A Quantitative Trait Locus Influencing Anxiety in the Laboratory Rat (original) (raw)
Abstract
A critical test for a gene that influences susceptibility to fear in animals is that it should have a consistent pattern of effects across a broad range of conditioned and unconditioned models of anxiety. Despite many years of research, definitive evidence that genetic effects operate in this way is lacking. The limited behavioral test regimes so far used in genetic mapping experiments and the lack of suitable multivariate methodologies have made it impossible to determine whether the quantitative trait loci (QTL) detected to date specifically influence fear-related traits. Here we report the first multivariate analysis to explore the genetic architecture of rodent behavior in a battery of animal models of anxiety. We have mapped QTLs in an F2 intercross of two rat strains, the Roman high and low avoidance rats, that have been selectively bred for differential response to fear. Multivariate analyses show that one locus, on rat chromosome 5, influences behavior in different models of anxiety. The QTL influences two-way active avoidance, conditioned fear, elevated plus maze, and open field activity but not acoustic startle response or defecation in a novel environment. The direction of effects of the QTL alleles and a coincidence between the behavioral profiles of anxiolytic drug and genetic action are consistent with the QTL containing at least one gene with a pleiotropic action on fear responses. As the neural basis of fear is conserved across species, we suggest that the QTL may have relevance to trait anxiety in humans.
Both pharmacological and genetic studies suggest that the neural basis of fear in animals underpins anxiety in humans. Therefore, major advances in our understanding of the neuronal basis of anxiety in humans followed the successful development of behavioral tests for investigating fear responses in rodents (Gray and McNaughton 2000; Lang et al. 2000; LeDoux 2000; McNaughton and Gray 2000). As a first step toward identifying the genetic basis of individual differences in response to fear-provoking stimuli in rodents, we, and others, have shown that by using crosses between inbred rodents, it is possible to map genetic loci that influence behavior in rodent models of anxiety (Flint et al. 1995; Moisan et al. 1996; Caldarone et al. 1997; Gershenfeld and Paul 1997; Wehner et al. 1997; Turri et al. 2001). However, in every genetic mapping experiment carried out to date, variation in rodent fear responses has been inferred from a limited number of behavioral tests.
Although it is often assumed that genetic effects on fear have a broad influence and that the loci so far detected will account for variation in conditioned responses such as the fear-potentiated startle and conditioned-avoidance paradigms favored in neurobiological investigation of emotion (LeDoux 2000; McNaughton and Gray 2000), this hypothesis has been difficult to test for a number of reasons. First, genetic mapping in rodents has, until recently, been easiest to carry out in the mouse, whereas investigation of the neuronal basis of fear and anxiety is based primarily on behavioral tests developed in the rat. Equivalent behavioral tests in the mouse can be found (Falls et al. 1997), but they are, in general, time consuming to carry out and not suited to the genetic mapping of fear, which requires analyzing large numbers of animals to detect the small genetic effects involved (Darvasi 1998). Tests of an animal's response to a novel, hence potentially threatening, environment (the open field, the elevated plus maze, and the light-dark box) can be relatively easily carried out on hundreds of mice, so most available genetic mapping data are for tests of this type. Mapping results derived from these tests alone may have limited applicability, as anxiety disorders in humans consists of more than pathological responses to fear of the unknown.
Second, it has been difficult to determine whether the quantitative trait loci (QTL) so far detected specifically influence fear and anxiety or other unrelated traits. For example, measures taken in the open field and elevated plus maze rely on differences in locomotor activity so that they reflect individual variation in both fear responses and spontaneous activity (Turri et al. 2001). The requisite multivariate analytical techniques have not been available to disentangle the genetic architecture of the traits and to test whether, as predicted for a genetic effect on fear responses (Ramos and Mormede 1998), a locus has a joint action on several behavioral measures or whether fear is multidimensional, consisting of independent traits, each with a limited domain of action.
With the development of dense genetic maps for the rat (McCarthy et al. 2000) and of appropriate multivariate tools (Knott and Haley 2000; Korol et al. 2001), it is now possible to ask whether the same genetic loci contribute to variation in different behavioral models of anxiety, including both conditioned fear and tests of novelty. Therefore, we set out to map QTL influencing fear-related behaviors in one of the most thoroughly documented animal models of anxiety, the Roman high and low avoidance rats (RHA/Verh and RLA/Verh, respectively), the product of bidirectional selection for two-way active avoidance acquisition in a shuttle box (Bignami 1965). Starting with a foundation population of commercially obtained Wistar albinos, rats were selected over five generations for speed of acquisition and retention of a conditioned-avoidance response. Compared to the unselected mean value of 105 avoidance response (out of 250 trials), selection increased the RHA rats' scores to a mean value of 171 and decreased the RLA scores to 51, a difference of about three standard deviations (Broadhurst and Bignami 1965). Inbreeding of the RHA and RLA rats was begun in 1993 and animals used in the experiments reported here were fully inbred.
The behavioral differences of the Roman rat strains are consistent with an interstrain variation in response to fear stimuli. In the shuttle box, RHA/Verh rats quickly acquire the active avoidance response, whereas RLA/Verh rats display much freezing and escape responses during the acquisition phase (Driscoll and Battig 1982; Escorihuela et al. 1995; Fernandez-Teruel et al. 1997). Results from other models of anxiety (the open field, elevated plus maze, and light-dark box and freezing to a conditioned stimulus) concur: Both inbred and outbred RHA/Verh rats are less anxious than their inbred or outbred RLA/Verh counterparts (Steimer et al. 1997; Driscoll et al. 1998; Escorihuela et al. 1999). Furthermore, differences in neuroendocrine responses support the view that the RLA/Verh animals are more susceptible to environmental stressors than the RHA/Verh rats, as would be expected from the strain that is more responsive to fear-provoking stimuli (Driscoll et al. 1998).
We used two approaches to define genetic influences on fear. First, we expect genetic effects on fear responses to work in a theoretically predictable fashion. Therefore, a QTL that influences two-way active avoidance should not only influence variation in conditioned fear as well, but the allele that decreases avoidance response in the shuttle box should also increase freezing to the conditioned stimulus. Additionally, the same QTL should increase fear-related behavior in the open field and elevated plus maze. Within these tests, the animal is presented with a choice between threatening and nonthreatening environments and the allelic effects of the QTL are expected to reflect this distinction. In the elevated plus maze, rats have a choice between two relatively fear-provoking regions (the open arms) and two relatively safe regions (the closed arms; Pellow et al. 1985; Hogg 1996; Rodgers and Dalvi 1997). Within the open field, there are thought to be distinctions in the level of threat the exposed area provides: The periphery is safer than the center. Consequently, an allele that decreases the number of entries and time spent on the open arms of the elevated plus maze should decrease activity in the center of the open field.
Second, we expect the genetic effects to be specific to fear responses and not to other behaviors. For instance, in the elevated plus maze, a QTL with a putative effect on fear responses is expected to have little or no influence on entries into the closed arms of the apparatus, a measure of activity. Nor should this QTL have an influence on spontaneous activity, which we measured in the home cage.
Therefore, we aimed to measure responses to fear-provoking stimuli from different perspectives and to employ multivariate techniques to determine whether loci act pleiotropically across all or a subset of the animal models of anxiety. We set out to determine specificity of action by including measures of spontaneous activity and by using multiple measures within each apparatus. Our experiment sought to identify common genetic effects that could be interpreted as influencing fear in rodents and, consequently, fear and anxiety in humans.
RESULTS
Univariate Analyses
In most cases we found that correlations between measures taken in the same test are highly significant and exceed 0.4, whereas correlations between tests are low, never exceeding 0.4 (see Table 1). These results, consistent with previous reports, indicate that common genetic effects, if present, are likely to be small. Because of the very high intratest correlations, for subsequent multivariate analyses we chose a subset of measures from the elevated plus maze (percentage of time spent on the open arms and number of entries into the closed arms) and shuttle box (avoidance responses).
Table 1.
Correlations between Phenotypes
| Open field activity in center | Open field activity in periphery | Elevated plus maze open arm entries | Elevated plus maze open arm time | Elevated plus maze closed arm entries | Elevated plus maze closed arm time | Acoustic startle response | Fear conditioning to context | Fear conditioning to cue | Shuttle box avoidances | Shuttle box intertrial crosses | Shuttle box latency | Defecation | Time grooming | Time rearing | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open field activity in periphery | −0.42 | ||||||||||||||
| Elevated plus maze open arm entries | 0.08 | 0.02 | |||||||||||||
| Elevated plus maze open arm time | 0.16 | 0.02 | 0.83 | ||||||||||||
| Elevated plus maze closed arm entries | 0.27 | −0.02 | −0.06 | 0.30 | |||||||||||
| Elevated plus maze closed arm time | −0.08 | −0.07 | −0.67 | −0.79 | −0.21 | ||||||||||
| Acoustic startle response | 0.05 | 0.00 | 0.05 | 0.01 | 0.01 | 0.00 | |||||||||
| Fear conditioning to context | −0.07 | 0.02 | −0.06 | −0.06 | 0.05 | 0.3 | 0.10 | ||||||||
| Fear conditioning to cue | −0.06 | 0.05 | −0.09 | −0.09 | −0.04 | 0.07 | 0.01 | 0.62 | |||||||
| Shuttle box avoidances | 0.06 | −0.03 | −0.02 | −0.05 | 0.03 | 0.01 | 0.03 | −0.15 | −0.17 | ||||||
| Shuttle box intertrial crosses | 0.08 | −0.05 | 0.03 | 0.02 | 0.04 | −0.06 | −0.01 | −0.10 | −0.17 | 0.75 | |||||
| Shuttle box latency | −0.04 | 0.01 | 0.02 | 0.05 | −0.02 | −0.02 | −0.04 | 0.14 | 0.17 | −0.87 | −0.72 | ||||
| Defecation | −0.10 | 0.09 | −0.02 | −0.10 | −0.13 | 0.08 | 0.09 | 0.14 | 0.15 | −0.05 | −0.12 | 0.07 | |||
| Time grooming | −0.06 | −0.01 | 0.03 | −0.10 | −0.22 | 0.25 | 0.07 | −0.09 | −0.09 | 0.07 | −0.05 | −0.07 | 0.11 | ||
| Time rearing | 0.35 | −0.05 | 0.11 | 0.15 | 0.20 | −0.06 | 0.04 | −0.23 | −0.20 | 0.14 | 0.17 | −0.15 | −0.12 | −0.04 | |
| Spontaneous activity | 0.00 | 0.03 | −0.01 | 0.00 | 0.03 | 0.01 | 0.05 | −0.12 | −0.13 | 0.20 | 0.19 | −0.18 | −0.23 | −0.12 | 0.14 |
A total of 908 F2 rats were genotyped. From 436 markers that amplified DNA from the parental strains, 82 polymorphic markers were obtained. The distribution of polymorphic markers across the genome was not random: Only 10% of 49 markers tested on chromosome 1 were polymorphic, compared to a third of those on chromosome 5 and half of those tested on chromosomes 12 and 13. Despite screening all available markers, only two were polymorphic on chromosomes 11 and 18. Overall, 75% of the genome was covered at a resolution of 15 cM or less.
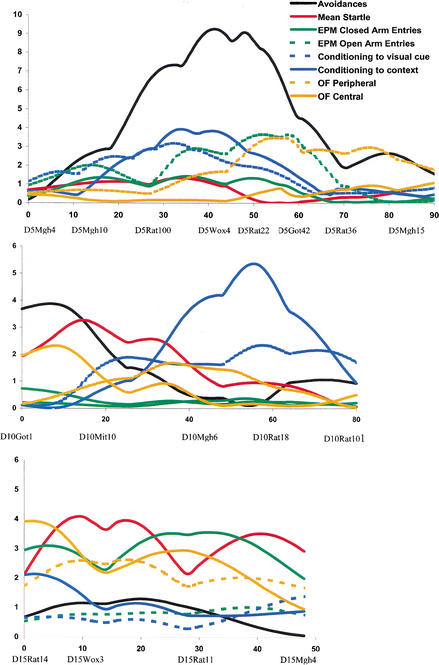
Table 2 shows the logarithm of odds (LOD) scores (in bold) for all behavioral measures on chromosomes with at least one chromosome exceeding a 5% significance level, as determined by a permutation test (Churchill and Doerge 1994). Eight loci were identified using MAPMAKER-QTL (Lincoln et al. 1992), with a variety of effects across tests. On chromosome 1, the QTL appears to influence rearing time only, whereas a QTL on chromosome 5 influences nine measures in six tests. Figure 1 shows LOD plots (MAPMAKER-QTL output; Lincoln et al. 1992) for several of the traits on chromosomes 5, 10, and 15. QTLs on chromosomes 5 and 10 account for 5.7% and 3.0%, respectively, of the total phenotypic variance of the avoidance response in the shuttle box, the measure on which the Roman rats were originally selected (Table 2). We found no evidence for significant dominance effects; all effects reported in Table 2 are additive.
Table 2.
LOD scores, additive effect sizes (“effect”), and percentage of the phenotypic variation explained (% var) for each quantitative trait loci
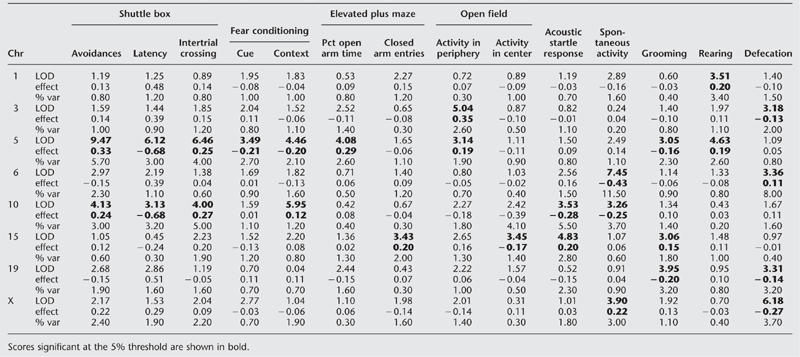
Figure 1.
LOD plots for single measures on chromosomes 5, 10, and 15. The horizontal distance shows the distance along the chromosome in centimorgans (cM) and the markers used in the study are shown.
Multivariate Analyses and a Test of Pleiotropy
We next mapped the traits jointly, using Multi-QTL (http://www.multiqtl.com). All traits were used in an initial analysis and significance was estimated by permutation (5000). Table 3 shows the results for chromosomes with a significance of <0.05. Eight QTLs were identified on the same chromosomes identified in the univariate analyses. However, these data do not address the question of the relative contribution of each trait to the QTL.
Table 3.
Multitrait Quantitative Trait Loci Mapping
| Chr | LOD | _P_-value | Position |
|---|---|---|---|
| 1 | 12.39 | 0.008 | 62 |
| 3 | 13.85 | 0.002 | 15 |
| 5 | 22.07 | <0.00001 | 44 |
| 6 | 18.23 | <0.00001 | 90 |
| 10 | 18.65 | <0.00001 | 24 |
| 15 | 15.55 | <0.00001 | 20 |
| 19 | 13.98 | 0.001 | 58 |
| X | 17.29 | <0.00001 | 28 |
To estimate the significance of a locus's contribution for the detection of a QTL and to test the significance of the QTL effect for each of the traits, we used methods that combine multivariate analysis with permutation techniques (Korol et al. 2001). The individual values of each phenotype are reshuffled relative to the other traits and genotypes, and the resulting data set is reanalyzed. Then, over a large number of permuted data sets (10,000 in our case) for each phenotype, the proportion of analyses is calculated in which the estimated QTL effect is greater than or equal to the QTL effect obtained with unpermuted data. The procedure is applied in a stepwise fashion, excluding the insignificant traits by creating a new data set without them and repeating the permutation.
Using this procedure, we took each QTL identified by the multivariate analysis and estimated the probability that each measure contributes to the LOD score. For example on chromosome 1, when all measures are analyzed together, activity in the periphery of the open field had the lowest probability (p = 0.84; see Table 4). Following the procedure of Korol et al. (2001), a new trait complex was constructed without this measure and the permutation test was repeated until the only remaining traits made significant contributions to the LOD score (at a 0.05 level). Table 4 shows the _P_-values for these analyses for all eight chromosomes bearing a QTL but omits the intermediate steps. Two columns are shown for each chromosome: The first displays the results when all traits are included in the analysis and the second when all but traits making a significant contribution have been removed (Korol et al. 2001).
Table 4.
Permutation Tests of Significance of the Contribution to a Multitrait LOD Score of Individual Measures
| Chr 1 | Chr 3 | Chr 5 | Chr 6 | |||
|---|---|---|---|---|---|---|
| Shuttle box Avoidances | 0.662 | 0.888 | 0.021 | 0.000 | 0.561 | |
| Fear conditioning Cue | 0.800 | 0.731 | 0.121 | 0.000 | 0.732 | |
| Context | 0.682 | 0.661 | 0.228 | 0.002 | 0.790 | |
| Elevated plus maze Pct open arm time | 0.552 | 0.551 | 0.203 | 0.000 | 0.832 | |
| Closed arm entries | 0.202 | 0.981 | 0.711 | 0.411 | ||
| Open field Activity in periphery | 0.842 | 0.222 | 0.000 | 0.129 | 0.000 | 0.953 |
| Activity in center | 0.732 | 0.718 | 0.841 | 0.289 | ||
| Acoustic startle response | 0.478 | 0.732 | 0.879 | 0.154 | ||
| Spontaneous activity | 0.017 | 0.030 | 0.863 | 0.861 | 0.014 | 0.000 |
| Grooming | 0.427 | 0.232 | 0.093 | 0.000 | 0.143 | |
| Rearing | 0.000 | 0.000 | 0.920 | 0.678 | 0.920 | |
| Defecation | 0.118 | 0.000 | 0.000 | 0.456 | 0.000 | 0.000 |
| Chr 10 | Chr 15 | Chr 19 | Chr X | |||
| Shuttle box Avoidances | 0.000 | 0.000 | 0.747 | 0.765 | 0.621 | |
| Fear conditioning Cue | 0.471 | 0.562 | 0.881 | 0.881 | ||
| Context | 0.555 | 0.000 | 0.444 | 0.831 | 0.923 | |
| Elevated plus maze Pct open arm time | 0.647 | 0.447 | 0.518 | 0.921 | ||
| Closed arm entries | 0.522 | 0.161 | 0.759 | 0.929 | ||
| Open field Activity in periphery | 0.059 | 0.645 | 0.800 | 0.038 | 0.000 | |
| Activity in center | 0.133 | 0.060 | 0.021 | 0.561 | 0.677 | |
| Acoustic startle response | 0.027 | 0.000 | 0.412 | 0.011 | 0.627 | 0.621 |
| Spontaneous activity | 0.237 | 0.761 | 0.929 | 0.510 | 0.019 | |
| Grooming | 0.521 | 0.000 | 0.000 | 0.033 | 0.000 | 1.000 |
| Rearing | 0.242 | 0.012 | 0.145 | 0.666 | 0.321 | |
| Defecation | 0.027 | 0.000 | 0.432 | 0.479 | 0.000 | 0.000 |
The multivariate analysis indicates that only three loci (on chromosomes 5, 10, and 15) have broad effects across different test measures. At other locations significant contributions to the LOD scores derive from a single (chromosome 19) or two phenotypes (chromosomes 1, 3, and 6). Both defecation and activity in a novel environment contribute to the LOD score on the X chromosome, but there is no significant contribution from the other measures of fear. Of the three potential candidates as loci influencing fear, the one on chromosome 15 has the most circumscribed effect. The evidence is strongest for an effect on grooming and there is no significant contribution from shuttle box, fear conditioning, or elevated plus maze to the LOD score.
The analyses do not distinguish a joint effect due to physical linkage of two QTL from the pleiotropic action of a single locus. We cannot use the location of the LOD scores for the univariate analyses to help here because the resolution of the F2 intercross is too low. The 95% confidence intervals for the QTLs of the size we have detected are in the region of 40 cM (Darvasi 1998). Therefore, we sought evidence for pleiotropic action on chromosomes 5, 10, and 15 using the multivariate regression method of Knott and Haley (2000). We chose those traits known to make a significant contribution to the LOD score for loci on chromosomes 5, 10, and 15 and tested the hypothesis of one QTL for each trait versus one QTL influencing all traits. The test statistic is based on the ratio of the determinants of the residual sum of squares matrix from the best pleiotropic QTL model to the residual sum of squares matrix from fitting a model in which a QTL affects each trait individually. In this test the null hypothesis is a single pleiotropic QTL. The estimates obtained from the best pleiotropic QTL model were used as parameters for replicate simulations. A test statistic was calculated from each of 1000 replicates and a significance threshold obtained. The test statistic from the original data set was compared with this threshold to determine significance. Table 5 gives the results of these analyses. We were able to reject the hypothesis of a single pleiotropic QTL on chromosome 10 at the 5% threshold.
Table 5.
Test of Pleiotropy Compared to Close Linkage
| Chr 5 | Chr 10 | Chr 15 | ||||
|---|---|---|---|---|---|---|
| Likelihood ratio | 5% Threshold | Likelihood ratio | 5% Threshold | Likelihood ratio | 5% Threshold | |
| One QTL for each | ||||||
| trait versus on QTL | ||||||
| influencing all traits | 9.38 | 23.24 | 20.81 | 18.53 | 12.72 | 16.72 |
Direction of Allelic Effects
The multivariate analyses indicated that the loci on chromosomes 5 and 15 have pleiotropic effects on fear responses. Next, we asked whether the alleles of these loci act in a manner consistent with this interpretation across all tests. For each QTL we looked at the direction of effect of the allele from the RHA/Verh strain. The direction of allelic effects is given by the sign associated with the effect size for each phenotype in Table 2.
On chromosome 5 the allele from the RHA/Verh rats increases avoidance responses and intertrial crossing while decreasing escape latency, consistent with the allele's origin from that strain and with a role in determining variation in fear. The allele's influence on other measures is also consistent with the hypothesis that it influences fear. It decreases conditioned freezing in response to both context and cue and increases the time spent in and number of entries into the open arms of the elevated plus maze. It increases rearing and activity in the periphery of the open field arena while decreasing time spent grooming in novel environments.
On chromosome 15 the allele increases acoustic startle responses, time spent grooming, and entries into the closed arms of the elevated plus maze but decreases activity in the center of the open field. Therefore, the QTL influences one measure of activity (entry into the closed arms of the elevated plus maze), as well as three measures of fear. It has little effect on other measures in the shuttle box or fear conditioning.
On chromosome 10, by contrast to the results on chromosome 5, the direction of allelic effects is inconsistent with a QTL that influences fear responses. The same allele that increases two-way active avoidance increases contextual conditioning and decreases the startle response. Presumably, the allele operating to increase avoidance response belongs to a different QTL, from that which influences fear conditioning. However, we cannot determine how many individual loci are operating and if any have pleiotropic action.
DISCUSSION
Our study is the first to exploit multivariate analyses to explore the genetic architecture of a battery of behavioral tests, all of which are used as animal models of anxiety. We have identified eight QTL, of which three (on chromosomes 5, 10, and 15), influence more than one behavioral measure of fear. Multivariate approaches were used to establish the significance of the contribution to the LOD score of each trait. These analyses provide evidence that loci on chromosomes 1, 3, 6, 19, and X have effects inconsistent with an influence on fear responses (for example, the QTL on chromosome 6 affects spontaneous activity and defecation in a novel environment), whereas the loci on chromosomes 5, 10, and 15 influence a broad range of measures of fear, as would be expected if they contain genes involved in determining a response to fear-provoking stimuli.
At each of the loci we have detected, multivariate analysis has been used to set the significance of the contribution from each trait (Korol et al. 2001) and show that, with the exception of the loci on chromosomes 5, 10, and 15, QTL effects are relatively specific. However, our analysis cannot exclude the existence of other QTL that have pleiotropic influences on fear but with such small effects that they are undetectable with the number of animals we have used.
A more difficult problem is to decide whether a joint genetic effect is due to the presence of a single pleiotropic QTL or to multiple linked genes. However, again using a novel multivariate statistic (Knott and Haley 2000), we have been able to show that multiple linked genes are more likely than pleiotropy on chromosome 10. At the other loci, on chromosomes 5 and 15, the test could not rule out pleiotropy.
Examination of the direction of QTL effects provides additional support for a QTL's influence on fear. The direction of allelic effects can be interpreted as indicating the presence of a gene that determines variation in fear responses at only one locus, on chromosome 5. Here, the allele that increases two-way active avoidance also decreases cue and contextual fear conditioning and grooming while increasing time in the open arms of the elevated plus maze and activity in the open field, as well as rearing. It has no discernible influence on spontaneous activity, the acoustic startle response, or defecation. This pattern is consistent with the action of a gene influencing an animal's reaction to a fear stimulus and parallels the effects of drugs used to treat anxiety disorders in humans (for reviews, see Gray 1977; Simon and Soubrie 1979; Fernandez-Teruel et al. 1991), which improve two-way active avoidance, block the acquisition of conditioned freezing, increase the time spent on and number of entries into the open arms of the elevated plus maze, and increase activity in the open field. Neither anxiolytic drugs nor the QTL affect acoustic startle response and defecation. The finding that genetic effects on defecation can be dissociated from other tests of fear is supported by our analysis of Maudsley rat strains, derived by selection for differences in open field defecation (Paterson et al. 2001).
How can the influence of other QTL be explained? Some of the inconsistencies of action are likely to be due to linked genes, as we have shown for chromosome 10, where the presence of at least two loci is required to explain an effect that decreases acoustic startle response and increases contextual fear conditioning. Multivariate analysis supports such a division but does not reveal how many genes there might be at this locus. Our analysis failed to rule out a pleiotropic locus on chromosome 15, but the fact that the QTL influences measures that do not cohere in any way predicted by current theories of the neuropsychology of anxiety suggests that the genetic effect may be due to multiple linked genes. At other locations, the QTL's influences are far too restricted to fit expectations. Using the results of the multivariate analyses, we find a QTL on chromosome 6 that influences defecation and spontaneous activity and loci on chromosomes 1 and 19 specific for rearing and grooming, respectively.
It is interesting that comparison with the genetic mapping carried out in mice for tests of novelty and conditioned fear shows no apparent overlap in the locations of QTL. In the mouse, QTL influencing behavior in the open field, elevated plus maze, light-dark box, and fear conditioning have been mapped to chromosomes 1, 3, 10, 12, and 15 (Caldarone et al. 1997; Gershenfeld and Paul 1997; Wehner et al. 1997; Turri et al. 2001). The syntenic regions in rat are chromosomes 13, 2, 7, 6, and 7, respectively (Blake et al. 2001), chromosomes devoid of QTL that have an effect on the same behaviors in rats. Crosses between inbred mouse and rat strains sample only a fraction of the genetic variation in the two species, so we cannot at this point say whether the failure to detect syntenic locations represents a difference in the genetic architecture of anxiety in mouse and rat or merely a failure to choose the right strain combination.
In summary, our results give rise to two conclusions. First, it is possible to detect QTL that have a consistent pattern of effects across a broad range of relevant tests of animal behavior. This is important because, despite many years of research, evidence that genetic effects operate in this way has been lacking. Based on the phenotypes that the QTL influences, the direction of effects of the QTL alleles, and a coincidence between the behavioral profiles of anxiolytic drug and genetic action, we argue that the QTL on rat chromosome 5 harbors a gene that influences fear behavior and that identification of the homologous gene in humans may lead to a better understanding of the neural basis of human anxiety. Second, our results are important for showing that many loci have narrow, often test-specific, ranges of influence, precluding ready functional interpretation. Our data show that a limited behavioral repertoire cannot be used reliably to infer a genetic effect on fear, whether that gene is a transgene or is contained within a QTL.
METHODS
F2 Intercross
The F2 generation Roman rats, derived from inbred RHA/Verh and RLA/Verh and equally divided between males and females, were bred in three batches over an 18-mo period. Behavioral testing was carried out separately for each batch. No significant effects were observed for batch or day-to-day variation in testing. Rats were maintained under controlled conditions of humidity (60% ± 10%) and temperature (22°C ± 2°C), a 12-h cycle (lights on at 8:00 am and lights off at 8:00 pm), and with free access to food and water. They were housed in groups of two (males) or three (females). Rats were tested at the age of 4 mo and male and females were evaluated together in a counterbalanced manner. A period of 10–20 d was allowed between consecutive tests. The experimental sequence was as follows.
Open Field
The apparatus was a beige circular arena (diameter = 83 cm), enclosed by white wood walls (height = 34 cm) and divided into 19 equal sectors. It was illuminated by a white 200-W bulb placed 90 cm over the center of the arena. Rats were placed in the center of the open field arena for a 5-min recording period. A computerized image analysis system (SMART, Panlab) was used to record distance covered in the center and the periphery of the open field and the latency to leave the center. Defecation was scored manually.
Elevated Plus Maze
The apparatus, made of black wood, consisted of two opposing open arms (50 × 10 cm), two opposing enclosed arms, (50 × 10 × 40 cm), and an open 10 × 10-cm square in the center, the whole being set 50 cm above the ground. Testing was carried out in ambient light. Rats were placed in the center of the plus maze facing an enclosed arm and behavior was measured for a 5-min period. The percentage of entries and time spent in the arms (open and enclosed) and defecations were scored.
Spontaneous Activity
Motor activity was measured in a multicage actimeter system (three cages simultaneously, Interface PANLAB 40035, Sensor Unit PANLAB 0603). Testing cages (transparent Plexiglas, 35 × 35 × 25 cm) were slightly different from the home cage and contained clean sawdust. Activity was automatically scored over a 30-min period.
Acoustic Startle Response
A Startle Response System (San Diego Institute) was used. Each animal was first placed in a Plexiglas cylinder (located within a 35 × 33 × 39-cm sound-attenuated chamber lit by a 20-W bulb). Cylinder movements resulting from startle responses were detected by an accelerometer. Acoustic stimuli of 110 dB for 50 msec were delivered by a loudspeaker, mounted at a distance of 23 cm above the Plexiglas cylinder. A fan located inside the sound-attenuated chamber provided background noise. Startle response amplitude was defined as the maximum accelerometer voltage during the first 200 msec following the startle-stimulus onset. Animals were tested pairwise, using two identical startle-response chambers. Each animal was given a 5-min acclimatization period before the first acoustic startle stimulus. Each testing session consisted of 20 startle stimuli, with an interstimulus interval of 30 sec. The mean startle response amplitude for the 20 trials was calculated for each animal.
Classical Fear Conditioning
The apparatus was a white chamber divided into two equal compartments (23 × 12 × 20 cm). A 1 mA scrambled electric footshock (0.5 sec; the unconditioned stimulus [US]) was administered through a grid floor. A 15-sec duration light from a 20-W bulb located in the upper part of a wall was the conditioned stimulus (CS). Training consisted of five CS-US pairings that started with the onset of the CS. US and CS terminated simultaneously. A 120-sec pseudorandom intertrial interval was used, along with a shock-free interlude of 1 min. After 24 h the rats were placed in the training chamber and freezing behavior was monitored for 10 min. For the first 5-min period the light was absent (to evaluate contextual fear conditioning). The light was then switched on for 5 min to measure fear conditioning to the CS.
Two-Way Active Avoidance Conditioning
The experiment was carried out with three identical shuttle boxes (Letica Institute), each one of them placed in independent, sound-attenuating boxes constructed of plywood. A fluorescent lamp provided dim and diffuse illumination. The shuttle boxes consisted of two equally sized compartments (25 × 25 cm, 28 cm), connected by an opening (8 × 10 cm). A 2400-Hz, 63-dB tone, plus a light (from a small 7-W lamp), functioned as the CS. The US, which started at the end of the CS, was a scrambled electric footshock of 0.7 mA delivered through the grid floor. Once rats were placed into the shuttle box, a 4-min familiarization period elapsed before starting training. After this period, 40 acquisition trials were administered. Each trial consisted of a 10-sec CS, followed by a 20-sec US. The CS or US were terminated when the animal crossed to the other compartment, with crossings during the CS being considered avoidance responses and crossings during the intertrial interval (ITI) considered as intertrial crossings. Once a crossing had been made and/or the shock (US) discontinued, a 1-min fixed ITI was presented.
Defecation, Rearing, and Grooming Time
Time spent self-grooming and number of fecal boli were recorded in the open field, in the elevated plus maze, and during habituation to the shuttle box. Time spent rearing (both free and against the wall) was recorded for the duration of the open field test and in the habituation phase of the shuttle box. Mean scores for each of the three were used in subsequent analyses.
Genotyping
DNA was extracted from tails and genotyped using standard techniques (Flint et al. 1995). We chose markers from the radiation hybrid map (Watanabe et al. 1999), aiming for intervals of between 20- and 30-cM intervals. The order of all markers was determined using the MAPMAKER software package (Lincoln et al. 1992) and results were compared with radiation hybrid maps (Watanabe et al. 1999).
Statistics
Data were analyzed by regression to assess mean differences as a function of sex and weight. Data were corrected for weight and sex by multiple regression. Standardized residuals were used in all subsequent analyses. We performed univariate analyses on each measure using the map distances derived from MAPMAKER by interval mapping (Lander and Botstein 1989) in QTL-MAPMAKER (Lincoln et al. 1992). Effect sizes and standard deviations were derived by bootstrapping the data (1000 bootstraps) in Multi-QTL (http://www.multiqtl.com; Korol et al. 2001). Significance levels were evaluated by permutation using the method of Churchill and Doerge (1994).
Multivariate analyses were performed using Multi-QTL. Significance levels were evaluated by permutation, carried out in Multi-QTL. To test between two linked QTL or one pleiotropic QTL, we used the method of Knott and Haley (2000). The test was carried out in a Fortran program kindly provided by Dr. Sara Knott. Traits for testing were chosen on the basis of whether they made a significant contribution to the LOD score, as determined by the Multi-QTL results. Genotype probabilities were generated using the program HAPPY (Mott et al. 2000).
Acknowledgments
This work was funded by an MRC LINK award in collaboration with Merck Sharp and Dohme, by the Wellcome Trust (J.F., A.B., A.N., R.M.), and by Dirección general de Investigaciones Científicas y Técnicas (DGICYT), Subdirección de Investigacion (SGR), and Fondo de Investigaciones Sanitarias (FIS) grants (A.T., A.F.-T.). R.A., L.G., and L.G.-Ll. are supported by a Dirección general de Investigaciones (DGR) fellowship, a Becas de Formación de Personal Investigador (BFPI) fellowship, and a Dirección general de Universidades (DGU) contract, respectively. Dr. Sara Knott kindly provided the Fortran program for performing the test of close linkage compared to pleiotropy. We thank M.-T. Bihoreau for providing markers for this study.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jf@molbiol.ox.ac.uk; FAX (44) 1861 287501.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.203402\. Article published online before print in March 2002.
REFERENCES
- Bignami G. Selection for high rates and low rates of avoidance conditioning in the rat. Anim Behav. 1965;13:221–227. doi: 10.1016/0003-3472(65)90038-2. [DOI] [PubMed] [Google Scholar]
- Blake JA, Eppig JT, Richardson JE, Bult CJ, Kadin JA, Group MGD. The Mouse Genome Database (MGD): Integration nexus for the laboratory mouse. Nucleic Acids Res. 2001;29:91–94. doi: 10.1093/nar/29.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst PL, Bignami G. Correlative effect of psychogenetic selection: A study of the Roman high and low avoidance strains of rats. Behav Res Ther. 1965;2:273–280. doi: 10.1016/0005-7967(64)90033-6. [DOI] [PubMed] [Google Scholar]
- Caldarone B, Saavedra C, Tartaglia K, Wehner JM, Dudek BC, Flaherty L. Quantitative trait loci analysis affecting contextual conditioning in mice. Nat Genet. 1997;17:335–337. doi: 10.1038/ng1197-335. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvasi A. Experimental strategies for the genetic dissection of complex traits in animal models. Nat Genet. 1998;18:19–24. doi: 10.1038/ng0198-19. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Battig K. Behavioral, emotional and neurochemical profiles of rats selected for extreme differences in active two way avoidance. In: Lieblich I, editor. Genetics of the Brain. Amsterdam, Netherlands: Elsevier; 1982. pp. 95–123. [Google Scholar]
- Driscoll P, Escorihuela RM, Fernandez-Teruel A, Giorgi O, Schwegler H, Steimer T, Wiersma A, Corda MG, Flint J, Koolhaas JM, et al. Genetic selection and differential stress responses—The Roman lines/strains of rats. Ann NY Acad Sci. 1998;851:501–510. doi: 10.1111/j.1749-6632.1998.tb09029.x. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Tobena A, Driscoll P, Fernandez-Teruel A. Effects of training, early handling, and perinatal flumazenil on shuttle box acquisition in Roman low-avoidance rats: Toward overcoming a genetic deficit. Neurosci Biobehav Rev. 1995;19:353–367. doi: 10.1016/0149-7634(94)00051-2. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Teruel A, Gil L, Aguilar R, Tobena A, Driscoll P. Inbred Roman high- and low-avoidance rats: Differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol Behav. 1999;67:19–26. doi: 10.1016/s0031-9384(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner JG, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behav Neurosci. 1997;111:855–861. [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Nunez JF, Zapata A, Boix F, Salazar W, Tobena A. The early acquisition of two-way (shuttle-box) avoidance as an anxiety-mediated behavior: Psychopharmacological validation. Brain Res Bull. 1991;26:173–176. doi: 10.1016/0361-9230(91)90205-x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Castellano B, Gonzalez B, Tobena A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: Focus on the Roman rat lines. Behav Genet. 1997;27:513–526. doi: 10.1023/a:1021400830503. [DOI] [PubMed] [Google Scholar]
- Flint J, Corley R, DeFries JC, Fulker DW, Gray JA, Miller S, Collins AC. A simple genetic basis for a complex psychological trait in laboratory mice. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- Gershenfeld HK, Paul SM. Mapping quantitative trait loci for fear-like behaviors in mice. Genomics. 1997;46:1–8. doi: 10.1006/geno.1997.5002. [DOI] [PubMed] [Google Scholar]
- Gray JA. Drug effects on fear and frustration: Possible limbic sites of action of minor tranquilizers. In: Iversen LL, et al., editors. Handbook of psychopharmacology. New York: Plenum; 1977. pp. 433–529. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Knott SA, Haley CS. Multitrait least squares for quantitative trait loci detection. Genetics. 2000;156:899–911. doi: 10.1093/genetics/156.2.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol AB, Ronin YI, Itskovich AM, Peng J, Nevo E. Enhanced efficiency of quantitative trait loci mapping analysis based on multivariate complexes of quantitative traits. Genetics. 2001;157:1789–1803. doi: 10.1093/genetics/157.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: Animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lincoln S, Daly M, Lander E. Mapping genes controlling quantitative traits with MAPMAKER/QTL 1.1. Cambridge, MA: Whitehead Institute Technical Report ; 1992. [Google Scholar]
- McCarthy LC, Bihoreau MT, Kiguwa SL, Browne J, Watanabe TK, Hishigaki H, Tsuji A, Kiel S, Webber C, Davis ME, et al. A whole-genome radiation hybrid panel and framework map of the rat genome. Mamm Genome. 2000;11:791–795. doi: 10.1007/s003350010132. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Courvoisier H, Bihoreau MT, Gauguier D, Hendley ED, Lathrop M, James MR, Mormede P. A major quantitative trait locus influences hyperactivity in the Wkha rat. Nat Genet. 1996;14:471–473. doi: 10.1038/ng1296-471. [DOI] [PubMed] [Google Scholar]
- Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. A method for fine mapping quantitative trait loci in outbred animal stocks. Proc Natl Acad Sci. 2000;97:12649–12654. doi: 10.1073/pnas.230304397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A, Whiting PJ, Gray JA, Flint J, Dawson GR. Lack of consistent behavioural effects of Maudsley reactive and non-reactive rats in a number of animal tests of anxiety and activity. Psychopharmacology. 2001;154:336–342. doi: 10.1007/s002130000640. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File S, Briley M. Validation of open:closed arms entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Meth. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Ramos A, Mormede P. Stress and emotionality: A multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Simon P, Soubrie P. Behavioral studies to differentiate anxiolytic and sedative activity of the tranquilizing drugs. In: Boisser JR, editor. Modern problems of pharmacopsychiatry: Differential psychopharmacology of anxiolytics and sedatives. Vol. 14. Basel, Switzerland: S. Karger; 1979. pp. 138–152. [PubMed] [Google Scholar]
- Steimer T, Driscoll P, Schulz PE. Brain metabolism of progesterone, coping behaviour and emotional reactivity in male rats from two psychogenetically selected lines. J Neuroendocrinol. 1997;9:169–175. doi: 10.1046/j.1365-2826.1997.t01-1-00571.x. [DOI] [PubMed] [Google Scholar]
- Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol. 2001;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Watanabe TK, Bihoreau MT, McCarthy LC, Kiguwa SL, Hishigaki H, Tsuji A, Browne J, Yamasaki Y, Mizoguchi-Miyakita A, Oga K, et al. A radiation hybrid map of the rat genome containing 5,255 markers. Nat Genet. 1999;22:27–36. doi: 10.1038/8737. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA, Rosmann ST, Christensen SC, Rasmussen DL, Fulker DW, Wiles M. Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet. 1997;17:331–334. doi: 10.1038/ng1197-331. [DOI] [PubMed] [Google Scholar]