Administration of plasmids expressing interleukin-4 and interleukin-10 causes BALB/c mice to induce a T helper 2-type response despite the expected T helper 1-type response with a low-dose infection of Leishmania major (original) (raw)
Abstract
BALB/c mice are susceptible to developing an infection with Leishmania major as a result of a fatal T helper 2 (Th2)-type response. However, mice infected with a low dose of parasites are reported to be able to overcome the lesions associated with a T helper 1 (Th1)-type response. To clarify why a difference in the dose of parasites induces a difference in the polarization of the Th phenotype, we first attempted to measure cytokine production. Soon after infection, the mice given high doses of parasites produced elevated levels of both Th1 [interferon-γ (IFN-γ)] and Th2 [interleukin (IL)-4 and IL-10] cytokines. However, when assessed at 1 and 2 weeks after infection, no significant difference in the balance of Th1 and Th2 cytokines could be detected between mice infected with low or high doses of L. major. These results support the notion that the Th2 cytokine levels at an early phase of infection could be a key factor for the induction of a Th2 response. In order to assess the efficacy of Th2 cytokines, the mice infected with low doses of L. major were co-administered IL-4 plasmid and IL-10 plasmid. Consequently, the mice (which originally exhibited a Th1 response) showed progressive disease and developed a Th2 response. However, administration of these plasmids at 7 days postinfection could not alter the Th polarization. Furthermore, production of IL-12 from the spleen cells stimulated by L. major was suppressed in the presence of IL-4 and IL-10. These results strongly suggest that the susceptibility to L. major in BALB/c mice depends on the persistence of Th2 cytokine levels at an early phase of infection.
Introduction
The differential expansion of functionally distinct CD4+ T-cell subsets in response to infection with Leishmania major determines the outcome of disease in murine hosts.1–3 A resistant C57BL/6 mouse strain is capable of controlling L. major infection as a result of an ability to expand T helper 1 (Th1)-type cells and thus increase their production of interferon-γ (IFN-γ), but susceptible BALB/c mice develop interleukin (IL)-4-secreting T helper 2 (Th2)-type cells rather than IFN-γ-producing Th1-type cells.4–6 It is well known that IL-4 and IFN-γ/IL-12 play a critical role in achieving Th2 and Th1 polarization, respectively. However, there are various factors influencing the development of the functionally polarized Th effector responses. For example, variations in the antigen doses can be a regulatory factor for T-cell differentiation, and the length of the antigen stimulation can also determine the elicited Th phenotype.7,8 In this context, high doses of antigen in vivo have been reported to favour the development of delayed-type hypersensitivity, presumably driven by Th1 polarization, while low doses of antigen tend to favour antibody production driven by Th2 polarization.9–12 However, the mouse strain also has a major impact on polarization and could reverse the antigen dosage effect. In this context, despite the marked susceptibility of BALB/c mice to infection with L. major, mice infected with a low dose of L. major have been shown to be able to control the infection and establish a long-lasting immunity.13 This finding is also consistent with the observation that in such mice a strong response of delayed-type hypersensitivity and immunoglobulin (Ig)G2a production is observed as a typical indicator of the Th1 response.14,15
In the present study, the role of cytokines was investigated as a trigger for the expansion of appropriate Th phenotypes in two distinct models of BALB/c mice. One model (the susceptible mice) revealed a susceptibility to high doses of L. major infection, while the other model (the resistant mice) acquired resistance with a low dose of parasites.15,16 From these models, preliminary data were obtained that the levels of Th2 cytokines (such as IL-4 and IL-10) in the early response may be related to the regulatory mechanisms of leishmanial diseases. To confirm whether IL-4 and/or IL-10 play a key role in triggering the Th2 response, an IL-4- and an IL-10-expressing plasmid were injected into BALB/c mice in which the Th1 response was expected to be induced with a low dose of L. major. The effects of these plasmids are discussed in the context of Th subtype polarization.
Materials and methods
Animals and parasites
Specific pathogen-free female BALB/c mice (6 weeks of age) were purchased from SLC (Shizuoka, Japan). Leishmanial infections were induced using stationary-phase promastigotes of L. major (MHOM/SU73/5KSKH) grown at 27° in Schneider medium, pH 6·5, (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 20% heat-inactivated fetal calf serum (FCS) (Lot no. AGD6389; HyClone, Logan, UT). The mice were injected in the left hind footpad with 1 × 102−1 × 106 stationary-phase promastigotes, and the course of the disease was monitored by weekly measurements of the footpad thickness using a dial gauge caliper. The experiments were performed according to the Guidelines of Animal Care from the Experimental Animal Center (National Defense Medical College, Tokorozawa, Saitama, Japan). Soluble leishmanial antigen (SLA) was prepared from promastigotes of L. major by four freeze–thaw cycles in phosphate-buffered saline (PBS) followed by centrifugation at 20 000 g for 10 min. The antigen was passed through a 0·2-µm filter and stored at −80° until use.
Plasmid
Murine IL-4 and IL-10 expression plasmids (designated pCAGGS IL-4 and pCAGGS IL-10, respectively) were constructed by inserting IL-4 DNA and IL-10 DNA into pCAGGS, an expression vector which contains the chicken β-actin promoter and rabbit β-globulin poly A.17 Unmanipulated pCAGGS was used as a control.
In vivo treatment with IL-4- and/or IL-10-expressing plasmids
BALB/c mice were administered IL-4 and IL-10 plasmids intradermally 10 mm distal from the tail base, as described previously,18 either 0 or 7 days after challenge with L. major. The groups of mice (n = 6–10 per group) received:
- 50 µl of PBS alone on day 0;
- 10 µg of control plasmid on day 0;
- IL-4 plasmid on day 0 (10 µg/50 µl of PBS);
- IL-10 plasmid on day 0 (10 µg/50 µl of PBS);
- IL-4 and IL-10 plasmids on day 0;
- IL-4 plasmid on day 0 and IL-10 plasmid at 7 days postinfection (p.i.);
- IL-10 plasmid on day 0 and IL-4 plasmid at 7 days p.i.; or
- IL-4 and IL-10 plasmids at 7 days p.i., respectively.
In vitro stimulation of lymph node cells (LNC)
Popliteal LNC were prepared from individual mice 1, 2, 4 and 15 weeks after the primary infection with L. major. Syngenic spleen cells γ-irradiated at 2000 rads were used as the antigen-presenting cells (APC). The LNC (2 × 106) from individual mice and APC (6 × 106) were dispensed in triplicate onto 24-well plates and cultured in 1 ml of RPMI-1640 with 10% FCS in the presence or absence of 50 µg/ml SLA. Seventy-two hours after culture at 37° in 5% CO2, the supernatants were collected and stored at −80° until required for cytokine assay. The levels of IFN-γ, IL-2, IL-4 and IL-10 in the culture supernatants were quantified by using a commercial enzyme-linked immunosorbent assay (ELISA) (PerSeptive Diagnostics, Cambridge, MA), according to the manufacturer's instructions.
Antibody isotype assay
The mice were bled 15 weeks after the primary infection, and the serum from individual mice was stored at −80° until use. For assay of antibodies, flat-bottom 96-well microtitre plates were precoated with 50 µl of 10 µg/ml SLA overnight at 4°, and the levels of IgG1 and IgG2a in the serum were evaluated on the plates by using a commercial mouse IgG isotyping kit (Zymed Laboratories, San Francisco, CA), according to the manufacturer's instructions.
In vitro assay of IL-12 production
Single-cell suspensions were prepared from the spleen of naive BALB/c mice after lysing the erythrocytes with ammonium chloride. The splenocytes (1 × 106 cells/ml), in 0·2 ml of RPMI-1640 supplemented with 10% charcoal adsorbed–heat-inactivated FCS, were placed onto a 96-well plate and then stimulated with live L. major (1 × 106/ml) for 24 hr at 37° in 5% CO2. Similarly, the cells were also stimulated with lipopolysaccharide (LPS) (10 ng/ml) as a control. To determine the inhibition efficacy of IL-4 and IL-10 on the production of IL-12, the cultures were maintained in the presence of 5 ng/ml of recombinant (r)IL-4 and/or rIL-10 (Pepro Tech, London, UK). After culture, the supernatants were collected for quantification of IL-12 (p40) using a commercial ELISA (BioSource, Camarillo, CA).
Statistical analyses
All data were expressed as the mean ± standard error of the mean (SEM) for each group. To assess the treatments, Dunnett's _t_-test was performed for comparison of the means by the General Linear Models of the Statistical Analysis System (SAS Institute Inc., Cary, NC). The reported _P_-values were two-sided, and _P_-values<0·05 were regarded as statistically significant.
Results
BALB/c mice infected with a low dose of L. major developed a protective Th1 response
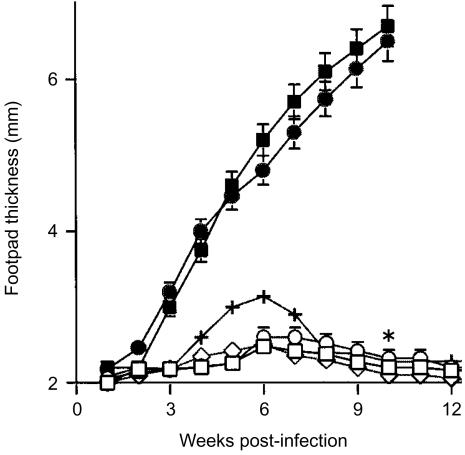
To confirm the effects of different levels of infectious doses on the response to L. major infection, BALB/c mice were infected at doses ranging from 1 × 102 to 1 × 106 parasites in the left hind footpad. As shown by Bretscher et al.13 and Doherty & Coffman,15 the mice infected with high doses of L. major developed a significant primary lesion, but in the groups of mice infected with low doses of parasites the lesion eventually ceased to increase in size at ≈6 weeks p.i. and thereafter resolved by 12 weeks p.i. (Fig. 1). Thus, the BALB/c mice were able to control low-level infections with L. major. In these mice, a typical Th1-type response was established, as judged from the following observations: (1) delayed type-hypersensitivity against SLA was inducible; (2) these mice were completely resistant to rechallenge with a lethal dose of parasites; and (3) the LNC from these mice produced a significant amount of IFN-γ but only a low level of IL-4 when stimulated with SLA (data not shown).
Figure 1.
BALB/c mice infected with low doses of Leishmania major induced a protective response to the disease. Groups of mice (n = 6–10 per group) were infected in the left hind footpad with 1 × 102 (□), 1 × 103 (○), 1 × 104 (+), 1 × 105 (▪) or 1 × 106 (•) L. major, and C57BL/6 mice were also infected with 1 × 106 parasites (◊) as a control for the protective response. The weekly measurements of footpad thickness represent the average score ± standard error of the mean (SEM) of at least six mice per group. *P<0·001 for the group of mice infected with 1 × 102 L. major compared to mice infected with 1 × 106 parasites 10 weeks postinfection, based on Dunnett's _t_-test.
Variations in the antigen dose are considered to be one of the factors influencing T-cell subset development.9 To explore whether or not the antigen doses themselves play an important role, irrespective of infectious doses of live L. major, we administered four times of 0, 0·5, 5·0 or 50 µg of SLA to groups of BALB/c mice intraperitoneally a week interval prior to infection. Ten days after the fourth injection, the mice were infected with a lethal dose of L. major. However, none of the immunized mice could alter the progressive pathology, nor induce a Th1 profile, as assessed by the cytokine milieu (data not shown). These results indicate that the protective response was governed by a low dose of live L. major, but not by the antigen load.
Elevations in the level of Th2 cytokines at an early phase of infection are essential for the induction of a Th2 response in _L. major_-infected mice
To assess the type of Th responses induced by infection of mice with different doses of L. major, antigen-specific cytokines from LNC were assayed at 4 weeks p.i. (Fig. 2). At this time-point, the cytokine responses already showed divergence, with IL-4 dominating in mice infected with high doses of L. major, and IFN-γ dominating in those infected with low doses. We next examined how the Th1 and Th2 phenotypes of the immune response were dependent on the cytokines produced during the course of the development of appropriate phenotypes. As the mice infected with L. major already exhibited appropriate Th responses at 4 weeks p.i., we assessed whether or not the responses were a result of the balance of cytokines that were produced in mice at relatively early infectious periods. At 1 and 2 weeks p.i., the antigen-specific IFN-γ, IL-2, IL-4 and IL-10 levels were compared with those in LNC from mice infected with low and high doses of L. major, respectively (Fig. 3). The relative balance of IL-4/IFN-γ was similar during these time-periods in both groups of mice, while their quantitative levels were elevated in the mice infected with high doses of parasites compared with those infected with low doses. In addition, at these time-points the cytokine profiles in neither group of mice indicated a Th1 or a Th2 phenotype. From these profiles, two points are suggested. First, the relative level of IFN-γ production following a high-dose infection invariably decreased after infection. Second, at around 4 weeks p.i., the IL-4 level had already significantly increased in the mice infected with high doses of parasites. These results indicate that the Th2 cytokine levels during the early infectious period may account for the ability to induce a Th2 phenotype in the _L. major_-infected mice.
Figure 2.
A T helper 1 (Th1)-type response in BALB/c mice, which had been infected with low doses of Leishmania major, was already established 4 weeks after infection. Single-cell suspensions from the popliteal lymph nodes of individual mice in the designated groups which had been infected with 1 × 102 (striped bars), 1 × 103 (dotted bars) or 1 × 106 (open bars) of L. major were prepared 4 weeks after infection. The cells were cultured in triplicate for 72 hr in the presence of soluble leishmanial antigen (SLA) and X-ray-irradiated splenocytes from syngenic mice as antigen-presenting cells (APC). After culture, the supernatants were collected and assayed for interferon-γ (IFN-γ) and interleukin (IL)-4 using an enzyme-linked immunosorbent assay (ELISA). Each bar represents the average ± standard error of the mean (SEM) for six mice per group. *P<0·001 compared with the group of mice infected with 1 × 106 parasites.
Figure 3.
At an early stage of infection, cytokine profiles of mice that had received low doses of Leishmania major were comparable to those of mice that had received high doses, except for the quantitative levels. Single-cell suspensions from the popliteal lymph nodes were prepared from mice infected with low (1 × 102) or high (1 × 106) doses of L. major at 1 and 2 weeks postinfection, respectively, and the cells were stimulated with soluble leishmanial antigen (SLA) in the presence of antigen-presenting cells (APC). After culture for 72 hr, the supernatants were collected and cytokine levels determined using an enzyme-linked immunosorbent assay (ELISA). Each bar represents the average ± standard deviation (SD) of triplicate analyses.
Administration of exogenous IL-4 contributed to the progression of leishmanial disease, but only transiently
As both IFN-γ and IL-4 were produced at relatively low levels during the early stages of infection in mice infected with low doses of L. major, an attempt to elevate the Th2 cytokine levels by exogenous IL-4 would be expected to induce Th2 responses. The mice were treated with continuous administration of rIL-4, for a 10-day period by a mini-osmotic pump implanted subcutaneously on the day of infection. The mice receiving IL-4 transiently showed disease progression for up to 6 weeks p.i., but the mice eventually healed by around 12 weeks p.i. (data not shown). The transient effects of exogenous IL-4 suggest that the mice appear to require some other factor for the development of a Th2 response or suppression of the Th1 response.
The combination of IL-4 plasmid and IL-10 plasmid played a critical role in the Th2 response during the initial infectious period
The lesions in mice infected with a low dose of L. major, and which received both IL-4 and IL-10 plasmids simultaneously on day 0, showed a linear increase in size for up to 15 weeks p.i., demonstrating the probable profiles of mice infected with a lethal dose of parasites (Fig. 4). However, the mice receiving IL-4 and IL-10 plasmids on day 0 showed a slightly slower lesion development in comparison to that seen in the mice infected with a high dose of parasites. This delay may be based on the doses of L. major in these groups of mice. In contrast, the co-administration of the two plasmids 7 days p.i. did not alter the disease progression. In addition, combinations of IL-4 plasmid on day 0 and IL-10 plasmid on day 7, or vice versa, did not affect lesion development. The treatment of mice with either IL-4 plasmid or IL-10 plasmid alone on day 0 had also little discernible effect on the disease (data not shown). These results indicate that IL-4 and IL-10 play a crucial role in the pathogenesis of this disease in mice.
Figure 4.
Co-administration of pCAGGS interleukin (IL)-4 and pCAGGS IL-10 significantly increased lesion development in BALB/c mice infected with a low dose of Leishmania major. BALB/c mice (n = 6 per group) infected with a low dose of L. major were injected intradermally with IL-4 and IL-10 plasmids (10 µg/mouse) via the tail at 0 or 7 days postinfection. The untreated mice were also infected with low or high doses of parasites in the same manner as the respective controls. The weekly measurements of footpad thickness represent the average score ± standard error of the mean (SEM) of six mice per group. *P < 0·001 for values of group of mice co-administered IL-4 and IL-10 plasmids in comparison to the control mice which received the control plasmid (unmanipulated pCAGGS) on day 0.
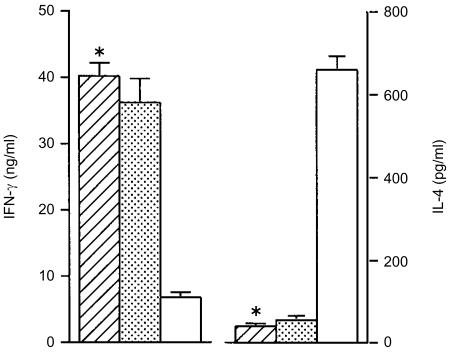
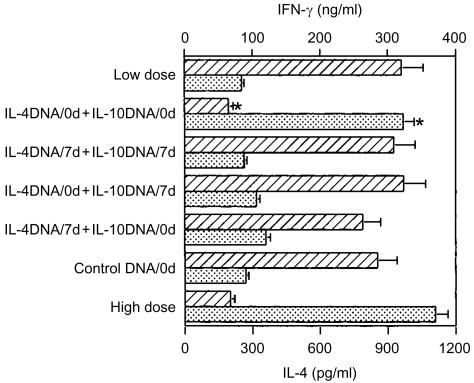
To determine whether or not the increasing course of the disease in the mice receiving IL-4 and IL-10 plasmids correlated with an induction of the Th2 response, we measured IFN-γ and IL-4 production from the LNC of individual mice at 15 weeks p.i. The mice which received both IL-4 and IL-10 plasmids at the time of infection developed a typical Th2 response with relatively high levels of IL-4 and a low level of IFN-γ (Fig. 5). Similarly, control BALB/c mice, infected with a lethal dose of L. major, showed a Th2 response with an IL-4-dominant profile. In contrast, the groups of mice which received plasmids 7 days p.i. or IL-4 plasmid or IL-10 plasmid alone did not show any features indicating a Th2-type response, with IFN-γ levels higher than those of IL-4, indicating that the composition of plasmids and the timing of administration are critical.
Figure 5.
Co-administration of interleukin (IL)-4 and IL-10 plasmids converted the cytokine profiles to T helper 2 (Th2) type in BALB/c mice in which the T helper 1 (Th1) type was expected to be induced with a low dose of Leishmania major. The single-cell suspensions from the popliteal lymph nodes of individual mice in the designated groups were prepared 15 weeks postinfection and then cultured in triplicate for 72 hr in the presence of soluble leishmanial antigen (SLA) and X-ray-irradiated splenocytes from syngenic mice as antigen-presenting cells (APC). The supernatants were collected and assayed for interferon-γ (IFN-γ) (striped bars) and IL-4 (dotted bars) using an enzyme-linked immunosorbent assay (ELISA). Each bar represents the average ± standard error of the mean (SEM) for six mice per group. *P < 0·001 compared to the group of control mice that received control (unmanipulated pCAGGS) plasmid.
The Th1 response is thought to be accompanied by the preferential production of IgG2a antibodies, whereas the dominating IgG1 antibodies characterize the Th2 response. Figure 6 shows the results of an antigen-specific serum IgG antibody analysis of the groups of mice which received IL-4 and IL-10 plasmids, IL-4 plasmid, IL-10 plasmid, an empty plasmid, or PBS alone, at 15 weeks p.i. The groups of mice receiving IL-4 and IL-10 plasmids and which were simultaneously infected with low doses of L. major, were found to have high levels of SLA-specific IgG1, similar to those of the mice infected with a lethal dose of parasites. On the other hand, the groups of mice that received both plasmids 7 days p.i. and healed the disease showed dominant levels of IgG2a, indicating a Th1 response. In addition, upon receiving the IL-4 and IL-10 plasmids 7 days p.i., or IL-4 plasmid or IL-10 plasmid alone on day 0, all groups of these mice developed a minimal antigen-specific IgG1 response. These results indicate a role for both IL-4 and IL-10 at the initial infectious period to enable L. major to induce a Th2 response in BALB/c mice.
Figure 6.
Co-administration of interleukin (IL)-4 and IL-10 plasmids enhanced the antigen-specific immunoglobulin (Ig)G1 antibody levels in BALB/c mice that had been infected with a low dose of Leishmania major. Soluble leishmanial antigen (SLA), specific serum IgG2a (striped bars) and IgG1 (dotted bars) levels were assessed by enzyme-linked immunosorbent assay (ELISA) 15 weeks after infection. The assays were performed at 1250-fold dilutions in triplicate for each serum sample. Serum from the BALB/c mice infected with a high dose of L. major was used as the standard for the IgG1-dominant profile. The values shown represent the mean ± standard error of the mean (SEM) of six mice per group. *P < 0·001 when comparing the levels of IgG1 and IgG2a, respectively, from the mice receiving plasmids versus those from the control mice which received empty plasmid (unmanipulated pCAGGS).
Combination of IL-4 and IL-10 suppressed the production of IL-12 in vitro
We next investigated the assumption that induction of the Th2 response with both IL-4 and IL-10 in BALB/c mice (infected with L. major) may be associated with the suppression of IL-12 production (Fig. 7). Significant IL-12 production was observed in live _L. major_-stimulated or LPS-stimulated splenocytes from BALB/c mice, whereas control cells without stimulation showed poor IL-12 production. The addition of rIL-4 and rIL-10 to the culture significantly inhibited the secretion of IL-12 from the _L. major_-stimulated splenocytes, while IL-4 or IL-10 alone partially decreased the production of IL-12. This observation supports the idea that IL-4 and IL-10 inhibit the induction of a Th1 response by suppressing IL-12 production while also promoting initiation of the Th2 response.
Figure 7.
Combination of interleukin (IL)-4 and IL-10 suppressed the production of IL-12 in vitro. The single cell suspensions of splenocytes from naive BALB/c mice were stimulated with live Leishmania major in complete RPMI-1640 media for 24 hr. The cells were also stimulated with lipopolysaccharide (LPS) as the control. For the inhibitory efficacy of IL-4 and IL-10 on the production of IL-12, the cells were cultured in the presence of recombinant IL-4 and rIL-10. After culture, the supernatants were collected and the IL-12 (p40) levels were quantified using an enzyme-linked immunosorbent assay (ELISA). The data shown represent the mean ± standard deviation (SD) of triplicate experiments. *P < 0·001 in comparison to the controls which were stimulated with L. major in the absence of IL-4 and IL-10.
Discussion
Th differentiation can be efficiently polarized into Th1 or Th2 phenotypes in vitro,19,20 as well as in vivo.21,22 In the present report, we demonstrated that differences in the inoculated dose of L. major induce distinct Th responses in BALB/c mice (i.e. Th2 responses were induced with a high dose of L. major, whereas a Th1 response was induced with a low dose). These results agree with those reported by Doherty & Coffman.15 Furthermore, and as suggested by Doherty & Coffman, we confirmed that this distinct polarization of Th phenotype does not simply reflect the difference in antigen load.15 There is an assumption that the profiles of Th1 and Th2 are determined at the early stages of infection. However, our results indicated that cytokine profiles at the early infectious period were not predictive of the subsequent adaptive immune response, Th2 and Th1 responses (Fig. 3). This raises the question in leishmanial diseases as to what is the key cytokine for developing a Th2 response or suppressing the Th1 response in susceptible BALB/c mice. As resistant C57BL/6 mice transiently produce IL-4 at the early infectious stage of an L. major infection,23–25 the possibility of suppressing the nascent Th2 response should be a pivotal event contributing to resistance in mice infected with a low dose of L. major. On the other hand, an elevated level of IFN-γ in BALB/c mice, which were infected with a lethal dose of parasites, was found at the early stages of infection, suggesting that IFN-γ could not affect the development of the Th2 response. Indeed, within the early infectious period it has become evident (from previous studies) that susceptible and resistant mice do not differ considerably regarding their levels of IFN-γ.26 These findings may also explain why the administration of rIFN-γ did not induce an improvement in the leishmanial disease in BALB/c mice.27 These results may thus indicate that the key factor in susceptibility to L. major is not a decrease in the IFN-γ production but an expansion of IL-4. Actually, an excessive quantity of IL-4 has been shown to be deleterious for the host in models of intracellular infections.28,29 These findings are supported by data reporting that depletion of IL-4 with anti-IL-4 antibody at the time of infection induces lesion healing and the development of a Th1 response in BALB/c mice.3,26 As a result, the early IL-4 response may play a decisive role in the lethal outcome of infection in BALB/c mice. We therefore expect that the continuous administration of rIL-4 by a mini-osmotic pump will lead to a non-healing state in BALB/c mice which are infected with a low dose of L. major. However, rIL-4 only increased the lesion development transiently, and all mice eventually healed under our experimental conditions. Similar findings have been reported previously,21 namely that infusion of exogenous IL-4 did not establish a Th2 response in genetically resistant mice that were infected with L. major. These data indicate that exogenous IL-4 failed to support a simple causal relationship between the expected function of IL-4 and the susceptibility to L. major infection in BALB/c mice.
On the other hand, IL-10 acts as an inhibitor for proinflammatory cytokines and IL-12 production, both in vitro30 and in vivo.31 Indeed, IL-10 was described as a Th2 cytokine that inhibits macrophage-dependent cytokine synthesis by Th1 cells.32,33 As the combination of IL-4 and IL-10 was reported to be able to inhibit Th1-cell development,33 we assessed whether exogenous IL-4 and IL-10 (administered together) could alter the Th balance in mice that were infected with low doses of L. major. The plasmid DNA encoding cytokine allows the cytokine to maintain its constant level of bioactivity and to be distributed at the site of infection.18,34 Therefore, a single dose of cytokine-expressing plasmid may help to produce an effective model for evaluating the cytokine function in murine leishmaniasis. We herein demonstrated that the administration of two plasmids changed resistant mice to susceptible mice, i.e. they demonstrated classical Th2 profiles with progressive footpad swelling and a cytokine milieu. These results demonstrate that IL-4 or IL-10 alone do not induce the mice to produce a Th2 phenotype, but together IL-4 and IL-10 may be sufficient to induce a Th2 response in the early infectious period and also maintain the response thereafter.
A question that emerges from the results of this report is why the combination of IL-4 and IL-10 is able to contribute to the Th2 response in the resistant mice while IL-4 or IL-10 alone was ineffective in altering the Th2 response. One possible explanation could be that IL-4 triggered Th2 cell expansion and IL-10 suppressed Th1 cell activity.31,35 However, a single injection of adenovirus expressing IL-4 has been reported to induce a progressive disease in a strain of mice resistant to L. major infection.36 This discrepancy may be related to differences in the mice models between the resistance-acquired BALB/c mice, which were infected with low doses of L. major in our study and the innate resistant strains of mice, B10D2, which were infected with high doses of parasites. In our report it is also possible that IL-4 and IL-10, when expressed in the resistant acquired mice may suppress the production of IL-12 and thus influence the Th2 response. Regarding the stimulation of naive T cells with Th2 cytokines in vitro, the Th2-polarized cells were observed to no longer be converted to a Th1 phenotype by restimulation with IL-12,37,38 probably owing to a defect in IL-12 signal transduction.39,40 In this context, the loss of IL-12 responsiveness by T cells in the early infectious period can critically determine the immune response which develops later during an L. major infection.41 In fact, we herein demonstrated that the addition of IL-4 and IL-10 suppressed the production of IL-12 from splenocytes stimulated with L. major.
In summary, this study demonstrated that for induction and maintenance of the Th2 response the levels of both IL-4 and IL-10 must be maintained from the initial infectious stage. We must further elucidate the functions of the Th2 cytokines involved in initiating and maintaining the Th2 response in order to develop appropriate therapies for treating infectious diseases where a Th2 response is detrimental to the host.
Abbreviations
APC
antigen-presenting cells
ELISA
enzyme-linked immunosorbent assay
IFN-γ
interferon-γ
IL
interleukin
LNC
lymph node cells
p.i.
postinfection
SLA
soluble leishmanial antigen
Th1
T helper 1
Th2
T helper 2
References
- 1.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Launois O, Conceicao-Silva F, Himmerlich H, Parra-Lopez C, Tacchini-Cottier F, Louis JA. Setting in motion the immune mechanisms underlying genetically determined resistance and susceptibility to infection with Leishmania major. Parasite Immunol. 1998;20:223–30. doi: 10.1046/j.1365-3024.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- 3.Chatelain R, Mauze S, Varkila K, Coffman RL. Leishmania major infection in interleukin-4 and interferon-γ depleted mice. Parasite Immunol. 1999;21:423–31. doi: 10.1046/j.1365-3024.1999.00240.x. 10.1046/j.1365-3024.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- 4.Constantinescu CS, Hondowicz BD, Elloso MM, Wysocka M, Trinchieri G, Scott P. The role of IL-12 in the maintenance of an established Th1 immune response in experimental leishmaniasis. Eur J Immunol. 1998;28:2227–33. doi: 10.1002/(SICI)1521-4141(199807)28:07<2227::AID-IMMU2227>3.0.CO;2-N. 10.1002/(sici)1521-4141(199807)28:07<2227::aid-immu2227>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Heinzel FP, Rerko RM, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–9. [PubMed] [Google Scholar]
- 6.Jones D, Elloso MM, Showe L, Williams D, Trinchieri G, Scott P. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major. Infect Immun. 1998;66:3818–24. doi: 10.1128/iai.66.8.3818-3824.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163:1205–13. [PubMed] [Google Scholar]
- 8.Tao X, Constant S, Jorritisma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–63. [PubMed] [Google Scholar]
- 9.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guery JC, Galbiati F, Smiroldo S, Adorini L. Selective development of T helper (Th)2 cells induced by continuous administration of low dose soluble proteins to normal and beta(2)-microglobulin-deficient BALB/c mice. J Exp Med. 1996;183:485–97. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruedl C, Bachmann MF, Kopf M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur J Immunol. 2000;30:2056–64. doi: 10.1002/1521-4141(200007)30:7<2056::AID-IMMU2056>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Morokata T, Ishikawa J, Yamada T. Antigen dose defines T helper 1 and T helper 2 responses in the lungs of C57BL/6 and BALB/c mice independently of splenic responses. Immunol Lett. 2000;72:119–26. doi: 10.1016/s0165-2478(00)00188-7. [DOI] [PubMed] [Google Scholar]
- 13.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohman H. Establishment of stable, cell-mediated immunity that makes ‘susceptible’ mice resistant to Leishmania major. Science. 1992;257:539–42. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 14.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-αβ-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty TM, Coffman RL. Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol. 1996;84:124–35. doi: 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 16.Menon JN, Bretscher PA. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol. 1998;28:4020–8. doi: 10.1002/(SICI)1521-4141(199812)28:12<4020::AID-IMMU4020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Nitta Y, Tashiro F, Tokui M, Shimada A, Takei I, Tabayashi K, Miyazaki J. Systemic delivery of interleukin 10 by intramuscular injection of expression plasmid DNA prevents autoimmune diabetes in nonobese diabetic mice. Hum Gene Ther. 1998;9:1701–7. doi: 10.1089/hum.1998.9.12-1701. [DOI] [PubMed] [Google Scholar]
- 18.Raz E, Carson DA, Parker SE, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–23. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primarily T cell proliferation in vitro. Eur J Immunol. 1999;29:1803–11. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. 10.1002/(sici)1521-4141(199906)29:06<1803::aid-immu1803>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Gomes NA, Barreto-de-Souza V, DosReis GA. Early in vitro priming of distinct Th cell subsets determines polarized growth of visceralizing Leishmania in macrophages. Int Immunol. 2000;12:1227–33. doi: 10.1093/intimm/12.9.1227. [DOI] [PubMed] [Google Scholar]
- 21.Sadick MD, Street N, Mosmann TR, Locksley RM. Cytokine regulation of murine leishmaniasis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect Immun. 1991;59:4710–4. doi: 10.1128/iai.59.12.4710-4714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sypeck JP, Chung CL, Mayor SEH, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–56. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1. Eur J Immunol. 1995;25:3298–307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 25.Launois P, Swihart KG, Milon G, Louis JA. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–24. [PubMed] [Google Scholar]
- 26.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171:115–27. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran TM, Isobe H, Fernandez-Sesma A, Schulman JL. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996;70:5230–5. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Shuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–67. [PubMed] [Google Scholar]
- 32.Chatelain R, Mauze S, Coffman RL. Experimental Leishmania major infection: role of IL-10. Parasite Immunol. 1999;21:211–8. doi: 10.1046/j.1365-3024.1999.00224.x. 10.1046/j.1365-3024.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 33.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:3043–9. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Z, Ertl HCJ. Manipulation of the immune response to plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–35. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 35.Himmelrich H, Launois P, Maillard I, Biedermann T, Taccini-Cottier F, Locksley RM, Röcken M, Louis JA. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol. 2000;164:4819–25. doi: 10.4049/jimmunol.164.9.4819. [DOI] [PubMed] [Google Scholar]
- 36.Gabaglia CR, Pedersen B, Hitt M, Burdin N, Sercarz EE, Graham FL, Gauldie J, Braciak TA. A single intramuscular injection with an adenovirus-expressing IL-12 protects BALB/c mice against Leishmania major infection, while treatment with an IL-4-expressing vector increases disease susceptibility in B10.D2 mice. J Immunol. 1999;162:753–60. [PubMed] [Google Scholar]
- 37.Perez VL, Lederer JA, Lichtman AH, Abbas AK. Stability of Th1 and Th2 populations. Int Immunol. 1995;7:869–75. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- 38.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–13. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broek MVD, Bachman MF, Köhler G, Barner M, Escher R, Zinkernagel R, Kopf M. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-γ and nitric oxide synthetase 2. J Immunol. 2000;164:371–8. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 40.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:666–75. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 41.Güler ML, Gorham JD, Hsieh CS, Mackey AJ, Steen RG, Dietrich WF, Murphy KM. Genetic susceptibility to Leishmania: IL-12 responsiveness in Th1 cell development. Science. 1996;271:984–90. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]