. 1991. In vivo analysis of integration of membrane proteins in Escherichia coli. Mol. Microbiol. 5**:**2243-2253. [DOI] [PubMed] [Google Scholar]
- 11.Klein, M., J. Meens, and R. Freudl. 1995. Functional characterization of the Staphylococcus carnosus SecA protein in Escherichia coli and Bacillus subtilis secA mutant strains. FEMS Microbiol. Lett. 131**:**271-277. [DOI] [PubMed] [Google Scholar]
- 12.Lill, R., K. Cunningham, L. A. Brundage, K. Ito, D. Oliver, and W. Wickner. 1989. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 8**:**961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manting, E. H., C. van der Does, H. Remigy, A. Engel, and A. J. M. Driessen. 2000. SecYEG assembles into a tetramer to form the active protein translocation channel. EMBO J. 19**:**852-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manting, E. H., and A. J. M. Driessen. 2000. Escherichia coli translocase: the unraveling of a molecular machine. Mol. Microbiol. 37**:**226-238. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto, G., T. Yoshihisa, and K. Ito. 1997. SecY and SecA interact to allow SecA insertion and protein translocation across the Escherichia coli plasma membrane. EMBO J. 16**:**6384-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto, G., H. Mori, and K. Ito. 1998. Roles of SecG in ATP- and SecA-dependent protein translocation. Proc. Natl. Acad. Sci. USA 95**:**13567-13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto, G., H. Nakatogawa, H. Mori, and K. Ito. 2000. Genetic dissection of SecA: suppressor mutations against the secY205 translocase defect. Genes Cells 5**:**991-999. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo, E., and K. Ito. 1998. Genetic analysis of an essential cytoplasmic domain of Escherichia coli SecY based on resistance to Syd, a SecY-interacting protein. Mol. Gen. Genet. 258**:**240-249. [DOI] [PubMed] [Google Scholar]
- 19.Matsuo, E., H. Mori, T. Shimoike, and K. Ito. 1998. Syd, a SecY-interacting protein excludes SecA from the SecYE complex with an altered SecY24 subunit. J. Biol. Chem. 273**:**18835-18840. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Mori, H., and K. Ito. 2001. An essential amino acid residue in the protein translocation channel revealed by targeted random mutagenesis of SecY. Proc. Natl. Acad. Sci. USA 98**:**5128-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori, H., and K. Ito. 2001. The Sec protein-translocation pathway. Trends Microbiol. 9**:**494-500. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama, K., M. Hanada, and H. Tokuda. 1994. Disruption of the gene encoding p12 (SecG) reveals the direct involvement and important function of SecG in the protein translocation of Escherichia coli at low temperature. EMBO J. 13**:**3272-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiyama, K., T. Suzuki, and H. Tokuda. 1996. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell 85**:**71-81. [DOI] [PubMed] [Google Scholar]
- 25.Pogliano, K. J., and J. Beckwith. 1993. The Cs sec mutants of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics 133**:**763-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramamurthy, V., and D. Oliver. 1997. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J. Biol. Chem. 272**:**23239-23246. [DOI] [PubMed] [Google Scholar]
- 27.Shimoike, T., Y. Akiyama, T. Baba, T. Taura, and K. Ito. 1992. SecY variants that interfere with Escherichia coli protein export in the presence of normal secY. Mol. Microbiol. 6**:**1205-1210. [DOI] [PubMed] [Google Scholar]
- 28.Shimoike, T., T. Taura, A. Kihara, T. Yoshihisa, Y. Akiyama, K. Cannon, and K. Ito. 1995. Product of a new gene, syd, functionally interacts with SecY when overproduced in Escherichia coli. J. Biol. Chem. 270**:**5519-5526. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, H., K. Nishiyama, and H. Tokuda. 1998. Coupled structure change of SecA and SecG revealed by the synthetic lethality of the secAcsR11 and ΔsecG::kan double mutant. Mol. Microbiol. 29**:**331-341. [DOI] [PubMed] [Google Scholar]
- 30.Swaving, J., K. H. M. van Wely, and A. J. M. Driessen. 1999. Preprotein translocation by a hybrid translocase composed of Escherichia coli and Bacillus subtilis subunits. J. Bacteriol. 181**:**7021-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takamatsu, H., S. Fuma, K. Nakamura, Y. Sadaie, A. Shinkai, S. Matsuyama, S. Mizushima, and K. Yamane. 1992. In vivo and in vitro characterization of the secA gene product of Bacillus subtilis. J. Bacteriol. 174**:**4308-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taura, T., T. Baba, Y. Akiyama, and K. Ito. 1993. Determinants of the quantitiy of the stable SecY complex in the Escherichia coli cell. J. Bacteriol. 175**:**7771-7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taura, T., T. Yoshihisa, and K. Ito. 1997. Protein translocation functions of Escherichia coli SecY: in vitro characterization of cold-sensitive secY mutants. Biochimie 79**:**517-521. [DOI] [PubMed] [Google Scholar]
- 34.Ueguchi, C., M. Wittekind, M. Nomura, Y. Akiyama, and K. Ito. 1989. The secY-rpmJ region of the spc ribosomal protein operon in Escherichia coli: structural alterations affecting secY expression. Mol. Gen. Genet. 217**:**1-5. [DOI] [PubMed] [Google Scholar]
- 35.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218**:**97-106. [PubMed] [Google Scholar]
- 36.Yatvin, M. B., K. M. Smith, and F. L. Siegel. 1986. Translocation of nascent non-signal sequence protein in heated Escherichia coli. J. Biol. Chem. 261**:**8070-8075. [PubMed] [Google Scholar]